Abstract
Natural killer (NK) cells are a subset of lymphocytes that contribute to innate immunity through cytokine secretion and target cell lysis. NK cell function is regulated by a multiplicity of activating and inhibitory receptors. The advance in instrumentation for multi-color flow cytometry and the generation of specific mAbs for different epitopes related to phenotypic and functional parameters have facilitated our understanding of NK cell responses. Here, we provide protocols for flow cytometric evaluation of degranulation and cytokine production by human NK cells from peripheral blood at the single cell level. In addition to offering insight into the regulation of human NK cell responses, these techniques are applicable to the assessment of various clinical conditions, including the diagnosis of immunodeficiency syndromes.
Keywords: Human, natural killer cells, immunophenotyping, polychromatic flow cytometry, CD107a, lysosomal-associated membrane protein-1, chemokines, IFN-γ, MIP-1β, TNF-α
1. Introduction
Natural killer (NK) cells are a subset of lymphocytes that participate in innate resistance to infected and neoplastic cells (1). Moreover, NK cells interface with adaptive immunity through physical interactions with dendritic cells and T cells, and by their ability to secrete specific cytokines (2, 3). NK cell function is regulated by a multiplicity of activating and inhibitory receptors (4). Upon activation, NK cells kill sensitive target cells by directed exocytosis of perforin-containing secretory lysosomes, also called cytotoxic granules. In addition to their cytolytic function, NK cells may secrete chemokines and cytokines. The soluble factors secreted by NK cells, such as MIP-1β, TNF-α and IFN-γ, recruit other immune cells, promote cellular resistance to infection, and influence adaptive immunity. Due to the important role of NK cells in immunity, analysis of NK cell responses is emerging as a valuable tool for assessment of various clinical conditions, e.g. post-stem cell transplantation to assess the risk of infection and in the diagnosis of immunodeficiency syndromes.
In this section, we present protocols and provide advice regarding flow cytometric assessment of NK cell functional read-outs. Flow cytometry represents a sensitive and quantitative technique readily applicable to phenotypic and functional characterization of lymphocytes. Several well-characterized mAbs to receptors and other surface molecules have been generated that facilitate assessment of NK cell responses and discrimination of NK cell subsets. Specifically, secretory lysosome release can be quantified by the induced cell surface expression of transmembrane proteins that usually reside in secretory lysosomes. In unactivated cytotoxic lymphocytes, CD63 (lysosomal-associated membrane protein 3, LAMP-3), CD107a (LAMP-1), CD107b (LAMP-2), and CD178 (Fas ligand) reside in secretory lysosomes (5, 6). In NK cells from healthy donors, CD107a co-localizes with perforin and appearance of CD107a at the NK cell surface occurs upon lysis of susceptible target cells (7, 8). Defective induction of CD107a surface expression is associated with certain subtypes of hyper-inflammatory immunodeficiency syndromes (9, 10). Combined assessment of de novo transcriptional responses, such as chemokine and cytokine synthesis, with degranulation promises to unravel novel signaling components associated with human immunodeficiencies affecting transcriptional responses or globally impairing lymphocyte effector functions. In addition, these tools should provide insight into how NK cell responses are regulated during other clinical and pathological conditions.
2. Materials
2.1. Cells, media, and solutions
Whole blood collected in sodium heparin vials (3 to 10 mL is sufficient for multiple functional experiments), or buffy coats.
Lymphoprep (Axis-Shield, Oslo, Norway) stored at room temperature and protected from light.
Optional: NK cell negative isolation kit (Miltenyi, Bergisch Gladbach, Germany).
Complete culture medium: RPMI-1640, supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1 mM L-glutamine (all Invitrogen, Carlsbad, CA).
Target cells: The human erythroleukemia cell line K562 and the murine Fc receptor+ mastocytoma cell line P815 (both American Tissue Type Collection, Manassas, VA) are maintained in complete culture medium.
Staining solution: Phosphate-buffered saline (PBS) supplemented with 2% heat-inactivated FBS and 2 mM ethylenediamine tetraacetic acid (EDTA).
Fixation solution: PBS supplemented with 2% (w/v) paraformaldehyde (Sigma, St. Louis, MO).
Permeabilization solution: PBS supplemented with 2% heat-inactivated FBS, 2 mM EDTA, and 0.5% saponin (Sigma).
2.2. Antibodies and fluorescent reagents
2.2.1. Stimulating mAbs for cellular assays
Anti-CD3 mAb (clone SK7, for stimulation of T cell responses) purified (BD Bioscience).
Anti-CD3 mAb (clone SK7) PerCP (BD Bioscience).
Anti-CD16 mAb (clone 3G8) purified (BD Bioscience).
2.2.2. Staining mAbs for 2-hour degranulation assay
Anti-CD3 mAb (clone SK7) PerCP (BD Bioscience, Franklin Lakes, NJ).
Anti-CD56 mAb (clone NCAM 16.2) PE (BD Bioscience).
Anti-CD107a mAb (clone H4A3) FITC (BD Bioscience).
Optional: Anti-CD8 mAb (clone SK1) APC (BD Bioscience).
2.2.3. Staining mAbs and fluorescent reagents for 6-hour multiple response assay
Anti-CD3 mAb (clone UCHT1) Cascade Yellow (Dako, Glostrup, Denmark).
