The column in this issue is supplied by Dr. Juan Jose Olivero, M.D., a nephrologist at Houston Methodist Hospital and a member of the Nephrology Training Program. Dr. Olivero obtained his medical degree from the University of San Carlos School of Medicine in Guatemala, Central America, and completed his residency and nephrology fellowship at Baylor College of Medicine in Houston, Texas.
Case 1: Phosphorus
A 54-year-old woman with short bowel syndrome secondary to complications of chronic ulcerative colitis was admitted to Houston Methodist Hospital because of worsening diarrhea, profound weakness, and shortness of breath. She had been taking sucralfate for chronic gastritis but denied the use of any other medications for several weeks prior to her admission.
On admission, she was noted to have poor skin turgor, dry mucous membranes, resting tachycardia (HR 100 bpm), hypotension (BP 95/60), and tachypnea (20 resp/min), all features of dehydration. Initial laboratory tests revealed mild hyperchloremic acidosis (chloride 109 mEq/L and HCO3 18 mEq/L), sodium level of 138 mEq/L, and potassium of 3.6 mEq/L; she also showed impaired kidney function manifested by a creatinine level of 1.6 mg/dL and BUN of 35 mg/dL. Chest X-ray showed mild cardiomegaly. The patient was begun on 5% dextrose in .45% normal saline with 75 mEq/L of NaHCO3 at 100 mL/h. Due to persistent symptoms, additional tests were ordered and revealed normal liver function except for albumin levels of 3.2 g/dL, uncorrected calcium of 7.9 mg/dL, and phosphorus of .9 mg/dL.
In this case, the combination of chronic malabsorption (phosphorus included), decreased oral intake of nutrients, use of a phosphate binder (aluminum-containing sucralfate), and the subsequent administration of intravenous dextrose resulted in life-threatening hypophosphatemia. This lead to myocardial dysfunction from low ATP, which was directly related to total body phosphorus depletion.
The systemic consequences of severe hypophosphatemia can be summarized as followed:
The patient was started on intravenous (IV) phosphorus replacement with boluses of sodium phosphate 10 mmol/h. Close follow-up of phosphorus levels occurred every 4 hours for 24 hours until levels were restored to normophosphatemia, paralleling dramatic improvement in the patient's condition. At times, if a patient is simultaneously phosphorus and potassium depleted, potassium phosphate 10 mmol IV over 1 hour can be used instead of sodium phosphate. Similarly, potassium phosphate would be preferred over sodium phosphate if the patient is hypernatremic (sodium levels > 150 mEq/L), provided that potassium administration is not contraindicated.
Figure 1.
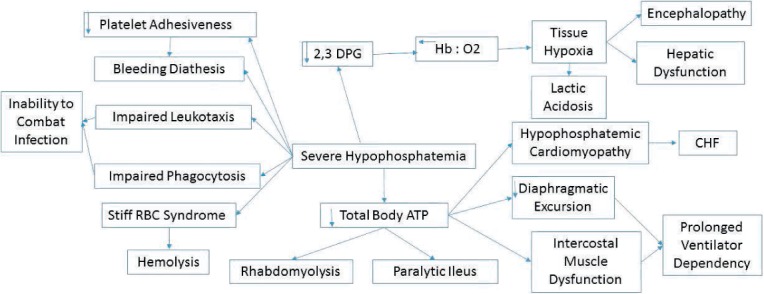
Systemic consequences of severe hypophosphatemia. DPG: diphosphoglycerate; CHF: congestive heart failure; Hb: hemoglobin.
Case 2: Potassium
A 75-year-old man was admitted to the hospital's emergency department with shortness of breath and palpitations. He was noted to have stable vital signs, including a blood pressure of 140/90 while taking amlodipine 10 mg and metoprolol 50 mg twice a day. He had a heart rate of 76/bpm, respiration rate of 16/min, and temperature of 97° F. Chest X-ray was normal except for borderline left ventricular hypertrophy. A 12-lead electrocardiogram showed premature atrial contraction and only occasional premature ventricular contraction.
