Abstract
Objectives
(1) To compare the systemic (non-urologic) symptoms between OAB patients and subjects without OAB; (2) compare the urinary symptoms, quality of life, and psychosocial measures between the two subgroups of OAB patients who had high versus low systemic symptom burden.
Materials and Methods
Patients diagnosed with OAB (n=51) and age-matched individuals without OAB (n=30) were administered the Polysymptomatic, Polysyndromic Questionnaire (PSPS-Q) to assess the numbers and distribution of systemic symptoms across multiple organ systems. Validated instruments were administered to evaluate their urinary symptoms (ICIQ-UI, ICIQ-OAB, OAB-q, USS), quality of life (UDI-6, IIQ-7, OAB-q), and psychosocial difficulties (depression, anxiety, stress, sexual trauma, sleep, fatigue). OAB patients were divided into two subgroups (with and without widespread systemic symptoms) and their responses were compared.
Results
OAB patients reported significantly more systemic (non-urologic) symptoms compared to controls (17.5 ± 12.3 versus 6.4 ± 7.9 symptoms, p<0.001). Differences were observed across multiple organ systems (neurological, cardiopulmonary, gastrointestinal, sexual, musculoskeletal, and gynecological, p<0.05). About a third of OAB patients (31.4%) reported widespread systemic symptoms across multiple organ systems (mean 32.0 symptoms). The presence of widespread systemic symptoms among OAB patients was correlated with worse incontinence/OAB symptoms (ICIQ-UI, OAB-q), poorer quality of life (UDI-6, IIQ-7, OAB-q), and more psychosocial difficulties (depression, anxiety, fatigue, and higher stress, p<0.05).
Conclusions
The increased presence of non-urologic symptoms in OAB suggests an underlying systemic etiology and pathogenetic mechanisms that may contribute to OAB. This study highlights the importance of understanding systemic factors in urologic conditions otherwise thought to be organ-specific.
Introduction
Overactive bladder (OAB) is defined by bladder-centric symptoms, characterized by the presence of urgency, with or without urgency incontinence, usually with frequency and nocturia.1 However, anecdotal observation suggested that many OAB patients also have non-urologic symptoms outside the lower urinary tract. In fact, a few recent studies have begun to examine the relationship between OAB and other systemic (non-urologic) conditions.
Chung et al (2013) showed that individuals with fibromyalgia were more likely to report OAB-like symptoms compared to individuals without fibromyalgia (40% versus 12%, OR=3.4).2 The association between fibromyalgia and the severity of OAB symptoms was highly significant (p<0.0001). Matsumoto et al (2013) showed that about a third of individuals with OAB symptoms (assessed using the OAB-SS questionnaire3) had gastrointestinal symptoms consistent with irritable bowel syndrome as defined using the ROME III criteria.4 The difficulties with these studies were two-fold: 1) they were both population-based community surveys that lacked clinical evaluation to distinguish OAB from IC/BPS (interstitial cystitis/bladder pain syndrome). As we know, IC/BPS symptoms can mimic OAB (both have frequency and urgency symptoms),5 and IC/BPS is known to be associated with fibromyalgia and irritable bowel syndrome.6 2) the symptom score threshold used by the two studies to categorize subjects as having OAB (OAB-SS3 ≥3 out of a total score of 15) was deemed too lax and lacked specificity.
Coyne et al (2009) compared the comorbid conditions between men and women with voiding, storage and post-micturition symptoms (VSPM) with those with no or minimal lower urinary tract symptoms (LUTS).7 The study showed that the VSPM group was significantly associated with arthritis, asthma, chronic anxiety, depression, heart diseases, irritable bowel syndrome, neurological conditions, and sleep disorders compared to individuals with no or minimal LUTS. A follow up study that specifically compared subjects with OAB symptoms versus those with no or minimal OAB symptoms also showed differences in comorbid conditions.8 The authors stated that: “LUTS are not organ specific; growing evidence supports the association of LUTS with a multitude of comorbid conditions sharing common pathophysiological pathways.”7 The potential link between LUTS/OAB and systemic conditions or symptoms warrants further investigation.
In this case control study, we compared the systemic (non-urologic) symptoms between clinic patients who seek care for their OAB against age-matched controls who reported no OAB symptoms. Unlike some of the previous population-based studies, our cohort underwent clinical evaluation and had a clinical diagnosis of OAB. The urinary symptoms, quality of life, and psychosocial measures between the two subgroups of OAB patients who had high versus low systemic symptom burden were also compared.
