Abstract
INTRODUCTION
Reducing racial/ethnic disparities is a primary objective of the National Alzheimer’s Plan (NAPA), yet direct comparisons within large samples representing diversity of the United States are lacking.
METHODS
Dementia incidence from 1/1/2000-12/31/2013 and 25-year cumulative risk in 274,283 healthcare members aged 64+ (n=18,778 African-American, n=4,543 American Indian/Alaska Native (AIAN), n=21,000 Latino, n=440 Pacific Islander, n=206,490 White, n=23,032 Asian-Americans). Cox proportional hazard models were adjusted for age, sex, medical utilization, and comorbidities.
RESULTS
Dementia incidence (N=59,555) was highest for African-Americans (26.6/1,000 person-years) and AIANs (22.2/1,000 person-years); intermediate for Latinos (19.6/1,000 person-years), Pacific Islanders (19.6/1,000 person-years), and Whites (19.3/1,000 person-years); and lowest among Asian-Americans (15.2/1,000 person-years). Risk was 65% greater for African-Americans (hazard ratio=1.65; 95% confidence interval=1.58-1.72) versus Asian-Americans. Cumulative 25-year risk at age 65 was: 38% African-Americans, 35% AIANs, 32% Latino, 25% Pacific Islanders, 30% White, and 28% Asian-Americans.
DISCUSSION
Dementia rates varied over 60% between groups, providing a comprehensive benchmark for the NAPA goal of reducing disparities.
Keywords: Dementia, race, ethnicity, disparities, cohort, epidemiology
1. Background
Reducing racial/ethnic disparities in dementia has been identified as a national priority by both the National Alzheimer’s Plan [1] and the Alzheimer’s Disease-Related Dementias Conference [2] roadmaps. It is widely believed there are racial/ethnic disparities in dementia incidence in the United States [3, 4], and social and behavioral factors are thought to be major drivers of these inequalities [5-8]. However, the currently available evidence is incomplete and difficult to interpret. No prior research has directly compared dementia incidence in a single population representing the diversity of the United States, including African Americans, American Indians and Alaska Natives, Latinos, Pacific Islanders, Whites, and Asian Americans. Most research on racial/ethnic inequalities in dementia includes only one or two racial/ethnic group or compares dementia prevalence or incidence across studies. This is problematic because variability in diagnostic criteria can strongly influence estimates [9, 10] and geographic patterns may also contribute to differences across studies [11]. Furthermore, to our knowledge, no population-based studies have included Pacific Islanders or American Indians and Alaska Natives. Dementia risk is reported to be up to twice as high for African Americans compared with Whites [5, 12-15]. Evidence on dementia risk among Latinos is less consistent, but suggests elevated dementia risk among Caribbean Latinos in New York City [12], but not among Mexican Americans in California [16], compared with Whites. Dementia incidence rates among Japanese Americans have been reported to be similar or lower compared with Whites in other studies [17, 18]. To our knowledge, there is not work examining dementia incidence among other Asian American populations.
The present study leverages 14 years of prospective data on a large, diverse older adult cohort in Northern California with equal access to healthcare. Our primary objective was to evaluate racial/ethnic inequalities in dementia incidence among African Americans, American Indians and Alaska Natives, Latinos, Pacific Islanders, Whites, and Asian Americans in this sample. Since dementia risk increases with age and some studies have shown dementia risk to be higher among women than men [4] and racial/ethnic disparities often vary by sex and age[19], we examined inequalities overall and also stratified by sex and age. Finally, because higher annual incidence rates of dementia may be offset by differences in mortality due to other causes, we estimated the 25-year cumulative incidence of dementia for each group.
2. Methods
2.1 Study population
Kaiser Permanente Northern California (KPNC) is a large, integrated healthcare delivery system providing comprehensive medical care to over 3 million members (30% of the geographic region). The member population is generally representative of the overall regional population, but underrepresents individuals at extreme tails of the income distribution [20-22]. Seniors (age ≥65) covered by KPNC are similar to the general population of seniors residing in Northern California with respect to history of chronic conditions, including diabetes, hypertension, heart disease, and asthma, and lifestyle factors, including smoking, obesity, and sedentary lifestyle [22]. California Health Interview Survey [23] results show similar patterns of racial inequalities for major chronic conditions as those observed in KPNC (authors’ calculation). The present study includes KPNC members who were enrolled and age ≥60 years as of 1/1/1996 (the year KPNC implemented electronic medical records). To ensure dementia diagnoses detected were incident cases, we included a four-year washout period (1/1/1996-12/31/1999). The present analyses include members who were still alive, KPNC members, and had no dementia diagnosis as of 1/1/2000. Cohort members were followed for incident dementia until end of health plan membership (defined as a gap in membership of ≥3 months), death, or 12/31/2013 (end of study period). On average, 1.2% of the baseline population ended health plan membership annually; this number was slightly higher among Pacific Islanders, for whom the rate was 2.3% annually.
2.2 Measures
Exposure
Self-reported race/ethnicity was retrieved from health plan membership databases and categorized into six groups: African American or Black, American Indian and Alaska Native (AIAN), Native Hawaiian or other Pacific Islander, Latino or Hispanic, non-Latino White, and Asian American. Members who identified as other or multi-racial were excluded from analyses due to small sample sizes. The primary Latino subgroup represented in health plan members is Mexican Americans. The primary Asian American subgroups represented in health plan members are Chinese Americans, Filipino Americans, and Japanese Americans, but we did not evaluate subgroups separately.