Anti-CD14 mAb (clone MΦP3) APC-Cy7 (BD Bioscience).
Anti-CD19 mAb (clone SJ25C1) APC-Cy7 (BD Bioscience).
Anti-CD56 mAb (clone NCAM 16.2) PE-Cy7 (BD Bioscience).
Anti-CD107a mAb (clone H4A3) biotin (BD Bioscience).
Anti-IFN-γ mAb (clone 25723.11) FITC (BD Bioscience).
Anti-MIP-1β mAb (clone D21-1351) PE (BD Bioscience).
Anti-TNF-α mAb (clone MAb11) Pacific Blue (eBioscience, San Diego, CA).
Qdot 605 Streptavidin-conjugate (Invitrogen).
LIVE/DEAD Fixable Far Red Dead Cell Stain Kit (Invitrogen).
2.3. Flow cytometry hardware and software
For analysis of degranulation with the three-color flow cytometry panel outlined in this chapter, a FACS Calibur (BD Bioscience) with a 488 nm laser is sufficient. Table 1 provides a detailed description of the filter setup and utilized detectors.
Analysis of multiple functional responses with the seven-color flow cytometry staining panel described here is optimized for a CyAn ADP 9 Color Analyzer (Beckman Coulter, Fullerton, CA) equipped with a 405 nm laser, a 488 nm laser, and a 635 nm laser. Table 2 provides a detailed description of the filter setup and utilized detectors (11).
FlowJo software (version 8.7, TreeStar, Ashland, OR) for analysis of acquired raw data.
Simplified Presentation of Incredibly Complex Evaluations (SPICE) software (version 4.1.6, courtesy of Mario Roederer, Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD) for processing and presentation of analyzed raw data.
Table 1.
Instrument configuration and antibody panel
| Laser (nm) | Detector | Filter (nm) | Fluorochrome | Utilized panel | Dilution |
|---|---|---|---|---|---|
| 488 | FL1 | 530/30 | FITC | CD107a | 1:50 |
| 488 | FL2 | 585/42 | PE | CD56 | 1:50 |
| 488 | FL3 | 670/LP | PerCP | CD3 | 1:50 |
| 635 | FL4 | 661/16 | APC | available |
Table 2.
Instrument configuration and antibody panel
| Laser (nm) | Detector | Filter (nm) | Fluorochrome | Utilized panel | Dilution |
|---|---|---|---|---|---|
| 488 | FL1 | 530/40 | FITC | IFN-γ | 5:50 |
| 488 | FL2 | 575/25 | PE | MIP-1β | 2:50 |
| 488 | FL3 | 613/20 | Qdot 605 | CD107a | 2:50 |
| 488 | FL4 | 630/80 | PerCP | available | |
| 488 | FL5 | 750LP | PE-Cy7 | CD56 | 1:50 |
| 405 | FL6 | 450/50 | PacB | TNF-α | 3:50 |
| 405 | FL7 | 550/30 | CasY | CD3 | 1:50 |
| 635 | FL8 | 665/20 | APC | available | |
| 635 | FL9 | 750LP | APC-Cy7 | CD14/19/DCM | 2:50 |
2.4. Other material
GolgiPlug (protein transport inhibitor containing brefeldin A, BD Bioscience).
GolgiStop (protein transport inhibitor containing monensin, BD Bioscience).
Anti-mouse Ig κ compensation beads (BD Bioscience).
3. Methods
Upon stimulation by sensitive target cells, NK cells rapidly release cytotoxic proteins by polarized fusion of secretory lysosomes with the plasma membrane. Secretion of chemokines and cytokines is a slower process, requires transcription and de novo protein synthesis, and follows different vesicular pathways. Thus, the temporal kinetics of responses must be taken into consideration when designing experiments that assess distinct NK cell functional parameters.
As a note of caution, although NK cell degranulation is a prerequisite for NK cell cytotoxicity, assessment of degranulation does not necessarily correlate with target cell lysis. Target cell lysis depends not only on the extent of effector cell degranulation, but also on the content of secretory lysosomes, target cell structures that facilitate adhesion, and polarized secretion of secretory granules, in addition to the target cell’s intrinsic sensitivity to NK cell-mediated death pathways (8).
Here we provide detailed instructions for a 2-hour assay that quantifies human NK cell degranulation by assessing CD107a surface expression, which is based on the use of peripheral blood mononuclear cells (PBMC) and standard target cell lines. This assay has been used for the differential diagnosis of defects in cellular cytotoxicity (10, 12). Furthermore, instructions are presented for a comprehensive 6-hour assay in which human NK cell degranulation, as evaluated by CD107a surface expression, is assessed simultaneously with chemokine and cytokine production, detected by intracellular staining of MIP-1β, TNF-α, and IFN-γ. Induction of MIP-1β production is generally robust in NK cells and can be measured as early as 30 min after target cell mixing, whereas TNF-α and IFN-γ are delayed. Depending on the available flow cytometer, additional antibodies can successfully be combined, facilitating increasingly detailed analysis of other functional parameters or responses in specific NK cell subsets. Although PBMCs are used as effector cells in the assays described here, the assays are also applicable to purified NK cells.
3.1. Preparation of peripheral blood mononuclear cells
Isolate PBMCs from heparinized whole blood samples by density gradient centrifugation with Lymphoprep according to the manufacturer’s instructions (See Note 1).
