Abstract
The leucocyte antigen CD23 is expressed by B lymphocytes following activation by a number of stimuli and functions as an IgE receptor, and in its soluble form, as a putative B cell growth factor. The expression of CD23 on the surface of lymphocytes in paraffin wax sections of synovial biopsy specimens was studied using a novel mouse monoclonal antibody, BU38. Specimens were investigated from nine cases of rheumatoid arthritis, six cases of osteoarthritis, and eight cases of chronic inflammation in articular and non-articular tissues. CD23 was expressed on a high proportion of lymphocytes in all forms of chronic inflammation and was not specific for rheumatoid arthritis. It may be a characteristic feature of any chronic inflammatory response. As CD23 was found on the surface of lymphocytes in many cases of these arthritides, sCD23 in serum or synovial fluid may yet prove a useful marker for the severity of the inflammatory infiltrate.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cairns J., Flores-Romo L., Millsum M. J., Guy G. R., Gillis S., Ledbetter J. A., Gordon J. Soluble CD23 is released by B lymphocytes cycling in response to interleukin 4 and anti-Bp50 (CDw40). Eur J Immunol. 1988 Mar;18(3):349–353. doi: 10.1002/eji.1830180305. [DOI] [PubMed] [Google Scholar]
- Combe B., Andary M., Klein B., Clot J., Sany J. Regulation of interleukin-2 production in rheumatoid arthritis. J Rheumatol. 1987 Apr;14(2):226–229. [PubMed] [Google Scholar]
- Combe B., Pope R. M., Fischbach M., Darnell B., Baron S., Talal N. Interleukin-2 in rheumatoid arthritis: production of and response to interleukin-2 in rheumatoid synovial fluid, synovial tissue and peripheral blood. Clin Exp Immunol. 1985 Mar;59(3):520–528. [PMC free article] [PubMed] [Google Scholar]
- Depper J. M., Bluestein H. G., Zvaifler N. J. Impaired regulation of Epstein-Barr virus-induced lymphocyte proliferation in rheumatoid arthritis is due to a T cell defect. J Immunol. 1981 Nov;127(5):1899–1902. [PubMed] [Google Scholar]
- Depper J. M., Zvaifler N. J. Epstein-Barr virus. Its relationship to the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 1981 Jun;24(6):755–761. doi: 10.1002/art.1780240601. [DOI] [PubMed] [Google Scholar]
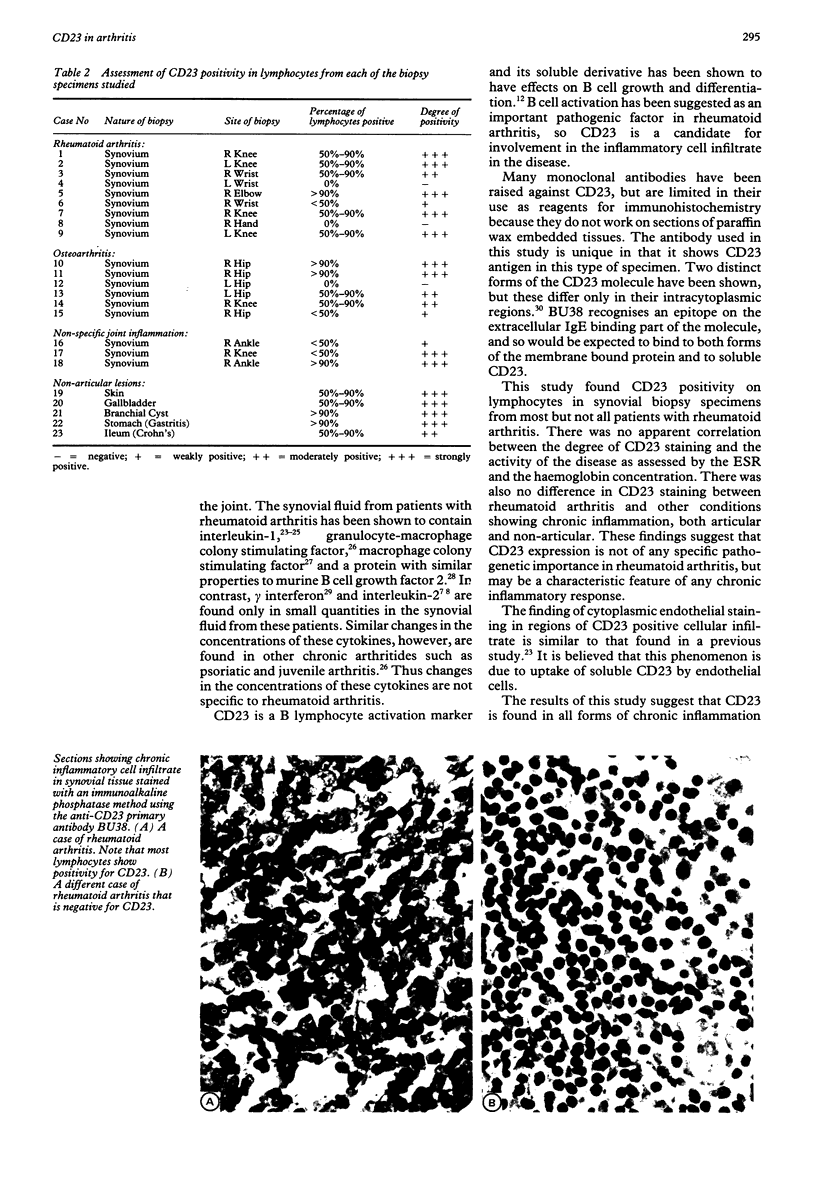
- Duke O., Panayi G. S., Janossy G., Poulter L. W. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982 Jul;49(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Xu W. D., Townsend K., Broide D., Alvaro-Gracia J., Glasebrook A., Zvaifler N. J. Cytokines in chronic inflammatory arthritis. I. Failure to detect T cell lymphokines (interleukin 2 and interleukin 3) and presence of macrophage colony-stimulating factor (CSF-1) and a novel mast cell growth factor in rheumatoid synovitis. J Exp Med. 1988 Nov 1;168(5):1573–1586. doi: 10.1084/jem.168.