Abstract
Accumulating studies demonstrate that interleukin (IL)-21 modulates the differentiation of various CD4 and CD8 T cell subsets and provide insights into the underlying cellular and molecular processes that are influenced by this cytokine. Intriguingly, the effects of IL-21 on T cells can be complex and vary depending upon the experimental system used. We review our current understanding of the roles of IL-21 in the generation of phenotypically distinct CD4 and CD8 T cell populations and discuss the potential environmental cues, cellular factors, and molecular mediators that impact the actions of IL-21. We propose that IL-21 acts in a context-dependent manner to accentuate T cell subset development.
T cell responses and the multifaceted roles of IL-21
The differentiation of functionally diverse T cell subsets helps confer immunological protection against pathogens and cancers, but also contributes to autoimmunity, chronic inflammation, and transplant rejection. The development of distinct T cell populations is guided by antigenic, costimulatory, and cytokine signals. The amalgamation of these multiple immunological instructions configure transcriptional networks that regulate gene expression patterns which dictate cell fate decisions, developmental flexibility, and survival. Here we review the range of impacts that one intriguing cytokine, IL-21, has on these processes that shape the phenotype and functions of CD4 and CD8 T cell pools.
IL-21 has been shown to be produced by natural killer T (NKT) cells [1]. Additionally, IL-21 is synthesized by various CD4 T cell subsets including Th17 cells, follicular helper T (Tfh) cells and Th9 cells, as well as by CD8 T cells under certain conditions such as during HIV infection [2–4]. The manufacture of IL-21 is induced by T cell receptor (TCR) signaling, costimulation, and also by cytokines including IL-1β, IL-6, IL-27, as well as by IL-21 itself, and is controlled by the transcription factors c-Maf and interferon regulatory factor 1 (IRF1) [4–8]. Notably, the timing, longevity, and levels of IL-21 production can vary as, for example, increased and prolonged IL-21 synthesis is observed during chronic compared with acute lymphocytic choriomeningitis virus (LCMV) infections [9]. Moreover, IL-21 transcript levels are upregulated in antigen-specific CD8 T cells by 12 hours following Listeria monocytogenes infection, suggesting that IL-21 is transiently induced early during the activation process [10]. IL-21 signals through Janus kinase (JAK)-signal transducer and activator of transcription (STAT), mitogen-activated protein kinase (MAPK), and phosphoinositide 3-kinase (PI3K)-AKT pathways and primarily activates STAT3 but can also activate STAT1, STAT5A, and STAT5B [11], as well as STAT4 in human T cells [12]. Notably, IL-21 has broad immunological actions and can regulate NK cells, macrophages, and dendritic cells, as well as plays a key role in promoting B cell and antibody responses [2, 3]. In this review we focus on the diverse roles of IL-21 in the differentiation of CD4 and CD8 T cell populations.
IL-21 has been documented to regulate the differentiation and function of several CD4 T cell subsets including Th1 cells [13–15], Th2 cells [16–19], Th17 cells [20–22], regulatory T (Treg) cells [23, 24], type 1 regulatory T (Tr1) cells [7, 25], and Tfh cells [26, 27]. Moreover, IL-21 also plays roles in the differentiation of Th9 cells [28] and follicular regulatory T (Tfr) cells [29], as well as the production of IL-22 by CD4 T cells [30–32]. The functional significance of IL-21 in regulating CD8 T cell responses is highlighted by its essential role in sustaining anti-viral CD8 T cells during chronic LCMV infections [9, 33, 34]. Additionally, IL-21 cooperates with IL-10 to promote the maturation of memory CD8 T cells via the transcription factor STAT3 [35]. Following certain infections, IL-21 is also required for the generation of effector CD8 T cells [36] and for the optimal recall responses of memory CD8 T cells [37–39].
Although IL-21 clearly plays pivotal roles in peripheral T cell differentiation, mixed and sometimes conflicting results have been reported. For example, several studies have questioned the stringency for the requirements of IL-21 for the generation of Th2, Th17, and Tfh cells [40–47]. Furthermore, a growing body of work demonstrates that IL-21 may play opposing or dispensable roles in influencing CD8 T cell responses during various infections including LCMV, vaccinia virus, adenovirus, influenza virus, and Encephalitozoon cuniculi [9, 33–39, 47–49]. These studies raise the possibility that the effects of IL-21 may be modulated by additional variables such as environmental cues and the differentiation state of responding T cell populations. This implies that cell-intrinsic and extrinsic parameters guide the biochemical interpretation of IL-21 signals by T cells and thereby direct downstream changes in transcriptional regulators that control cell fate decisions and developmental outcomes. We discuss recent publications that have begun to decipher the factors that influence the outcome of IL-21 signaling in T cells and anticipate future studies into the mechanisms underlying the apparently complex functions of IL-21.
IL-21 and the differentiation of CD4 T cell subsets
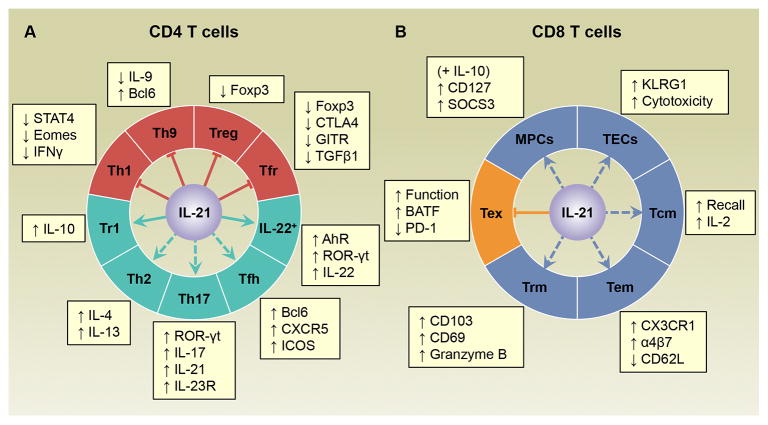
IL-21 plays important roles in the differentiation of almost every major CD4 T cell subset characterized so far (Figure 1A). In order to achieve this IL-21 signals must be integrated with other lineage-specific pathways that regulate the developmental fates of the responding CD4 T cells.
Figure 1.