After centrifugation, harvest the PBMCs in a Falcon tube and add PBS for washing.
Centrifuge the cells at 450 g for 10 min.
Discard the supernatant and wash the cell pellet twice in PBS.
Optional: Purify NK cells by negative selection using a NK cell isolation kit according to the manufacturer’s instructions.
Resuspend cells in culture medium at a concentration equal to or less than 5 × 106 cells/mL.
After isolation, maintain effector cells overnight in complete culture medium in an incubator set at 37°C and 5% CO2.
3.2. Stimulation and staining of effector cells
3.2.1 Two-hour degranulation assay
Spin down the PBMC and resuspend in complete medium at 2 × 106 cells/mL (or 1 × 106 cells/mL if purified NK cells are used) (See Note 2).
Spin down target cells and resuspend at 2 × 106 cells/mL in complete medium. For redirected antibody-dependent cellular cytotoxicity, add stimulating mAbs at a concentration of 5 μg/mL to target cell suspensions. Mix well. Pipet 100 μL of target cell suspension per well into a V-bottom 96-well plate, as indicated. Table 3 provides a schematic representation of how effector cells and target cells are distributed in an assay designed to evaluate cytotoxic lymphocyte degranulation induced by receptors for natural cytotoxicity, Fc receptors, and the T cell receptor in a diagnostic setting (See Note 3).
Add 100 μL of the effector cell suspension per well to the V-bottom 96-well plate, as indicated. Table 3 provides a schematic representation of the assay.
Centrifuge the cells at 30 g for 3 min.
Place the cells in an incubator at 37°C for 2 hours (See Note 4).
During the incubation period, prepare a master mix containing fluorochrome-conjugated anti-CD3, anti-CD56, and anti-CD107a mAbs in staining solution. Table 1 provides a summary of the suggested antibody panel including recommended dilutions of the fluorochrome-conjugated mAbs (See Notes 5, 6, 7, 8).
Centrifuge the cells at 450 g for 3 min.
Promptly flick off the supernatant.
Resuspend the cells in 50 μL staining solution containing the appropriate combination and dilution of fluorochrome-conjugated mAbs. Incubate samples for 30 min in the dark at 4°C.
Centrifuge the cells at 450 g for 3 min.
Discard the supernatant and wash the cells once with 200 μL staining solution.
Resuspend the cells in 200 μL staining solution and transfer the cells to cytometer tubes.
Analyze cells on a flow cytometer, e.g. a FACS Calibur (See Note 9).
Table 3.
Layout of effector and target cells in wells for diagnostic evaluation of cytotoxic lymphocyte degranulation
| Medium | K562 | P815 | P815 anti-CD16 | P815 anti-CD3 | P815 anti-CD3* | |
|---|---|---|---|---|---|---|
| Control | ||||||
| Patient | ||||||
PerCP-conjugated mAb
The top row indicates the target cells mixed with stimulating mAbs, as indicated. The left column indicates the effector cells.
3.2.2 Six-hour multiple response assay
For cell stimulation, follow Methods 3.2.1, steps 1 through 4.
Place the cells in an incubator at 37°C for 1 hour.
To each well, add 20 μL of culture medium supplemented with GolgiPlug diluted 1:100 and GolgiStop diluted 1:150. Immediately return the cells to the incubator at 37°C for 5 more hours. (See Notes 6 and 10).
After a total of 6 hours, centrifuge the cells at 450 g for 3 min (See Note 11).
Promptly flick off the supernatant.
Prepare a master mix of staining solution for cell surface staining containing fluorochrome-conjugated anti-CD3, anti-CD14, anti-CD19, and anti-CD56, in addition to biotinylated anti-CD107a mAbs and LIVE/DEAD Cell Stain diluted 1:200. Table 2 provides a summary of the suggested antibody panel including recommended dilutions of the fluorochrome-conjugated mAbs. (See Notes 6, 7, 12, and 13).
Resuspend the cells in 50 μL staining solution containing an appropriate combination and dilution of fluorochrome-conjugated mAbs. Incubate samples for 30 min in the dark at 4°C.
Centrifuge the cells at 450 g for 3 min.
Discard the supernatant and wash the cells twice with 200 μL staining solution.
Resuspend the cells in 50 μL staining solution supplemented with streptavidin-Qdot 605 (recommended dilution 1:500). Incubate samples for 30 min at 4°C in the dark.
Centrifuge the cells at 450 g for 3 min.
Discard the supernatant and wash the cells twice with 200 μL staining solution.
Fix the cells in 100 μL fixation solution for 10 min at room temperature in the dark.
Centrifuge the cells at 450 g for 3 min.
Discard the supernatant and wash the cells once with 200 μL staining solution.
Resuspend the cells in 25 μL permeabilization solution. Incubate the cells for 10 min at 4°C in the dark.
Prepare a master mix of permeabilization solution for intracellular staining containing fluorochrome-conjugated anti-IFN-γ, anti-MIP-1β, and anti-TNF-α mAbs (See Note 13).
Add 25 μL permeabilization solution supplemented with an appropriate combination and dilution of fluorochrome-conjugated mAbs for intracellular staining. Mix well. Incubate the cells for 30 min at 4°C in the dark.
