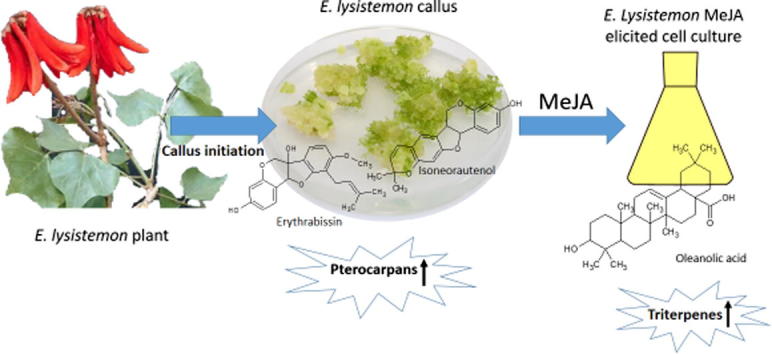
Graphical abstract
Keywords: Cell culture, Erythrina lysistemon, Metabolomics, Methyl jasmonate, Oleanolic acid, Ultra-performance liquid chromatography
Abstract
An MS-based metabolomic approach was used to profile the secondary metabolite of the ornamental plant Erythrina lysistemon via ultra-performance liquid chromatography coupled to photodiode array detection and high resolution q-TOF mass spectrometry (UPLC-PDA-MS). Cultures maintained the capacity to produce E. lysistemon flavonoid subclasses with pterocarpans amounting for the most abundant ones suggesting that it could provide a resource of such flavonoid subclass. In contrast, alkaloids, major constituents of Erythrina genus, were detected at trace levels in suspension cultures. Methyl jasmonate (MeJA), phytohormone, was further supplied to culture with the aim of increasing secondary metabolites production and with metabolite profiles subjected to multivariate data analysis to evaluate its effect. Results revealed that triterpene i.e. oleanolic acid and fatty acid i.e. hydroxy-octadecadienoic acid were elicited in response to methyl jasmonate, whereas pterocarpans i.e., isoneorautenol showed a decline in response to elicitation suggesting for the induction of terpenoid biosynthetic pathway and concurrent with a down regulation of pterocarpans. In conclusion, a total of 53 secondary metabolites including 3 flavones, 12 isoflavones, 4 isoflavanones, 4 alkaloids, 11 pterocarpans, and 5 phenolic acids were identified.
Introduction
The genus Erythrina constitutes 115 species in the pea family “Fabaceae” which are distributed worldwide in tropical and subtropical regions growing as trees, often recognized in agriculture for its bright red flowers as coral or flame trees [1]. Alkaloids and phenolics are among the most widely distributed constituents in these flowering trees mostly localized in stem bark [2], [3], roots [4] and seeds [5], [6]. Erythrina alkaloids are tetracyclic spiroamines possessing an erythrinane skeleton. Over 90 Erythrina alkaloids have been isolated [7], [8], often classified as dienoid or lactonic alkaloids. Interest in Erythrina alkaloids is mostly driven by its curare-like neuromuscular blocking effect. Moreover, Erythrina spp. possess a broad-spectrum of physiological activities such as anti-plasmodial activity due to the flavonoids and isoflavonoids [9], anti-oxidant and anti-inflammatory activities due to pterocarpans [10] and fungicidal activity associated with its alkaloidal content [11].
Erythrina genus has been extensively examined in terms of its taxonomy and chemical composition. However, very little information is available concerning biotechnological attempts for natural products production within that genus. Garcia-Mateos et al., showed that an unexpected profile of oxygenated alkaloids was observed in undifferentiated callus of Erythrina Coralloides and Erythrina americana [12]. Furthermore, San Miguel-Chavez et al., showed that jasmonic acid elicited E. americana cell culture has led to reduction in alkaloid accumulation [13]. Among the most common and effective elicitors used for stimulating secondary metabolites production in plant cell culture are the carbohydrate fractions of fungal and plant cell walls, MeJA, chitosan and/or heavy metal salts. In particular, jasmonates have been long regarded as transducers of elicitor signals for the production of plant secondary metabolites. Application of elicitors results in the induction of signaling compounds, including jasmonic acid and or MeJA, as well as the downstream up regulation of secondary metabolites. In contrast to salicylic acid being an elicitor of limited secondary metabolite classes, jasmonates seem to be general natural products inducer via the activation and de novo biosynthesis of transcription factors that up regulate genes involved in secondary metabolites production [14]. For example, jasmonates induce the accumulation of terpenoids, flavonoids, alkaloids and phenylpropanoids [15]. The aim of this work was to examine MeJA elicitation effect on cell suspension culture of Erythrina lysistemon regarding the accumulation of alkaloids, flavonoids, pterocarpans and phenolic acids using an MS-based metabolomic approach.
Material and methods
Plant material
Seeds of E. lysistemon were collected in January 2012 from trees previously authenticated by Professor Nabil El Hadidi, College of Science, Cairo University, Egypt. Voucher specimens of the flowers and seeds are deposited at Faculty of Pharmacy, Alexandria University, Egypt.
Callus initiation
Seeds were surface sterilized in 20% sodium hypochlorite solution for 30 min, washed three times in sterile purified water and placed on agarized Murashige and Skoog (MS; Caisson, Smithfield, USA) germination medium (1962) and incubated under 12 h light period and a temperature of 23 °C ± 1 °C in a culture room [16]. Leaves were excised from 28 d-old seedlings. The leaves were then scored on their abaxial sides with a sterile scalpel blade and cut into 1 cm2 pieces. Explants were cultured on 25 mL aliquots of MS supplemented with either 1 mg L−1 or 2 mg L−1 of each of kinetin (Kin; Acros, Geel, Belgium) and 2,4-dichlorophenoxyacetic acid (2,4D; Acros, Belgium) in addition to 30 g L−1 sucrose (El-Nasr, Alexandria, Egypt), and semi-solidified with 0.8% (w/v) agar (Roko, Llanera – Asturias, Spain), pH 5.6, in a 9 cm diameter Petri dishes. The explants were transferred onto fresh media, until callus was produced [16].
