Abstract
Objectives
This two-stage open-label pilot study evaluated the safety and potential efficacy of naltrexone plus bupropion as a pharmacotherapy for methamphetamine (MA) use disorder.
Methods
The study was conducted in two stages of recruitment across 3 sites; 20 participants were enrolled in Stage 1, and 29 participants were enrolled in Stage 2. Eight weeks of open-label pharmacotherapy with a combination of extended-release injectable naltrexone (XR-NTX; Vivitrol®) plus extended-release oral bupropion (BRP; Wellbutrin® XL) were provided with a smartphone-assisted medication adherence platform. Participants met DSM-5 criteria for severe MA use disorder, self-reported ≥20 days of MA use in the 30 days prior to consent, and submitted 3 MA-positive urine drug screens (UDS) out of 4 collected during screening. Participants attended clinic twice weekly for observed BRP dosing, UDS testing, assessments, and medical management; XR-NTX was administered at weeks 1 and 5. A BRP taper and follow-up visit occurred in Week 9.
Results
Analyses evaluated effects of XR-NTX+BRP to determine the number of “responders” according to a statistically pre-defined response criterion (6 of 8 MA-negative UDS during the last four weeks of medication). The two-stage design required that Stage 1 yield ≥3 responders to continue to Stage 2; 11 of the 49 participants met responder criteria across both stages (5 in Stage 1, 6 in Stage 2).
Conclusions
Under the statistical analysis plan, study “success” required ≥9 responders. With 11 responders, the study demonstrated sufficient potential of naltrexone plus bupropion as a combination pharmacotherapy for MA use disorder to warrant further study.
INTRODUCTION
Methamphetamine (MA) use is associated with medical, psychiatric, and socioeconomic consequences and remains a significant public health problem. In the United States, rates of lifetime MA use peaked in 2006, and an estimated 12.25 million people had used MA (SAMHSA, 2014). There are no approved medications to help reduce relapse from MA use. Several promising candidates have shown preliminary clinical utility in reducing use, including bupropion and naltrexone (Elkashef et al., 2008; Jayaram-Lindstrom et al., 2008; McCann & Li, 2012). However, prior pharmacotherapy trials have suffered from poor medication adherence rates, which may limit detection of clinical efficacy (Anderson et al., 2015; Heinzerling et al., 2014).
Bupropion has been approved for the treatment of major depression and smoking cessation. Bupropion acts by increasing the levels of catecholamines, including norepinephrine and dopamine (Richmond & Zwar, 2003), and blocking the nicotinic acetylcholine receptor (Slemmer et al., 2000). The stimulant and antidepressant properties of bupropion may benefit MA users by alleviating dysphoria and cravings experienced during early abstinence (Netwon et al., 2004), thus helping to prevent relapse. Bupropion has been shown to reduce MA self-administration in animals (Reichel et al., 2008; Reichel et al., 2009; Schindler et al., 2011) and to reduce craving and the subjective “high” from MA in humans (Newton et al., 2006). Naltrexone, an opioid receptor antagonist, has been shown to reduce cue- (Anggardiredia et al., 2004) and amphetamine-induced reinstatement of MA (Haggkvist et al., 2009) in rodents, reduce subjective effects of amphetamine in humans (Jayaram-Lindstrom et al., 2008a), and reduce relapse to amphetamine, possibly via modulating effects of mu opioid receptors on dopamine (Jayaram-Lindstrom et al., 2008b).
A recent advance in pharmacotherapy research has been the study of medication combinations, such as those tested for the treatment of major depression (e.g., Zisook et al., 2011; Trivedi et al., 2006) and a recent study of extended-release naltrexone in combination with buprenorphine+naloxone for cocaine dependence (Mooney et al., 2012). The rationale for combining bupropion and naltrexone is predicated on their potentially complementary effects as shown in clinical research and as postulated in mechanistic arguments for naltrexone (e.g., Jayaram-Lindstrom et al, 2008), for bupropion, (e.g., Ascher et al., 1995; Newton et al., 2006), and for the combination, which could regulate the mesolimbic reward pathways (Ornellas & Chavez, 2011). The combination of naltrexone and bupropion has also been studied as a treatment for obesity (e.g., Greenway et al., 2010, Wadden et al., 2011), and was recently approved by the FDA for this indication. A recent human laboratory study demonstrated the safety and tolerability of combination bupropion and naltrexone in MA users (Stoops et al., 2015).
This Phase II open-label clinical trial sponsored by the National Drug Abuse Treatment Clinical Trials Network (CTN) utilized a two-stage design to investigate extended-release injectable naltrexone (XR-NTX, as Vivitrol® 380mg) in combination with extended-release oral bupropion (BRP, as Wellbutrin® XL 450mg/day) as a potential pharmacotherapy for MA use disorder. The primary objective of this study was to evaluate the safety and preliminary efficacy of the medication combination to determine if the combination warrants further investigation.