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. Peripheral blood and synovial fluid monocyte activation in inflammatory arthritis. II. Low levels of synovial fluid and synovial tissue interferon suggest that gamma-interferon is not the primary macrophage activating factor. Arthritis Rheum. 1987 Aug;30(8):864–871. doi: 10.1002/art.1780300804. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. The pathogenesis of rheumatoid arthritis. A critical assessment of the role of autologous stimulation in the perpetuation of rheumatoid synovitis. Rheum Dis Clin North Am. 1987 Dec;13(3):447–461. [PubMed] [Google Scholar]
- Fontana A., Hengartner H., Weber E., Fehr K., Grob P. J., Cohen G. Interleukin 1 activity in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int. 1982;2(2):49–53. doi: 10.1007/BF00541245. [DOI] [PubMed] [Google Scholar]
- Gordon J., Flores-Romo L., Cairns J. A., Millsum M. J., Lane P. J., Johnson G. D., MacLennan I. C. CD23: a multi-functional receptor/lymphokine? Immunol Today. 1989 May;10(5):153–157. doi: 10.1016/0167-5699(89)90171-0. [DOI] [PubMed] [Google Scholar]
- Gordon J., Rowe M., Walker L., Guy G. Ligation of the CD23,p45 (BLAST-2,EBVCS) antigen triggers the cell-cycle progression of activated B lymphocytes. Eur J Immunol. 1986 Sep;16(9):1075–1080. doi: 10.1002/eji.1830160908. [DOI] [PubMed] [Google Scholar]
- Kikutani H., Inui S., Sato R., Barsumian E. L., Owaki H., Yamasaki K., Kaisho T., Uchibayashi N., Hardy R. R., Hirano T. Molecular structure of human lymphocyte receptor for immunoglobulin E. Cell. 1986 Dec 5;47(5):657–665. doi: 10.1016/0092-8674(86)90508-8. [DOI] [PubMed] [Google Scholar]
- Kurosaka M., Ziff M. Immunoelectron microscopic study of the distribution of T cell subsets in rheumatoid synovium. J Exp Med. 1983 Oct 1;158(4):1191–1210. doi: 10.1084/jem.158.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. W., Simmons C. F., Jr, Wileman T., Geha R. S. Intracellular cleavage of newly synthesized low affinity Fc epsilon receptor (Fc epsilon R2) provides a second pathway for the generation of the 28-kDa soluble Fc epsilon R2 fragment. J Immunol. 1989 Mar 1;142(5):1614–1620. [PubMed] [Google Scholar]
- Lindblad S., Hedfors E. Arthroscopic and immunohistologic characterization of knee joint synovitis in osteoarthritis. Arthritis Rheum. 1987 Oct;30(10):1081–1088. doi: 10.1002/art.1780301001. [DOI] [PubMed] [Google Scholar]
- Lindblad S., Hedfors E. The synovial membrane of healthy individuals--immunohistochemical overlap with synovitis. Clin Exp Immunol. 1987 Jul;69(1):41–47. [PMC free article] [PubMed] [Google Scholar]
