Summary
Borrelia burgdorferi , the etiologic agent of Lyme disease, adapts to the mammalian hosts by differentially expressing several genes in the BosR and Rrp2-RpoN-RpoS dependent pathways, resulting in a distinct protein profile relative to that seen for survival in the Ixodes spp. tick. Previous studies indicate that a putative lipoprotein, BBA33, is produced in an RpoS-dependent manner under conditions that mimic the mammalian component of the borrelial lifecycle. However, the significance and function for BBA33 is not known. Given its linkage to the BosR/Rrp2-RpoN-RpoS regulatory cascade, we hypothesized that BBA33 facilitates B. burgdorferi infection in the mammalian host. The deletion of bba33 eliminated B. burgdorferi infectivity in C3H mice, which was rescued by genetic complementation with intact bba33. With regard to function, a combinatorial peptide approach, coupled with subsequent in vitro binding assays, indicated that BBA33 binds to collagen type VI and, to a lesser extent, collagen type IV. Whole cell binding assays demonstrated BBA33-dependent binding to human collagen type VI. Taken together, these results suggest that BBA33 interacts with collagenous structures and may function as an adhesin in a process that is required to prevent bacterial clearance.
Introduction
The spirochetal bacterium Borrelia burgdorferi, the etiologic agent of Lyme disease, infects mammalian hosts via the bite of Ixodes spp. ticks (Samuels, 2011; Radolf et al., 2012). When humans become infected, they present with nondescript flu-like symptoms with most developing a characteristic skin lesion referred to as a erythema migrans (recently reviewed in Shapiro, 2014). If untreated, the infection can progress to a multistage disorder as the spirochete disseminates to distant organ sites, resulting in carditis, neurological disorders and arthritis. Recently, the CDC reported that more than 300,000 cases of Lyme disease are diagnosed annually in the United States, indicating that Lyme borreliosis is a significant, re-emerging infectious disease (Hinckley et al., 2014). Furthermore, the lack of an effective vaccine underscores the need to better understand factors that contribute to the pathogenic potential of B. burgdorferi.
Borrelia burgdorferi establishes infection by interaction with connective tissue of the skin and disseminates to deeper tissues following colonization (Cabello et al., 2007). Early in the infectious process, B. burgdorferi is known to align with host collagen (Barthold et al., 1991) and interact with other structures found within the extracellular matrix (ECM) in a process that involves the function of borrelial adhesins. Known surface-exposed adhesins that recognize host structures found in the ECM include the following: the lipoproteins DbpA and DbpB that bind decorin and glycosoaminoglycans (GAGs) (Guo et al., 1995; Fischer et al., 2003; Blevins et al., 2008; Weening et al., 2008); BBK32 that interacts with fibronectin and GAGs (Probert and Johnson, 1998; Kim et al., 2004; Fischer et al., 2006; Seshu et al., 2006; Norman et al., 2008); BmpA and ErpX that engage laminin (Brissette et al., 2009; Verma et al., 2009); P66 and BBB07 that adhere to integrin proteins (Behera et al., 2008; Ristow et al., 2012); and CspA and CspZ that bind to complement proteins, fibronectin, laminin and collagens (Rogers and Marconi, 2007; Kenedy et al., 2009; Hallström et al., 2010; Hallström et al., 2013). Several of these proteins are categorized as MSCRAMMs (for microbial surface component that recognize adhesive matrix molecules) (Patti et al., 1994), and some (Dbp’s, BBK32, and P66) are linked to borrelial pathogenesis (Seshu et al., 2006; Weening et al., 2008; Lin et al., 2012; Ristow et al., 2012).
It is well established that B. burgdorferi modulates its gene expression as it cycles between the tick vector and the mammalian hosts they infect (Akins et al., 1998; Ohnishi et al., 2001; Liang et al., 2002; Revel et al., 2002; Brooks et al., 2003; Narasimhan et al., 2003; Ojaimi et al., 2003; Samuels, 2011), including genes that encode infection-associated lipoproteins (Zhang et al., 1997; Grimm et al., 2004; Pal et al., 2004; Seshu et al., 2006; Weening et al., 2008; Radolf et al., 2012). For mammalian infection, the Rrp2-RpoN-RpoS regulatory pathway plays an important role in this process (Hubner et al., 2001; Boardman et al., 2008; Samuels, 2011; Radolf et al., 2012) as this regulatory cascade is required for the expression of ospC, dbpBA and bbk32, all of which are required for optimal mammalian infection (Grimm et al., 2004; Pal et al., 2004; Seshu et al., 2006; Weening et al., 2008; Samuels, 2011; Radolf et al., 2012). In addition to these pathogenesis-associated genes, several additional genes are regulated in a similar manner; however, the importance and function of the proteins they encode are not known. One such gene that is RpoS-dependent is bba33, which is found on the 54 kb linear plasmid of B. burgdorferi strain B31 (Fraser et al., 1997). Previous studies showed that bba33 was up-regulated 29-fold in implanted dialysis membrane chambers and 8.2-fold in vitro in an RpoS-dependent manner (Caimano et al., 2007), and required Rrp2 for maximal expression (Boardman et al., 2008), suggesting that BBA33 is produced under mammalian-like conditions. In support of this contention, Kumar et al. (2010) found that bba33 is induced during a blood meal in ticks and is expressed in infected mouse skin. Furthermore, microarray and RT-PCR experiments revealed that bba33 transcripts were significantly up-regulated in heart tissue but poorly expressed in the central nervous system in B. burgdorferi infected nonhuman primates (Narasimhan et al., 2003), suggesting that bba33 expression is spatially regulated and thus may contribute to the dissemination and secondary colonization of B. burgdorferi to targeted organs. Taken together, these data suggested that BBA33 was linked to B. burgdorferi virulence; however, its function was unknown.
By analogy to other infection-associated lipoprotein-encoding genes that are regulated in a similar manner [e.g. dbpA or bbk32 (Seshu et al., 2006; Blevins et al., 2008; Weening et al., 2008)], we hypothesized that the protein encoded by bba33 could contribute to pathogenesis-associated functions. We now report that the loss of bba33 renders B. burgdorferi essentially noninfectious. A combinatorial peptide approach suggested that BBA33 recognizes a sequence that maps to human collagen type VI. Given its abundance in the skin and other sites colonized by B. burgdorferi, as well as the known association of B. burgdorferi with collagen (Barthold et al., 1991), we evaluated the ability of BBA33 to bind collagen and mediate bacterial attachment to collagen substrates. The results presented herein demonstrate that BBA33 plays an important role in early stages of borrelial infection due presumably to its ability to interact with collagenous substrates.
Results
Molecular analysis of bba33
Given the significance of several RpoS-regulated B. burgdorferi genes in mammalian infection (Hubner et al., 2001; Pal et al., 2004; Blevins et al., 2008; Weening et al., 2008; Radolf et al., 2012), including several that map to the linear 54 kb plasmid (lp54) of B. burgdorferi strain B31 (Blevins et al., 2008; Weening et al., 2008; Gilmore et al., 2010; Kumar et al., 2010), we sought to determine whether RpoS-dependent BBA33 contributed to borrelial pathogenesis. As a first step, we genetically deleted B. burgdorferi bba33 as depicted in Fig. 1A. Briefly, a construct that replaced the entire bba33 locus with a streptomycin-resistant (StrR) cassette was made and designated pHZ001. This construct was transformed into strain ML23, and transformants were screened by PCR for allelic exchange of the native bba33 gene with the StrR marker (Fig. 1B). ML23 is a strain that lacks the 25 kb linear plasmid that encodes a restriction/modification gene (bbe02); as such, this strain is more readily transformable via allelic exchange (Seshu et al., 2006; Weening et al., 2008) but is also noninfectious since the bbe22/pncA locus is essential for survival following experimental infection (Purser and Norris, 2000; Labandeira-Rey and Skare, 2001; Purser et al., 2003). Infectivity can be restored via transcomplementation of bbe22/pncA on the shuttle vector pBBE22 (Purser et al., 2003).
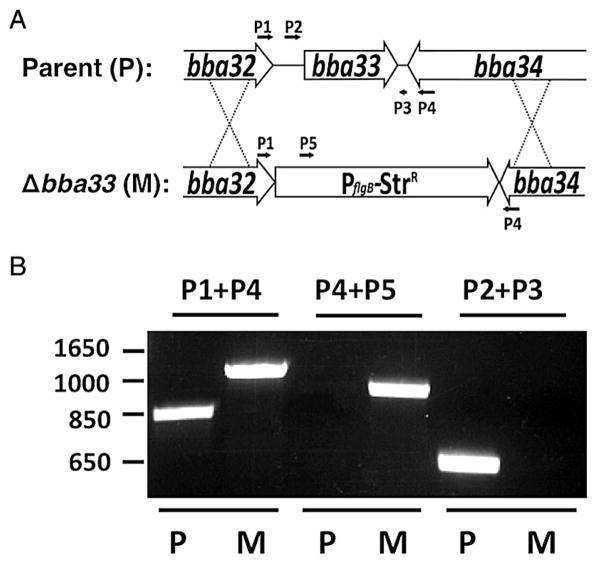
Fig. 1.
A. Schematic representation of the bba33 deletion strategy. The bba33 locus and its flanking region were replaced by a PflgB-StrR cassette by homologous recombination.
B. Primer pairs P1/P4 and P4/P5 (Table 3) were used to confirm the presence of PflgB-StrR cassette in the bba33 deletion mutant strain HZ001 (M, Δbba33 mutant) relative to its parent strain ML23 (P, parent) by PCR. Likewise, PCR with the primer pair P2/P3 (Table 3) confirmed that ML23 alone carried intact bba33 (P).
To assess the status of the StrR transformants relative the parent strain, PCR was employed. Consistent with the deletion of bba33, a 1296 bp PCR product was amplified from the putative Δbba33 strain using primers 1 and 4, relative to the expected 841 bp fragment from the parent strain (Fig. 1B). As predicted, primers 4 and 5 failed to amplify any fragment from the parent strain ML23 pBBE22luc, whereas a StrR-cassette containing 995 bp fragment was amplified from the putative Δbba33 mutant (Fig. 1B). Furthermore, primers 2 and 3 amplified a 672 bp fragment from the parent, but not from the putative Δbba33 mutant (Fig. 1B). The resulting Δbba33 mutant strain was designated HZ001 and then transformed with pBBE22luc to both complement mammalian infectivity with bbe22, which is necessary in strains lacking lp25 (Purser et al., 2003), and provide a constitutively expressed borrelial codon-optimized luciferase (luc) gene (Blevins et al., 2007).