Centrifuge the cells at 450 g for 3 min.
Discard the supernatant and wash the cells once with 200 μL staining solution.
Resuspend the cells in 200 μL staining solution and transfer the cells to cytometer tubes.
Analyze cells on a flow cytometer. The panel is optimized for a CyAn ADP 9 Color Analyzer (See Note 8). However, the staining panel can be adapted to most multi-color flow cytometry instruments with three or four lasers and at least nine fluorescence detectors (See the chapter by Björkström et al. for means of adapting staining panels to a four laser BD LSR II System).
3.2.3 Control samples for compensation of spectral overlap
Either cells or anti-mouse Ig κ compensation beads can be used for compensation of spectral overlap among different fluorophores (See Note 14). Cells or compensation beads are plated in a 96-well V-bottom plate and saturating amounts of fluorochrome-conjugated mAb are added followed by a 30 min incubation at 4°C in the dark. Typically, for compensation beads, 1 μL of mAb per staining is sufficient. Incubate the cells or beads for 30 min at 4°C in the dark.
Follow Methods 3.2.1, steps 9 to 11 for instructions on the washing procedure.
Acquire the stained anti-mouse Ig κ beads on the flow-cytometer instrument used for acquisition of the cellular assay. Identical photo multiplier tube (PMT) voltages should be used for acquisition of PBMC samples and compensation control samples. Acquisition of compensation control samples should typically be performed in the same session as the functional assay.
3.3. Flow cytometry compensation and analysis
3.3.1 Two-hour degranulation assay
Compensation matrixes can be generated automatically in FlowJo after acqusition of data, or manually with CellQuest software upon acquisition on a FACS Calibur instrument. For a more extensive discussion of compensation for multi-parameter flow cytometry, we refer the reader to previously published guidelines on standard operating procedures (13).
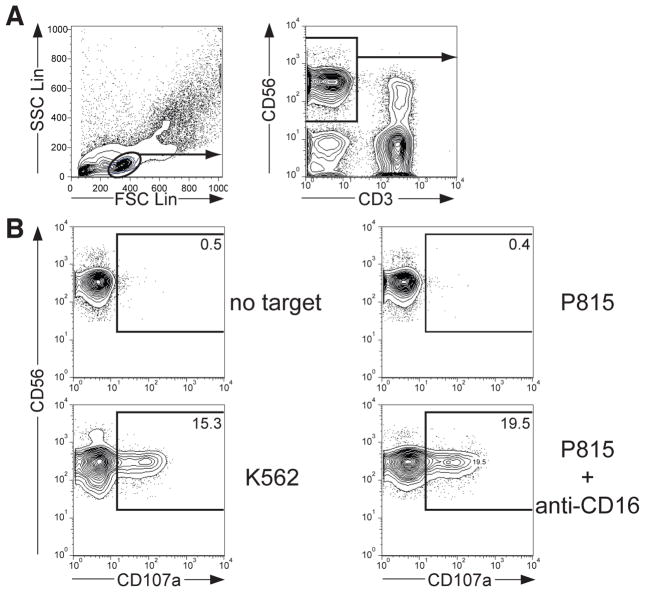
After compensation of all samples, the cell population of interest is identified and data are acquired on parameters of interest. In this example, the functional response of CD56dim NK cells has been assessed. Figure 1A shows a schematic representation of a gating strategy. Briefly, create a “lymphocyte gate” on a forward scatter / side scatter plot. Apply the “lymphocyte gate” to all samples. Thereafter, gate on CD56+ CD3− cells on an anti-CD3 / anti-CD56 plot. Apply this “NK cell gate” to all samples.
To quantify the percentage of degranulating cells, gate on CD107a+ cells on a histogram displaying anti-CD107a staining from a sample of effector cells with no target cells added. Without target cells, the frequency of CD107a+ NK cells should be less than 1%. Figure 1B exemplifies NK cell degranulation after stimulation with different target cells.
Figure 1.
Analysis of human NK cell degranulation by flow cytometric analysis. PBMC were incubated with target cells for 2 hours at 37°C, surface stained with fluorochrome-conjugated anti-CD3, anti-CD56, and anti-CD107a mAbs. (A) Profiles demonstrate the gating strategy [side scatter (SSC) vs. forward scatter (FCS) and CD56 vs. CD3] for identification of CD3− CD56+ NK cells with FlowJo software. (B) Profiles show CD56 versus CD107a staining on CD3− CD56+ NK cells for one representative donor after incubation of PBMC with indicated target cells.
3.3.2 Six-hour degranulation assay
For generation of a compensation matrix, software based compensation is recommended for multi-color experiments, here performed with FlowJo (See ref. (11) for a discussion on how selection of fluorochromes can influence degree of compensation).
