Abstract
With meta-analysis, participant-level data from five text messaging-based smoking cessation intervention studies were pooled to investigate cessation patterns across studies and participants. Individual participant data (N = 8,315) collected in New Zealand (2001-2003; n = 1,705), U.K. (2008-2009; n = 5,792), U.S. (2012; n = 503; n = 164) and Turkey (2012; n = 151) were collectively analyzed in 2014. The primary outcome was self-reported 7-day continuous abstinence at 4 weeks post-quit day. Secondary outcomes were: (1) self-reported 7-day continuous abstinence at 3 months and (2) self-reported continuous abstinence at 6 months post-quit day. Generalized linear mixed models were fit to estimate the overall treatment effect, while accounting for clustering within individual studies. Estimates were adjusted for age, sex, socioeconomic status, previous quit attempts, and baseline Fagerstrom score. Analyses were intention to treat. Participants lost to follow-up were treated as smokers. Twenty-nine percent of intervention participants and 12% of control participants quit smoking at 4 weeks (adjusted odds ratio [aOR] = 2.89, 95% CI [2.57, 3.26], p < .0001). An attenuated but significant effect for cessation for those in the intervention versus control groups was observed at 3 months (aOR = 1.88, 95% CI [1.53, 2.31]) and 6 months (aOR = 2.24, 95% CI [1.90, 2.64]). Subgroup analyses were conducted but few significant findings were noted. Text messaging-based smoking cessation programs increase self-reported quitting rates across a diversity of countries and cultures. Efforts to expand these low-cost and scalable programs, along with ongoing evaluation, appear warranted.
Introduction
Cigarette smoking continues to be a significant contributor to morbidity and mortality across the world1-5 and accounts for 12% of all deaths among adults ages 30 years and older.6 In the United States (U.S.), despite notable declines in smoking rates since 1965, almost one in five adults (19.3%), aged 18 years and older, were current smokers in 2010.7 Among young adults, the rate was slightly higher at 20.1%.8 Rates in other English-speaking developed countries and cultures are comparable: Although smoking prevalence rates have decreased over the last fifty years in Great Britain, rates have remained stable at 20% of adults 16 years of age and older over the past several years.9 Rates among younger smokers are higher in Great Britain: More than one in four (29%) adults, aged 20 to 24 years, are smokers.9 Rates in New Zealand are slightly lower: Recent studies suggest that 17.2% of adults, aged 15 years and older, are current smokers.1 In contrast, rates of cigarette smoking in Middle Eastern countries, such as Turkey, are higher. Almost one in three (31%) adults were current smokers in Turkey in 2008, although recent tobacco control efforts decreased prevalence to 27%.10
Across countries and settings, smokers express a desire to quit smoking.11-17 Mobile phone-based smoking cessation programs that use text messaging to deliver content have emerged as an important tool in the arsenal of tobacco control efforts.18-33 Text messaging overcomes structural issues (e.g., lack of services, transportation) of face-to-face programs. They are cost-effective and easy to scale up with the ever-increasing use of text messaging across the world. Reviews and meta-analyses suggest that text messaging-based programs are effective in affecting cessation and other health behaviors.34-39
Despite the growing evidence that text messaging-based smoking cessation programs can positively affect quitting rates, gaps in our knowledge remain. Firstly, our understanding is limited about for whom these programs work best, for example, whether these programs work better for heavier smokers than for lighter smokers. Second, while previous meta-analyses were based on analyses of overall effect sizes for each study and did not include analyses at the participant level,34,35,40 there is a dearth of meta-analyses that analyze data at the individual participant level. This methodology, sometimes referred to as “integrative data analysis” has several advantages, including the ability to better study the effects of participant subgroups and characteristics on outcomes, and is considered the gold standard of meta-analyses.41,42 As such, the purpose of this study was to pool data at the individual level across five text messaging for smoking cessation studies21,28,32,33,43 conducted in four different countries (U.S., United Kingdom [U.K.], New Zealand, and Turkey). Once pooled, data were analyzed for overall predictors of smoking cessation, the effects of subgroups on quitting, and the effects of specific text messaging-based interventions on quitting.
Methods
Five text messaging-based smoking cessation programs were included in the meta-analysis: STOMP in New Zealand,28 txt2stop in the U.K.,21 Text2Quit43 and Stop My Smoking (SMS) USA33 in the U.S., and SMS Turkey in Ankara, Turkey.32 All studies were randomized controlled trials of interventions delivered primarily by text messages and compared with respective standard care. Outcomes included point prevalence and continuous abstinence at approximately 4 weeks and 3 months post-quit day, depending on when the participant responded to the assessment. Individual study results have been previously published.21,28,32,33,43 Studies were included by convenience: All authors agreed to share their respective entire data sets with the current study's biostatistician. Moreover, at the time of that request, there were no other published studies of RCTs of stand-alone SMS interventions with similar cessation outcome measures. Thus, these five studies were the most similar in terms of design, outcomes, and intervention and therefore most amenable to inclusion in the integrative data analysis.
Table 1 provides an overview of each cessation program and its evaluation. Text2Quit was developed independently, txt2stop was adapted from the original STOMP program, and SMS USA and SMS Turkey were developed by the same research team. Inclusion criteria were similar across the five trials. Control programs varied (e.g., pamphlets, referral to existing services, unrelated and/or infrequent messages) but intent was similar (i.e., to provide an inactive representation of standard care). Participants received program messages most frequently during the first four weeks following the quit day, which then reduced in frequency and intensity for the rest of the intervention period. All were based on known effective cessation techniques (e.g., setting a quit day) and, to some degree, behavior change theories, including cognitive behavioral therapy.44-49 Points of difference include: the degree and methods for personalization and tailoring of the interventions, with Text2Quit being the most highly personalized program (e.g., messages include participant's first name, quit date, their top three reasons for quitting, money saved by quitting40); the frequency and scheduling of messages, although all start prior to the scheduled quit day; and the inclusion of a relapse program, with the exception of STOMP.
