Abstract
Objective
To determine the impact of recent relocation prior to a cancer diagnosis on cancer-specific outcomes.
Methods
We identified 272,718 patients with two different entries in the Surveillance, Epidemiology, and End Results database within three years of each other. Those who had relocated to a different county between entries were identified and we determined the risk of stage IV disease or cancer-specific mortality among relocators and non-relocators after adjusting for other patient-specific demographic and clinical factors.
Results
A total of 4,639 (1.7%) patients relocated to a new county within three years prior to a second cancer diagnosis and 268,079 (98.3%) patients did not. Patients who had relocated to a new area were more likely to be diagnosed with stage IV cancer (25.2% vs. 20.8%; adjusted odds ratio = 1.27; 95% confidence interval [CI], 1.18–1.37; P < 0.001), and had an increased risk of 10-year cancer-specific mortality (20.9% vs. 17.9%; adjusted hazard ratio 1.26; 95% CI, 1.17–1.36; P < 0.001).
Conclusion
These results suggest that recent relocation to a new county prior to a cancer diagnosis is associated with an increased risk of late-stage presentation and worse cancer-specific mortality.
Keywords: Relocation, psychosocial stress, cancer-specific mortality, healthcare disparities, metastatic cancer
INTRODUCTION
Cancer patients are a vulnerable population at high risk of mortality for whom psychosocial stressors may affect the timing or delivery of life-saving treatment [1]. Although traditional measures of SES such as low income or low education have been previously associated with poor health or cancer-specific outcomes [2–4], these relatively static measures may not adequately capture the volatility of life changes that may affect the treatment and caregiving processes or long-term survivorship. One such form of psychosocial stress is relocation to a new area shortly preceding or following a cancer diagnosis [5]. Relocation has previously been shown to affect mental and physical health outcomes [6,7], but data on the effects of relocation shortly preceding diagnosis among cancer patients are lacking.
Relocation shortly before a cancer diagnosis may have a significant effect on cancer-specific outcomes. In particular, recent relocation, which represents a relatively acute form of psychosocial stress, may have a larger impact on cancer-specific outcomes than more chronic psychosocial stressors such as poverty. Cancer survivors reporting psychosocial stress have been shown to have higher levels of health care utilization and medical expenditures compared to those without cancer [8]; yet, patients who relocate may also experience a break in care continuity [9]. Relocation can also lead to social disconnectedness by disrupting social networks, which has been shown to have negative health effects [10].
Research suggests that relocation can also be a proxy for household financial hardship, which has been associated with negative cancer-specific consequences [11–15]; and, not surprisingly, cancer survivors reporting financial problems may be likely to delay or forego care compared to cancer survivors not reporting such problems [16]. Financial stress can arise from the cost of medical treatment, other medical supplies, and health support needs that are not covered by health insurance. Among patients without adequate health insurance coverage, these factors may cause even greater financial stress. In addition to the costs directly associated with treatment, a cancer diagnosis may lead to in inability to work and earn income as has been demonstrated to occur in 7–70% of patients diagnosed with cancer in previous studies [17,18].
In this study, we employ a novel approach using the Surveillance, Epidemiology, and End Results (SEER) national cancer database to study the effect of recent relocation on cancer-specific outcomes. The primary aims of the present study were to determine the associations between recent relocation and diagnosis with late-stage cancer and between recent relocation and cancer-specific mortality. A second aim was to determine racial differences in the association between recent relocation and cancer-specific mortality, as other research has previously suggested racial differences in the response to psychosocial stressors [19,20]. Finally, the third aim was to compare the effect of recent relocation to the effect of baseline poverty on cancer-specific mortality. We hypothesized that recent relocation would be associated with an increased risk of stage IV disease at diagnosis as well as increased cancer-specific mortality, even after controlling for other patient characteristics. We also hypothesized that the effect of recent relocation would be stronger among white patients than non-white patients and stronger than the negative consequences of baseline poverty.