Cell suspension culture initiation
Cell suspensions were established by transferring 1–2 g fresh wt. of callus and maintained in 100 mL MS liquid medium supplemented with same growth regulators, but no agar was added, pH 5.6 in 100 mL Erlenmeyer flasks. Cultures were maintained on a rotary shaker at 100 rpm and incubated under a 12 h photoperiod, with day and night temperatures of 23 °C ± 1 °C until the stock suspension was produced.
Elicitation
Aliquots of 2.5 mL packed cell volume (PCV) with 2.5 mL spent medium of the stock suspension were transferred to six 100 mL Erlenmeyer flasks, each containing 45 mL of fresh media and maintained under same conditions for two weeks. Methyl jasmonate (MeJA; Sigma Aldrich, Poole, UK) was then added into five flasks to produce a concentration 1 mM L−1 MeJA. The dose 1 mM L−1 MeJA was previously optimized to elicit secondary metabolic pathways in cell cultures [17], [18]. Furthermore, an increase in the concentration of MeJA resulted in retarded callus growth. The remaining flask was used as control by the addition of the same volume of sterile water. Cultures were kept at 23 °C ± 1 °C, with a 12 h photoperiod and maintained on a rotary shaker at 100 rpm. Cell culture samples were harvested at 0, 6, 12, 24 and 48 h post elicitation and kept at −80 °C until being analyzed.
Extraction and UPLC-MS analysis of cell culture extracts
Metabolites extraction followed the protocol developed for similar metabolite classes [18], [19]. Briefly, lyophilized E. lysistemon cultures (20 ± 0.06 mg) were extracted with 1.8 mL aq.80% MeOH for 10 h using an orbital shaker in the dark. Extracts were centrifuged at 10,000g for 15 min and 1.4 mL of the supernatant was aliquot and evaporated under nitrogen till complete dryness. The dried residue was resuspended in 300 μL 45% aq. MeOH. For comparative analysis, the extracts were spiked with 2 μg umbelliferone as an internal standard (IS) and quantifications were determined from peak areas normalized based on the amount of recovered IS peak. The residue was re-suspended in 300 μL methanol and used for UPLC-MS analysis following the exact chromatographic conditions described by Farag et al. [20].
Identification and quantification of metabolites and MS data multivariate analysis
File Converter tool in X-Calibur software was used to convert UPLC–MS files to NetCDF file format and then further processed by AMDIS software for background subtraction and peak deconvolution. Metabolite identification was done via UV-VIS spectra (220–600 nm), retention times relative to external standards, mass spectra, and comparison to both the reference literature and phytochemical dictionary of natural product database. Quantification of alkaloids was calculated from the calibration curve of erythraline, pterocarpans using medicarpin standard, and for oleanolic acid using that of oleanolic acid standard detected using MS detector. Standard calibration curves were constructed for each standard using 4 concentrations spanning from 0.1, 1, 10 and 200 μg/mL. Assays were carried out in triplicate.
MS data processing for multivariate analysis
Relative quantification and comparison of metabolites profiles after UPLC-MS were performed using XCMS data analysis software, which can be downloaded for free as an R package from the Metlin Metabolite Database (http://137.131.20.83/download/) [21].
Results and discussion
E. lysistemon cell culture metabolite profile
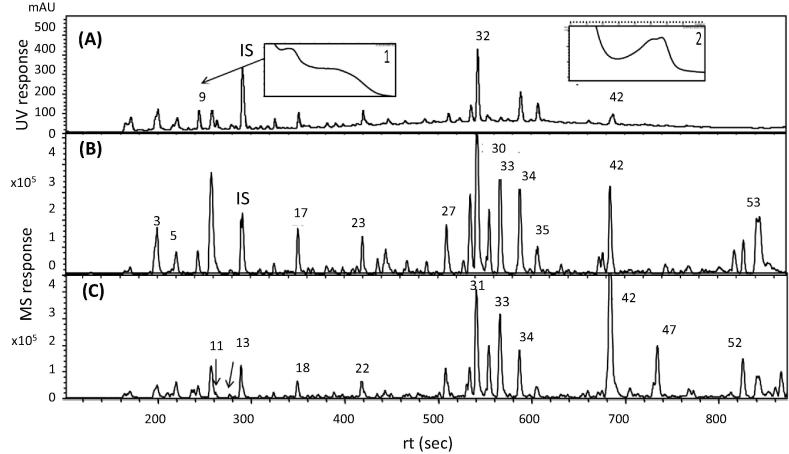
Callus was produced from cut ends of scored E. lysistemon explants after 3 weeks. Chemical constituents of callus extracts were analyzed via UPLC/PDA/(−)ESI-qTOF-MS that allowed for the elution of cinnamates, flavonoids, alkaloids and fatty acids within 13 min (ca. 800 s). The elution order of secondary metabolites followed a sequence of decreasing polarity, whereby cinnamates and alkaloids eluted first, followed by flavonoid glycosides, free aglycones, prenylated aglycones and finally triterpenes and fatty acids. Simultaneously acquired UPLC–PDA and UPLC–MS total ion chromatograms of E. lysistemon cell culture extracts in positive and negative ionization mode are presented in Fig. 1. The identities, retention times, UV and MS spectral data observed for secondary metabolites are presented in Table 1 with a total of 53 identified metabolites. It is worth noting that this is the first comprehensive metabolite profile of E. lysistemon plant. Identified metabolites belonged to various classes (Table 1, Suppl. Fig. 1) including phenolic acids (cinnamates) i.e. N-caffeoyl aspartic acid (2), alkaloids i.e. erysotrine (6), pterocarpans i.e. isoneorautenol (30), isoflavones i.e. lysisteisoflavone (44), triterpenes i.e. oleanolic acid (53) and fatty acid i.e. hydroxy-9,11-octadecadienoic acid (45), with isoflavones and pterocarpans as the most abundant classes in cell culture extract. The structures of major metabolites identified in E. lysistemon and discussed throughout the manuscript are shown in Suppl. Fig. 1.