Table 1. Description of Smoking Cessation Studies (n = 5).
| STOMP | SMS USA | SMS Turkey | txt2stop | Text2Quit | ||
|---|---|---|---|---|---|---|
| Year fielded | 2001-2003 | 2012 | 2012 | 2008-2009 | 2012 | |
| 4-week self-reported cessation | ||||||
| Risk Ratio | 2.23*** | 1.61* | 1.07 | 2.43*** | 2.10*** | |
| Odds Ratio (95% CI) | 2.72 (2.12, 3.50) | 2.09 (1.06, 4.13) | 1.08 (0.46, 2.56) | 2.99 (2.60, 3.44) | 2.59 (1.66, 4.03) | |
| Participants | 1,705 participants were randomized to the intervention (n = 853) or control group (n = 853) | 164 young adults were randomized to the intervention (n = 101) or control group (n = 63) | 151 participants were randomized to the intervention (n = 76) or control group (n = 75) | 5,792 participants were randomized to the intervention (n = 2,911) or control group (n = 2,881) | 503 participants were randomized to the intervention (n = 262) or control group (n = 241) | |
| Inclusion criteria | Age | Aged 16 years and older | Aged between 18 and 25 years | Aged 18 years or older | Aged 16 years or older | Aged 18 or older |
| Smoking frequency | Currently smoking cigarettes daily | Smoking 24 cigarettes or more per week (at least 4/day on at least 6 days/ week) | Daily smokers | Currently smoking cigarettes daily | Smoking five or more cigarettes a day | |
| Quitting intention | Interested in quitting | Seriously thinking about quitting in the next 30 days | Seriously thinking about quitting in the next 15 days | Willing to make an attempt to quit smoking in the next month | Interested in quitting in the next month | |
| Language/residency | English-speaking, living in New Zealand | English-speaking, living in the U.S. | Turkish-speaking, living in Ankara, Turkey | English-speaking, living in the U.K. | English-speaking, living in the U.S. | |
| Text familiarity | Able to send and receive messages | Able to send and receive messages | Sent or received at least 1 text message in the past year | |||
| Type of phone | Current owner of a Vodafone mobile phone | Owning a mobile phone | Owning a mobile phone | Owning a mobile phone | Owning a cell phone for personal use | |
| Text plan | Currently or planning to enroll in an unlimited text messaging plan | Enrolled in an unlimited text messaging plan | ||||
| Other | Agree to verification of smoking cessation status by a significant other | No chronic or serious illness | An email address for personal use | |||
| Other | Not being pregnant | |||||
| Comparison/control group | One message every two weeks unrelated to quitting | Attention-matched on number and frequency of program messages. Content focused on the benefits of improving sleep and fitness to assist in quitting. | Quitting brochure | One message every two weeks unrelated to smoking | Occasional text messages encouraging user to visit http://smokefree.gov | |
| Components | Cessation support, motivation & tips | X | X | X | X | X |
| Smoking facts | X | X | X | X | X | |
| On-demand tips to get through craving | X | X | X | X | ||
| Quit buddy | X (if requested) | X | ||||
| Polls & quizzes | X | X | X | |||
| Trivia messages for distraction | X | X | ||||
| Tailoring | Personalized to concerns at baseline (e.g., weight) | Type of message changes according to pre-quit, early quit, late quit, relapse, and post-quit | Type of message changes according to pre-quit, early quit, late quit, relapse, and post-quit | Personalized to concerns at baseline (e.g., weight) | Personalized to goals, reasons, triggers, social support, gender, money saved, medications used | |
| Promotes use of helpline & nicotine replacement therapy | X | X | X | X | X | |
| Relapse program | X | X | X | X | ||
| Messages to check in on smoking status | Daily during quit week | 2-day and 7-day post-quit | X | |||
| Schedule | Pre-quit | 1-2 weeks pre-quit: 1-2 text/day | 2 weeks pre-quit: 3-5 texts/day | 2 weeks pre-quit: 3-5 texts/day | 1-2 weeks pre-quit: 1-2 text/day | 2-4 weeks pre-quit: 8 texts/week. Week prior to quit day: 30/week |
| 4 weeks post-quit | 5/day | 8/day on quit day, then reduced from 6/day to 1/day in the last week | 8/day on quit day, then reduced from 6/day to 1/day in the last week | 3-5/day | Week post-quit: 34. 2-3 weeks post-quit: 3/wk. 3+ weeks post-quit: 1/ wk | |
| 5+ weeks post-quit | 5-24 weeks: 1 every 3 days | 5-26 weeks: 1 every 3 days | 4/month | |||
| Duration | 26 weeks | 6 weeks (depending on pathing) | 6 weeks (depending on pathing) | 3 months | ||
| Number of messages (core program) | Approximately 198 | Approximately 140 | Approximately 120 | 186 | Approximately 120 | |
Fidelity of Implementation
As reported previously,33 allocation concealment was broken for the last 8 participants enrolled in SMS USA because of an imbalance in the arm allocations. Otherwise, the RCT was implemented as intended. In SMS Turkey, two serious issues with the software program were noted during the RCT32: The software program failed to send at least one program message to 58% (n = 44) of intervention group participants; duplicate text messages were sent to 66% (n = 50) of intervention participants. Neither appeared to affect cessation rates. In the Text2Quit trial,43 a significant proportion of those randomized were later excluded (n = 1242) because they did not provide handset verification via SMS, did not have a valid cell phone number, or the cell phone number given was a wrong number. Additionally, the control material was changed during the trial when the source of the control material (Smokefree.gov) began offering an SMS-based smoking cessation program. After this point, control group participants received an electronic brochure. There were no reported issues with the fidelity of implementing the STOMP and txt2stop interventions.
Measures
Smoking outcomes
The primary outcome was self-reported 7-day continuous abstinence at 4 weeks post-quit day. In each study, participants were asked whether he/she had ever smoked in the last seven days. Self-reported 7-day continuous abstinence at 3 months was also queried in four trials and is therefore a secondary outcome. Self-reported continuous abstinence at 6 months (i.e., whether a participant has smoked no more than five cigarettes since their quit day50) was measured in two trials and is a secondary outcome. As described above, the actual timing of data collection for these measures are approximate and depend on when the participant responded to the survey request. For simplicity, we will refer to these measures by their main timeframe (e.g., 4 weeks post-quit).