METHODS
Design
A study design involving two stages of recruitment (Simon, 1989) was planned in which Stage 2 would proceed only if a successful outcome criterion, defined as meeting a pre-determined number of “responders,” was met in Stage 1. A responder was defined as a participant who provided at least 6 of 8 (75%) MA-negative urine drug screens during the evaluation period (i.e., last four weeks of the active medication phase, week 5–8), including the final UDS, which had to be obtained in week 8. Based upon findings from prior pharmacotherapy trials for MA dependence in high severity users (e.g., Elkashef et al., 2008), the placebo response rate (P0) was hypothesized to be 10% or less. If the medication combination were associated with a true response rate (P1) of 30% or more, a cutoff determined to be greater than what could be expected from placebo response (e.g. Heinzerling et al., 2014), then it would be worthy of advancing to further definitive evaluations. It was hypothesized that the combination of XR-NTX+BRP would be well-tolerated and associated with reductions in MA-positive urine drug screens (UDS) from baseline to treatment-end.
The study analysis plan required 20 participants to be enrolled in Stage 1, with ≥3 participants meeting responder criteria in Stage 1 before Stage 2 was conducted. If Stage 1 criteria was not met, the study would be terminated for lack of efficacy. If ≥3 participants met Stage 1 responder criteria, 29 additional participants would be enrolled in Stage 2. A total of 9 or more responders of the 49 total participants (in both Stages) would indicate sufficient promise to warrant further investigation.
Participants
The sample was recruited from three study sites in California, Hawaii, and Texas and included 49 men and women between age 18 to 65 who met all eligibility criteria, including DSM-5 criteria for severe stimulant use disorder (MA type), ≥20 days of self-reported MA use in the 30 days prior to consent, and 3 MA-positive UDS each collected at least 3 days apart in a 14-day period during screening; criteria were consistent with other studies that enrolled high severity MA users (e.g. Shoptaw et al., 2008; Dean et al., 2009). Other eligibility criteria were intended to ensure safe participation, including exclusion of pregnancy or lactation or unstable medical or psychiatric conditions. All participants signed informed consent.
Study Intervention and Procedures
Stages 1 and 2 included the same intervention and followed the same procedures.
Intervention
The intervention included 8 weeks of open-label pharmacotherapy with extended-release naltrexone (XR-NTX; as Vivitrol®) plus oral extended-release bupropion (BRP; as Wellbutrin XL®).
Procedures
Following a maximum 30-day screening phase to establish eligibility, including absence of opioids based on naloxone challenge, participants received a first monthly injection of XR-NTX, followed by initial dose of BRP. The standard 380mg XR-NTX injection was provided at Weeks 1 and 5. The dose of BRP was titrated as follows: 150mg provided on Days 1 and 2, 300mg on Days 3 and 4, and the 450mg on Day 5. Dosing continued for 8 weeks. A reduction to 300mg was permitted to alleviate BRP-related adverse effects. Take-home oral medication (BRP) was dispensed weekly for dosing on non-clinic days. Participants attended clinic twice weekly for observed BRP dosing, collection and testing of urine samples, assessments, and medical management. Participants used study-supplied smartphones to record and submit dosing videos of oral medication taken at home on non-clinic days. A taper occurred during the first four days of Week 9 (Days 57–60), reducing the daily BRP dose to 300mg for Days 57 and 58, and to 150mg for Days 59 and 60. Compensation up to $980 was provided for visit attendance and assessments, including $10 for each dosing video demonstrating medication adherence. Participants received $30 if they returned the smartphone at the end of the trial.
Measures
Methamphetamine use
Screening/baseline assessments included self-reported use and on-site UDS (QuickTox® immunoassay test), collected twice weekly at least 3 days apart. Urine samples collected during the active medication phase were analyzed by a central laboratory and results were not made available to study staff; a cutoff of 500 ng/mL was used to determine MA-negative urines.
Medication adherence
On clinic days, oral medication dosing was observed. Participants were provided with smartphones and instructions on how to video-record oral medication dosing on non-clinic days. Dosing videos were transmitted to the study team, which were then reviewed to ensure that the video clearly showed dosing adherence based on objective criteria. Blood samples were taken at weeks 5 and 8 to test for blood levels of bupropion and hydroxybupropion, its primary metabolite.
Safety
Screening/baseline safety and medical assessments included a medical and psychiatric history, physical examination, clinical laboratories (blood chemistry, hematology, and urinalysis), 12-lead electrocardiogram, vital signs, and pregnancy tests for females. Safety of study participants and intervention tolerability were assessed throughout the study by self-reports of treatment-emergent adverse events (AEs). Participants who experienced an AE that compromised safety were discontinued from medication and provided referrals for medical care. All AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 16.1, 17.0, and 17.1. The safety endpoint was assessed by the frequency, severity, and nature of study medication-related AEs.
Quality of life
Functional outcomes were measured by quality-of-life assessments from baseline to end of treatment. Quality of life was measured with the Treatment Effectiveness Assessment (TEA; Ling, 2012), a four-item, patient-centered instrument for evaluating participant progress and recovery from the participant’s perspective. The TEA uses a Likert scale to document level of functioning in substance use, health, lifestyle/personal responsibility, and community domains, with higher scores indicating improvement in outcomes.