Fig. 1.
UPLC–UV at 250 nm (A), UPLC–negative-ion ESI–MS (B) and UPLC–positive-ion ESI–MS chromatogram of E. lysistemon cell culture extracts (C). Chromatographic conditions are described under Material and methods. Insets 1 and 2 represent UV spectra of peak 9 (dihydroxyflavone hexoside) and peak 42 (sandwicensin, a pterocarpan), respectively. The identities, rt-value, UV and MS spectra of all peaks are listed in Table 1. IS = spiked internal standard (umbelliferone). Chromatographic conditions followed that were described in [20].
Table 1.
Metabolites identified in E. lysistemon L. cell suspension methanol extract using UPLC–PDA–MS/MS in negative/positive ionization modes.
| Peak | rt (s) | UV (nm) | Metabolite | Class | Molecular ion | Error | Composition | MS/MS |
|---|---|---|---|---|---|---|---|---|
| m/z (±) | ppm | |||||||
| 1 | 122 | 285 | Dihydroxybenzoic acid pentosylhexoside | Phenolic acid | 447.1139[M − H]− | 1.2 | C18H23O13 | 378, 304 |
| 2 | 161 | 294, 325 | N-Caffeoylaspartic acid | Phenolic acid | 294.0592[M − H]− | 9.1 | C13H12NO7 | 175, 132 |
| 3 | 198 | 294, 326 | N-p-Coumaroylaspartic acid | Phenolic acid | 278.0661[M + H]+ | 3.2 | C13H12NO6 | 163, 132 |
| 4 | 209 | 287, 312 | N-(Hydroxycinnamoyl) tyraminehexoside | Phenolic acid | 476.187[M + H]+ | 9.3 | C24H30NO9 | 314 |
| 5 | 220 | 294, 325 | N-Feruloylaspartic acid | Phenolic acid | 308.0758[M − H]− | 5.9 | C14H14NO7 | 193, 132 |
| 6 | 237 | 282 | Erysotrine | Alkaloid | 314.1756[M + H]+ | −1.6 | C19H24NO3 | 280 |
| 7 | 238 | 272, 340 | Apigeninpentosyl hexoside | Flavone | 563.1423[M − H]− | −3 | C26H27O14 | 269, 253 |
| 8 | 238 | 280 | DOPA methyl ether | Alkaloid | 226.1073[M + H]+ | 0.6 | C11H16NO4 | |
| 9 | 245 | 270, 332 | Dihydroxyflavone hexoside | Flavone | 415.102 [M − H]− | 3.5 | C21H19O9 | 253 |
| 10 | 252 | 325 | Erythrartine/11-Methoxyerysodine | Alkaloid | 330.1696[M + H]+ | 1.3 | C19H24NO4 | 312, 280 |
| 11 | 262 | nd | Apigeninhexosylmalonate | Flavone | 517.1702[M − H]− | 4.0 | C24H21O13 | 269, 253 |
| 12 | 263 | nd | Erysotramidine | Alkaloid | 328.1534[M + H]+ | 2.9 | C19H22NO4 | 313 |
| 13 | 277 | 282, 286 | Demethylmedicarpin hexosylmalonate | Pterocarpan | 503.1158[M + H]+ | 5.1 | C24H23O12 | 255 |
| 14 | 278 | 280, 308 | Diacetoxy benzoic acid | – | 237.0397[M − H]− | 3.3 | C11H9O6 | 215, 174 |
| 15 | 289 | 282, 286 | Demethylmedicarpin hexosylmalonate | Pterocarpan | 503.1162[M + H]+ | 5.1 | C24H23O12 | 255 |
| 16 | 292 | 272, 319 | N-Cinnamoyl-Aspartic acid | – | 262.0717[M − H]− | 1.5 | C13H12NO5 | 218, 146 |
| 17 | 350 | 262, 308 | Dihydroxyisoflavone | Isoflavone | 253.0497[M − H]− | 3.5 | C15H9O4 | |
| 18 | 350 | 282, 286 | Demethylmedicarpin | Pterocarpan | 255.0637[M + H]+ | 5.7 | C15H11O4 | 174 |
| 19 | 357 | 232, 285 | Erythribyssin B | Pterocarpan | 283.0598[M − H]− | 4.9 | C16H11O5 | 269, 253, 214 |
| 20 | 369 | 280, 335 | Eryvarin D | Pterocarpan | 335.1264[M − H]− | 7.3 | C21H19O4 | 271, 266, 241 |
| 21 | 380 | 284 | Unknown isoflavone | Isoflavone | 355.1173[M − H]− | 4.0 | C20H19O6 | 333, 267 |
| 22 | 395 | 280 | Unknown isoflavone | Isoflavone | 369.1324[M − H]− | 5.4 | C21H21O6 | 321 |
| 23 | 408 | 280, 335 | Apigenin | Flavone | 269.0448[M − H]− | 2.9 | C15H9O5 | |
| 24 | 485 | 280, 310 | Unknown isoflavone | Isoflavone | 397.1288[M − H]− | 1.3 | C22H21O7 | 353 |
| 25 | 485 | 230, 287 | Vogelin A | Isoflavanone | 369.0999[M − H]− | 5.2 | C20H17O7 | 329, 269 |
| 26 | 507 | nd | Oleanolic acid trihexoside | Triterpene | 943.5253[M + H]+ | −5.4 | C48H79O18 | 457 |
| 27 | 510 | 270, 307 | 5-Deoxyglyasperin F/5-Deoxylicoisoflavanone | Isoflavanone | 337.1085[M − H]− | −1 | C20H17O5 | |
| 28 | 531 | 287, 330 | Unknown isoflavanone | Isoflavanone | 353.1014[M + H]+ | 1.6 | C20H17O6 | |
| 29 | 531 | 287. 320 | Licoisoflavanone/Ficuisoflavone | Isoflavone | 353.104[M − H]− | 2.6 | C20H17O6 | 319 |
| 30 | 537 | 287, 311 | Isoneorautenol | Pterocarpan | 321.1147[M − H]− | 4.5 | C20H17O4 | 269, 252, 174 |