IL-21 modulates the differentiation of CD4 and CD8 T cell subsets in a context-dependent manner. (A) IL-21 suppresses the differentiation of several CD4 T cell subsets including Th1, Th9, regulatory T (Treg), and follicular regulatory T (Tfr) cells, while it promotes the differentiation of IL-22-producing CD4 T cells as well as type 1 regulatory T (Tr1), Th2, Th17, and follicular helper T (Tfh) cells. However, the roles of IL-21 in Th2, Th17 and Tfh cell differentiation may vary between different disease models. (B) IL-21 has been reported to promote the development of memory precursor cells (MPCs) (in conjunction with IL-10), terminal effector cells (TECs), central memory T (Tcm), effector memory T (Tem), and tissue-resident memory T (Trm) cells, and is stringently required to alleviate exhausted T cell (Tex) responses during chronic LCMV infections. Dashed lines indicate that conflicting results have been reported regarding the function of IL-21 in the differentiation of the indicated T cell populations. Abbreviations: AhR, aryl hydrocarbon receptor; BATF, basic leucine transcription factor ATF-like; Bcl6, B cell lymphoma 6; CTLA4, cytotoxic T-lymphocyte associated antigen 4; Eomes, Eomesodermin; Foxp3, forkhead box P3; GITR, glucocorticoid-induced TNF receptor family related protein; ICOS, inducible costimulatory receptor; IFNγ, interferon- γ; KLRG1, killer cell lectin-like receptor G1; ROR-γt, retinoic acid receptor-related orphan receptor (ROR)-γt; SOCS, suppressor of cytokine signaling; STAT4, signal transducer and activator of transcription 4; TGFβ1, transforming growth factor β1.
IL-21, Treg cells, and Th17 cells
IL-21 plays opposing roles in the formation of Th17 and Treg cells. IL-21 cooperates with transforming growth factor-β (TGFβ) to accentuate the development of Th17 cells while restricting the differentiation of Treg cells [20–22]. This role can also be fulfilled by IL-6 [50], which functions in part by inducing the production of IL-21 by Th17 cells, which in turn enforces the Th17 differentiation program independently of IL-6 [20–22]. In contrast, IL-21 can act directly on Treg cells to suppress their expansion and also indirectly by inhibiting IL-2 production by non-Treg cells [23, 24]. Thus, IL-21 can promote potentially pathogenic responses by both facilitating Th17 differentiation and curtailing Treg expansion. Conversely, induction of IL-21 by IL-27 can elicit IL-10 production by Tr1 cells, which exert immunosuppressive effects [7, 25]. Thus, the priming conditions can influence the actions and importance of IL-21.
The necessity for IL-21 to drive pathogenic Th17 responses is also variable and influenced by the inflammatory setting. It was initially reported that the incidence of experimental autoimmune encephalomyelitis (EAE) and levels of Th17 responses were reduced in IL-21-deficient mice following immunization with myelin oligodendrocyte glycoprotein (MOG) peptide and complete Freund’s adjuvant (CFA) to induce active disease [20]. Nevertheless, subsequent reports suggested that IL-21 is dispensable for the differentiation of Th17 cells and that the loss of IL-21 signals worsens rather than alleviates the disease [41, 42]. Intriguingly, a recent publication suggests that IL-21 is required for the development of spontaneous but not active EAE [32]. Since CFA is required to induce active EAE but not spontaneous disease this highlights the potential role of the inflammatory milieu in modifying or bypassing the influence of IL-21 signals.
IL-21, Tfh cells, and Tfr cells
The production of high-affinity antibodies relies on optimal germinal center (GC) reactions to allow B cells to undergo somatic hypermutation, affinity-based selection, and class switch recombination [51]. Tfh cells are essential for the formation and maintenance of GCs, and their differentiation relies on the transcriptional regulators B cell lymphoma 6 (Bcl6) and STAT3, which are both mobilized by IL-21 [52]. Accordingly, it has been suggested that IL-21 is essential for Tfh cell development [26, 27]; however, other studies indicate that IL-21 has modest, if any, impact on the formation of this subset following protein immunization or viral infections [43–47]. Tfh cell differentiation is also regulated by IL-6 which likely compensates for IL-21 under certain circumstances [52]. Indeed, during acute LCMV and influenza virus infections Tfh cell development is more severely impaired by the absence of both IL-21 and IL-6, than by the loss of either cytokine alone [46, 47]. Moreover, stronger TCR signals may also partially rescue the IL-21-dependent induction of Tfh cells [26]. Collectively, these findings further demonstrate the interplay between IL-21 and other cellular, inflammatory and antigenic signals in dictating the developmental direction of the response.
Tfr cells are a subset of Treg cells which share certain properties with Tfh cells and also localize to the GC, but unlike their Tfh counterparts, Tfr cells exert suppressive effects on GC reactions [53–55]. Consistent with the role in restricting Treg responses, Ding et al. showed using the autoimmune-prone BXD2 mouse model that IL-21 selectively supports Tfh cells while restricting Tfr cell responses, resulting in higher Tfh to Tfr cell ratios, which may contribute to the generation of autoimmune antibody responses [29]. Further studies are necessary to determine whether IL-21 influences the balance of Tfh and Tfr cells during infections and whether this influences pathogen-specific humoral immunity.
IL-21 and Th9 cells
Th9 cells are characterized by their production of their signature cytokine, IL-9, and contribute to allergic and inflammatory diseases as well as provide protective immunity against helminth infections and cancer [56]. IL-21 has been shown to counteract IL-2-induced IL-9 production, under polarizing conditions in vitro, by promoting the expression of the transcriptional regulator Bcl6, which may inhibit the generation of Th9 cells by competing with STAT5 [28]. Since Th9 cells can be induced to produce IL-21 they may self-regulate by manufacturing this cytokine [4]. Although the production of IL-21 by Th9 cells may inhibit IL-9 synthesis, it can also indirectly augment their anti-tumor activities by stimulating the output of interferon- γ (IFNγ) by NK and CD8 T cells [4], highlighting the pleiotropic roles of IL-21 in positively and negatively regulating immune responses.
IL-21 and IL-22-producing CD4 T cells
IL-21 promotes the production of IL-22 by CD4 T cell populations [30–32]. Yeste et al. demonstrated that IL-21 induces the expression of the transcription factors retinoic acid receptor-related orphan receptor (ROR)-γt and aryl hydrocarbon receptor (AhR), which drive the production of IL-22 [31]. In addition, STAT3 activated by IL-21 facilitates histone acetylation of the Il22 promoter and AhR recruitment [31]. Thus, IL-21 can indirectly leverage the expression of target genes such as Il22 and thus influence T cell differentiation and function by modulating chromatin accessibility. Conversely, the outcome of IL-21 signaling may also rely on the chromatin landscape of the responding cell, which impacts the binding of IL-21-activated transcription factors such as STAT3.
