Abstract
We evaluated the effects of repetitive Transcranial Magnetic Stimulation (rTMS) in the treatment of phantom limb pain (PLP) in landmine victims. Fifty-four patients with PLP were enrolled in a randomized, double-blinded placebo-controlled parallel group, single center trial. The intervention consisted in real or sham rTMS of M1 contralateral to the amputated leg. RTMS was given in series of 20 trains of 6s of duration (54 s inter-train, intensity 90% of Motor Threshold) at a stimulation rate of 10 Hz (1200 pulses), 20 minutes per day, during 10 days. For the control group, a sham coil was used. The administration of active rTMS induced a significantly greater reduction in pain intensity (Visual Analogue Scale scores) fifteen days after treatment when compared to sham stimulation (−53.38±53.12% vs −22.93±57.16%; mean between-group difference=30.44%, 95%CI 0.30, 60.58; p=0.03). This effect was not significant 30 days after treatment. In addition, 70.3% (19) of subjects attained a clinically significant pain reduction (>30%) in the active group compared to 40.7% (11) in the sham group fifteen days after treatment (p=0.03). The administration of 10 Hz rTMS on the contralateral primary motor cortex for 2 weeks in traumatic amputees with PLP induces significant clinical improvement in pain.
Trial registry
www.clinicaltrials.gov: Code Number: NCT01872481
Perspective
High frequency rTMS on the contralateral primary motor cortex of traumatic amputees induces a clinically significant pain reduction up to 15 days after treatment without any major secondary effect. These results indicate that rTMS is a safe and effective therapy in patients with phantom limb pain caused by landmine explosions.
Keywords: Phantom limb pain, Landmine victims, rTMS, Neurophatic pain, Non-invasive brain stimulation
INTRODUCTION
Landmines are one of the world’s most disabling public health hazards causing devastating injuries such as traumatic limb amputations and associated psychological disorders.13,45 The exact number of worldwide landmine victims is currently unknown as there is no systematic collection of reliable data. However, it is widely estimated that landmines result in 15,000 to 25,000 victims each year.43 Following trauma-related limb amputation for landmine injury, one of the significant causes of disability is the presence of Phantom Limb Pain (PLP).38,42,48 PLP is a neuropathic syndrome characterized by pain felt in the patients remaining perception of the amputated limb after partial or complete deafferentation. This pain is usually described as a stabbing, throbbing, burning or cramping sensation.14,24,33 PLP is present in up to 87% of all amputees24 and is considered a challenging condition because of its negative impact in quality of life and lack of treatment response, particularly in those patients with traumatic-related amputations.1,15
Maladaptive plasticity seems to play a major role in the mechanisms of PLP. Reorganization of the primary sensorimotor cortex, including changes in motor cortex excitability and peripheral factors such as nociceptive inputs from the residual limb have been implicated in the development of this condition.1,16,39 Additionally, psychological factors may affect pain duration and severity.23 The high prevalence of PLP after amputation and its lack of treatment response have resulted in major efforts to look for interventions to decrease the pain in those affected patients.11 Given PLP mechanisms, repetitive transcranial magnetic stimulation (rTMS) has been tested in this condition as a tool to block the maladaptive plasticity in the sensorimotor cortex.1 rTMS applied daily over the primary motor cortex (M1) has shown pain relief effects in other neuropathic pain syndromes such as post-stroke pain and spinal cord injury pain.22,28,49 Some previous reports have also suggested analgesic effects of rTMS in subjects with PLP.1,10 There have been only three trials testing rTMS in PLP – two of them were small pilot studies10,46 and the other was a randomized clinical trial (RCT) with 27 subjects.1 The RCT showed that 5 consecutive sessions of rTMS induce a significant analgesic effect as compared to sham rTMS, lasting up to two months in 39% of the subjects. However, a recent meta-analysis judged this trial as a high risk of bias study due to a deficient randomization method, which led to an unbalanced distribution between the intervention groups.36 Furthermore, the conclusion of the cited meta-analysis, after including 56 trials using non-invasive brain stimulation techniques for chronic pain treatment, was that although single doses of high-frequency rTMS of the motor cortex may have small short-term effects on chronic pain; these effects do not meet the predetermined threshold of minimal clinical significance, and there is therefore a need for larger, rigorously designed studies, particularly of longer courses of stimulation.
Given these results, we aimed to assess in a larger sample size study and properly designed RCT, the immediate and sustained effects of a larger dose of real rTMS of M1 – 10 sessions – on PLP as compared to sham rTMS in landmine victims. We hypothesized that 10 Hz rTMS for two weeks over M1 contralateral to the PLP could significantly decrease the level of pain compared with sham stimulation.
METHODS
Study design
This was a single center, double-blinded, sham-controlled, randomized, parallel-group trial that consisted of three main phases: (1) a baseline evaluation consisting of a week period of observation to establish baseline measurements for pain levels, depression and anxiety symptomatology (2) a treatment phase consisting of daily sessions with active or sham rTMS for five days a week during two consecutive weeks, and (3) a follow-up evaluation after 15 and 30 days of treatment completion. In the baseline evaluation, we recorded demographic data, medical history, medications and other therapies used for the treatment of PLP.