| 31 | 552 | 286, 323 | Dihydroisoneorautenol | Pterocarpan | 323.1288[M − H]− | 0.3 | C20H19O4 | |
| 32 | 562 | 287,322 | Eryvarin I/Erypoegin B | Isoflavone | 337.1419[M + H]+ | 4.6 | C21H21O4 | |
| 33 | 567 | 282 | Erythrabissin I | Pterocarpan | 353.1409[M − H]− | 4.1 | C21H21O5 | 338, 309, 269 |
| 34 | 596 | 287, 304 | 5-Deoxylicoisoflavanone | Isoflavanone | 337.1084[M − H]− | 0.8 | C20H17O5 | |
| 35 | 601 | 285, 340 | Erysubin A | Isoflavone | 351.0878[M − H]− | 1.2 | C20H15O6 | 311 |
| 36 | 621 | 282 | Unknown | – | 339.1237[M − H]− | 0.2 | C20H19O5 | 315, 248 |
| 37 | 630 | 281 | Unknown | – | 353.1392[M − H]− | 0.6 | C21H21O5 | 316, 239 |
| 38 | 642 | 280 | Erystagallin B | Pterocarpan | 437.1993[M − H]− | −5.4 | C26H29O6 | 368, 299 |
| 39 | 655 | 283 | Unknown isoflavone | Isoflavone | 351.1223[M − H]− | 4.3 | C21H19O5 | 316, 248, 174 |
| 40 | 659 | 272 | Unknown | – | 335.0922[M − H]− | 0.8 | C20H15O5 | 316 |
| 41 | 665 | 286 | Unknown triterpene | Triterpene | 471.3500[M − H]− | 4.4 | C30H47O4 | 316, 284 |
| 42 | 678 | 281, 287 | Sandwicensin | Pterocarpan | 337.1445[M − H]− | 0 | C21H21O4 | 295, 268, 112 |
| 43 | 680 | 287,322 | Dimethoxyisoflavone | Isoflavone | 283.0962[M + H]+ | 1 | C17H15O4 | 253 |
| 44 | 689 | 285, | Lysisteisoflavone | Isoflavone | 421.1664[M − H]− | 1.7 | C25H25O6 | 337, 295, 293 |
| 45 | 691 | 280 | Hydroxy-9,11-octadecadienoic acid | Fatty acid | 295.2269[M − H]− | 3.4 | C18H31O3 | 248, 174 |
| 46 | 714 | 280 | Unknown | – | 643.2375[M − H]− | 9.5 | C36H35O11 | 471, 365, 297 |
| 47 | 721 | 280 | 4′-O-Methylalpinumisoflavone | Isoflavone | 349.108[M − H]− | 0.5 | C21H17O5 | 335, 297, 248 |
| 48 | 748 | 280 | Erylysin A | Pterocarpan | 405.1709[M − H]− | −0.5 | C25H25O5 | |
| 49 | 762 | nd | Unknown sterol | Sterol | 463.3013[M + H]+ | 3.2 | C27H43O6 | |
| 50 | 779 | 282 | Hydroxy-9,11-octadecadienoic acid isomer | Fatty acid | 295.2274[M − H]− | 1.8 | C18H31O3 | 248, 180 |
| 51 | 785 | 287,330 | Unknown | – | 311.1682[M − H]− | −9.5 | C20H23O3 | |
| 52 | 806 | nd | Unknown | – | 421.2047[M + H]+ | 0.2 | C25H25O6 | 377 |
| 53 | 838 | nd | Oleanolic acid | Triterpene | 455.3551[M − H]− | −4.4 | C30H47O3 | 384, 297 |
rt, retention time; nd, not detected.
Flavonoids
Photodiode array detection provided an overview of the main flavonoid constituents (Fig.1A). UV spectra (200–600 nm) were measured for flavonoid sub-classes including 12 isoflavones, 3 flavones, 4 isoflavanones and 11 pterocarpans. Each sub-class exhibits a characteristic UV spectrum. For example, flavones have a maximum absorbance near 265 nm with a second maximum between 320 and 340 nm (peak 9), whereas pterocarpans have λ max around 280–290 nm (42). Extracts were analyzed in positive and negative ion electrospray ionization (ESI) MS modes to provide a comprehensive overview of the metabolite composition. Compared to the positive-ion ESI mode (Fig.1C), negative-ion MS spectra (Fig.1B) revealed better sensitivity than in positive mode, especially in the elution range of flavonoids (200–500 s). In addition, negative-ion MS spectral characteristics showed strong [M − H]− ions and lower chemical noise and consequently better sensitivity [22]. The positive ion ESI mass spectra were characterized by cations corresponding to [M + H]+, [M + Na]+ and fragment ions attributed to the sequential losses of isoprenyl (69 amu), malonyl (86 amu) and hexosyl (162 amu) groups. Few minor isoflavone peaks 13, 15, 18, 32 and 43 were only detected in positive ionization mode warranting the importance of acquiring data in both ionization modes. Two major flavone glycosides including dihydroxyflavone hexoside (m/z 415.102, [M − H]− peak 9) and apigenin hexosylmalonate (m/z 517.1702, [M − H]− peak 11) were identified in cell culture. With regard to isoflavanone subclass, vogelin A (m/z 369.0999, [M − H]− peak 25) and 5-deoxyglyasperin F/5-deoxylicoisoflavanone (m/z 337.1085, [M − H]− peak 27) exhibiting UV max around 310–320 nm typical for flavanones were measured.