Outcome
Dementia diagnoses were identified from electronic medical records of inpatient and outpatient encounters between 1/1/2000 and 12/31/2013 based on International Classification of Diseases, Ninth Revision (ICD-9) diagnostic codes for Alzheimer’s disease (331.0), vascular dementia (290.4x), and nonspecific dementia (290.0, 290.1x, 290.2x, 290.3, 294.2x, 294.8). Dementia case identification using ICD-9 codes has been used successfully in other publications in this population [24-27]. Dementia diagnosis in neurology, memory clinic, and neuropsychology departments in the KPNC system is typically based on information from medical history, physical examination, mental status examination, blood test, functional ability, and imaging. A similar battery of ICD-9 codes for diagnosis of dementia was found to have a sensitivity of 77% and a specificity of 95% compared with a consensus diagnosis of dementia in a healthcare system in Seattle, Washington [28]. A similar battery of ICD-9 codes from Medicare claims data had a sensitivity of 87% for a sample of Alzheimer’s disease patients ages 65+ who participated in the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) [29]. There were a small number of diagnoses of frontotemporal dementia (331.1x), dementia with Lewy bodies (331.82), and Parkinson’s dementia (332.0 + 294.1x) in the KPNC records (cumulative incidence <2% over the entire 18-year washout and follow-up period), and we did not consider these as cases in the current analyses, unless the patient was also diagnosed with a more common diagnostic code.
Mortality was identified from electronic medical records for deaths that occurred within the healthcare system and the California Automated Mortality Linkage System, which captured deaths from the California State Mortality File and Social Security Death Records. This mortality ascertainment method is standard for epidemiologic studies of KPNC members [25, 30].
Covariates
Age and sex were retrieved from KPNC health plan membership databases. We measured baseline characteristics, including healthcare utilization and comorbidities, during the washout period (1/1/1996-12/31/1999). As a measure of healthcare utilization, we calculated a dichotomous variable for whether participants had ≥1 healthcare visit (inpatient or outpatient) per year during the washout period. We identified comorbidities, including diabetes, depression, hypertension, stroke (ischemic stroke, transient ischemic attack, and hemorrhagic stroke), and cardiovascular disease (myocardial infarction, heart failure, ischemic heart disease, and peripheral arterial disease) from inpatient and outpatient visits (Table A.1).
2.3 Statistical Analysis
Age-adjusted dementia incidence rates by race/ethnicity were estimated by standardization to the 2000 United States Census population. Dementia incidence rates were also estimated for each racial/ethnic group by sex and age group (64-69, 70-74, 75-79, 80-84, 85-89, or 90+ years). We specified Cox proportional hazards models to estimate the association between race/ethnicity and dementia risk. Because of the strong association between age and dementia incidence, we used age as the timescale, starting from age as of 1/1/2000 until age at dementia diagnosis, death, end of health plan membership, or 12/31/2013 (end of study period). We examined both overall and sex-specific hazard ratios for dementia. We first examined racial/ethnic differences in dementia risk after adjustment for age (as timescale) and sex (Model 1). Next, we additionally adjusted for healthcare utilization (≥1 annual healthcare visit) to account for potential differences in diagnosis rates arising from unequal healthcare utilization (Model 2). To examine whether proximal comorbidities could explain racial/ethnic differences in dementia, we additionally adjusted for depression, hypertension, diabetes, stroke, and cardiovascular disease (Model 3). To formally test whether dementia incidence rates differed by sex within a racial/ethnic group, we evaluated whether the 84% confidence intervals overlapped to test whether the incidence rates differ at p-value<0.05 [31]. To formally test whether racial disparities in dementia incidence differ by sex, we specified a Cox proportional hazards model including both females and males with a multiplicative interaction term between race/ethnicity and sex.
We estimated cumulative incidence of dementia (at 5-year intervals from 5 to 35 years) adjusted for the competing risk of death for individuals who survived dementia-free to age 65 using the Practical Incidence Estimator Macro developed by Framingham Study investigators [32]. Cumulative incidence is interpreted as the percent of individuals expected to develop dementia prior to death over a hypothetical a follow-up period. Analyses were performed with SAS version 9.3 (SAS Institute Inc., Cary, NC).
To contextualize the magnitude of inequalities, we applied incidence rates in each race/ethnicity and age stratum to 2013 United States Census Population Estimates. We then estimated how many dementia cases could be eliminated if all race/ethnic groups had the incidence rate achieved by the lowest risk group.
3. Results
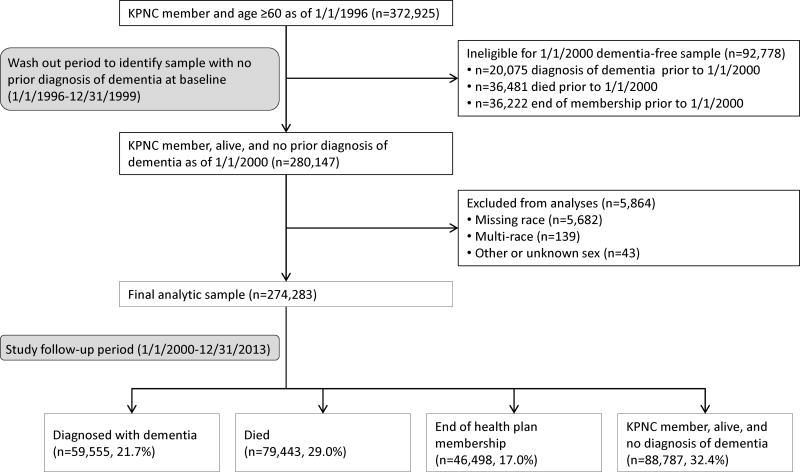
Of the n=372,925 KPNC members who were age ≥60 years as of 1/1/1996, n=280,147 met eligibility criteria after the washout. We excluded n=5,864 members who were missing race, mixed race, or were recorded as other or unknown sex, leaving a final analytic cohort of n=274,283 members (Figure 1). At baseline, mean age was 73.4 years and 54.6% of the sample was female. Regular contact with the healthcare system was common: over 80% of members had ≥1 annual healthcare visit. The proportion of members with ≥1 annual healthcare visit was highest among AIANs and lowest among Pacific Islanders. The proportion of individuals with ≥1 comorbidity was highest among African Americans and lowest among Pacific Islanders (Table 1).