Study Population
Fifty-four patients (mean age, 33.9 ± 8.41 years; 4 female patients) were included in the study. The participants were prospectively selected from the rehabilitation department of the Regional Military Hospital and local Non-Governmental Organizations (NGOs) in Bucaramanga, Colombia. Patients were included if they fulfilled the following criteria: adults aged 18 years or over, who had amputation at any level of one lower limb by anti-personnel landmines with symptoms compatible with PLP. PLP was defined as a sensation of shooting, stabbing, boring, squeezing, throbbing and burning or paresthesia or any other pain sensation in a limb that did not exist anymore.34
We excluded patients with diagnosis of complex regional pain syndrome, any pathology that could alter the course of PLP (diagnosis of cancer, immunological disorders, renal insufficiency requiring dialysis treatment, etc.), pregnancy, neuropsychiatric disorders that can affect the patient ability to consent the study participation and contraindications to TMS, such as cardiac pacemaker, medical pumps or implanted metals in the scalp.47 This study was performed in accordance with the Declaration of Helsinki (1964).8 Written informed consent was obtained from each participant before inclusion in the study, which was approved by the local institutional review board.
Intervention: Repetitive Transcranial Magnetic Stimulation (rTMS)
Patients received rTMS on the primary motor cortex (M1) contralateral to the amputated leg using a figure-of-eight coil connected to a Magstim Rapid2 magnetic stimulator, which provides a biphasic pulse (Magstim Company Ltd, Whitland, UK). The coil was positioned tangentially to the scalp, approximately at a 45° angle from the midline. The resting motor threshold (RMT) (of the first dorsal interosseous) was defined as the minimal intensity to induce motor evoked potentials of 50µV peak-to-peak amplitude in at least 5 of 10 trials. Twenty trains of 6 seconds each (inter-train interval 54 s), using an intensity of 90% of RMT and 10 Hz frequency, were applied in each patient for 10 days during a two-week period. For the sham treatment group, stimulation parameters were the same (location and duration), but a sham coil (Magstim Company Ltd, Whitland, UK) was used. This coil has similar appearance to the active coil in shape and weight, produces a similar sound artifact but does not induce a scalp skin sensation nor emit a magnetic pulse within the cortex.30 All sessions were administered by only one investigator who was not blinded to the intervention and did not participate in the outcome assessments. Participants and investigators who performed the pain assessments were blinded to treatment allocation.
Randomization
A computer-generated randomization method with a permuted block size of 6 was used to allocate subjects to the sham or active rTMS interventions. The randomization code was only given to the treating investigator on the first day of treatment session by an independent investigator not involved with any other aspect of the trial.
The blinding integrity was assessed at the end of the study. Participants were asked to guess their treatment allocation. We did not assess blinding in 2 patients due to early trial withdrawal from the study. The blinded investigators were also asked to guess the patient allocation.
Study Outcomes
All evaluations were performed by an investigator blinded to treatment allocation. The primary endpoint of the study was the score change in the Visual Analogue Scale (VAS) for pain. Response was defined as a reduction of 30% or more as compared to baseline (at 15 and 30 days after treatment).12,21 The other outcome measures were considered secondary.
Pain measurement
Visual Analogue scale (VAS) for pain
The response to the stimulation was evaluated by measuring the pain intensity using the VAS. This self-evaluation scale ranges from 0 to 10 as visually described in centimeter units, 0 cm indicates no pain and 10 cm the worst pain possible. This scale has been widely used in studies that evaluate pain as an outcome, and both validity and reproducibility have been demonstrated.18 As we expected daily variability in pain levels, pain was self-assessed daily at baseline for 1 week before treatment, and twice during the follow-up period (at 15 and 30 days after completing the treatment scheme). The patients were asked to continue their routine medication regimen during the study development. If a patient required a change in medication dose, it was recorded and considered in the analysis.
Anxiety and depression symptomatology
Because depression and anxiety might be confounders of pain relief, we measured these two domains by using the following instruments:
Zung self-rating depression scale
This is a 20-item self-report scale that measures the four common characteristics of depression: pervasive affect, physiological equivalents, other disturbances, and psychomotor activities. The minimum score is 20 and the maximum score is 80. Four categories ranging from “normal” to “severely depressed” are based on specific ranges of the score.51
Zung Self-Rating Anxiety Scale
This is a 20 item questionnaire based on scoring in 4 groups of manifestations: cognitive, autonomic, motor and central nervous system symptoms. The total scores range from 20 to 80, meaning normal range to extreme anxiety levels.50 Depressive and anxiety symptoms were measured once at baseline, and twice during the follow-up period (at 15 and 30 days after completing the treatment scheme).
Sample Size and Statistical analysis
A sample size of 54 patients (27 in each arm) was calculated expecting that 60% of subjects in the active group would obtain a significant pain reduction (decrease >30% in pain level) after finishing the intervention compared to 20% in the sham group. This was based on the results of a previous study.27 It was considered a power of 80%, type I error of 0.05 (double-sided) and an adjustment for a dropout rate of 5%.
The data is presented as mean and Standard Deviation (SD) and also proportion of responders in each group. Continuous variables were subjected to a Shapiro–Wilk test to determine whether the data fitted normal distribution. Baseline characteristics of patients randomized to active and sham therapies were compared using Student’s t-test or Wilcoxon rank-sum test for continuous variables and chi-square test or Fisher's exact test for categorical data. We analyzed the endpoint of the study using the intention-to-treat method including patients who attended at least one of the rTMS sessions. The missing data was considered at random, thus we used a regression imputation method to handle this issue. Such technique allowed us to substitute missing VAS values at the first (15 days after treatment, 2 subjects) and second (30 days after treatment, 6 subjects) follow-up visits, for values derived from a regression model using baseline variables as well as VAS scores from all other participants.