Pterocarpans
Among flavonoid subclasses, pterocarpans amounted for the major forms in cell culture (11 peaks), exhibiting λ max around 280–290 nm with isoneorautenol (m/z 321.1147, [M−H]− peak 30) as the most abundant (Table 1). Other identified pterocarpans include erythribyssin B (m/z 283.0598, [M − H]− peak 19), eryvarin D (m/z 335.1264, [M − H]− peak 20), dihydroisoneorautenol (m/z 323.1288, [M − H]− peak 31) and sandwicensin (m/z 337.1445, [M − H]− peak 42). The predominant loss of 69 amu (–C5H9, prenyl group) in the MSn spectrum of pterocarpans is diagnostic for the presence of the isoprenyl group; a total of 6 peaks showed this pattern. For example, erystagallin B (m/z 437.1993, [M − H]− peak 38) showed 2 mass fragments at m/z 368 and 299 indicative for 2 isoprenyl losses (−2 × 69 amu). The abundance of isoprenylated pterocarpans in cell culture suggests for the presence of isoprenyl transferase enzyme with higher affinity toward pterocarpans. This is the first report for the accumulation of pterocarpans in E. lysistemon cell culture and suggests that it could provide a resource of that flavonoid subclass.
Alkaloids
With an increased sensitivity for detection of nitrogenous metabolites in positive mode, alkaloids could only be detected in that mode. Alkaloids that are known to predominate E. lysistemon plant extracts were almost absent in cell culture, except for few alkaloid peaks present at trace levels including erysotrine (m/z 314.1756 [M + H]+, peak 6), erythrartine/11-methoxyerysodine (m/z 330.1696, [M + H]+, peak 10) and erysotramidine (m/z 328.1534 [M + H]+, peak 12). In contrast, DOPA methyl ether (m/z 226.1073 [M + H]+, peak 8) was present as the major nitrogenous secondary metabolite identified in culture. No UV absorbance could be traced for alkaloid peaks, except for DOPA methyl ether showing distinct UV max at 270 nm. In tandem MS, alkaloids showed methyl losses from methoxy group (−15 Da).
Phenolic acid (cinnamates)
The most abundant nitrogenous compounds detected in cell culture were amino acyl hydroxycinnamic acid conjugates. A total of 5 peaks (2–5, 16) not previously reported in E. lysistemon plant tissue were identified in cell culture suggesting for an activation toward the production of acylcinnamates in cell culture. The predominant fragment of cinnamic acid derivatives in the MSn spectrum and characteristic UV max values at 298 and 325 nm are diagnostic for cinnamates; a total of 5 peaks showed similar UV (Table 1). MS/MS analysis confirmed the structure of N-p-coumaroylaspartic acid (3) m/z 278 and N-feruloylaspartic acid (5) m/z 308 from their respective product ions at m/z 163 and 193 indicative of a p-coumaroyl and feruloyl moieties, respectively, whereas N-caffeoylaspartic acid (2) gave a [M–H]− at m/z 294 with product ions m/z 132 for the aspartic acid moiety.
Differences in metabolites composition observed in E. lysistemon callus from its native plant are likely to be the result of genetic variation and/or lack of differentiation [22], [23]. It is worth mentioning that there was no obvious qualitative or quantitative difference in the metabolite profile of the 2 different treatments of the calli (1 mg l−1 or 2 mg l−1 of Kinetin and 2,4D), results not shown.
PCA of E. lysistemon MeJA elicited and control suspension culture observed in negative ionization mode
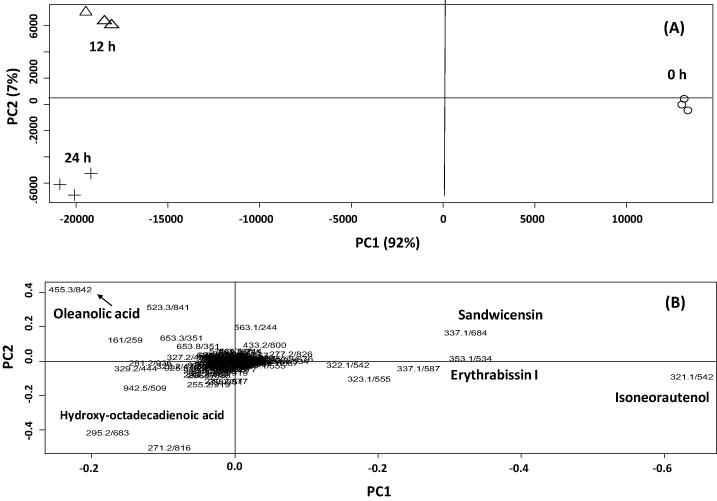
Cell culture was further subjected to MeJA treatment to determine its impact on reprogramming of secondary metabolites as revealed via UPLC-MS analysis. To assess for changes in metabolite composition in response to elicitation as monitored via UPLC-MS traces of the different callus samples harvested at 0, 12 and 2 h post MeJA elicitation (Suppl. Fig. 2), principal component analysis (PCA) was further adopted to classify samples in a more holistic way. From all samples, a total of 3152 mass signals were extracted by XCMS from the UPLC-MS data set acquired in negative ionization mode. The main principal component (PC) to differentiate between samples, i.e. PC1, accounted for 92% of the variance. The multivariate data analysis performed on MS data revealed a significant separation among samples (Fig.2A) with cells harvested at 0 h clearly distinguished (positive PC1 values) from cells treated with the MeJA at 12 and 24 h (negative PC1 values, Fig.2A right side of the score plot). Loading plot that exposes the most variant MS signals among samples revealed for enrichment of pterocarpans, sandwicensin (42), isoneorautenol (30) and erythrabissin I (33) in unelicited cultures. In contrast, cell culture samples harvested at 12 and 24 h were found more enriched in triterpenes and fatty acids namely oleanolic acid (53) and hydroxy-octadecadienoic acid (45) and suggestive for a suppression effect on pterocarpan biosynthetic branch in E. lysistemon cell culture. The induction of oleanolic acid is consistent with reports on MeJA up regulation of terpenoid biosynthetic pathways in planta [24]. MeJA induction of β-amyrin synthase gene associated with oleanolic acid (54) production was also previously reported in Gentiana straminea [25].