Figure 1. Flow of study participants.
Table 1.
Baseline characteristics of the sample by race/ethnicity.
| Variable | African American | American Indian and Alaska Native | Latino | Pacific Islander | White | Asian American |
|---|---|---|---|---|---|---|
| n | 18,778 | 4,543 | 21,000 | 440 | 206,490 | 23,032 |
| Age (years), mean (SD) | 72.7 (6.5) | 73.5 (6.3) | 71.9 (5.9) | 71.5 (7.0) | 73.9 (6.7) | 71.7 (5.9) |
| Female, % | 54.9 | 54.3 | 52.4 | 49.8 | 54.9 | 53.1 |
| ≥1 healthcare visit per year, % | 80.5 | 87.5 | 81.6 | 58.0 | 82.3 | 78.9 |
| Depression, % | 8.2 | 14.0 | 11.4 | 3.6 | 11.6 | 5.5 |
| Hypertension, % | 68.4 | 54.4 | 52.5 | 44.3 | 49.9 | 53.8 |
| Diabetes, % | 35.6 | 29.6 | 37.2 | 29.1 | 21.3 | 33.5 |
| Stroke, % | 9.2 | 9.5 | 7.3 | 7.5 | 8.5 | 6.2 |
| Cardiovascular disease*, % | 24.6 | 28.2 | 20.4 | 17.7 | 24.1 | 16.8 |
| Myocardial infarction, % | 2.3 | 3.2 | 2.5 | 2.5 | 2.8 | 2.1 |
| Heart failure, % | 10.0 | 8.9 | 6.6 | 7.5 | 8.3 | 5.2 |
| Ischemic heart disease, % | 16.3 | 21.2 | 15.7 | 13.6 | 17.7 | 13.4 |
| Peripheral arterial disease, % | 7.7 | 7.8 | 5.3 | 4.1 | 6.8 | 3.2 |
| Any comorbidity+ | 79.7 | 74.2 | 72.0 | 59.3 | 66.7 | 69.2 |
Baseline characteristics collected between 1/1/1996-12/31/1999.
Cardiovascular disease = myocardial infarction, heart failure, ischemic heart disease, and peripheral arterial disease.
Any comorbidity (depression, diabetes, stroke, or cardiovascular disease.
Over an average of 8.6 years (SD=4.9) follow-up, 59,555 members (21.7%) were diagnosed with dementia, 79,443 (29.0%) died, 46,498 (17.0%) were lost to follow-up (ended KPNC membership), and 88,787 (32.4%) were still alive and KPNC members without a diagnosis of dementia at end of follow-up.
Age-adjusted dementia incidence rates were highest among African Americans and AIANs, lowest among Asian Americans-, and intermediate among Latinos, Pacific Islanders, and Whites (Table 2). Hazard ratios (95% CIs) adjusted for age (as timescale), sex, and healthcare utilization, relative to Asian Americans were 1.73 (1.66-1.81) for African Americans, 1.42 (1.33-1.51) for AIAN, 1.29 (1.23-1.35) for Latinos, 1.28 (1.00-1.65) for Pacific Islanders, and 1.25 (1.21-1.29) for Whites (Table 2). Results remained similar after adjustment for comorbidities.
Table 2.
Dementia incidence by race/ethnicity, 2000-2013.
| Race/ethnicity | Events | Person-years | Age-adjusted incidence rate per 1,000 person-years (95% CI) | Hazard ratio (95% CI) | ||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| African American | 4,942 | 157,118 | 26.60 (25.83, 27.37) | 1.73 (1.66, 1.81) | 1.73 (1.66, 1.81) | 1.65 (1.58, 1.72) |
| American Indian and Alaska Native | 1,224 | 41,182 | 22.18 (20.85, 23.52) | 1.43 (1.34, 1.52) | 1.42 (1.33, 1.51) | 1.32 (1.24, 1.41) |
| Latino | 4,371 | 195,686 | 19.59 (18.97, 20.20) | 1.29 (1.24, 1.35) | 1.29 (1.23, 1.35) | 1.24 (1.19, 1.29) |
| Pacific Islander | 61 | 3,246 | 19.63 (14.51, 24.75) | 1.26 (0.98, 1.63) | 1.28 (1.00, 1.65) | 1.23 (0.95, 1.58) |
| White | 45,110 | 1,750,252 | 19.35 (19.16, 19.54) | 1.25 (1.21, 1.30) | 1.25 (1.21, 1.29) | 1.22 (1.18, 1.26) |
| Asian American | 3,847 | 224,120 | 15.24 (14.73, 15.74) | 1.00 ( ref) | 1.00 ( ref) | 1.00 ( ref) |
Age-adjusted dementia incidence rates use 2000 US Census as standard. Hazard ratios for dementia are from Cox proportional hazards models. Model 1: adjusted for age (as timescale) and sex; Model 2: Model 1 + healthcare utilization (≥1 heathcare visit per year); Model 3: Model 2 + depression, diabetes, hypertension, stroke, and cardiovascular disease.
