Abstract
Primary cardiac sarcomas are rare and carry a grave prognosis. Improved survival requires a complete margin negative resection of the tumor. These surgical resections are often large and complex, requiring extensive reconstructive procedures. The appropriate material for cardiac reconstruction is not known. We have used glutaraldehyde-fixed bovine pericardium in our early series but have recently employed the MatriStem® Surgical Matrix PSMX membrane (ACell®, Inc.; Columbia, MD), a unique proprietary urinary bladder matrix derived from porcine urinary bladder with the potential for viability and tissue ingrowth. In our study of six patients at this institution, all six underwent successful surgical resection and repair with the MatriStem acellular porcine urinary bladder membrane (ACell). The postoperative course was uncomplicated in all patients, and they are still alive at this time. An aggressive surgical approach to cardiac tumors can possibly lead to complete resection but often requires reconstruction of the cardiac tissue with a membrane. We were able to achieve acceptable results in our cardiac reconstruction by using the ACell extracellular matrix to reconstruct the defect following tumor resection. Longer-term follow-up in these patients, including imaging studies, will be necessary to demonstrate the durability and integrity of the reconstruction.
Keywords: atrium, right ventricle, cardiac tumor, cardiac reconstruction, ACell
Introduction
Primary cardiac tumors are rare, with an incidence of .056% to 1.23% in early autopsy series.1 Of these, 75% are benign, and 75% of the benign tumors are myxomas. Sarcomas represent 75% of the primary cardiac malignant tumors,2 and these tumors require complete resection for improved survival.3 Our group has pursued an aggressive resection approach for primary cardiac sarcomas that requires complex and large reconstruction techniques. Our primary material used for these repairs has been bovine pericardium, which has provided adequate cardiac reconstruction following resection. However, the material is fixed in glutaraldehyde, which is nonviable and can show calcification over time. When biologic membranes became available, we began using the MatriStem membrane (ACell®) and found that it has acceptable use characteristics and the theoretical potential of stem cell migration and remodeling that might lead to better long-term integrity and function. More importantly, we wanted to ensure that this newly viable membrane was sturdy and could provide long-term cardiac reconstruction similar to the bovine pericardium we had used in the past. Herein, we present our initial experience with the MatriStem acellular porcine urinary bladder membrane following complex cardiac tumor resections.
Patients and Methods
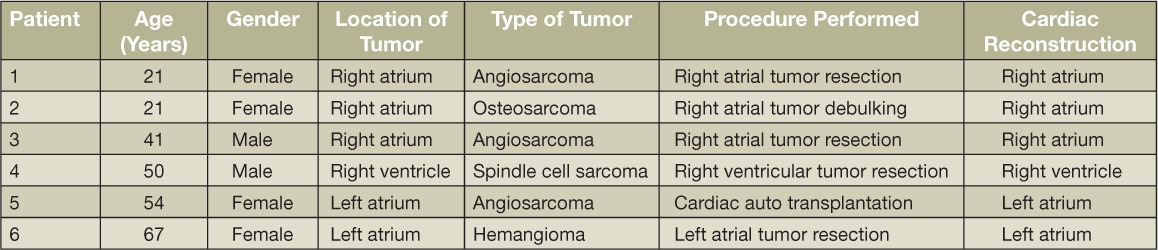
Six patients (4 females, 2 males, mean age: 42 years, range 21–67 years) were operated on between September 2013 and August 2014 at our institution—five with primary cardiac sarcomas and one with a giant left atrial hemangioma. Tumor pathology included angiosarcoma, spindle cell sarcoma, metastatic osteosarcoma, and hemangioma. Of the six patients, three had tumors in the right atrium, one in the right ventricle, and two in the left atrium. All patients had resection through a median sternotomy. A cardiac autotransplantation approach was used in one patient with a left atrial tumor, and the MatriStem acellular porcine urinary bladder xenograft (ACell) was used for cardiac repair in all six patients. The procedures were done on an IRB-approved protocol and the patients gave full consent. Patient demographics and procedural information are listed in Table.1.
Table 1.