Fig. 2.
UPLC-qTOF-negative ionization MS (m/z 100–1000) principal component analyses of E. lysistemon unelicited cell culture (○), cell cultures treated with 1.0 mM MeJA at 0 h (Δ), 12 h (Δ) and 24 h (+) (n = 3). The metabolome clusters are located at the distinct positions in two-dimensional space prescribed by two vectors of principal component 1 (PC1 = 92%) and principal component 2 (PC2 = 7%). (A) Score Plot of PC1 vs PC2 scores. (B) Loading plot for PC1 and PC2 contributing to mass peaks and their assignments, with each metabolite denoted by its mass/rt (s) pair.
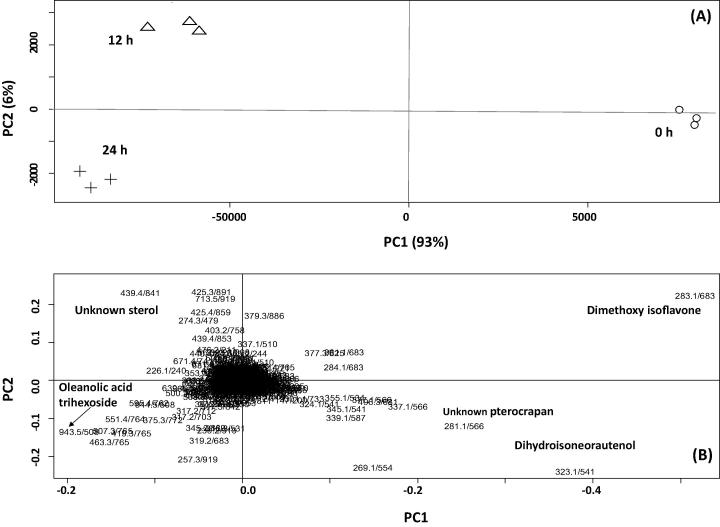
PCA of E. lysistemon MeJA elicited and control suspension culture observed in positive ionization mode
To provide more overview on the effects of elicitation on E. lysistemon cell culture metabolome, samples were also analyzed in positive ionization MS condition. PCA score plots derived from MS peaks in positive ionization mode were comparable to those in negative mode concerning segregation of samples at 0 h from 12 and 24 h. The PCA model (Fig.3A) explained 93% of the total variance in the first component, PC1, whereas the second principal component, PC2 presented 6% of the variance. Although comparable score plots in PCA were derived from both data sets, loading plots revealed a slightly different set of metabolites contributing for sample clustering. As revealed in Fig.3B, the major group that stood out in this plot corresponded to MS signals for dimethoxyisoflavone (43), isoneorautenol (30) and an unknown pterocarpan found more enriched in unelicited cell culture samples harvested at 0 h. In contrast, negative loading plot results along PC1 revealed that the triterpene glycoside “oleanolic acid tri-hexoside” (26) and an unknown sterol (49) (Fig.3B) levels were higher in the MeJA treated samples and accounting for its segregation at 12 h and 24 h from 0 h time point. The enrichment of the major pterocarpan “isoneorautenol” (30) in the untreated control cell culture samples (Fig.3B) concurs results derived from negative ionization mode and highlighting the negative impact of MeJA on pterocarpans biosynthetic branch. The decrease in pterocarpan levels in response to MeJA treatment is contrary to previous reports in Medicago truncatula cell culture [17] and lupines [26], suggesting that a differential response to MeJA exists in various legume species. This is the first report of MeJA differential effect on terpenoid accumulation versus pterocarpans in E. lysistemon cell culture (Fig. 4). Studies focused on the genetic bases of MeJA elicitation will help affirm induction hypothesis derived via metabolite profiling. It should be noted that oleanolic acid tri-hexoside conjugate was not detected by visual examination of unelicited cell culture chromatograms, suggesting that coupling of metabolomics for analysis of elicited samples presents a powerful methodology for identification of novel metabolites. Quantification of the major differential metabolites in elicited cell culture is presented in Table 2.
Fig. 3.