Materials and Methods
Population
Patients diagnosed with OAB were approached and prospectively enrolled by a single physician (HL) between October 2012 and March 2014. Eligibility criteria included a clinical diagnosis of OAB, and complained of urinary urgency, with or without urgency incontinence, usually with frequency and nocturia (in accordance to the 2002 International Continence Society definition), in the absence of infection or other identifiable causes.1 The clinical evaluation of OAB conformed to the published AUA guidelines,9 and in our cases consisted of history, physical, urinalysis, and post-void residual measurement. The comparison group (controls) was age-matched individuals who had no prior diagnosis of OAB or IC/BPS, no significant lower urinary tract symptoms (AUA symptom index <7), no significant bladder or pelvic pain, and no evidence of urinary infection. Controls were recruited by local advertisement and research database. 51 OAB patients and 30 individuals without OAB (controls) consented to participate (see Table 1). All participants signed an informed consent. The Washington University Institutional Review Board approved this study.
Table 1.
Comparison of urinary and psychosocial symptoms between OAB and controls
| OAB | Controls | p-value | |
|---|---|---|---|
| No. of subjects | 51 | 30 | |
| Age (mean ± SD) | 53.8 ± 11.9 | 54.2 ± 12.3 | 0.98 |
| Sex (% females) | 73% | 57% | 0.14 |
| Race (% white) | 43.1% | 63.3% | 0.08 |
| Age of diagnosis of OAB (mean ± SD) | 47.5 ± 15.2 | NA | |
| % with OAB symptoms less than one year? | 24% | NA | |
| Urinary symptoms: (mean ± SD) | |||
| No. of daytime void* (1-6,7-8,9-10,11-12,13 times or more) | 1.8 ± 1.3* | 0.2 ± 0.6* | <0.001 |
| No of nighttime void** | 2.6 ± 1.1 | 0.9 ± 0.7 | <0.001 |
| How often rush to bathroom to void?*** | 2.7 ± 0.9*** | 0.6 ± 0.7*** | <0.001 |
| How often does urine leak before getting to the bathroom?**** | 2.2 ± 0.9**** | 0.3 ± 0.5**** | <0.001 |
| Urologic questionnaires: (mean ± SD) | |||
| ICIQ-UI (urinary incontinence, 0-21) | 12.0 ± 4.9 | 1.4 ± 2.0 | <0.001 |
| ICIQ-OAB (overactive bladder, 0-16) | 9.3 ± 2.6 | 2.0 ± 1.5 | <0.001 |
| OAB-q symptom bother subscale (6-36) | 19.1 ± 6.6 | 2.2 ± 2.8 | <0.001 |
| OAB-q quality of life subscale (13-78) | 29.7 ± 16.9 | 2.0 ± 3.0 | <0.001 |
| UDI-6 (urogenital distress inventory, 0-24) | 12.6 ± 5.6 | 0.9 ± 1.4 | <0.001 |
| IIQ-7 (incontinence impact questionnaire, 0-28) | 8.8 ± 8.2 | 0.1 ± 0.4 | <0.001 |
| USS (urgency severity scale, 0-3) | 2.1 ± 0.7 | 0.5 ± 0.6 | <0.001 |
| Numeric rating scale of urgency (0-10) | 6.1 ± 2.6 | 0.4 ± 0.6 | <0.001 |
| Numeric rating scale of frequency (0-10) | 6.4 ± 2.6 | 0.6 ± 0.9 | <0.001 |
| Psychosocial measures: (mean ± SD) | |||
| Depression (HADS-D) | 5.3 ± 3.9 | 2.8 ± 3.9 | <0.001 |
| Anxiety (HADS-A) | 7.5 ± 4.5 | 3.3 ± 3.6 | <0.001 |
| Psychological stress level (PSS) | 17.3 ± 8.1 | 10.7 ± 8.5 | <0.001 |
| Exposure to childhood sexual trauma (CTES) | 29.4% | 6.7% | 0.022 |
| Sleep (PROMIS-8b) | 54.3 ± 10.3 | 43.8 ± 9.2 | <0.001 |
| Fatigue (PROMIS-7a) | 54.7 ± 9.6 | 46.0 ± 6.4 | <0.001 |
Based on the categories of response on the ICIQ-OAB questionnaire:
How many times do you urinate during the day? 0=1 to 6 times, 1=7 to 8 times 2=9 to 10 times, 3=11 to 12 times, 4=13 or more times.