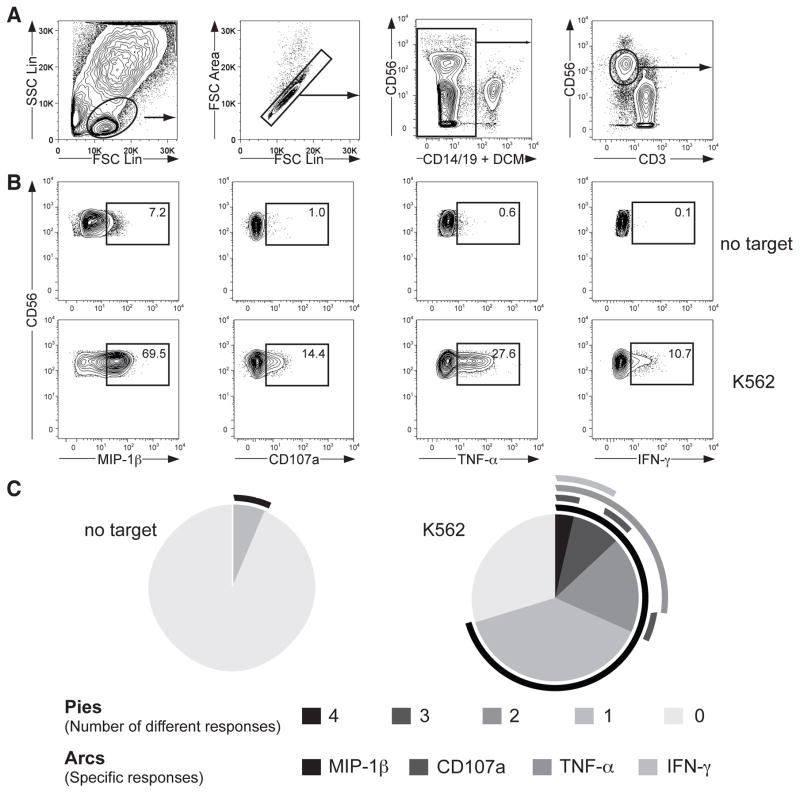
Following compensation of all samples, the cell population of interest is identified. In this example, the functional response of CD56dim NK cells has been assessed. Figure 2A provides a schematic representation of a gating strategy. Briefly, create a “lymphocyte gate” on a forward scatter / side scatter plot. Apply the “lymphocyte gate” to all samples. Exclude cells adhering to each other by creating a gate on single cells on a FSC Lin / FSC Area plot. Further, eliminate dead cells and cells that potentially may bind mAbs non-specifically by gating on LIVE/DEAD Cell Stain− CD14− CD19− cells. Finally, gate on CD56dim CD3− cells on an anti-CD3 / anti-CD56 plot. Apply this “CD56dim NK cell gate” to all samples. Alternatively, by different gating strategies CD56bright CD3− or CD56+ CD3− NK cell can be assessed.
To quantify the percentage of differentially responding CD56dim NK cells, a preferable method to identify the 16 (24) possible combinations generated with the four markers is to apply a Boolean gating strategy by identifying the positive population for each of the molecules/receptors of interest. Figure 2B exemplifies CD56dim NK cell responses after stimulation with K562 cells. An algorithm in FlowJo allows for automatic generation of the different subpopulations of interest.
The Boolean gating strategy presented above simplifies analysis of raw data. Software such as SPICE can be used for processing, organizing, and visualizing data from Boolean analysis. Figure 2C provides an example of presentation of data with SPICE. (See Note 15). SPICE enables the investigator to navigate through complex datasets allowing simplified data interpretation and presentation (14).
Figure 2.
Analysis of multiple human NK cell responses by flow cytometric analysis. PBMC were incubated with target cells for 6 hours at 37°C, surface stained with fluorochrome-conjugated anti-CD3, anti-CD14, anti-CD19, anti-CD56, and anti-CD107a mAbs, followed by intracellular staining with fluorochrome-conjugated anti-IFN-γ, anti-MIP-1β, and anti-TNF-α mAbs. (A) Gating strategy for identification of CD3− CD56dim NK cells. (B) Profiles show staining for multiple responses on CD3− CD56dim NK cells from one representative donor after incubation with target cells as indicated for 6 hours at 37°C. One representative donor is shown. (C) Pies represent the distribution of cells responding with different numbers of distinct responses, as indicated. Surrounding the pies, lines indicate the nature of individual responses.
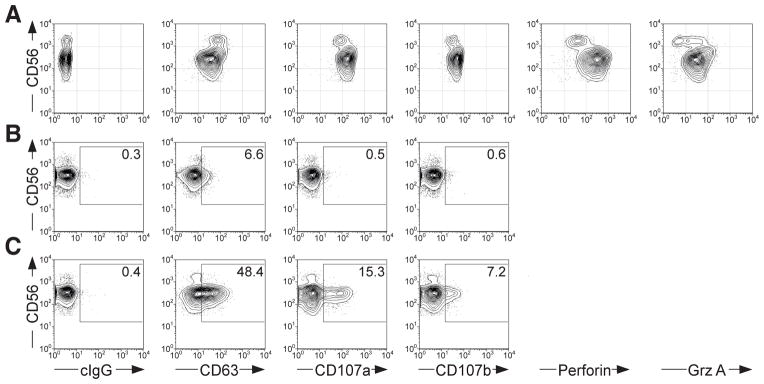
Figure 3.
Comparsion of different lysosomal markers for the identification of degranulating NK cells. (A) PBMC were surface stained with fluorochrome-conjugated anti-CD3 and anti-CD56 mAbs, followed by intracellular staining with fluorochrome-conjugated anti-CD63, anti-CD107a, anti-CD107b, anti-perforin, or anti-granzyme A mAbs, as indicated. Profiles show CD56 versus intracellular lysosomal protein staining, as indicated on CD3− CD56+ NK cells. PBMC were incubated either alone (B), or with K562 cells (C) for 2 hours at 37°C, surface stained with fluorochrome-conjugated anti-CD3 and anti-CD56 mAbs, in addition to anti-CD63, anti-CD107a, or anti-CD107b mAbs, as indicated. Profiles show CD56 versus surface staining of lysosomal proteins, as indicated on CD3− CD56+ NK cells.