UPLC-qTOF-positive ionization MS (m/z 100–1000) principal component analyses of E. lysistemon unelicited cell culture samples (○), cell cultures treated with 1.0 mM MeJA at 0 h (o), 12 h (Δ) and 24 h (+) (n = 3). The metabolome clusters are located at the distinct positions in two-dimensional space prescribed by two vectors of principal component 1 (PC1 = 93%) and principal component 2 (PC2 = 6%). (A) Score Plot of PC1 versus PC2 scores. (B) Loading plot for PC1 and PC2 contributing to mass peaks and their assignments, with each metabolite denoted by its mass/rt (s) pair.
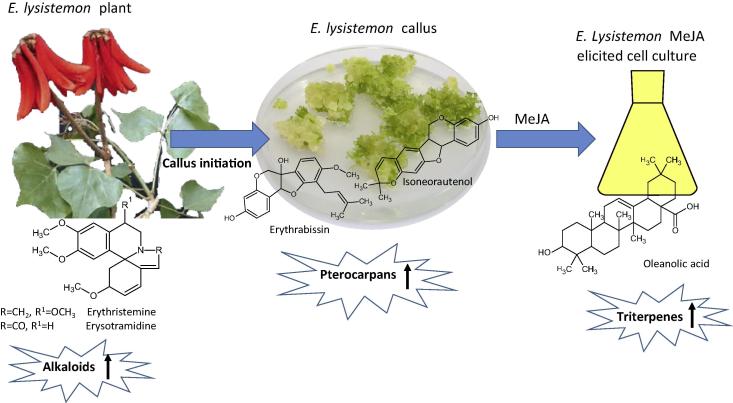
Fig. 4.
Diagram showing major secondary metabolite pathways with represented major structures that are up regulated in E. lysistemon plant, cell culture and in response to MeJA elicitation.
Table 2.
Quantification of metabolites identified in E. lysistemon cell suspension at 0, 12 and 24 h post 1.0 mM MeJA elicitation using MS detection in positivea and negativeb ionization modes. Values are expressed in μg g−1 as average for 3 biological replicates.
| Metabolites (μg g−1) |
E. lysistemon cell suspension |
||
|---|---|---|---|
| 0 h | 12 h | 24 h | |
| Erysotrinea | 2.8 ± 0.9 | 3.4 ± 2.1 | 0.6 ± 0.5 |
| Erysotramidinea | 10.8 ± 3.8 | 8.9 ± 2.5 | 6.0 ± 1.4 |
| ErylysinAb | 36.0 ± 8.2 | 22.2 ± 5.4 | 12.7 ± 2.6 |
| Sandwicensinb | 10.7 ± 2.9 | 3.4 ± 0.5 | 2.2 ± 0.5 |
| Oleanolic acidb | 406 ± 32.1 | 4907 ± 133 | 4838 ± 237 |
| ErythrabissinIb | 1268 ± 85 | 268 ± 59 | 243 ± 18 |
| Isoneorautenolb | 2217 ± 89 | 473 ± 16 | 564 ± 47 |
Conclusions
This study provides the first report on E. lysistemon cell suspension culture metabolite fingerprint via UPLC-MS. A metabolomic approach was used to investigate secondary metabolites viz. alkaloids, flavonoids and triterpenes and their reprogramming in response to MeJA elicitation. The results confirm MeJA elicitation effect on terpenoid accumulation and extend our knowledge base concerning secondary metabolism in other legume species [27]. Comparative metabolic profiling of E. lysistemon cell suspension culture and in response to elicitation using MeJA, revealed an activation in sterol/triterpenes formation, see model depicted in Fig. 4. The effect of other elicitors on secondary metabolites accumulation in Erythrina cell culture could also provide more holistic insight into elicitation effect within that genus and how it can reprogram its different secondary metabolite pathways.
Conflict of Interest
The authors declare that they have no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgments
Dr. Mohamed A. Farag acknowledges the funding received by Science and Technology Development Fund STDF, Egypt (grant number 12594), and the support of the Alexander von Humboldt Foundation, Germany. We also thank Dr. Christoph Böttcher, Leibniz Institute of Plant Biochemistry, Germany, for assistance with the UPLC-MS. We are grateful to Dr. Tilo Lübcken, University of Dresden, Germany, for providing R scripts for UPLC-MS data analysis.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jare.2016.07.002.
Appendix A. Supplementary material
Supplementary material contains Figs. 1 and 2.