During the night, how many times do you have to get up to urinate, on average? 0=none, 1=one time, 2=two times, 3=three times, 4=four or more times
Do you have to rush to the toilet to urinate? 0=never, 1=occasionally, 2=sometimes, 3=most of the time, 4=all of the time
Does urine leak before you can get to the toilet? 0=never, 1=occasionally, 2=sometimes, 3=most of the time, 4=all of the time
Assessment
Systemic (non-urologic) symptoms were assessed using the Polysymptomatic, Polysyndromic Questionnaire (PSPS-Q).10 The PSPS-Q is a self-reported checklist used to assess the numbers as well as the distribution of systemic symptoms. It is used to identify patients who report high symptom burden across multiple organ systems in a pattern that is characteristic of widespread systemic symptoms. The checklist consists of items that inquired about the presence or absence of 59 symptoms across 10 symptom groups (see Appendix). The PSPS-Q has been utilized in two previous studies to characterize systemic symptoms in patients with IC/BPS.10, 11 For this and previous studies,10, 11 widespread systemic symptoms were operationalized as having ≥25 symptoms in ≥9 symptom groups in women, or ≥20 symptoms in ≥8 symptom groups in men. The operational definition was different between men and women because men do not have gynecological symptoms.
Participants also completed the following validated questionnaires at the same time to assess their OAB: 1) international consultation on incontinence – urinary incontinence short form (ICIQ-UI)12, 2) international consultation on incontinence – overactive bladder (ICIQ-OAB)13, 3) OAB-q short form,14 4) urogenital distress inventory short form (UDI-6)15, 5) incontinence impact questionnaire short form (IIQ-7)15, 6) urgency severity scale (USS)16, 7) numeric rating scale (0 to 10) of the severity of their urgency, and 8) frequency symptom.
In addition, the following psychosocial measures were assessed: 1) depressive symptoms using the HADS-D (Hospital Anxiety and Depression Scale),17 2) anxiety symptoms using the HADS-A,17 3) psychological stress levels using the Perceived Stress Scale (PSS),18 and 4) exposure to childhood sexual trauma using the Childhood Traumatic Event Scale (CTES).19 PROMIS-Sleep20 and PROMIS-Fatigue20 were used to assess sleep disturbance and the impact of fatigue on daily life. All data were collected prospectively from the questionnaires completed by the participants.
Statistical Analyses
Wilcoxon rank-sum tests (continuous variables) and Chi-square tests (categorical variables) were used for univariate comparisons between OAB and controls, and between the two OAB subgroups (with versus without widespread systemic symptoms). Linear regression models (continuous variables) and logistic regression models (categorical variables) were used for multivariate comparisons adjusting for age and sex. P<0.05 was considered significant difference. All statistical analysis was completed using the open source statistical package R v3.2.0.
Results
Population
Participant characteristics were presented in Table 1. There was no age or sex difference between the OAB cohort and the comparison group without OAB (controls) (p=0.98 and 0.14 respectively). The mean age of the OAB cohort was 53.9. About one-fourth of them were men. About one-fourth of them had OAB symptoms for less than a year. As shown in Table 1, patients reported worse urinary incontinence and OAB symptoms than controls as reflected in the ICIQ-UI, ICIQ-OAB, UDI-6, IIQ-7, OAB-q, and USS comparisons (all p<0.001).
Systemic symptom burden
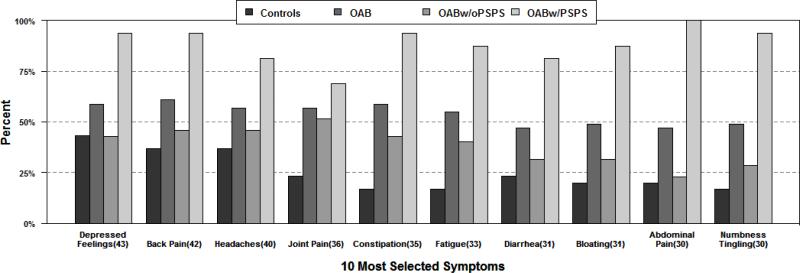
Of the 59 symptoms on the PSPS-Q checklist, OAB patients reported significant higher symptom burden compared to controls (17.5 ± 12.3 versus 6.4 ± 7.9 symptoms, mean ± SD, p<0.001, adjusted for age and sex, see Table 2). The top 10 individual symptoms reported are shown in Figure 1. When the symptoms are categorized based on the symptom groups (cardiopulmonary, gastrointestinal, etc., see Appendix), the distribution of symptoms was more widespread in OAB patients compared to controls. OAB patients were more likely to report neurological, cardiopulmonary, gastrointestinal, sexual, musculoskeletal, and gynecological symptoms compared to controls (p<0.05 for all comparisons, adjusted for age and sex). There was no difference in psychiatric symptoms between OAB and controls.
Table 2.