To genetically complement the bba33 deletion in trans, intact bba33 with both upstream (210 bp) and downstream (129 bp) sequences included was cloned into pBBE22luc (Hyde et al., 2011a). The resulting construct, designated pHZ300, was transformed into the bba33 deletion strain HZ001. PCR analysis of the transformed deletion strain (Fig. 2B) showed a 672 bp fragment similar to that seen for the initial parent strain (Fig. 1B). B. burgdorferi plasmid profiles of all strains were evaluated to ensure no further loss of plasmid DNA (Labandeira-Rey and Skare, 2001) (data not shown). All three strains made PncA/BBE22 protein and produced light, consistent with the presence of the pncA locus and firefly luc on pBBE22luc respectively (data not shown). Furthermore, all isolated strains grew at the same rate indicating that the loss of bba33 did not alter B. burgdorferi replication in vitro (Fig. S1).
Fig. 2.
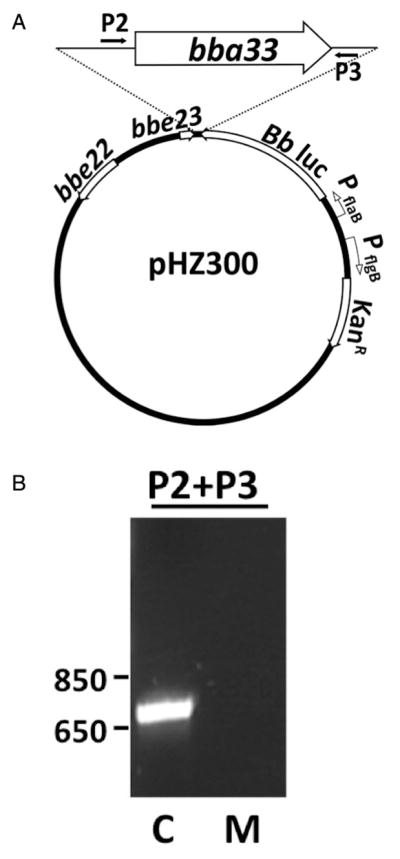
A. Schematic of the bba33 complementation construct pHZ300.
B. PCR using primer pairs P2/P3 confirmed the presence of bba33 in the complemented strain HZ001 pHZ300 (C; complement) relative the Δbba33 strain HZ001 pBBE22luc (M; Δbba33 mutant).
Conditions that mimic the mammalian host environment induce bba33
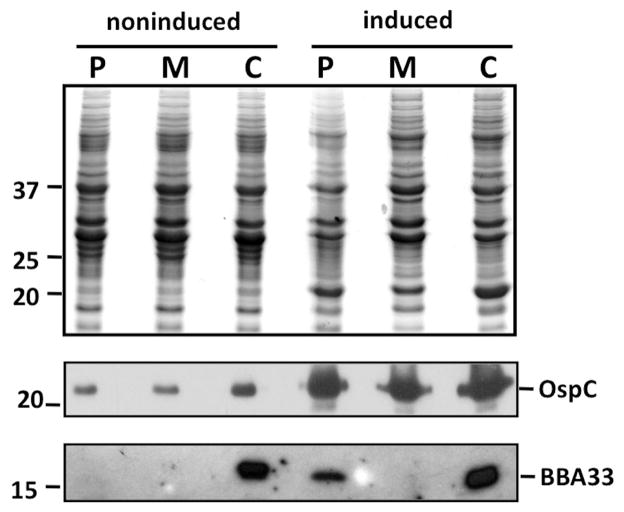
To determine whether BBA33 was produced in the deletion strain HZ001 pBBE22luc relative to its parent (ML23 pBBE22luc) and genetic complement (HZ001 pHZ300), we subjected protein lysates to Western immunoblot analysis. Prior studies indicated that temperature, pH and CO2 content affected gene expression in B. burgdorferi (Stevenson et al., 1995; Carroll et al., 1999; Hyde et al., 2007; Samuels, 2011). Furthermore, bba33 was induced under conditions that result in the production of several virulence-associated proteins, including OspC, BBK32 and DbpA (Grimm et al., 2004; Pal et al., 2004; Seshu et al., 2006; Weening et al., 2008), which require Rrp2/RpoS for their induction (Hubner et al., 2001; Yang et al., 2003; Caimano et al., 2007; Boardman et al., 2008). To assess whether the aforementioned variables contributed to bba33 induction, we subjected the aforementioned three strains to growth at either 32°C, 1% CO2, pH 7.6 (our conventional growth conditions) or 37°C, 5% CO2, pH 6.8 to mimic the mammalian host conditions in vitro. When equivalent amounts of B. burgdorferi proteins from these conditions were resolved by SDS-PAGE, lp54-encoded BBA33 was detected in the parent strain ML23 pBBE22luc grown at 37°C, 5% CO2, pH 6.8 (Fig. 3, bottom panel, right), consistent with the induction of mammalian-induced proteins, including OspC (Fig. 3, middle panel). In contrast, lp54-encoded BBA33 was not detectable in ML23 pBBE22luc when grown at 32°C, 1% CO2, pH 7.6 (Fig. 3, bottom panel, left). In fact, BBA33 levels were only observed at 37°C, 5% CO2, pH 6.8; no other combination of temperature, CO2 content or pH resulted in detectable levels of BBA33 (not shown). As expected, BBA33 was not detected in the Δbba33 mutant HZ001 pBBE22luc, regardless of growth condition (Fig. 3, lower panel). In contrast, when bba33 is expressed from a cp9-derived shuttle vector in the genetic complement strain HZ001 pHZ300 (Fig. 3, lower panel), the levels of BBA33 produced were high independent of the conditions imposed, presumably due to the increased copy number of the episomal DNA relative to its native location on lp54, which may titrate out regulatory components that affect bba33 expression. In support of this premise, previous studies estimated the copy number of cp9 at 5–10 per cell relative to linear replicons (Tilly et al., 2006; Beaurepaire and Chaconas, 2007). In addition, a similar effect was seen when dbpBA was complemented in trans; that is, shuttle-vector encoded DbpA was produced at levels that were approximately eightfold greater than the lp54-encoded version (Weening et al., 2008). Given that dbpBA and bba33 are similarly regulated (Blevins et al., 2008; Weening et al., 2008), it is not surprising that in trans encoded BBA33 is produced at a similarly high level.
Fig. 3.
BBA33 is produced under conditions that mimic the mammalian-like environment. Equivalent amount of protein were loaded for the B. burgdorferi parent strain ML23 pBBE22luc (P for parent), the Δbba33 strain HZ001 pBBE22luc (M for mutant) and the genetic complement HZ001 pHZ300 (C for complement) from cells that were either grown in media under ‘noninduced’ (32°C, 1% CO2, pH 7.6; left 3 lanes) or ‘induced’ conditions (37°C, 5% CO2, pH 6.8; right 3 lanes), separated by SDS-PAGE, and stained with Coomassie blue (top panel) or immunoblotted with anti-OspC (middle panel), or anti-BBA33 serum (bottom panel). Note that the only condition where the parent strain made BBA33 is shown; no other combination of temperature, CO2 content, or pH resulted in the detection of BBA33. The numbers on the side panel depict the molecular mass of protein markers (in kDa).
BBA33 is a surface-exposed lipoprotein
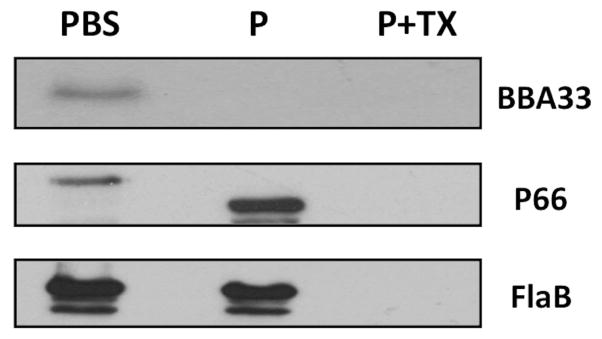
The primary sequence of B. burgdorferi BBA33 predicts a lipoprotein with a leader peptidase II signal peptide at its amino terminus. To provide supporting evidence that BBA33 was surface exposed, we subjected intact B. burgdorferi strain ML23 pBBE22luc to proteinase K treatment. These cells were grown at 37°C, 5% CO2, pH 6.8, to induce detectable levels of BBA33 (Fig. 3). The data showed that BBA33 was degraded in intact B. burgdorferi cells under conditions where P66 was accessible to protease [producing a known 50 kDa stable product; (Bunikis et al., 1998)], but subsurface endoflagella (FlaB) was not (Fig. 4). The addition of detergent Triton X-100 together with proteinase K resulted in the loss of all antigens tested providing further support that the cells treated with PBS or proteinase K alone were intact (Fig. 4). Additional studies confirmed that BBA33 partitioned with Triton X-114, consistent with its predicted status as a lipoprotein (not shown). Taken together, these results demonstrate that BBA33 is a surface-exposed outer membrane lipoprotein of B. burgdorferi.
Fig. 4.
BBA33 is surface exposed. Borrelia burgdorferi strain ML23 pBBE22luc was cultivated at 37°C, 5% CO2, pH 6.8 (inducing conditions) and harvested. The cells were then either resuspended in buffer (PBS), incubated with Proteinase K (P) or treated with both Proteinase K and Triton X-100 (P + TX). Following processing, the resulting samples were subjected to SDS-PAGE and immunoblotted with antiserum directed against either BBA33, the outer membrane P66 protein, or the subsurface FlaB protein.
