Abstract
Objective
To determine which factors are associated with higher urethroplasty procedural costs and whether they have been increasing or decreasing over time. Identification of determinants of extreme costs may help reduce cost while maintaining quality.
Materials and Methods
We conducted a retrospective analysis using the 2001–2010 Healthcare Cost and Utilization Project - Nationwide Inpatient Sample (HCUP-NIS). The HCUP-NIS captures hospital charges which we converted to cost using the HCUP Cost-to-Charge Ratio. Log cost linear regression with sensitivity analysis was used to determine variables associated with increased costs. Extreme cost was defined as the top 20th percentile of expenditure, analyzed with logistic regression and expressed as Odds Ratios (OR).
Results
A total of 2298 urethroplasties were recorded in NIS over the study period. The median (interquartile range) calculated costs was $7321 ($5677–$10000). Patients with multiple comorbid conditions were associated with extreme costs (OR 1.56 95% CI 1.19–2.04, p=0.02) compared to patients with no comorbid disease. Inpatient complications raised the odds of extreme costs OR 3.2 CI 2.14–4.75, p<0.001). Graft urethroplasties were associated with extreme costs (OR 1.78 95% CI 1.2–2.64, p=0.005). Variation in patient age, race, hospital region, bed size, teaching status, payer type, and volume of urethroplasty cases were not associated with extremes of cost.
Conclusion
Cost variation for perioperative inpatient urethroplasty procedures is dependent on preoperative patient comorbidities, postoperative complications and surgical complexity related to graft usage. Procedural cost and cost variation are critical for understanding which aspects of care have the greatest impact on cost.
Keywords: surgical cost, cost effectiveness, urethroplasty, utilization
Introduction
Urethral stricture disease affects roughly 5,000 new patients per year. [1] Common etiologies of urethral strictures include traumatic urethral injury, infections of the genitourinary tract, and/or prior lower urinary tract instrumentation. The clinical implications of stricture disease include lower urinary tract symptoms, pain, urinary infections and ejaculatory dysfunction. [2]
Urethral stricture disease may be managed with urethral dilation, urethrotomy, urinary diversion or urethroplasty. [3] Urethroplasty is considered the gold standard treatment for urethral stricture with high success rates. [4] Urethroplasty has also been shown to be a cost-effective management of urethral stricture disease. [5] Several studies have demonstrated that urethroplasty is cost-effective for long strictures, recurrent strictures following internal urethrotomy, and virgin bulbar strictures. [5–7] Increasing attention to the development of a high value low cost healthcare system is a priority of United States (U.S.) policy makers as projections of healthcare costs in the U.S. are shown to be unsustainable. [8] Attention on healthcare cost reduction has been a major focus of policy efforts. [9] Cost-transparency is an important first step in targeting cost-containment efforts.
In surgical subspecialties, procedural costs and surgical outcomes are under increased scrutiny.
Efforts to minimize cost and maximize quality care have led to the development of quality reporting clearinghouses such as the NIS and NSQIP (National Surgical Quality Improvement Program). [10] Such programs allow for critical appraisal of healthcare delivery with a focus on optimizing quality at reduced cost. Recently the American Urological Association (AUA) announced its quality registry termed AQUA whose focus will be quality improvement in prostate cancer management. [11] Utilizing this NIS data, we can critically evaluate the optimal drivers of cost associated with inpatient surgical procedures.
There remains a paucity of literature examining national urethroplasty trends and outcomes with most published data limited to single institutional series. [12] Furthermore, data on national variations in urethroplasty cost is lacking. Our objective is to examine this variation in urethroplasty cost and identify predictors of highest cost for urethroplasties admitted to U.S. hospitals. We hypothesize urethroplasty associated with extremes of cost is related to patient comorbidities and surgical complexity.
Methods
Data Source
We identified men in the National Inpatient Sample (NIS) database who underwent urethroplasty surgery between 2001 and 2010. The NIS is a database that captures 20% of hospital admissions within the U.S. Details on the methods of data capture and variance in NIS have been previously published. [13]
Inclusion and Exclusion Criteria
Male patients were included if they had both an International Classification of Disease (ICD-9) diagnosis code of urethral stricture disease and an ICD-9 procedural code for urethroplasty indicating that a urethroplasty was performed. The ICD-9 diagnosis codes utilized for urethral stricture were 598, 598.0, 598.01, 598.1, 598.2, 598.8, and 598.9. ICD-9 procedural codes for urethroplasty included 58.4 (repair of urethra), 58.42 (closure of urethrostomy), 58.44 (reanastomosis of urethra), 58.45 (repair of hypospadias), 58.46 (reconstruction of urethra), 58.47 (urethral meatoplasty), and 58.49 (other repair of urethra). Two separate urologists (CRH and BNB) individually evaluated all codings to ensure proper assignment of procedures.
We excluded patients who underwent a urethral dilation, urethral fistula repair, and those with an additional major surgical procedure(s) such as cystectomy. Patients were categorized based upon the type of urethroplasty performed: buccal mucosa grafting (ICD-9 procedural codes 7.49, 27.99, and 27.56), other graft or flap urethroplasty (83.43, 83.82, 86.63, 86.66, 86.69, 86.70, 86.71, 86.72, 86.74, and 86.91), or lack of graft or flap procedural codes i.e. excision and primary anastomosis [13]. If a procedural code for grafting was not utilized, then procedures were categorized as EPA procedures.