Comparison of systemic symptoms between OAB and controls (adjusted for age and sex)
| OAB | Controls | p-value, adjusted for age and sex (OAB vs. controls) | OAB with widespread systemic symptoms | OAB without widespread systemic symptoms | p-value, adjusted for age and sex (with vs. without systemic symptoms) | |
|---|---|---|---|---|---|---|
| No. of subjects | 51 | 30 | 16 | 35 | ||
| Systemic symptoms: (mean ± SD) | ||||||
| No. of symptoms checked on the PSPS-Q (up to 59) | 17.5 ± 12.3 | 6.4 ± 7.9 | <0.001* | 32.0 ± 4.5 | 10.8 ± 8.2 | <0.001* |
| % of subjects with widespread systemic symptoms | 31.4% (n=16) | 6.7% (n=2) | 0.027* | 100% | 0% | |
| No. of symptoms in each organ system group: (mean ± SD) | ||||||
| Neurological | 4.6 ± 3.5 | 1.2 ± 1.6 | <0.001* | 8.3 ± 2.2 | 2.9 ± 2.6 | <0.001* |
| Cardiopulmonary | 1.4 ± 1.4 | 0.5 ± 1.0 | 0.004* | 2.5 ± 1.5 | 0.9 ± 1.1 | <0.001* |
| Gastrointestinal | 4.0 ± 3.4 | 1.2 ± 2.1 | <0.001* | 7.6 ± 1.5 | 2.3 ± 2.6 | <0.001* |
| Gynecological (females only) | 1.9 ± 1.7 | 1.0 ± 0.9 | 0.017* | 2.6 ± 1.4 | 1.4 ± 1.7 | 0.042* |
| Sexual | 1.0 ± 1.1 | 0.4 ± 0.9 | 0.027* | 2.1 ± 1.3 | 0.6 ± 0.8 | <0.001* |
| Musculoskeletal | 2.1 ± 1.5 | 0.9 ± 1.3 | 0.001* | 3.6 ± 1.2 | 1.4 ± 1.1 | <0.001* |
| Psychiatric | 3.0 ± 3.1 | 1.7 ± 2.7 | 0.085 | 5.7 ± 2.4 | 1.8 ± 2.6 | <0.001* |
p<0.05
Figure 1.
Histogram showing the frequency of the top 10 individual specific symptom reported by controls and OAB patients (the overall OAB cohort, OAB with PSPS or widespread systemic symptoms, OAB without PSPS or widespread systemic symptoms)
Characterization of OAB patients with versus without systemic symptoms
About a third of OAB patients (31.4%) reported widespread systemic symptoms across multiple organ systems using the operational definition defined in the Methods section (≥25 symptoms in ≥9 symptom groups in women, or ≥20 symptoms in ≥8 symptom groups in men). The percentage of participants who reported widespread systemic symptoms was significantly higher in OAB than in controls (31.4% versus 6.7%, p=0.027, adjusted for age and sex).
To determine if systemic presentation influenced the presentation of OAB, OAB patients with widespread systemic symptoms (n=16, 31.4%, mean 32.0 symptoms out of a maximum of 59) and OAB patients without widespread systemic symptoms (n=35, 68.6%, mean 10.8 symptoms) were compared. There was no age or sex difference between the two OAB subgroups. As shown in Table 3, OAB patients with widespread systemic symptoms had worse urinary incontinence symptoms (ICIQ-UI), OAB symptoms (OAB-q), and urinary-specific quality of life (UDI-6, IIQ-7, OAB-q) than OAB patients without widespread systemic symptoms (p<0.05 for all comparisons, adjusted for age and sex). There was no difference in ICIQ-OAB or USS between the two subgroups of OAB patients.
Table 3.