MATERIALS AND METHODS
Patient Population
The SEER database is a population-based cancer registry that collects cancer diagnostic, treatment, and survival data along with patient demographic characteristics from 18 registries within the United States, representing 28.0% of the population [21]. SEER reports the county of residence at the time of a cancer diagnosis. Therefore, we studied patients who had two cancer diagnoses within three years of each other so we could determine whether they had recently moved to a different county. Like all recorded cancer diagnoses in SEER, second cancers are recorded at the registry level and patient data is sent to SEER with a patient-specific identification number and a sequence number describing the order of the tumor in question (e.g. 1st or 2nd). Patients were included if they were diagnosed between 1973 and 2011 and diagnosed with any malignancy at any stage, except for non-melanoma skin cancer, as these cases are not captured in SEER. Patients must have also resided in an area captured by one of the SEER registries (Alaska Native Tumor Registry, Arizona Indians, Cherokee Nation, Connecticut, Detroit, Georgia Center for Cancer Statistics, Greater Bay Area Cancer Registry, Greater California, Hawaii, Iowa, Kentucky, Los Angeles, Louisiana, New Jersey, New Mexico, Seattle-Puget Sound, and Utah) for both cancer diagnoses. This approach identified 272,718 patients. This study was approved by the institutional review board.
Definition of Variables
The primary outcome variables for this study included: (1) stage IV disease at presentation; and (2) 10-year cancer-specific mortality. Our primary predictor variable was recent relocation to a new county. We also extracted data on stage at presentation, county-wide median family income, marital status at diagnosis, race, age at diagnosis, and sex.
Statistical analyses
Stata/MP 13.1 was used for all statistical analyses. Multivariable logistic regression analysis, adjusting for marital status at diagnosis, change in marital status between diagnoses, sex, age, county-wide median income, and race, was used to model the odds ratio of stage IV disease at presentation among patients who moved to a new county compared to those who did not. A similar logistic regression analysis was used to compare the 10% of patients who lived in the poorest counties to the 10% of patients who lived in the wealthiest counties.
Differences between groups in the 10-year risk of cancer-specific mortality from the second diagnosis of cancer were estimated using the Fine & Gray model [22] for competing risks after adjusting for marital status at diagnosis, change in marital status between diagnoses, sex, age, county-wide median income level, and race. In a subsequent analysis, we also controlled for stage at diagnosis (stage I–III versus stage IV). Cancer-specific mortality was compared between patients who relocated to a new county and those who did not. This analysis was repeated following stratification by race (white versus non-white) and by cancer site among the four most common cancers (lung, prostate, breast, and colon).
To test the possible effect of cancer recurrences or sites of metastatic disease being incorrectly recorded as second primary malignancies, we also repeated our analyses after excluding patients who were recorded as having been diagnosed with a second primary malignancy at the same site as the first primary malignancy or for whom the second primary malignancy was in a common site of metastatic disease (liver, lung, bone, or brain/central nervous system). We also performed a separate analysis including insurance status and change in insurance status between diagnoses (private/Medicare to Medicaid or no insurance, or Medicaid to no insurance), which was available for 22.0% of patients, as covariates. Cancer-specific mortality was also compared between the 10% of patients who lived in the poorest counties compared to the 10% who lived in the wealthiest counties.
RESULTS
Patient Characteristics
Of the 272,718 patients we identified, 4,639 (1.7%) relocated to a new county within three years prior to their second cancer diagnosis, while 268,079 (98.3%) did not. Baseline characteristics of the patients are shown in Table 1.
Table 1.
Baseline patient characteristics.
| Patients who did not relocate to a new county | Patients who relocated to a new county | P-value | |
|---|---|---|---|
| Number | 268,079 | 4,639 | |
| Median age at time of first cancer diagnosis | 68 | 67 | < 0.001 |
| Median age at time of second cancer diagnosis | 69 | 68 | < 0.001 |
| Median county income at time of first cancer diagnosis | $54,020 | $53,880 | < 0.001 |
| Median county income at time of second cancer diagnosis | $54,020 | $54,020 | < 0.001 |
| % Men | 55.1% | 54.8% | 0.655 |
| % Married (at time of second diagnosis) | 61.2% | 47.7% | < 0.001 |
| % White | 85.7% | 83.3% | < 0.001 |
Recent Relocation is Associated with Later-Stage Diagnosis
Of patients who had recently relocated, 25.2% were diagnosed with stage IV disease, compared to 20.8% of those who had not recently relocated (adjusted odds ratio [AOR] 1.27; 95% confidence interval [CI] 1.18–1.37; P < 0.001) (Table 2).
Table 2.