Craving
Responses collected with a Visual Analog Scale (VAS) documented participants’ craving for MA. Response range is from 0 (no craving) to 100 (most intense craving possible), and the measure was completed at each screening visit, once weekly throughout the 8-week medication phase, and at the final post-medication visit during week 9.
Analyses
The primary efficacy endpoint was a binary assessment of success defined as meeting a pre-determined number of responders. Sample size was determined using Simon’s two-stage design (Simon, 1989). A sample of 49 participants was required to evaluate the hypothesis for true response rate (p1) of ≥30%, with Type I and II error rates of 5%. Under the null hypothesis, the response rate (p0) was hypothesized to be ≤10%.
The safety endpoint was measured by number and nature of study-related AEs. Descriptive analyses examined participants’ baseline characteristics, the success of efforts to enhance medication adherence, and adherence to other protocol parameters. Pre-/post-intervention quality-of-life analysis using the Wilcoxon test compared responder and non-responder groups to determine possible improvements across status and function domains. Pre-/post-intervention craving analysis using the Wilcoxon test were also performed to compare responder and non-responder groups.
RESULTS
Participant Characteristics
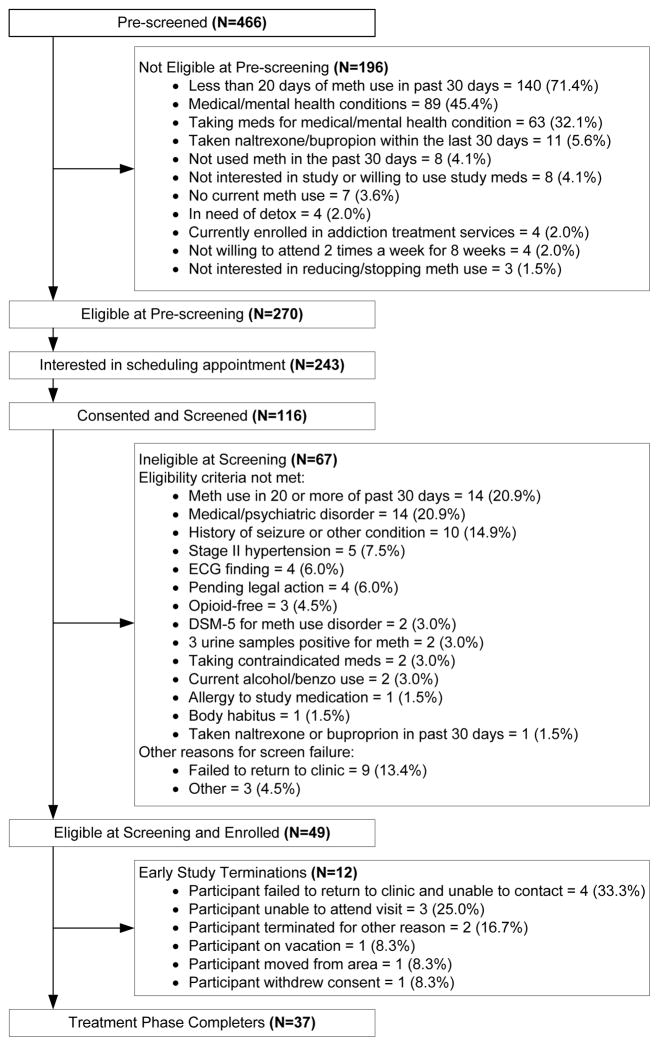
Figure 1 presents participant flow. After providing verbal consent, a total of 466 candidates were evaluated for preliminary eligibility using an IRB-approved pre-screening telephone interview script. As shown in Figure 1, 116 provided written consent and were screened, and 49 were enrolled and inducted onto study medications. Table 1 provides a summary of baseline characteristics of the enrolled sample.
Figure 1.
Participant Flow
Table 1.