IL-21 and Th1 and Th2 cells
IL-21 has been reported to inhibit the differentiation of IFNγ-producing Th1 cells in both human and murine systems, which has been attributed to the downregulation of the transcriptional regulator STAT4 and the T-box transcription factor eomesodermin (Eomes) [13–15]. Interestingly, IL-21 may enhance the expression of Th1-associated molecules including IFNγ and T-bet by human T cells pre-activated by TCR stimulation and IL-2, supporting the notion that the effects of IL-21 may vary according to the differentiation state of the responding T cells [57]. By contrast, IL-21 has been implicated in promoting Th2 responses during infections with the helminth parasites Schistosoma mansoni and Nippostrongylus brasiliensis [16, 17]. Additionally, in the house dust mite (HDM) challenge model of asthma, IL-21 is produced by both Tfh and non-Tfh cells and drives Th2 responses in a cell-intrinsic manner [19]. Notably, recent studies suggest that Th2 cells which arise following exposure to HDM antigens are derived from IL-4-committed IL-21+ Tfh cells [58]. Accordingly, whether IL-21-producing non-Tfh cells also originate from Tfh/Tfh-like precursors or develop independently of the Tfh lineage warrants further investigation. Conflicting findings showing that IL-21 is and is not required for the production of the Th2 signature cytokine IL-4 have, however, been reported during Heligmosomoides polygyrus infections [17, 40]. Since STAT3 cooperates with STAT6 to enhance Th2 differentiation [59] it will be interesting to determine whether IL-21 acts via STAT3 to elicit Th2 immunity and whether this accentuating ability is dependent upon STAT6 activity.
IL-21 and CD8 T cell differentiation and maintenance
Paralleling the effects on CD4 T cell responses, IL-21 also has variable effects on the proliferation, differentiation, function, and survival of CD8 T cell subsets (Figure 1B). The requirements for IL-21 are more rigid during certain chronic infections and under homeostatic conditions, suggesting that IL-21 is of greater importance when the availability of other differentiation factors is limited or perhaps when antigenic activation is sustained.
IL-21 and CD8 T cell differentiation during acute infections
The impact of IL-21 on CD8 T cell responses during acute infections is pathogen-dependent and generally limited. The development of effector and memory CD8 T cells is largely IL-21-independent following acute LCMV infection [9, 33, 34]; however, IL-21 can enhance the production of IL-2 and promotes the secondary recall responses upon rechallenge under competitive conditions in mixed bone marrow chimeras [37]. Although Fröhlich et al. demonstrated that IL-21 is dispensable for priming vaccinia virus-specific CD8 T cell responses [34], other studies reported that it increases the abundance of memory CD8 T cells and regulates their ability to mount secondary responses [38]. Additionally, IL-21 may promote the survival of CD8 T cells during the early phase of vaccinia virus infection by upregulating the expression of B cell lymphoma 2 (Bcl2) and Bcl-xL in a STAT1- and STAT3-dependent manner [39]. IL-21 also bolsters anti-viral CD8 T cells and programs recall responses during adenovirus infections [38]. Notably, CD8 T cells primed by adenovirus infection in the absence of IL-21 signaling express higher levels of tumor-necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) which likely contributes to their poor ability to accumulate following rechallenge [38], mirroring the phenotype of “helpless” CD8 T cells generated under CD4 T cell-deficient conditions [60].
IL-21 has also been shown to act in conjunction with IL-10 to promote memory CD8 T cell development. In the absence of both IL-21 and IL-10, reduced frequencies of CD127high, killer cell lectin-like receptor G1 (KLRG1)low memory precursor cells (MPCs) are detected following acute LCMV infection, indicative of impaired maturation of memory CD8 T cells [35]. These studies suggest that IL-21 and IL-10 play redundant roles in activating STAT3, which drives the differentiation of memory CD8 T cells [35]. Although IL-6 also activates STAT3 and cooperates with IL-21 to promote the differentiation of CD4 Tfh cells, the loss of IL-6, IL-21, or both does not impair the development of anti-viral CD8 T responses following influenza infection, further illustrating the context-dependent requirements for IL-21 [34, 47, 48].
IL-21 and CD8 T cell exhaustion
Sustained antigenic activation associated with persistent infections and tumor outgrowth may drive the development of T cell exhaustion which is characterized by the progressive deterioration of T cell functions and the expression of multiple inhibitory receptors, which can result in the deletion of the T cell population [61–63]. Several reports have shown that IL-21, likely produced by CD4 T cells, acts directly on anti-viral CD8 T cells to support their responsiveness and limit exhaustion during chronic LCMV infection [9, 33, 34]. Since exhausted CD8 T cells downregulate CD127 (IL-7Rα) and CD122 (IL-15Rβ) [64, 65] this suggests that IL-21 may be crucial for the maintenance of CD8 T cells when IL-7 and IL-15 signals are insufficient.
During hepatitis B virus (HBV) and hepatitis C virus (HCV) infections increased IL-21 levels and IL-21-producing CD4 T cells are associated with better anti-viral CD8 T cell responses and viral control [66–68]. Similarly, during HIV-1 infections higher levels of IL-21 production by CD4 and CD8 T cells are associated with enhanced CD8 T cell functions and lower viral loads [69–72]. IL-21 enhances the cytotoxicity of HIV-specific CD8 T cells in vitro by promoting their production of perforin and degranulation [72, 73], and the administration of exogenous IL-21 promotes the effector functions of anti-viral CD8 T cells in rhesus macaques during simian immunodeficiency virus (SIV) infection, although no impact on viral loads were detected [74]. Interestingly, HIV-specific IL-21-producing CD4 T cells in the peripheral blood resemble Tfh cells and enhance CD8 T cell and B cell responses in vitro [75]. Thus, during certain persistent infections IL-21 levels regulate the quantity and quality of the CD8 T cell response.
Recent studies by Xin et al. revealed that IL-21 maintains functional CD8 T cells during chronic LCMV infection by sustaining the expression of the transcription factor basic leucine transcription factor, ATF-like (BATF) via the activation of STAT3 [76]. In vitro studies further indicate that IL-21-induced BATF cooperates with IRF4 to induce the expression of the transcriptional regulator B lymphocyte-induced maturation protein 1 (Blimp1) [76], which can both enhance the effector functions of CD8 T cells [77–79], as well as exacerbate exhaustion and the expression of inhibitory receptors [78]. Indeed, BATF-deficient virus-specific CD8 T cells express lower levels of the inhibitory receptors PD-1 and 2B4 [76]. Therefore, it will be important to pinpoint how the IL-21-STAT3-BATF axis controls the levels of Blimp1 and how its regulatory activities are influenced by the degree of antigenic stimulation and presence of other activating or inhibitory signals.
