Abstract
Cancer and its treatment can lead to a myriad of adverse events and negatively impact quality of life of older cancer patients and survivors. Unmet physical activity needs vary across the cancer continuum and remain an important yet understudied area of research in this population. Exercise interventions have been shown to be effective in treating both the physical and psychological declines associated with cancer and its treatment, with a potential to improve cancer-related outcomes. Despite the current evidence, exercise is clearly underutilized due to several barriers and knowledge gaps in existing trials that include appropriate population identification, design, and outcome measures selection. The benefits of regular exercise in both the primary and secondary prevention of chronic conditions are well established in the non-cancer population. In older cancer patients and survivors, further research is needed before exercise gains widespread acceptance. The Cancer and Aging Research Group convened experts in exercise, aging and cancer to evaluate current scientific evidence and knowledge gaps in geriatric exercise oncology. This report summarizes these findings and provides future research directions.
Keywords: Exercise, Cancer, Older patients, Geriatric recommendations
1. Introduction
By the year 2024, there will be nearly 19 million cancer patients and survivors in the United States. The majority (63%) of these cancer patients and survivors will be age 65 and older.1,2 These trends have far-reaching implications for an increased burden on the U.S. healthcare system, because of the limited capacity of the system to handle the unique needs of this growing population. Unfortunately, limited knowledge is available to guide providers in effectively managing the complexity of the older cancer patients and survivors.
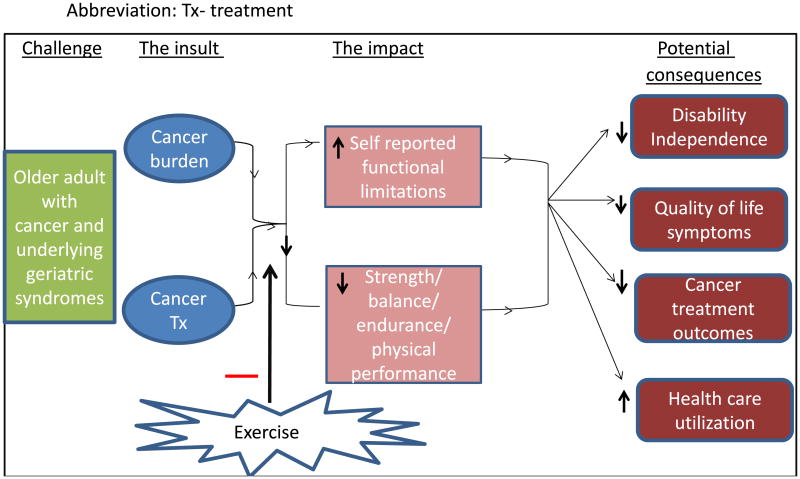
Natural aging is associated with a decline in both physical and psychological function in the absence of a cancer history.3 A diagnosis of cancer and its accompanying treatments accelerate these physical and emotional declines observed in older adults (Fig. 2). In the American Cancer Society Study for Cancer Survivors-II, a national cross-sectional survey of cancer survivors post-treatment, 38.2% of survivors reported unmet physical needs. Older adults constituted a significant proportion (48.5%) of those reporting these concerns.4 In another study, 41% of older adults with cancer reported significant psychological distress, and this was directly related to reduced physical function and loss of independence.5 These studies highlight the urgent need to address the physical and psychological needs of the older adult with cancer. Declines in physical and psychological function significantly limit therapeutic options to treat the disease, instigate poorer health outcomes, increase economic burden for patients and healthcare systems, and portend an inflated social burden for patients, families and providers.6 Fig. 1 illustrates some of the challenges cancer patients and survivors may face when they seek out an exercise regimen.
Fig. 2. Benefits of exercise intervention upon functional decline cascade Abbreviation: Tx—treatment.
Fig. 1. Exercise oncology clinical vignette.
A growing body of literature demonstrates that maintaining physical activity (defined as any skeletal muscle movement that increases metabolic expenditure above a resting rate) through formal exercise interventions (defined as physical activity performed in a structured, repetitive, organized manner with the objective of changing physical or psychological fitness or health-related outcomes [i.e., cancer or cancer-treatment-related toxicities and side effects]) is an effective method for treating both the physical and psychological declines experienced by cancer patients and survivors.7–10 Research shows that adults with cancer decrease their physical activity levels after diagnosis and during treatment and often do not return to their pretreatment activity levels without formal exercise interventions.11–13 This tendency for declining physical activity is reinforced as patients are often advised to limit activities when fatigued or experiencing other toxicities and side effects.14,15
Exercise improves a wide array of physical and psychological toxicities including muscle atrophy and weakness, fatigue, obesity, immune function, insomnia, anxiety, cognitive decline and impaired quality of life, among others.16–20 Epidemiological data also suggest that increased physical activity via regular exercise reduces the risk of cancer recurrence and cancer mortality.21–23 The American College of Sports Medicine (ACSM) published public health recommendations for exercise among cancer patients and survivors. ACSM recommends, “patients and survivors exercise at least 150 minutes per week of aerobic activity on most days of the week at a moderate intensity level or 30 minutes of vigorous aerobic activity 3 days of the week, accompanied by at least 20–30 minutes of resistance activity 2 or more days per week.”24 ACSM also recommends cancer patients and survivors obtain information regarding their baseline levels of physical and psychological function, start low, progress slowly and get help from qualified professionals. Despite these published public health guidelines, it is estimated up to 70% of cancer survivors do not meet these ACSM public health recommendations.25 The two main reasons for this include 1) a lack of awareness among health care providers, patients, and survivors of these exercise recommendations and 2) a lack of understanding about how to provide precise exercise prescriptions for individuals to effectively treat specific cancer and treatment-related outcomes.
