Abstract
The cerebral cortex is organized into morphologically distinct areas that provide biological frameworks underlying perception, cognition, and behavior. Profiling mouse and human cortical transcriptomes have revealed temporal-specific differential gene expression modules in distinct neocortical areas during cortical map establishment. However, the biological roles of spatiotemporal gene expression in cortical patterning and how cortical topographic gene expression is regulated are largely unknown. Here, we characterize temporal- and spatial-defined expression of serotonin (5-HT) transporter (SERT) in glutamatergic neurons during sensory map development in mice. SERT is transiently expressed in glutamatergic thalamic neurons projecting to sensory cortices and in pyramidal neurons in the prefrontal cortex (PFC) and hippocampus (HPC) during the period that lays down the basic functional neural circuits. We previously identified that knockout of SERT in the thalamic neurons blocks 5-HT uptake by their thalamocortical axons, resulting in excessive 5-HT signaling that impairs sensory map architecture. In contrast, here we show that selective SERT knockout in the PFC and HPC neurons does not perturb sensory map patterning. These data suggest that transient SERT expression in specific glutamatergic neurons provides area-specific instructions for cortical map patterning. Hence, genetic and pharmacological manipulations of this SERT function could illuminate the fundamental genetic programming of cortex-specific maps and biological roles of temporal-specific cortical topographic gene expression in normal development and mental disorders.
Keywords: Serotonin transporter (SERT), Serotonin (5-HT)-absorbing neurons, SERT conditional knockout mice, Spatiotemporal gene expression, Cortical map architecture
1. Introduction
Development of the mammalian central nervous system (CNS) follows a highly stereotyped series of histogenic processes during which an enormous variety of cell types form unique arrangements and oriented connectivity — the cortical maps in distinct brain areas each with unique function such as sensation, perception, decision making, and motivation (Rakic, 1988; Rash and Grove, 2006; O’Leary and Sahara, 2008). The basic principles of brain development are conserved in all mammals, although cortical size, complexity and detailed architecture have evolved to confer species-specific cognition and behavior (Goldman-Rakic, 1987; Mountcastle, 1995; Geschwind and Rakic, 2013). Alterations in processes regulating cortical map patterning have been implicated in neurological and psychiatric disorders (State and Levitt, 2011; Ecker et al., 2012; Parikshak et al., 2015). Further, maternal/early life exposure to physiological stressors and psychotropic drugs has been associated with increased risks for developing psychiatric traits (Harrington et al., 2013; Lewis et al., 2014), suggesting that mechanisms involved in cortical map formation could underscore an intersection of genetic and environmental modulation of behavioral circuits and an origin of circuit disruption in mental disorders. Very little is known, however, about the mechanisms of genetic programming of cortical maps and how area-specific architectures are established in normal brain and perturbed in the disorders.
Recent work in characterizing cortical transcriptomes, coupled with in situ hybridization and immunohistochemical analysis, has identified temporal-specific differential gene expression clusters (modules) forming islands in prospective neocortical subdivisions (Kang et al., 2011; Miller et al., 2014; Pletikos et al., 2014). In human, the most striking cortical topographic gene expression modules display in the midfetal brain, where expression levels of disparate genes are dramatically elevated in specific neurons in a small number of cortical areas, including primary sensory cortices and subdivisions of the prefrontal cortex (PFC) (Pletikos et al., 2014). Moreover, spatially patterned gene expression in these cortical areas also manifests from adolescence onward but involves different sets of genes (Miller et al., 2014; Pletikos et al., 2014), suggesting that these two phases of the cortices involve distinct molecular and cellular processes. In line with this idea, work from several groups mapped many autism risk genes co-expressed only in the midfetal modules (Voineagu et al., 2011; Parikshak et al., 2013; Willsey et al., 2013). For example, nine high confidence autism-associated genes (hcASD) identified based on de novo loss-of-function mutations were found co-enriched specifically in glutamatergic neurons in PFC layer 5/6 and in somatosensory cortex in midfetal human brain and the first two postnatal weeks in mice (Willsey et al., 2013). Since cortical events in midfetal human brain occur in neonatal mice (Romijn et al., 1991), the temporal-specific topographic gene expression could represent certain critical developmental processes that are shared between humans and rodents and perturbed in common by the mutations of disparate genes associated with ASD.