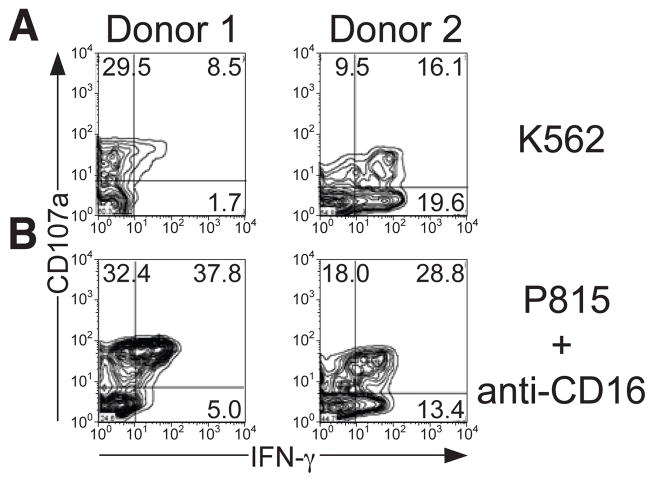
Figure 4.
Donor variation in NK cell responses. NK cells were stimulated with (A) K562 cells or (B) P815 cells supplemented with anti-CD16 mAb and incubated for 6 hours at 37°C. Profiles show CD107a versus IFN-γ staining on CD3− CD56dim NK cells from two different donor.
Acknowledgments
Our research is supported by the Swedish Foundation for Strategic Research, Research Council and Cancer Society (to H.-G.L.) and the Intramural Research Program of the NIH, NIAID (to E.O.L.). Y.T.B is supported by a grant from Mary Beve’s Foundation.
Footnotes
Vials for the collection of blood must contain an anticoagulant. Sodium-heparin vials are recommended for collecting blood samples to be analyzed for cellular function. Other anticoagulants such as EDTA and citrate deplete intracellular calcium stores. Therefore, cells collected in sample vials with such anticoagulants may display functional hyporesponsiveness, unless the cells are sufficiently rested in culture medium to replenish intracellular calcium stores.
If the samples require transport to a laboratory for diagnostic evaluation, we recommend shipping them at room temperature. Furthermore, for obtaining comparable results of cellular responses from shipped blood relative to results of cells isolated directly from fresh blood, the duration from sample acquisition to isolation of PBMCs should not exceed 24 hours.
The assays presented in this chapter are designed for analysis of human NK cells in PBMC populations. However, the assays are applicable to, and similar results are obtained with, freshly isolated, purified NK cells. To exclude the possibility of NK cell cross-talk with other cells that might confound interpretation of data on NK cell responses, investigators may wish to use purified NK cells in certain experimental settings. The use of purified NK cells also eliminates the need to stain with anti-CD3 mAbs. Thus, in the place of anti-CD3 mAbs, mAbs facilitating analysis of other parameters of NK cell responses or phenotype can be added to the panel when purified NK cells are used as effectors.
Moreover, these assays are applicable to frozen PBMC samples, the use of which may reduce inter-assay variation when performing a clinical investigation of different patient cohorts. For consistent results, we recommend that frozen PBMC samples be thawed one day prior to stimulation and rested overnight in culture medium.
Efficient expression of DNA delivered into primary NK cells is very difficult to achieve. Similarly, expression knock-down with siRNA is inefficient. Therefore, to study mechanisms of NK cell activation, many investigators use NK cell-like cell lines, which are more receptive to DNA or RNA delivery. Unfortunately, we have not been able to obtain satisfactory results with the anti-CD107a-based degranulation assay using NK cell lines NK92, NKL, or YTS. Instead, assays quantifying release of secretory lysosome contents, such as perforin and granzyme, can be used to assess the response of cell lines.
In addition to experiments addressing basic questions of NK cell biology, these assays are applicable to several clinical settings. In regards to diagnosis of immunodeficiencies, we routinely evaluate NK cell function based on their responses to the prototypical NK cell-sensitive cell line K562, in addition to redirected antibody-dependent responses with P815 cells and anti-CD16 mAbs. As outlined in this chapter, anti-CD3 mAbs can also be used to evaluate the function of effector T cells. CD8+ effector T cells are the predominant T cell subset that express surface CD107a in response to anti-CD3 stimulation (10). In addition, subsets of CD16+ CD8+ effector T cells may also respond to Fc receptor stimulation (10). Thus, the capacity of NK cells and T cells to respond to triggers of natural, Fc receptor-dependent, and T cell receptor-dependent degranulation can be quantified with the suggested assays.
In the foreseeable future, tumor cells from cancer patients might be evaluated for susceptibility to autologous or allogeneic NK cells in clinical settings (15, 16). The assays presented here could provide useful tools to evaluate tumor cell recognition and to assess the potential efficacy of various NK cell-mediated immunotherapies in individual patients.