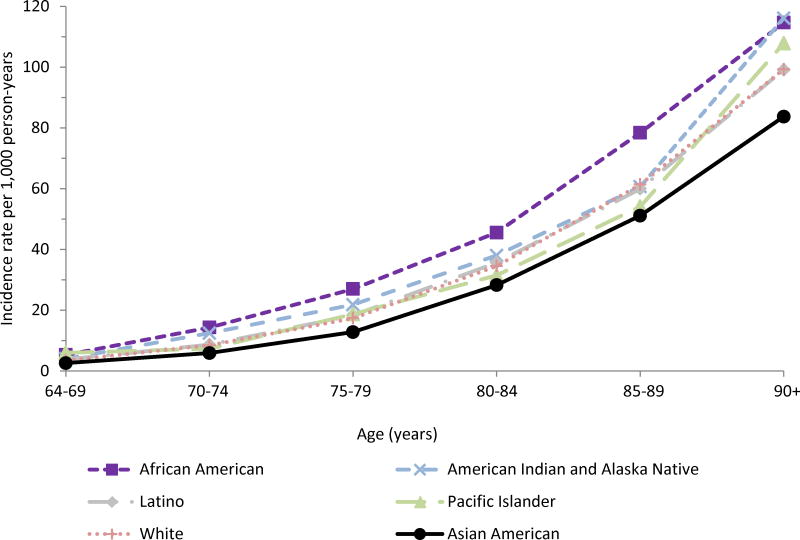
Dementia incidence was highest among African Americans, followed by AIANs, and lowest among Asian Americans at all ages (Figure 2, Table A.2). Absolute differences in dementia incidence (comparisons based on subtraction) increased with age, but relative differences (comparisons based on ratios) decreased with age. For example, at ages 70-74 years, the incidence rate among African Americans compared with Asian Americans was 14 versus 6 cases per 1,000 person-years (absolute difference of 8 cases/1,000 person-years; relative excess of 133%), while at ages 90+ years, incidence among African Americans compared with Asian Americans was 115 versus 84 cases per 1,000 person years (absolute difference of 31 cases/1,000 person-years; relative excess of 37%).
Figure 2. Dementia incidence rates per 1,000 person-years by age and race-ethnicity, 2000-2013.
Racial/ethnic inequalities were observed for both sexes, although the magnitude of associations tended to be stronger for men (Table 3, Table A.3). For example, dementia rates were 60% higher among African American women compared with Asian American women, while dementia rates were 93% higher among African American men compared with Asian American men (interaction p-value <0.001). In most racial/ethnic groups, dementia incidence rates were similar for women and men until ages 90+ years, when rates were higher for women in some racial/ethnic groups; this difference was statistically significant for Whites (Table A.3, Figure A.1).
Table 3.
Dementia incidence by race/ethnicity and sex, 2000-2013.
| Female | ||||||
|---|---|---|---|---|---|---|
| Race/ethnicity | Events | Person-years | Age-adjusted incidence rate per 1,000 person-years (95% CI) | Hazard ratio (95% CI) | ||
| Model 1 | Model 2 | Model 3 | ||||
| African American | 2,921 | 89,670 | 26.29 (25.29, 27.28) | 1.60 (1.51, 1.69) | 1.60 (1.51, 1.69) | 1.50 (1.42, 1.58) |
| American Indian and Alaska Native | 711 | 22,920 | 22.17 (20.42, 23.92) | 1.35 (1.24, 1.46) | 1.34 (1.23, 1.45) | 1.24 (1.14, 1.35) |
| Latino | 2,497 | 106,277 | 20.14 (19.32, 20.97) | 1.26 (1.19, 1.33) | 1.25 (1.18, 1.32) | 1.19 (1.12, 1.26) |
| Pacific Islander | 29 | 1,798 | 17.67 (10.74, 24.59) | 1.03 (0.72, 1.49) | 1.04 (0.72, 1.51) | 0.98 (0.68, 1.42) |
| White | 27,070 | 984,055 | 19.71 (19.46, 19.97) | 1.20 (1.15, 1.25) | 1.19 (1.14, 1.25) | 1.16 (1.11, 1.21) |
| Asian American | 2,219 | 122,844 | 16.03 (15.33, 16.73) | 1.00 ( ref) | 1.00 ( ref) | 1.00 ( ref) |
| Male | ||||||
| Race/ethnicity | Events | Person-years | Age-adjusted incidence rate per 1,000 person-years (95% CI) | Hazard ratio (95% CI) | ||
| Model 1 | Model 2 | Model 3 | ||||
| African American | 2,021 | 67,448 | 27.05 (25.82, 28.28) | 1.93 (1.81, 2.06) | 1.93 (1.81, 2.06) | 1.88 (1.76, 2.01) |
| American Indian and Alaska Native | 513 | 18,261 | 21.98 (19.93, 24.03) | 1.54 (1.40, 1.71) | 1.53 (1.39, 1.69) | 1.45 (1.31, 1.60) |
| Latino | 1,874 | 89,409 | 18.87 (17.96, 19.78) | 1.34 (1.25, 1.43) | 1.34 (1.25, 1.43) | 1.31 (1.23, 1.40) |
| Pacific Islander | 32 | 1,447 | 23.08 (14.95, 31.22) | 1.58 (1.12, 2.25) | 1.63 (1.15, 2.31) | 1.58 (1.12, 2.25) |
| White | 18,040 | 766,197 | 18.76 (18.47, 19.05) | 1.33 (1.26, 1.40) | 1.32 (1.25, 1.39) | 1.29 (1.23, 1.36) |
| Asian American | 1,628 | 101,276 | 14.29 (13.56, 15.02) | 1.00 ( ref) | 1.00 ( ref) | 1.00 ( ref) |
Age-adjusted dementia incidence rates use 2000 US Census as standard. Hazard ratios for dementia are from sex-specific Cox proportional hazards models. Model 1: adjusted for age (as timescale); Model 2: Model 1 + healthcare utilization (≥1 heathcare visit per year); Model 3: Model 2 + depression, diabetes, hypertension, stroke, and cardiovascular disease. P-values for multiplicative interaction terms between race/ethnicity and sex from a pooled-sex model adjusted for age (as timescale) to test whether racial inequalities in dementia incidence differ by sex: African American vs. Asian American p<0.001; American Indian Alaska Native vs. Asian American p=0.043; Latino vs. Asian American p=0.141; Pacific Islander vs. Asian American p= 0.097; White vs. Asian American p=0.003.
