Supplemental Digital Content is available in the text.
Background:
Air travel is associated with the spread of influenza through infected passengers and potentially through in-flight transmission. Contact tracing after exposure to influenza is not performed systematically. We performed a systematic literature review to evaluate the evidence for influenza transmission aboard aircraft.
Methods:
Using PubMed and EMBASE databases, we identified and critically appraised identified records to assess the evidence of such transmission to passengers seated in close proximity to the index cases. We also developed a bias assessment tool to evaluate the quality of evidence provided in the retrieved studies.
Results:
We identified 14 peer-reviewed publications describing contact tracing of passengers after possible exposure to influenza virus aboard an aircraft. Contact tracing during the initial phase of the influenza A(H1N1)pdm09 pandemic was described in 11 publications. The studies describe the follow-up of 2,165 (51%) of 4,252 traceable passengers. Altogether, 163 secondary cases were identified resulting in an overall secondary attack rate among traced passengers of 7.5%. Of these secondary cases, 68 (42%) were seated within two rows of the index case.
Conclusion:
We found an overall moderate quality of evidence for transmission of influenza virus aboard an aircraft. The major limiting factor was the comparability of the studies. A majority of secondary cases was identified at a greater distance than two rows from the index case. A standardized approach for initiating, conducting, and reporting contact tracing could help to increase the evidence base for better assessing influenza transmission aboard aircraft.
In the past decades, air travel has increased worldwide.1,2 The higher number of people traveling, frequently in close proximity to others, enhances the likelihood for transmission of infectious diseases, in particular of airborne pathogens. In addition, the better connection between distant regions poses an increasing risk for rapid global spread of infectious diseases, causing pandemics.3,4 Air travel has been shown to be associated with the intercontinental spread of new emerging viruses, both via importation of cases and through in-flight transmission.5–8 Airplanes have been predicted to act as a major vector when the next pandemic occurs.9–11
As in other closed/semiclosed settings, on board transmission of influenza virus is facilitated by direct person-to-person contact or contact with contaminated surfaces.12–14 Contact tracing after possible exposure to an infectious disease is a common means of trying to limit subsequent transmission.15 The current World Health Organization (WHO) guidance recommends contact tracing of passengers seated within two rows of a case of A(H1N1) influenza.16
The European Centre for Disease Prevention and Control has published disease-specific guidance assessing the risk of aboard transmission of several pathogens.17–19 As part of the risk assessment for the guidance on infectious diseases transmitted on aircraft (RAGIDA) project,17 a first literature review of transmission aboard aircraft was undertaken in 2009 for 10 prioritized infectious diseases, including influenza.20
As basis for the expert consultation, we conducted a systematic literature review to critically appraise the published literature and assess the evidence for influenza transmission aboard aircraft. Weaknesses and gaps of all the contact tracing investigations included in the review were systematically evaluated using a self-developed bias assessment tool and the quality of evidence was assessed. A secondary objective was to investigate the association between influenza transmission and proximity to an index case.
METHODS
Literature Search Strategy and Selection Criteria
We systematically searched PubMed (1960–2015) and EMBASE (1974–2015) for articles containing information on the transmission of influenza virus or influenza-like illness aboard aircraft. The date of search was July 18, 2013 with regular updates until October 2015. In addition, bibliographies of relevant articles, including reviews, were screened for other related studies, and to validate the electronic database search. No language restriction was applied in the first steps, but publications in other than European languages were excluded for the final analysis. For details of the search strategies developed for each database, see eAppendix 1 (http://links.lww.com/EDE/B6). First, titles and abstracts of all articles were independently screened by the two authors for potential inclusion in this review. Discrepancies were resolved through discussion between the authors. For all references that passed as potentially relevant for investigating influenza or influenza-like illness transmission during flight, a full-text copy of the article was obtained and reviewed by both authors. Studies investigating on board transmission of influenza virus or influenza-like illness, from passengers and/or crew to passengers and/or crew, were included. Studies estimating transmission with modeling techniques or experimental studies in mock-up settings were excluded.
Data Extraction and Quality Assessment of Relevant Literature
Relevant data from each publication were extracted independently by both the authors and cross-checked. Discrepancies were resolved by further study evaluation by the two authors. Data were extracted in a summary table (see eAppendices 2 and 3; http://links.lww.com/EDE/B6) which included publication details (year, authors, location); flight characteristics (flight origin and destination, flight duration, aircraft type, ground delays, information on ventilation system); details on the index case(s) (number, age, gender, country of residence or nationality, seating, symptoms during the flight, pathogen/strain found, laboratory confirmation of diagnosis); study type and details on contact tracing (definition of contact, contact tracing strategy, methods used to identify contacts, methods used for contacting contacts, total number of contacts identified, total number of successfully traced contacts, seating of contacts in relation to index case); exposure of primary and secondary cases (before, during, and after flight); conclusion on disease transmission (number of cases/number of contacted passengers, and/or crew excluding index cases), and intervention.