Comparison of urinary symptoms, quality of life, and psychosocial measures among OAB patients with and without widespread systemic symptoms (adjusted for age and sex)
| OAB Only | OAB with widespread systemic symptoms | OAB without widespread systemic symptoms | p-value, adjusted for age and sex |
|---|---|---|---|
| No. of OAB subjects | 16 | 35 | |
| % of OAB subjects | 31.4% | 68.6% | |
| Age (mean ± SD) | 54.8 ± 9.2 | 53.3 ± 13.1 | 0.79 (not adjusted) |
| Sex (% females) | 88% | 66% | 0.18 (not adjusted) |
| Age of diagnosis of OAB (mean ± SD) | 49.0 ± 13.8 | 47.0 ± 16.1 | 0.78 |
| % with OAB symptoms less than one year? | 33.3% | 18.2% | 0.25 |
| No. of symptoms checked on the PSPS-Q (up to 59) | 32.0 ± 4.5 | 10.8 ± 8.2 | <0.001* |
| Urinary symptoms: (mean ± SD) | |||
| ICIQ-UI (urinary incontinence, 0-21) | 15.1 ± 4.2 | 10.6 ± 4.5 | 0.003* |
| ICIQ-OAB (overactive bladder, 0-16) | 10.1 ± 2.8 | 8.9 ± 2.5 | 0.11 |
| OAB-q symptom bother subscale (6-36) | 22.5 ± 6.4 | 17.6 ± 6.1 | 0.010* |
| OAB-q quality of life subscale (13-78) | 38.5 ± 17.3 | 25.5 ± 6.4 | 0.006* |
| UDI-6 (urogenital distress inventory, 0-24) | 16.4 ± 5.7 | 10.9 ± 4.7 | 0.002* |
| IIQ-7 (incontinence impact questionnaire, 0-28) | 13.3 ± 8.7 | 6.8 ± 7.1 | 0.002* |
| USS (urgency severity scale, 0-3) | 2.3 ± 0.7 | 2.1 ± 0.7 | 0.34 |
| Numeric rating scale of urgency (0-10) | 6.5 ± 3.0 | 6.0 ± 2.4 | 0.43 |
| Numeric rating scale of frequency (0-10) | 7.4 ± 2.0 | 5.9 ± 2.7 | 0.057 |
| Psychosocial measures: (mean ± SD) | |||
| Depression (HADS-D) | 6.9 ± 3.5 | 4.5 ± 3.8 | 0.011* |
| Anxiety (HADS-A) | 9.3 ± 3.4 | 6.6 ± 4.8 | 0.034* |
| Psychological stress level (PSS) | 22.1 ± 7.3 | 15.0 ± 7.5 | 0.002* |
| Exposure to childhood sexual trauma (CTES) | 38% | 26% | 0.41 |
| Sleep (PROMIS-8b) | 57.1 ± 8.9 | 53.0 ± 10.8 | 0.17 |
| Fatigue (PROMIS-7a) | 60.5 ± 7.1 | 52.1 ± 9.5 | 0.011* |
Note: Widespread systemic symptoms were operationalized as having ≥25 symptoms in ≥9 symptom groups in women, or ≥20 symptoms in ≥8 symptom groups in men among the symptoms listed in the Appendix. The operational definition was different between men and women because men do not have gynecological symptoms.
p<0.05
When the psychological measures were compared, OAB patients with widespread systemic symptoms had worse depressive symptoms (HADS-D), anxiety symptoms (HADS-A), fatigue symptoms (PROMIS-Fatigue), and higher psychological stress (PSS) than OAB patients without widespread systemic symptoms (p<0.05 for all comparisons, adjusted for age and sex). There was no difference in sleep disturbance (PROMIS-Sleep) or history of childhood sexual trauma (CTES) between the two subgroups of OAB patients who had high versus low systemic symptom burden.
Discussion
There are four major findings in this study: 1) OAB patients reported significantly more systemic (non-urologic) symptoms compared to an age-matched comparison group of individuals without OAB; 2) differences were observed across multiple organ systems; 3) in fact, about a third of OAB patients reported widespread systemic symptoms with high symptom burden across multiple organ systems; 4) patients with more non-urologic (systemic) presentation also had worse urinary (incontinence/OAB) symptoms, poorer urinary-specific quality of life, and more psychosocial difficulties.
Overactive bladder (OAB) is defined by bladder-centric symptoms, requiring the presence of urgency, with or without urgency incontinence, usually with frequency and nocturia.1 Traditionally OAB is thought of as a “bladder-specific condition.” Here we provided evidence showing that OAB patients can have significant systemic (non-urologic) manifestation of their syndrome.
Recent studies have alluded to the potential associations between OAB and systemic conditions including fibromyalgia, irritable bowel syndrome, arthritis, hypertension, diabetes, and heart disease, and neurologic conditions.2, 4, 7 The caveat of these population studies was that they lacked a clinical evaluation to distinguish OAB from other diagnosis such as IC/BPS. Since OAB and IC/BPS shared many overlapping symptoms (e.g., frequency, urgency),5 it was not easy to distinguish between these conditions using symptom-based questionnaires without a clinical evaluation. This is an important consideration since previous studies have also demonstrated systemic presentation among IC/BPS patients, and association between IC/BPS and fibromyalgia and irritable bowel syndrome.10, 21 Here we focused on patients who received a clinical evaluation and a diagnosis of OAB. The validity of our OAB cohort is further supported by high incontinence scores (mean ICIQ-UI of 12.0), in addition to having high urgency scores. Another strength of our study was that we have also taken a systematic approach to assess symptoms across multiple organ systems and administered the questionnaires to an age-matched comparison group without OAB/incontinence.