Preliminary laboratory tests were unremarkable except for a potassium level of 3 mEq/L. The patient denied having diarrhea or using a diuretic. Before the patient received intravenous potassium, particularly since there was no obvious etiology for hypokalemia, simultaneous blood and urine samples were sent for potassium and osmolality to calculate the transtubular potassium gradient (TTKG). This is a simple, quick, and inexpensive test designed to assess the etiology of both hypokalemia and hyperkalemia. The TTKG formula is as follows:
In the presence of hypokalemia, results < 2 mEq/L = GI losses and results > 4mEq/L = renal losses and excess of mineralocorticoids.
In our patient, the test yielded results of > 6 consistent with mineralocorticoid excess, while a CT scan of his adrenal glands failed to show any abnormalities. He was begun on an aldosterone receptor blocker (spironolactone) at progressively increasing doses of 50 mg three times a day, resulting in normalization of blood pressure (off amlodipine and beta blocker) and potassium levels. If a male patient develops gynecomastia, eplerenone could be the preferred aldosterone blocker since supposedly this compound has less gynecomastia effect, although cost can be a limiting factor.
This case underscores the importance of trying to determine the etiology of hypokalemia to treat it more rationally and not simply giving potassium supplements. Aldosterone receptor blockers, independently of reducing blood pressure (particularly in some cases of refractory hypertension), have cardioprotective and nephroprotective effects, presumably as a consequence of inhibiting the profibrotic effect of collagen factor IV. In the case of hyperkalemia, TTKG can also provide important information to initiate a rational treatment approach. The same parameters are entered into the formula, and in the case of hyperkalemia, TTKG < 6 mEq/L implies mineralocorticoid deficit, a condition that can be easily corrected by using fludrocortisone .2 mg/d; TTKG > 10 indicates hyperkalemia of a non-renal etiology.
It is important to remember that these calculations are only useful in the steady state, when the patient is not taking diuretics or potassium supplements and is not having acute intercurrent events such as diarrhea.
Case 3: Magnesium
A 36-year-old woman had a sarcoma of the left tibia when she was 11 years old and received aggressive chemotherapy with a successful outcome. She did well until approximately 10 years ago, when she complained of cramping and “skip beats.” An extensive evaluation disclosed severe hypomagnesemia (Mg level of .4 mg/dL), at times complicated by symptomatic hypocalcemia (Ca level < 7 mg/dL) and on occasion hypokalemia (K level 3.4 mEq/L). A 24-hr urine collection disclosed magnesium levels > 30 mg, which is diagnostic of renal magnesium wasting, in this case presumably related to cisplatin. Other drugs implicated in magnesium wasting include diuretics, amphotericin B, aminoglycosides, cyclosporine, and pentamidine. Magnesium handling can also be affected by hypercalcemia, hypokalemia, and hypophosphatemia, resulting in impaired magnesium reabsorption and in turn leading to magnesuria. Diabetic ketoacidosis can also promote magnesuria due to osmotic diuresis (glycosuria) and could result in profound magnesium depletion. Hypomagnesemia is known to cause cardiac arrhythmia (torsades de pointes, ventricular, and supraventricular), increased digitalis sensitivity, and electrocardiogram changes including widening QRS, prolonged PR/QR intervals, and T-wave changes.
Total body magnesium deficit can lead to intractable hypocalcemia caused by resistance to parathyroid hormone (PTH), thus interfering with gastrointestinal calcium and bone mobilization. As a result, even providing exogenous PTH infusion, the hypercalcemic and hyperphosphaturic effects of PTH are blunted. The correlation between hypomagnesemia and hypokalemia is less well understood but appears to be mediated by aldosterone. Hypokalemia is difficult to correct at times unless magnesium levels normalize.
Amiloride, a diuretic known to inhibit some of the magnesium secretory channels, can be tried at a dose of 5 to 10 mg/d (maximal dose 20 mg). Most of the time, as with our patient, correction of hypomagnesemia that is not achieved with magnesium supplements alone can be significantly improved by combining amiloride with magnesium supplements in the form of magnesium oxide 400 mg 3–4/d according to response.
Large doses of oral magnesium can result in diarrhea and cause a vicious cycle whereby magnesium is not well absorbed in the GI tract, thus perpetuating hypomagnesemia.