Multivariable logistic regression for predictors of diagnosis with stage IV disease. AOR = adjusted odds ratio, CI = confidence interval.
| Covariate | AOR | 95% CI |
|---|---|---|
| No relocation | 1 | |
| Relocation | 1.27 | 1.18–1.37 |
|
| ||
| Median family income (by $1000) | 0.99 | 0.99–0.99 |
|
| ||
| Unmarried | 1 | |
| Married | 0.90 | 0.88–0.92 |
|
| ||
| No change in marital status | 1 | |
| Change in marital status | 0.98 | 0.95–1.01 |
|
| ||
| Non-white | 1 | |
| White | 0.81 | 0.78–0.83 |
|
| ||
| Female | 1 | |
| Male | 1.18 | 1.16–1.21 |
|
| ||
| Age at diagnosis (years) | 1.01 | 1.01–1.01 |
Recent Relocation is Associated with Increased Cancer-Specific Mortality
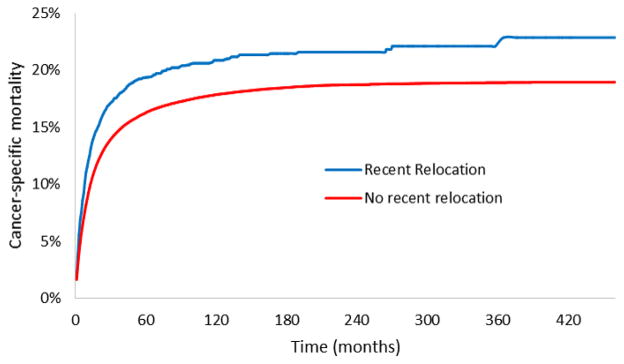
Patients who relocated within 3 years of a second cancer diagnosis had an increased risk of 10-year cancer-specific mortality compared to those who did not, even after adjusting for patient-specific demographic factors (20.9% vs. 17.9%; adjusted hazard ratio [HR] 1.26; 95% CI, 1.17–1.36; P < 0.001; Figure 1). When stage at diagnosis was included in the model, the effect of relocation on cancer-specific mortality was attenuated, but remained statistically significant (adjusted HR 1.16; 95% CI, 1.07–1.25; P < 0.001) (Table 3).
Figure 1.
Cancer-specific mortality among patients with or without recent relocation.
Table 3.
Multivariable competing risks regression for cancer-specific mortality, without stage (Model 1) or with stage (Model 2) included as a covariate. AHR = adjusted hazard ratio, CI = confidence interval.
| Covariate | Model 1
|
Model 2
|
||
|---|---|---|---|---|
| AHR | 95% CI | AHR | 95% CI | |
| No relocation | 1 | 1 | ||
| Relocation | 1.26 | 1.17–1.36 | 1.16 | 1.07–1.25 |
|
| ||||
| Stage I–III | - | 1 | ||
| Stage IV | - | 3.77 | 3.69–3.85 | |
|
| ||||
| Median family income (by $1000) | 0.999 | 0.999–1.001 | 1.001 | 1.001–1.002 |
|
| ||||
| Unmarried | 1 | 1 | ||
| Married | 0.98 | 0.96–1.01 | 1.007 | 0.98–1.03 |
|
| ||||
| No change in marital status | 1 | 1 | ||
| Change in marital status | 0.83 | 0.79–0.86 | 0.82 | 0.79–0.86 |
|
| ||||
| Non-white | 1 | 1 | ||
| White | 0.89 | 0.86–0.91 | 0.94 | 0.92–0.97 |
|
| ||||
| Female | 1 | 1 | ||
| Male | 1.38 | 1.35–1.41 | 1.36 | 1.33–1.39 |
|
| ||||
| Age at diagnosis (years) | 1.02 | 1.02–1.02 | 1.02 | 1.02–1.02 |
In a sensitivity analysis to test the possible effect of cancer recurrences or sites of metastatic disease being incorrectly recorded as second primary malignancies, we observed a similar increase in the rate of cancer-specific mortality after excluding the 31.2% of patients for whom their second cancer was at the same site as the first cancer or for whom the second cancer was diagnosed at a common site of metastatic disease (adjusted HR 1.24; 95% CI, 1.13–1.37; P < 0.001).
Among the 22.0% of patients with complete insurance information, insurance status and changes in insurance status were not significantly associated with cancer-specific mortality (p > 0.05 in all cases), nor did inclusion of these factors in multivariable analysis reduce the estimated effect of relocation on cancer-specific mortality (not shown).