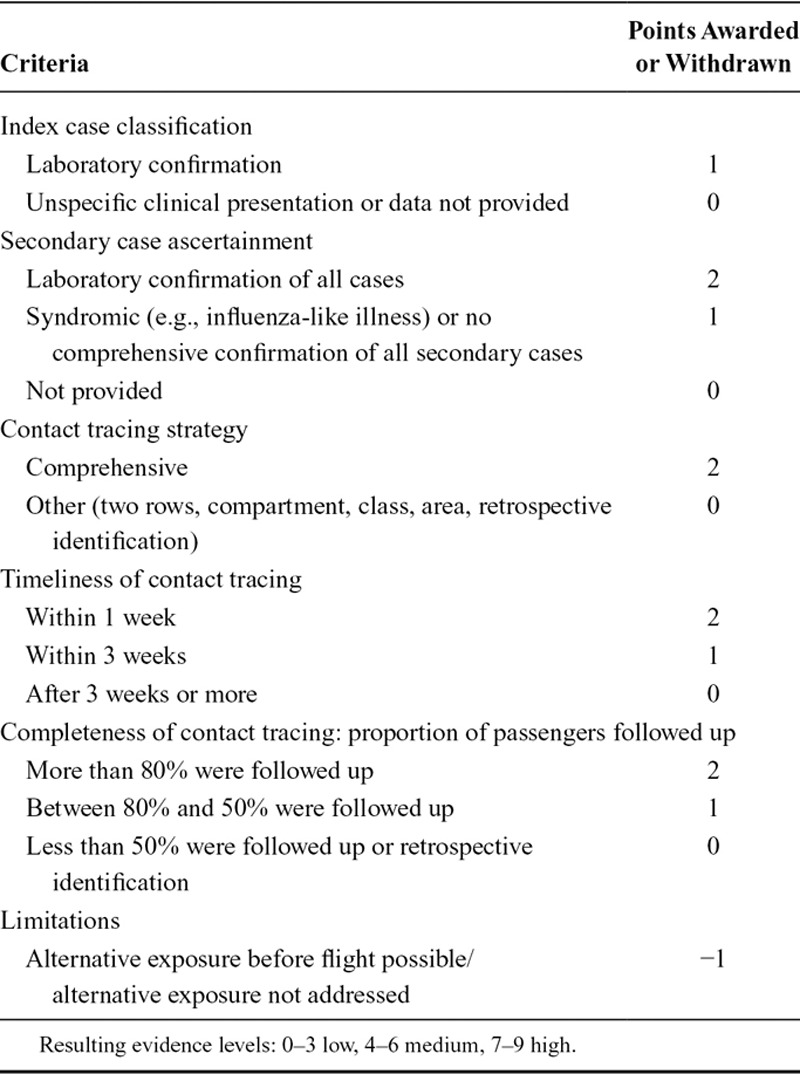
The quality and the strength of evidence for each investigation described within one study was assessed with a self-developed bias assessment tool which used elements of the PRISMA statement21 and the Newcastle-Ottawa scale.22 Using the tool (Table 1), the quality of the case classification (definition of index and secondary case/s); quality of contact tracing (tracing strategy, timeliness of contact tracing, completeness of follow-up), the possibility of alternative exposures/means of infection (before, during, and after the flight), as well as other limitations (e.g., circulation of the virus at the place of origin/destination of the passengers) were reviewed in a structured manner. Based on the bias assessment, we made a determination on the evidence level for influenza transmission aboard the aircraft for each of the individual flights described in a study. Each flight could earn a maximum number of nine points. The three evidence categories were defined as low (0–3 points), medium (4–6 points), and high (7–9 points). We graded favorably if the case definition included a laboratory confirmation of the diagnosis to reduce misclassification. Points were also awarded to studies with high quality of index and secondary case ascertainment, comprehensiveness, timeliness, and completeness of the contact tracing limiting the potential for selection and recall bias. We deducted one point if studies failed to control for confounders like other possible exposure before and/or after the flight.
Table 1.
Bias Assessment Tool for Evaluating the Evidence of Influenza Transmission Aboard Aircraft
Data Analysis
We estimated the proportion of secondary cases seated within two rows of the index case out of all traced passengers. This estimate was based on the numbers of all passengers on board, the numbers of passengers followed up, and the numbers of secondary cases as well as their seating distance from the index case. In the first analysis, we used all data irrespective of limitations/case definitions/contact tracing strategies, and so on. We restricted a subsequent analysis to secondary laboratory-confirmed influenza A(H1N1)pdm09 cases only.
For an estimate of the completeness of the contact tracing, the percentage of successfully traced passengers among all passengers on board was used. The attack rate among all passengers followed up was calculated by the number of secondary cases by all successfully traced passengers (overall number of secondary cases/overall number of successfully traced passengers). The percentage of secondary cases seated within two rows of the index was calculated as the number of secondary cases seated within two rows by the number of secondary cases identified in the contact tracing.
RESULTS
Literature Search/Study Selection
After deleting duplicates, the search criteria yielded 402 potentially relevant articles, 371 of which were excluded due to nonrelevant title, and/or after abstract review (Fig.). Of the remaining 31 articles, one (in Chinese) was excluded due to lack of translation capacity. Of the remaining 30 potentially relevant articles, 14 articles included sufficient data for inclusion in the final systematic review. Two investigations (one historical cohort study and one retrospective investigation) apparently reported about the same flight.23,24 However, both studies were included in our analysis, as different contact tracing strategies were applied and resulting data differed (e.g., the number of index cases, secondary cases, passengers traced, and passengers aboard). The other 16 articles were excluded as they reported findings using modeling techniques and experimental approaches rather than analyzing actual transmission events aboard aircraft. Among them the study of Perz et al.,25 although repeatedly cited as relevant, solely describes the transmission of a respiratory illness among members of a tour group returning from Ireland to the US on three different flights without following up with any of the passengers. As contact tracing was limited to the tour group members for which intensive exposure was described before the flight, this study was excluded from further analysis.
FIGURE.
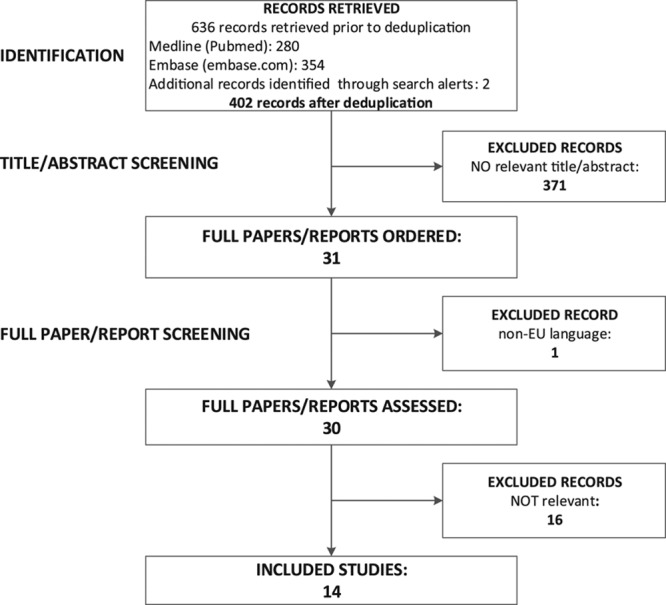
Flow diagram of the literature review process and results of article selection.