Observed rates of dementia and mortality predict that among individuals who survive dementiafree to age 65, 38% (36%-39%) of African Americans, 35% (32%-36%) of AIANs, 32% (31%-33%) of Latinos, 25% (15%-31%) of Pacific Islanders, 30% (29%-30%) of Whites, and 28% (27%-29%) of Asian Americans would be diagnosed with dementia over the next 25 years (Table A.4 displays cumulative risk estimates for other time periods). Applying the observed dementia incidence rates to the 2013 United States Census population indicates that if these racial/ethnic differences hold nationally, reducing dementia incidence in all groups to the rate among Asian Americans would prevent more than 190,000 dementia cases annually in the United States.
4. Discussion
In a large population-based sample with equal access to healthcare followed for 14 years, we found dementia incidence was highest among African Americans and AIANs, lowest among Asian Americans, and intermediate among Latinos, Pacific Islanders, and Whites. These inequalities in dementia incidence were observed among women and men and across all ages. Adjustment for comorbidities and healthcare utilization did not substantially explain differences in dementia incidence. Estimated cumulative incidence of dementia over 25 years was high for all groups. This study provides the most comprehensive view of racial/ethnic inequalities in dementia risk to date.
Prior evidence on racial/ethnic disparities in dementia incidence has relied heavily on comparisons of dementia rates across studies. Because dementia rates are extremely sensitive to diagnostic criteria adopted in each study [9] and geographic patterns may also cause differences across studies [11] it has been difficult to establish the magnitude of racial/ethnic inequalities in dementia incidence. The results of the present study substantiate findings from prior studies with dementia incidence estimates for African Americans, Mexican Americans, and Japanese Americans. Most research on disparities in dementia incidence has focused on African Americans and Whites. These studies suggest dementia risk is up to twice as high among African Americans compared with Whites [5, 12-14]. African Americans experienced the highest dementia rates of the racial/ethnic groups included in this study and had approximately 40% higher dementia risk compared with Whites, which is more modest than the estimated disparity in some prior studies [12, 13] and similar to disparities estimated in other prior work [5, 14]. Among Latinos, prior studies suggest dementia risk differs between Latino subgroups, with elevated risk among Caribbean Latinos in New York City [12] but not among Mexican Americans in California [16, 33] compared with Whites [16]. Evidence from Hispanic/Latino Hispanic Community Health Study/Study of Latinos (HCHS/SOL) provides additional evidence that dementia risk likely varies markedly across Latino subgroups in the United States: neurocognitive test performance varied significantly across Latino subgroups in HCHS/SOL, with significantly higher odds of low performance on a neuropsychological test battery among Dominican and Puerto Rican than Mexican origin participants [34]. Although HCHS/SOL did not assess dementia, low performance on neuropsychological tests in population samples strongly predicts future dementia risk [35]. Mexican Americans are the predominant Latino group represented in the current California-based study and make up over 60% of the Latino population in the United States [36]. Our study supports prior evidence suggesting similar dementia rates among Mexican Americans and Whites [16, 33]. To our knowledge, the only work examining dementia incidence among Asian Americans has been conducted in Japanese American cohorts. A study of Japanese American men in Hawaii and a study of Japanese Americans in Washington reported similar or lower dementia incidence among Japanese Americans compared with dementia incidence among Whites in other studies [17, 18]. The low incidence of dementia among Asian Americans in the present study corroborates these prior findings.
To our knowledge, no prior population-based studies examine dementia incidence among Pacific Islanders or AIANs. Our findings differ from the limited research on dementia in these populations. A recent hospital-based study estimated higher dementia rates among Native Hawaiians and Japanese Americans compared with Whites in Hawaii [37]. A small cross-sectional study reported low prevalence of dementia among Cree living on reservations in Northern Manitoba, Canada [38]. Differences in our results compared with these prior studies may reflect differences in study design or true variations in dementia rates among these diverse groups in different geographic locations. The patterning of social, behavioral, and clinical risk factors for dementia may be distinct in Hawaii and Canadian First Nations reservations compared with Northern California.