We then conducted subgroup analyses for the four most common cancer types, including lung cancer (N=704 relocated), prostate cancer (N=404 relocated), breast cancer (N=571 relocated), and colon cancer (N=355 relocated). We observed an increase in cancer-specific mortality associated with recent relocation among patients with lung cancer (adjusted HR 1.26; 95% CI, 1.11–1.42; P < 0.001) or prostate cancer (adjusted HR 1.64; 95% CI 1.22–2.19; P = 0.001), but not colon cancer (adjusted HR 1.06; 95% CI, 0.79–1.43; P = 0.701) or breast cancer (adjusted HR 1.12; 95% CI, 0.77–1.63; P = 0.555).
Racial Differences in the Association between Recent Relocation and Mortality
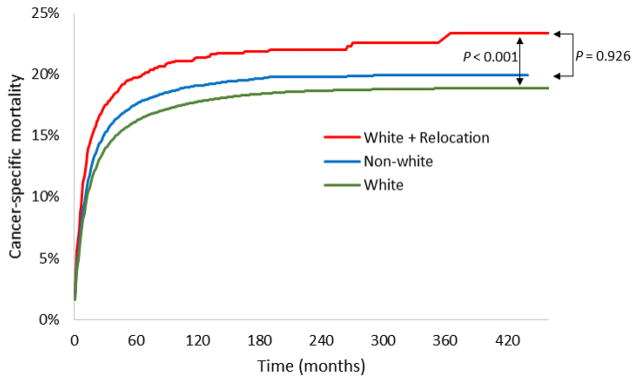
We found that at baseline, white patients in our cohort had a lower risk of cancer-specific mortality than non-white patients (adjusted HR 0.89; 95% CI, 0.86–0.91; P < 0.001). When we repeated our initial analysis stratified by race, we found that white patients who recently relocated had worse 10-year cancer-specific mortality compared to white patients who did not, like our overall cohort (21.3% vs. 17.7%; adjusted HR 1.30; 95% CI, 1.20–1.41; P < 0.001). In contrast, we found that among non-white patients, relocation was not associated with a significantly increased risk of cancer-specific mortality at 10 years (18.8% vs. 19.1%; adjusted HR 1.10; 95% CI, 0.91–1.33; P = 0.315). Interestingly, the survival advantage observed for white versus non-white patients was lost among white patients who relocated to a new area (Figure 2; adjusted HR for white patients who relocated vs. all non-white patients 1.00; 95% CI, 0.92–1.09; P = 0.926).
Figure 2.
Cancer-specific mortality among all white patients (N = 232,984), white patients who relocated (N = 3,855), and all non-white patients (N = 39,084).
Mortality is More Closely Associated with Recent Relocation than with County-Wide Median Income Level
Lastly, as hypothesized, we found that the 10% of patients in the least wealthy counties in our cohort had a higher risk of 10-year cancer-specific mortality than the 10% in the wealthiest counties (18.4% vs. 17.3%; P < 0.005), but the difference was smaller than the one observed with recent relocation (absolute risk difference of 1.1% for baseline poverty vs. 3.0% for recent relocation). The 10% of patients in the poorest counties were more likely to be diagnosed with stage IV disease than the 10% in the wealthiest counties (24.0% vs. 19.3%; P < 0.001), a similar difference to what was observed with recent relocation.
DISCUSSION
In this population-based study of cancer patients, we found that recent relocation to a new county was associated with an increased risk of advanced-stage disease at diagnosis and worse cancer-specific mortality. These effects persisted even after controlling for patient-specific demographic factors, including sex, race, age, marital status, change in marital status between diagnoses, and county-wide income level. The mortality difference was only partially explained by the increased risk of stage IV disease among relocators. Our findings for the overall cohort were confirmed in the subgroups of patients with lung or prostate cancer, but not breast or colon cancers, although we may have been limited in our ability to detect differences within subgroups due to the relatively small numbers of patients who relocated in each subgroup, especially given that the estimated hazard ratios remained greater than 1 in the breast and colon cancer subgroups. Additionally, the survival decrement associated with recent relocation was worse than the survival gap between those in the poorest and wealthiest counties by median family income, suggesting that the negative effect associated with recent relocation was larger than the chronic effect of living in a lower-income area. While our methodology only allowed us to identify a small number of patients (4,639 patients, or 1.7% of the whole cohort) who relocated to a new county, our findings may be relevant to the 30–43% of cancer patients who experience similar forms of acute psychosocial stress such as relocation within the same county or financial hardship around the time of a cancer diagnosis [23–25].