IL-21 and CD8 T cell differentiation under homeostatic and lymphopenic conditions
Early studies indicated that IL-21 supports the expansion of T cells in lymphopenic non-obese diabetic mice [80]. Our laboratory has further demonstrated that IL-21 can directly promote the generation and/or maintenance of effector-phenotype CD8 T cell populations during homeostasis or lymphopenia [49]. This is consistent with the recently reported role of IL-21 in supporting the differentiation of terminal effector CD8 T cells (TECs) which express high levels of KLRG1 during the acute phase of Encephalitozoon cuniculi infection [36].
Under lymphopenic and homeostatic conditions IL-21 also increases the accumulation of CD8 T cells in non-lymphoid organs as well as the differentiation of CD8 T cells in the small intestine which express CD69, CD103, and granzyme B, properties associated with tissue-resident memory T (Trm) cell populations [49]. Similar to exhausted CD8 T cells, Trm cells have been reported to express low levels of CD122 and CD127 [81–83], again supporting the notion that IL-21 may more significantly contribute to T cell differentiation and maintenance when IL-7 and IL-15 signals are limiting.
Interestingly, IL-21 is associated with increased expression of the chemokine receptor CX3CR1 and integrin α4β7 on T cells, which may influence their migratory patterns, and in vitro studies suggest that IL-21 may amplify or substitute for retinoic acid (RA) signals to induce α4β7 [49], which is further supported by the observation that IL-21 and RA synergistically promote α4β7 expression on B cells [84]. These requirements for IL-21 can be overridden by acute LCMV infection, but LCMV-primed memory CD8 T cells still require IL-21 for optimal accumulation in tissues during lymphopenia-induced homeostatic proliferation [49]. This further highlights how the levels of antigenic signaling and presence of other homeostatic and/or inflammatory cytokines, as well as the differentiation state of the responding cells, likely modulate the requirements for, and effects of, IL-21.
Integrating and interpreting IL-21 signals
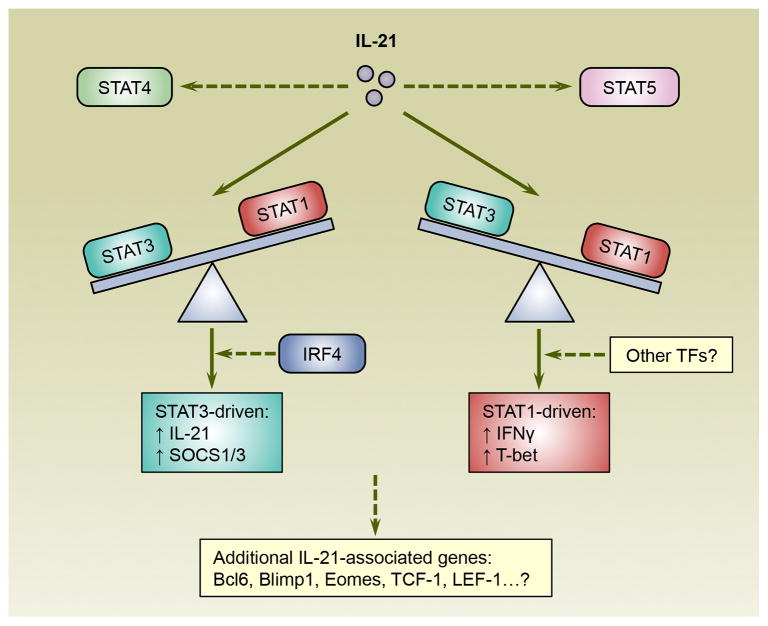
The presence of IL-21 can clearly sway the establishment of both CD4 and CD8 T cell subsets but the impact and stringency varies. This implies that IL-21 commands can be directed, amplified, or perhaps nullified by the local environmental milieu and cellular conditions. In addition, the anatomical placement of IL-21 producing cells dictates the regional availability and concentrations of this cytokine, further influencing its potential effects. The engagement of IL-21 with its receptor activates several signaling and transcriptional pathways in T cells including JAK-STAT cascades, involving STAT3 as well as STAT1, STAT5A, and STAT5B. The influence of IL-21 on STAT4 activity in T cells is more complex as some studies show that IL-21 activates STAT4 [12] while others suggest that IL-21 inhibits the expression and IL-12-induced activation of STAT4 [13, 35]. STAT transcription factors can have asymmetric actions [85] and recently Wan et al. reported that IL-21 has variegated effects on CD4 T cell gene expression via STAT1 and STAT3 (Figure 2) [86]. While IL-21 induces the phosphorylation of both STAT1 and STAT3, the activation of STAT1 is enhanced by the absence of STAT3, possibly due to decreased expression of suppressor of cytokine signaling (SOCS)-1 and 3 [86]. Consequently, in IL-21-activated CD4 T cells, ablation of STAT3 leads to higher expression of STAT1-stimulated genes that have been implicated in T cell differentiation and function including T-bet and IFNγ [86]. Conversely, in the absence of STAT1, the expression of STAT3 regulated genes, such as IL-21 itself, are increased [86]. Thus, the interplay between STAT1 and STAT3 is one mechanism which governs the outcome of IL-21 signaling in CD4 T cells. Notably, the collaborative and differential effects of STAT3 and STAT1 on gene expression have also been elegantly demonstrated during IL-6 and IL-27 signaling in CD4 T cells [85].
Figure 2.
The reciprocal balance between STAT1 and STAT3 influences the expression of IL-21-regulated genes in CD4 T cells. IL-21 activates both STAT1 and STAT3, which can differentially regulate gene expression and counteract each other. Additionally, IL-21 has also been shown to impact STAT4 and STAT5 activities. STAT3 induces SOCS1 and SOCS3, as well as the expression of IL-21-driven genes including IL-21 itself. Conversely, the expression of STAT1-regulated genes such as T-bet and interferon- γ (IFNγ) is further elevated in IL-21 activated CD4 T cells by the absence of STAT3. Thus, the actions of IL-21 may depend on the relative expression and activity of STAT1 and STAT3. Since the binding of STAT3 to many of its target sites relies on IRF4, other transcription factors also influence the interpretation of IL-21 signals. Notably, IL-21 also leverages the expression of other transcription regulators that control T cell fate decisions and lineage fitness including B cell lymphoma 6 (Bcl6), B lymphocyte-induced maturation protein-1 (Blimp1), eomesodermin (Eomes), T cell factor-1 (TCF-1) and lymphoid enhancer-binding factor-1 (LEF-1). Abbreviations: IRF4, interferon regulatory factor 4; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription; TFs, transcription factors.