Our data suggest that in at least a subset of OAB patients (up to a third of patients), OAB may not be only a “bladder specific condition”, and that systemic mechanisms and common pathophysiology pathways may modify the severity of OAB or be shared between OAB and a number of systemic conditions. About a third of OAB patients (31.4%) reported widespread systemic symptoms across multiple organ systems (neurological, cardiopulmonary, gastrointestinal, sexual, musculoskeletal, and gynecological). The disproportionate systemic involvement is surprising. Moreover, there appears to be a dosage effect: OAB patients with more non-urologic (systemic) presentation also had the most severe urologic (OAB/incontinence) symptoms.
OAB patients reported more gastrointestinal, sexual and gynecological symptoms compared to controls (Table 2). The association between lower urinary tract, gastrointestinal, or gynecological symptoms may be explained by pelvic organ cross-talk.22 The pelvic organs share nerve innervations at the afferent, efferent, and spinal cord levels. Viscero-visceral convergence (e.g., between bladder and bowel), viscero-somatic convergence (e.g., between bladder and pelvic floor), and central sensitization occurring at the level of the spinal cord may contribute to pelvic organ cross talk. Neurogenic inflammation and peripheral sensitization may also contribute to pelvic organ cross talk. This pelvic cross-sensitization is manifested clinically in the overlap between bladder and bowel dysfunctions.8 Urodynamic studies in irritable bowel syndrome patients revealed detrusor overactivity, providing evidence that irritable bowel syndrome may be associated with dysfunction of bladder smooth muscle.23 Besides neurological cross talk and smooth muscle dysfunction, the serotonin pathway may also be a common link between LUTS/OAB and IBS. Serotonin is one of the key neurotransmitters in the urinary and gastrointestinal tracts. In fact, Steers et al (2007) have shown that the serotonin uptake inhibitor duloxetine was more effective than placebo in reducing OAB symptoms.24
In our study, OAB patients also reported more systemic symptoms outside the pelvis (e.g., neurological, cardiopulmonary, musculoskeletal) compared to controls (Table 2). The pathophysiology behind this systemic presentation is unknown. Perhaps a subset of OAB patients had underlying global sensory hypersensitivity due to central sensitization occurring at the supraspinal level (e.g., at the insula and thalamus where sensory signals from multiple body sites including the bladder are processed and integrated before the signals are relayed to higher cortical processing centers). With central sensitization, a stimulus that is normally non-noxious might now be interpreted as noxious or bothersome by the brain. Supraspinal central sensitization is manifested as global sensory hypersensitivity to various bodily stimuli across multiple organ systems. This sensory hypersensitivity is reflected in increased systemic symptom burden reported by the patient.10 Global sensory hypersensitivity could be evaluated in OAB patients in the future by quantitative sensory testing (QST) at a body site outside the pelvis.
Besides sensory hypersensitivity, there are other potential explanations of the systemic presentation of OAB. A link between OAB and metabolic syndrome, or components of metabolic syndrome such as diabetes, may explain some of the cardiovascular and neurological symptoms.25 In a large population-based study, Coyne et al (2011) showed that men and women with LUTS/OAB had higher prevalence of hypertension, diabetes, heart disease, arthritis, and neurologic conditions compared to individuals with no or minimal LUTS/OAB symptoms.7, 8 We noticed high rates of hypertension (37%), diabetes (8%) and stroke/transient ischemic attack (8%) among our OAB cohort, however the rates were not statistically different from controls (33%, 3% and 7% respectively), due to small sample size. An association between OAB and systemic pro-inflammatory states may also explain the multitudes of systemic presentation. There is growing evidence that systemic pro-inflammatory markers such as serum C-reactive protein and cytokines (e.g., IL-1β, IL-6, IL-8) are elevated in OAB compared to controls.26 These are important areas of research, but there have been a paucity of studies.
Our study does not dispute the involvement of the bladder and the lower urinary tract in OAB. Rather we raise the possibility that in at least a subset of patients, additional systemic mechanisms and pathophysiology pathways may also contribute to the overall presentation of OAB. Perhaps the bladder is a victim of systemic processes in some patients, and there are interactions between local and systemic factors. Non-urologic conditions may modify the OAB syndrome. Alternatively, the condition of multisystem phenotype including OAB may be classified as a distinct syndrome. Understanding, inquiring, and addressing these non-urologic issues could be important in the management of OAB, particularly for patients who do not improve with bladder-centric treatments (e.g., anticholinergics, beta-agonist, onabotulinum A).