Quit day was defined as the day after the participant's last cigarette or the nominated quit day.
Fagerstrom score
All studies included the 6-item Fagerstrom test51 for nicotine dependence, which is a standard instrument for assessing the intensity of physical nicotine addiction. Scores ranged from 0 to 10, with a score of 5 or more indicating a high level of nicotine dependence.
Socioeconomic status (SES)
SES was measured differently across studies. In each case, low/high SES was categorized using predefined cut-off points. Household income was collected in STOMP (<15,000 New Zealand dollar versus higher), SMS USA (<15,000 U.S. dollar versus higher), and SMS Turkey (<2,000 Turkish lira versus higher); education level was collected in Text2Quit (some high school/college versus higher); and age when the participant left full-time education in txt2stop (≤16 years versus older).
Statistical Analysis
Data analyses were performed on the principle of intention to treat using statistical software SAS version 9.352 and R version 3.0.53 Participants with missing smoking status were considered to be smokers in the analyses. First, descriptive summaries of baseline demographic and smoking characteristics were tabulated for each study as well as the combined sample. Heterogeneity across studies was tested in the fixed-effect model using the Mantel-Haenszel method54 and in the random-effects mixed model using generalized linear regression with a binomial distribution. The overall treatment effect on primary and secondary smoking outcomes was evaluated using the multilevel mixed models, adjusting for predefined baseline confounding variables (i.e., age in years, biological sex, SES, previous quit attempts, and total Fagerstrom score). Individual studies were fitted as a random effect in the model to control for clustering of data. Adjusted odds ratios (aOR) and adjusted relative risks (aRR) were estimated using the logit and log links, respectively. The effects of intervention on specific subgroups of interest, sex, and level of nicotine dependence were estimated and tested using their interaction with the treatment groups in the main model (i.e., intervention × sex; intervention × nicotine dependence).
Results
A total of 8,315 randomized participants from the five individual trials were included in the meta-analysis: 4,202 participants in the treatment group and 4,113 participants in the control group. Participant characteristics within and across studies are shown in Table 2. The two groups were similar with respect to age, sex, SES, baseline number of previous quit attempts, and baseline nicotine dependence.
Table 2. Participant characteristics within and across studies.
| Overall (N = 8,315) | STOMP (N = 1,705) | Text2Quit (N = 503) | SMS USA (N = 164) | SMS Turkey (N = 151) | txt2stop (N = 5,792) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | |
|
N = 4,202 |
N = 4,113 | N = 852 | N = 853 | N = 262 | N = 241 | N = 101 | N = 63 | N = 76 | N = 75 | N = 2,911 | N = 2,881 | |
| Smoking behavior | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) |
| Age | 33.6 (11.51) | 33.66 (11.61) | 24.86 (8.78) | 24.64 (8.63) | 35.85 (10.72) | 35.53 (10.6) | 21.63 (2.11) | 21.56 (2.12) | 36.13 (9.51) | 35.64 (10.31) | 36.31 (10.98) | 36.38 (11.09) |
| Previous quit attempts | 4.58 (8.17) | 4.35 (7.86) | 3.04 (2.55) | 2.78 (2.42) | 5.34 (7.07) | 6.89 (16.14) | 3.66 (1.61) | 2.62 (1.69) | 2.38 (1.51) | 2.36 (1.53) | 5.06 (9.42) | 4.67 (7.94) |
| Fagerstrom score | 4.63 (2.42) | 4.62 (2.44) | 4.6 (2.16) | 4.52 (2.23) | 5.36 (2.28) | 5.3 (2.33) | 3.92 (2.06) | 3.83 (2.23) | 1.87 (1.25) | 1.96 (1.49) | 4.67 (2.48) | 4.68 (2.48) |
| Demographic characteristics | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Female | 2062 (49.1) | 1995 (48.5) | 500 (58.7) | 497 (58.3) | 180 (68.7) | 150 (62.8) | 44 (43.6) | 28 (44.4) | 35 (46) | 24 (32) | 1303 (44.8) | 1296 (45) |
| SES | ||||||||||||
| Low | 1872 (44.6) | 1830 (44.5) | 364 (42.7) | 349 (40.9) | 165 (63) | 152 (63.1) | 46 (45.5) | 32 (50.8) | 23 (30.3) | 37 (49.3) | 1274 (43.8) | 1260 (43.7) |
| High | 2109 (50.2) | 2068 (50.3) | 267 (31.3) | 289 (33.9) | 97 (37) | 89 (36.9) | 55 (54.5) | 31 (49.2) | 53 (69.7) | 38 (50.7) | 1637 (56.2) | 1621 (56.3) |
| Missing | 221 (5.3) | 215 (5.2) | 221 (25.9) | 215 (25.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Smoking outcomes | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| 4 weeks (PP) | ||||||||||||
| Quit smoking | 1200 (28.6) | 507 (12.3) | 243 (28.5) | 109 (12.8) | 80 (30.5) | 35 (14.5) | 44 (43.6) | 17 (27) | 13 (17.1) | 12 (16) | 820 (28.2) | 334 (11.6) |
| Smoking | 2794 (66.5) | 3441 (83.7) | 563 (66.1) | 709 (83.1) | 133 (50.8) | 183 (75.9) | 43 (42.6) | 38 (60.3) | 46 (60.5) | 48 (64) | 2009 (69) | 2463 (85.5) |
| Missing | 208 (5.0) | 165 (4.0) | 46 (5.4) | 35 (4.1) | 49 (18.7) | 23 (9.5) | 14 (13.9) | 8 (12.7) | 17 (22.3) | 15 (20) | 82 (2.8) | 84 (2.9) |
| 3 months (PP) | N = 1,291 | N = 1,232 | ||||||||||
| Quit smoking | 380 (29.4) | 231 (18.8) | 247 (29) | 160 (18.8) | 87 (33.2) | 48 (19.9) | 36 (35.6) | 19 (30.2) | 10 (13.2) | 4 (5.3) | NA | NA |
| Smoking | 691 (53.5) | 842 (68.3) | 499 (58.6) | 628 (73.6) | 123 (47.0) | 159 (66.0) | 45 (44.6) | 32 (50.8) | 24 (31.6) | 23 (30.7) | NA | NA |
| Missing | 220 (17.0) | 159 (12.9) | 106 (12.4) | 65 (7.6) | 52 (19.8) | 34 (14.1) | 20 (19.8) | 12 (19) | 42 (55.3) | 48 (64) | NA | NA |
NA = not applicable; measures are not available.