Baseline Participant Characteristics
| Characteristic | % (n) or M (SD) |
|---|---|
| Gender % (n) | |
| Male | 53.1 (26) |
| Female | 46.9 (23) |
| Mean Age (SD) | 39.9 (10.76) |
| Age Range | 24–65 |
| Race/Ethnicity % (n) | |
| White | 49.0 (24) |
| Hispanic or Latino | 30.6 (15) |
| Multiracial | 16.3 (8) |
| Black or African American | 10.2 (5) |
| Asian | 4.1 (2) |
| Native Hawaiian or Pacific Islander | 2.0 (1) |
| Other | 18.4 (9) |
| Education Completed % (n) | |
| Less than high school diploma | 14.3 (7) |
| High school graduate, or GED | 28.6 (14) |
| Some college, no degree | 34.7 (17) |
| Associate degree | 12.3 (6) |
| Bachelor degree | 6.1 (3) |
| Masters degree | 4.1 (2) |
| Marital Status % (n) | |
| Married/Living with partner | 10.2 (5) |
| Divorced/Separated | 26.6 (13) |
| Never married | 61.2 (30) |
| Don't know | 2.0 (1) |
| Employment % (n) | |
| Employed | 32.7 (16) |
| Unemployed | 49.0 (24) |
| Temporarily laid off, sick leave, or maternity leave | 2.0 (1) |
| Retired | 4.1 (2) |
| Disabled (permanently and temporarily) | 6.1 (3) |
| Student | 6.1 (3) |
| Mean MA-Positive UDS during Screening (SD) | 98.5 (7.92) |
| Mean Self-Report MA Use Days in 30 Days Prior to Consent (TLFB) | 27.0 (3.44) |
Methamphetamine Use
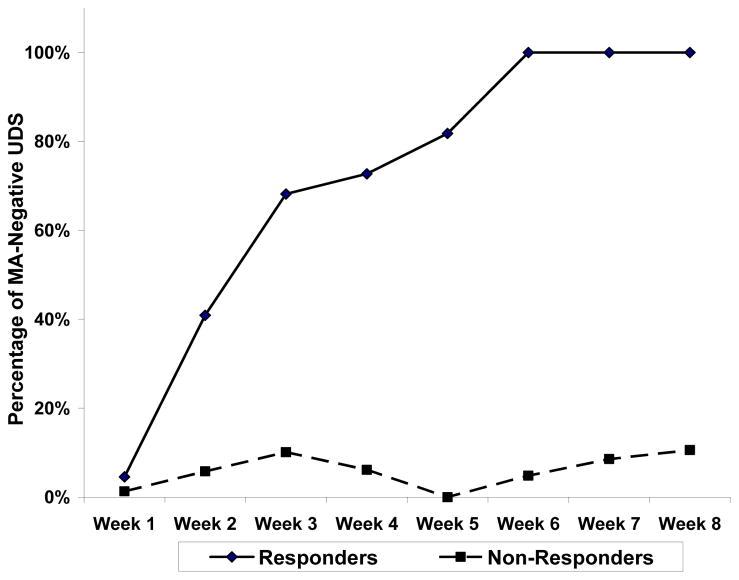
The criteria to advance from Stage 1 to Stage 2 were met; therefore, all 49 participants were included in the analysis of primary efficacy endpoint. The unbiased point estimate, confidence interval, and trial p-value were calculated to determine response rate (responders) using methods discussed by Koyama and Chen (2008). Of the 49 participants, 11 were responders (5 in Stage 1, 6 in Stage 2), thus meeting the primary efficacy endpoint criterion of 9 or more responders and yielding an unbiased response rate of 24% (p=0.0075) with 95% lower confidence limit of 13%. No χ2 differences were found in MA use by gender (p=0.42); site (p=0.66), race (p=0.34), or ethnicity (p=0.63). Analysis of the medication-adherent participants (defined as receiving both naltrexone injections and at least 80% adherent to prescribed daily doses of bupropion confirmed by video or in-clinic observation), shows a 33% (95% exact CI = 17%-53%) response rate in participants who were adherent with medication; 32% (95% exact CI = 17%–51%) response rate in participants with detectable bupropion blood levels (> 0.5 ng/ml); and 30% (95% exact CI = 16%–47%) response rate in participants with detectable hydroxybupropion blood levels (> 1 ng/ml). Figure 2 shows the percentage of MA-negative UDS by study week for all 49 participants by responder group, and highlights the pattern of MA use across the trial. The two trajectories (responder vs non-responder) begin differentiating by week 2 and continued through week 8. An exploratory χ2 test showed that the two groups differ significantly in the proportion of MA-negative urines at each week except week 1.
Figure 2. Percentage Methamphetamine-Negative Urine Drug Screen by Responder Status by Study Week*.
*The proportion of MA-negative urines was significantly higher at each week for weeks 2–8 for the responder group as compared to the non-responder group (p=<0.05).
Retention
A total of 833 clinic visits were expected for the entire medication phase (49 participants, 2 visits each for 8 weeks, with a follow-up visit at week 9). Of those, 88 visits were missed for a 10.6% missed visit rate. In all, 20 participants missed at least 1 visit, including 6 participants who, upon missing a first visit, never returned to the clinic (1 missed in week 1, 2 in week 2, and 1 each in weeks 4, 5, and 6). None of the participants in the responder group missed a clinic visit.
Medication Adherence
Medication adherence was assessed by self-reported doses taken, observed twice-weekly in-clinic dosing, smartphone dosing videos of bupropion taken at home on non-clinic days, and assessment of bupropion and hydroxybupropion blood levels at weeks 5 and 8. All participants were administered the first XR-NTX injection, and 41 (83.7%) received the second injection. Table 2 shows the results of individual adherence assessments.
Table 2.