To advance patient and survivor care, as well as research in the field of geriatric oncology, the Cancer and Aging Research Group held a U13 conference in May 2015 in collaboration with the National Cancer Institute (NCI), the National Institute on Aging (NIA) and the University of Rochester Cancer Center-NCI Community Oncology Research Program (NCORP) Research Base. Primary goals of the conference included bringing together expert providers and scientists in cancer and aging to 1) review current practices and knowledge, 2) identify gaps in care and knowledge about exercise safety, prescription and effectiveness for managing cancer and cancer treatment toxicities among older cancer patients and survivors, and 3) to identify important areas for future research. This article summarizes conference proceedings related specifically to physical activity and exercise among older cancer patients and survivors. The recommendations in the manuscript are based on the review of existing literature and expert consensus opinion from the discussion at the meeting.
2. Overarching Trial Design Issues in Exercise Oncology for the Older Adult with Cancer
To better understand the evolution of exercise trials in geriatric oncology, it is important to review the current literature on exercise interventions, which exists among frail older adults without cancer. A recently published review of randomized interventional trials that include only frail older patients identified 38 trials, of which 25 included some physical activity intervention (13 exercise alone, 7 exercise plus nutritional supplements and five multi-component programs including exercise).26 Most of the trials included an objective physical performance test as the primary endpoint, and few were designed to evaluate mortality, functional capacity and quality of life. These results suggest in frail older adults exercise is safe, feasible, and improves functional outcomes. However, simply extracting results from the larger body of geriatric exercise trials is not sufficient to inform how exercise is prescribed for geriatric oncology patients. Exercise intervention trials are also required with older cancer patients and survivors to inform how we use exercise to treat the toxicities and side effects stemming from cancer and its treatments.27
Studies evaluating exercise in older adults with cancer are scarce. A literature search using PubMed identified 111 papers that discuss physical activity and exercise in the older adult with cancer. However, only a small number of these articles reported on a clinical intervention study. We provide a summary of a few of these clinical trials, where the mean age was greater than 60 years in Table 1. Analysis of these studies identified a growing body of literature supporting the benefit of exercise in the older patient with cancer; however, there are methodological issues that can be improved upon in the future. Research in this field is at an adolescent stage and needs to move beyond the “one size fits all” exercise dose approach. The majority of the studies have assumed that cancer is physiologically homogeneous. There is a paucity of data evaluating the safety and efficacy of different methods of exercise delivery. There is a lack of phase I–II clinical trials to inform the a priori design of phase III randomized clinical trials. The development of synchronous clinical trials is warranted when it is established that cancer or its treatment will have significant effects on physical or psychological status. Traditional phase 1 parameters to evaluate the safety and efficacy of therapeutic drugs have not been applied with the same rigor to exercise oncology trials. Trials evaluating the effects of exercise on tumor biology are lacking. Tolerability and safety data for the intervention, as assessed by attrition, compliance, dose modifications, and adverse events have seldom been reported in the current trials. Also, there was limited assessment of surrogate markers of antitumor efficacy in any of these studies.
Table 1. Physical intervention randomized control trials among older cancer survivors.
| Author | Sample # | Age: Mean (Range) | Cancer | Design | Intervention | Intervention timing | Outcomes/Measures | Results |
|---|---|---|---|---|---|---|---|---|
| Demark-Wahnefried et al.28 (2006) | 182 | 71.5 (65–86) | Breast Prostate | RCT | 6 month (20 to 30 min bimonthly) Home based diet and exercise interventions (telephone and mail-based counseling versus general health education) |
Diagnosis to 18 months |
|
↑ functional status ↑Physical activity (CHAMPS) ↑↑Diet quality index -6 months post intervention there was no difference in outcomes between the two groups |
| Morey et al.29 (2009) | 641 | 73 (65–91) | Breast Colorectal Prostate And BMI>25 -early stage |
RCT | 12 month (30 min-initial weekly×3, then bimonthly×2 and subsequently monthly) home-based diet and exercise interventions (telephone and mail counseling regarding diet and exercise) Immediate versus delayed until after 12 months |
≥5 years since diagnosis |
|
↑↑Physical Function (SF-36 subscale) ↑↑Physical activity ↑↑QoL ↓↓BMI |
| Winters-Stone et al.31 (2012) | 106 | 62.3 (53–83) | Breast -early stage |
RCT | 12 month[3 – 1-h sessions/week(2 clinic and I home based)]Progressive resistance + impact exercise versus stretching placebo program | ≥1 year since chemotherapy or RT |
|
↑↑Bench press and leg press strength |
| Campo et al.30 (2013) | 40 | 72 (58–93) | Prostate cancer | RCT | 12 week (2× week Qigong) versus stretch | 5 years median from diagnosis. |
|
↑↑FACIT-fatigue ↑↑Distress (BSI-18) |
| Cormie et al.43 (2015) | 63 | 69.6 (NR) | Prostate cancer | RCT | 3 month(2 supervised sessions/week, 60 min each-aerobic and resistance exercise regimen) versus normal care | Within 10 days of first hormonal therapy |
|
Preservation of lean mass ↑↑Muscular strength ↑Lower body function ↓Fatigue ↑↑Social functioning ↓Psychological distress |
| Winters-Stone et al.49 (2015) | 51 | 70.2 (NR) | Prostate cancer | RCT | 12 month[3 – 1 h sessions/week(2 clinic and I home based)]Progressive resistance + impact exercise versus stretching placebo program | On hormonal therapy |
|
↑↑ Bench press and leg strength ↑ objective and self-reported physical function ↓ disability |
| Sprod et al.50 (2015) | 97 | 67 (NR) | Breast Other cancers | RCT | 4 week (2 sessions per week each lasting 75 min) yoga intervention + Standard of care versus standard of care | 2 months to 2 years post cancer treatment |
|
↓Cancer related fatigue ↓global side effect burden |
Notes to Table 1
Abbreviation: RCT—randomized controlled trials.