There are many possible explanations for our findings. First, one possibility is that relocation shortly before a cancer diagnosis is a proxy for other forms of psychosocial stress caused by financial hardship, which may be especially likely in our patient population as all patients had been diagnosed with two cancers within 3 years, which may have caused reduced employment, income, and significant health care costs [26]. Such psychosocial stressors may lead to delays in cancer screening or diagnostic workup of worrisome symptoms, possibly because patients are preoccupied by financial concerns or have lapses in insurance coverage in the wake of financial insecurity [12–14]. We found that patients with evidence of recent relocation were at increased risk of late-stage presentation, consistent with delayed diagnosis. However, in multivariable regression analysis, the increased rate of late-stage diagnosis among patients who had relocated did not completely explain their increased risk of cancer-specific mortality, suggesting that other factors may have a role. If patients relocated due to financial hardship, they may have delayed or refused treatment due to cost concerns, which may partially explain their worse outcomes [25]. For example, patients may be hesitant to initiate a 6–8 week course of radiation that can interfere with their ability to earn income, but delays in the receipt of radiation therapy for cancer have been associated with an increased risk of locoregional recurrence and mortality [27]. Others have previously found that financial hardship and other psychosocial stressors are also associated with an increased risk of depression, anxiety, and worse overall health [28–30], which can lead to a reduced ability to tolerate definitive cancer therapy and decreased survival [31,32]. Future work will be needed to determine the specific factors that mediate the effect of recent relocation on the risk of late-stage presentation and increased cancer-specific mortality.
Second, recent relocation may lead to a disruption in social supports without sufficient time to re-build those networks prior to diagnosis [33]. Cancer patients who relocate away from caregivers and support networks might experience worse survival outcomes due to lose of instrumental support, such as assistance in going to appointments, reminders to take medication, and assistance with nutrition and mobility [34–36]. Similarly, the loss of emotional support from nearby friends and family has also been associated with worse cancer-specific survival [37]. Alternatively, it is also possible that patients who relocate do so to be closer to family members or other caregivers, but that the concomitant change in their social networks might still represent a disruptive psychosocial stressor that leads to worse outcomes despite increased family support.
Third, patients who relocated to a new county may have experienced gaps in health care continuity. A recent study found that over a third of Medicaid enrollees that moved to a new state experienced gaps in health care coverage [9]. Such gaps in care might lead to delayed cancer workup and diagnosis. In addition, based on our study design, all patients in our cohort had been diagnosed with two cancers, so many may have already had relationships with oncologic providers to rely on at the time of second diagnosis. Those who relocated to a new county may have forgone this potential benefit.
The effects of relocation might be partially addressed through interventions at the societal and clinical levels. To ameliorate the negative effects of relocation at a societal level, there is a need to help ensure that patients with recent financial and psychosocial hardship can continue to receive appropriate care and avoid delayed diagnoses. Universal coverage for cancer treatment or at least screening, as the American Cancer Society has suggested [38], may help to address part of these issues. Consistent with a focus on patient centered care [39], at the clinical practice level, clinicians caring for cancer patients should implement distress screeners to detect patients experiencing recent relocation [40], doing so may uncover additional sources of psychosocial stress that patients might not otherwise mention. Cancer patients experiencing financial hardship, loss of social support, or a break in health care continuity may especially benefit from multidisciplinary coordinated assistance, including from social workers, psychologists, pharmacists, and hospital financial counselors to help ensure they receive appropriate and timely services to address their psychosocial needs along with their physical health needs, which may improve their chances of surviving their cancer.
We also found that non-white patients did not experience increased cancer-specific mortality following recent relocation, while white patients did. This result may seem counterintuitive, but adjustment for multiple indicators of socioeconomic context in previous studies has been shown to eliminate racial survival differences [41]. Others have suggested that older minority patients may be more resilient to financial or psychosocial hardship due to having weathered more psychosocial hardships previously [19]. In addition, strong racial identity among racial minority groups such as African Americans has also been reported to potentially buffer the effects of financial stress [20].
Our results are consistent with the work of others showing that psychosocial stressors, in addition to the influence of traditional measures of socioeconomic status such as low income or education [2,3], are associated with poorer all-cause mortality and cardiac-specific outcomes [30,41–45]. Our study adds an important dimension to this literature. This study is the first to our knowledge that specifically assesses the effect of recent relocation on cancer-specific mortality. In addition, most of the current literature on the effect of psychosocial stressors on cancer-specific outcomes has either focused on acute stressors following diagnosis, which may be confounded by the presence of a new diagnosis, or on chronic stressors, such as baseline poverty preceding diagnosis. Our novel operationalization of recent relocation using the SEER database may capture additional aspects of patients’ socioeconomic context not previously measured in prior studies.