In addition to STAT proteins, IL-21 influences the expression of multiple downstream transcription factors that dictate the differentiation state and lineage fitness of CD4 and CD8 T cells, including T-bet, Eomes, Bcl6, and Blimp1 [14, 35, 86–90]. In CD4 T cells, T-bet and Eomes promote the production of IFNγ, with T-bet being essential for the differentiation of Th1 cells [14, 91], whereas Bcl6 and Blimp-1 reciprocally regulate the differentiation of Tfh cells [92]. In terms of CD8 T cells, while Eomes and Bcl6 promote the differentiation of central memory CD8 T cells, T-bet and Blimp-1 drive the terminal differentiation of effector CD8 T cells [93]. Therefore, IL-21 can play diverse roles in the differentiation and fate decisions of CD4 and CD8 T cells via these transcription factors, and the outcome of IL-21 signaling may rely on their relative abundance. Moreover, IL-21 also increases the expression of T cell factor-1 (TCF-1) and lymphoid enhancer-binding factor-1 (LEF-1) [88], which upregulate Eomes in CD8 T cells and cooperate to promote memory formation [94] and are also required for the differentiation of CD4 Tfh cells by increasing the expression of Bcl6 while concurrently suppressing Blimp1 expression [95].
Distinct cell-type dependent signaling scenarios are further highlighted by the observation that the increase in IL-21-induced STAT1 activation is much less pronounced in STAT3-deficient CD8 T cells compared with CD4 T cells [86]. Thus, other mechanisms must be more dominant in CD8 T cells to direct the actions of IL-21. Additional players may include STAT5A and STAT5B, which regulate the differentiation of both CD4 and CD8 T cells. In CD4 T cells STAT5 has been reported to compete with STAT3 binding to the Il17a-Il17f and Bcl6 loci, and consequently limiting their expression [96, 97], which is consistent with the observation that STAT3 and STAT5 play opposing roles in the differentiation of Th17 and Tfh cells [96, 98, 99]. For CD8 T cell responses, STAT5 is critical for the expansion of effector cells and promotes the survival of both MPCs and TECs [100–102], while STAT3 preferentially supports the maturation of MPCs [35]. Thus, it is plausible that the asymmetric actions of STAT3 and STAT5 may also dictate the outcome of IL-21 signals.
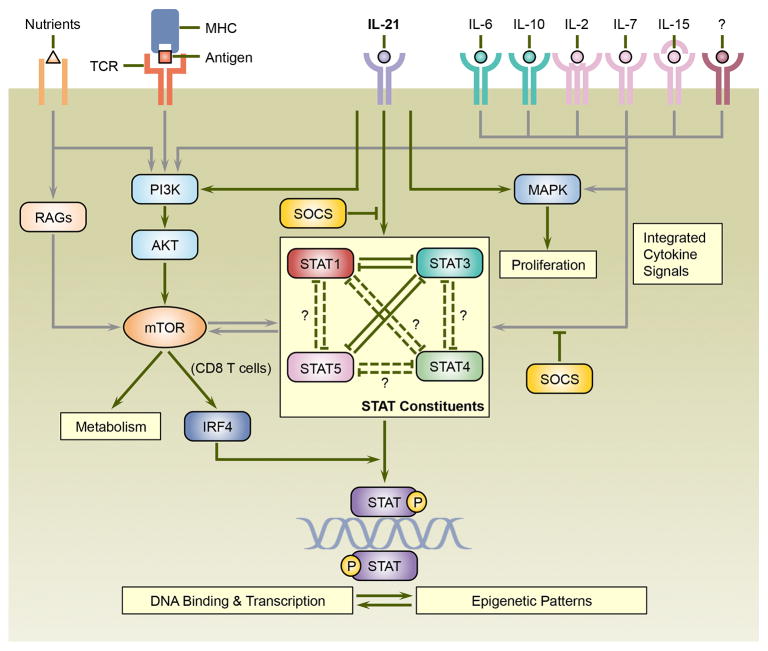
IL-21 not only operates via the JAK-STAT pathway, but also activates PI3K-AKT and MAPK signaling cascades, both of which are involved in IL-21-induced CD8 T cell proliferation [11]. Intriguingly, the PI3K-AKT pathway activates mammalian target of rapamycin (mTOR) which regulates T cell metabolism and differentiation, and the crosstalk between mTOR and STAT proteins play important roles in T cell differentiation [103]. Thus, it is plausible that IL-21 may exert some of its effects on T cell differentiation and potentially metabolism via PI3K-AKT-mTOR pathway. Interestingly, IL-21-induced STAT3 binding to DNA is severely curtailed in the absence of IRF4 [89]. Since TCR signals regulate the expression of IRF4 at least partially via mTOR in CD8 T cells [104, 105], this may couple the actions of IL-21 with the strength and duration of antigenic activation. Taken together, we propose a model where transcriptional regulators including STAT proteins integrate IL-21 signals with numerous cellular and environmental parameters, and ultimately direct the T cell differentiation program (Figure 3, Key Figure).
Figure 3.
The actions of IL-21 on T cells are context-dependent and shaped by cellular and environmental parameters. IL-21 signals are interpreted and integrated via a network that includes STAT, MAPK, and PI3K-AKT transduction pathways. STAT proteins are central constituents of this network, and a wide array of cytokines including IL-6, IL-10, IL-2, IL-7 as well as IL-15 activate overlapping and distinct STAT proteins that cooperate with, counteract, or compensate for IL-21. Suppressor of cytokine signaling (SOCS) proteins, which attenuate STAT activation also provide an additional level of regulation. IL-21 may integrate with TCR-signals via the PI3K-AKT pathway which can culminate in the activation of mammalian target of rapamycin (mTOR), which is also sensitive to nutrient levels and plays important roles in T cell metabolism and differentiation. In addition, mTOR has been shown in CD8 T cells to promote the expression of interferon regulatory factor 4 (IRF4), which is necessary for STAT3 binding to many of its target sties. IL-21 can also promote proliferative responses by activation of MAPK. Thus, antigenic signals, other cytokines, and the metabolic status of the responding cell all likely contribute to the eventual outcome of IL-21 signaling. Finally, the epigenetic profiles of target genes and the binding of transcriptional regulators may mutually influence each other, adding to the cell-type dependent control of the magnitude and patterns of gene expression that dictate the differentiation, plasticity, and survival of the responding cells. Abbreviations: MAPK, mitogen-activated protein kinase; MHC, major histocompatibility complex; PI3K, phosphoinositide 3- kinase; RAGs, RAS-related GTP-binding protein family of small GTPases; STAT, signal transducer and activator of transcription; TCR, T cell receptor.