The loss of bba33 results in the rapid clearance of B. burgdorferi
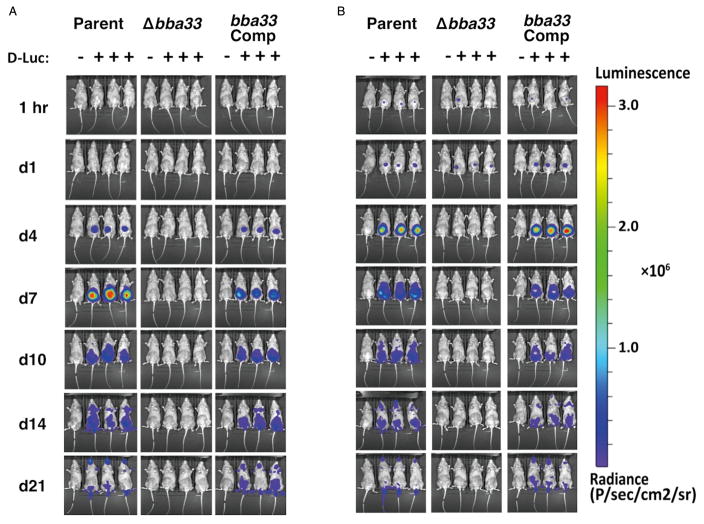
Next, we asked if B. burgdorferi cells lacking bba33 were attenuated using the murine infection model. To track the active infection of cells lacking BBA33, we infected C3H/HeN mice with the parent strain (ML23 pBBE22luc), the Δbba33 strain (HZ001 pBBE22luc) and the bba33 complement strain (HZ001 pHZ300), all containing borrelial codon-optimized firefly luciferase at doses of 103 and 105 cells and measured light emission from these cells over time (Figs 5 and S2). At the lower inoculum dose, a signal is not detected above background levels for any of the strains prior to day 4. At day 4, a clear signal is observed in mice infected with the parent and complement strain (Fig. 5). The signal expanded with a peak at day 7 and then began to wane commensurate with the development of the adaptive immune response (Figs 5 and S2). At the low dose, the Δbba33 mutant did not emit light above background for any of the time points tested (Fig. 5). For the high dose, an equivalent light signal was detected at 24 hours following infection for all strains tested (Figs 5 and S2). However, at day 4 and beyond, no signal above background was detected in the Δbba33 strain HZ001 pBBE22luc (Fig. 5). Conversely, the parent and bba33 complement strains exhibited similar light emission and dissemination profiles (Figs 5 and S2).
Fig. 5.
Temporal and spatial tracking of Borrelia burgdorferi strains following infection with 103 and 105 spirochetes. C3H mice were infected with the parent (Parent; ML23 pBBE22luc), the Δbba33 mutant (Δbba33; HZ001 pBBE22luc) or the bba33 complemented strain (bba33 Com., HZ001 pHZ300) at a dose of 103 (A) and 105 (B). Mice denoted with ‘+’ were treated with D-luciferin and imaged at the times listed on the left. For each image shown, the mouse on the far left (denoted as ‘−’) was infected with B. burgdorferi but did not receive D-luciferin to serve as a background control. All images were normalized to the same scale (shown on the right).
After 21 days, the mice infected with the parent, Δbba33, and bba33 complement, at both inoculum doses, were sacrificed and organ harvested for cultivation and quantitative PCR (qPCR) analysis. Consistent with the imaging data, the absence of bba33 resulted in the inability of B. burgorferi to survive or colonize mice from any organ site tested at any inoculum dose tested including up to an infection dose of 107 Δbba33 B. burgdorferi (Table 1). This is in stark contrast to the parent and complement strains, which were recovered from all organs independent of dose (Table 1).
Table 1.
Infectivity of the Δbba33 mutant strain relative to its parent and genetic complement.
| Strain | Number of culture positive/total number
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Inoculum dose | Lymph node | Skin | Heart | Spleen | Bladder | Joint | All sites | |
| ML23 pBBE22luc (parent) | 103 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 24/24 |
| 105 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 24/24 | |
| HZ001 pBBE22luc (Δbba33) | 103 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/24 |
| 105 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/24 | |
| 107 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/18 | |
| HZ001 pHZ300 (Δbba33/bba33 Comp) | 103 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 24/24 |
| 105 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 24/24 | |
To quantify the bacterial burden, qPCR was performed on total DNA extracted from the skin, lymph node, tibiotarsal joint and heart from mice infected with the parent, the Δbba33 mutant, and bba33 complemented strains harvested at the same time as the in vitro cultivated samples. Consistent with the cultivation data shown in Table 1, few genomic equivalents were detected in tissues infected with the Δbba33 mutant HZ001 pBBE22luc at a 103 or 105 inoculum (Fig. 6A and B respectively). Also in line with the prior infection data (Fig. 5 and Table 1), the parent and complemented strains exhibited similar colonization phenotypes indicating that the defect observed in the mutant was due to the loss of BBA33 and not a secondary mutation (Fig. 6A and B). Interestingly, the overproduction of BBA33 in complemented strains resulted in lower colonization in joint tissue relative to the parent strain independent of infectious dose, yet exhibited similar bacterial burden in heart tissue (Fig. 6A and B). Taken together, these data indicate that BBA33 is required for infectivity in this genetic background. Furthermore, the absence of BBA33 from the surface of B. burgdorferi leads to rapid clearance, suggesting that BBA33 is required for the establishment of a localized infection and resistance to the host innate immune response.
Fig. 6.
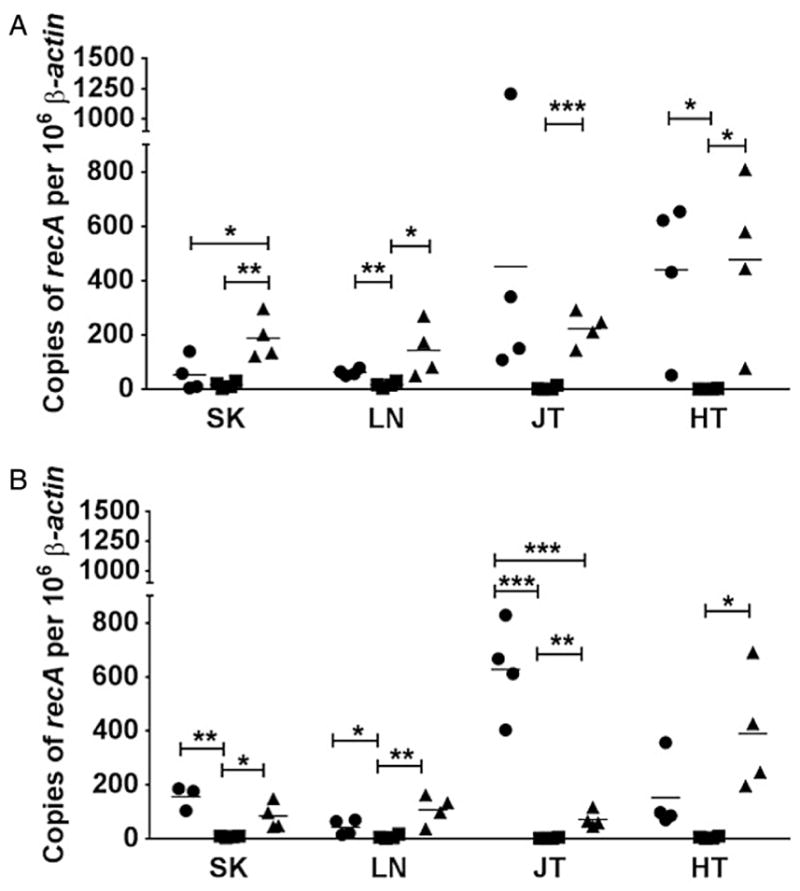
Quantitative assessment of Borrelia burgdorferi infection in C3H mice. Real-time PCR (qPCR) of the parental strain B. burgdorferi ML23 pBBE22luc (circles), the Δbba33 derivative HZ001 pBBE22luc (squares) and the genetic complement HZ001 pHZ300 (triangles) was used to enumerate borrelial genomic equivalents from mouse tissues. Mice were infected with either 103 (A) or 105 (B) B. burgdorferi strains for 21 days before total DNA was obtained from skin (SK), lymph nodes (LN), joints (JT) and heart (HT) tissues. The results are represented as the number of borrelial genome copies per 106 mouse β-actin copies. The horizontal line depicts the mean value. Each data point shown represents an independent sample from a single mouse tissue assayed in triplicate and averaged. * P < 0.05; ** P < 0.01; *** P < 0.001.
Recombinant BBA33 mediates binding to collagens
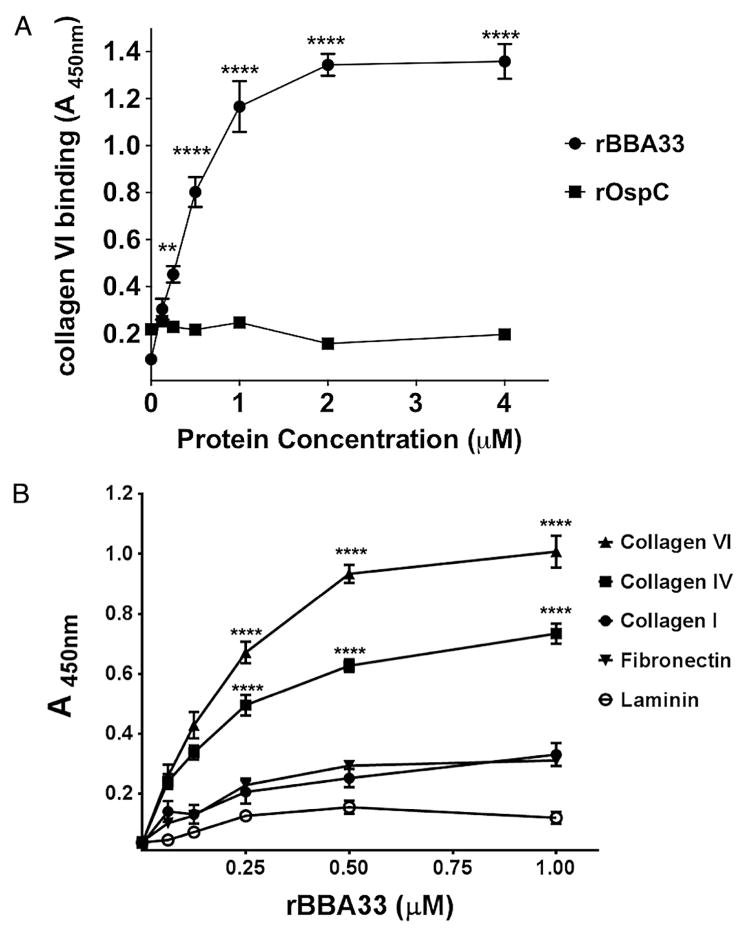
Given that BBA33 was an infectivity-associated surface-exposed lipoprotein, we were next interested in identifying a function for this protein. To this end, we screened random cyclic peptide phage display libraries to identify peptide sequences that would selectively recognize recombinant BBA33 (rBBA33; Mullen et al., 2006; Sergeeva et al., 2006; Barbu et al., 2010). We found that the number of phage binding to immobilized rBBA33 increased relative to background after several rounds of panning (based on BSA binding), suggesting a specific enrichment for BBA33. After the aforementioned panning, the DNA corresponding to the peptide insert from 100 randomly selected plaques was sequenced. An alignment of the deduced amino acids revealed enrichment for the sequence PGEPGLN, which is found in the human collagen type VI alpha 3 chain. However, in collagen, this sequence is present in the triple helix collagenous domains, and it is unlikely that all residues identified in the cyclic peptide would be available for interactions in the structurally restricted triple helix. To explore the possibility that BBA33 can bind collagens, we used an ELISA-type binding assay where increasing concentrations of purified rBBA33 protein were incubated in wells coated with different collagen types and other ECM proteins. A concentration-dependent binding of BBA33 to type VI collagen was noted with saturation at 2 μM and half maximum binding (e.g. apparent KD) at approximately 350 nM (Fig. 7A). In contrast, purified OspC did not bind to collagen type VI at any concentration tested (Fig. 7A) indicating that the BBA33::collagen type VI interaction is specific. BBA33 showed dose-dependent binding to collagen type IV but not to human collagen type I, fibronectin and murine laminin, indicating specificity for a subset of targets (Fig. 7B). When BBA33 was subjected to heat inactivation, the binding to collagen observed was eliminated, suggesting that the native conformation was required for dose-dependent binding (not shown).