Upon stimulation of cytotoxic cells by sensitive target cells, secretory lysosomes rapidly fuse with the plasma membrane, as detected by CD107a surface expression (7, 8, 17). With freshly isolated NK cells, CD107a surface expression typically peaks at 1 to 2 hours after mixing NK cells with sensitive target cells (8).
A number of transmembrane proteins are confined to secretory lysosomes and appear at the cell surface upon secretory lysosome exocytosis. These proteins include CD63, CD107a, CD107b, and FasL. Comparing intracellular staining of a panel of fluorochrome-conjugated mAbs to CD63, CD107a, and CD107b, we find that anti-CD107a mAbs provide the highest staining intensity (Figure 3A). Both CD107a and CD107b expression correlates with intracellular expression of perforin and granzymes, whereas CD63 expression does not (10). In accord with the intracellular staining intensities, surface staining for CD107a provides the most sensitive marker for NK cell degranulation (Figure 3B and C).
To enhance the sensitivity for detection of degranulating cells, mAbs to different secretory lysosome proteins may be combined (18, 19). However, in diagnostic settings, combining mAbs for assessment of cytotoxic lymphocyte degranulation capacity may confound results. Tentatively, a mutation that selectively affects the binding of one mAb epitope can be misinterpreted as a diminished capacity for degranulation. To avoid such misinterpretations and unequivocally ascertain degranulation deficits in patients, we recommend using anti-CD107a mAbs exclusively for assessment of degranulation and supplementing such assays with intracellular staining of CD107a to verify the presence of the CD107a epitope.
CD107a, once exposed on the cell surface after degranulation, becomes internalized by endocytosis. A strategy to enhance the sensitivity for detection of degranulating cells is to include fluorochrome-conjugated anti-CD107a mAb during the stimulation assay, allowing internalization of complexes of CD107a and fluorochrome-conjugated mAb (7, 19). To prevent degradation of internalized CD107a, monensin can be added to such assays (7, 19). Monensin is a polyether ionophore that blocks the acidification of endocytic vesicles. Arguably, in experiments studying NK cell function, it is desirable to avoid the use of such ionophores, which may perturb cellular signaling and function. Moreover, in assays lasting two hours or less, the internalization of surface expressed CD107a on resting NK cells is negligible (8).
Investigators are advised to titrate each fluorochrome-conjugated mAb for optimal staining. The most desirable mAb concentration is the one that provides the brightest signal of a positive subset together with the dimmest background signal of the negative subset. The titration procedure is to be repeated for each new batch of fluorochrome-conjugated mAb.
Prepare the mAbs (as listed in Table 1 and Table 2) by mixing them in a total volume of 50 μL of staining solution for each sample to be stained. This can, if necessary, be prepared the day before. To limit inter-experimental variability, a master mix covering several days of experiments can be prepared. Store the mAb master mix at 4°C in the dark until usage, and for a maximum of five days.
For analysis on a FACS Calibur, the suggested mAb panel has one available fluorescence channel. To assess degranulation by CD8+ T cells, APC-conjugated anti-CD8 mAb can be added to the panel. Alternatively, this channel can be used for markers that discriminate NK cell subsets, such as inhibitory receptors that can influence the education of human NK cells (20), or mAbs that quantify functional parameters, such as MIP-1β that can be detected at early time-points after stimulation of NK cells.
We recommend acquiring samples the same day as running the cell stimulation and staining experiments. However, samples may be fixed in staining solution supplemented with 1% formaldehyde and acquired at later timepoints.
Flow cytometric detection of cellular cytokine production is enhanced by inhibitors of the constitutive pathway for secretion of newly synthesized proteins. Brefeldin A is an inhibitor of the guanine exchange activity of ADP-ribosylation factor (ARF) proteins and interferes with anterograde protein transport from the endoplasmic reticulum to the Golgi apparatus. Thus, addition of Brefeldin A retains cytokines within the responding cells, facilitating sensitive detection of cytokine-producing cells.
As previously alluded to in Note 4, the duration of the assay is critical in determining the frequency of cells responsive to distinct functional parameters. Whereas CD107a surface expression and MIP-1β synthesis are rapidly induced upon NK cell activation, the synthesis of TNF-α and particularly IFN-γ are protracted. Therefore, for analysis of IFN-γ by NK cells, we recommend at least 6 hours of stimulation. For production of IFN-γ induced by exogenous cytokines, secretion cannot be detected until more than 12 hours after stimulation (Y.T.B, unpublished observation).
Dead cells can nonspecifically bind mAb conjugates, leading to erroneous conclusions. Recently developed amine-reactive viability dyes are useful dead cell exclusion markers, as they reproducibly identify dead cells even after subsequent fixation and permeabilization of the cells (21). Dissolve the LIVE/DEAD Cell Stain in 500 μL of dehydrated DMSO. Aliquot and store at −20°C. Dilute the aliquoted LIVE/DEAD Cell Stain at 1:200 in staining solution prior to cell staining. LIVE/DEAD Cell Stain aliquots can be repeatedly freeze-thawed 4 times. The LIVE/DEAD Cell Stain is light sensitive and must be protected from light.