BMI—body mass index.
QoL –quality of life.
BMI-body mass index.
RT—radiotherapy.
Pca—Prostate cancer.
FACIT—Fatigue: Functional assessment of chronic illness therapy—Fatigue; BSI-18—Brief symptom inventory.
CHAMPS: Community Healthy Activities Models Program for Seniors, Short Form-36 subscale.↑—Trend towards improvement;
↑↑—significant improvement.
NA—not available.
NR—not reported.
Thus far, there are limited exercise translational studies that evaluate clinical oncology endpoints, and no genomic/biomarker-guided trials. In the era of personalized medicine, the understanding of the molecular and genetic complexity of human cancers has aided in the development of successful novel therapeutic agents. Likewise, in exercise oncology, the prerequisites to optimize the efficacy of an exercise intervention would be to elucidate the biologic mechanisms of the exercise, identify the biologically effective dose, and determine the predictors of response to guide patient selection. Studies utilizing these methods can help elucidate the scientific knowledge to improve outcomes for older adults with cancer.
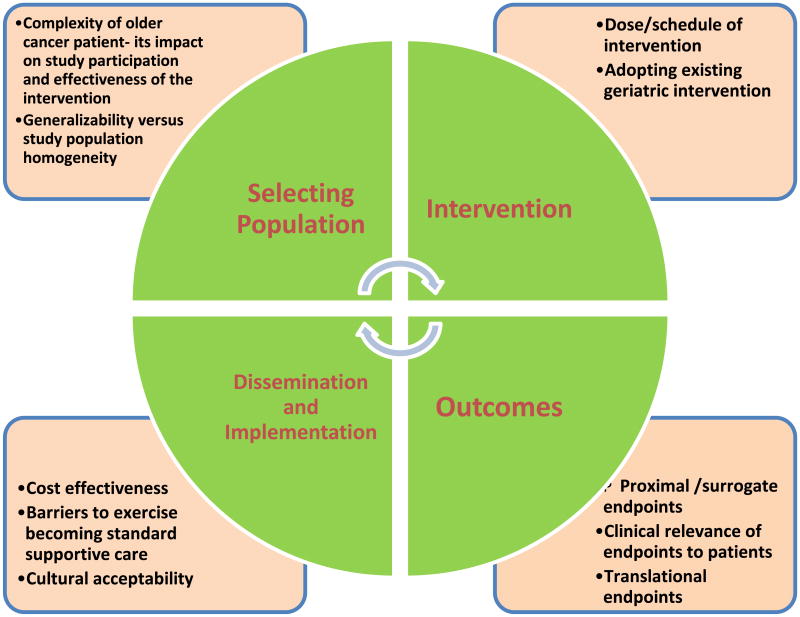
During the conference, a research framework was developed to address four key areas in trial design: 1) Selection of patient population, 2) Interventions, 3) Outcome Measures, and 4) Dissemination and Implementation (Fig. 3). Subsequent sections of the paper will utilize this framework to address these particular components of exercise oncology trial design. The proposed framework, though applicable to the cancer population of all ages, mainly attempts to address the unique needs of the older adults with cancer.
Fig. 3. Framework for discussion.
3. Section 1: Patient Selection
3.1. Summary of Prior Research
In this section, we summarize the characteristics of patients included in existing geriatric oncology exercise trials. The participant's age, type of cancer, and phase of survivorship are all well-described. The studies largely comprise breast and prostate cancer survivors of European descent. Enrollment was largely limited to academic centers in urban areas. Many of the studies enrolled only post-treatment patients and excluded patients on active therapy, yet fatigue is known to be worst during treatment. Recruitment was often challenging. A large number of patients had to be screened to meet target accrual; Project LEAD accrued 182 of the required 420 patients.28 Of the screened 2010 patients, only 26% were considered eligible. In Project RENEW,29 over 26,000 letters were posted, and more than 2000 initial telephone calls made, to reach the accrual target. The resources required for accrual in both these studies were substantial. In another study, family members were invited to participate, to aid in the enrollment and retention. However, only 40 of the 502 screened participated in the program.30 In a breast cancer survivors' study, participants who withdrew from the program were closer to their diagnosis and reported more difficulties with activities of daily living, and lower mental health scores.31 Scrutiny of the population in these studies demonstrates the need to improve upon current methods used for population selection.
3.2. Gaps, Barriers, and Challenges
Several gaps exist as far as current patient selection in geriatric oncology exercise studies (Table 2). Participants' socio-demo-graphics, cancer treatment course, stage and overall baseline health status were seldom reported. In almost all the studies, patients who had a poor baseline physical function were excluded. Description of the accrual barriers and reasons for exclusion/attrition in both the general and high-risk population were not reported. However, knowledge of these factors could enhance the future trial design and are likely to improve outcomes. All the described issues limit the generalizability of the current trial results. The following section discusses some of the barriers and challenges to the identification, and the inclusion of older adults with cancer in exercise research.