Descriptive Smoking Data
Compared to one in four participants in the treatment group (n = 1,200; 28.6%), one in ten in the control group (n = 507, 12.3%) reported smoking abstinence during the previous seven days at 4 weeks post-quit day (Table 2). The unadjusted relative risk (RR) was 2.32 (p < 0.0001). Among the four trials that measured 7-day continuous abstinence at 3 months post-quit, 29.4% of the participants in the treatment group (n = 380/1,291) reported not smoking, compared to 18.8% in the control group (n = 231/1,232) with an unadjusted RR of 1.57 (p <0.0001). Based upon the two studies that measured continuous abstinence at 6 months post-quit, 13.1% of the participants in the treatment group (n = 493/3,763) versus 6.4% in the control group (n = 237/3,734) had quit smoking long-term with an unadjusted RR of 2.06 (p <0.0001).
Smoking Cessation Outcomes
Heterogeneity of the treatment effect across studies was not supported by analyses: There was no significant interaction between treatment group and individual study in the random-effect mixed model using outcomes data at 4 weeks (p = 0.32), 3 months (p = 0.34), and 6 months (p = 0.48). Similar results were observed using the fixed-effect Mantel-Haenszel method for test of heterogeneity at 4 weeks (Chi-squared = 6.41, p = 0.17), 3 months (Chi-squared = 1.67, p = 0.64), and 6 months (Chi-squared = 0.28, p = 0.60). An overall treatment effect was therefore evaluated across all studies (i.e., the interaction term reflecting heterogeneity was therefore dropped from subsequent multivariate regression models).
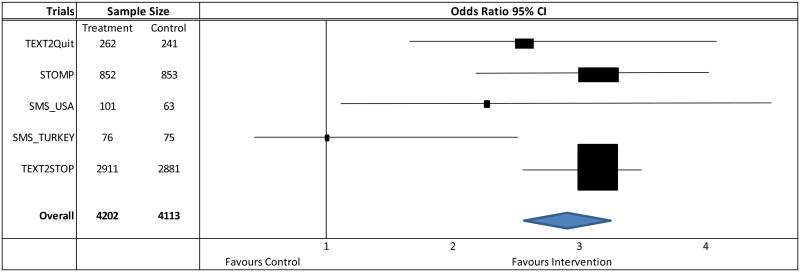
Adjusting for age, sex, SES, previous quit attempts, and baseline Fagerstrom score, the odds ratio of 7-day continuous abstinence at 4 weeks was over 2.0 for four of the five studies included in the meta-analysis (Figure 1). Across studies, after adjusting for underlying differences in personal characteristics, those in the intervention groups were more than twice as likely as their control group peers (aOR = 2.89, 95% CI [2.57, 3.26]; aRR = 2.34, 95% CI [2.12, 2.58]) to report being abstinent in the past seven days at 4 weeks post-quit (Table 3). Although attenuated, the likelihood of cessation continued to be significantly higher for smokers with similar demographic and baseline smoking characteristics in the intervention versus control groups in the four studies that measured 7-day continuous abstinence at 3 months post-quit day (aOR = 1.88, 95% CI [1.53, 2.31]; aRR = 1.60, 95% CI [1.37, 1.87]). Findings persisted at 6 months post-quit in the two studies that measured long-term continuous abstinence (aOR = 2.24, 95% CI [1.90, 2.64]; aRR = 2.07, 95% CI [1.91, 2.40]).
Figure 1. Forest plot of per-program adjusted odds ratios associated with 7-day continuous abstinence at 4 weeks post-quit.
Table 3. Self-reported abstinence across studies.
| Timeframe | aOR (95% CI) | p value | aRR (95% CI) | p value |
|---|---|---|---|---|
| 4-weeks post quit (n = 8,315) | ||||
| Intervention | 2.89 (2.57, 3.26) | <0.0001 | 2.34 (2.12, 2.58) | <0.0001 |
| Age (in years) | 1.01 (1.01, 1.02) | <0.0001 | 1.01 (1.006, 1.014) | <0.0001 |
| Female | 0.99 (0.88, 1.11) | 0.8124 | 0.99 (0.91, 1.08) | 0.7904 |
| High SES | 0.94 (0.84, 1.06) | 0.2971 | 0.96 (0.88, 1.04) | 0.3064 |
| Previous quit attempts | 1.00 (0.99, 1.01) | 0.7199 | 1.00 (0.99, 1.004) | 0.7549 |
| Fagerstrom score | 0.98 (0.96, 1.00) | 0.0748 | 0.98 (0.97, 1.00) | 0.0640 |
| 3 months post-quit (n = 2,523) | ||||
| Intervention | 1.88 (1.53, 2.31) | <0.0001 | 1.60 (1.37, 1.87) | < 0.0001 |
| Age (in years) | 0.99 (0.98, 1.00) | 0.0862 | 0.99 (0.98, 1.00) | 0.0715 |
| Female | 1.03 (0.83, 1.27) | 0.8140 | 1.02 (0.87, 1.19) | 0.8426 |
| High SES | 0.93 (0.75, 1.15) | 0.4935 | 0.95 (0.81, 1.12) | 0.5420 |
| Previous quit attempts | 1.00 (0.98, 1.02) | 0.8292 | 1.00 (0.98, 1.01) | 0.8523 |
| Fagerstrom score | 0.96 (0.92, 1.01) | 0.0967 | 0.97 (0.94, 1.01) | 0.1139 |
aOR = adjusted Odds Ratio; aRR = adjusted Risk Ratio. Six multivariate models are shown: two for each time point, one for odds and the other for risk ratio. Point estimates are adjusted for the five covariates included in the model
Investigation of Outcomes by Subpopulations
Subsequent analyses suggested that men in the intervention group (aOR = 2.95, 95% CI [2.50, 3.48]) were just as likely as women (aOR = 2.83, 95% CI [2.39, 3.36]) to quit at 4 weeks post-quit (p value for the interaction term = 0.73; data not shown). Similarly no difference was observed between men (aOR = 1.66, 95% CI [1.20, 2.30]) and women (aOR = 2.05, 95% CI [1.56, 2.68]) at 3 months post-quit among the four studies that collected these data (p value for interaction term = 0.33). In the two studies that collected continuous abstinence at 6 months post-quit day, however, data suggested that intervention group men (aOR = 2.86, 95% CI [2.26, 3.61]) were more likely than women (aOR = 1.70, 95% CI [1.34, 2.16]) to achieve long-term abstinence (p value for interaction term = 0.002).