Study Characteristics
The 14 articles included in the analysis were published between July 1979 and January 2014 and reported events taking place between March 1977 and April 2009.9–11,23–35 An overview of the extracted data and a summary of the main characteristics and limitations of each study are presented in eAppendices 2 and 3 (http://links.lww.com/EDE/B6).
Flights Details
The total number of flights investigated (n = 23) exceeded the number of articles reviewed, because five studies referred to multiple or stop-over flights (eAppendix 2; http://links.lww.com/EDE/B6).11,26,27,29,33 The flight duration ranged from 45 minutes to 20 hours 20 minutes (long-distance flight with stop-over during which all passengers remained seated). The majority of flights (n = 11) were short-haul, ranging between 45 minutes and 3 hours 20 minutes. Eight single long-haul flights were described lasting between 5 hours 50 minutes and 14 hours (the legs of the 20 hours 20 minutes stop-over flight were calculated separately). Flight duration was not provided for three flights. One study reported a ground delay of more than 3 hours due to an engine failure with an inoperative ventilation system.9 No other study reported any ground delay, and in some the ventilation system was described as fully operational.
Study Types and Contact Tracing
All investigations described retrospective follow-up of passengers after identifying one or more index cases with influenza or symptoms of influenza-like illness aboard aircraft (eAppendices 2 and 3; http://links.lww.com/EDE/B6). Follow-up strategies either focused on passengers seated within two rows of the index case, passengers in the same section or class (economy), or used a comprehensive approach. Seven articles reported the assessment of multiple incidents.9,26,27,29,33,35 Among the few studies that provided details on the contact tracing method, the most frequently used method was active case finding through telephone interviews. The time span for initiating follow-up ranged between 1 day and 106 days after the flight.24,35 In four studies, contact tracing was initiated within the first 3 days after the flight.28,31,32,35 The start of contact tracing activities was described in most of the studies, however without specifying its overall duration. In four studies, additional retrospective data from the national disease notification system were retrieved and included several weeks after the flight.23,24,28,29 Five studies analyzed travel groups sharing flights and tour activities before or after the flights.11,24,28,33,34 In two studies, all passengers were staff of the same company.10,11
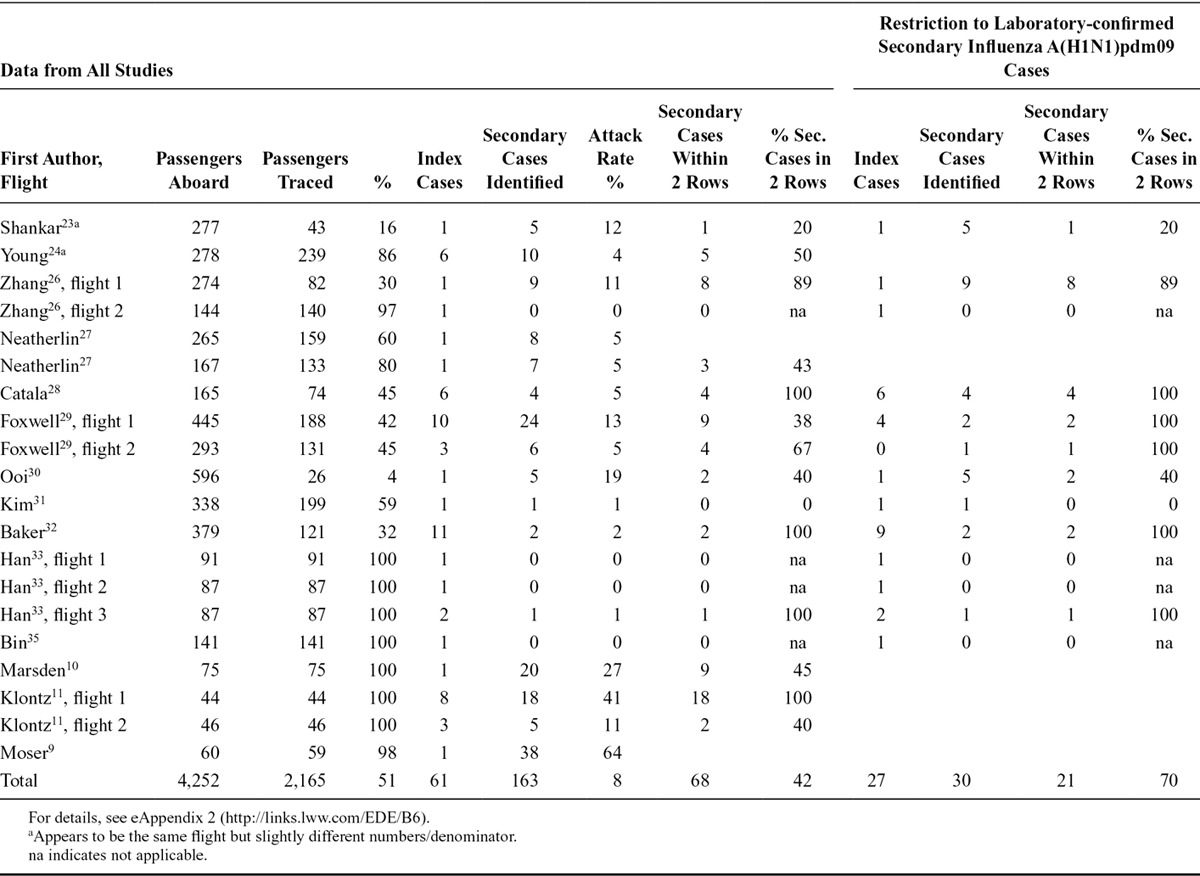
The proportion of contacts identified and traced ranged from 4 to 100 percent of all the passengers of the flight. One study described the follow-up of contacts as better for passengers remaining in the country than for transit passengers.31 For eight of the flights described, a complete or near-complete follow-up of contacts in a comprehensive contact tracing,9–11,32,33,35 or in an approach restricted to two rows from the index cases23 was achieved. In total, 2,165 (51%) of 4,252 passengers were followed up (Table 2). Restricting this calculation to confirmed influenza A(H1N1)pdm09 cases during the pandemic, the percentage of followed up passengers decreased to 43% with 1,410 of 3,317 passengers traced.