Table 2. Gaps identified in the current exercise literature with regards to patient selection, intervention, and outcomes.
| Patient selection |
| Predominant recruitment of breast and prostate cancer survivors |
| Exclusion of patients with poor baseline functional status |
| Lack of individual physical activity needs-based approach to patient selection |
| Racially and ethnically homogenous population |
| Limited number of studies during active treatment of cancer |
| Lack of information regarding reasons for attrition |
| Lack of information regarding barriers to patient accrual to exercise studies |
| Intervention |
| Not tailored to the type, and stage of cancer or treatment |
| Tends to be of short duration (typically 4–12 weeks) |
| Often cumbersome and expensive |
| Often not personalized to patient needs |
| Not integrated into cancer care or patient lifestyle |
| Not reinforced after the completion of the study |
| Adverse events often not considered or known |
| Not designed with patient input |
| Outcomes |
| Functional outcomes (i.e. driveway walk) not routinely measured |
| Impact of exercise intervention on cancer outcomes not regularly assessed |
| Patient-centered outcomes not prioritized |
| Individualized change from baseline not assessed |
| Biologic pathways are non-existent |
| Not sustained after intervention is complete |
| Long-term effect of the intervention on outcomes is unknown |
| Relation of functional outcomes to the quality of cancer survivorship, cancer treatment, and health care are largely unknown. |
3.2.1. Patient Perception/Factors
Although a high percentage of older cancer patients and survivors report concerns related to balance, mobility issues, and fatigue,32 little has been done to adequately address these problems. More importantly, most survey respondents did not identify a need for education, regarding these concerns, suggesting that it is crucial to recognize older adult's perceptions of needs before the design of a study.33 It is also important to recruit patients to studies, based on their need for improvement in the selected outcome, rather than the “all comers” approach. Interventional studies often entail an intense supervised regimen, without taking into account patient's lifestyle. Due to lack of awareness of the potential benefit of exercise interventions, patients may consider these studies burdensome, leading to a reluctance to participate. Additionally, many patients may find the experience of participating in clinical trials disappointing and disempowering if they are not given appropriate feedback and are considered passive subjects. Providing a comprehensive informed consent, selecting meaningful outcomes, and involving the patient actively in the study, may help change these perceptions. While the current practice is not to reveal baseline assessment information to study participants, involving patients in reviewing their results to individualize the intervention plan may allow for more successful completion of the intervention.
3.2.2. Provider Perception
An “activity prescription” remains underutilized in clinical practice, potentially due to time constraints, and limited physician training in prescribing such interventions. In pharmacological trials, clinician fears of toxicity represent a barrier to recruitment, and this may also be the case with physical activity studies. Additionally, age alone has also been cited as a reason for not including older adults in therapeutic clinical trials.
34 The role of an allied health professional such as an exercise physiologist or a physiotherapist in this setting largely remains untapped, and if utilized appropriately, can potentially translate research evidence into broadly available interventions to support physical activity participation. To achieve better recruitment, and retention, all the personnel involved in clinical trials should be appropriately trained.35
3.2.3. Complexity of the Older Adult with Cancer
Understanding the complexity of an older cancer survivor can help improve exercise adherence. Older patients with cancer tend to have not only multimorbidity but also a significantly higher prevalence of geriatric syndromes, compared to those without cancer.36 Poor baseline health status, functional limitations, comorbidity, cognitive decline, and limited social support not only correlate with toxicity to therapy, and cancer outcome but also can limit their ability to participate in physical activity interventions.37 Additionally, it is important to highlight that by 2050, racial/ethnic minorities will make up the majority of older adults in the United States, which will further add to the complexity.38
3.3. Recommendations for Patient Selection
Table 3 summarizes the recommendations to help with improved patient participation in geriatric exercise oncology trials, and the below section discusses some of these recommendations.
Table 3. Recommendations for future geriatric oncology research, with regards to patient selection, intervention, and outcomes.
| Patient selection |
|
| Intervention |
|
| Outcome selection |
|
3.3.1. Comprehensive Inclusion Criteria
To generalize results of studies, a wider and more representative population should be evaluated in future trials. Criteria that are too stringent jeopardize the generalizability of a study; however, criteria that are overly expansive can also jeopardize patient safety and generate an exceedingly heterogeneous study population that interferes with detecting a treatment effect. To overcome this issue, different approaches have been proposed, including designing large trials with different cohorts, eligibility criteria, and outcomes. Depending on the phase and focus of the research, larger phase III trials with broad inclusion and few exclusion criteria may be best for generalizability in the post-treatment survivorship setting, whereas smaller, more focused trials may be best for specific patient populations (e.g. older women with lymphedema post breast cancer treatment).