Predictor Variables
NIS captures the total charges rendered for perioperative and inpatient hospital admission. This does not reflect how much hospital services actually cost or the specific amounts that hospitals received in payment. Such costs represent the actual expenses incurred during a hospital admission ie. supplies and utility costs. The NIS charges can be converted to dollar amount using the HCUP Cost-to-Charge Ratio based on hospital accounting reports from the Centers for Medicare & Medicaid Services. [14] This report contains hospital-specific cost-to-charge ratios based upon all-payer inpatient costs for NIS participating hospitals. To obtain cost estimates, we multiplied total charges with the appropriate cost-to-charge ratio.
We evaluated the following patient demographic characteristics: age (18–45, 45–65, and >65), race (Caucasian, African American, other), household income quartile (extrapolated from a patient’s ZIP code), number of comorbidities (0, 1, 2, 3 or more), and type of comorbidity (e.g. diabetes, hypertension, obesity). We assessed the following hospital characteristics: hospital location (rural or urban), region of the hospital (Northeast, Midwest, South, West), hospital bed size (small, medium, large), and teaching status of the hospital (yes/no). We also examined payer status (Medicaid, private insurer, self-pay, or Medicare), urethroplasty volume of the surgeon (1, 2–9, or greater than 10 per year), the year the urethroplasty was performed, type of urethroplasty procedure, presence and type of a perioperative complication(s), and length of hospital stay. Demographic definitions were all congruent with the current descriptions of data elements utilized by HCUP-NIS.
Outcome Variables
The primary outcome variable was urethroplasty cost, specifically variables associated with the top 20th percentile in urethroplasty cost, which was defined as Extreme Cost. Extreme cost was set at the top 20th percentile of cost a priori. As a post-hoc sensitivity analysis, we compared the outcome of the top 20th percentile and 10th percentile of cost and demonstrated no differences.
Statistical Analysis
Data analysis was performed using SAS (Version 9.2, SAS Institute Inc, Cary NC). Relative median cost (RMC) was used to compare differences in increased cost and Odds Ratios (OR) were used to compare extremes of cost. A log cost linear regression model was used to assess for variables associated with more costly urethroplasties. Multivariate analysis controlling for patient age, race, and year of urethroplasty was performed. Extremes of cost was defined as the top 20th percentiles of all reported urethroplasties and were analyzed using multiple logistic regression with the same predictors as for log cost. A comparison was then performed between extreme of cost and increased cost to determine which variables were major drivers of cost.
Results
Predictors of High Cost Urethroplasty
A total of 2298 male urethroplasties were reported in NIS between 2001–2010 representing an estimated total 12,389 (95% CI 8750–16029) procedures performed in the U.S. The median charges (inter-quartile range) were $19866 ($14346–$29382) with calculated costs of $7321 ($5677–$10000). The mean cost of urethroplasty did not vary by year over time. (p=0.58) The median increase in U.S. dollars per year of urethroplasty was $616.
Cost of urethroplasty was higher in patients age 45–65 years relative to patients age 18–45 years (RMC 1.1, 95% CI 1.0–1.1, p=0.03). Conversely, patients older than 65 years were not more costly (RMC 1.0, 95% CI .95–1.10, p=.74). Patient race, patient income, and payor type were not independently associated with increased cost on univariate analysis. (Table 1)