Racial/ethnic inequalities are widely observed for many health outcomes, and hypothesized explanations for inequalities include socioeconomic disparities, psychosocial pathways related to discrimination, behavioral norms, comorbidities, and genetic profiles. In the case of dementia, the most compelling explanations relate to established risk factors for dementia, such as education and vascular comorbidities [8, 39]. In two prior population-based studies in the United States, racial/ethnic differences in dementia incidence were substantially explained by literacy (a marker of quality of education) and comprehensive measures of socioeconomic status [5, 6]. However, the patterns we report defy simple explanations. Although vascular disease is an appealing explanation for elevated dementia risk in African Americans, the observed racial/ethnic inequalities in dementia incidence remained after adjustment for vascular comorbidities. Prior studies have demonstrated that associations between midlife vascular risk factors and late-life dementia risk are stronger than associations for vascular risk factors measured in late-life [26, 40-42]. Differences in midlife vascular comorbidities may help explain the observed racial/ethnic disparities in dementia. We did not have measures of education in the present study, but educational patterns among older Californians do not strictly follow the observed patterns of dementia risk. For example, Whites have the highest average education levels, whereas Latinos have the lowest (authors’ calculations from California Health Interview Survey [43]). There is currently substantial uncertainty on the relative importance of genetic differences in explaining racial/ethnic patterns in dementia [5]. For example, the prevalence of the APOE-ε4 allele is higher among African Americans than among Whites, but the relative risk of dementia associated with the ε4 allele is smaller among African Americans compared with Whites [14, 44-47]. The fact that we observe these disparities in this population with equal healthcare access indicates access to healthcare in late-life cannot eliminate disparities in dementia incidence. This supports the need for characterization of lifecourse risk factors [8] to unravel the sources of disparities and identify strategies to eliminate them.
Because mortality is strongly patterned by race/ethnicity, selective survival may contribute to patterns of dementia incidence. However, the patterns we observed were consistent across age groups, and African Americans and AIANs have notably higher mortality rates compared with Asian Americans [19]. Thus, selective survival would likely attenuate, rather than explain, the observed racial/ethnic patterns in dementia incidence. Selective survival is a likely explanation for why dementia incidence rates were slightly higher among women than men at ages 90+ in some racial/ethnic groups [48].
The most salient limitation in this study is reliance on clinical diagnosis of dementia from medical records and lack of neuroimaging or post mortem pathology. Given cultural differences in expectations for healthy aging and the potential for differential under-diagnosis by clinicians, it is possible there were differences in diagnosis patterns by race/ethnicity, which could contribute to observed trends, including low incidence of dementia among Asian Americans. Some studies have reported later and more missed diagnoses in African Americans [49] and Latinos in Southern California [50] compared with Whites. The majority of members in our cohort had regular contact with the healthcare system, which may mitigate this potential source of bias. Dementia incidence was highest in African Americans, and if there were under-diagnosis in this group, we could be underestimating the burden of dementia in this group. Ensuring consistency of diagnostic criteria is an important challenge for the field of dementia research, given concerns that even very comprehensive neuropsychological batteries are sensitive to educational and cultural differences [9, 10]. Regular standardized dementia screening of all older health plan members, as is commonly utilized in cohort studies, could provide one approach to partially address this challenge, but such a protocol was not implemented. Neuroimaging measures could provide another approach to partially address this challenge and help distinguish more specific dementia phenotypes (e.g., Alzheimer’s disease versus vascular dementia), but neuroimaging was not available for this cohort. An additional limitation in our approach is that, although our study provides more detailed racial/ethnic patterns than any previous population-based cohort, we nonetheless combine diverse individuals within broad groups, such as Asian American or Latino.
Exploring potential heterogeneity in dementia risk between subgroups within these broad categories is an important future research step. Another limitation is that healthcare utilization and censoring due to end of health plan membership were slightly higher among Pacific Islanders than other racial/ethnic groups; we do not know if this censoring is related to dementia risk, but it could influence dementia incidence estimates for this group. Finally, although we assessed whether proximal risk factors for dementia explained observed racial/ethnic inequalities, we could not examine whether these differences remain after adjustment for education, socioeconomic status, and APOE genotype.
This study is the first to directly compare dementia incidence in six racial/ethnic groups and the first population-based study to examine dementia incidence among AIANs and Pacific Islanders. The stability of membership among KPNC members allowed us to follow members for incidence of dementia over a prolonged time period, with a substantial washout period to ensure we identified incident cases. The population is representative of the community, rather than recruited from selected clinics or volunteers, making our results generalizable to the geographic region of Northern California.
In a longitudinal study of healthcare members with nearly 60,000 incident dementia cases, we observed major racial/ethnic inequalities in dementia incidence, with highest risk among African Americans and AIANs, lowest risk among Asian Americans, and intermediate risk among Latinos, Pacific Islanders, and Whites. Adjustment for comorbidities did not account for these differences. Cumulative incidence rates indicate that, in every racial/ethnic group, over one in four individuals who survive to age 65 can expect to be diagnosed with dementia. Reducing dementia rates in all racial/ethnic groups to rates observed among Asian Americans would prevent more than 190,000 dementia cases annually in the United States and have a major public health impact. These findings demonstrate that there are major inequalities in dementia incidence and provide a benchmark for progress towards the National Alzheimer’s Plan goal of decreasing disparities [1]. It is unclear if these differences are due to genetic or social and behavior factors, but if social and behavior factors are the primary pathways, these findings suggest substantial reductions in dementia incidence are possible. Critical next steps include identifying lifecourse determinants of the observed disparities, examining the correspondence of disparities in dementia incidence with neuroimaging measures, evaluating heterogeneity within racial/ethnic groups, and examining dementia outcomes after diagnosis.
Supplementary Material
Research in Context.
Systematic review: In a literature review using PubMed, most studies on racial/ethnic inequalities in dementia included only 1-2 racial/ethnic groups, for example only Whites and African Americans. Dementia studies within a single sample representing the full racial/ethnic diversity of the United States are needed.
Interpretation: We found marked racial/ethnic disparities in dementia incidence, with highest rates among African Americans, American Indians and Alaska Natives, lowest rates among Asian Americans, and intermediate rates among Latinos, Pacific Islanders, and Whites. It is unclear if these differences are due to genetic or social and behavioral factors; if social and behavioral factors are primary pathways, these findings suggest substantial reductions in dementia incidence are possible.