3.3.2. Necessity-Based Design
When designing an exercise clinical trial, researchers should first acknowledge the characteristics of the population they wish to study (tumor type, stage, current treatment, comprehensive geriatric assessment results, baseline functional needs), and the specific outcomes they desire to address. Fostering patient and community engagement in the design phase itself is essential for the success of exercise trials. This strategy will provide vital information, as well as help with successful recruitment and retention into clinical trials, and is also likely to help with building trust between the community and the researchers.39
4. Section 2: Design of Exercise Interventions
4.1. Summary of Prior Research
Salient characteristics of the intervention in the discussed studies (Table 2) are reported here. In most studies, participants were randomized to either an exercise regimen or standard of care/stretching exercises. The intervention was usually four weeks long, and never more than 12 months. The exercise regimen tended to be of moderate-vigorous intensity, consisting of both aerobic and resistance training. The exercise regimen was scheduled for anywhere between one to three times a week and lasted approximately 30 min per session. Adverse events were infrequently reported and rarely attributed to the physical intervention itself. Often the intervention was in a supervised setting. Some studies integrated supervised and home-based regimens for patient convenience. Home based interventional studies were comparatively large and of longer duration, and when prescribed reinforced through telephone and mail counseling.28 Adherence to the home based sessions was significantly higher, underlying the importance of this strategy as a potent tool to improve compliance. Alternative exercise regimens like Yoga and Tai Chi were also evaluated.
4.2. Gaps and Design Issues
The significant underrepresentation of frail older adults with cancer in exercise trials and minimal stakeholder input in trial design has led to a dearth of information regarding the optimal exercise regimen for this population. Several gaps have been identified (Table 2) and include, the use of moderate to high-intensity regimens in most of the studies to date rather than lower intensity regimens, and the relative absence of studies during and immediately after active cancer treatment. Though adverse events have been linked to exercise40,41 none of the trials discuss the potential side effects of exercise during trial design or report them during the study. Exercise modalities in the current studies differ in content, frequency, intensity,and duration, thereby making it impossible for generalization. Some of the key design issues about currently selected interventions are discussed below.
4.2.1. Appropriate Intervention Selection
In patients receiving chemotherapy, the initial, as well as subsequent doses, are modified based on functional age, comorbidities, and tolerance. However, this approach is not typically adopted in the design of an exercise intervention. With the exercise interventions, the adage “start low and go slow” may improve patient accrual, compliance, and sustainability across patients with cancer of all age groups. Further research into exactly where to start and how to progress (e.g., frequency, intensity, mode, duration, etc.) is needed for patients with cancer and survivors. Developing risk-adapted strategies for the optimal exercise regimen is important. For example baseline geriatric assessment (GA) could be included in future studies to identify predictors of exercise capacity. Furthermore, elements of the GA could be collected in longitudinal follow-up to understand the impact of exercise on elder-relevant outcomes such as functional status.
4.2.2. Lack of Stakeholder Input
In most studies, the stakeholder's input is seldom taken into account. However, exercise interventions should be designed with feedback from all involved parties, including cancer survivors, physicians, care team, payers and policy makers. Understanding the patient's baseline health status, limitations to activity and needs during treatment are of utmost priority. This design is likely to empower patients to participate in trials to help themselves. Similarly, adequate training of study team to prescribe and modify exercise intervention, along with the integration of allied health professions to care teams will enhance exercise utilization.
4.2.3. Delivery of Intervention
Ideally exercise interventions should be cost-effective and not burdensome to the patient/payer/society. Institution-based exercise programs offers the opportunity to conduct exercise in a structured, supervised and group setting with direct monitoring. It has been long considered the standard. Home-based exercise programs, on the contrary, offer minimal to modest contact with a physical therapist/exercise physiologist. The potential benefits include higher participation, increased adherence due to greater flexibility, decreased cost, better ability to individualize the regimen, and the potential to continue with the intervention beyond the study. Pitfalls include difficulty measuring compliance to the exercise program, potential for injury if exercises are performed incorrectly. The integration of institution-based exercise programs transitioning to home-based programs is currently being investigated. Mostly exercise interventions have been limited to a supervised setting that involves considerable cost, time and travel commitments. In Project RENEW29 where a home based intervention was assessed, the costs for materials (including development), telephone counseling, and postage alone were $1000 per person, highlighting the need to consider cost/implementation issues in the design of studies. Financial matters(paying for transport, parking, etc.) are considered one of the main barriers to the delivery of physical activity interventions and should be routinely taken into account.42
4.3. Recommendations for Intervention Design
Table 3 summarizes the recommendations for the design of an intervention in geriatric exercise oncology trials, and the below section discusses some of these recommendations.
4.3.1. Individualization of Interventions
It is fundamental for researchers to ascertain the baseline activity and fitness levels (e.g. 1-repetition maximum, peak heart rate or submaximal heart rate) of the target population when designing an exercise intervention. Optimal intervention should target observed deficits. Understanding what amount of incremental changes in physical activity is feasible for patients, is a vital component for the individualization of any lifestyle intervention, including physical activity. In the RENEW trial, for instance, participants answered a baseline survey to establish their current physical activity. The intensity of the intervention as well as the planned increments was tailored using this information. A custom report was mailed every 12 weeks for visual reinforcement. Similarly, in a few other studies, changes in the training program based on survivor's baseline health status and responses were made.31,43 Published guidelines for both older adults and cancer survivors from the American College of Sports Medicine44 can be of help when designing these interventions. It is also important to integrate exercise into the patient's lifestyle, as standard supportive care from the time of cancer diagnosis.