Concluding remarks
Since its identification in 2000 [106], much has been discovered about the pleiotropic effects of IL-21 on CD4 and CD8 T cell differentiation. The functions of IL-21 are varied, context-dependent, and dispensable in certain settings. From a molecular perspective, the framework of STAT proteins and their collaborators are important for dictating the effects of IL-21. Therefore, environmental and cellular conditions that alter the balance between STAT constituents and other transcription factors may have a strong impact on the interpretation of IL-21 signals. Thus, a detailed definition of the cellular and molecular elements that steer the effects of IL-21 is now required (see Outstanding Questions). Addressing this is important, as IL-21 is associated with a diverse range of diseases, including several allergic, autoimmune, and inflammatory diseases such as systemic lupus erythematosus, rheumatoid arthritis, and Crohn’s disease [3]. Thus, targeting IL-21 may be beneficial in such settings and this is being clinically tested [3]. Blocking IL-21 may also be advantageous for combating certain tumors such as multiple myeloma and Hodgkin’s lymphoma as IL-21 has been shown to serve as a proliferative and/or pro-survival factor for these malignant cells [107–109]. Conversely, IL-21 plays a role in controlling infectious diseases, many cancers and maintaining host immunocompetence. The administration of recombinant IL-21 to certain cancer patients has had mixed outcomes [3]. Nevertheless, since the functions of IL-21 are influenced by multiple parameters, combination therapies, which consider the context, are worthwhile exploring. Such strategies could include the incorporation of checkpoint inhibitors, additional cytokines, or other approaches. Indeed, the combined use of IL-21 with the B cell depleting antibody rituximab (anti-CD20) achieved an overall response rate of 42% in patients with indolent B-cell malignancies [110]. In sum, future investigations into the regulation of T cell differentiation by IL-21 may reveal strategies for fine-tuning the actions of IL-21 that can be practically applied to improve immunity or curb pathogenic responses.
Outstanding Questions.
How do the immunological disruptions caused by infections and inflammation influence the actions and requirements for IL-21?
When is IL-21 induced during distinct immune responses to influence T cell differentiation?
What are the spatial locations and physiologically relevant cellular sources of IL-21 within lymphoid and non-lymphoid organs?
How does immunization-induced inflammation offset the necessity for IL-21 to drive pathogenic Th17 responses and EAE?
What factors are induced during acute infections to overcome the requirements for IL-21 to promote the differentiation of Tem and Trm cells and induce the expression of the chemokine receptor CX3CR1 and integrin α4β7?
How do the relative levels of STAT and SOCS proteins within normal T cell populations direct the outcome of IL-21 signaling?
How do the epigenetic features of IL-21-associated genes influence the outcomes of IL-21 signaling?
Why are the requirements for IL-21 more stringent during persistent infection that cause T cell exhaustion? Is it because the balance between STAT3 and STAT5 is skewed due to decreased IL-7 and IL-15 signals that mainly activate STAT5? In addition to BATF, does IL-21 control the levels of other transcriptional regulators that alleviate exhaustion?
Does IL-21 modulate mTOR activity via the PI3K-AKT pathway in T cells and thus influence their metabolism?
Does IL-21 act in conjunction with other STAT activators such as type I IFN, to modulate T cell responses during persistent viral infections?
Does IL-21 promote anti-viral antibody responses by modulating Tfr cells? What are the functions of Tfr cells in the generation of protective virus-specific antibodies?
Trends.
IL-21 accentuates the differentiation of multiple CD4 and CD8 T cell subsets, including the more recently characterized Th9, Tfr, IL-22-producing CD4 T cells populations, and also possibly CD8 Trm cells.
Emerging evidence suggests that the effects of IL-21 on T cell differentiation is context-dependent and varies with infection, inflammation, and cytokine milieu. Certain cytokines such as IL-6 and IL-10, as well as antigenic signals may compensate for IL-21 under certain circumstances.
The reciprocal balance between STAT1 and STAT3 is important in dictating the outcome of IL-21 signaling and the expression of IL-21-associated genes.
The function of IL-21 in CD8 T cells is especially prominent during chronic viral infections. IL-21 sustains CD8 T cells in chronically infected hosts in part by upregulating the expression of the transcription factor BATF.