For analysis on a CyAn ADP 9 Color Analyzer, the suggested mAb panel has two available fluorescence channels, which can be used for antibodies conjugated to the fluorochromes PerCP and APC. These are commonly used fluorochromes and a variety of mAbs to different cellular markers that are directly conjugated to these fluorochromes are available. Thus, these channels can be used for markers that discriminate NK cell subsets, such as inhibitory receptors, markers of cellular activation or senescence, or markers that provide measures of additional functional parameters. Alternatively, these channels can be used to discriminate other lymphocyte subsets, e.g. with fluorochrome-conjugated anti-CD8 to identify cytotoxic T lymphocytes, so that the responses of such subsets can be assessed concurrent with the responses of NK cells.
Compensation for spectral overlap among different fluorophores is required for analysis of any multi-color flow cytometry experiment. Controls should encompass single staining of all fluorochromes used, in addition to stainings where all aside from one fluorochrome is included. The signal intensity of compensation controls should be at least as high as for the signals obtained with cellular stainings. Ideally, the compensation controls comprise two equal populations of stained and unstained particles. We recommend using anti-mouse Ig κ compensation beads for spectral compensation staining that includes an equal number of positive and negative beads. For a more extensive discussion of compensation for multi-parameter flow cytometry, we refer the reader to previously published standard operating procedures (13).
Considerable donor variation in NK cell responses is to be expected, both in terms of the magnitude and the kinetics of responses. We have also noted that the relationship among responses may vary among donors. In most donors (approximately 75%), after 6 hours of stimulation IFN-γ expression is confined to a subset of CD107a+ cells, whereas in some donors IFN-γ expression does not correlate with CD107a surface expression (Figure 4). Similar variations in TNF-α responses are also observed (J.M.N. and Y.T.B., unpublished observations). Such variation in different cellular responses could be attributed to previous stimulation of cells in vivo. Cells that have been subject to inflammatory stimuli may produce cytokines more readily upon ex vivo stimulation.
References
- 1.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 2.Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5:996–1002. doi: 10.1038/ni1114. [DOI] [PubMed] [Google Scholar]
- 3.Strowig T, Brilot F, Munz C. Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J Immunol. 2008;180:7785–91. doi: 10.4049/jimmunol.180.12.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryceson YT, Long EO. Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol. 2008 doi: 10.1016/j.coi.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters PJ, Borst J, Oorschot V, Fukuda M, Krahenbuhl O, Tschopp J, Slot JW, Geuze HJ. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991;173:1099–109. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med. 1999;5:90–6. doi: 10.1038/4779. [DOI] [PubMed] [Google Scholar]
- 7.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–12. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcenaro S, Gallo F, Martini S, Santoro A, Griffiths GM, Arico M, Moretta L, Pende D. Analysis of natural killer-cell function in familial hemophagocytic lymphohistiocytosis (FHL): defective CD107a surface expression heralds Munc13-4 defect and discriminates between genetic subtypes of the disease. Blood. 2006;108:2316–23. doi: 10.1182/blood-2006-04-015693. [DOI] [PubMed] [Google Scholar]
- 10.Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, Bechensteen AG, Boelens JJ, Celkan T, Farah RA, Hultenby K, Winiarski J, Roche PA, Nordenskjold M, Henter JI, Long EO, Ljunggren HG. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110:1906–15. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez VD, Björkström NK, Malmberg KJ, Moll M, Kuylenstierna C, Michaëlsson J, Ljunggren HG, Sandberg JK. Application of nine-color flow cytometry for detailed studies of the phenotypic complexity and functional heterogeneity of human lymphocyte subsets. J Immunol Methods. 2008;330:64–74. doi: 10.1016/j.jim.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudd E, Bryceson YT, Zheng C, Edner J, Wood SM, Ramme K, Gavhed S, Gurgey A, Hellebostad M, Bechensteen A, Ljunggren HG, Fadeel B, Nordenskjold M, Henter JI. Spectrum, and clinical and functional implications of UNC13D mutations in familial haemophagocytic lymphohistiocytosis. J Med Genet. 2008;45:134–41. doi: 10.1136/jmg.2007.054288. [DOI] [PubMed] [Google Scholar]
- 13.Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry. 2001;45:194–205. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 14.Lamoreaux L, Roederer M, Koup R. Intracellular cytokine optimization and standard operating procedure. Nat Protoc. 2006;1:1507–16. doi: 10.1038/nprot.2006.268. [DOI] [PubMed] [Google Scholar]
- 15.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–39. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 17.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 18.Enders A, Zieger B, Schwarz K, Yoshimi A, Speckmann C, Knoepfle EM, Kontny U, Muller C, Nurden A, Rohr J, Henschen M, Pannicke U, Niemeyer C, Nurden P, Ehl S. Lethal hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type II. Blood. 2006;108:81–7. doi: 10.1182/blood-2005-11-4413. [DOI] [PubMed] [Google Scholar]
- 19.Andre P, Anfossi N. Clinical analysis of human natural killer cells. Methods Mol Biol. 2008;415:291–300. doi: 10.1007/978-1-59745-570-1_17. [DOI] [PubMed] [Google Scholar]
- 20.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagne F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–42. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 21.Perfetto SP, Chattopadhyay PK, Lamoreaux L, Nguyen R, Ambrozak D, Koup RA, Roederer M. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J Immunol Methods. 2006;313:199–208. doi: 10.1016/j.jim.2006.04.007. [DOI] [PubMed] [Google Scholar]