4.3.2. Tailoring Interventions to Specific Endpoints
The goals of exercise prescription should be well defined before designing the intervention. Various outcomes can potentially be targeted using an exercise intervention, and are described in the next section. The type of exercise recommended, as well as the intensity needs to be tailored to fit the planned outcome.9
4.3.3. Designing and Testing Interventions for the Community
While most academic centers have access to the resources, personnel, and infrastructure needed to implement complex exercise interventions; this may not be true for oncologists working in the community. To implement a community-based delivery approach, researchers should include a plan for the training of the personnel required to implement the intervention. A multidisciplinary team is needed to foster adherence and participation in exercise in cancer survivors. Thus, when designing and testing an exercise intervention, researchers must always keep in mind whether such an intervention is feasible in a real world situation at the community level.
5. Section 3: Design of Outcomes in Exercise Trials
5.1. Summary of Prior Research
Most exercise intervention trials in older adults with cancer have selected functional parameters, such as physical activity, or anthropometric measures as their core outcomes. These were all validated measures. There was no uniformity in endpoints amongst studies. Cancer-specific outcomes, including reducing the rates of recurrence and attenuating or preventing symptoms caused by either the disease or its treatment,45–47 were rarely studied.
5.2. Gaps and Design Issues
Within the current studies, several gaps have been identified relating to outcomes selection (Table 2). Some of the relevant design issues are described here.
5.2.1. Lack of Intervention–Outcome Concordance
Exercise has diverse effects on multiple domains that can be used as outcomes (physiologic measures, objective performance indicators, self-reported functioning and symptoms, psychological well-being, and overall health-related quality of life). In many trials, outcomes are not appropriate for the selected population or do not fit the proposed intervention's dosing. For instance, while some outcomes, such as weight loss, are appropriate for overweight patients who have achieved disease remission post treatment, this may not be appropriate for underweight patients during active treatment.
5.2.2. Defining Relevant Outcomes for Patients and Payers
Outcomes need to be pertinent to the targeted patients. In Table 2, most endpoints involve improvements in physical function tests, but few have used a more comprehensive assessment of physical well-being and quality of life.
5.3. Recommendations for Exercise Research Outcomes Selection
Functional outcomes are relevant to patients as they are related to improvement in the quality of life, as well as cancer and treatment-related symptoms. However, to justify changes in health care policy and practice, and to have exercise interventions become a part of standard oncological care, robust clinical trials that are powered to show improvement in endpoints such as decreased hospitalizations, reduced cancer treatment toxicity, disease prevention, disease-free survival or overall survival and cost saving should be designed. A good example of such a study is the ongoing Colon Health and Life-Long Exercise Change (CHALLENGE) trial, which is a randomized study of a supervised physical activity intervention aimed at improving disease-free survival in patients 18 years of age and older with high-risk stage II and III colorectal cancer.48 Table 3 summarizes these recommendations. As with other components of exercise trials, researchers should engage the relevant stakeholders, (patients, providers, insurance companies, and granting organizations) upfront when choosing an outcome in order to identify what is relevant. The inclusion of functional end points can aid in the shared decision making by physicians and patients for identifying the most important areas of intervention.
6. Section 4: Dissemination and Implementation
Despite multiple factors that potentially contribute to the low physical activity levels in cancer patients and survivors, the lack of widely available disseminable evidence should not be overlooked. Dissemination is defined as “the targeted distribution of information and intervention materials to a specified clinical practice audience.” Implementation is “the use of strategies to adopt and integrate evidence-based heath intervention and change practice patterns within specified settings.” Dissemination and implementation of exercise oncology research findings into practice are necessary to improve health outcomes in a broader population, and will also help with obtaining a return on investment in research. Dissemination and implementation to be effective should take into account multiple factors including characteristics and needs of the older cancer patient and survivor and the type of evidence needed. Barriers to dissemination and implementation to exercise include the focus on discovery, limited study relevance, and efficiency, and insufficient collaboration among stakeholders. Referral pathways and reimbursements mechanisms will also help with more widespread adoption of exercise prescription. Finally epidemiologic studies that correlate exercise with cancer outcomes are essential in convincing payers, patients, and providers alike, to incorporate exercise into standard oncology care.
7. Summary
Exercises interventions have a positive effect on health outcomes in older cancer patients and survivors. More studies are necessary throughout the cancer continuum in all cancer sub-types and with robust outcomes for the physical activity prescription to be implemented routinely. It is imperative to develop studies based on current knowledge gaps, and tailored to the needs of cancer survivors, in such a way that the intervention should focus on the need to improve the targeted outcome. With adequate research, there may be a potential to integrate exercise regimens into anticancer therapy. The Cancer and Aging Research Group hopes that the current paper will provide the platform for constructive discussion and interdisciplinary collaboration for future design of exercise oncology trials.
Acknowledgments
This work was funded by a U13 AG038151 from the National Institute on Aging. The work was also funded by the American Cancer Society and a Patient-Centered Outcomes Research Institute (PCORI) Program contract (4634). The work received support from the James Wilmot Cancer Institute (WCI), the Alliance for Clinical Trials in Oncology (National Cancer Institute of the National Institutes of Health under Award Numbers U10CA18082 and 1UG1CA189823), and UG1 CA189961 from the National Cancer Institute. This work was made possible by the generous donors to the WCI geriatric oncology philanthropy fund. All statements in this report, including its findings and conclusions, are solely those of the authors, do not necessarily represent the official views of the funding agencies, and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
Footnotes
Disclosures and Conflict of Interest Statements: The authors have no conflicts of interest to disclose.