Acknowledgments
We thank Preeyam Patel and Arthur Totten for helpful discussions, as well as the members of the Harrington and Zajac laboratories for critical reading of this manuscript. Some of the findings described were supported in part by grants AI049360, and AI109962 from the National Institutes of Health (to A.J.Z.). As a result of the space constraints, we apologize that we were unable to cite all our colleagues who have advanced our understanding of IL-21 and T cell differentiation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coquet JM, et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–2834. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 2.Yi JS, et al. Interleukin-21: a multifunctional regulator of immunity to infections. Microbes and infection / Institut Pasteur. 2010;12:1111–1119. doi: 10.1016/j.micinf.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nature reviews. Drug discovery. 2014;13:379–395. doi: 10.1038/nrd4296. [DOI] [PubMed] [Google Scholar]
- 4.Vegran F, et al. The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat Immunol. 2014;15:758–766. doi: 10.1038/ni.2925. [DOI] [PubMed] [Google Scholar]
- 5.Kim HP, et al. Calcium-dependent activation of interleukin-21 gene expression in T cells. J Biol Chem. 2005;280:25291–25297. doi: 10.1074/jbc.M501459200. [DOI] [PubMed] [Google Scholar]
- 6.Bauquet AT, et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pot C, et al. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suto A, et al. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205:1369–1379. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsaesser H, et al. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Best JA, et al. Transcriptional insights into the CD8(+) T cell response to infection and memory T cell formation. Nat Immunol. 2013;14:404–412. doi: 10.1038/ni.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng R, et al. The molecular basis of IL-21-mediated proliferation. Blood. 2007;109:4135–4142. doi: 10.1182/blood-2006-10-054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strengell M, et al. IL-21 in synergy with IL-15 or IL-18 enhances IFN-gamma production in human NK and T cells. J Immunol. 2003;170:5464–5469. doi: 10.4049/jimmunol.170.11.5464. [DOI] [PubMed] [Google Scholar]
- 13.Wurster AL, et al. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J Exp Med. 2002;196:969–977. doi: 10.1084/jem.20020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suto A, et al. IL-21 inhibits IFN-gamma production in developing Th1 cells through the repression of Eomesodermin expression. J Immunol. 2006;177:3721–3727. doi: 10.4049/jimmunol.177.6.3721. [DOI] [PubMed] [Google Scholar]
- 15.Kastirr I, et al. IL-21 is a central memory T cell-associated cytokine that inhibits the generation of pathogenic Th1/17 effector cells. J Immunol. 2014;193:3322–3331. doi: 10.4049/jimmunol.1400775. [DOI] [PubMed] [Google Scholar]
- 16.Pesce J, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frohlich A, et al. IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood. 2007;109:2023–2031. doi: 10.1182/blood-2006-05-021600. [DOI] [PubMed] [Google Scholar]
- 18.Lajoie S, et al. IL-21 receptor signalling partially mediates Th2-mediated allergic airway responses. Clin Exp Allergy. 2014;44:976–985. doi: 10.1111/cea.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coquet JM, et al. Interleukin-21-Producing CD4(+) T Cells Promote Type 2 Immunity to House Dust Mites. Immunity. 2015;43:318–330. doi: 10.1016/j.immuni.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 21.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 23.Attridge K, et al. IL-21 inhibits T cell IL-2 production and impairs Treg homeostasis. Blood. 2012;119:4656–4664. doi: 10.1182/blood-2011-10-388546. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz I, et al. IL-21 restricts virus-driven Treg cell expansion in chronic LCMV infection. PLoS Pathog. 2013;9:e1003362. doi: 10.1371/journal.ppat.1003362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spolski R, et al. IL-21 mediates suppressive effects via its induction of IL-10. J Immunol. 2009;182:2859–2867. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogelzang A, et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao W, et al. Opposing actions of IL-2 and IL-21 on Th9 differentiation correlate with their differential regulation of BCL6 expression. Proc Natl Acad Sci U S A. 2014;111:3508–3513. doi: 10.1073/pnas.1301138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ding Y, et al. Interleukin-21 promotes germinal center reaction by skewing the follicular regulatory T cell to follicular helper T cell balance in autoimmune BXD2 mice. Arthritis & rheumatology. 2014;66:2601–2612. doi: 10.1002/art.38735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basu R, et al. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeste A, et al. IL-21 induces IL-22 production in CD4+ T cells. Nature communications. 2014;5:3753–3765. doi: 10.1038/ncomms4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Y, et al. IL-21R signaling is critical for induction of spontaneous experimental autoimmune encephalomyelitis. J Clin Invest. 2015;125:4011–4020. doi: 10.1172/JCI75933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi JS, et al. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frohlich A, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 35.Cui W, et al. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35:792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moretto MM, Khan IA. IL-21 Is Important for Induction of KLRG1+ Effector CD8 T Cells during Acute Intracellular Infection. J Immunol. 2016;196:375–384. doi: 10.4049/jimmunol.1501258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yi JS, et al. IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J Immunol. 2010;185:4835–4845. doi: 10.4049/jimmunol.1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barker BR, et al. Critical role for IL-21 in both primary and memory anti-viral CD8+ T-cell responses. Eur J Immunol. 2010;40:3085–3096. doi: 10.1002/eji.200939939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novy P, et al. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J Immunol. 2011;186:2729–2738. doi: 10.4049/jimmunol.1003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King IL, et al. A nonredundant role for IL-21 receptor signaling in plasma cell differentiation and protective type 2 immunity against gastrointestinal helminth infection. J Immunol. 2010;185:6138–6145. doi: 10.4049/jimmunol.1001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coquet JM, et al. Cutting edge: IL-21 is not essential for Th17 differentiation or experimental autoimmune encephalomyelitis. J Immunol. 2008;180:7097–7101. doi: 10.4049/jimmunol.180.11.7097. [DOI] [PubMed] [Google Scholar]
- 42.Sonderegger I, et al. IL-21 and IL-21R are not required for development of Th17 cells and autoimmunity in vivo. Eur J Immunol. 2008;38:1833–1838. doi: 10.1002/eji.200838511. [DOI] [PubMed] [Google Scholar]
- 43.Linterman MA, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zotos D, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poholek AC, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185:313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eto D, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS One. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karnowski A, et al. B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. J Exp Med. 2012;209:2049–2064. doi: 10.1084/jem.20111504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moser EK, et al. IL-21R signaling suppresses IL-17+ gamma delta T cell responses and production of IL-17 related cytokines in the lung at steady state and after Influenza A virus infection. PLoS One. 2015;10:e0120169. doi: 10.1371/journal.pone.0120169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian Y, et al. A Context-Dependent Role for IL-21 in Modulating the Differentiation, Distribution, and Abundance of Effector and Memory CD8 T Cell Subsets. J Immunol. 2016;196:2153–2166. doi: 10.4049/jimmunol.1401236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 51.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 52.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Linterman MA, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wollenberg I, et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol. 2011;187:4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- 56.Kaplan MH, et al. The development and in vivo function of T helper 9 cells. Nat Rev Immunol. 2015;15:295–307. doi: 10.1038/nri3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strengell M, et al. IL-21 up-regulates the expression of genes associated with innate immunity and Th1 response. J Immunol. 2002;169:3600–3605. doi: 10.4049/jimmunol.169.7.3600. [DOI] [PubMed] [Google Scholar]