Fig. 7. BBA33 binds to type VI collagen and type IV collagen in a dose-dependent manner.
A. Various concentrations of purified BBA33 were incubated with type VI collagen. Identical concentrations of OspC were tested for type VI collagen interaction as well and served as a negative control.
B. Binding of BBA33 to collagens type I, IV, and VI, as well as fibronectin and laminin, was evaluated as in panel A. Binding to collagens type VI and IV was dose dependent, whereas binding to fibronectin and laminin did not demonstrate specific binding to BBA33. All assays were done independently three times, each time in duplicate. **P < 0.01; ****P < 0.0001.
Native BBA33 binds to type VI collagen
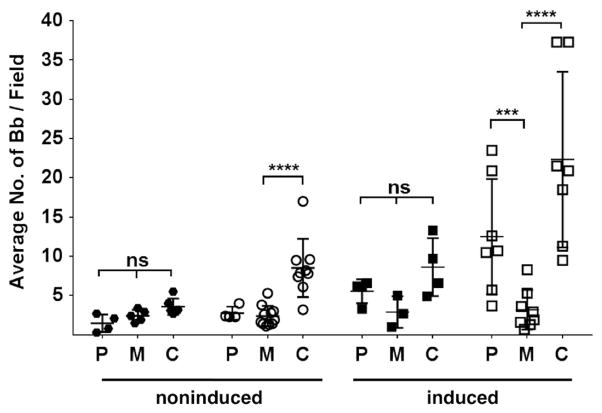
We next sought to determine whether native BBA33, presented on the surface of B. burgdorferi cells, could promote bacterial attachment to the identified BBA33 targets. A significant difference in borrelial cell attachment was observed to collagen type VI when the bba33 complement (HZ001 pHZ300; designated C) was compared with the parent strain ML23 pBBE22luc (designated P) and Δbba33 strain HZ001 pBBE22luc (designated M) when the cells are grown at 32°C, 1% CO2, pH 7.6 (Fig. 8, left); in contrast, no difference was seen in the attachment to laminin to any of the strains tested (Fig. 8). Under these conditions, the levels of BBA33 are low in the parent strain (Fig. 3); therefore, the low level of binding observed for the parent strain ML23 pBBE22luc is consistent with the lack of BBA33 produced when the parent is grown under noninducing conditions and thus, not surprisingly, comparable with the Δbba33 mutant (Fig. 8, left). However, when the cells were grown under conditions that promote the production of BBA33 (Fig. 3; e.g., 37°C, 5% CO2, pH 6.8), a significant difference in collagen type VI binding was observed between the parent ML23 pBBE22luc and the Δbba33 mutant HZ001 pBBE22luc; no significant differences to laminin binding were noted (Fig. 8, right). As expected, the bba33 complement HZ001 pHZ300 strain binds collagen type VI under inducing conditions (Fig. 8, right) as shuttle vector encoded BBA33 is synthesized at high levels independent of growth parameters (Fig. 3). These data are consistent with the induction of bba33 under the ‘mammalian-like’ induction conditions and provide further support that surface-exposed native BBA33 specifically recognizes collagen type VI.
Fig. 8. Borrelia burgdorferi.
whole cells that produce surface exposed BBA33 mediate specific binding to type VI collagen. B. burgdorferi strain ML23 pBBE22luc (P; parent), HZ001 pBBE22luc (M; Δbba33 mutant) and HZ001 pHZ300 (C; complement) were grown either under ‘noninducing’ conditions (32°C, 1% CO2, pH 7.6; left side) or ‘inducing’ conditions (37°C, 5% CO2, pH 6.8; right side) and incubated on cover slips containing either laminin (filled circles or squares) or type VI collagen (open circles or squares). B. burgdorferi cells binding was scored via dark field microscopy. Each data point represents the average number of spirochetes visualized in 10 random fields. Each strain was tested a minimum of three times, with each sample assayed in duplicate. ***P < 0.001; ****P < 0.0001; ns, nonsignificant difference.
Discussion
Interaction with the host ECM is an essential part of the pathogenic process of B. burgdorferi. B. burgdorferi is transmitted from ticks to mammalian host during a blood meal and, as a result, is deposited within the dermal layer of the skin where collagen and other components of the ECM reside (Cabello et al., 2007; Radolf et al., 2012). Some of the first micrographs of B. burgdorferi from infected tissue showed spirochetal bacteria aligned with collagen fibrils (Barthold et al., 1991; Cabello et al., 2007). Subsequent infectivity studies demonstrated that the association of B. burgdorferi with ECM tissue is required for persistence (Cabello et al., 2007; Brissette and Gaultney, 2014), although the basis for all B. burgdorferi–ECM interactions was not completely defined.
In this study, we describe a collagen binding lipoprotein from B. burgdorferi, designated BBA33, which is required for experimental Lyme borreliosis. To date, the only data associated with bba33 is its dependence on the BosR/Rrp2-RpoN-RpoS regulatory pathways for expression (Caimano et al., 2007). Despite the observation that bba33 was subject to the same degree of regulation observed for other borrelial virulence determinants (Hubner et al., 2001; Yang et al., 2003; Caimano et al., 2007; He et al., 2007; Boardman et al., 2008), notably ospC, dbpBA and bbk32 (Hubner et al., 2001; He et al., 2007), no function was known for BBA33. Using phage display, we identified a sequence found in type VI collagen as a target for BBA33. To biochemically validate the predicted BBA33–collagen interaction, we used an ELISA-based assay and found that BBA33 binds to collagen types IV and VI in a dose-dependent manner but does not bind to collagen I, laminin or fibronectin (Fig. 7B). To address the ability of surface-exposed native BBA33 to recognize ECM proteins, we incubated whole cells producing BBA33 with human collagen type VI and laminin. The results in Fig. 8 show that the genetic complement strain HZ001 pHZ300, which produces large amounts of BBA33, significantly enhances the ability of the spirochete to adhere to collagen type VI relative to the parent strain (ML23 pBBE22luc) and Δbba33 strain (HZ001 pBBE22luc) that are grown under conditions where BBA33 is not produced (Fig. 3). These data appear to contradict earlier findings that saw no binding of B. burgdorferi to collagens (Guo et al., 1995). However, upon reflection, this observation may be consistent with our findings as lp54-encoded bba33 is only expressed in the parent strain under conditions that mimic mammalian host adapted conditions (Fig. 3). The prior work was done using conventionally grown B. burgdorferi (Guo et al., 1995), which represents experimental conditions that do not yield detectable levels of BBA33 (Fig. 3). In addition, when the parent strain ML23 pBBE22luc is grown under inducing conditions, the level of collagen type VI binding increases significantly consistent with the production of BBA33 within this same environment (Fig. 3).
The ability of bacteria to adhere to collagen type VI is known for a few pathogens, including Legionella and some Staphylococcus spp. (Liu et al., 2004; Wagner et al., 2007; Bober et al., 2010), but is still poorly characterized relative to other pathogen–collagen interactions (Zong et al., 2005; Liu et al., 2007). It is important to note that, in the case of collagen type VI binding by the Staphylococcus capitis protein SdrX, the recombinant protein bound to several types of collagens in ELISA-based binding assays but only type VI collagen when S. capitis cells were used to query collagen binding (Liu et al., 2004). As such, the ability of B. burgdorferi cells to bind to collagen type VI provides similar support for BBA33-type VI collagen interactions.
The recognition of collagen type VI by BBA33 is of further interest given the recent reports demonstrating the ability of activated macrophages to secrete this particular collagen in a nonfibrillar form as is normally seen in fibroblasts (Schnoor et al., 2008). Two molecules that stimulate the production and secretion of type VI collagen include IL-10 and TGF-β1, both of which are induced early in B. burgdorferi infection (Codolo et al., 2008; Lazarus et al., 2008). In addition, both of these cytokines negate the inflammatory response of cells, including activated macrophages (Bogdan and Nathan, 1993; Oosting et al., 2014) and, by virtue of their ability to secrete collagens, notably type VI collagen (Schnoor et al., 2008), might create an additional platform for B. burgdorferi to bind. Based on the concurrent deactivation of previously activated macrophages, this microenvironment may promote localized colonization of B. burgdorferi and assist in the dissemination of spirochetes to distal sites. Based on our current data, it is unclear if this is operative in vivo; however, the dramatic attenuation of cells lacking BBA33 suggests that this lipoprotein plays an important role early in B. burgdorferi infection.
Type VI collagen also interacts with both biglycan and decorin, two proteoglycans that B. burgdorferi recognizes via the surface exposed lipoproteins, DbpA and DbpB (Brown et al., 1999; Fischer et al., 2003). It is tempting to speculate that B. burgdorferi infection stimulates the production of type VI collagen locally within the skin via innate immune cells and fibroblasts to generate a scaffold that borrelial cells can actively engage via a number of adhesins that bind to ECM targets. Further experimentation is required to test this hypothesis.