Table 2.
Number and Percentage of Successfully Traced Passengers After In-flight Exposure to Influenza, Number of Index, and Secondary Cases, and Percentage of Secondary Cases Seated Within Two Rows of an Index Case, With and Without Restriction to Laboratory-confirmed Secondary Cases Infected with influenza A(H1N1)pdm09, by Study/Flight
Pathogens, Case Definitions, and Index Cases
The majority (11/14) of studies described potential transmission of influenza A(H1N1)pdm09 virus during the initial phase of the pandemic in 2009 (eAppendix 2; http://links.lww.com/EDE/B6).23–33,35 One study investigated the transmission of influenza A/Taiwan/1/86 (H1N1) [5], one the transmission of influenza A/Alaska/1–8/77 (H3N2) [3], and one the transmission of an undetermined influenza-like illness-causing pathogen.10 Different index case definitions were applied in the studies based on (1) influenza-like illness or respiratory symptoms; (2) clinical (respiratory) symptoms and PCR confirmation; (3) clinical (respiratory) symptoms and positive culture; or (4) symptoms and positive serology (eAppendix 3; http://links.lww.com/EDE/B6). The case definitions for secondary cases also differed across the studies and were based on clinical (respiratory) symptoms only and/or additional laboratory confirmation (PCR or serology). Two studies did not provide any specific case definition for secondary cases. Five studies described incidents with more than one index case.11,24,28,29,32
On Board Transmission, Seating Proximity, and Attack Rate
Four studies did not clearly describe the number of infected passengers and attack rates, mixing symptomatic and confirmed secondary cases.10,24,27,29 Two studies reported evidence that transmission of influenza did not occur during flight (eAppendix 2; http://links.lww.com/EDE/B6).33,35 Studies identifying secondary cases reported attack rates between 0% and 64%. The flight time was not obviously associated with the attack rate: there were high number of secondary cases reported for short-distance flights10,32 and a lower number of secondary cases for long-haul flights.31,32 In total, investigators traced 2,165 (51%) of 4,252 passengers and identified 163 secondary cases for an estimated overall attack rate of the successfully traced passengers of 8%. Sixty-eight (42%) of these 163 secondary cases had been seated within two rows of an index case, whereas this proportion ranged between 0% and 100% in the respective studies (Table 2). In 13 events, each index case was associated with one or more than one secondary case.
Restricting the analysis to laboratory-confirmed secondary cases of influenza A(H1N1)pdm09 (nine studies), 1,410 (43%) of 3,317 passengers were traced and 30 secondary cases were identified resulting in an attack rate among the traced passengers of 2%. Of these secondary cases, 21 (70%) passengers that could be followed up were seated within two rows of an index case (Table 2).
Intervention After Contact Tracing
Nine of the 14 studies described an intervention following contact tracing (eAppendix 3; http://links.lww.com/EDE/B6): antiviral treatment and isolation (n = 5), only isolation of symptomatic cases or all passengers (n = 3), and assessment of the need for antiviral medication (n = 1). The remaining studies did not report any public health intervention after contact tracing.
Results of the Bias Assessment/Evaluation of the Evidence Level
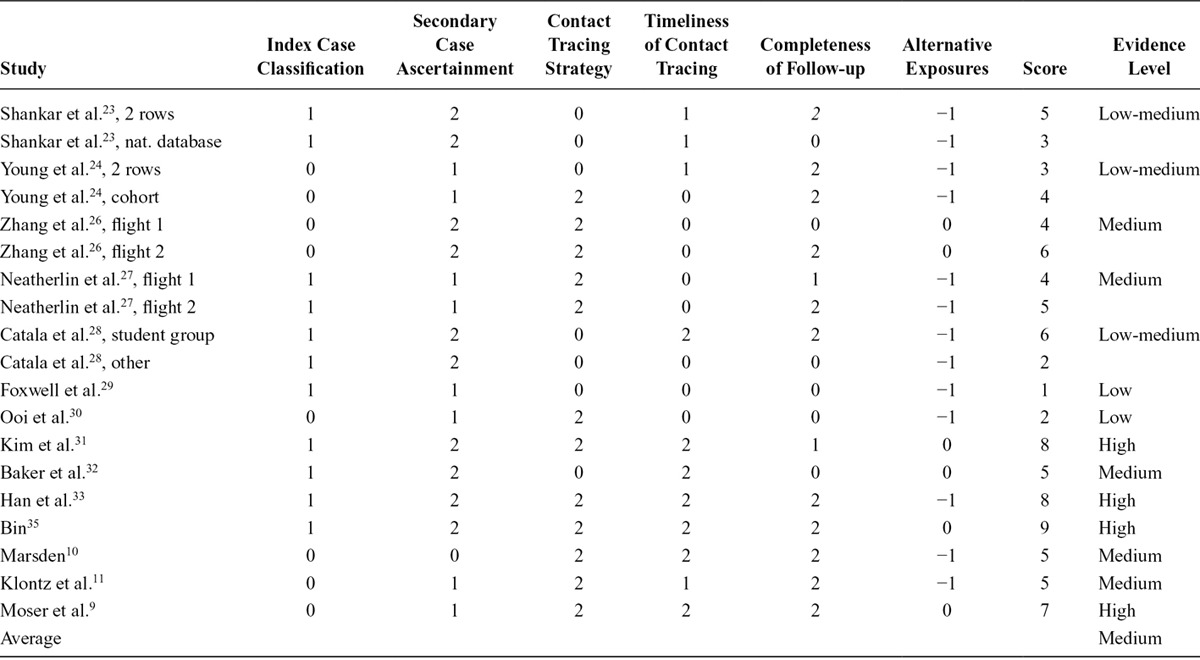
In nine (64%) of the 14 studies, the evidence level for influenza transmission aboard aircraft was low to medium (Table 3). The highest score of nine points was only reached by the study of Bin et al.,35 which describes a timely, comprehensive contact tracing with a high number of follow-ups, assuring high quality of case ascertainment by laboratory confirmation and ruling out alternative exposures. The average score of all studies was five, indicating an overall moderate quality of the evidence. In nine studies, alternative exposure was described or considered to be possible for example due to extensive circulation of the virus in the country/region of departure or travelling in a group with contact to the index case(s) before and/or after the flight. The lower scores also reflect the missing description of index and secondary case identification and delayed contact tracing with a low number of passengers being followed up. Overall, due to the major limitations discussed below, the evidence for transmission of influenza during air travel was of moderate quality.