- 58.Ballesteros-Tato A, et al. T Follicular Helper Cell Plasticity Shapes Pathogenic T Helper 2 Cell-Mediated Immunity to Inhaled House Dust Mite. Immunity. 2016;44:259–273. doi: 10.1016/j.immuni.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stritesky GL, et al. The transcription factor STAT3 is required for T helper 2 cell development. Immunity. 2011;34:39–49. doi: 10.1016/j.immuni.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Janssen EM, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 61.Zajac AJ, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kahan SM, et al. T cell exhaustion during persistent viral infections. Virology. 2015;479–480:180–193. doi: 10.1016/j.virol.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuller MJ, et al. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J Immunol. 2005;174:5926–5930. doi: 10.4049/jimmunol.174.10.5926. [DOI] [PubMed] [Google Scholar]
- 65.Shin H, et al. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li L, et al. HBcAg-specific IL-21-producing CD4+ T cells are associated with relative viral control in patients with chronic hepatitis B. Scand J Immunol. 2013;78:439–446. doi: 10.1111/sji.12099. [DOI] [PubMed] [Google Scholar]
- 67.Publicover J, et al. IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B. J Clin Invest. 2011;121:1154–1162. doi: 10.1172/JCI44198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kared H, et al. Galectin-9 and IL-21 mediate cross-regulation between Th17 and Treg cells during acute hepatitis C. PLoS Pathog. 2013;9:e1003422. doi: 10.1371/journal.ppat.1003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iannello A, et al. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J Immunol. 2010;184:114–126. doi: 10.4049/jimmunol.0901967. [DOI] [PubMed] [Google Scholar]
- 70.Yue FY, et al. HIV-specific IL-21 producing CD4+ T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control. J Immunol. 2010;185:498–506. doi: 10.4049/jimmunol.0903915. [DOI] [PubMed] [Google Scholar]
- 71.Williams LD, et al. Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. J Virol. 2011;85:2316–2324. doi: 10.1128/JVI.01476-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chevalier MF, et al. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J Virol. 2011;85:733–741. doi: 10.1128/JVI.02030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White L, et al. Differential effects of IL-21 and IL-15 on perforin expression, lysosomal degranulation, and proliferation in CD8 T cells of patients with human immunodeficiency virus-1 (HIV) Blood. 2007;109:3873–3880. doi: 10.1182/blood-2006-09-045278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pallikkuth S, et al. Interleukin-21 administration to rhesus macaques chronically infected with simian immunodeficiency virus increases cytotoxic effector molecules in T cells and NK cells and enhances B cell function without increasing immune activation or viral replication. Vaccine. 2011;29:9229–9238. doi: 10.1016/j.vaccine.2011.09.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schultz BT, et al. Circulating HIV-Specific Interleukin-21(+)CD4(+) T Cells Represent Peripheral Tfh Cells with Antigen-Dependent Helper Functions. Immunity. 2016;44:167–178. doi: 10.1016/j.immuni.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 76.Xin G, et al. A Critical Role of IL-21-Induced BATF in Sustaining CD8-T-Cell-Mediated Chronic Viral Control. Cell reports. 2015;13:1118–1124. doi: 10.1016/j.celrep.2015.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rutishauser RL, et al. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shin H, et al. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kallies A, et al. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 80.King C, et al. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 81.Casey KA, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang X, et al. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Masopust D, et al. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 84.Cao AT, et al. Interleukin (IL)-21 promotes intestinal IgA response to microbiota. Mucosal Immunol. 2015;8:1072–1082. doi: 10.1038/mi.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hirahara K, et al. Asymmetric Action of STAT Transcription Factors Drives Transcriptional Outputs and Cytokine Specificity. Immunity. 2015;42:877–889. doi: 10.1016/j.immuni.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wan CK, et al. Opposing roles of STAT1 and STAT3 in IL-21 function in CD4+ T cells. Proc Natl Acad Sci U S A. 2015;112:9394–9399. doi: 10.1073/pnas.1511711112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sutherland AP, et al. IL-21 promotes CD8+ CTL activity via the transcription factor T-bet. J Immunol. 2013;190:3977–3984. doi: 10.4049/jimmunol.1201730. [DOI] [PubMed] [Google Scholar]
- 88.Hinrichs CS, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwon H, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 92.Johnston RJ, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou X, Xue HH. Cutting edge: generation of memory precursors and functional memory CD8+ T cells depends on T cell factor-1 and lymphoid enhancer-binding factor-1. J Immunol. 2012;189:2722–2726. doi: 10.4049/jimmunol.1201150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi YS, et al. LEF-1 and TCF-1 orchestrate T(FH) differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat Immunol. 2015;16:980–990. doi: 10.1038/ni.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang XP, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oestreich KJ, et al. Molecular mechanisms that control the expression and activity of Bcl-6 in TH1 cells to regulate flexibility with a TFH-like gene profile. Nat Immunol. 2012;13:405–411. doi: 10.1038/ni.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Johnston RJ, et al. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ray JP, et al. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity. 2014;40:367–377. doi: 10.1016/j.immuni.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hand TW, et al. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc Natl Acad Sci U S A. 2010;107:16601–16606. doi: 10.1073/pnas.1003457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin JX, et al. Critical Role of STAT5 transcription factor tetramerization for cytokine responses and normal immune function. Immunity. 2012;36:586–599. doi: 10.1016/j.immuni.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tripathi P, et al. STAT5 is critical to maintain effector CD8+ T cell responses. J Immunol. 2010;185:2116–2124. doi: 10.4049/jimmunol.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saleiro D, Platanias LC. Intersection of mTOR and STAT signaling in immunity. Trends Immunol. 2015;36:21–29. doi: 10.1016/j.it.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Man K, et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat Immunol. 2013;14:1155–1165. doi: 10.1038/ni.2710. [DOI] [PubMed] [Google Scholar]
- 105.Yao S, et al. Interferon regulatory factor 4 sustains CD8(+) T cell expansion and effector differentiation. Immunity. 2013;39:833–845. doi: 10.1016/j.immuni.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parrish-Novak J, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 107.Brenne AT, et al. Interleukin-21 is a growth and survival factor for human myeloma cells. Blood. 2002;99:3756–3762. doi: 10.1182/blood.v99.10.3756. [DOI] [PubMed] [Google Scholar]
- 108.Menoret E, et al. IL-21 stimulates human myeloma cell growth through an autocrine IGF-1 loop. J Immunol. 2008;181:6837–6842. doi: 10.4049/jimmunol.181.10.6837. [DOI] [PubMed] [Google Scholar]
- 109.Scheeren FA, et al. IL-21 is expressed in Hodgkin lymphoma and activates STAT5: evidence that activated STAT5 is required for Hodgkin lymphomagenesis. Blood. 2008;111:4706–4715. doi: 10.1182/blood-2007-08-105643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Timmerman JM, et al. A phase I dose-finding trial of recombinant interleukin-21 and rituximab in relapsed and refractory low grade B-cell lymphoproliferative disorders. Clin Cancer Res. 2012;18:5752–5760. doi: 10.1158/1078-0432.CCR-12-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]