Risk ratios were calculated to evaluate statistically significant differences between treatment groups in the proportion of subjects attaining a clinical important pain reduction (>30%) 15 and 30 days after finishing the treatment protocol. The differences between groups in the proportion of subjects attaining a substantial clinical benefit (pain reduction >50%) were also explored. We also conducted additional analyzes treating pain as a continuous variable and also for the other secondary continuous outcomes (depression and anxiety scores). For these analyzes, a repeated-measure analysis of variance (RM-ANOVA) was performed using a two group (active versus sham) by three-time periods (baseline, 15 and 30 days after finishing treatment) design. Post hoc comparisons were carried out using a Scheffe test for multiple comparisons. Statistical significance was defined as p less than 0.05. All analyzes were conducted using Stata statistical software, release 11.0 (Stata Corporation, College Station, TX).
RESULTS
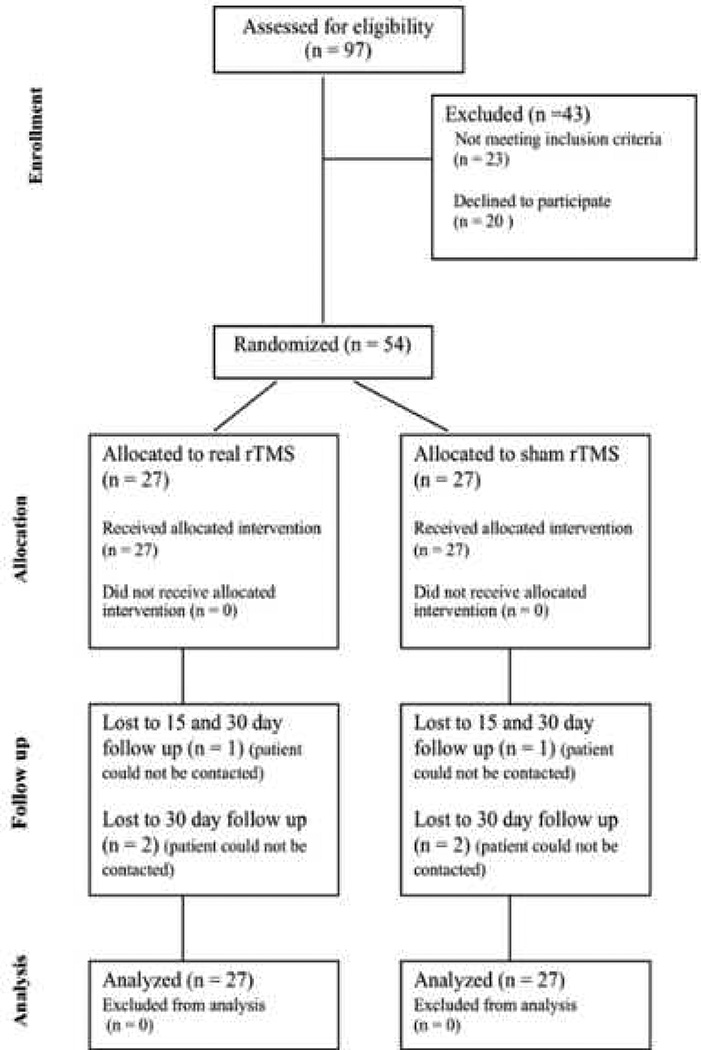
Fifty-four patients (n=27 in the active group and n=27 in the sham group) were included in the study. A participant flow diagram is shown in Figure 1. There were no significant differences in demographic and clinical characteristics at baseline between the groups (table 1). All patients tolerated the rTMS without experiencing any serious adverse effect. Some patients experienced minor adverse effects such as headache (11.1%), neck pain (5.5%), and sleepiness (18.5%) without significant differences between groups. There were also no differences in relation to the current use of NSAIDs (28.5% versus 37.0%, p=0.12), and the participation in a physical rehabilitation program (88.8% versus 85.1%, p=0.68) or psychological therapy (88.8% versus 77.7%, p=0.27).
Figure 1.
CONSORT 2010 patient flow diagram (It is a 1.5 column-fitting image)
rTMS = repetitive Transcranial Magnetic Stimulation
Table 1.
Baseline characteristics of the study participantsa
| Variable | Active rTMS group (n = 27) | Sham rTMS group (n = 27) |
|---|---|---|
| Age (years) | 33.1 ± 6.6 | 34.7 ± 9.9 |
| Gender (Female/Male) | 2/25 | 2/25 |
| Years since amputation | 7.4 ± 5.6 | 8.2 ± 6.3 |
| Zung depression scaleb | 26.7 ± 5.7 | 25.6 ± 6.8 |
| Zung anxiety scalec | 27.8 ± 7.7 | 26.9 ± 9.3 |
| VAS baselined | 4.9 ± 1.9 | 4.8 ± 1.9 |
| BMI (kg/m2) | 25.6 ± 4.4 | 25.2 ± 3.5 |
Abbreviations: rTMS = repetitive Transcranial Magnetic Stimulation; VAS = Visual Analogue Scale; BMI = Body Mass Index.