References
- 1.Gledhill D. 4th ed. Cambridge University Press; Cambridge, UK: 2008. The names of plants; p. 157. [Google Scholar]
- 2.El-Masry S., Amer M.E., Abdel-Kader M.S., Zaatout H.H. Prenylated flavonoids of Erythrina lysistemon grown in Egypt. Phytochemistry. 2002;60(8):783–787. doi: 10.1016/s0031-9422(02)00202-9. [DOI] [PubMed] [Google Scholar]
- 3.El-Masry S., Hamoda H.M., Zaatout H.H., Abdel-Kader M.S. Constituents of Erythrina caffra stem bark grown in Egypt. Natural Product Sciences. 2010;16(4):211–216. [Google Scholar]
- 4.El-Masry S., Amer M.E., Dawood H.M., Radwan M.M., ElSohly M.A., Abou-Karam M. Bioassay-guided isolation of cytotoxic agents from Erythrina caffra root bark. Planta Med. 2014;80(10):807–808. [Google Scholar]
- 5.Amer M.E., El-Masry S., Shamma M., Freyer A.J. Three novel glycodienoid alkaloids from Erythrina lysistemon. J Nat Prod. 1990;54(1):161–166. [Google Scholar]
- 6.Iranshahi M., Vu H., Pham N., Zencak D., Forster P., Quinn R.J. Cytotoxic evaluation of alkaloids and isoflavonoids from the Australian tree Erythrina vespertilio. Planta Med. 2012;78(7):730–736. doi: 10.1055/s-0031-1298310. [DOI] [PubMed] [Google Scholar]
- 7.Wanjala C.C.W., Majinda R.R.T. Two novel glucodienoid alkaloids from Erythrina latissima seeds. J Nat Prod. 2000;63:871–873. doi: 10.1021/np990540d. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka H., Etoh H., Shimizu H., Oh-Uchil T., Terada Y., Tateishi Y. Erythrina alkaloids and isoflavonoids from Erythrina poeppigiana. Planta Med. 2001;67:871–872. doi: 10.1055/s-2001-18852. [DOI] [PubMed] [Google Scholar]
- 9.Yenesew A., Derese S., Irungu B., Midiwo J.O., Waters N.C., Liyala P. Flavonoids and isoflavonoids with antiplasmodial activities from the root bark of Erythrina abyssinica. Planta Med. 2003;69:658–661. doi: 10.1055/s-2003-41119. [DOI] [PubMed] [Google Scholar]
- 10.Njamen D., Talla E., Mbafor J.T., Fomum Z.T., Kamanyi A., Mbanya J.C., Cerda Anti-inflammatory activity of erycristagallin, a pterocarpene from Erythrina mildbraedii. Eur J Pharmacol. 2003;468:67–74. doi: 10.1016/s0014-2999(03)01664-9. [DOI] [PubMed] [Google Scholar]
- 11.San Miguel-Chávez R., Soto-Hernández M. Antifungal activity of the alkaloid extract of Erythrina americana miller seedlings. In: Vinciery F., editor. Proceedings of the 53rd annual congress of medicinal plant research, Florence. 2005. p. 326. [Google Scholar]
- 12.García-Mateos R., Soto-Hernández M., Martínez-Vázquez M., Villegas-Monter A. Isolation of alkaloids of Erythrina from tissue culture. Phytochem Anal. 1999;10(1):12–16. [Google Scholar]
- 13.San Miguel-Chávez R., Soto-Hernández M., Ramos-Valdivia A.C., Kite G. Alkaloid production in elicited cell suspension cultures of Erythrina americana miller. Phytochem Rev. 2007;6:167–173. [Google Scholar]
- 14.Baenas N., García-Viguera C., Moreno D.A. Elicitation: a tool for enriching the bioactive composition of foods. Molecules. 2014;19:13541–13563. doi: 10.3390/molecules190913541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J., Davisb L.C., Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005;23:283–333. doi: 10.1016/j.biotechadv.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Mekky H., Mohamed M., Lazarus C., Power J.B., Davey M.R. Biosynthesis of very long chain polyunsaturated fatty acids in the leafy vegetable chicory. Agric Food Sci. 2011;20:327–340. [Google Scholar]
- 17.Farag M.A., Huhman D.V., Dixon R.A., Sumner L.W. Metabolomics reveals novel pathways and differential mechanistic and elicitor specific responses in phenylpropanoid and isoflavonoid biosynthesis in Medicago truncatula cell cultures. Plant Physiol. 2008;146(2):387–402. doi: 10.1104/pp.107.108431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farag M.A., El Sayed A.M., El Banna A. Metabolomics reveals distinct methylation reaction in MeJA elicited Nigella sativa callus via UPLC-MS and chemometrics. Plant Cell Tiss Org. 2015;122:453–463. [Google Scholar]
- 19.El Senousy A.S., Farag M.A., Al-Mahdy D.A., Wessjohann L.A. Developmental changes in leaf phenolics composition from three artichoke cvs. (Cynara scolymus) as determined via UHPLC-MS and chemometrics. Phytochemistry. 2014;108:67–76. doi: 10.1016/j.phytochem.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Farag M.A., EL-Ahmady S., Alian F., Wessjohann L.A. Metabolomics driven analysis of artichoke leaf and its commercial products via UHPLC-q-TOF-MS. Phytochemistry. 2013;95:177–187. doi: 10.1016/j.phytochem.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Smith C.A., Want E.J., O’Maille G., Abagyan R., Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78:779–787. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 22.Farag M.A., Huhman D.V., Lei Z., Sumner L.W. Metabolic profiling and systematic identification of flavonoids and isoflavonoids in roots and cell suspension cultures of Medicago truncatula using HPLC-UV-ESIMS and GC-MS. Phytochemistry. 2007;68:342–354. doi: 10.1016/j.phytochem.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 23.Jalal M.A.F., Collin H.A. Polyphenols of mature plant, seedling and tissue cultures of Theobroma cacao. Phytochemistry. 1977;16:1377–1380. [Google Scholar]
- 24.Scholz M., Lipinski M., Leupold M., Luftmann H., Harig L., Ofir R. Methyl jasmonate induced accumulation of kalopanaxsaponin I in Nigella sativa. Phytochemistry. 2009;70(4):517–522. doi: 10.1016/j.phytochem.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y., Cai Y., Zhao Z., Wang J., Li J., Xin W. Cloning and functional analysis of a beta-amyrin synthase gene associated with oleanolic acid biosynthesis in Gentiana straminea MAXIM. Biol Pharm Bull. 2009;32(5):818–824. doi: 10.1248/bpb.32.818. [DOI] [PubMed] [Google Scholar]
- 26.Katagiri Y., Hashidoko Y., Ibrahim R.K., Tahara S. Activation of isoflavone biosynthesis in excised cotyledons of Lupinus seedlings by jasmonoids and excess light. Z Naturforsch C. 2001;56(11–12):1038–1046. doi: 10.1515/znc-2001-11-1222. [DOI] [PubMed] [Google Scholar]
- 27.Dixon R.A., Sumner L.W. Legume natural products. Understanding and manipulating complex pathways for human and animal health. Plant Physiol. 2003;131:878–885. doi: 10.1104/pp.102.017319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material contains Figs. 1 and 2.