Differences in cessation rates at 4 weeks post-quit were not apparent for intervention group smokers with high (aOR = 2.95, 95% CI [2.51, 3.47]) versus low (aOR = 2.82, 95% CI [2.37, 3.36]) levels of baseline nicotine dependence as measured on the Fagerstrom score (p value for the interaction term = 0.72; data not shown). The trend at 3 months post-quit suggested that intervention group smokers with high levels of nicotine dependence (aOR = 2.29, 95% CI [1.71, 3.06]) might be more likely to quit than intervention group smokers with lower levels of dependence (aOR = 1.53, 95% CI [1.13, 2.06]; p value for interaction term = 0.06). In the two studies that measured continuous abstinence at 6-months post-quit, however, those with low levels of addiction may have been more likely to quit (aOR = 2.52, 95% CI [1.98, 3.21]) than those with high levels of addiction (aOR = 2.0, 95% CI [1.59, 2.51]), although the interaction term was not significant (p = 0.17).
Additional Characteristics Predictive of Quitting
Beyond exposure to text messaging-based smoking cessation programs, increasing age was slightly but significantly associated with 7-day continuous abstinence at 4 weeks post-quit (aOR = 1.01, 95% CI [1.01, 1.02], p < 0.0001; Table 3). On the other hand, trends suggested that as Fagerstrom score increased, the relative odds of quitting at 4 weeks decreased (aOR = 0.98, 95% CI [0.96, 1.00], p = 0.07). Patterns persisted for age (aOR = 0.99, 95% CI [0.98, 1.00]) and Fagerstrom score (aOR = 0.96, 95% CI [0.92, 1.01]) at 3 months post-quit day, although neither was statistically significant (p = 0.1). Both were significantly associated with quitting in the two studies that reported continuous abstinence at 6 months post-quit day, however (age: aOR = 1.02, 95% CI [1.01, 1.02], p < 0.0001; Fagerstrom score: aOR = 0.95, 95% CI [0.92, 0.98], p = 0.003).
Discussion
In one of the first individual-level meta-analyses of smoking cessation outcomes associated with text messaging-based programming, findings across four countries suggest that text messaging-based programs are associated with a two-fold increased odds of quitting. This is true for both short-term and long-term cessation. Findings are also persistent after adjusting for participants' sex, age, SES, smoking dependence, and previous quit attempts at baseline. Using the gold standard of meta-analyses,41,42 this study therefore adds to literature reporting positive cessation outcomes associated with text messaging-based interventions.34,35
It is intriguing that control group quit rates increase in the short-term from 4-week to 3-month post-quit day across studies (12% at 4 weeks versus 19% at 3 months post-quit; Table 2). This does not seem to persist through 6 months, however. Perhaps because these control participants are unable to quit in the short term (i.e., at 4 weeks) and try again. While some may be briefly successful (i.e., at 3-month follow-up) they appear to be unable to sustain cessation over time (i.e., by 6-month follow-up). This is purely speculative, but the pattern deserves greater attention in future research. Indeed, it may be that control content, which by its nature is less intense, may invigorate cessation behavior (e.g., by resulting in people seeking out other methods of quitting) that could, for some smokers, represent an important opportunity to engage them before they experience another relapse.
Although not entirely clear, the findings suggest that perhaps, among those exposed to the intervention, men compared to women may be more likely to benefit from text messaging-based interventions and sustain long-term abstinence. The findings are also unclear about how nicotine dependence may moderate the intervention effect: In the short term, there is some suggestion that highly dependent people were more likely to quit; in the long term, there is some suggestion that lesser dependent people were more likely to quit. Nonetheless, these data highlight the importance for future research to examine when and for whom different types of cessation programs have more potential to invigorate cessation rates, particularly among subpopulations with higher smoking rates.
Limitations
Findings should be interpreted within the study limitations. Although long-term effects would have been the optimal outcome, the main outcome was at 4-weeks post-quit because this time point was measured in all of the studies. Only studies to which the biostatistician had access to the full data set were included. It is possible that findings may have been different if a larger number of studies had been included. Given the statement released by the National Institutes of Health on sharing research data,55 it may be feasible to conduct such a study in the future.
Also too, a potential time effect was not taken into account in the analyses: Studies ranged in field from 2001 to 2012. This is particularly important for Turkey, New Zealand, and U.K., which were each at varying stages of implementing the World Health Organization's Framework Convention on Tobacco Control56 during their respective randomized controlled trials. That said, in the analysis, “study” was fit as a random cluster effect. There was no observed difference between studies when it was also considered as a fixed effect in the model. This could indicate that there was no time effect associated with individual studies.
It should also be noted that in countries where text message interventions have been studied, a national tobacco control program of some kind had been implemented to varying degrees. There are yet to be any published trials in developing countries that have little organized response, including mass media, promoting the harms of smoking and benefits of quitting. The generalizability of the current findings to these lesser resourced environments is unknown.