Assessments of Medication Adherence and Quality of Life (TEA) Functional Outcomes
| Adherence Measure | Responder (n=11) | Non-Responder (n=38) | Total (N=49) |
|---|---|---|---|
| % of dispensed BRP doses taken based on participant self-report | 97.6% | 92.1% | 93.6% |
| % of dispensed BRP doses* taken as confirmed by dosing video or in- person observation | 95.2% | 83.4% | 86.6% |
| % Participants who received XR-NTX injection #2 | 100% | 78.9% | 83.7% |
| % Participants with detectable BRP blood levels (>0.50 ng/mL) at weeks 5 and 8** | 100% | 69.7% | 76.5% |
| % Participants with detectable hydroxybupropion blood levels (>1.00 ng/mL) at weeks 5 and 8** | 100% | 75.0% | 80.6% |
| Functional Outcomes: Quality of Life (TEA): | |||
| TEA Item(s) | Responder (n=11) | Non-Responder(n=31)+ | P-value |
| Baseline Assessments: | |||
| Total Score | 16.4 (7.2) | 17.7 (7.1) | 0.54 |
| Substance Abuse | 3.5 (1.9) | 3.8 (2.7) | 0.87 |
| Health | 3.7 (2.1) | 4.5 (2.2) | 0.28 |
| Lifestyle/Personal Responsibility | 3.5 (2.5) | 4.6 (2.2) | 0.10 |
| Community | 5.5 (2.3) | 4.8 (2.4) | 0.26 |
| Week 9 Assessments: | |||
| Total TEA Score | 36.9 (3.5) | 23.7 (9.1) | 0.00002 |
| Substance Abuse | 9.7 (0.65) | 5.4 (2.8) | 0.004 |
| Health | 9.4 (1.0) | 6.0 (2.6) | 0.0002 |
| Lifestyle/Personal Responsibility | 8.8 (1.2) | 5.7 (2.6) | 0.0007 |
| Community | 9.0 (1.7) | 6.6 (2.4) | 0.002 |
Dose dispensed is adjusted as a result of dose reductions and early medication discontinuation.
There were 11 responders for weeks 5 & 8; Non-responders in weeks 5 & 8 are 34 and 31, respectively.
Seven participants in the non-responder group missed the week 9 visit. P-values were obtained from Wilcoxon test.
Functional Outcome: Quality of Life
Analyses of the TEA include comparisons across responder and non-responder groups for each of four domains as well as a summed score (Table 2). The total scores did not differ between responder and non-responder groups at baseline (p=0.54), but were significantly different at treatment end (p<0.001). Similarly, no difference in any of the four individual domains was found at baseline between responder and non-responder groups, but all item scores were significantly different at treatment end (p≤0.004).
Craving
Exploratory analyses of participants’ craving for MA showed that craving for both responder and non-responder groups decreased over the medication phase. Comparison of weekly craving scores between the responder and non-responder groups indicates that craving was significantly lower at each week for weeks 2–8 for the responder group as compared to the non-responder group (p=<0.05).
Adverse Events
Treatment-emergent adverse events (AEs) and serious adverse events (SAEs) are listed in Table 3. The table provides overall treatment-emergent AE frequency, severity and causal relationship with study medications. Forty-five participants reported AEs with 249 treatment-emergent AEs occurred between the signing of informed consent and the final study visit. Serious AEs (SAEs) were documented for two participants (convulsion and acute amphetamine intoxication). Most AEs were deemed causally unrelated to either study drug (66.3%). One SAE was causally related to bupropion, a single generalized seizure occurring four days after the participant reached the 450mg maintenance dose, suggesting both a plausible temporal sequence and a dose-dependence relationship. Because the participant reported prior use of bupropion at therapeutic doses without occurrence of seizure, other factors lowering the seizure threshold likely contributed to this event, including concurrent use of medication that may also lower the seizure threshold, possible electrolyte disturbances due to dehydration, and recent cessation of alcohol use.
Table 3.
Summary of Treatment-Emergent Adverse Events
| Participants with Adverse Events % (n) | 91.8 (45) |
| Number of Adverse Events | 249 |
| Severity of Adverse Event % (n) | |
| Grade 1 - Mild | 79.4 (197) |
| Grade 2 - Moderate | 16.5 (41) |
| Grade 3 - Severe | 4.0 (10) |
| Relationship of Adverse Event % (n) | |
| Not Related | 66.3 (165) |
| Related to XR-NTX only | 11.2 (28) |
| Related to Bupropion only | 13.7 (34) |
| Related to Both | 8.8 (22) |
| Types of Study Medication-Related Adverse Events (of a total 84 Medication-Related AEs) % (n) | |
| Nausea | 33.33 (28) |
| Headache | 7.14 (6) |
| Anxiety | 5.95 (5) |
| Insomnia | 5.95 (5) |
| Vomiting | 4.76 (4) |
| Dry mouth | 4.76 (4) |
| Fatigue | 3.57 (3) |
| Dizziness | 3.57 (3) |
| Tremor | 3.57 (3) |
| Nightmare, abnormal dreams, parasomnia | 3.57 (3) |
| Depressed mood, anhedonia | 2.38 (2) |
| Decreased appetite | 2.38 (2) |
| Diarrhea | 2.38 (2) |
| Hot flush | 2.38 (2) |
| Panic attack | 1.19 (1) |
| Eye disorders | 1.19 (1) |
| Back pain | 1.19 (1) |
| Irritability | 1.19 (1) |
| Somnolence, lethargy | 1.19 (1) |
| Renal and urinary disorders | 1.19 (1) |
| Blood pressure increased | 1.19 (1) |
| Convulsion* | 1.19 (1) |
| Libido decreased | 1.19 (1) |
| Dysphonia | 1.19 (1) |
| Thirst | 1.19 (1) |
| Illusion | 1.19 (1) |
As per protocol, classified as a Serious Adverse Event (SAE)
Early medication terminations occurred in 8 participants, with four due to intolerable symptoms or side effects (headache, nausea/dizziness, fatigue, parasomnia, and seizure described above), one due to worsening substance use disorder, and three due to logistical issues such as moving from the area or otherwise not being available for the second XR-NTX injection. A total of seven participants had dose reductions from 450mg to 300mg, with three due to symptoms or side effects (panic attack, nausea, and anhedonia/depressed mood). Nine of the 11 responders remained on the full 450mg dose throughout the study.