Our study has some limitations and should be interpreted with caution. First, there are likely many complex factors at play when patients relocate to a new county, and we were not able to determine the reasons for relocation for our cohort. We attempted to control for patient-specific factors that may be related to the reasons for relocation, such as race, marital status, age, sex, and county income level, but it is possible that there are other biases or factors present within this subgroup of patients that are partially responsible for the differences in stage at diagnosis and cancer-specific mortality that we observed. In particular, patient comorbidity, which is not available in the SEER database, may be an important cause of both relocation (e.g. to a retirement home or to be closer to family caregivers) and increased cancer-specific mortality. Similarly, change in health insurance status, which was only available for 22.0% of our cohort, might partially explain the association between relocation and mortality, despite the lack of an effect seen in our possibly underpowered analysis of insurance status.
Second, because we needed to be able to identify patients who relocated between two points in time and because patients are only entered into the SEER database once per cancer diagnosis, all patients in our study were diagnosed with two malignancies, and our analysis was focused on the second cancer. Based on the possible biases associated with this study design, our findings may have limited generalizability. Future work is needed to validate our findings in other cohorts of patients diagnosed with cancer.
Third, because the SEER database only provides county-level data for patient location, we could not distinguish between short-range relocations versus long-range relocations, which could potentially be more disruptive and have a larger effect on health outcomes. Furthermore, we could only control for income at the county level, and it is possible that variation in patient income level is able to explain some of the association between recent relocation and mortality, and that baseline differences in individual-level income better predict survival than differences in county-level income.
CONCLUSION
Using a large national cancer database of cancer patients, we found that relocation to a new county shortly preceding a cancer diagnosis was associated with an increased risk of late stage at presentation and increased cancer-specific mortality. Our results highlight the importance of social, economic, and provider stability for cancer patients and suggest that the negative effects of relocation might be even larger than the negative effects of poverty at baseline. Clinicians caring for cancer patients should seek to identify those who have recently relocated and offer access to additional resources, including from social workers, psychologists, pharmacists, and hospital financial counselors, as the associated psychosocial stress may have a significant negative impact on their odds of surviving their disease.
HIGHLIGHTS.
Recent relocation prior to a cancer diagnosis may negatively affect outcomes.
Recent relocation was associated with an increased rate of advanced disease at diagnosis.
Recent relocators were also more likely to die from cancer than non-relocators.
Further work is required to determine the reasons for worse outcomes among relocators.
Acknowledgments
This work was supported by grants from the IDEA2 Program supported by the Peter C. Farrell (1967) Fund, The Prostate Cancer Foundation, Fitz’s Cancer Warriors, David and Cynthia Chapin, Hugh Simons in Honor of Frank and Anne Simons, The Scott Forbes and Gina Ventre Fund, and an anonymous grant. Dr. Tucker-Seeley is funded by a K01 career development award (Grant# CA169041-02) from the National Cancer Institute. The funding sources had no role in the study.
Footnotes
Disclosures: PLN has worked as a consultant for Medivation, GenomeDx, and Ferring Pharmaceuticals. VM and RTS have no relationships to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Vinayak Muralidhar, Email: vinayak_muralidhar@hms.harvard.edu.
Paul L. Nguyen, Email: pnguyen@LROC.harvard.edu.
Reginald D. Tucker-Seeley, Email: retucker@hsph.harvard.edu.