Future Directions: Future research is needed to identify lifecourse determinants of the observed disparities, examine the correspondence of disparities in dementia incidence with neuroimaging measures, evaluate heterogeneity within racial/ethnic groups, and examine dementia outcomes after diagnosis.
Acknowledgments
This work was supported by a grant from the Kaiser Community Benefits Health Policy and Disparities Research Program Foundation and grant number P30-AG15272 from the National Institute on Aging, National Institutes of Health. The funding organizations played no role in the design and conduct of the study; in the management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author contributions: ERM was responsible for study concept and design, analysis and interpretation of data, drafting and revising of the manuscript, and obtaining funding. MMG was responsible for the study concept and design, interpretation of data, and revising of the manuscript. CPQ was responsible for interpretation of the data and revising of the manuscript. RAW is the guarantor and was responsible for the study concept and design, interpretation of data, revising of the manuscript, study supervision, and obtaining funding.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.US Department of Health and Human Services. National plan to address Alzheimer’s disease: 2014 update. Washington, DC: 2014. [Google Scholar]
- 2.Montine TJ, Koroshetz WJ, Babcock D, Dickson DW, Galpern WR, Glymour MM, et al. Recommendations of the Alzheimer’s Disease–Related Dementias Conference. Neurology. 2014;83:851–60. doi: 10.1212/WNL.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lines LM, Wiener JM. RACIAL AND ETHNIC DISPARITIES IN ALZHEIMER’S DISEASE: A Literature Review. 2014 [Google Scholar]
- 4.Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2015;11:332–84. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster A, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. Bmj. 2013;347:f7051. doi: 10.1136/bmj.f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrd DA, Sanchez D, Manly JJ. Neuropsychological test performance among Caribbean-born and Us-born African American elderly: The role of age, education and reading level. Journal of Clinical and Experimental Neuropsychology. 2005;27:1056–69. doi: 10.1080/13803390490919353. [DOI] [PubMed] [Google Scholar]
- 7.Meng X, D’Arcy C. Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One. 2012;7:e38268. doi: 10.1371/journal.pone.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology review. 2008;18:223–54. doi: 10.1007/s11065-008-9064-z. [DOI] [PubMed] [Google Scholar]
- 9.Erkinjuntti T, Ostbye T, Steenhuis R, Hachinski V. The effect of different diagnostic criteria on the prevalence of dementia. The New England journal of medicine. 1997;337:1667–74. doi: 10.1056/NEJM199712043372306. [DOI] [PubMed] [Google Scholar]
- 10.Weuve J, Proust-Lima C, Power MC, Gross AL, Hofer SM, Thiébaut R, et al. Guidelines for reporting methodological challenges and evaluating potential bias in dementia research. Alzheimer’s & Dementia. 2015;11:1098–109. doi: 10.1016/j.jalz.2015.06.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glymour MM, Kosheleva A, Wadley VG, Weiss C, Manly JJ. Geographic distribution of dementia mortality: elevated mortality rates for black and white Americans by place of birth. Alzheimer disease and associated disorders. 2011;25:196–202. doi: 10.1097/WAD.0b013e31820905e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang M-X, Cross P, Andrews H, Jacobs D, Small S, Bell K, et al. Incidence of AD in African-Americans, Caribbean hispanics, and caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 13.Shadlen MF, Siscovick D, Fitzpatrick AL, Dulberg C, Kuller LH, Jackson S. Education, Cognitive Test Scores, and Black-White Differences in Dementia Risk. Journal of the American Geriatrics Society. 2006;54:898–905. doi: 10.1111/j.1532-5415.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- 14.Evans DA, Bennett DA, Wilson RS, Bienias JL, Morris MC, Scherr PA, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Archives of Neurology. 2003;60:185–9. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 15.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. Journal of the American Geriatrics Society. 2003;51:169–77. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- 17.White L, Petrovitch H, Ross GW, Masaki KH, Abbott RD, Teng EL, et al. Prevalence of dementia in older Japanese-American men in Hawaii: the Honolulu-Asia aging study. JAMA : the journal of the American Medical Association. 1996;276:955–60. [PubMed] [Google Scholar]
- 18.Borenstein AR, Wu Y, Bowen JD, McCormick WC, Uomoto J, McCurry SM, et al. Incidence rates of dementia, Alzheimer disease, and vascular dementia in the Japanese American population in Seattle, WA: the Kame Project. Alzheimer disease and associated disorders. 2014;28:23–9. doi: 10.1097/WAD.0b013e3182a2e32f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hummer RA, Benjamins MR, Rogers RG. Racial and Ethnic Disparities in Health and Mortality Among the U.S. Elderly Population. In: Anderson N, Bulatao R, Cohen B, editors. Critical perspectives on racial and ethnic differences in health in late life. Washington D.C: National Academies Press; 2004. p. 53. [PubMed] [Google Scholar]
- 20.Gordon NP, Kaplan GA. Some evidence refuting the HMO “favorable selection” hypothesis: the case of Kaiser Permanente. Advances in health economics and health services research. 1991;12:19–39. [PubMed] [Google Scholar]
- 21.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. American journal of public health. 1992;82:703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon NP. Similarity of the Kaiser Permanente Senior Member Population in Northern California to the Non-Kaiser Permanente Covered and General Population of Seniors in Northern California: Statistics from the 2009 California Health Interview Survey. Kaiser Permanente Northern California Division of Research. 2012 [Google Scholar]