Table 3.
Summary of the Bias Assessment Concluding on the Level of Evidence for Influenza Transmission on Aircraft
The analysis also allowed us to identify challenges, risk of biases, and limiting factors of contact tracing of influenza transmission events. Major issues identified during the analysis of the studies retrieved limiting the comparability and the evidence of influenza transmission were related to the:
Contact tracing strategy: selection bias, limitation of the representativeness of the cases due to the exclusion of passengers beyond two rows distance/section/compartment/class
Timeliness of contact tracing: recall bias due to delayed investigation and passenger identification
The proportion of passengers successfully traced: selection bias, low number of follow-ups underestimating the true number of secondary cases due to the unknown status of the nonresponders
Exclusion of crew from the investigation: selection bias, underestimating the true number of secondary cases and potential role of the crew as source of transmission
Using influenza-like illness as clinical case definition might lead to misclassification of index and secondary cases with a potential under/overestimation of the secondary attack rate among traced passengers
Use of different case definitions: selection/measurement bias: specificity and sensitivity are different using clinical and/or laboratory-confirmed (PCR or serology) cases
Testing strategy and case ascertainment might introduce selection bias, as only clinical cases or a subset of passengers were tested with more specific methods (e.g., PCR or serology)
Alternative exposure: misclassification, overestimating the number of secondary cases, and secondary attack rate among traced passengers due to transmission event before or after flight.
DISCUSSION
To our knowledge, no comprehensive review of the literature has been undertaken assessing the evidence for transmission of influenza aboard aircraft. Although influenza transmission is very common, only a very small number of articles describe contact tracing of influenza cases aboard aircraft; we found only 14 articles in 35 years. This might be due to the fact that contact tracing is not performed during a “normal” influenza season, but is increasingly used early in a pandemic, based on extraordinary objectives. The data extraction was challenging due to missing, incomplete, or unclear description of the investigation. Thus, the conclusions that can be reached regarding transmission aboard aircraft are limited.
Contact tracing of influenza is specifically challenging due to the characteristics and natural history of the influenza virus. Existing evidence supports the potential role of several means for transmission of influenza: droplets, aerosols, and contact. The differently sized particles in which the virus can be transmitted and their relative importance is considered to depend on the setting at a given time.36 Direct (airborne, large droplet, direct contact) and indirect (indirect contact via contaminated surfaces and fomites) modes of transmission have been assumed for influenza.13,37–39 Influenza virus can survive in air for periods long enough to allow transmission.12 The complex transmission dynamic of influenza make a clear identification of those at risk and a decision about consequent contact tracing difficult.
Investigation of influenza transmission is challenging as most influenza infections are asymptomatic, symptoms of influenza-like illness are nonspecific with the majority of respiratory diseases not being influenza, and the short incubation period of 0.7–2.8 days makes timely intervention difficult.40,41 Recent findings of the Flu Watch group showed that, on average, influenza infected 18% of unvaccinated people each winter with up to three-quarters of infections being asymptomatic.41 Quick and accurate diagnosis of early stages of influenza is limited and renders epidemiologic studies of transmission dynamics challenging. Additionally taking into account that transmission from asymptomatic or presymptomatic persons cannot be ruled out at all and natural immunity as well as vaccination coverage might impact transmission pattern.42–45 The possibility of asymptomatic index case transmitting virus as well as asymptomatic secondary cases not being tested due to lack of any or of specific symptoms is a major limitation of all contact tracing investigations reducing the quality of case ascertainment.
Given the short incubation time of influenza, the timeliness of contact tracing is the key for preventing further spread. Although the time point of initiation of contact tracing was reported in most of the reviewed studies, its duration was not. During the influenza pandemic it was already assessed that contact tracing was not timely enough for public health measures and incomplete of follow-ups.46 Likewise, the identification of secondary cases via the national database might be too slow for any effective public health intervention. Delayed investigations potentially bias the results toward an underestimation of cases with milder symptoms and could also hinder secondary case identification based on molecular detection (e.g., PCR method).
The main limiting factors for the comparability of the studies analyzed were different secondary case definitions, contact-tracing strategy employed, and numbers of passengers successfully followed-up. Broader unspecific case definitions, e.g., based on influenza-like illness symptoms, might lead to misclassification and overestimation of transmission. Previous studies have shown that the incidence of confirmed influenza A(H1N1)pdm09 among persons with respiratory illness is low: in a study of 79 symptomatic travelers (90% with ARI, 66% with fever) who flew on a commercial airplane from North America to Sweden, only 5% were confirmed to be infected with A(H1N1)pdm09 and 34% were rhinovirus-positive.47
The assumption that the risk of transmission increases with the length of flight time due to a higher exposure, as shown for tuberculosis48,49 and modeled for influenza,50 was not corroborated in the studies analyzed here: there were short and long flights with high and low numbers of secondary cases. In a modeling attempt, the risk of infection posed by a single case of influenza A(H1N1)pdm09 was estimated at 5–10 new infections over an 11-hour flight.50 The same study estimated that the risk for transmission is lower in first compared with economy class which is in line with some but not all studies, as transmission occurred in small aircraft with one compartment as well. Furthermore, the number of infected passengers on board seemed not to be directly associated with the number of secondary cases. In-flight influenza transmission therefore seems to be rather a multifactorial event including the number of index cases, infectivity, and proximity to the index case as well as other factors not investigated so far, e.g., immune status, age, contaminated surfaces, etc.