References
- 1.Timmons A, Gooberman-Hill R, Sharp L. “It’s at a time in your life when you are most vulnerable”: a qualitative exploration of the financial impact of a cancer diagnosis and implications for financial protection in health. PLoS ONE. 2013;8(11):e77549. doi: 10.1371/journal.pone.0077549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassuk SS, Berkman LF, Amick BC. Socioeconomic status and mortality among the elderly: findings from four US communities. Am J Epidemiol. 2002;155(6):520–33. doi: 10.1093/aje/155.6.520. [DOI] [PubMed] [Google Scholar]
- 3.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 4.Singh GK, Miller BA, Hankey BF, Edwards BK. Area Socioeconomic Variations in US Cancer Incidence, Mortality, Stage, Treatment, and Survival 1975–1999. Bethesda, MD: National Cancer Institute; 2003. NCI Cancer Surveillance Monograph Series, Number 4. NIH Publication No. 03-5417. [Google Scholar]
- 5.Mcgrath P, Rawson N. The experience of relocation for specialist treatment for Indigenous women diagnosed with vulvar cancer in East Arnhem Land. J Psychosoc Oncol. 2013;31(5):540–55. doi: 10.1080/07347332.2013.822051. [DOI] [PubMed] [Google Scholar]
- 6.Lix LM, Hinds A, Deverteuil G, Robinson JR, Walker J, Roos LL. Residential mobility and severe mental illness: a population-based analysis. Adm Policy Ment Health. 2006;33(2):160–71. doi: 10.1007/s10488-006-0035-5. [DOI] [PubMed] [Google Scholar]
- 7.Lin KC, Huang HC, Bai YM, Kuo PC. Lifetime residential mobility history and self-rated health at midlife. J Epidemiol. 2012;22(2):113–22. doi: 10.2188/jea.JE20110055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han X, Lin CC, Li C, et al. Association between serious psychological distress and health care use and expenditures by cancer history. Cancer. 2015;121(4):614–22. doi: 10.1002/cncr.29102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baugh DK, Verghese S. Migration patterns for Medicaid enrollees 2005–2007. Medicare Medicaid Res Rev. 2013;3(4) doi: 10.5600/mmrr.003.04.b02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornwell EY, Waite LJ. Social disconnectedness, perceived isolation, and health among older adults. J Health Soc Behav. 2009;50(1):31–48. doi: 10.1177/002214650905000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanratty B, Holland P, Jacoby A, Whitehead M. Financial stress and strain associated with terminal cancer--a review of the evidence. Palliat Med. 2007;21(7):595–607. doi: 10.1177/0269216307082476. [DOI] [PubMed] [Google Scholar]
- 12.Coulton C, Theodos B, Turner MA. Residential mobility and neighborhood change: real neighborhoods under the microscope. Cityscape. 2012;14(3):55–90. [Google Scholar]
- 13.Robinson JM, Shavers V. The role of health insurance coverage in cancer screening utilization. J Health Care Poor Underserved. 2008;19(3):842–56. doi: 10.1353/hpu.0.0048. [DOI] [PubMed] [Google Scholar]
- 14.Pornet C, Dejardin O, Morlais F, Bouvier V, Launoy G. Socioeconomic determinants for compliance to colorectal cancer screening. A multilevel analysis. J Epidemiol Community Health. 2010;64(4):318–24. doi: 10.1136/jech.2008.081117. [DOI] [PubMed] [Google Scholar]
- 15.Barry J, Breen N, Barrett M. Significance of increasing poverty levels for determining late-stage breast cancer diagnosis in 1990 and 2000. J Urban Health. 2012;89(4):614–27. doi: 10.1007/s11524-011-9660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119(20):3710–7. doi: 10.1002/cncr.28262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewitt M, Greenfield S, Stovall E, editors. IOM; NRC. From cancer patient to cancer survivor: Lost in transition. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 18.Spelten ER, Sprangers MAG, Verbeek JHAM. Factors reported to influence the return to work of cancer survivors: A literature review. Psycho-Oncology. 2002;11(2):124–131. doi: 10.1002/pon.585. [DOI] [PubMed] [Google Scholar]
- 19.Keyes CL. The Black-White paradox in health: flourishing in the face of social inequality and discrimination. J Pers. 2009;77(6):1677–706. doi: 10.1111/j.1467-6494.2009.00597.x. [DOI] [PubMed] [Google Scholar]
- 20.Hughes M, Kiecolt KJ, Keith VM. How Racial Identity Moderates the Impact of Financial Stress on Mental Health among African Americans. Society and Mental Health. 2014;4(1):38–54. [Google Scholar]
- 21.Surveillance, Epidemiology, and End Results (SEER) Program. ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2013 Sub (1973–2011) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission.