- Miossec P., Dinarello C. A., Ziff M. Interleukin-1 lymphocyte chemotactic activity in rheumatoid arthritis synovial fluid. Arthritis Rheum. 1986 Apr;29(4):461–470. doi: 10.1002/art.1780290402. [DOI] [PubMed] [Google Scholar]
- Rowlands D. C., Hansel T. T., Crocker J. Immunohistochemical determination of CD23 expression in Hodgkin's disease using paraffin sections. J Pathol. 1990 Mar;160(3):239–243. doi: 10.1002/path.1711600310. [DOI] [PubMed] [Google Scholar]
- Stastny P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N Engl J Med. 1978 Apr 20;298(16):869–871. doi: 10.1056/NEJM197804202981602. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Mann K. P. Early events in Epstein-Barr virus infection provide a model for B cell activation. J Exp Med. 1985 Jul 1;162(1):45–59. doi: 10.1084/jem.162.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Swendeman S. L., Edson C. M. Biochemical analysis suggests distinct functional roles for the BLAST-1 and BLAST-2 antigens. J Immunol. 1986 Mar 1;136(5):1745–1751. [PubMed] [Google Scholar]
- Tosato G., Steinberg A. D., Yarchoan R., Heilman C. A., Pike S. E., De Seau V., Blaese R. M. Abnormally elevated frequency of Epstein-Barr virus-infected B cells in the blood of patients with rheumatoid arthritis. J Clin Invest. 1984 Jun;73(6):1789–1795. doi: 10.1172/JCI111388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchibayashi N., Kikutani H., Barsumian E. L., Hauptmann R., Schneider F. J., Schwendenwein R., Sommergruber W., Spevak W., Maurer-Fogy I., Suemura M. Recombinant soluble Fc epsilon receptor II (Fc epsilon RII/CD23) has IgE binding activity but no B cell growth promoting activity. J Immunol. 1989 Jun 1;142(11):3901–3908. [PubMed] [Google Scholar]
- Wessels H. P., Spiess M. Insertion of a multispanning membrane protein occurs sequentially and requires only one signal sequence. Cell. 1988 Oct 7;55(1):61–70. doi: 10.1016/0092-8674(88)90009-8. [DOI] [PubMed] [Google Scholar]
- Wood D. D., Ihrie E. J., Dinarello C. A., Cohen P. L. Isolation of an interleukin-1-like factor from human joint effusions. Arthritis Rheum. 1983 Aug;26(8):975–983. doi: 10.1002/art.1780260806. [DOI] [PubMed] [Google Scholar]
- Yukawa K., Kikutani H., Owaki H., Yamasaki K., Yokota A., Nakamura H., Barsumian E. L., Hardy R. R., Suemura M., Kishimoto T. A B cell-specific differentiation antigen, CD23, is a receptor for IgE (Fc epsilon R) on lymphocytes. J Immunol. 1987 Apr 15;138(8):2576–2580. [PubMed] [Google Scholar]
- Zoschke D., Segall M. Dw subtypes of DR4 in rheumatoid arthritis: evidence for a preferential association with Dw4. Hum Immunol. 1986 Jan;15(1):118–124. doi: 10.1016/0198-8859(86)90322-8. [DOI] [PubMed] [Google Scholar]