Data are expressed as mean ± SD.
Depression scores range from 20 to 80, with higher scores indicating more severe anxiety symptoms.
Anxiety scores range from 20 to 80, with higher scores indicating more severe anxiety symptoms.
Scores range from 0 to 10, with higher scores indicating more severe symptoms.
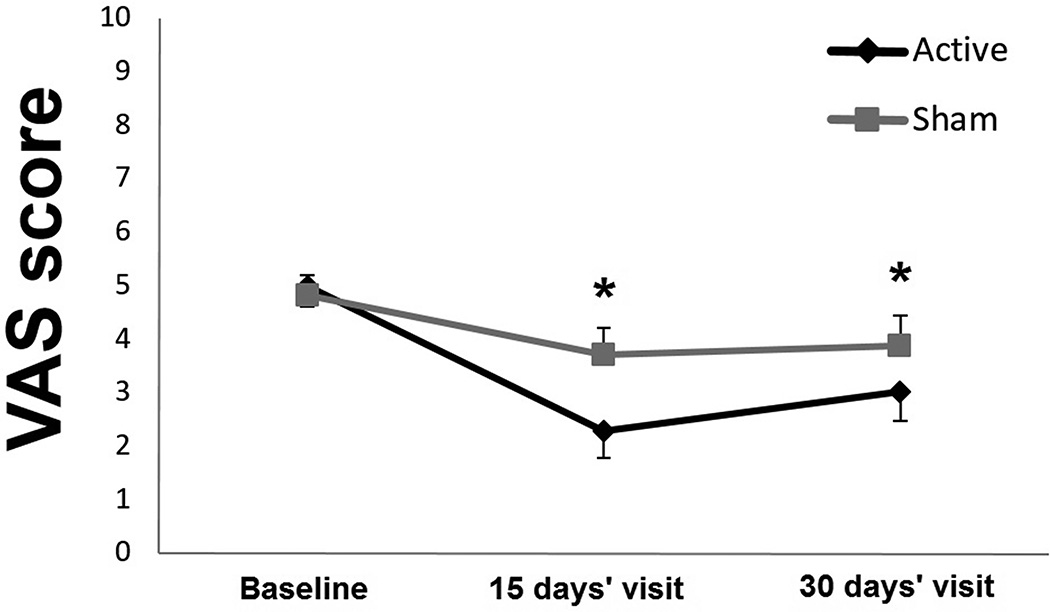
A significantly greater mean percentage reduction in pain intensity (VAS score) was found 15 days after treatment in the active group when compared to sham stimulation (−53.38 ± 53.12% vs −22.93 ± 57.16%; mean between-group difference=30.44%, 95% CI 0.30, 60.58; p=0.03). However, no significant differences between groups were found 30 days after treatment (−37.74 ± 52.39% vs −14.97 ± 53.88%; mean between-group difference=22.76%, 95% CI −6.25, 51.79; p=0.12)
Nineteen subjects (70.3%) attained a significant clinical response (pain reduction > 30%) in the active group compared to 11 (40.7%) in the sham group 15 days after treatment (RR 1.72, 95% CI 1.03, 2.89). However, no statistically significant between-group difference was found 30 days after treatment [15 (55.5%) vs 9 (33.3%); RR 1.66, 95% CI 0.88, 3.13]. A higher proportion of subjects obtaining a substantial clinical benefit (pain reduction >50%) was also found in the active treatment group when compared to sham stimulation 15 days after treatment [17 (62.9%) vs 9 (33.3%); RR 1.88, 95% CI 1.02, 3.46]. This difference also showed a statistical trend toward significance when evaluated 30 days after treatment [13 (48.1%) vs 6 (22.2%); RR 2.16, 95% CI 0.96, 4.85]
For our secondary analyzes, we assessed the effects of rTMS on pain level using a RM-ANOVA. We found a significant main effect of group of treatment and time (F1, 104=7.54, p<0.01; F2, 104=19.49, p<0.0001). The analysis also showed a significant interaction term (group per time; F2, 104=3.25, p=0.04). Post-hoc tests revealed a significant decrease in VAS scores 15 and 30 days after finishing the intervention in the active group, whereas no significant change was noted with sham stimulation (figure 2). No statistically significant between-group difference was found when comparing the absolute VAS scores at day 15 (mean between-group difference=1.42, 95% CI −0.07, 2.93; p=0.06) or day 30 (mean between-group difference=0.86, 95% CI −0.59, 2.31; p=0.24) after treatment (Table 2). In relation to the scores of the depression and anxiety scales a main effect of time was found (F2, 104=4.55, p=0.01, F2, 104=7.91, p<0.0001), without significant group of treatment effects (F1, 104=0.11, p=0.7; F1, 104=0.07, p=0.7) or interactions terms (F2, 104=1.32, p=0.27; F2, 104=0.2, p=0.81). No statistically significant between-group difference was found when comparing the absolute scores of the depression and anxiety scales at day 15 or day 30 after treatment (Table 2).
Figure 2.
Variations in VAS score according to treatment group (It is a 1.5 column-fitting image) *p Value less than 0.05 compared to baseline score. The error bars represent standard errors. VAS = Visual Analogue Scale.
Table 2.