Conclusions
The increased presence of non-urologic symptoms in a subset of OAB patients suggests an underlying systemic etiology and pathogenetic mechanisms that may contribute to OAB. This study highlights the importance of understanding systemic factors in urologic conditions otherwise thought to be organ-specific.
Acknowledgement
The study was partly supported by the National Institutes of Health grants P20-DK-097798 (HL, GA, SJ) and K08-DK-094964 (HL). We would like to thank all the subjects who participated in the study, Vivien Gardner for recruiting the subjects, Alexandra Kim for protocol development, Kristen Aubuchon for assistance with IRB procedures, Alethea Paradis for data management, and Dr. Robert Gereau IV in the Department of Anesthesiology for critical review of the manuscript. We thank the support of kidney translational research core (KTRC) and the renal division at Washington University. Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
ABBREVIATIONS
- IC/BPS
interstitial cystitis/bladder pain syndrome
- ICIQ
international consultation on incontinence
- IIQ-7
incontinence impact questionnaire short form
- LUTS
lower urinary tract symptoms
- OAB
overactive bladder
- PSPS
polysymptomatic, polysyndromic
- UDI-6
urogenital distress inventory short form
- UI
urinary incontinence
Appendix: PSPS-Questionnaire
For the following symptom list, we would like to know if you have ever had a lot of trouble with the symptom (circle Yes or No).
Group 1:
Headache
Feeling generally sick
Group 2:
Blindness
Paralysis
Numbness and tingling (anesthesia)
Inability to speak (aphonia)
Convulsions or seizures
Unconsciousness
Amnesia (periods of time without memory)
Deafness
Hallucinations (e.g., seeing visions, hearing voices)
Difficulty urinating
Trouble walking
Other conversion symptoms (e.g., any unusual spells)
Group 3:
Fatigue
Lump in throat or inability to swallow
Fainting spells
Blurred vision (not just due to needing glasses)
Unexplained weakness of body or limbs
Painful urination
Group 4:
Breathing difficulty
Palpitation or irregular heartbeat
Anxiety attacks
Chest pain
Dizziness (without fainting)
Group 5:
Lack of appetite (anorexia)
Unintentional weight loss
Marked fluctuation in weight
Nausea
Abdominal bloating
Inability to tolerate several kinds of food
Diarrhea
Constipation
Group 6:
Abdominal pain
Vomiting
Group 7:
Painful menstruation (dysmenorrhea)
Menstrual irregularities
Amenorrhea (don't count menopause)
Excessive bleeding with menstrual periods
Vomiting all 9 months of pregnancy or hospitalized for vomiting during pregnancy
Group 8:
Loss of interest in sex
Frigidity or impotence
Painful sexual intercourse (dyspareunia)
Other difficulties with sex or sexual organs
Group 9:
Back pain
Joint pain without swelling or redness in more than 1 joint
Painful extremities (limbs, hands, feet, not counting joints)
Burning pain of the sexual organs or rectum
Other bodily pains
Group 10:
Nervousness
Fears
Depressed feelings
Need to quit work or inability to carry regular duties because of feeling sick
Crying easily
Feeling life is hopeless
Thinking a good deal about dying
Wanting to die
Thinking of suicide
Suicidal attempts
Symptom grouping:
Neurological = groups 1, 2, 3.
Cardiopulmonary = group 4.
Gastrointestinal = groups 5, 6.
Gynecological = group 7 (women only).
Sexual = group 8.
Musculoskeletal = group 9.