- 22.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 23.Zabora J, Brintzenhofeszoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Zafar SY, Peppercorn JM, Schrag D, et al. The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist. 2013;18(4):381–390. doi: 10.1634/theoncologist.2012-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shankaran V, Jolly S, Blough D, Ramsey SD. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer: a population-based exploratory analysis. J Clin Oncol. 2012;30(14):1608–14. doi: 10.1200/JCO.2011.37.9511. [DOI] [PubMed] [Google Scholar]
- 26.Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119(20):3710–7. doi: 10.1002/cncr.28262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pajak TF, Laramore GE, Marcial VA, et al. Elapsed treatment days–a critical item for radiotherapy quality control review in head and neck trials: RTOG report. Int J Radiat Oncol Biol Phys. 1991;20:13–20. doi: 10.1016/0360-3016(91)90132-n. [DOI] [PubMed] [Google Scholar]
- 28.Larson NI, Story MT, Nelson MC. Neighborhood environments: disparities in access to healthy foods in the U.S. Am J Prev Med. 2009;36(1):74–81. doi: 10.1016/j.amepre.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Sareen J, Afifi TO, McMillan KA, Asmundson GJ. Relationship between household income and mental disorders: findings from a population-based longitudinal study. Arch Gen Psychiatry. 2011;68(4):419–27. doi: 10.1001/archgenpsychiatry.2011.15. [DOI] [PubMed] [Google Scholar]
- 30.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5(8):466–75. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 31.Cohen L, Cole SW, Sood AK, et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PLoS ONE. 2012;7(8):e42324. doi: 10.1371/journal.pone.0042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peairs KS, Barone BB, Snyder CF, et al. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol. 2011;29(1):40–6. doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dupuis-Blanchard S, Neufeld A, Strang VR. The significance of social engagement in relocated older adults. Qual Health Res. 2009;19(9):1186–95. doi: 10.1177/1049732309343956. [DOI] [PubMed] [Google Scholar]
- 34.Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol. 2006;24(7):1105–11. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- 35.Mendes de leon CF, Glass TA, Beckett LA, Seeman TE, Evans DA, Berkman LF. Social networks and disability transitions across eight intervals of yearly data in the New Haven EPESE. J Gerontol B Psychol Sci Soc Sci. 1999;54(3):S162–72. doi: 10.1093/geronb/54b.3.s162. [DOI] [PubMed] [Google Scholar]
- 36.Haley WE. Family caregivers of elderly patients with cancer: understanding and minimizing the burden of care. J Support Oncol. 2003;1(4 Suppl 2):25–9. [PubMed] [Google Scholar]
- 37.Ell K, Nishimoto R, Mediansky L, Mantell J, Hamovitch M. Social relations, social support and survival among patients with cancer. J Psychosom Res. 1992;36(6):531–41. doi: 10.1016/0022-3999(92)90038-4. [DOI] [PubMed] [Google Scholar]
- 38.American Cancer Society. [Accessed 2 Feb 2015];Colorectal Cancer Screening—Insurance Coverage. http://www.cancer.org/cancer/colonandrectumcancer/moreinformation/colonandrectumcancerearlydetection/colorectal-cancer-early-detection-screening-coverage-laws.
- 39.American College of Surgeons Commission on Cancer. Cancer Program Standards 2012: Ensuring Patient-Centered Care. Available: https://www.facs.org/quality%20programs/cancer/coc/standards.
- 40.Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120:2946–2954. doi: 10.1002/cncr.28750. [DOI] [PubMed] [Google Scholar]
- 41.Tucker-Seeley RD, Li Y, Subramanian SV, Sorensen G. Financial hardship and mortality among older adults using the 1996–2004 Health and Retirement Study. Ann Epidemiol. 2009;19(12):850–7. doi: 10.1016/j.annepidem.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosengren A, Hawken S, Ounpuu S, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):953–62. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 43.Ferrie JE, Martikainen P, Shipley MJ, Marmot MG. Self-reported economic difficulties and coronary events in men: evidence from the Whitehall II study. Int J Epidemiol. 2005;34(3):640–8. doi: 10.1093/ije/dyi063. [DOI] [PubMed] [Google Scholar]
- 44.Georgiades A, Janszky I, Blom M, László KD, Ahnve S. Financial strain predicts recurrent events among women with coronary artery disease. Int J Cardiol. 2009;135(2):175–83. doi: 10.1016/j.ijcard.2008.03.093. [DOI] [PubMed] [Google Scholar]
- 45.Russ TC, Stamatakis E, Hamer M, Starr JM, Kivimäki M, Batty GD. Association between psychological distress and mortality: individual participant pooled analysis of 10 prospective cohort studies. BMJ. 2012;345:e4933. doi: 10.1136/bmj.e4933. [DOI] [PMC free article] [PubMed] [Google Scholar]