Table 1.
Univariate Analysis of Mean Costs Stratified by Patient, Operative, Hospital and Comorbid/Complication Characteristics
| # of pts | % of pts | Mean Cost $ | Univariate Relative Median Cost (CI) | p-value | ||
|---|---|---|---|---|---|---|
| All Patients | 12389 | 8569 | ||||
|
| ||||||
| Patient characteristics | ||||||
|
| ||||||
| Age | ||||||
| 18–45 | 6070 | 49.0 | 8355 | reference | – | |
| 45–65 | 4595 | 37.1 | 8980 | 1.06 (1.01–1.11) | 0.03 | |
| 65+ | 1725 | 13.9 | 8439 | 1.01 (.95–1.08) | 0.74 | |
| Race | ||||||
| White | 6712 | 70.3 | 8498 | reference | – | |
| Black | 1406 | 14.7 | 8423 | 0.98 (.82–1.17) | 0.84 | |
| Other | 1424 | 14.9 | 8785 | 1.02 (.94–1.12) | 0.6 | |
| Payor | ||||||
| Medicaid | 937 | 7.6 | 8852 | 1.05 (.93–1.18) | 0.42 | |
| No charge | 101 | 0.8 | 11816 | 1.13 (.75–1.71) | 0.56 | |
| Other | 953 | 7.7 | 8760 | 1.04 (.94–1.14) | 0.44 | |
| Private insurance | 7610 | 61.5 | 8552 | 1.00 (.92–1.08) | 0.95 | |
| Self Pay | 578 | 4.7 | 8370 | .95 (.78–1.16) | 0.62 | |
| Medicare | 2196 | 17.7 | 8513 | reference | – | |
| Median Income by Zipcode | ||||||
| Quartile 1 | 2383 | 23.3 | 8505 | reference | – | |
| Quartile 2 | 2328 | 22.7 | 8499 | 1.01 (.94–1.09) | 0.79 | |
| Quartile 3 | 2594 | 25.3 | 8920 | 1.04 (.95–1.15) | 0.39 | |
| Quartile 4 | 2930 | 28.6 | 9663 | 1.09 (.9–1.33) | 0.36 | |
| Number of Comorbidities | ||||||
| 0 | 7167 | 57.8 | 8107 | reference | – | |
| 1 | 3068 | 24.8 | 8988 | 1.12 (1.07–1.17) | <.0001 | |
| 2 | 1578 | 12.7 | 9442 | 1.12 (1.04–1.20) | 0.003 | |
| 3 or more | 577 | 4.7 | 10263 | 1.27 (1.12–1.43) | 0.0001 | |
|
| ||||||
| Operative characteristics | ||||||
|
| ||||||
| Graft Use | ||||||
| No graft | 9930 | 80.2 | 8276 | reference | – | |
| Buccal graft | 1824 | 14.7 | 10015 | 1.2 (1.14–1.30) | <.0001 | |
| Other graft | 635 | 5.1 | 9466 | 1.12 (1.02–1.24) | 0.02 | |
| Hospital teaching status | ||||||
| Teaching | 2394 | 68.5 | 7512 | 1.13 (1.02–1.24) | 0.02 | |
| Nonteaching | 1099 | 31.5 | 6735 | reference | – | |
| Annual hospital urethroplasty volume | ||||||
| 1 | 2757 | 22.3 | 6756 | reference | – | |
| 2–9 | 4212 | 34.0 | 8113 | 1.22 (1.12–1.34) | <.0001 | |
| 10+ | 5420 | 43.7 | 9813 | 1.45 (1.17–1.83) | 0.001 | |
|
| ||||||
| Hospital characteristics | ||||||
|
| ||||||
| Hospital location | ||||||
| rural | 377 | 3.1 | 8730 | 1.01 (.81–1.25) | 0.95 | |
| urban | 11880 | 96.9 | 8604 | reference | – | |
| Hospital region | ||||||
| Northeast | 679 | 19.4 | 7168 | .86 (.73–1.02) | 0.09 | |
| Midwest | 719 | 20.5 | 7040 | .92 (.81–1.04) | 0.17 | |
| South | 1214 | 34.6 | 7015 | .90 (.79–1.01) | 0.08 | |
| West | 895 | 25.5 | 7893 | reference | – | |
| Hospital bedsize | ||||||
| Small | 537 | 4.4 | 7686 | reference | – | |
| Medium | 1659 | 13.5 | 7444 | .97 (.83–1.14) | 0.69 | |
| Large | 10061 | 82.1 | 8835 | 1.12 (.94–1.33) | 0.2 | |
| Length of Hospital Stay | ||||||
| 0 | 117 | 0.9 | 4966 | .75 (.59–.96) | 0.02 | |
| 1 | 3929 | 31.7 | 6321 | reference | – | |
| 2 | 3579 | 28.9 | 7899 | 1.24 (1.15–1.34) | <.0001 | |
| 3 | 2177 | 17.6 | 8121 | 1.32 (1.18–1.46) | <.0001 | |
| 4 | 1087 | 8.8 | 9796 | 1.58 (1.32–1.89) | <.0001 | |
| 5+ | 1499 | 12.1 | 15744 | 2.33 (1.70–3.20) | <.0001 | |
|
| ||||||
| Comorbid and Complications characteristics | ||||||
|
| ||||||
| Number of Comorbidities | ||||||
| 0 | 7167 | 57.8 | 8107 | reference | – | |
| 1 | 3068 | 24.8 | 8988 | 1.12 (1.07–1.17) | <.0001 | |
| 2 | 1578 | 12.7 | 9442 | 1.12 (1.04–1.20) | 0.003 | |
| 3 or more | 577 | 4.7 | 10263 | 1.27 (1.12–1.43) | 0.0001 | |
| Comorbidities Type | ||||||