The ventilation system on board of aircraft might also play a crucial role. A functional ventilation system, high-efficiency particulate air filters, and a high air exchange rate (15 times per hour) is assumed to be even more preventive than negative-pressure isolation rooms for multidrug-resistant tuberculosis cases.51 A study comparing the risk for an upper respiratory infection during air travel in 50% recirculated versus 100% fresh air found no difference between the two groups.52 The currently used environmental control system managing the air flow should minimize risks for passengers other than sitting within close proximity to an index patient.53 While the in-flight risk for airborne transmission seems to be minimal, ground delays without adequate ventilation do pose a risk. This is also reflected in a statement by the US Department of Transportation: “If the ventilation system is not operating, passengers should not stay aboard the plane for long time (i.e., more than 30 minutes).”54 The study of Moser described potential transmission influenza via aerosol with a high attack rate associated with a ground delay of 3 hours without operational ventilation system.9 Another study showed that the concentration of microorganisms in the cabin air is much lower than in shopping malls and the air terminal.55
According to WHO and others, the risk of acquiring influenza infections in aircraft appears to be similar to the risk in other situations when people are in close proximity to one another over a certain period of time, such as on a train or bus, in office buildings, theatres, etc.56,57 For example, the tracing of contacts of a woman with influenza A(H1N1)pdm09 during a long-distance bus trip revealed a low attack rate of 2% among the 72% of passengers that could be followed up.14
It is assumed that the likelihood of influenza transmission increases in close proximity to the index case, and WHO recommends contact tracing of passengers seated within two rows of a case of influenza.16 The studies included in this review indicate that 42% of secondary cases were seated within two rows of an index case, increasing to 70% when restricting the analysis to confirmed influenza A(H1N1)pdm09 cases. These findings suggest a higher risk in close proximity. The identification of secondary cases greater distance to the index case, however, limits the evidence for restricting contact tracing to those in close proximity especially when aiming for containment of the disease. In the analysis restricted to confirmed influenza A(H1N1)pdm09, the secondary attack rate among traced passengers was 2% and thus lower than the overall estimate of 8%. The completeness of contact tracing investigation is key for the reliability of the attack rate estimation. This is important in particular when analyzing the association between influenza transmission to passengers and the proximity to an index case. Furthermore, this association is biased by a possible common exposure before (e.g., waiting hall), within (lavatory rooms), or after the flight (e.g., queuing to exit the aircraft, lining at border entry, security checkpoints). Many studies also failed to include the flight crew in the contact tracing, although a previous study showed that many crew members were flying while sick.58
Possibilities to become infected before or after the flight are manifold, especially if influenza activity is high in the country of origin. In some of the studies reviewed, prior and posthoc exposure cannot be clearly separated from in-flight exposure. Systematic studies on the transmission of influenza on aircraft (e.g., the mode and extent/effectiveness including also seroepidemiologic and environmental analyses) are limited.59,60 To determine the real extent of influenza transmission, serologic testing to identify seroconversion of asymptomatic cases would be required.
In many studies, the purpose of contact tracing and the intervention measure were not clear. With all the limitations described, the decision on the initiation of contact tracing should be based on clear objectives. Contact tracing is of limited value if an appropriate public health intervention after identification of infected individuals cannot be offered (i.e., vaccine, antivirals, and isolation).
The expert group for the RAGIDA influenza chapter considered that the decision on contact tracing has to be based on a situational risk assessment, taking into account specific limitations for influenza (i.e., the time period during which it is possible to intervene with public health measures).18 The experts made recommendations for contact tracing, taking the index case’s classification (laboratory confirmation), infectivity during flight, the time factor (between flight and identification of index case less than 10 days) and the epidemiologic situation in the country of destination (no evidence of transmission) as well as country of departure (no evidence of transmission) into consideration: three specific scenarios were identified, differentiated on the bases of the type of influenza virus, for which the recommendations were
(1) Seasonal influenza: no contact tracing
(2) Novel influenza virus with pandemic potential OR seasonal influenza virus with increased virulence (emergence of a novel influenza virus in humans with known or suspected sustained human-to-human transmission OR a seasonal virus with increased virulence): comprehensive contact tracing of all passengers
(3) Influenza virus with zoonotic potential (e.g., avian, swine influenza): contact tracing of close contacts and passengers seated two seats away in all directions from the index
CONCLUSIONS
Overall, the quality of evidence in the published literature of transmission of influenza virus during flight is moderate. There is evidence that influenza transmission aboard aircraft occurs, but the published data do not permit any conclusive assessment of the likelihood and extent. The studies were often biased by other potential exposures before or after the flight. There might be an association between seating and transmission to passengers in proximity to an index case, but the evidence is not robust enough to propose a single standard approach for contact tracing of passengers following potential transmission of influenza on board aircraft. The decision on the initiation of contact tracing needs to be taken case-by-case and based on clear objectives. Our findings and those of others underline the need for a standardized approach to investigation and reporting once the decision on initiation of contact tracing has been taken.46
Supplementary Material
Footnotes
This study has been supported by internal funding of the European Centre for Disease Prevention and Control (ECDC).
The authors Katrin Leitmeyer and Cornelia Adlhoch contributed equally to this study.
The authors report no conflicts of interest.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
REFERENCES
- 1.EUROSTAT. Available at: http://epp.eurostat.ec.europa.eu/portal/page/portal/transport/data/main_tables. Accessed April 9, 2014.
- 2.Federal Aviation Administration. Terminal area forecast reports. Available at: https://www.faa.gov/data_research/aviation/taf/media/taf_summary_fy2014-2040.pdf. Accessed February 2016.