Perhaps the more striking result presented herein is the absolute loss of infectivity seen for the Δbba33 mutant strain HZ001 pBBE22luc. The isolation and characterization of additional isogenic mutants in other B. burgdorferi genetic backgrounds will be important to further validate the findings reported herein. In this regard, a prior transposon mutant library screen demonstrated that bba33 transposon mutants are infectious in only 16% of the tissues evaluated (Lin et al., 2012). This degree of attenuation for bba33 mutants is comparable with transposon insertion mutants in the dbpBA locus (Lin et al., 2012), which encode the virulence-associated decorin binding protein adhesins (Guo et al., 1995; Cabello et al., 2007). Mutants in dbpBA exhibit a significant infectivity defect with an approximate three log increase in ID50 (Blevins et al., 2008; Weening et al., 2008) and are cleared early in the host as assessed by in vivo imaging in a manner that is indistinguishable from the Δbba33 strain (Fig. 5 and Hyde et al., 2011a). Given the similarities of the dbpBA and bba33 mutant phenotypes, the transposon mutant screen provides additional genetic evidence that BBA33 is involved in B. burgdorferi pathogenesis.
From our imaging data of bioluminescent spirochetes (Fig. 5), it is clear that the Δbba33 mutant is cleared early in the infectious process at time points where the ML23 pBBE22luc parent and the resulting genetic complement are replicating in vivo, suggesting that BBA33 may assist in the resistance to innate immune clearance. It is curious that the complement protein, C1q, contains a collagen-like motif (Kishore and Reid, 2000). Although C1q is involved in the classical pathway of complement, a serum-sensitive B. burgdorferi sensu lato isolate, B. garinii, is resistant to serum when C1q is selectively depleted (van Dam et al., 1997), suggesting that the interaction of C1q and Borrelia isolates is key for innate survival. If and how BBA33 and C1q interact are currently being evaluated.
A diverse set of pathogens are known to bind to mammalian collagen-rich tissues to promote colonization (Umemoto et al., 1997; Bober et al., 2010; Foster et al., 2014). The structure of several of these interactions has been resolved yielding important insight into the binding process (Zong et al., 2005). The best characterized is the Cna collagen binding protein from Staphylococcus aureus, which uses a collagen-hug mechanism to bind host collagen (Zong et al., 2005). This interaction is important for pathogenesis as Cna is required for several pathogenic features associated with experimental S. aureus infections, including ocular keratitis, osteomyelitis and arthritis (Foster et al., 2014). The portion of Cna that mediates collagen binding is composed of two subdomains, each adopting an IgG-like fold (Zong et al., 2005). This same beta-pleated sheet rich structure is seen or presumed for the homologous collagen binding protein from Streptococcus and Enterococcus spp (Lannergard et al., 2003; Liu et al., 2007). Several algorithms predict that BBA33 is devoid of beta-pleated sheet structure and instead is largely alpha helical in nature. If this is true, then the mechanisms that BBA33 employs to bind collagen are likely to be novel relative to that seen for Cna and related collagen binding proteins.
In summary, herein, we demonstrate that B. burgdorferi BBA33 is a surface-exposed lipoprotein that is expressed preferentially under conditions that mimic the mammalian host environment. In addition, we show that BBA33 mediates specific interaction of B. burgdorferi with collagen substrates (i.e. human collagen type VI and murine type IV collagen). Despite a long history of B. burgdorferi–collagen interactions, to our knowledge, BBA33 represents the first B. burgdorferi protein that recognizes human collagens in a dose-dependent manner. Imaging of bioluminescent Δbba33 B. burgdorferi showed that the loss of BBA33 results in rapid clearance, which suggests a role in resistance to innate immune mechanisms. Further studies are warranted to investigate the significance of this interaction in vivo.
Experimental procedures
Bacterial strains and culture conditions
Bacterial strains and plasmids used in this study are described in Table 2. Escherichia coli strains were grown with aeration in Luria broth media at 37°C. Concentrations of antibiotics used in E. coli for selective pressure are as follows: kanamycin, 50 μg ml−1; spectinomycin, 50 μg ml−1. B. burgdorferi B31 ML23 (Labandeira-Rey and Skare, 2001) and derivative strains were grown in BSK-II media supplemented with 6% normal rabbit serum (Pel-Freez Biologicals, Rogers, AR, USA) under either conventional microaerobic conditions at 32°C, at pH 6.8 or pH 7.6, under a 1% or 5% CO2 atmosphere, or at 37°C, pH 6.8 or pH 7.6, under a 1% or 5% CO2 atmosphere. For most experiments, B. burgdorferi cultures were grown at either 32°C, 1% CO2, pH 7.6 (noninducing or conventional conditions) or at 37°C, 5% CO2, pH 6.8 (inducing conditions). Growth was scored daily by dark field microscopy. Antibiotics used for the selective pressure in B. burgdorferi are as follows: kanamycin at 300 μg ml−1 and/or streptomycin at 50 μg ml−1, depending on genetic composition. The use of infectious B. burgdorferi in this study was reviewed and approved by the Institutional Biosafety Committee at Texas A&M University.
Table 2.
Strains and plasmid constructs used in this study.
| Strain or plasmid | Genotype and/or characteristics | Source |
|---|---|---|
| E. coli strains | ||
| Mach-1™-T1R | ϕ80lacZΔM15 ΔlacX74 hsdR (rK−, mk+) ΔrecA1498 endA1 tonA | Invitrogen |
| BL21 Star™ (DE3) | F− ompT hsdSB (rB−mB−) gal dcm rne131 (DE3) | Invitrogen |
| BL21 Star™ (DE3) pLysS | F− ompT hsdSB (rB−mB−) gal dcm rne131 (DE3) pLysS (CamR) | Invitrogen |
| Top10 | F− mcrA Δ (mrr-hsdRMS-mcrBC) Φ80lacZΔM15 ΔlacX74 recA1 araD139, Δ (ara leu)7697 galU galK rpsL (StrR) endA1 nupG | Invitrogen |
| B. burgdorferi strains | ||
| ML23 | B. burgdorferi strain B31 clonal isolate missing lp25 | Labandeira-Rey and Skare (2001) |
| ML23 pBBE22luc | Clonal isolate of strain B31 lacking lp25 containing bbe22 and B. burgdorferi codon-optimized luc gene under the control of a strong borrelial promoter (PflaB-luc); parent strain | Hyde et al. (2011a) |
| HZ001 | ML23 Δbba33::StrR | This study |
| HZ001 pBBE22luc | ML23 Δbba33::StrR, containing bbe22 and the B. burgdorferi codon-optimized luc gene under the control of a strong borrelial promoter (PflaB-luc) | This study |
| HZ001 pHZ300 | ML23 Δbba33::StrR, containing bbe22, bba33, both under the control of their native promoters, and the B. burgdorferi codon-optimized luc gene under the control of a strong borrelial promoter (PflaB-luc) | This study |
| Plasmids | ||
| pCR2.1 | pCR™2.1-TOPO® vector; AmpR, KanR | Invitrogen |
| pKFSS1 | B. burgdorferi shuttle vector containing PFlgB-StrR cassette; SpcR in E. coli, StrR in B. burgdorferi | Frank et al. (2003) |
| pBBE22Gate | pBBE22 modified to be a gateway destination vector containing attL and attR sites; CamR, KanR | Weening et al. (2008) |
| pBBE22luc | Borrelial shuttle vector containing bbe22 and B. burgdorferi codon-optimized luc gene under the control of a strong borrelial promoter (PflaB-luc) | Hyde et al. (2011a) |
| pCR2.1Bactin | β-actin gene cloned into pCR2.1 vector; KanR | Hyde et al. (2011a) |
| pCR2.1recA | 1119 bp fragment, containing the recA gene, cloned into pCR2.1 vector; KanR | Hyde et al. (2011a) |
| pET15b | Cloning vector for overexpression of genes to generate His-tagged recombinant protein; AmpR | EMD Millipore |
| pSS008 | bba33 lacking its leader peptide (including the cysteine residue) was cloned into the NdeI and BamHI sites of pET15b in order to produce His-tagged recombinant BBA33 | This study |
| pHZ001 | PCR amplicons containing sequences 1526 bp upstream of the start of B. burgdorferi bba33, the PflgB-StrR cassette from pKFSS1 and sequences 1527 bp downstream of the bba33 stop codon, as well as pCR2.1, were specifically configured using Gibson assembly | This study |
| pHZ300 | Intact bba33 containing sequences 210 bp upstream and 129 bp downstream were cloned into the BamHI and SalI sites of pBBE22luc | This study |
Genetic modification of B. burgdorferi
Borrelia burgdorferi deleted for bba33 was generated via homologous recombination by replacing all of bba33 with a PflgB-aadA (StrR) antibiotic cassette (Frank et al., 2003). To accomplish this, a 1526 bp fragment upstream of bba33 was amplified using the primer pairs BamHI-A33us-recF and flgB-A33us-recR (Table 3) that created a 5′ overhang to pCR2.1 with the BamHI site and a 3′ overhang homologous to PflgB sequences from pKFSS1 (Table 3). An additional 1527 bp PCR product, which was directed to sequences downstream from bba33, was engineered with primers aadA-A33ds-recF and A33ds-XhoI-recR (Table 3) with overlapping sequences to sequences downstream to the stop codon of the aadA locus (StrR) at the 5′ end, and sequences homologous to pCR2.1, including the XhoI site. Finally, a PCR product containing the PflgB-StrR cassette was amplified from plasmid pKFSS1 using primer set FlgB-F and aadA-R (Table 3). All three PCR products, each containing homologous sequences, along with pCR2.1 digested with BamHI and XhoI, were combined and subjected to Gibson assembly as outlined by the manufacturer (New England Biolabs). The resulting construct was designated pHZ001 (Table 3). Transformation of strain ML23 with linearized pHZ001 was done as previously described (Samuels, 1995; Weening et al., 2008; Hyde et al., 2011b). Transformants were selected for their resistance to streptomycin and screened by PCR to confirm the deletion of bba33 using primers listed in Table 3 and as depicted in Fig. 1. Candidates were screened for borrelial plasmid content as before (Labandeira-Rey and Skare, 2001), and those that mirrored strain ML23 were transformed with pBBE22luc and transformants were selected for resistance to kanamycin (Hyde et al., 2011a). Putative transformants were screened for the presence of pBBE22luc and total borrelial plasmids using methods described previously (Labandeira-Rey and Skare, 2001; Hyde et al., 2011a).
Table 3.