Sample sizes (Range: 151-5,792) greatly varied between the trials. Given that the analyses are at the individual level, this limitation might be considered similar to subgroup analyses with varying sample sizes. Thus, data in the larger samples may have overshadowed those in the smaller samples in the same way that subgroup analyses may be underpowered in analyses where the subgroups are limited in sample size.
Furthermore, although outcomes were adjusted for several important characteristics (e.g., number of quit attempts, sex), other characteristics were not measured in the same manner across all studies (e.g., SES) or were excluded because each study used a unique schedule of assessments.
Additionally, although we were lacking in power to examine it, differences in study components (Table 1) might have possibly helped contextualize the results.
Also, as noted above, the comparison groups differed across the studies. This may have introduced bias to the pooled effect sizes, although this bias is unlikely to be more than is introduced in all meta-analyses that include studies with different study populations, inclusion/exclusion criteria, and intervention/control strategies. Indeed, the individual patient data analysis used in the current study could potentially improve the efficiency and reduce some bias compared to the summary level meta-analysis. Further research work may still be needed to evaluate the potential impact of different intervention and standard care strategies implemented in different studies on the size of overall treatment effect before this could be said definitively.
Finally, not all studies included in the meta-analysis used biochemical verification.28 To align the data across studies, only self-reported cessation was used in the current study. Although self-report is vulnerable to misreporting, review groups have advised that in population-based studies with limited contact face-to-face, biochemical verification should not necessarily be seen as required nor desirable.57,58
Next Steps
Despite some crossover (e.g., txt2stop was developed from STOMP; SMS USA and SMS Turkey were developed by the same research team), each intervention was developed in a different context and included unique content. This leads to interesting questions about the efficacy of specific program components and mechanisms of text messaging that support behavior changes, including smoking cessation: Is the actual content of the messages less important than the inclusion of key smoking cessation techniques (e.g., setting a quit day, preparing to quit) and a strong behavior change model, such as cognitive behavioral therapy? This is an important point for further research to explore. That said, we do not believe that simply reminding people of the importance of quitting smoking is enough to significantly affect quitting rates. Ybarra's team tested this directly in the SMS USA study using a control group that received the same number of messages as the intervention group.33 Content provided guidance on quitting smoking within the context of improving one's sleep and fitness. Control messages were not based upon a theoretical model and also lacked key components known to invigorate cessation (e.g, preparing to quit, etc.). There is still much to be learned from this very simple technology with its minimal content. We encourage future trials to collect data about the mechanisms of action so that these may be included in future meta-analyses.
Aside from SMS USA, the studies used passive, low-contact control groups that may not have been blinded to their condition. Understanding how these programs compare to more active controls, including perhaps other smoking cessation programs (e.g., telephone quit lines), would help contextualize the relative impact of mobile health programming compared to other evidence-based smoking cessation options. Future research could also examine the potential impact of different frequencies and intensities of text messaging-based interventions on cessation rates.
Conclusion
In one of the first integrative data analyses of text messaging-based smoking cessation programming, multi-national data suggest that text messaging-based smoking cessation programs increase the chance of quitting. Efforts to expand these low-cost and scalable programs to other countries appear warranted, but should be combined with ongoing evaluation of impact. As the field of public health moves from proving the effectiveness of text messaging-based programs to exploring their optimization for specific population groups, an important next step will be to identify differences in effects. Such information could inform the development of interventions in different contexts and for priority groups, such as pregnant women, underserved populations, and individuals of low SES.
Highlights.
This is the first multi-national, integrative data analysis of mHealth cessation programs.
Cessation rates both short- and longer-term were higher for intervention than control.
Increased short-term cessation in the control groups was noted across studies.
Cessation rates were largely similar across subgroups suggesting similar effects.
Efforts to expand mHealth cessation programs to additional countries appear warranted.
Acknowledgments
The studies included in the meta-analysis were supported: National Heart Foundation of New Zealand, the Cancer Society of New Zealand, Vodafone NZ, Alcatel, and Auckland UniServices (STOMP NZ); MRC UK grant ID 80492 (Text2Stop); 5K07CA124579 (text2quit); R01 TW007918 (Stop My Smoking Turkey); and R21 CA135669 (Stop My Smoking USA). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of any of the funders. We also would like to thank Ms. Ying Huang, at the University of Auckland, for her help coding the analysis.
Abbreviations
- SMS
Stop My Smoking
- SES
socioeconomic status
- aRR
adjusted relative risk
Footnotes
Conflicts of interest: Dr. Ybarra reports that the SMS USA program is publicly available at StopMySmoking.com. Dr. Lorien Abroms receives royalties for the licensing of Text2Quit from Voxiva Inc. Dr. Whittaker reports that Auckland Uniservices Ltd received a small amount of royalties from the licensing of STOMP program. Drs. Free and Jiang have no conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yannan Jiang, Email: y.jiang@auckland.ac.nz.
Caroline Free, Email: Caroline.Free@lshtm.ac.uk.
Lorien C. Abroms, Email: lorien@gwu.edu.
Robyn Whittaker, Email: r.whittaker@nihi.auckland.ac.nz.
References
- 1.Ministry of Health. [Accessed September 23, 2015];Tobacco smoking. 2014 http://www.stats.govt.nz/browse_for_stats/snapshots-of-nz/nz-social-indicators/Home/Health/tobacco-smoking.aspx.
- 2.Health & Social Care Information Centre. [Accessed September 23, 2015];Hospital Episodes Statistics. http://www.hscic.gov.uk/hes.
- 3.Ministry of Health of Turkey. [Accessed September 23, 2015];Global Adult Tobacco Survey (GATS) 2010 http://www.who.int/tobacco/surveillance/en_tfi_gats_turkey_2009.pdf.