- 23.UCLA Center for Health Policy Research. 2001 California Health Interview Survey. Los Angeles, CA: [Google Scholar]
- 24.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA : the journal of the American Medical Association. 2009;301:1565–72. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayeda ER, Karter AJ, Huang ES, Moffet HH, Haan MN, Whitmer RA. Racial/ethnic differences in dementia risk among older type 2 diabetic patients: the diabetes and aging study. Diabetes care. 2014;37:1009–15. doi: 10.2337/dc13-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitmer R, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–81. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 27.Exalto LG, Biessels GJ, Karter AJ, Huang ES, Katon WJ, Minkoff JR, et al. Risk score for prediction of 10 year dementia risk in individuals with type 2 diabetes: a cohort study. The Lancet Diabetes & Endocrinology. 2013;1:183–90. doi: 10.1016/S2213-8587(13)70048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katon WJ, Lin EH, Williams LH, Ciechanowski P, Heckbert SR, Ludman E, et al. Comorbid depression is associated with an increased risk of dementia diagnosis in patients with diabetes: a prospective cohort study. Journal of general internal medicine. 2010;25:423–9. doi: 10.1007/s11606-009-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor DH, Fillenbaum GG, Ezell ME. The accuracy of medicare claims data in identifying Alzheimer’s disease. Journal of clinical epidemiology. 2002;55:929–37. doi: 10.1016/s0895-4356(02)00452-3. [DOI] [PubMed] [Google Scholar]
- 30.Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA internal medicine. 2014;174:251–8. doi: 10.1001/jamainternmed.2013.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Julious SA. Using confidence intervals around individual means to assess statistical significance between two means. Pharmaceutical Statistics. 2004;3:217–22. [Google Scholar]
- 32.Beiser A, D’Agostino RB, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study. The Practical Incidence Estimators (PIE) macro. Statistics in medicine. 2000;19:1495–522. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1495::aid-sim441>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 33.Haan MN, Miller JW, Aiello AE, Whitmer RA, Jagust WJ, Mungas DM, Homocysteine B, et al. vitamins, and the incidence of dementia and cognitive impairment: results from the Sacramento Area Latino Study on Aging. The American journal of clinical nutrition. 2007;85:511–7. doi: 10.1093/ajcn/85.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez HM, Tarraf W, Gouskova N, Gallo LC, Penedo FJ, Davis SM, et al. Neurocognitive function among middle-aged and older Hispanic/Latinos: results from the Hispanic Community Health Study/Study of Latinos. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2015;30:68–77. doi: 10.1093/arclin/acu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elias MF, Beiser A, Wolf PA, Au R, White RF, D’Agostino RB. The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham Cohort. Archives of Neurology. 2000;57:808–13. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 36.Ennis SR, Rios-Vargas M, Albert NG. 2010 Census Briefs. US Department of Commerce, Economics, and Statistics Administration, US Census Bureau; Washington D.C.: 2011. This Hispanic population: 2010. [Google Scholar]
- 37.Sentell TL, Valcour N, Ahn HJ, Miyamura J, Nakamoto B, Chow D, et al. High Rates of Native Hawaiian and Older Japanese Adults Hospitalized with Dementia in Hawai ‘i. Journal of the American Geriatrics Society. 2014 doi: 10.1111/jgs.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hendrie HC, Hall KS, Pillay N, Rodgers D, Prince C, Norton J, et al. Alzheimer’s disease is rare in Cree. International psychogeriatrics / IPA. 1993;5:5–14. doi: 10.1017/s1041610293001358. [DOI] [PubMed] [Google Scholar]
- 39.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet neurology. 2011;10:819–28. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kivipelto M, Helkala E-L, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. Bmj. 2001;322:1447–51. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–8. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.UCLA Center for Health Policy Research. 2011-2012 California Health Interview Survey. Los Angeles, CA: [Google Scholar]
- 44.Burchard EG, Ziv E, Coyle N, Gomez SL, Tang H, Karter AJ, et al. The importance of race and ethnic background in biomedical research and clinical practice. New England Journal of Medicine. 2003;348:1170–5. doi: 10.1056/NEJMsb025007. [DOI] [PubMed] [Google Scholar]
- 45.Tang M-X, Stern Y, Marder K, Bell K, Gurland B, Lantigua R, et al. The APOE-∈ 4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA : the journal of the American Medical Association. 1998;279:751–5. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 46.Maestre G, Ottman R, Stern Y, Gurland B, Chun M, Tang MX, et al. Apolipoprotein E and Alzheimer’s disease: ethnic variation in genotypic risks. Annals of neurology. 1995;37:254–9. doi: 10.1002/ana.410370217. [DOI] [PubMed] [Google Scholar]
- 47.Marden JR, Walter S, Tchetgen Tchetgen EJ, Kawachi I, Glymour MM. Validation of a polygenic risk score for dementia in black and white individuals. Brain and behavior. 2014;4:687–97. doi: 10.1002/brb3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chêne G, Beiser A, Au R, Preis SR, Wolf PA, Dufouil C, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimer’s & Dementia. 2015;11:310–20. doi: 10.1016/j.jalz.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark PC, Kutner NG, Goldstein FC, Peterson-Hazen S, Garner V, Zhang R, et al. Impediments to timely diagnosis of Alzheimer’s disease in African Americans. Journal of the American Geriatrics Society. 2005;53:2012–7. doi: 10.1111/j.1532-5415.2005.53569.x. [DOI] [PubMed] [Google Scholar]
- 50.Fitten LJ, Ortiz F, Pontón M. Frequency of Alzheimer’s disease and other dementias in a community outreach sample of Hispanics. Journal of the American Geriatrics Society. 2001;49:1301–8. doi: 10.1046/j.1532-5415.2001.49257.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.