Author Contributions: Manuscript concept: D Kilari, E Soto, SG Mohile, K Mustian
Data acquisition: D Kilari, E Soto
Manuscript preparation: D Kilari, E Soto, SG Mohile, K Mustian
Manuscript editing and review: D Kilari, E Soto, SG Mohile, S Alibhai, CJ Presley, TM Wildes, HD Klepin, W Demark-Wahnefried, A Jatoi, R Harrison, E Won, K Mustian.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20(10):1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawkins SA, Wiswell RA. Rate and mechanism of maximal oxygen consumption decline with aging: implications for exercise training. Sports Med. 2003;33(12):877–888. doi: 10.2165/00007256-200333120-00002. [DOI] [PubMed] [Google Scholar]
- 4.Burg MA, Adorno G, Lopez EDS, Loerzel V, Stein K, Wallace C, et al. Current unmet needs of cancer survivors: analysis of open-ended responses to the American Cancer Society Study of Cancer Survivors II. Cancer. 2015;121(4):623–630. doi: 10.1002/cncr.28951. [DOI] [PubMed] [Google Scholar]
- 5.Hurria A, Li D, Hansen K, Patil S, Gupta R, Nelson C, et al. Distress in older patients with cancer. J Clin Oncol. 2009;27(26):4346–4351. doi: 10.1200/JCO.2008.19.9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohile SG, Xian Y, Dale W, Fisher SG, Rodin M, Morrow GR, et al. Association of a cancer diagnosis with vulnerability and frailty in older medicare beneficiaries. J Natl Cancer Inst. 2009;101(17):1206–1215. doi: 10.1093/jnci/djp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mustian KM, Sprod LK, Palesh OG, Peppone LJ, Janelsins MC, Mohile SG, et al. Exercise for the management of side effects and quality of life among cancer survivors. Curr Sports Med Rep. 2009;8(6):325–330. doi: 10.1249/JSR.0b013e3181c22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spence RR, Heesch KC, Brown WJ. Exercise and cancer rehabilitation: a systematic review. Cancer Treat Rev. 2010;36(2):185–194. doi: 10.1016/j.ctrv.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Mustian KM, Sprod LK, Janelsins M, Peppone LJ, Mohile S. Exercise recommendations for cancer-related fatigue, cognitive impairment, sleep problems, depression, pain, anxiety, and physical dysfunction: a review. Oncol Hematol Rev. 2012;8(2):81–88. doi: 10.17925/ohr.2012.08.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 11.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, et al. Physical activity levels before and after a diagnosis of breast carcinoma. Cancer. 2003;97(7):1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med (New York, NY) 1997;3(3):215–226. doi: 10.1089/acm.1997.3.215. [DOI] [PubMed] [Google Scholar]
- 13.Mustian KM, Griggs JJ, Morrow GR, McTiernan A, Roscoe JA, Bole CW, et al. Exercise and side effects among 749 patients during and after treatment for cancer: a University of Rochester Cancer Center Community Clinical Oncology Program Study. Support Care Cancer. 2006;14(7):732–741. doi: 10.1007/s00520-005-0912-6. [DOI] [PubMed] [Google Scholar]
- 14.Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. The Oncologist. 2000;5(5):353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 15.Winningham ML, Nail LM, Burke MB, Brophy L, Cimprich B, Jones LS, et al. Fatigue and the cancer experience: the state of the knowledge. Oncol Nurs Forum. 1994;21(1):23–36. [PubMed] [Google Scholar]
- 16.Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(1):123–133. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- 17.Irwin ML, Alvarez-Reeves M, Cadmus L, Mierzejewski E, Mayne ST, Yu H, et al. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity. 2009;17(8):1534–1541. doi: 10.1038/oby.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 19.Tang MF, Liou TH, Lin CC. Improving sleep quality for cancer patients: benefits of a home-based exercise intervention. Support Care Cancer. 2010;18(10):1329–1339. doi: 10.1007/s00520-009-0757-5. [DOI] [PubMed] [Google Scholar]
- 20.Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin Psychol Rev. 2001;21(1):33–61. doi: 10.1016/s0272-7358(99)00032-x. [DOI] [PubMed] [Google Scholar]
- 21.Betof AS, Dewhirst MW, Jones LW. Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain Behav Immun. 2013;30(0):S75–S87. doi: 10.1016/j.bbi.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(11):815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bittoni MA, Harris RE, Buckworth J, Clinton SK, Focht BC. Abstract 5043: Physical activity and the risk of lung cancer death: results from the Third National Health and Nutrition Examination Survey. Cancer Res. 2014;74(19 Supplement):5043. [Google Scholar]
- 24.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):242–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 25.Irwin ML. Physical activity interventions for cancer survivors. Br J Sports Med. 2009;43(1):32–38. doi: 10.1136/bjsm.2008.053843. [DOI] [PubMed] [Google Scholar]
- 26.Bibas L, Levi M, Bendayan M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Prog Cardiovasc Dis. 2014;57(2):134–143. doi: 10.1016/j.pcad.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Chin APMJ, van Uffelen JG, Riphagen I, van Mechelen W. The functional effects of physical exercise training in frail older people: a systematic review. Sports Med (Auckland, NZ) 2008;38(9):781–793. doi: 10.2165/00007256-200838090-00006. [DOI] [PubMed] [Google Scholar]