| Hypertension | 3236 | 26.1 | 9111 | 1.08 (1.03–1.13) | 0.0009 | |
| Diabetes | 1205 | 9.7 | 9174 | 1.04 (.97–1.12) | 0.27 | |
| Diabetes Chronic | 99 | 0.8 | 10785 | 1.18 (.91–1.53) | 0.22 | |
| Chronic Lung Disease | 871 | 7.0 | 8563 | 1.02 (.95–1.10) | 0.52 | |
| Obesity | 885 | 7.1 | 10303 | 1.20 (1.10–1.32) | 0.0001 | |
| Depression | 410 | 3.3 | 9097 | 1.09 (.97–1.22) | 0.15 | |
| Renal failure | 293 | 2.4 | 12234 | 1.41 (1.22–1.64) | <.0001 | |
| Alcohol abuse | 175 | 1.4 | 8972 | 1.08 (.91–1.28) | 0.41 | |
| Arthritis | 169 | 1.4 | 10961 | 1.25 (.99–1.58) | 0.06 | |
| Psychiatric illnesses | 162 | 1.3 | 10015 | 1.17 (.97–1.42) | 0.09 | |
| Liver disease | 156 | 1.3 | 8918 | 1.03 (.88–1.21) | 0.7 | |
| Drug use | 139 | 1.1 | 9612 | 1.10 (.92–1.32) | 0.31 | |
| Perivascular disease | 84 | 0.7 | 11919 | 1.21 (.85–1.72) | 0.29 | |
| Coagulopathy | 78 | 0.6 | 10882 | 1.15 (.83–1.62) | 0.39 | |
| Valvular disease | 58 | 0.5 | 9091 | 1.12 (.92–1.36) | 0.27 | |
| Congestive heart failure | 34 | 0.3 | 12594 | 1.56 (1.19–2.05) | 0.002 | |
| Lymphoma | 29 | 0.2 | 8769 | 1.04 (.74–1.47) | 0.82 | |
| AIDS | 11 | 0.1 | 5209 | .59 (.27–1.31) | 0.2 | |
| Pulmonary/Circulatory disease | 10 | 0.1 | 16613 | 2.05 (1.18–3.56) | 0.01 | |
| Weight loss | 10 | 0.1 | 8984 | 1.15 (.78–1.70) | 0.47 | |
| 12380 | 99.9 | 8599 | ||||
| Complication | 829 | 6.7 | 11550 | 1.29 (1.16–1.42) | <.0001 | |
| 11560 | 93.3 | 8391 | ||||
| Complication Type | ||||||
| Genitourinary | 364 | 2.9 | 12082 | 1.29 (1.12–1.48) | 0.0003 | |
| Surgical | 156 | 1.3 | 9816 | 1.19 (1.02–1.38) | 0.03 | |
| Wound | 143 | 1.2 | 12281 | 1.46 (1.21–1.75) | <.0001 | |
| Cardiovascular | 88 | 0.7 | 17678 | 1.69 (1.24–2.31) | 0.001 | |
| Respiratory | 77 | 0.6 | 19645 | 1.85 (1.18–2.88) | 0.007 | |
| Gastrointestinal | 46 | 0.4 | 7923 | .97 (.74–1.26) | 0.82 | |
| Neurologic | 30 | 0.2 | 6871 | .89 (0.70–1.12) | 0.31 | |
| Medical | 16 | 0.1 | 6224 | .83 (.75–.92) | 0.0003 | |
| Musculoskeletal | 15 | 0.1 | 34118 | 4.5 (4.05–5.10) | <.0001 | |
On multivariate analysis after controlling for age, race, and year of urethroplasty performed, there was a significantly higher cost of urethroplasty in higher volume urethroplasty centers. Compared to hospitals performing 1 urethroplasty/year, hospitals performing 2–9 and ≥10 urethroplasty/year were most costly (RMC 1.2, 95% CI 1.1–1.3, p=0.002; RMC 1.5, 95% CI 1.2–1.8, p=0.01, respectively). Use of buccal or other graft was also associated with increased cost (RMC 1.2, 95% CI 1.1–1.3, p<0.001; RMC 1.2, 95% CI 1.1–1.3, p=0.003, respectively). There was no significant difference in urethroplasty cost by hospital setting, hospital region, teaching hospital status, or hospital size. (Table 2) The presence of any medical comorobidity was associated with increased urethroplasty cost (RMC 1.1, 95% CI 1.07–1.2), with increasing cost with increasing numbers of comorbities (RMC 1.1, 95% CI 1.05–1.24, RMC 1.2, 95% CI 1.03–1.33, all p<0.03 for patients with 1, 2, and 3 or more comorobidites, respectively). Specific cormorbidites that increase urethroplasty cost include hypertension (RMC 1.1, 95% CI1.0–1.1, p=0.02), obesity (RMC 1.1, 95% CI 1.1–1.2, p=001), renal failure (RMC 1.4, 95% CI 1.1–1.6, p=0.002), psychiatric illness (RMC 1.3, 95% CI 1.0–1.6, p=0.04), and congestive heart failure (RMC 1.6, 95% CI 1.1–2.2, p=0.01). Diabetes, chronic lung disease, depression, alcohol abuse, arthritis, liver disease, drug use, perivascular disease, coagulopathy, valvular disease, lymphoma, aids, circulatory disease, and weight loss were not associated with increased cost on multivariate analysis. (Table 2)
Table 2.