- 4.Centers for Disease Control and Prevention. Vital signs: Current cigarette smoking among adults aged ≥18 years --- United States, 2009. 2010 [PubMed] [Google Scholar]
- 5.Ministry of Health. Annual Report for the year ended 30 June 2013 including the Director-General of Health's Annual Report on the State of Public Health. Wellington: Ministry of Health; 2013. [Google Scholar]
- 6.World Health Organization. WHO global report: mortality attributable to tobacco. WHO Press; 2012. [Google Scholar]
- 7.Centers for Disease Control and Prevention. Vital Signs: Current Cigarette Smoking Among Adults Aged ≥18 Years — United States, 2005–2010. 2011 [Google Scholar]
- 8.Centers for Disease Control and Prevention. Trends in Current Cigarette Smoking among High School Students and Adults, United States, 1965-2011. [Accessed September 23, 2015];2014 http://www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking/index.htm.
- 9.Office for National Statistics. Opinions and Lifestyle Survey, Smoking Habits Amongst Adults, 2012. 2013 [Google Scholar]
- 10.Ministry of Health Turkey. Global Adult Tobacco Survey: Comparison fact sheet Turkey 2008 & 2012. [Accessed November 27, 2015];2013 http://www.who.int/tobacco/surveillance/survey/gats/gats_turkey_2008v2012_comparison_fact_sheet.pdf.
- 11.Reeder AL, Williams S, McGee R, Poulton R. Nicotine dependence and attempts to quit or cut down among young adult smokers. N Z Med J. 2001;114(1139):403–406. [PubMed] [Google Scholar]
- 12.Lamkin L, Davis B, Kamen A. Rationale for tobacco cessation interventions in youth. Prev Med. 1998;27(5 Pt 3):A3–A8. doi: 10.1006/pmed.1998.0386. [DOI] [PubMed] [Google Scholar]
- 13.Stone SL, Kristeller JL. Attitudes of adolescents toward smoking cessation. Am J Prev Med. 1992;8(4):221–225. [PubMed] [Google Scholar]
- 14.Ybarra ML, Bağci Bosi AT, Bilir N, Holtrop JS, Korchmaros J, Emri AKS. Interest in technology-based and traditional smoking cessation programs among adult smokers in Ankara, Turkey. Tob Induc Dis. 2011;9(1):10. doi: 10.1186/1617-9625-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thyrian JR, Panagiotakos DB, Polychronopoulos E, West R, Zatonski W, John U. The relationship between smokers' motivation to quit and intensity of tobacco control at the population level: a comparison of five European countries. BMC Public Health. 2008;8:2. doi: 10.1186/1471-2458-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Footman K, Roberts B, Stickley A, Kizilova K, Rotman D, McKee M. Smoking cessation and desire to stop smoking in nine countries of the former Soviet Union. Nicotine Tob Res. 2013;15(9):1628–1633. doi: 10.1093/ntr/ntt034. [DOI] [PubMed] [Google Scholar]
- 17.Sriha Belguith A, Elmhamdi S, Bouanene I, et al. Smoking cessation attitudes among adult smokers. Tunis Med. 2015;93(3):142–147. [PubMed] [Google Scholar]
- 18.Borland R, Balmford J, Benda P. Population-level effects of automated smoking cessation help programs: a randomized controlled trial. Addiction. 2013;108(3):618–628. doi: 10.1111/j.1360-0443.2012.04091.x. [DOI] [PubMed] [Google Scholar]
- 19.Buller DB, Borland R, Bettinghaus EP, Shane JH, Zimmerman DE. Randomized trial of a smartphone mobile application compared to text messaging to support smoking cessation. Telemed J E Health. 2014;20(3):206–214. doi: 10.1089/tmj.2013.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Free C, Knight R, Robertson S, et al. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet. 2011;378(9785):49–55. doi: 10.1016/S0140-6736(11)60701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Free C, Whittaker R, Knight R, Abramsky T, Rodgers A, Roberts IG. Txt2stop: a pilot randomised controlled trial of mobile phone-based smoking cessation support. Tob Control. 2009;18(2):88–91. doi: 10.1136/tc.2008.026146. [DOI] [PubMed] [Google Scholar]
- 22.Gritz ER, Danysh HE, Fletcher FE, et al. Long-term outcomes of a cell phone-delivered intervention for smokers living with HIV/AIDS. Clin Infect Dis. 2013;57(4):608–615. doi: 10.1093/cid/cit349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haug S, Meyer C, Schorr G, Bauer S, John U. Continuous individual support of smoking cessation using text messaging: a pilot experimental study. Nicotine Tob Res. 2009;11(8):915–923. doi: 10.1093/ntr/ntp084. [DOI] [PubMed] [Google Scholar]
- 24.Hertzberg JS, Carpenter VL, Kirby AC, et al. Mobile contingency management as an adjunctive smoking cessation treatment for smokers with posttraumatic stress disorder. Nicotine Tob Res. 2013;15(11):1934–1938. doi: 10.1093/ntr/ntt060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naughton F, Prevost AT, Gilbert H, Sutton S. Randomized controlled trial evaluation of a tailored leaflet and SMS text message self-help intervention for pregnant smokers (MiQuit) Nicotine Tob Res. 2012;14(5):569–577. doi: 10.1093/ntr/ntr254. [DOI] [PubMed] [Google Scholar]
- 26.Naughton F, Jamison J, Boase S, et al. Randomized controlled trial to assess the short-term effectiveness of tailored web- and text-based facilitation of smoking cessation in primary care (iQuit in practice) Addiction. 2014;109(7):1184–1193. doi: 10.1111/add.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollak KI, Lyna P, Bilheimer A, et al. A pilot study testing SMS text delivered scheduled gradual reduction to pregnant smokers. Nicotine Tob Res. 2013;15(10):1773–1776. doi: 10.1093/ntr/ntt045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodgers A, Corbett T, Bramley D, et al. Do u smoke after txt? Results of a randomised trial of smoking cessation using mobile phone text messaging. Tob Control. 2005;14(4):255–261. doi: 10.1136/tc.2005.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi HJ, Jiang XX, Yu CY, Zhang Y. Use of mobile phone text messaging to deliver an individualized smoking behaviour intervention in Chinese adolescents. J Telemed Telecare. 2013;19(5):282–287. doi: 10.1177/1357633x13495489. [DOI] [PubMed] [Google Scholar]