Average scores of pain, depression and anxiety scales in subjects included in the studya
| Baseline | 15 days’ visit | 30 days’ visit | |
|---|---|---|---|
| VAS scoreb | |||
| Active rTMS | 4.98 ± 1.97 | 2.28 ± 2.51 | 3.02 ± 2.64 |
| Sham rTMS | 4.82 ± 1.98 | 3.71 ± 2.97 | 3.88 ± 2.68 |
| Zung depression scalec | |||
| Active rTMS | 26.7 ± 5.72 | 25.1 ± 5.87 | 24.9 ± 9.05 |
| Sham rTMS | 25.6 ± 6.82 | 24.2 ± 4.39 | 23.2 ± 2.99 |
| Zung anxiety scaled | |||
| Active rTMS | 27.8 ± 7.71 | 25.8 ± 7.02 | 23.8 ± 7.27 |
| Sham rTMS | 26.9 ± 9.32 | 25.1 ± 5.52 | 24.4 ± 4.24 |
Abbreviations: rTMS = repetitive Transcranial Magnetic Stimulation; VAS = Visual Analogue Scale.
Data are expressed as mean ± SD.
Scores range from 0 to 10, with higher scores indicating more severe symptoms.
Depression scores range from 20 to 80, with higher scores indicating more severe anxiety symptoms.
Anxiety scores range from 20 to 80, with higher scores indicating more severe anxiety symptoms.
Subjects and investigators did not guess correctly the treatment allocation beyond chance (p = 0.704; p=0.571).
DISCUSSION
The present study shows that the treatment with 10 Hz rTMS of contralateral M1 during two weeks in traumatic amputees with PLP induces a clinically significant pain reduction up to 15 days after treatment compared with sham stimulation. In addition, no serious adverse effects were found during the study indicating that rTMS was a safe and effective therapy in patients with PLP caused by landmine explosions.
Previous studies had shown some beneficial effects of rTMS on PLP.1,10,46 These reports have evaluated either the effects of low frequency rTMS (< 1 Hz), which have demonstrated to decrease cortical network excitability, or high frequency (>1 Hz), which may induce an opposite effect.2,40 In an initial case-report study, Topper et al.46 evaluated the effect of rTMS series on phantom pain-like syndrome in two patients with long-lasting brachial plexus avulsion, who underwent 10 and 1 Hz rTMS during 15 days, separated by 4 and 6 weeks respectively [at 110% of RMT, 12 minutes duration] over the contralateral posterior parietal cortex to the injured limb. The authors reported a maximum pain reduction of approximately 60% and 23.6%, during the rTMS treatment compared to baseline; however, the pain decrease was not maintained in the long term. Similarly, Di Rollo et al.10 in one patient with PLP of traumatic origin, applied 15 sessions of low frequency rTMS (thirty 20 second trains at 80% of RMT, 15 minutes) over the ipsilesional motor cortex, showing a pain reduction of 33.3% at the end of the third week of treatment and a slightly decrease (16.6%) at the follow-up visit (three weeks after the last session). In a recent clinical trial, Ahmed et al.1 evaluated the analgesic effect of rTMS for chronic PLP by assigning subjects to active (n=17) or sham (n=10) stimulation of the contralateral M1 for 5 consecutive days (200 pulses at 20 Hz, 10 seconds trains, at 80% of RMT). The authors found a significant reduction on VAS in the real stimulation group immediately after the fifth session (55%) that was maintained after two months (39%) in comparison with the sham group. Although these studies showed promising effects of rTMS in PLP, there were some methodological limitations that could have affected their results, such as a low sample size, unbalanced distribution in the treatment groups, low number of sessions (i.e., 5 sessions) and the lack of standardized criteria for placebo stimulation.30 In addition, the population included in these studies was heterogeneous, with different amputation locations and etiologies, which is of particular importance given that these factors could be related with different pathophysiological mechanisms and/or treatment responses.9,31,37 Given the challenges to recruit a population of PLP with similar characteristics, our study included, in a 10-day stimulation protocol, a homogeneous population consisting of 100% of subjects with traumatic lower limb amputation caused by landmine explosions. Thus our findings extend beyond previous rTMS studies on PLP and provide a more reliable estimate of effect size. The NNT for 30% pain reduction with rTMS as compared to sham rTMS was 4. This result indicates that 1 patient in every 4 treated with rTMS will benefit from this treatment as compared to sham treatment. This effect size (NNT of 4, 95% (CI 1.8 to 23.1) is similar to tricyclic antidepressants for the treatment of central pain.14
The pain relief found in the present study could be explained by the potential effect of rTMS over the central pathophysiological mechanisms related to PLP. After a traumatic amputation, the main factors associated with PLP include maladaptive reorganization of the sensorimotor cortex, which involves a reduction in intracortical inhibition mechanisms, an imbalance between inhibitory and excitatory aminoacids (GABA and glutamate) and an increase in the excitability of corticospinal neurons.6,32,48 It has been hypothesized that the administration of high-frequency rTMS over the motor cortex enhances its excitability leading to indirect activation of inhibitory projections towards the thalamus, resulting in a modulation of ascending nociceptive signal pathways.3,20 Additionally, the modulation of thalamic activity generated by the enhancement of motor cortex excitability may influence other brain pain related networks such as the orbitofrontal, anterior cingulate gyri and the periaqueductal gray matter (PAG), which are related with the affective-emotional components of nociception.7,26,32,48