Patient demographics.
Of the three patients with tumors in the right atrium, two had involvement of the atrioventricular groove that required more extensive resection and a larger piece of acellular membrane. All three patients received preoperative chemotherapy. The one patient whose tumor involved the right ventricle received preoperative chemotherapy before resection. Magnetic resonance imaging done at the 1-year follow-up showed no evidence of breakdown or loss of integrity of the ventricular wall and no recurrence of the tumor. Of the two patients who had tumors involving the left atrial wall, one had to undergo an autotransplant procedure in which the tumor was completely resected and the defect completely sealed. The other patient had the largest tumor in our series, measuring 8.7 × 7.7 cm and arising from the roof of the left atrium all the way to the left and right pulmonary veins. The patient received preoperative chemotherapy and underwent complete resection and reconstruction of not only the left atrial tissue but also the two pulmonary veins.
Discussion
Very little has been published in the literature about cardiac tumors in general, let alone the presentation, management, and outcome of patients with these primary cardiac sarcomas. In a 17-year retrospective study, Hamidi found that median overall survival for cardiac sarcoma patients was 6 months.4 In addition, cardiac sarcoma patients who underwent surgery had a median survival of 12 months, whereas those who did not undergo surgery had a median survival of 1 month.4 Bakaeen and colleagues reported a 2-year survival rate of 62% among 27 primary cardiac sarcoma patients who received aggressive multimodal therapy.5 In our own published experience, benefit has been shown in the resection of left atrial sarcomas and pulmonary artery sarcomas, but resection of right atrial sarcomas has not shown overall improvement in survival.6–8 However, increased survival rates were observed in patients when negative surgical margins were achieved in right-sided resections.3
Cardiac tumors typically present with symptoms that are dependent upon the tumor's size and location.9 Patients can present with congestive heart failure, arrhythmias, systemic embolization, or constitutional symptoms. Surgical resection is imperative for long-term survival of primary cardiac sarcoma patients, and several studies have shown that the median overall survival of patients with complete resection is twice as long or longer (17–24 months) than the median overall survival of patients with incomplete resections (6–10 months).10,11 Complex and large resections are often needed to achieve complete tumor removal in primary cardiac sarcoma. These large resections then require complex and extensive reconstruction of the removed cardiac tissue. In the past, we have preferentially used glutaraldehyde-fixed bovine pericardium, a nonviable tissue that is known to calcify and become rigid over time.12 However, we have recently started using the MatriStem acellular porcine urinary bladder membrane to reconstruct the atria and ventricles of the heart after tumor resection. The intact basement membrane surface of this graft is hypothesized to contribute to epithelial and progenitor cell attachment and proliferation. In addition, the xenograft allows for the possibility of endogenous seeding with native cardiac tissue for a more viable reconstructed area of the heart. We found this material to have the same ease of use and successful repair as bovine pericardium (Figure 1). We encountered no technical difficulty when sewing the patch to our patients' native tissue, and we noted that the patch was hemostatic during protamine administration after cardiopulmonary bypass.
Figure 1.
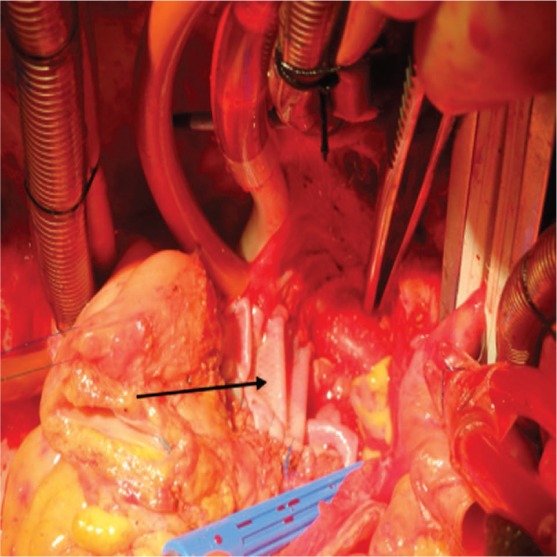
The arrow shows the MatriStem surgical matrix (ACell, Inc., Columbia, MD) used to reconstruct the lateral wall of the left atrium.