Multivariate Analysis of Variables Associated with Increased Cost Urethroplasty after adjusting for age, race and year
| Number of patients | Percent of patients | Mean Cost ($) | Relative Median Cost (CI) | p-value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Graft Use | ||||||
|
| ||||||
| No graft | 9930 | 80.2 | 8276 | – | – | |
| Buccal graft | 1824 | 14.7 | 10015 | 1.21 (1.13–1.29) | <.0001 | |
| Other graft | 635 | 5.1 | 9466 | 1.18 (1.06–1.31) | 0.003 | |
|
| ||||||
| Annual hospital urethroplasty volume | ||||||
|
| ||||||
| 1 | 2757 | 22.3 | 6756 | – | – | |
| 2–9 | 4212 | 34.0 | 8113 | 1.20 (1.07–1.34) | 0.002 | |
| 10+ | 5420 | 43.7 | 9813 | 1.32 (1.07–1.64) | 0.01 | |
|
| ||||||
| Length of Hospital Stay | ||||||
|
| ||||||
| 0 | 117 | 0.9 | 4966 | .71(.57–.88) | 0.002 | |
| 1 | 3929 | 31.7 | 6321 | – | – | |
| 2 | 3579 | 28.9 | 7899 | 1.31 (1.20–1.43) | <.0001 | |
| 3 | 2177 | 17.6 | 8121 | 1.44 (1.28–1.63) | <.0001 | |
| 4 | 1087 | 8.8 | 9796 | 1.78 (1.47–2.16) | <.0001 | |
| 5+ | 1499 | 12.1 | 15744 | 2.61 (1.86–3.66) | <.0001 | |
|
| ||||||
| Number of Comorbidities | ||||||
|
| ||||||
| 0 | 7167 | 57.8 | 8107 | – | – | |
| 1 | 3068 | 24.8 | 8988 | 1.13 (1.07–1.20) | <.0001 | |
| 2 | 1578 | 12.7 | 9442 | 1.14 (1.05–1.24) | 0.002 | |
| 3 or more | 577 | 4.7 | 10263 | 1.17 (1.03–1.33) | 0.02 | |
|
| ||||||
| Comorbidities Type | ||||||
|
| ||||||
| Hypertension | 3236 | 26.1 | 9111 | 1.07 (1.01–1.13) | 0.02 | |
| Obesity | 885 | 7.1 | 10303 | 1.13 (1.05–1.22) | 0.001 | |
| Renal failure | 293 | 2.4 | 12234 | 1.35 (1.12–1.63) | 0.002 | |
| Psychiatric illnesses | 162 | 1.3 | 10015 | 1.29 (1.02–1.64) | 0.04 | |
| Congestive heart failure | 34 | 0.3 | 12594 | 1.56 (1.10–2.21) | 0.01 | |
|
| ||||||
| Complication | ||||||
|
| ||||||
| 829 | 6.7 | 11550 | 1.29 (1.15–1.45) | <.0001 | ||
|
| ||||||
| Complication Type | ||||||
|
| ||||||
| Genitourinary | 364 | 2.9 | 12082 | 1.33 (1.11–1.61) | 0.003 | |
| Surgical | 156 | 1.3 | 9816 | 1.20 (1.00–1.43) | 0.05 | |
| Wound | 143 | 1.2 | 12281 | 1.51 (1.26–1.81) | <.0001 | |
| Cardiovascular | 88 | 0.7 | 17678 | 1.68 (1.09–2.60) | 0.02 | |
| Respiratory | 77 | 0.6 | 19645 | 2.0 (1.21–3.38) | 0.007 | |
| Gastrointestinal | 46 | 0.4 | 7923 | 1.02 (.82–1.27) | 0.88 | |
| Neurologic | 30 | 0.2 | 6871 | .85 (.67–1.08) | 0.19 | |
| Musculoskeletal | 15 | 0.1 | 34118 | – | – | |
The presence of a complication was associated with higher cost urethroplasty (RMC 1.3, 95% CI 1.2–1.5, p<0.001). Among complications, genitourinary (RMC 1.3, 95% CI 1.1–1.6, p=0.003), surgical (RMC 1.2, 95% CI 1.0–1.4, p=0.05), wound (RMC 1.5, 95% CI 1.3–1.8, p<0.001), cardiovascular (RMC 1.7, 95% CI 1.1–2.6, p=0.02), and respiratory complications (RMC 2.0, 95% CI 1.2–3.4, p=0.007) were more costly. Gastrointestinal, neurologic, and medical complications were not associated with a significant increase in cost. (Table 2)
Extremes of Cost Analysis
Multiple factors were associated with extreme cost which we categorized as the top 20th percentile of costs. A rural hospital setting had more than three-fold higher odds of having extreme cost compared to an urban hospital setting (OR 3.5, 95% CI 1.3–9.3, p=0.01). Other graft use was associated with highest cost urethroplasty (OR 1.8, 95% CI 1.2–2.6, p=0.005) however buccal graft was not associated with extreme cost (OR 1.3, 95% CI 0.9–1.9) (Table 3) Patient factors of age, race, and payer type as well as hospital region, size, teaching status, and volume of urethroplasty cases were not associated with extremes of cost.
Table 3.
Multivariate Analysis of Variables Associated with Extreme Cost (top 20%) Urethroplasty after adjusting for age, race and year
| Multivariate OR of Extreme Cost (CI) | p-value | |
|---|---|---|
|
| ||
| Graft Use | 0.005 | |
|
| ||
| No graft | – | |
| Buccal graft | 1.30 (.88–1.93) | |
| Other graft | 1.78 (1.20–2.64) | |
|
| ||
| Hospital location | 0.01 | |
|
| ||
| rural vs. urban | 3.50 (1.31–9.31) | |
|
| ||
| Length of Hospital Stay | <.0001 | |
|
| ||
| 0 | – | – |
| 1 | – | |
| 2 | 5.45 (2.40–12.40) | |
| 3 | 5.22 (1.71–15.94) | |
| 4 | 14.29 (4.15–49.25) | |
| 5+ | 61.13 (15.10–247.54) | |
|
| ||
| Number of Comorbidities | 0.02 | |
|
| ||
| 0 | – | |
| 1 | 1.56 (1.19–2.04) | |
| 2 | 1.32 (.85–2.03) | |
| 3 or more | 1.56 (.78–3.12) | |
|
| ||
| Complications | ||
|
| ||
| yes vs. no | 3.19 (2.14–4.75) | <.0001 |
|
| ||
| Complication Type | ||
|
| ||
| Genitourinary | 3.03 (1.72–5.32) | 0.0001 |
| Surgical | 3.85 (1.84–8.07) | 0.0004 |
| Wound | 8.66 (3.14–23.94) | <.0001 |
| Respiratory | 8.44 (2.06–34.60) | 0.003 |
In evaluating comorbidities, univariate analysis showed that patients with a single comorbidity had increased odds of highest cost urethroplasties compared to patients with no comorbid disease (OR 1.4, 95% CI 1.1–1.7, p<0.001). The odds increased even further in patients with three or more comorbidities (OR 2.2, 95%1.3–3.8, p<0.001). On multivariate analysis findings were consistent that patients with one comorbid disease had increased odds of highest cost urethroplasties compared to patients with no comorbid disease (OR 1.6, 95% CI 1.2–2.0, p=0.02). However, there was no further increase in extreme cost in patients with 3 or more comorbidities (OR 1.6, 95% CI .8–3.12). Patients with obesity (OR 1.8, 95% CI 1.2–2.5, p=0.001) and renal failure (OR 2.3, 95% CI 1.12–4.6, p=0.02) were most likely to have extremes of cost compared to other comorbidities on univariate analysis, but on multivariate analysis there was no single comorbidity found to be associated with extremes of cost. (Table 3, Fig. 1) Increasing length of inpatient hospital stay was significantly associated with extreme cost. Greater than five days was associated with extreme cost (OR 61.1, CI 15.1–247.5, p<0.001). (Table 3, Fig. 1)
Fig. 1.