DISCUSSION
The primary objective of this single-arm, open–label, pilot study was to evaluate the safety and preliminary efficacy of a combination pharmacotherapy, XR-NTX+BRP, as a potential treatment for MA use disorder. The two medications have little or no abuse potential and the combination has been shown to regulate reward-based behavior and have anti-craving properties in other populations, including individuals with craving for food in an obesity study (Billes et al., 2014).
Findings from this study support the potential efficacy of the combination pharmacotherapy as determined by having met the a priori minimum of at least 3 “responders” at the completion of Stage 1 of the trial and a cumulative total of at least 9 responders at the conclusion of Stage 2. Successful response criteria were established according to a statistically determined and clinically meaningful primary outcome analysis, defined as having 6 of 8 (75%) MA-free urine tests during the evaluation period (the last four weeks of the medication phase, including the final test).
Findings from this study support the general safety and tolerability of this medication combination in MA users. Nausea was the most commonly reported medication-related AE and comprised one-third of all study medication-related AEs; this is consistent with studies of naltrexone plus BRP as a combination treatment for obesity in which nausea has been reported as the most commonly associated side effect (Verpeut & Bello, 2014). Headache was the second most commonly reported study medication-related AE, also consistent with prior research. Importantly, AEs related to XR-NTX and BRP were typically mild to moderate in severity, transient, and the BRP-related AEs occurred most commonly during dose escalation and typically did not lead to discontinuation of study medication. Seizure, which occurred in one participant, has been reported in association with BRP (Foley, et al., 2006) and may also be associated with MA intoxication (Winslow, et al., 2007).
This study had high rates of retention and medication adherence. Medication adherence was facilitated by twice-weekly observed medication dosing and submission of smartphone videos of each take-home dose to ensure adequate exposure to study medication. While smartphone video technology has been used in monitoring adherence to infectious disease treatments (Bashshur, Shannon, & Smith, 2014; Wang et al., 2014) and in interventions for tobacco use (Whittaker et al., 2012), to our knowledge this is the first time it has been used in a study involving stimulant users, demonstrating the acceptability of implementing novel smartphone-based adherence procedures. Adherence may have been additionally strengthened by our use of extended-release bupropion, which requires only once-daily dosing, as opposed to prior investigations that used the sustained-release formulation requiring twice-daily dosing. The inclusion of three geographically distinct study sites broadened the generalizability of study findings and demonstrated the feasibility of treating MA users with a medication combination not previously used in an outpatient context.
These results are in contrast to a recent human laboratory study that failed to show decreased reinforcement effect of MA after short-term bupropion, naltrexone, or the combined treatment in non-treatment seeking MA users (Stoops et al., 2015). However, the present study included MA users seeking to stop or reduce MA use and a larger sample size, and participants were monitored for MA use as opposed to psychological measures of reinforcement. Furthermore, our data appear to support the conclusions of Brensilver et al. (2012), whose retrospective analyses of other bupropion research (Elkashef et al., 2008 and Shoptaw et al., 2008) suggest that treatment response can be predicted after two to three weeks (Figure 2); responders and non-responders in this study appeared to diverge in terms of MA-negative UDS by week 3.
The findings and conclusions of this study were limited by the open-label pilot design. Lack of a comparator group and knowledge of treatment assignment may have introduced bias via the “placebo effect” beyond the expected placebo response rate as predetermined and accounted for in the statistical analysis plan. This effect could have been enhanced by selection of motivated, treatment-seeking participants and use of smartphone video adherence procedures that could have introduced additional therapeutic effects. However, inclusion of individuals with only severe MA use disorder enabled within-subject detection of clinically meaningful reduction of MA use. Attempts to minimize potential bias by research staff included blinding of twice-weekly UDS results via analysis by a central laboratory. Additionally, this study could not assess whether the combination might also be helpful in the treatment of less severe MA users, as was previously shown for bupropion alone (Elkashef et al., 2008; McCann & Li, 2012). The study design did not enable determination of potential differential or independent contributions of each medication to study outcomes, as they were examined only in combination. As this study did not include a dose-finding design, it is unknown if less than the maximum tolerated doses may be as effective at reducing MA use.