Psychiatric = group 10.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 2.Chung JH, Kim SA, Choi BY, et al. The association between overactive bladder and fibromyalgia syndrome: a community survey. Neurourol Urodyn. 2013;32:66. doi: 10.1002/nau.22277. [DOI] [PubMed] [Google Scholar]
- 3.Homma Y, Yoshida M, Seki N, et al. Symptom assessment tool for overactive bladder syndrome--overactive bladder symptom score. Urology. 2006;68:318. doi: 10.1016/j.urology.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto S, Hashizume K, Wada N, et al. Relationship between overactive bladder and irritable bowel syndrome: a large-scale internet survey in Japan using the overactive bladder symptom score and Rome III criteria. BJU Int. 2013;111:647. doi: 10.1111/j.1464-410X.2012.11591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai HH, Vetter J, Jain S, et al. The Overlap and Distinction of Self-Reported Symptoms between Interstitial Cystitis/Bladder Pain Syndrome and Overactive Bladder: A Questionnaire Based Analysis. J Urol. 2014;192:1679. doi: 10.1016/j.juro.2014.05.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krieger JN, Stephens AJ, Landis JR, et al. Relationship between chronic nonurological associated somatic syndromes and symptom severity in urological chronic pelvic pain syndromes: baseline evaluation of the MAPP study. J Urol. 2015;193:1254. doi: 10.1016/j.juro.2014.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyne KS, Kaplan SA, Chapple CR, et al. Risk factors and comorbid conditions associated with lower urinary tract symptoms: EpiLUTS. BJU Int. 2009;103(Suppl 3):24. doi: 10.1111/j.1464-410X.2009.08438.x. [DOI] [PubMed] [Google Scholar]
- 8.Coyne KS, Cash B, Kopp Z, et al. The prevalence of chronic constipation and faecal incontinence among men and women with symptoms of overactive bladder. BJU Int. 2011;107:254. doi: 10.1111/j.1464-410X.2010.09446.x. [DOI] [PubMed] [Google Scholar]
- 9.Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2012;188:2455. doi: 10.1016/j.juro.2012.09.079. [DOI] [PubMed] [Google Scholar]
- 10.Lai HH, North CS, Andriole GL, et al. Polysymptomatic, polysyndromic presentation of patients with urological chronic pelvic pain syndrome. J Urol. 2012;187:2106. doi: 10.1016/j.juro.2012.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai HH, North CS, Andriole GL, et al. Urological symptoms in a subset of patients with urological chronic pelvic pain syndrome and a polysymptomatic, polysyndromic pattern of presentation. J Urol. 2014;191:1802. doi: 10.1016/j.juro.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avery K, Donovan J, Peters TJ, et al. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23:322. doi: 10.1002/nau.20041. [DOI] [PubMed] [Google Scholar]
- 13.Jackson S, Donovan J, Brookes S, et al. The Bristol Female Lower Urinary Tract Symptoms questionnaire: development and psychometric testing. Br J Urol. 1996;77:805. doi: 10.1046/j.1464-410x.1996.00186.x. [DOI] [PubMed] [Google Scholar]
- 14.Coyne K, Revicki D, Hunt T, et al. Psychometric validation of an overactive bladder symptom and health-related quality of life questionnaire: the OAB-q. Qual Life Res. 2002;11:563. doi: 10.1023/a:1016370925601. [DOI] [PubMed] [Google Scholar]
- 15.Uebersax JS, Wyman JF, Shumaker SA, et al. Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Research Group. Neurourol Urodyn. 1995;14:131. doi: 10.1002/nau.1930140206. [DOI] [PubMed] [Google Scholar]
- 16.Nixon A, Colman S, Sabounjian L, et al. A validated patient reported measure of urinary urgency severity in overactive bladder for use in clinical trials. J Urol. 2005;174:604. doi: 10.1097/01.ju.0000165461.38088.7b. [DOI] [PubMed] [Google Scholar]
- 17.Snaith RP, Zigmond AS. The hospital anxiety and depression scale. Br Med J (Clin Res Ed) 1986;292:344. doi: 10.1136/bmj.292.6516.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385. [PubMed] [Google Scholar]
- 19.Pennebaker JW, Susman JR. Disclosure of traumas and psychosomatic processes. SOCIAL SCIENCE AND MEDICINE (OXFORD) 1988;26:327. doi: 10.1016/0277-9536(88)90397-8. [DOI] [PubMed] [Google Scholar]
- 20.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63:1179. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naliboff BD, Stephens AJ, Afari N, et al. Widespread Psychosocial Difficulties in Men and Women With Urologic Chronic Pelvic Pain Syndromes: Case- control Findings From the Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network. Urology. 2015;85:1319. doi: 10.1016/j.urology.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malykhina AP. Neural mechanisms of pelvic organ cross-sensitization. Neuroscience. 2007;149:660. doi: 10.1016/j.neuroscience.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 23.Whorwell PJ, Lupton EW, Erduran D, et al. Bladder smooth muscle dysfunction in patients with irritable bowel syndrome. Gut. 1986;27:1014. doi: 10.1136/gut.27.9.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steers WD, Herschorn S, Kreder KJ, et al. Duloxetine compared with placebo for treating women with symptoms of overactive bladder. BJU Int. 2007;100:337. doi: 10.1111/j.1464-410X.2007.06980.x. [DOI] [PubMed] [Google Scholar]
- 25.Bunn F, Kirby M, Pinkney E, et al. Is there a link between overactive bladder and the metabolic syndrome in women? A systematic review of observational studies. Int J Clin Pract. 2015;69:199. doi: 10.1111/ijcp.12518. [DOI] [PubMed] [Google Scholar]
- 26.Bhide AA, Cartwright R, Khullar V, et al. Biomarkers in overactive bladder. Int Urogynecol J. 2013;24:1065. doi: 10.1007/s00192-012-2027-1. [DOI] [PubMed] [Google Scholar]