Oligonucleotides used in this study.
| Oligonucleotide | Sequence (5′ to 3′) | Description |
|---|---|---|
| BamHI-A33us-recF | CCAAGCTTGGTACCGAGCTCGGATCCACACGCGCTAGCATCCTTTAAATT | Primer pair used to amplify 1526 bp region upstream of bba33 with a 22 bp 5′ flanking region homologous to pCR2.1 upstream of the BamHI site and a 3′ flanking region homologous to the 5′ region of the flgB promoter (PflgB) |
| flgB-A33us-recR | TAGGAAATCTTCCACGCCAATTGTTAAAGCTTTCTGTTTCTACGATATCG | |
| aadA-A33ds-recF | GTGAAAAAGTTTAAAAATCAGTTATTCTTCTATAGGTTTTATTTCTG | Primer pair used to amplify the 1527 bp region downstream from bba33 with a 21 bp 5′ flanking region homologous to the aadA 3′ end and a 32 bp 3′ flanking region homologous to sequences downstream of the XhoI site of pCR2.1 |
| A33ds-XhoI-recR | CGAATTGGGCCCTCTAGATGCATGCTCGAGCGGGTGTTGGAATTGGAAACGAACC | |
| flgB-F | CAATTGGCGTGGAAGATTTCC | Primer pair used to amplify PflgB-StrR cassette from pKFSS1 |
| aadA-R | CTGATTTTTAAACTTTTTCACAAATAGG | |
| P1 | GCCAGAGAGCGATATCGTAGAAAC | Primers P1 through P5 were used to confirm the bba33 deletion in B. burgdorferi as well as complementation construct (see Figs 1 and 2). |
| P2 | AATAATAGGTTCATGAAAAATTTGTATG | |
| P3 | CACAAATAGGGTCAAAATTTTGACC | |
| P4 | TGCCCTATCAGAAATAAAACCTATAGAAG | |
| P5 | GAAGTGCCTGGCAGTAAGTTG | |
| A33-NdeI-5′F | ACGCCATATGTATTTAAATGATTTTTCTGGTATG | Primer pair used to amplify bba33 lacking sequences that encode its leader peptide through the stop codon with NdeI (underlined) and BamHI (bold) sites engineered for cloning into pET15b expression vector |
| A33-BamHI-3′R | ACGCGGATCCTTATTTTTGAATTAGTGCTAAAGCAC | |
| A33up210-BamHI-F | ACGCGGATCCGCCAGAGAGCGATATCGTAGAAAC | Primer pair used to amplify a region from 210 bp upstream and 129 downstream of bba33 with BamHI (bold) and SalI (underlined) sites; cloned into pBBE22luc |
| A33dn129-SalI-R | ACGCGTCGACAAATGGCTAGGGTGGAATACAAAC | |
| b-Actin-F | ACGCAGAGGGAAATCGTGCGTGAC | Primer pair used for enumerating copies of mouse β-actin via qPCR (Pal et al., 2008) |
| b-Actin-R | ACGCGGGAGGAAGAGGATGCGGCAGTG | |
| nTM17FrecA | GTGGATCTATTGTATTAGATGAGGCT | Primer pair used for enumerating copies of B. burgdorferi recA via qPCR (Liveris et al., 2002; Weening et al., 2008) |
| nTM17RrecA | GCCAAAGTTCTGCAACATTAACACCT |
For genetic complementation, bba33 and 210 bp 5′ to the translation start site were cloned into the BamHI and SalI sites in the pBBE22luc shuttle vector to yield the final construct, pHZ300, using the oligonucleotides used are listed in Table 3. HZ001 was then made competent and electroporated with pHZ300 as described (Samuels, 1995). Transformants were selected for resistance to kanamycin, and candidates were confirmed by PCR as indicated in Fig. 2. As before, borrelial plasmid DNA was accounted for using previously published methods (Labandeira-Rey and Skare, 2001). Only strains that contained the DNA profile of transformed ML23 were used for subsequent analyses.
Infectivity studies and bioluminescent imaging
Infectivity studies were performed as previously described (Hyde et al., 2009). Described briefly, 8-week-old C3H/HeN mice were inoculated with either 103 or 105 organisms of the B. burgdorferi parent strain ML23/pBBE22luc, the Δbba33 strain HZ001/pBBE22luc, or the genetic complement strain HZ001/pHZ300 by intradermal injection. For each dose and strain used, four mice were infected per group. Imaging of infected mice to detect bioluminescence in the aforementioned B. burgdorferi strains was done as described in Hyde et al. (Hyde et al., 2011a). After 21 days, the mice were sacrificed, and inguinal lymph node, skin, heart, spleen, bladder and tibiotarsal joint tissues from each mouse were aseptically collected for in vitro cultivation and qPCR analysis of B. burgdorferi burden as described (Weening et al., 2008; Hyde et al., 2011a). All animal experiments were performed in accordance to the Association for Assessment and Accreditation of Laboratory Animal Care guidelines. The Texas A&M University Institutional Animal Care and Use Committee (IACUC) approved all animal procedures used in this study.
DNA extraction of B. burgdorferi from infected tissues and qPCR analysis
Total DNA was isolated from mice skin, lymph node, heart and tibiotarsal joint samples using Roche High Pure PCR template preparation kit as previously described (Weening et al., 2008). Approximately 100 ng of total DNA was used for each qPCR reaction. Quantitative real-time PCR analysis was conducted using the Applied Biosystems ABI 7900 HT system. B. burgdorferi genome copies and mammalian cell equivalents were determined using either the oligonucleotides nTM17FRecA and nTM17RRecA (Liveris et al., 2002; Weening et al., 2008) and primer set βactin-F and βactin-R (Pal et al., 2008) respectively. The bacterial burden was depicted as the number of B. burgdorferi recA per 106 β-actin copies.
Purification of rBBA33
To overproduce and purify rBBA33, a construct lacking the first 51 bp of the bba33 open reading frame (corresponding to the leader peptide) was amplified from B. burgdorferi genomic DNA using primer set A33-NdeI-5′F and A33-BamHI-3′R (Table 3) and cloned in-frame into pET15b following its digestion with NdeI and BamHI. The resulting construct, designated pSS008, encodes an amino terminal His-tagged version of BBA33. To affinity purify His-tagged BBA33, pSS008 was transformed into BL21 Star™(DE3) pLysS, grown to early log phase and the His-tagged BBA33 produced following induction with 0.2 mM IPTG for 16 h at 15°C. The cells were harvested by centrifugation, the pellet frozen and lysed via French pressure cell treatment. The soluble fraction, containing the His-tagged BBA33 protein, was applied to HisPur Cobalt resin (Thermo scientific) and affinity purified as outlined by the manufacturer.
Rabbit polyclonal antiserum to BBA33 was obtained following immunization of New Zealand white rabbits via an IACUC approved regimen at the Comparative Medicine Program, Texas A&M University.
SDS-PAGE and immunoblotting
Borrelia burgdorferi protein lysates were resolved by SDS-PAGE (Laemmli, 1970), and gels were either stained with Coomassie Brilliant Blue R-250 (Sigma Aldrich, St. Louis, MO, USA) or transferred to PVDF membranes and immunoblotted as described (Weening et al., 2008). Primary antibodies were used at the following dilutions: anti-BBA33 at 1:2000; anti-His6 (GE Healthcare) at 1:6000; anti-OspC at 1:4 × 106 (a kind gift from R. Gilmore); anti-PncAat 1:1500 (generously provided by J. Seshu); anti-P66 at 1:1000 (kindly provided by Sven Bergström); or anti-FlaB at 1:20,000 (Affinity Bioreagent, Golden, CO, USA). Prior to its use for Western immunoblotting, the BBA33 antiserum was adsorbed against HZ001 borrelial lysates to reduce the amount of nonspecific antibodies, as reported previously (Skare et al., 1999). Appropriate secondary antibodies, with horseradish peroxidase (HRP) conjugates [anti-mouse HRP (Invitrogen, Carlsbad, CA, USA) or anti-rabbit HRP (Amersham, Piscataway, NJ, USA), both diluted 1:4000] were used to detect immune complexes, the membranes washed extensively in PBS, 0.2% Tween-20, and developed using the Western Lightning Chemiluminescent Reagent plus system (Perkin Elmer, Waltham, MA, USA).
Proteinase K accessibility assay
Borrelia burgdorferi strain ML23 pBBE22luc was grown under inducing conditions (37°C, 5% CO2, pH 6.8) and harvested by centrifugation at 5,800 × g, and washed twice with PBS. The cell pellet was resuspended in 0.5 ml of either PBS alone, PBS with proteinase K (to a final concentration of 200 μg ml−1) or PBS/proteinase K with 0.05% Triton X-100. All samples were incubated at 20°C for 40 min. Reactions were terminated by the addition of phenylmethylsulfonyl fluoride (PMSF) to a final concentration of 1 mM. Cells were again pelleted by centrifugation (9,000 × g for 10 min at 4°C), washed twice with PBS containing 1 mM PMSF and resuspended in Laemmli sample buffer (Laemmli, 1970). Samples were run on SDS-PAGE gel and probed with anti-BBA33, anti-P66 and anti-FlaB antibodies respectively.
Triton X-114 phase partitioning
Borrelia burgdorferi strain ML23 pBBE22luc was grown under inducing conditions (37°C, 5% CO2, pH 6.8) and subjected to Triton X-114 phase partitioning as previously described (Skare et al., 1995).
Solid-phase phage display screening
A phage library displaying random cyclic 8-mer peptides (CX8C; X is any amino acid) was used for the screening against rBBA33 immobilized onto microtiter wells overnight at 4°C (at 1 μg per well in PBS). After the wells were washed twice with PBS and blocked with PBS containing 3% BSA for 1 h at 23°C, 109 PFU in 50 μl PBS containing 2% BSA were added to the wells (Barbu et al., 2010). After 2 h at 23°C, the wells were washed six times with PBS containing 0.1% Tween 20 and three times with PBS, and phage were recovered by bacterial infection as described (Smith and Scott, 1993). Phage recovered after the second and third round of selection were subjected to dideoxy sequencing and the resulting deduced amino acid sequences compared in the existing NCBI protein database.