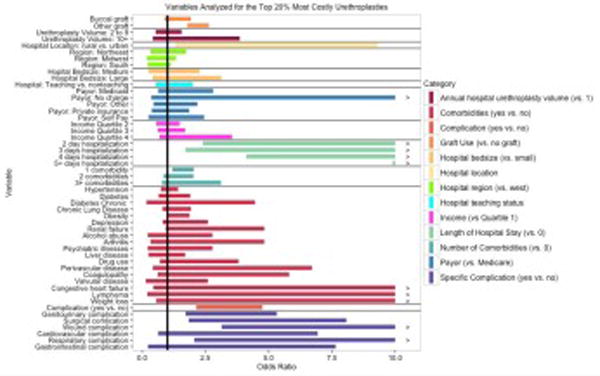
Multivariate analysis of Variables Associated with Extreme Cost (top 20%) Urethroplasty after adjusting for age, race and year
On multivariate analysis, inpatient complications increased the odds of high hospital costs (OR 3.1, 95% CI 1.7–5.3, p<0.001), with wound (OR 8.6, 95% CI 3.1–23.9, p<0.001) and respiratory (OR 8.4, 95%CI 2.1–34.6, p=0.003) complications contributing to the highest odds of cost. (Table 3, Fig. 1)
When we examined the top 10th percentile of urethroplasty costs as compared with the top 20th percentile we found similar extremes of cost with graft usage, length of hospital stay, and presence of postoperative complications. Interestingly, cardiovascular complications were drivers of cost in the top 10th percentile (OR 4.1, 95% CI 1.01–16.88, p=0.05) whereas this was not significant in the 20th percentile. (OR 2.08, 95% CI 0.63–6.94, p=0.23)
Discussion
To our knowledge this is the first report on cost variation in a national sample of urethroplasties. Two prior studies have tackled cost evaluation from the perspective of optimum surveillance strategies for the postoperative management of urethral strictures. [15, 16] Here we analyze and begin to understand cost variations, specifically which factors are most strongly associated with increased urethroplasty cost among admitted patients, as this has important implications for healthcare resource utilization.
We demonstrate that patient comorbidities and postoperative complications are strong predictors of a more costly urethroplasty. This highlights the importance of optimizing patient comorbidities preoperatively in an effort to decrease postoperative complications and cost. We found that postoperative complications were major drivers of extereme cost urethroplasties. Supporting efforts to optimize patients for surgery in order to maximize outcomes and thus minimze complications is warranted.
Buccal graft and other grafting use was also associated with a higher cost urethroplasty and other graft use was associated with extreme cost urethroplasty. This is reflective of more complex urethral stricture disease that drives up procedural cost, complication rate, and length of hospital stay. We hypothesize that urethroplasty in patients over 65 were not more costly due to selection bias toward simpler urethral reconstructive techniques such as urethrotomy or urethral dilations which were excluded in this analysis and have been shown to be the most common procedure done for Medicare beneficiaries with urethral stricture disease over 65 years of age. [17]
We hypothesize that hospitals with an annual urethroplasty volume of two or more are associated with higher cost urethroplasty as they are more likely to perform complex urethral surgery and are more likely to utilize graft/flap procedures compared to hospitalize performing only one urethroplasty per year. Also, hospitals coded as performing one urethroplasty a year may suffer from accidental mis-coding.
When examining the extremes of cost, a rural hospital setting was more than three times the cost compared to an urban hospital setting. We hypothesize that this is the result of rural settings having less specialty trained urethral reconstructive surgeons and lower volume of complex urethral surgical cases compared to larger referral center which are usually located in urban or suburban settings. [18] Studies have consistently shown increased length of stay and hospital costs for complex procedures performed in rural settings.[19] Interestingly, hospital bed size e.g. a smaller number of beds was not found to be predictive of a urethroplasty of higher cost or extreme cost.