Besides rTMS, other noninvasive and invasive brain stimulation methods have been explored for the treatment of chronic PLP. Transcranial direct current stimulation (tDCS) is a noninvasive method that modulates spontaneous neuronal activity with anodal stimulation enhancing cortical excitability and cathodal inducing an opposite effect.35 Recently, tDCS has been explored as a neurorehabilitatory tool for the treatment of chronic PLP.3–5 Bolognini et al.,4,5 studied the effect of anodal tDCS (1.5 mA, 15 min for 5 days) over M1 contralateral to the amputated limb in eight patients with unilateral lower and upper limb amputation of different etiologies. The authors demonstrated a pain relief immediately after the five sessions and up to 1 week of the last stimulation session (−41%, p = 0.04). Among the invasive stimulation methods, epidural motor cortex stimulation (MCS) has emerged as an alternative therapy for refractory neuropathic pain.25,29 However, its analgesic effects in patients with PLP have been described only in case series.41 Fontaine et al.17 performed a systematic review of the effects of MCS on chronic neuropathic pain. The authors showed that 40% (4/10) of the patients with PLP reported pain relief (>70%). In our study we found that 63% of the patients with PLP experienced pain relief >50%, results that are comparable with those reported in MCS studies, furthermore these effects were present 15 days after finishing the treatment, which extend beyond the findings in patients using tDCS. It is worth noting that a recent meta-analysis evaluating the use of rTMS for the treatment of chronic pain demonstrated significant heterogeneity, reporting a short-term analgesic effect but failing to reach pre-established criteria for a minimal clinically important difference.36. One strength of our study was the inclusion of a very homogeneous population and the administration of the stimulation for 10 days, which could have contributed to the lasting and clinically significant pain reduction.
We also observed a significant reduction of depressive and anxiety symptoms after 30 days post-intervention without any differences between treatment groups, indicating an effect not attributable to rTMS. A recent meta-analysis showed that high-frequency rTMS over the dorsolateral prefrontal cortex is associated with clinically relevant antidepressant effects with a safe profile, whereas no consistent effects have been found when stimulating the motor cortex,19 which could explain the lack of differential treatment effects in our study.
This study has some limitations. Although our main outcome was the proportion of subjects attaining a clinically significant pain reduction, other non-painful phantom phenomena such as phantom limb awareness, telescoping and phantom sensations were not assessed and could have been confounding factors in the evaluation of rTMS effects. Despite this, our results support the notion that rTMS induces a clinically significant pain relief in subjects with PLP after traumatic amputation. In addition, although we found a significant difference between groups when analyzing mean percentage reduction (difference in pain scores from baseline), there was no statistically significant between-groups differences when analyzing absolute VAS scores after treatment. Although randomization, in theory allows for a balanced baseline measure in both treatment groups, this balance is often not seen in small randomized trials such as ours. Therefore, analyses of differences can correct for baseline imbalances, providing a more precise estimation of treatment effects in small randomized trials.44 In addition, as for any small randomized clinical trial, our results need to be confirmed in large randomized trials. An additional limitation is that we stimulated the motor cortex corresponding to the first dorsal interosseous muscle of the hand contralateral to pain instead of the area corresponding to the lower limb. However, previous studies using rTMS over the hand motor cortex have reported analgesic effects in patients with chronic neuropathic pain of diverse anatomical origin,25,26 results that were confirmed by our study. Although the sham coil used in this trial might induce a slightly different scalp sensation when compared to the real stimulation coil, it is noteworthy that both coils are similar in appearance (shape and weight) and auditory artifacts. In addition, although the investigator performing the stimulation was not blinded to the intervention, she did not participate in the outcome evaluation of the subjects; therefore, it is unlikely that this fact could have influenced the obtained results. Finally, this study consisted of a small sample size, which can compromise the generalization of the results. This was in part due to the challenges of recruiting such a homogeneous study population. In addition, although we found a clinically significant effect of the stimulation on pain reduction 15 days after finishing the treatment, further studies will be necessary to determine if longer rTMS stimulation protocols could derive in even more long-lasting and maintained analgesic effects in patients with PLP.
Highlights.