- 3.Tatem AJ. The worldwide airline network and the dispersal of exotic species: 2007-2010. Ecography (Cop) 2009;32:94–102. doi: 10.1111/j.1600-0587.2008.05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colizza V, Barrat A, Barthélemy M, Vespignani A. The role of the airline transportation network in the prediction and predictability of global epidemics. Proc Natl Acad Sci USA. 2006;103:2015–2020. doi: 10.1073/pnas.0510525103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flint J, Burton S, Macey JF, et al. Assessment of in-flight transmission of SARS–results of contact tracing, Canada. Can Commun Dis Rep. 2003;29:105–110. [PubMed] [Google Scholar]
- 6.Lycett S, McLeish NJ, Robertson C, et al. Origin and fate of A/H1N1 influenza in Scotland during 2009. J Gen Virol. 2012;93(Pt 6):1253–1260. doi: 10.1099/vir.0.039370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bajardi P, Poletto C, Ramasco JJ, Tizzoni M, Colizza V, Vespignani A. Human mobility networks, travel restrictions, and the global spread of 2009 H1N1 pandemic. PLoS One. 2011;6:e16591. doi: 10.1371/journal.pone.0016591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desenclos JC, van der Werf S, Bonmarin I, et al. Introduction of SARS in France, March-April, 2003. Emerg Infect Dis. 2004;10:195–200. doi: 10.3201/eid1002.030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moser MR, Bender TR, Margolis HS, Noble GR, Kendal AP, Ritter DG. An outbreak of influenza aboard a commercial airliner. Am J Epidemiol. 1979;110:1–6. doi: 10.1093/oxfordjournals.aje.a112781. [DOI] [PubMed] [Google Scholar]
- 10.Marsden AG. Outbreak of influenza-like illness related to air travel. Med J Aust. 2003;179:172–173. doi: 10.5694/j.1326-5377.2007.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 11.Klontz KC, Hynes NA, Gunn RA, Wilder MH, Harmon MW, Kendal AP. An outbreak of influenza A/Taiwan/1/86 (H1N1) infections at a naval base and its association with airplane travel. Am J Epidemiol. 1989;129:341–348. doi: 10.1093/oxfordjournals.aje.a115137. [DOI] [PubMed] [Google Scholar]
- 12.Weber TP, Stilianakis NI. Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. J Infect. 2008;57:361–373. doi: 10.1016/j.jinf.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piso RJ, Albrecht Y, Handschin P, Bassetti S. Low transmission rate of 2009 H1N1 Influenza during a long-distance bus trip. Infection. 2011;39:149–153. doi: 10.1007/s15010-011-0084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Low CL, Chan PP, Cutter JL, Foong BH, James L, Ooi PL. International health regulations: lessons from the influenza pandemic in Singapore. Ann Acad Med Singapore. 2010;39:325–323. [PubMed] [Google Scholar]
- 16.World Health Organization. WHO technical advice for case management of Influenza A(H1N1) in air transport. May 13, 2009; Available at: http://www.who.int/ihr/travel/A(H1N1)_air_transport_guidance.pdf. Accessed February 3, 2016.
- 17.Leitmeyer K. European risk assessment guidance for infectious diseases transmitted on aircraft–the RAGIDA project. Euro Surveill. 2011;16 [PubMed] [Google Scholar]
- 18.European Centre for Disease Prevention and Control (ECDC) Risk assessment guidelines for infectious diseases transmitted on aircraft (RAGIDA) - Influenza. Available at: http://www.ecdc.europa.eu/en/publications/Publications/influenza-RAGIDA-2014.pdf. Accessed August 2015.
- 19.European Centre for Disease Prevention and Control (ECDC) Risk assessment guidelines for diseases transmitted on aircraft. PART 2: operational guidelines for assisting in the evaluation of risk for transmission by disease. Available at: http://ecdc.europa.eu/en/publications/Publications/0911_GUI_Risk_Assessment_Guidelines_for_Diseases_Transmitted_on_Aircraft.pdf. Accessed August 2015.
- 20.European Centre for Disease Prevention and Control (ECDC) Risk assessment guidelines for infectious diseases transmitted on aircraft. Available at: http://ecdc.europa.eu/en/publications/Publications/0906_TER_Risk_Assessment_Guidelines_for_Infectious_Diseases_Transmitted_on_Aircraft.pdf. Accessed August 2015.
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009;3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 22.Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for assessing non randomised studies in meta-analysis. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp, 2014. Accessed January 2014.
- 23.Shankar AG, Janmohamed K, Olowokure B, et al. Contact tracing for influenza A(H1N1)pdm09 virus-infected passenger on international flight. Emerg Infect Dis. 2014;20:118–120. doi: 10.3201/eid2001.120101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young N, Pebody R, Smith G, et al. International flight-related transmission of pandemic influenza A(H1N1)pdm09: an historical cohort study of the first identified cases in the United Kingdom. Influenza Other Respir Viruses. 2014;8:66–73. doi: 10.1111/irv.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perz JF, Craig AS, Schaffner W. Mixed outbreak of parainfluenza type 1 and influenza B associated with tourism and air travel. Int J Infect Dis. 2001;5:189–191. doi: 10.1016/s1201-9712(01)90068-2. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Peng Z, Ou J, et al. Protection by face masks against influenza A(H1N1)pdm09 virus on trans-Pacific passenger aircraft, 2009. Emerg Infect Dis. 2013;19 doi: 10.3201/eid1909.121765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neatherlin J, Cramer EH, Dubray C, et al. Influenza A(H1N1)pdm09 during air travel. Travel Med Infect Dis. 2013;11:110–118. doi: 10.1016/j.tmaid.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catala L, Rius C, García de Olalla P, et al. Pandemic A/H1N1 influenza: transmission of the first cases in Spain. Enferm Infecc Microbiol Clin. 2012;30:60–63. doi: 10.1016/j.eimc.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Foxwell AR, Roberts L, Lokuge K, Kelly PM. Transmission of influenza on international flights, may 2009. Emerg Infect Dis. 2011;17:1188–1194. doi: 10.3201/eid1707.101135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ooi PL, Lai FY, Low CL, et al. Clinical and molecular evidence for transmission of novel influenza A(H1N1/2009) on a commercial airplane. Arch Intern Med. 2010;170:913–915. doi: 10.1001/archinternmed.2010.127. [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Lee DH, Shin SS, et al. In-flight transmission of novel influenza A (H1N1). Epidemiol Health. 2010;32:e2010006. doi: 10.4178/epih/e2010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker MG, Thornley CN, Mills C, et al. Transmission of pandemic A/H1N1 2009 influenza on passenger aircraft: retrospective cohort study. BMJ. 2010;340:c2424. doi: 10.1136/bmj.c2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han K, Zhu X, He F, et al. Lack of airborne transmission during outbreak of pandemic (H1N1) 2009 among tour group members, China, June 2009. Emerg Infect Dis. 2009;15:1578–1581. doi: 10.3201/eid1510.091013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perz JF, Craig AS, Schaffner W. Mixed outbreak of parainfluenza type 1 and influenza B associated with tourism and air travel. Int J Infect Dis. 2001;5:189–191. doi: 10.1016/s1201-9712(01)90068-2. [DOI] [PubMed] [Google Scholar]