- 30.Skov-Ettrup LS, Ringgaard LW, Dalum P, Flensborg-Madsen T, Thygesen LC, Tolstrup JS. Comparing tailored and untailored text messages for smoking cessation: a randomized controlled trial among adolescent and young adult smokers. Health Educ Res. 2014;29(2):195–205. doi: 10.1093/her/cyt112. [DOI] [PubMed] [Google Scholar]
- 31.Whittaker R, Dorey E, Bramley D, et al. A theory-based video messaging mobile phone intervention for smoking cessation: randomized controlled trial. J Med Internet Res. 2011;13(1):e10. doi: 10.2196/jmir.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ybarra M, Bağcı Bosi AT, Korchmaros J, Emri S. A text messaging-based smoking cessation program for adult smokers: randomized controlled trial. J Med Internet Res. 2012;14(6):e172. doi: 10.2196/jmir.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ybarra ML, Holtrop JS, Prescott TL, Rahbar MH, Strong D. Pilot RCT results of Stop My Smoking USA: A text messaging-based smoking cessation program for young adults. Nicotine Tob Res. 2013;15(8):1388–1399. doi: 10.1093/ntr/nts339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whittaker R, McRobbie H, Bullen C, Borland R, Rodgers A, Gu Y. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2012;(11):CD006611. doi: 10.1002/14651858.CD006611.pub3. [DOI] [PubMed] [Google Scholar]
- 35.Free C, Phillips G, Galli L, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guide to Community Preventive Services. Reducing tobacco use and secondhand smoke exposure: mobile phone-based cessation interventions. [Accessed November 27, 2015];2011 http://thecommunityguide.org/tobacco/mobilephone.html.
- 37.Cole-Lewis H, Kershaw T. Text messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev. 2010;32(1):56–69. doi: 10.1093/epirev/mxq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmadvand A, Whittaker R, Lim MSC. Placing Prevention in the Pockets: The Role of mHealth in Preventive Medical Services. In: Adibi S, editor. mHealth Multidisciplinary Verticals. CRC Press; 2015. pp. 11–36. [Google Scholar]
- 39.Spohr SA, Nandy R, Gandhiraj D, Vemulapalli A, Anne S, Walters ST. Efficacy of SMS text message interventions for smoking cessation: A meta-analysis. J Subst Abuse Treat. 2015;56:1–10. doi: 10.1016/j.jsat.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Whittaker R, Borland R, Bullen C, Lin RB, McRobbie H, Rodgers A. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2009;(4):CD006611. doi: 10.1002/14651858.CD006611.pub2. [DOI] [PubMed] [Google Scholar]
- 41.About IPD meta-analyses. [Accessed May 11, 2015];Cochrane Individual Participant Data (IPD) Meta-analysis Methods Group. http://ipdmamg.cochrane.org/about-ipd-meta-analyses.
- 42.Bainter SA, Curran PJ. Advantages of integrative data analysis for developmental research. J Cogn Dev. 2015;16(1):1–10. doi: 10.1080/15248372.2013.871721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abroms LC, Boal AL, Simmens SJ, Mendel JA, Windsor RA. A randomized trial of Text2Quit: A text messaging program for smoking cessation. Am J Prev Med. 2014;47(3):242–250. doi: 10.1016/j.amepre.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. Clinical practice guideline. [Google Scholar]
- 45.Ossip-Klein DJ, McIntosh S. Quitlines in North America: Evidence base and applications. Am J Med Sci. 2003;326(4):201–205. doi: 10.1097/00000441-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Lancaster T, Stead L, Silagy C, Sowden A. Effectiveness of interventions to help people stop smoking: Findings from the Cochrane Library. BMJ. 2000;321(355):358. doi: 10.1136/bmj.321.7257.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lichtenstein E, Glasgow RE, Lando HA, Ossip-Klein DJ, Boles SM. Telephone counseling for smoking cessation: Rationales and meta-analytic review of evidence. Health Educ Res. 1996;11(2):243–257. doi: 10.1093/her/11.2.243. [DOI] [PubMed] [Google Scholar]
- 48.Wadland WC, Stoffelmayr B, Berger E, Crombach A, Ives K. Enhancing smoking cessation rates in primary care. J Fam Pract. 1999;48(9):711–718. [PubMed] [Google Scholar]
- 49.Wadland WC, Stoffelmayr B, Ives K. Enhancing smoking cessation of low-income smokers in managed care. J Fam Pract. 2001;50(2):138–144. [PubMed] [Google Scholar]
- 50.West R. Assessing smoking cessation performance in NHS Stop Smoking Services: The Russell Standard (Clinical) [Accessed March 16, 2015];2005 http://www.ncsct.co.uk/usr/pub/assessing-smoking-cessation-performance-in-nhs-stop-smoking-services-the-russell-standard-clinical.pdf.
- 51.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 52.SAS® 9.3 [computer program] Cary, NC: SAS Institute Inc.; 2011. [Google Scholar]
- 53.R: A language and environment for statistical computing [computer program] Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 54.Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. 5.0.1. John Wiley & Sons, Ltd.; 2008. [Google Scholar]
- 55.National Institutes of Health. Final NIH Statement on Sharing Research Data. [Accessed September 23, 2015];2003 NOT-OD-03-032: http://grants.nih.gov/grants/guide/notice-files/NOT-OD-03-032.html.
- 56.WHO Framework Convention on Tobacco Control. [Accessed April 13, 2016]; http://www.who.int/fctc/en/
- 57.Williams GC, McGregor H, Borrelli B, Jordan PJ, Strecher VJ. Measuring tobacco dependence treatment outcomes: A perspective from the behavior change consortium. Ann Behav Med. 2005;29(2):11–19. doi: 10.1207/s15324796abm2902s_4. [DOI] [PubMed] [Google Scholar]
- 58.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]