Ten days of rTMS over the motor cortex induces significant analgesia in PLP
rTMS is a well-tolerated technique for PLP caused by landmine explosions
Noninvasive brain stimulation might be a promising option for PLP rehabilitation
Acknowledgments
This study was partially supported by a grant from the Colombian Science and Technology Institute (COLCIENCIAS, project code: 6566-49-326169). Felipe Fregni is the principal investigator at Spaulding Rehabilitation Hospital of a research grant funded by NIH (5R01HD082302-02).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Ahmed MA, Mohamed SA, Sayed D. Long-term antalgic effects of repetitive transcranial magnetic stimulation of motor cortex and serum beta-endorphin in patients with phantom pain. Neurol Res. 2011;33:953–958. doi: 10.1179/1743132811Y.0000000045. [DOI] [PubMed] [Google Scholar]
- 2.Andre-Obadia N, Peyron R, Mertens P, Mauguiere F, Laurent B, Garcia-Larrea L. Transcranial magnetic stimulation for pain control. Double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clin Neurophysiol. 2006;117:1536–1544. doi: 10.1016/j.clinph.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Bolognini N, Olgiati E, Maravita A, Ferraro F, Fregni F. Motor and parietal cortex stimulation for phantom limb pain and sensations. Pain. 2013;154:1274–1280. doi: 10.1016/j.pain.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 4.Bolognini N, Spandri V, Ferraro F, Salmaggi A, Molinari ACL, Fregni F, Maravita A. Immediate and Sustained Effects of 5-Day Transcranial Direct Current Stimulation of the Motor Cortex in Phantom Limb Pain. J Pain. 2015;16:657–665. doi: 10.1016/j.jpain.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Bolognini N, Spandri V, Olgiati E, Fregni F, Ferraro F, Maravita A. Long-term analgesic effects of transcranial direct current stimulation of the motor cortex on phantom limb and stump pain: a case report. J Pain Symptom Manage. 2013;46:e1–e4. doi: 10.1016/j.jpainsymman.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Chen A, Yao J, Kuiken T, Dewald JPA. Cortical motor activity and reorganization following upper-limb amputation and subsequent targeted reinnervation. Neuroimage Clin. 2013;3:498–506. doi: 10.1016/j.nicl.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis KD, Kiss ZH, Luo L, Tasker RR, Lozano AM, Dostrovsky JO. Phantom sensations generated by thalamic microstimulation. Nature. 1998;391:385–387. doi: 10.1038/34905. [DOI] [PubMed] [Google Scholar]
- 8.Declaration of Helsinki. Recommendations guiding doctors in clinical research. Adopted by the World Medical Association in 1964. Wis Med J. 1967;66:25–26. [PubMed] [Google Scholar]
- 9.Di Pino G, Guglielmelli E, Rossini PM. Neuroplasticity in amputees: main implications on bidirectional interfacing of cybernetic hand prostheses. Prog Neurobiol. 2009;88:114–126. doi: 10.1016/j.pneurobio.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Di Rollo A, Pallanti S. Phantom limb pain: low frequency repetitive transcranial magnetic stimulation in unaffected hemisphere. Case Rep Med. 2011:130751. doi: 10.1155/2011/130751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang J, Lian Y, Xie K, Cai S. Pharmacological interventions for phantom limb pain. Chin Med J (Engl) 2013;126:542–549. [PubMed] [Google Scholar]
- 12.Farrar JT, Young JPJ, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson AD, Richie BS, Gomez MJ. Psychological factors after traumatic amputation in landmine survivors: the bridge between physical healing and full recovery. Disabil Rehabil. 2004;26:931–938. doi: 10.1080/09638280410001708968. [DOI] [PubMed] [Google Scholar]
- 14.Finnerup NB, Otto M, Jensen TS, Sindrup SH. An evidence-based algorithm for the treatment of neuropathic pain. MedGenMed. 2007;9:36. [PMC free article] [PubMed] [Google Scholar]
- 15.Flor H. Phantom-limb pain: characteristics, causes, and treatment. Lancet Neurol. 2002;1:182–189. doi: 10.1016/s1474-4422(02)00074-1. [DOI] [PubMed] [Google Scholar]
- 16.Flor H, Nikolajsen L, Staehelin Jensen T. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7:873–881. doi: 10.1038/nrn1991. [DOI] [PubMed] [Google Scholar]
- 17.Fontaine D, Hamani C, Lozano A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: critical review of the literature. J Neurosurg. 2009;110:251–256. doi: 10.3171/2008.6.17602. [DOI] [PubMed] [Google Scholar]
- 18.Fregni F, Boggio PS, Lima MC, Ferreira MJL, Wagner T, Rigonatti SP, Castro AW, Souza DR, Riberto M, Freedman SD, Nitsche MA, Pascual-Leone A. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122:197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Fregni F, Marcolin MA, Myczkowski M, Amiaz R, Hasey G, Rumi DO, Rosa M, Rigonatti SP, Camprodon J, Walpoth M, Heaslip J, Grunhaus L, Hausmann A, Pascual-Leone A. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2006;9:641–654. doi: 10.1017/S1461145705006280. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Larrea L, Peyron R. Motor cortex stimulation for neuropathic pain: From phenomenology to mechanisms. Neuroimage. 2007;37(Suppl 1):S71–S79. doi: 10.1016/j.neuroimage.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 21.Haanpaa M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, Cruccu G, Hansson P, Haythornthwaite JA, Iannetti GD, Jensen TS, Kauppila T, Nurmikko TJ, Rice ASC, Rowbotham M, Serra J, Sommer C, Smith BH, Treede R-D. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152:14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry. 2005;76:833–838. doi: 10.1136/jnnp.2004.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knotkova H, Cruciani RA, Tronnier VM, Rasche D. Current and future options for the management of phantom-limb pain. J Pain Res. 2012;5:39–49. doi: 10.2147/JPR.S16733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kooijman CM, Dijkstra PU, Geertzen JH, Elzinga A, van der Schans CP. Phantom pain and phantom sensations in upper limb amputees: an epidemiological study. Pain. 2000;87:33–41. doi: 10.1016/S0304-3959(00)00264-5. [DOI] [PubMed] [Google Scholar]