- 28.Demark-Wahnefried W, Clipp EC, Morey MC, Pieper CF, Sloane R, Snyder DC, et al. Lifestyle intervention development study to improve physical function in older adults with cancer: outcomes from project LEAD. J Clin Oncol. 2006;24(21):3465–3473. doi: 10.1200/JCO.2006.05.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. JAMA. 2009;301(18):1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campo R, Agarwal N, LaStayo P, O'Connor K, Pappas L, Boucher K, et al. Levels of fatigue and distress in senior prostate cancer survivors enrolled in a 12-week randomized controlled trial of Qigong. J Cancer Surviv. 2014;8(1):60–69. doi: 10.1007/s11764-013-0315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winters-Stone KM, Dobek J, Bennett JA, Nail LM, Leo MC, Schwartz A. The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: a randomized controlled trial. J Cancer Surviv. 2012;6(2):189–199. doi: 10.1007/s11764-011-0210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keating NL, Nørredam M, Landrum MB, Huskamp HA, Meara E. Physical and mental health status of older long-term cancer survivors. J Am Geriatr Soc. 2005;53(12):2145–2152. doi: 10.1111/j.1532-5415.2005.00507.x. [DOI] [PubMed] [Google Scholar]
- 33.Schlairet MC, Benton MJ. Quality of life and perceived educational needs among older cancer survivors. J Cancer Educ. 2012;27(1):21–26. doi: 10.1007/s13187-011-0279-y. [DOI] [PubMed] [Google Scholar]
- 34.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23(13):3112–3124. doi: 10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]
- 35.Herrera AP, Snipes SA, King DW, Torres-Vigil I, Goldberg DS, Weinberg AD. Disparate inclusion of older adults in clinical trials: priorities and opportunities for policy and practice change. Am J Public Health. 2010;100(Suppl 1):S105–S112. doi: 10.2105/AJPH.2009.162982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohile SG, Fan L, Reeve E, Jean-Pierre P, Mustian K, Peppone L, et al. Association of cancer with geriatric syndromes in older Medicare beneficiaries. J Clin Oncol. 2011;29(11):1458–1464. doi: 10.1200/JCO.2010.31.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohile S, Dale W, Hurria A. Geriatric oncology research to improve clinical care. Nat Rev Clin Oncol. 2012;9(10):571–578. doi: 10.1038/nrclinonc.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez AD, Hirschman C. The changing racial and ethnic composition of the US population: emerging american identities. Popul Dev Rev. 2009;35(1):1–51. doi: 10.1111/j.1728-4457.2009.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fouad MN, Partridge E, Green BL, Kohler C, Wynn T, Nagy S, et al. Minority recruitment in clinical trials: a conference at Tuskegee, researchers and the community. Ann Epidemiol. 2000;10(8 Suppl):S35–S40. doi: 10.1016/s1047-2797(00)00199-x. [DOI] [PubMed] [Google Scholar]
- 40.Falcone C, Buzzi MP, Klersy C, Schwartz PJ. Rapid heart rate increase at onset of exercise predicts adverse cardiac events in patients with coronary artery disease. Circulation. 2005;112(13):1959–1964. doi: 10.1161/CIRCULATIONAHA.105.545111. [DOI] [PubMed] [Google Scholar]
- 41.Sayers SP, Clarkson PM, Rouzier PA, Kamen G. Adverse events associated with eccentric exercise protocols: six case studies. Med Sci Sports Exerc. 1999;31(12):1697–1702. doi: 10.1097/00005768-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Josyula LK, Lyle RM. Barriers in the implementation of a physical activity intervention in primary care settings: lessons learned. Health Promot Pract. 2013;14(1):81–87. doi: 10.1177/1524839910392991. [DOI] [PubMed] [Google Scholar]
- 43.Cormie P, Galvao DA, Spry N, Joseph D, Chee R, Taaffe DR, et al. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: a randomised controlled trial. BJU Int. 2015;115(2):256–266. doi: 10.1111/bju.12646. [DOI] [PubMed] [Google Scholar]
- 44.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 45.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 46.Courneya KS, Segal RJ, Gelmon K, Reid RD, Mackey JR, Friedenreich CM, et al. Six-month follow-up of patient-rated outcomes in a randomized controlled trial of exercise training during breast cancer chemotherapy. Cancer Epidemiol Bio-markers Prev. 2007;16(12):2572–2578. doi: 10.1158/1055-9965.EPI-07-0413. [DOI] [PubMed] [Google Scholar]
- 47.Mock V, Frangakis C, Davidson NE, Ropka ME, Pickett M, Poniatowski B, et al. Exercise manages fatigue during breast cancer treatment: a randomized controlled trial. Psychooncology. 2005;14(6):464–477. doi: 10.1002/pon.863. [DOI] [PubMed] [Google Scholar]
- 48.Courneya KS, Booth CM, Gill S, O'Brien P, Vardy J, Friedenreich CM, et al. The Colon Health and Life-Long Exercise Change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol (Toronto, Ont) 2008;15(6):279–285. doi: 10.3747/co.v15i6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winters-Stone KM, Dobek JC, Bennett JA, Dieckmann NF, Maddalozzo GF, Ryan CW, et al. Resistance training reduces disability in prostate cancer survivors on androgen deprivation therapy: evidence from a randomized controlled trial. Arch Phys Med Rehabil. 2015;96(1):7–14. doi: 10.1016/j.apmr.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sprod LK, Fernandez ID, Janelsins MC, Peppone LJ, Atkins JN, Giguere J, et al. Effects of yoga on cancer-related fatigue and global side-effect burden in older cancer survivors. J Geriatr Oncol. 2015;6(1):8–14. doi: 10.1016/j.jgo.2014.09.184. [DOI] [PMC free article] [PubMed] [Google Scholar]