Despite a trend toward more costly urethroplasty being performed at teaching hospitals, multivariate analysis demonstrated no significant differences when compared to cost of urethroplasty at nonteaching institutions. This is congruent with current data suggesting that increased trainee oversight amounts to equal cost, quality and patient satisfaction at teaching institutions. [20] We hypothesize that despite more complex urethroplasties being performed at teaching hospitals, the cost may be offset by attending oversight rendering improved quality and/or patient satisfaction.
Cost reduction efforts are only part of the solution in optimizing healthcare delivery. Other policy efforts include increasing value of healthcare by improving the metrics of healthcare outcomes and patient satisfaction. Quality indicators of urethral stricture disease have previously been explored.[21] A shift in focus on cost-conscious, high value healthcare is a priority for U.S. policy makers as current healthcare expenditures are unsustainable. [22] Identifying which aspects of perioperative care and the impact of presurgical patient optimization will be criticial for cost reduction. Current data suggests that preoperative intervention strategies can reduce postoperative complications and therefore surgery-associated cost. In a randomized trial of multimodal preoperative patient optimization preceeding colorectal surgery, Gatt et al demonstrated less postoperative complications and shorter hospital length of stay. [23] This study identifies which aspects of urethroplasty are most costly in effort to improve cost-transparency – an important first step in cost-containment.
There are several study limitations within this study. First, NIS data is cross-sectional and based on procedural coding and thus does not include information on urethral stricture disease etiology. It is well known that stricture etiology such as lichen sclerosis, reoperative strictures, or longer stricture length are preoperative factors associated with complex flap/graft urethroplasty and increased complication rates and hospital stay.[24–27] Second, the NIS data is limited to the immediate inpatient stay after urethroplasty. Outpatient or short-stay related procedures may not be captured by this dataset depending on coding differences across hospitals. As a result, there is selection bias toward morbid patients who require admission following urethroplasty. Longer-term complications, types of complications, readmissions, and/or urethroplasty outcomes are also not accounted for such that NIS data is lacking granularity. However, long-term success rates of urethroplasty are high [12, 28] and delayed complications from urethroplasty are rare. [29] Therefore, the downstream cost of stricture recurrence is unlikely to be a major driver of total urethroplasty cost. Third, we subcategorized anastomotic urethroplasty if a buccal grafting procedural code was not utilized. This may falsely overestimate the number of anastomotic procedures rendered nationally. Lastly, only inpatient complications during the initial perioperative hospital admission period were captured thus missing complications from outpatient surgical centers and some 23-hour stay patients might not be included based on coding differences across hospitals. Similarly, readmissions following urethroplasty were not captured. Understanding which patients have such long term complicating features would allow for a more sensitive subgroup analysis to determine drivers of cost.
Conclusion
We demonstrate that cost variation for perioperative inpatient urethroplasty procedures is dependent on preoperative patient comorbidities, postoperative complications and usage of grafting. Identification of extreme cost variation has policy implications to reduce healthcare costs meanwhile maintaining quality. Further evaluation of long-term outcomes of outpatient urethroplasty is needed to fully understand predictors of extreme cost given that the majority of urethroplasties are performed on an outpatient basis.
Acknowledgments
None
Source of Funding
None
Abbreviations
- HCUP-NIS
Healthcare Cost and Utilization Project - Nationwide Inpatient Sample
- EPA
excision and primary anastomosis
- IQR
inter-quartile range
- CI
confidence interval
- U.S.
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Santucci RA, Joyce GF, Wise M. Male urethral stricture disease. The Journal of urology. 2007;177(5):1667–74. doi: 10.1016/j.juro.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 2.Lubahn JD, Zhao LC, Scott JF, Hudak SJ, Chee J, Terlecki R, et al. Poor quality of life in patients with urethral stricture treated with intermittent self-dilation. The Journal of urology. 2014;191(1):143–7. doi: 10.1016/j.juro.2013.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mundy AR. Management of urethral strictures. Postgrad Med J. 2006;82(970):489–93. doi: 10.1136/pgmj.2005.042945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbagli G, De Angelis M, Romano G, Lazzeri M. Long-term followup of bulbar end-to-end anastomosis: a retrospective analysis of 153 patients in a single center experience. The Journal of urology. 2007;178(6):2470–3. doi: 10.1016/j.juro.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Wright JL, Wessells H, Nathens AB, Hollingworth W. What is the most cost-effective treatment for 1 to 2-cm bulbar urethral strictures: societal approach using decision analysis. Urology. 2006;67(5):889–93. doi: 10.1016/j.urology.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Greenwell TJ, Castle C, Andrich DE, MacDonald JT, Nicol DL, Mundy AR. Repeat urethrotomy and dilation for the treatment of urethral stricture are neither clinically effective nor cost-effective. The Journal of urology. 2004;172(1):275–7. doi: 10.1097/01.ju.0000132156.76403.8f. [DOI] [PubMed] [Google Scholar]
- 7.Rourke KF, Jordan GH. Primary urethral reconstruction: the cost minimized approach to the bulbous urethral stricture. The Journal of urology. 2005;173(4):1206–10. doi: 10.1097/01.ju.0000154971.05286.81. [DOI] [PubMed] [Google Scholar]
- 8.Kronick R, Rousseau D. Is Medicaid sustainable? Spending projections for the program’s second forty years. Health affairs. 2007;26(2):w271–87. doi: 10.1377/hlthaff.26.2.w271. [DOI] [PubMed] [Google Scholar]
- 9.Antos J, Bertko J, Chernew M, Cutler D, Goldman D, McClellan M, et al. Bending the curve: effective steps to address long-term healthcare spending growth. The American journal of managed care. 2009;15(10):676–80. [PubMed] [Google Scholar]
- 10.Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44:251–67. doi: 10.1016/j.yasu.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Association AU. AUA Quality Registry 2014 [8/11/15] Available from: https://www.auanet.org/resources/quality-registry.cfm.