Our early results have been encouraging. As we continue to use the MatriStem in heart reconstruction procedures, we intend to perform semiannual imaging studies in those patients for up to 2 years in order to evaluate the membrane's long-term integrity.
Conclusion
We found that the MatriStem extracellular matrix membrane can be used for complex cardiac repair after resections for primary cardiac sarcoma. Our early results with the MatriStem in the heart are encouraging, and long-term outcomes using this membrane will be necessary to see if lasting benefits are realized.
Footnotes
Conflict of Interest Disclosure: The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
References
- 1.Lam KY, Dickens P, Chan AC. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med. 1993 Oct;117(10):1027–31. [PubMed] [Google Scholar]
- 2.Vander Salm TJ. Unusual primary tumors of the heart. Semin Thorac Cardiovasc Surg. 2000 Apr;12(2):89–100. doi: 10.1053/ct.2000.5080. [DOI] [PubMed] [Google Scholar]
- 3.Kim MP, Correa AM, Blackmon S et al. Outcomes after right-side heart sarcoma resection. Ann Thorac Surg. 2011 Mar;91(3):770–6. doi: 10.1016/j.athoracsur.2010.09.079. [DOI] [PubMed] [Google Scholar]
- 4.Hamidi M, Moody JS, Weigel TL, Kozak KR. Primary cardiac sarcoma. Ann Thorac Surg. 2010 Jul;90(1):176–81. doi: 10.1016/j.athoracsur.2010.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakaeen FG, Jaroszewski DE, Rice DC et al. Outcomes after surgical resection of cardiac sarcoma in the multimodality treatment era. J Thorac Cardiovasc Surg. 2009 Jun;137(6):1454–60. doi: 10.1016/j.jtcvs.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Reardon MJ. Malignant tumor overview. Methodist DeBakey Cardiovasc J. 2010 Jul–Sep;6(3):35–7. doi: 10.14797/mdcj-6-3-35. [DOI] [PubMed] [Google Scholar]
- 7.Vaporciyan A, Reardon MJ. Right heart sarcomas. Methodist DeBakey Cardiovasc J. 2010 Jul–Sep;6(3):44–8. doi: 10.14797/mdcj-6-3-44. [DOI] [PubMed] [Google Scholar]
- 8.Ramlawi B, Leja MJ, Abu Saleh WK et al. Surgical Treatment of Primary Cardiac Sarcomas: Review of a Single-Institution Experience. Ann Thorac Surg. 2016 Feb;101(2):698–702. doi: 10.1016/j.athoracsur.2015.07.087. [DOI] [PubMed] [Google Scholar]
- 9.Zhang PJ, Brooks JS, Goldblum JR et al. Primary cardiac sarcomas: a clinicopathologic analysis of a series with follow-up information in 17 patients and emphasis on long-term survival. Hum Pathol. 2008 Sep;39(9):1385–95. doi: 10.1016/j.humpath.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putnam JB, Jr, Sweeney MS, Colon R, Lanza LA, Frazier OH, Cooley DA. Primary cardiac sarcomas. Ann Thorac Surg. 1991 Jun;51(6):906–10. doi: 10.1016/0003-4975(91)91003-e. [DOI] [PubMed] [Google Scholar]
- 11.Simpson L, Kumar SK, Okuno SH et al. Malignant primary cardiac tumors: review of a single institution experience. Cancer. 2008 Jun;112(11):2440–6. doi: 10.1002/cncr.23459. [DOI] [PubMed] [Google Scholar]
- 12.Aimoli CG, Nogueira GM, Nascimento LS et al. Lyophilized bovine pericardium treated with a phenethylamine-diepoxide as an alternative to preventing calcification of cardiovascular bioprosthesis: preliminary calcification results. Artif Organs. 2007 Apr;31(4):278–83. doi: 10.1111/j.1525-1594.2007.00376.x. [DOI] [PubMed] [Google Scholar]