CONCLUSIONS
Prior pharmacotherapy trials for stimulant use disorder have suffered from poor medication adherence rates and inconsistent findings related to drug use outcomes. This is the first investigation of the combination of extended-release bupropion and long-acting injectable naltrexone as a potential pharmacotherapy for MA use disorder utilizing a video-based strategy to improve and monitor medication adherence. The medication combination safely resulted in a clinically meaningful outcome, demonstrated by the proportion of participants who met “responder” criteria. These findings support the need for further study using an adequately powered, randomized, placebo-controlled design to evaluate the combination pharmacotherapy for MA use disorder. The two-stage design could also be used to efficiently evaluate other interventions for stimulant use disorder and thus accelerate treatment development.
Acknowledgments
We are grateful for the dedication of staff at the three participating Community-based Treatment Programs and the Regional Research and Training Center (RRTC): Nexus Recovery Center in the Texas Node (Erica Adkins, Stacey Burns, Charles Eliot deGravelles, Brittany Eghaneyan, Tracy Greer, Kathy Shores-Wilson, Lisa Stephenson, Rameshwar Thapa, and Mark Vasquez ), the University of Hawaii (Daniel Alicata, Christine Cloak, Elizabeth “Mendy” Dunn, Erin Fukaya), and UCLA Integrated Substance Abuse Programs (Jeffrey Annon, Erendida Cano, Daniel Dickerson, Cindy Fernandez, Albert Hasson, Mark Oyama, Brian Perrochet, Susan Reed) in the Pacific Region Node. We would also like to thank our partners at the Center for the Clinical Trials Network of the National Institute on Drug Abuse, the Clinical Coordinating Center and Data and the Statistics Center at Emmes (Alex Borbely, Maria Campanella, Radhika Kondapaka), and Alkermes Pharmaceuticals.
Conflicts of Interest and Source of Funding
Alkermes Inc. (Waltham, MA, USA) provided monthly naltrexone (Vivitrol®).
Walter Ling, MD has received research support from Indivior and has served as consultant to Indivior, Alkermes, and USWorldMeds. For the remaining authors no conflicts of interest were declared.
Support for this research provided through the National Institute on Drug Abuse (DA13045)
Footnotes
ClinicalTrials.gov Identifier: NCT01982643
References
- Anderson AL, Li S-H, Markova D, et al. Bupropion for the treatment of methamphetamine dependence in non-daily users: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2000;150:170–174. doi: 10.1016/j.drugalcdep.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anggadiredga K, Sakimura K, Hiranita T, Yamanoto T. Naltrexone attenuates cue- but not drug-induced methamphetamine seeking: a possible mechanism for the dissociation of primary and secondary reward. Brain Res. 2004;24:272–276. doi: 10.1016/j.brainres.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Ascher JA, Cole JO, Colin JN, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56(9):395–401. [PubMed] [Google Scholar]
- Bashshur RL, Shannon GW, Smith BR. The empirical foundations of telemedicine interventions for chronic disease management. Telemedicine and e-Health. 2014;20(9):769–800. doi: 10.1089/tmj.2014.9981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billes SK, Sinnayah P, Cowley MA. Naltrexone/bupropion for obesity: An investigational combination pharmacotherapy for weight loss. Pharmacological Research. 2014;84:1–11. doi: 10.1016/j.phrs.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Brensilver M, Heinzerling KG, Swanson A-N, Shoptaw SJ. A retrospective analysis of two randomized trials of bupropion for methamphetamine dependence: Suggested guidelines for treatment discontinuation/augmentation. Drug Alcohol Depend. 2012;125:169–72. doi: 10.1016/j.drugalcdep.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, London ED, Sugar CA, et al. Predicting adherence to treatment for methamphetamine dependence from neuropsychological and drug use variables. Drug and Alcohol Dependence. 2009;105:48–55. doi: 10.1016/j.drugalcdep.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, et al. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162–70. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Foley KF, DeSanty KP, Kast RE. Bupropion: Pharmacology and therapeutic applications. Expert Rev Neurother. 2006;6(9):1249–65. doi: 10.1586/14737175.6.9.1249. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR–I): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- Haggkvist J, Lindholm K, Franck J. The opioid receptor antagonist naltrexone attenuates reinstatement of amphetamine drug-seeking in the rat. Behav Brain Res. 2009;197(1):219–224. doi: 10.1016/j.bbr.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Heinzerling KG, Gadzhyan J, van Oudheusden H, et al. Pilot randomized trial of bupropion for adolescent methamphetamine abuse/dependence. J Adolesc Health. 2013;52:502–5. doi: 10.1016/j.jadohealth.2012.10.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzerling KG, Swanson A-N, Hall TM, et al. Randomized, placebo-controlled trial of bupropion in methamphetamine-dependent participants with less than daily methamphetamine use. Addiction. 2014;109:1878–1886. doi: 10.1111/add.12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram-Lindström N, Konstenius M, Eksborg S, Beck O, Hammarberg A, Franck J. Naltrexone Attenuates the Subjective Effects of Amphetamine in Patients with Amphetamine Dependence. Neuropsychopharmacology. 2008;33(8):1856–63. doi: 10.1038/sj.npp.1301572. [DOI] [PubMed] [Google Scholar]