- 12.Blaschko SD, Harris CR, Zaid UB, Gaither T, Chu C, Alwaal A, et al. Trends, Utilization, and Immediate Perioperative Complications of Urethroplasty in the United States: Data From the National Inpatient Sample 2000–2010. Urology. 2015 doi: 10.1016/j.urology.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quality USDoHaHSAfHRa. Calculating National (Nationwide) Inpatient Sample (NIS) Variances Report # 2003-02 2014. [updated December 18, 2014]. Available from: http://www.hcup-us.ahrq.gov/reports/methods/2003_02.pdf.
- 14.Pfuntner A, Wier LM, Steiner C. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD); 2006. Costs for Hospital Stays in the United States, 2010: Statistical Brief #146. [Google Scholar]
- 15.Zaid UB, Hawkins M, Wilson L, Ting J, Harris C, Alwaal A, et al. The Cost of Surveillance After Urethroplasty. Urology. 2015 doi: 10.1016/j.urology.2014.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belsante MJ, Zhao LC, Hudak SJ, Lotan Y, Morey AF. Cost-effectiveness of risk stratified followup after urethral reconstruction: a decision analysis. The Journal of urology. 2013;190(4):1292–7. doi: 10.1016/j.juro.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 17.Anger JT, Buckley JC, Santucci RA, Elliott SP, Saigal CS, Urologic Diseases in America P Trends in stricture management among male Medicare beneficiaries: underuse of urethroplasty? Urology. 2011;77(2):481–5. doi: 10.1016/j.urology.2010.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenblatt RA, Hart LG. Physicians and rural America. West J Med. 2000;173(5):348–51. doi: 10.1136/ewjm.173.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 20.Wachter RM, Katz P, Showstack J, Bindman AB, Goldman L. Reorganizing an academic medical service: impact on cost, quality, patient satisfaction, and education. JAMA. 1998;279(19):1560–5. doi: 10.1001/jama.279.19.1560. [DOI] [PubMed] [Google Scholar]
- 21.Jackson MJ, Chaudhury I, Mangera A, Brett A, Watkin N, Chapple CR, et al. A prospective patient-centred evaluation of urethroplasty for anterior urethral stricture using a validated patient-reported outcome measure. European urology. 2013;64(5):777–82. doi: 10.1016/j.eururo.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Ghildayal NS, Shah RN. Examining quality and efficiency of the U.S. healthcare system. Int J Health Care Qual Assur. 2011;24(5):366–88. doi: 10.1108/09526861111139197. [DOI] [PubMed] [Google Scholar]
- 23.Gatt M, Anderson AD, Reddy BS, Hayward-Sampson P, Tring IC, MacFie J. Randomized clinical trial of multimodal optimization of surgical care in patients undergoing major colonic resection. Br J Surg. 2005;92(11):1354–62. doi: 10.1002/bjs.5187. [DOI] [PubMed] [Google Scholar]
- 24.Warner JN, Malkawi I, Dhradkeh M, Joshi PM, Kulkarni SB, Lazzeri M, et al. A Multi-institutional Evaluation of the Management and Outcomes of Long-segment Urethral Strictures. Urology. 2015 doi: 10.1016/j.urology.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 25.Breyer BN, McAninch JW, Whitson JM, Eisenberg ML, Mehdizadeh JF, Myers JB, et al. Multivariate analysis of risk factors for long-term urethroplasty outcome. The Journal of urology. 2010;183(2):613–7. doi: 10.1016/j.juro.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Kulkarni S, Barbagli G, Kirpekar D, Mirri F, Lazzeri M. Lichen sclerosus of the male genitalia and urethra: surgical options and results in a multicenter international experience with 215 patients. European urology. 2009;55(4):945–54. doi: 10.1016/j.eururo.2008.07.046. [DOI] [PubMed] [Google Scholar]
- 27.Levine LA, Strom KH, Lux MM. Buccal mucosa graft urethroplasty for anterior urethral stricture repair: evaluation of the impact of stricture location and lichen sclerosus on surgical outcome. The Journal of urology. 2007;178(5):2011–5. doi: 10.1016/j.juro.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 28.Levine MA, Kinnaird AS, Rourke KF. Revision urethroplasty success is comparable to primary urethroplasty: a comparative analysis. Urology. 2014;84(4):928–32. doi: 10.1016/j.urology.2014.05.047. quiz 32-3. [DOI] [PubMed] [Google Scholar]
- 29.Granieri MA, Webster GD, Peterson AC. Critical Analysis of Patient-reported Complaints and Complications After Urethroplasty for Bulbar Urethral Stricture Disease. Urology. 2015;85(6):1489–93. doi: 10.1016/j.urology.2015.03.002. [DOI] [PubMed] [Google Scholar]