- 25.Lefaucheur JP. Pain. Handb Clin Neurol. 2013;116:423–440. doi: 10.1016/B978-0-444-53497-2.00035-8. [DOI] [PubMed] [Google Scholar]
- 26.Lefaucheur JP, Drouot X, Ménard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology. 2006;67:1568–1574. doi: 10.1212/01.wnl.0000242731.10074.3c. [DOI] [PubMed] [Google Scholar]
- 27.Leo RJ, Latif T. Repetitive transcranial magnetic stimulation (rTMS) in experimentally induced and chronic neuropathic pain: a review. J Pain. 2007;8:453–459. doi: 10.1016/j.jpain.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Leung A, Donohue M, Xu R, Lee R, Lefaucheur J-P, Khedr EM, Saitoh Y, André-Obadia N, Rollnik J, Wallace M, Chen R. rTMS for suppressing neuropathic pain: a meta-analysis. J Pain. 2009;10:1205–1216. doi: 10.1016/j.jpain.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology. 2008;70:2329–2337. doi: 10.1212/01.wnl.0000314649.38527.93. [DOI] [PubMed] [Google Scholar]
- 30.Loo CK, Taylor JL, Gandevia SC, McDarmont BN, Mitchell PB, Sachdev PS. Transcranial magnetic stimulation (TMS) in controlled treatment studies: are some “sham” forms active? Biol Psychiatry. 2000;47:325–331. doi: 10.1016/s0006-3223(99)00285-1. [DOI] [PubMed] [Google Scholar]
- 31.Montoya P, Ritter K, Huse E, Larbig W, Braun C, Töpfner S, Lutzenberger W, Grodd W, Flor H, Birbaumer N. The cortical somatotopic map and phantom phenomena in subjects with congenital limb atrophy and traumatic amputees with phantom limb pain. Eur J Neurosci. 1998;10:1095–1102. doi: 10.1046/j.1460-9568.1998.00122.x. [DOI] [PubMed] [Google Scholar]
- 32.Navarro X, Vivó M, Valero-Cabré A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Nikolajsen L. Postamputation pain: studies on mechanisms. Dan Med J. 2012;59:B4527. [PubMed] [Google Scholar]
- 34.Nikolajsen L, Jensen TS. Phantom limb pain. Br J Anaesth. 2001;87:107–116. doi: 10.1093/bja/87.1.107. [DOI] [PubMed] [Google Scholar]
- 35.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 36.O’Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2011;4:CD008208. doi: 10.1002/14651858.CD008208.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pascual-Leone A, Peris M, Tormos JM, Pascual AP, Catalá MD. Reorganization of human cortical motor output maps following traumatic forearm amputation. Neuroreport. 1996;7:2068–2070. doi: 10.1097/00001756-199609020-00002. [DOI] [PubMed] [Google Scholar]
- 38.Perkins ZB, De’Ath HD, Sharp G, Tai NRM. Factors affecting outcome after traumatic limb amputation. Br J Surg. 2012;99(Suppl 1):75–86. doi: 10.1002/bjs.7766. [DOI] [PubMed] [Google Scholar]
- 39.Reilly KT, Sirigu A. The motor cortex and its role in phantom limb phenomena. Neuroscientist. 2008;14:195–202. doi: 10.1177/1073858407309466. [DOI] [PubMed] [Google Scholar]
- 40.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roux FE, Ibarrola D, Lazorthes Y, Berry I. Chronic motor cortex stimulation for phantom limb pain: a functional magnetic resonance imaging study: technical case report. Neurosurgery. 2001;48:681–687. doi: 10.1097/00006123-200103000-00050. [DOI] [PubMed] [Google Scholar]
- 42.Schans CP, Geertzen JHB, Schoppen T, Dijkstra PU. Phantom pain and health-related quality of life in lower limb amputees. J Pain Symptom Manage. 2002;24:429–436. doi: 10.1016/s0885-3924(02)00511-0. [DOI] [PubMed] [Google Scholar]
- 43.Shabila NP, Taha HI, Al-Hadithi TS. Landmine injuries at the Emergency Management Center in Erbil, Iraq. Confl Health. 2010;4:15. doi: 10.1186/1752-1505-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Senn SJ. Covariate imbalance and random allocation in clinical trials. Stat Med. 1989;8:467–475. doi: 10.1002/sim.4780080410. [DOI] [PubMed] [Google Scholar]
- 45.Smith WK, Wu Y, Pitkin M. Rehabilitation after landmine injury. Pain Med. 2006;7(Suppl 2):S218–S221. doi: 10.1111/j.1526-4637.2006.00234_8.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Töpper R, Foltys H, Meister IG, Sparing R, Boroojerdi B. Repetitive transcranial magnetic stimulation of the parietal cortex transiently ameliorates phantom limb painlike syndrome. Clin Neurophysiol. 2003;114:1521–1530. doi: 10.1016/s1388-2457(03)00117-2. [DOI] [PubMed] [Google Scholar]
- 47.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 48.Willoch F, Rosen G, Tölle TR, Oye I, Wester HJ, Berner N, Schwaiger M, Bartenstein P. Phantom limb pain in the human brain: unraveling neural circuitries of phantom limb sensations using positron emission tomography. Ann Neurol. 2000;48:842–849. [PubMed] [Google Scholar]
- 49.Yilmaz B, Kesikburun S, Yasar E, Tan AK. The effect of repetitive transcranial magnetic stimulation on refractory neuropathic pain in spinal cord injury. J Spinal Cord Med. 2014;37:397–400. doi: 10.1179/2045772313Y.0000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- 51.Zung WW. A SELF-RATING DEPRESSION SCALE. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]