- Jayaram-Lindström N, Hammarberg A, Beck O, Franck J. Naltrexone for the treatment of amphetamine dependence: A randomized , placebo-controlled trial. Am J Psychiatry. 2008;165:1442–1448. doi: 10.1176/appi.ajp.2008.08020304. [DOI] [PubMed] [Google Scholar]
- Koyama, Chen Proper inference from Simon’s two-stage designs. Stat Med. 2008;27:3145–3154. doi: 10.1002/sim.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Farabee D, Liepa D, Wu LT. The Treatment Effectiveness Assessment (TEA): an efficient, patient-centered instrument for evaluating progress in recovery from addiction. Subst Abuse Rehabil. 2012;3:129–136. doi: 10.2147/SAR.S38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann DJ, Li SH. A novel, nonbinary evaluation of success and failure reveals bupropion efficacy versus methamphetamine dependence: Reanalysis of a multisite trial. CNS Neurosci Ther. 2012;18(5):414–8. doi: 10.1111/j.1755-5949.2011.00263.x. Epub 2011 Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney LJ, Nielsen S, Saxon A, et al. Cocaine Use Reduction with Buprenorphine (CURB): Rationale, Design, and Methodology. Journal of Contemporary Clinical Trials. 2012;34(2):196–204. doi: 10.1016/j.cct.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TF, Kalechstein AD, Duran S, et al. Methamphetamine abstinence syndrome: preliminary findings. Am J Addict. 2004;13:248–55. doi: 10.1080/10550490490459915. [DOI] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, 2nd, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31:1537–44. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- Ornellas T, Chavez B. Naltrexone SR/Bupropion SR (Contrave): A new approach to weight loss in obese adults. Pharmacy and Therapeutics. 2011;36(5):255–256. 261–262. [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA. Bupropion differentially impacts acquisition of methamphetamine self-administration and sucrose-maintained behavior. Pharmacol Biochem Behav. 2008;89:463–72. doi: 10.1016/j.pbb.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Murray JE, Grant KM, Bevins RA. Bupropion attenuates methamphetamine self-administration in adult male rats. Drug Alcohol Depend. 2009;100:54–62. doi: 10.1016/j.drugalcdep.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev. 2003;22:203–20. doi: 10.1080/09595230100100642. [DOI] [PubMed] [Google Scholar]
- SAMHSA. 2014 http://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabsPDFWHTML2013/Web/HTML/NSDUH-DetTabsSect7peTabs1to45-2013.htm#tab7.1a.
- Schindler CW, Gilman JP, Panlilio LV, McCann DJ, Goldberg SR. Comparison of the effects of methamphetamine, bupropion and methylphenidate on the self-administration of methamphetamine by rhesus monkeys. Exp Clin Psychopharmacol. 2011;19:1–10. doi: 10.1037/a0022432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96:222–232. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. Optimal two-stage designs for Phase II clinical trials. Controlled Clinical Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295:321–7. [PubMed] [Google Scholar]
- Stoops WW, Pike E, Hays LR, et al. Naltrexone and bupropion, alone or combined, do not alter the reinforcing effects of intranasal methamphetamine. Pharmacology, Biochemistry and Behavior. 2015;129:45–50. doi: 10.1016/j.pbb.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: The COR–BMOD Trial. Obesity (Silver Spring) 2011;19(1):110–120. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang Y, Wei C, et al. Smartphone interventions for long-term health management of chronic diseases: an integrative review. Telemedicine and e-Health. 2014;20(6):570–83. doi: 10.1089/tmj.2013.0243. [DOI] [PubMed] [Google Scholar]
- Whittaker R, McRobbie H, Bullen C, et al. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2012 Nov 14;11:CD006611. doi: 10.1002/14651858.CD006611.pub3. [DOI] [PubMed] [Google Scholar]
- Winslow BT, Voorhees KI, Phel KA. Methamphetamine abuse. Am Fam Physician. 2007;76(8):1169–74. [PubMed] [Google Scholar]
- Zisook S, Lesser IM, Lebowitz B, et al. Effect of antidepressant medication treatment on suicidal ideation and behavior in a randomized trial: an exploratory report from the Combining Medications to Enhance Depression Outcomes Study. J Clin Psychiatry. 2011;72(10):1322–32. doi: 10.4088/JCP.10m06724. [DOI] [PubMed] [Google Scholar]