In vitro ECM binding assay
Maxinunc microtiter plates (eBiosciences) were coated with 0.5 μg human collagen I (Corning), human collagen VI (Abcam), bovine fibronectin (Corning), mouse collagen IV (Corning) and mouse laminin (Corning) at 4°C for overnight, and blocked with 3% BSA for 1 h at 37 °C. Recombinant His-tagged BBA33 protein was serially diluted 1:2 starting from 4 μM down to 62.5 nM in PBS, 0.1% Tween-20, and added to coated wells in triplicate and incubated for 1 h at 37 °C. After five washes in PBS, 0.1% Tween-20, a 1:6000 dilution of monoclonal antibody directed against poly-His (His6) was added to each well and incubated for 1 h at 37°C. Following five washes in PBS, 0.1% Tween-20, a 1:4000 dilution of HRP conjugated antimouse IgG was added to each well and incubated for 1 h at 37°C. The wells were then washed in PBS, 0.1% Tween-20, after which 3,3′,5,5′-tetramethylbenzidine was added as substrate. The enzymatic reaction was stopped after 3 min using 0.16 M sulfuric acid (Thermo scientific), and the absorbance at 450 nm was determined.
B. burgdorferi whole cell adherence assay
Poly-D-lysine precoated coverslips (Corning Biocoat) were coated with 2 μg human collagen VI or mouse laminin and incubated at 4°C overnight. The coverslips were washed thoroughly in PBS to remove excess unbound proteins. The coverslips were then blocked with 3% BSA at 37°C for 1 h. The B. burgdorferi parent strain ML23 pBBE22luc, the Δbba33 strain HZ001/pBBE22luc and the genetic complement strain HZ001/pHZ300 were all grown to exponential phase at either 32°C, 1% CO2, pH 7.6 (noninducing conditions) or 37°C, 5% CO2, pH 6.8 (inducing conditions). The resulting cells were then harvested by centrifugation at 5,800 × g and washed with BSK-II medium without serum three times, and diluted to 107 organisms per ml in BSK-II medium without serum. The resulting B. burgdorferi cells, in 0.1 ml volumes, were applied onto the coverslips and incubated for 2 h at 32°C. Unbound bacteria were removed from the coverslips by gentle washing with PBS; this wash step was repeated 10 times. The coverslips were applied to a glass slide, and attached bacteria were counted by dark field microscopy. Binding was scored as the average number of bacteria bound per field based on 10 random fields per slide.
Statistical analysis
For real-time qPCR analysis, a one-tailed Mann–Whitney t-test was performed between the strains indicated. For ELISA and bioluminescent assays, two-way analyses of variance (ANOVA) was performed respectively among variables. Tukey’s post-test was used to determine P values between coated proteins. Statistical significance was accepted when the P values were less than 0.05 for all statistical analyses employed.
Supplementary Material
Acknowledgments
We thank Beau Wager for helpful discussions and Sunita Seemanapalli for the initial purification of rBBA33. We are grateful to R. Gilmore, J. Seshu and S. Bergström for providing us with antiserum to OspC, PncA and P66, respectively. We also wish to thank Katherine Biancardi and Brian Bordelon for excellent technical assistance. We gratefully acknowledge the assistance of Texas A&M University’s Comparative Medicine Program with antibody production against rBBA33. This work was supported by Public Health Service, grant R01-AI042345 (to J.T.S.) and R01-AI058086 (to J.T.S. and M.H.), and a Texas A&M Health Science Center College of Medicine Training Grant Award (to H.Z.). The authors have no conflicts of interest to declare.
Footnotes
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Akins DR, Bourell KW, Caimano MJ, Norgard MV, Radolf JD. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Invest. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbu EM, Ganesh VK, Gurusiddappa S, Mackenzie RC, Foster TJ, Sudhof TC, Höök M. beta-Neurexin is a ligand for the Staphylococcus aureus MSCRAMM SdrC. PLoS Pathog. 2010;6:e1000726. doi: 10.1371/journal.ppat.1000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, Persing DH, Armstrong AL, Peeples RA. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- Beaurepaire C, Chaconas G. Topology-dependent transcription in linear and circular plasmids of the segmented genome of Borrelia burgdorferi. Mol Microbiol. 2007;63:443–453. doi: 10.1111/j.1365-2958.2006.05533.x. [DOI] [PubMed] [Google Scholar]
- Behera AK, Durand E, Cugini C, Antonara S, Bourassa L, Hildebrand E, et al. Borrelia burgdorferi BBB07 interaction with integrin alpha3beta1 stimulates production of pro-inflammatory mediators in primary human chondrocytes. Cell Microbiol. 2008;10:320–331. doi: 10.1111/j.1462-5822.2007.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JS, Revel AT, Smith AH, Bachlani GN, Norgard MV. Adaptation of a luciferase gene reporter and lac expression system to Borrelia burgdorferi. Appl Env Microbiol. 2007;73:1501–1513. doi: 10.1128/AEM.02454-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JS, Hagman KE, Norgard MV. Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol. 2008;8:82. doi: 10.1186/1471-2180-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman BK, He M, Ouyang Z, Xu H, Pang X, Yang XF. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect Immun. 2008;76:3844–3853. doi: 10.1128/IAI.00467-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bober M, Enochsson C, Collin M, Morgelin M. Collagen VI is a subepithelial adhesive target for human respiratory tract pathogens. J Innate Immun. 2010;2:160–166. doi: 10.1159/000232587. [DOI] [PubMed] [Google Scholar]
- Bogdan C, Nathan C. Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann N Y Acad Sci. 1993;685:713–739. doi: 10.1111/j.1749-6632.1993.tb35934.x. [DOI] [PubMed] [Google Scholar]
- Brissette CA, Gaultney RA. That’s my story, and I’m sticking to it–an update on B. burgdorferi adhesins. Front Cell Infect Microbiol. 2014;4:1–13. doi: 10.3389/fcimb.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissette CA, Verma A, Bowman A, Cooley AE, Stevenson B. The Borrelia burgdorferi outer-surface protein ErpX binds mammalian laminin. Microbiol. 2009;155:863–872. doi: 10.1099/mic.0.024604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CS, Hefty PS, Jolliff SE, Akins DR. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect Immun. 2003;71:3371–3383. doi: 10.1128/IAI.71.6.3371-3383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EL, Guo BP, O’Neal P, Hook M. Adherence of Borrelia burgdorferi. Identification of critical lysine residues in DbpA required for decorin binding. J Biol Chem. 1999;274:26272–26278. doi: 10.1074/jbc.274.37.26272. [DOI] [PubMed] [Google Scholar]
- Bunikis J, Luke CJ, Bunikiene E, Bergstrom S, Barbour AG. A surface-exposed region of a novel outer membrane protein (P66) of Borrelia spp. is variable in size and sequence. J Bacteriol. 1998;180:1618–1623. doi: 10.1128/jb.180.7.1618-1623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello FC, Godfrey HP, Newman SA. Hidden in plain sight: Borrelia burgdorferi and the extracellular matrix. Trends Microbiol. 2007;15:350–354. doi: 10.1016/j.tim.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, et al. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol. 2007;65:1193–1217. doi: 10.1111/j.1365-2958.2007.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JA, Garon CF, Schwan TG. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect Immun. 1999;67:3181–3187. doi: 10.1128/iai.67.7.3181-3187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codolo G, Amedei A, Steere AC, Papinutto E, Cappon A, Polenghi A, et al. Borrelia burgdorferi NapA-driven Th17 cell inflammation in lyme arthritis. Arthritis Rheum. 2008;58:3609–3617. doi: 10.1002/art.23972. [DOI] [PubMed] [Google Scholar]
- van Dam AP, Oei A, Jaspars R, Fijen C, Wilske B, Spanjaard L, Dankert J. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect Immun. 1997;65:1228–1236. doi: 10.1128/iai.65.4.1228-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JR, Parveen N, Magoun L, Leong JM. Decorin-binding proteins A and B confer distinct mammalian cell type-specific attachment by Borrelia burgdorferi, the Lyme disease spirochete. Proc Natl Acad Sci USA. 2003;100:7307–7312. doi: 10.1073/pnas.1231043100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JR, LeBlanc KT, Leong JM. Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans. Infect Immun. 2006;74:435–441. doi: 10.1128/IAI.74.1.435-441.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TJ, Geoghegan JA, Ganesh VK, Höök M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nat Rev Microbiol. 2014;12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank KL, Bundle SF, Kresge ME, Eggers CH, Samuels DS. aadA confers streptomycin resistance in Borrelia burgdorferi. J Bacteriol. 2003;185:6723–6727. doi: 10.1128/JB.185.22.6723-6727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia Burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- Gilmore RD, Howison RR, Dietrich G, Patton TG, Clifton DR, Carroll JA. The bba64 gene of Borrelia burgdorferi, the Lyme disease agent, is critical for mammalian infection via tick bite transmission. Proc Natl Acad Sci USA. 2010;107:7515–7520. doi: 10.1073/pnas.1000268107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, et al. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci USA. 2004;101:3142–3147. doi: 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo BP, Norris SJ, Rosenberg LC, Hook M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallström T, Haupt K, Kraiczy P, Hortschansky P, Wallich R, Skerka C, Zipfel PF. Complement regulator-acquiring surface protein 1 of Borrelia burgdorferi binds to human bone morphogenic protein 2, several extracellular matrix proteins, and plasminogen. J Infect Dis. 2010;202:490–498. doi: 10.1086/653825. [DOI] [PubMed] [Google Scholar]