- 35.Bin C, Xingwang L, Yuelong S, et al. National Influenza A Pandemic (H1N1) 2009 Clinical Investigation Group. Clinical and epidemiologic characteristics of 3 early cases of influenza A pandemic (H1N1) 2009 virus infection, People’s Republic of China, 2009. Emerg Infect Dis. 2009;15:1418–1422. doi: 10.3201/eid1509.090794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Killingley B, Nguyen-Van-Tam J. Routes of influenza transmission. Influenza Other Respir Viruses. 2013;7(Suppl 2):42–51. doi: 10.1111/irv.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. Transmission of influenza A in human beings. Lancet Infect Dis. 2007;7:257–265. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- 38.Cowling BJ, Ip DK, Fang VJ, et al. Aerosol transmission is an important mode of influenza A virus spread. Nat Commun. 2013;4:1935. doi: 10.1038/ncomms2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemieux C, Brankston G, Gitterman L, Hirji Z, Gardam M. Questioning aerosol transmission of influenza. Emerg Infect Dis. 2007;13:173–174; author reply 174. doi: 10.3201/eid1301.061202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DA. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9:291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayward AC, Fragaszy EB, Bermingham A, et al. Flu Watch Group. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med. 2014;2:445–454. doi: 10.1016/S2213-2600(14)70034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patrozou E, Mermel LA. Does influenza transmission occur from asymptomatic infection or prior to symptom onset? Public Health Rep. 2009;124:193–196. doi: 10.1177/003335490912400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thai PQ, Mai le Q, Welkers MR, et al. Pandemic H1N1 virus transmission and shedding dynamics in index case households of a prospective Vietnamese cohort. J Infect. 2014;68:581–590. doi: 10.1016/j.jinf.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freitas FT, Cabral AP, Barros EN, et al. Pre-symptomatic transmission of pandemic influenza H1N1 2009: investigation of a family cluster, Brazil. Epidemiol Infect. 2013;141:763–766. doi: 10.1017/S0950268812001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suess T, Remschmidt C, Schink SB, et al. Comparison of shedding characteristics of seasonal influenza virus (sub)types and influenza A(H1N1)pdm09; Germany, 2007-2011. PLoS One. 2012;7:e51653. doi: 10.1371/journal.pone.0051653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swaan CM, Appels R, Kretzschmar ME, van Steenbergen JE. Timeliness of contact tracing among flight passengers for influenza A/H1N1 2009. BMC Infect Dis. 2011;11:355. doi: 10.1186/1471-2334-11-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Follin P, Lindqvist A, Nyström K, Lindh M. A variety of respiratory viruses found in symptomatic travellers returning from countries with ongoing spread of the new influenza A(H1N1)v virus strain. Euro Surveill. 2009;14 doi: 10.2807/ese.14.24.19242-en. [DOI] [PubMed] [Google Scholar]
- 48.Dowdall NP, Evans AD, Thibeault C. Air travel and TB: an airline perspective. Travel Med Infect Dis. 2010;8:96–103. doi: 10.1016/j.tmaid.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Plotkin BJ, Hardiman MC. The international health regulations (2005), tuberculosis and air travel. Travel Med Infect Dis. 2010;8:90–95. doi: 10.1016/j.tmaid.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Wagner BG, Coburn BJ, Blower S. Calculating the potential for within-flight transmission of influenza A (H1N1). BMC Med. 2009;7:81. doi: 10.1186/1741-7015-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sehulster L, Chinn RY CDC; HICPAC. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR-10):1–42. [PubMed] [Google Scholar]
- 52.Zitter JN, Mazonson PD, Miller DP, Hulley SB, Balmes JR. Aircraft cabin air recirculation and symptoms of the common cold. JAMA. 2002;288:483–486. doi: 10.1001/jama.288.4.483. [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization. Tuberculosis and Air Travel: Guidelines for Prevention and Control. 3rd ed. Geneva, Switzerland: World Health Organization; 2008. Available at: http://www.who.int/tb/publications/2008/WHO_HTM_TB_2008.399_eng.pdf. Accessed February 2016. [PubMed] [Google Scholar]
- 54.Nagda NL FR, Loontz MD, et al. Washington, DC: US Department of Transportation; 1989. Airliner cabin environment: contaminant measurements, health risks ans mitigation options. [Google Scholar]
- 55.Wick RL, Jr, Irvine LA. The microbiological composition of airliner cabin air. Aviat Space Environ Med. 1995;66:220–224. [PubMed] [Google Scholar]
- 56.World Health Organization. Transmission of communicable diseases on aircraft. Available at: http://www.who.int/ith/mode_of_travel/tcd_aircraft/en/index.html. Accessed November 2014.
- 57.Cui F, Luo H, Zhou L, et al. Transmission of pandemic influenza A (H1N1) virus in a train in China. J Epidemiol. 2011;21:271–277. doi: 10.2188/jea.JE20100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartz MD, Macias-Moriarity LZ, Schelling J. Professional aircrews’ attitudes toward infectious diseases and aviation medical issues. Aviat Space Environ Med. 2012;83:1167–1170. doi: 10.3357/asem.3330.2012. [DOI] [PubMed] [Google Scholar]
- 59.Gupta JK, Lin CH, Chen Q. Risk assessment of airborne infectious diseases in aircraft cabins. Indoor Air. 2012;22:388–395. doi: 10.1111/j.1600-0668.2012.00773.x. [DOI] [PubMed] [Google Scholar]
- 60.Han Z, To GN, Fu SC, Chao CY, Weng W, Huang Q. Effect of human movement on airborne disease transmission in an airplane cabin: study using numerical modeling and quantitative risk analysis. BMC Infect Dis. 2014;14:434. doi: 10.1186/1471-2334-14-434. [DOI] [PMC free article] [PubMed] [Google Scholar]