- Hallström T, Siegel C, Mörgelin M, Kraiczy P, Skerka C, Zipfel PF. CspA from Borrelia burgdorferi inhibits the terminal complement pathway. mBio. 2013;4:e00481–13. doi: 10.1128/mBio.00481-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Boardman BK, Yan D, Yang XF. Regulation of expression of the fibronectin-binding protein BBK32 in Borrelia burgdorferi. J Bacteriol. 2007;189:8377–8380. doi: 10.1128/JB.01199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis. 2014;59:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner A, Yang X, Nolen DM, Popova TG, Cabello FC, Norgard MV. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc Natl Acad Sci USA. 2001;98:12724–12729. doi: 10.1073/pnas.231442498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JA, Trzeciakowski JP, Skare JT. Borrelia burgdorferi alters its gene expression and antigenic profile in response to CO2 levels. J Bacteriol. 2007;189:437–445. doi: 10.1128/JB.01109-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JA, Shaw DK, Smith R, III, Trzeciakowski JP, Skare JT. The BosR regulatory protein of Borrelia burgdorferi interfaces with the RpoS regulatory pathway and modulates both the oxidative stress response and pathogenic properties of the Lyme disease spirochete. Mol Microbiol. 2009;74:1344–1355. doi: 10.1111/j.1365-2958.2009.06951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JA, Weening EH, Chang M, Trzeciakowski JP, Höök M, Cirillo JD, Skare JT. Bioluminescent imaging of Borrelia burgdorferi in vivo demonstrates that the fibronectin-binding protein BBK32 is required for optimal infectivity. Mol Microbiol. 2011a;82:99–113. doi: 10.1111/j.1365-2958.2011.07801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JA, Weening EH, Skare JT. Curr Protoc Microbiol. Unit–12C.4. 2011b. Genetic manipulation of Borrelia burgdorferi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenedy MR, Vuppala SR, Siegel C, Kraiczy P, Akins DR. CspA-mediated binding of human factor H inhibits complement deposition and confers serum resistance in Borrelia burgdorferi. Infect Immun. 2009;77:2773–2782. doi: 10.1128/IAI.00318-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Singvall J, Schwarz-Linek U, Johnson BJ, Potts JR, Hook M. BBK32, a fibronectin binding MSCRAMM from Borrelia burgdorferi, contains a disordered region that undergoes a conformational change on ligand binding. J Biol Chem. 2004;279:41706–41714. doi: 10.1074/jbc.M401691200. [DOI] [PubMed] [Google Scholar]
- Kishore U, Reid KB. C1q: structure, function, and receptors. Immunopharmacology. 2000;49:159–170. doi: 10.1016/s0162-3109(00)80301-x. [DOI] [PubMed] [Google Scholar]
- Kumar M, Yang X, Coleman AS, Pal U. BBA52 facilitates Borrelia burgdorferi transmission from feeding ticks to murine hosts. J Infect Dis. 2010;201:1084–1095. doi: 10.1086/651172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labandeira-Rey M, Skare JT. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect Immun. 2001;69:446–455. doi: 10.1128/IAI.69.1.446-455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lannergard J, Frykberg L, Guss B. CNE, a collagen-binding protein of Streptococcus equi. FEMS Microbiol Lett. 2003;222:69–74. doi: 10.1016/S0378-1097(03)00222-2. [DOI] [PubMed] [Google Scholar]
- Lazarus JJ, Kay MA, McCarter AL, Wooten RM. Viable Borrelia burgdorferi enhances interleukin-10 production and suppresses activation of murine macrophages. Infect Immun. 2008;76:1153–1162. doi: 10.1128/IAI.01404-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FT, Nelson FK, Fikrig E. Molecular adaptation of Borrelia burgdorferi in the murine host. J Exp Med. 2002;196:275–280. doi: 10.1084/jem.20020770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Gao L, Zhang C, Odeh E, Jacobs MB, Coutte L, et al. Analysis of an ordered, comprehensive STM mutant library in infectious Borrelia burgdorferi: insights into the genes required for mouse infectivity. PLoS ONE. 2012;7:e47532. doi: 10.1371/journal.pone.0047532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Ponnuraj K, Xu Y, Ganesh VK, Sillanpaa J, Murray BE, et al. The Enterococcus faecalis MSCRAMM ACE binds its ligand by the collagen hug model. J Biol Chem. 2007;282:19629–19637. doi: 10.1074/jbc.M611137200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ames B, Gorovits E, Prater BD, Syribeys P, Vernachio JH, Patti JM. SdrX, a serine-aspartate repeat protein expressed by Staphylococcus capitis with collagen vi binding activity. Infect Immun. 2004;72:6237–6244. doi: 10.1128/IAI.72.11.6237-6244.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liveris D, Wang G, Girao G, Byrne DW, Nowakowski J, McKenna D, et al. Quantitative detection of Borrelia burgdorferi in 2-millimeter skin samples of erythema migrans lesions: correlation of results with clinical and laboratory findings. J Clin Microbiol. 2002;40:1249–1253. doi: 10.1128/JCM.40.4.1249-1253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen LM, Nair SP, Ward JM, Rycroft AN, Henderson B. Phage display in the study of infectious diseases. Trends Microbiol. 2006;14:141–147. doi: 10.1016/j.tim.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Caimano MJ, Liang FT, Santiago F, Laskowski M, Philipp MT, et al. Borrelia burgdorferi transcriptome in the central nervous system of non-human primates. Proc Natl Acad Sci USA. 2003;100:15953–15958. doi: 10.1073/pnas.2432412100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman MU, Moriarty TJ, Dresser AR, Millen B, Kubes P, Chaconas G. Molecular mechanisms involved in vascular interactions of the Lyme disease pathogen in a living host. PLoS Pathog. 2008;4:e1000169. doi: 10.1371/journal.ppat.1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi J, Piesman J, de Silva AM. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc Natl Acad Sci USA. 2001;98:670–675. doi: 10.1073/pnas.98.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojaimi C, Brooks C, Casjens S, Rosa P, Elias A, Barbour A, et al. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect Immun. 2003;71:1689–1705. doi: 10.1128/IAI.71.4.1689-1705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosting M, Buffen K, van der Meer JWM, Netea MG, Joosten LAB. Innate immunity networks during infection with Borrelia burgdorferi. Crit Rev Microbiol. 2014:1–12. doi: 10.3109/1040841X.2014.929563. [DOI] [PubMed] [Google Scholar]
- Pal U, Yang X, Chen M, Bockenstedt LK, Anderson JF, Flavell RA, et al. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest. 2004;113:220–230. doi: 10.1172/JCI19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal U, Wang P, Bao F, Yang X, Samanta S, Schoen R, et al. Borrelia burgdorferi basic membrane proteins A and B participate in the genesis of Lyme arthritis. J Exp Med. 2008;205:133–141. doi: 10.1084/jem.20070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti JM, Allen BL, McGavin MJ, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- Probert WS, Johnson BJ. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci USA. 2000;97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol Microbiol. 2003;48:753–764. doi: 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel AT, Talaat AM, Norgard MV. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc Natl Acad Sci USA. 2002;99:1562–1567. doi: 10.1073/pnas.032667699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow LC, Miller HE, Padmore LJ, Chettri R, Salzman N, Caimano MJ, et al. The β3-integrin ligand of Borrelia burgdorferi is critical for infection of mice but not ticks. Mol Microbiol. 2012;85:1105–1118. doi: 10.1111/j.1365-2958.2012.08160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EA, Marconi RT. Delineation of species-specific binding properties of the CspZ protein (BBH06) of Lyme disease spirochetes: evidence for new contributions to the pathogenesis of Borrelia spp. Infect Immun. 2007;75:5272–5281. doi: 10.1128/IAI.00850-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DS. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol Biol Clifton NJ. 1995;47:253–259. doi: 10.1385/0-89603-310-4:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DS. Gene regulation in Borrelia burgdorferi. Annu Rev Microbiol. 2011;65:479–499. doi: 10.1146/annurev.micro.112408.134040. [DOI] [PubMed] [Google Scholar]
- Schnoor M, Cullen P, Lorkowski J, Stolle K, Robenek H, Troyer D, et al. Production of type VI collagen by human macrophages: a new dimension in macrophage functional heterogeneity. J Immunol. 2008;180:5707–5719. doi: 10.4049/jimmunol.180.8.5707. [DOI] [PubMed] [Google Scholar]
- Sergeeva A, Kolonin MG, Molldrem JJ, Pasqualini R, Arap W. Display technologies: application for the discovery of drug and gene delivery agents. Adv Drug Deliv Rev. 2006;58:1622–1654. doi: 10.1016/j.addr.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshu J, Esteve-Gassent MD, Labandeira-Rey M, Kim JH, Trzeciakowski JP, Höök M, Skare JT. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol Microbiol. 2006;59:1591–1601. doi: 10.1111/j.1365-2958.2005.05042.x. [DOI] [PubMed] [Google Scholar]
- Shapiro ED. Lyme disease. N Engl J Med. 2014;370:1724–1731. doi: 10.1056/NEJMcp1314325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare JT, Shang ES, Foley DM, Blanco DR, Champion CI, Mirzabekov T, et al. Virulent strain associated outer membrane proteins of Borrelia burgdorferi. J Clin Invest. 1995;96:2380–2392. doi: 10.1172/JCI118295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skare JT, Foley DM, Hernandez SR, Moore DC, Blanco DR, Miller JN, Lovett MA. Cloning and molecular characterization of plasmid-encoded antigens of Borrelia burgdorferi. Infect Immun. 1999;67:4407–4417. doi: 10.1128/iai.67.9.4407-4417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GP, Scott JK. Libraries of peptides and proteins displayed on filamentous phage. Methods Enzymol. 1993;217:228–257. doi: 10.1016/0076-6879(93)17065-d. [DOI] [PubMed] [Google Scholar]
- Stevenson B, Schwan TG, Rosa PA. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, et al. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun. 2006;74:3554–3564. doi: 10.1128/IAI.01950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto T, Li M, Namikawa I. Adherence of human oral spirochetes by collagen-binding proteins. Microbiol Immunol. 1997;41:917–923. doi: 10.1111/j.1348-0421.1997.tb01950.x. [DOI] [PubMed] [Google Scholar]
- Verma A, Brissette CA, Bowman A, Stevenson B. Borrelia burgdorferi BmpA is a laminin-binding protein. Infect Immun. 2009;77:4940–4946. doi: 10.1128/IAI.01420-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C, Khan AS, Kamphausen T, Schmausser B, Unal C, Lorenz U, et al. Collagen binding protein Mip enables Legionella pneumophila to transmigrate through a barrier of NCI-H292 lung epithelial cells and extracellular matrix. Cell Microbiol. 2007;9:450–462. doi: 10.1111/j.1462-5822.2006.00802.x. [DOI] [PubMed] [Google Scholar]
- Weening EH, Parveen N, Trzeciakowski JP, Leong JM, Höök M, Skare JT. Borrelia burgdorferi Lacking DbpBA Exhibits an Early Survival Defect during Experimental Infection. Infect Immun. 2008;76:5694–5705. doi: 10.1128/IAI.00690-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Alani SM, Norgard MV. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc Natl Acad Sci USA. 2003;100:11001–11006. doi: 10.1073/pnas.1834315100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JR, Hardham JM, Barbour AG, Norris SJ. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- Zong Y, Xu Y, Liang X, Keene DR, Höök A, Gurusiddappa S, et al. A ‘Collagen Hug’ model for Staphylococcus aureus CNA binding to collagen. EMBO J. 2005;24:4224–4236. doi: 10.1038/sj.emboj.7600888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.