Evidence has been mounting in recent years that excessive serotonin (5-HT) signaling perturbs early life programming of neural circuits leading to increased risks for psychiatric traits (Oberlander et al., 2009; Harrington et al., 2013; Booij et al., 2015). In the brain, the levels of 5-HT signals are regulated by 5-HT uptake transporter (SERT), which removes 5-HT from the extracellular space thereby limiting 5-HT availability to 5-HT receptors at downstream targets. Complete SERT knockout mice display elevated brain 5-HT, disrupted sensory maps, altered PFC synaptic architecture and enhanced anxiety/depressive behavior (Upton et al., 1999; Persico et al., 2001; Wellman et al., 2007; Fox et al., 2008). Consistently, polymorphisms that reduce SERT gene functionality in human cause altered PFC structure, reduced PFC inhibition of amygdala, exaggerated response to fear stimuli and increased anxiety (Hariri et al., 2002; Heinz et al., 2005; Pezawas et al., 2005; Canli and Lesch, 2007). In addition, early life exposure to selective 5-HT reuptake inhibitors (SSRIs) impairs PFC synaptic architecture and enhances anxiety-like behavior in mice (Ansorge et al., 2004; Rebello et al., 2014) and has been implicated in increased risks for autism and anxiety-related traits in humans, in contrast to SSRI effects in alleviating anxiety/depression in adults (Oberlander et al., 2009; Harrington et al., 2013). Consequently, defining the mechanisms of SERT gene function in early life programming of mouse cortical architecture could contribute to a better understanding of fundamental principles of cortical map development, and discern the specific biological processes regulated by SERT, thus 5-HT and SSRIs, in developing and adult brain.
Our laboratory is using a systematic genetic approach to determine SERT mechanisms in early life programming of cortical maps by generating SERT conditional knockout mice (Chen et al., 2015). In the mammalian CNS, SERT is primarily expressed in 5-HT-producing neurons, which are located in the brainstem raphe nuclei, release 5-HT via axons projecting to nearly every region of the brain, and constitutively express SERT along their axons to regulate 5-HT levels at synapses (Rudnick et al., 2014). In addition, SERT is also expressed in a unique set of glutamatergic neurons, which do not synthesize 5-HT but take up extrasynaptic or trophic 5-HT, and are thus termed “5-HT-absorbing neurons” (Gaspar et al., 2003; Jafari et al., 2011). A unique feature of the mammalian brain is that 5-HT-absorbing neurons are present only transiently during a specific period of development (Gaspar et al., 2003). In rodents, SERT is expressed in thalamic glutamatergic neurons projecting to sensory cortices and in pyramidal neurons in the PFC and hippocampus (HPC) from E17 to P10 (Hansson et al., 1998; Lebrand et al., 1998), coinciding with a period of exuberant synaptogenesis that lays down the basic functional neural circuits (Hensch, 2005). To determine the biological role of SERT expressed in the 5-HT-absorbing neurons, we utilized the Cre/LoxP recombinase system to generate SERT conditional knockout mice, and analyzed aspects of sensory maps in the somatosensory barrel cortex. We have identified that the transient SERT expression in the 5-HT-absorbing neurons is essential for elaborating the sensory maps and oriented dendritic patterning in the barrel cortex, whereas SERT expressed in the raphe neuron axons is not required for the sensory map establishment, indicating that SERT expressed in the 5-HT-absorbing neurons regulates the processes involved in cortical map construction (Chen et al., 2015). In the present study, we further investigated the relation of transient SERT expression in distinct brain areas to spatiotemporal cortical map development.
2. Materials and methods
2.1. Animals
Animal procedures were approved by the Albert Einstein College of Medicine Institutional Animal Care and Use Committee. SERTfl/fl mice were generated as previously described (Chen et al., 2015). Specifically, two loxP sites were inserted in introns 2 and 4 of the SERT gene Slc6a4, and Cre recombinase-mediated deletion eliminates SERT function (Chen et al., 2015). ePet1-Cre (Scott et al., 2005), Vglut2-Cre (Vong et al., 2011) and Emx1-Cre (Gorski et al., 2002) were utilized to knock out SERT in raphe, thalamic and cortical neurons, respectively. Two complete SERT knockout mouse alleles, one was generated by targeted deletion (Bengel et al., 1998) and the other by crossing SERTfl/fl into transgenic female mice expressing Cre in the germline (Ella-Cre) (Chen et al., 2015), produced equivalent phenotypes and are referred here generally as SERTNull mice. All mouse lines were backcrossed to WT C57BL/6 (Taconic Farms Inc. NY) for at least seven generations. Every effort was made to minimize animal suffering and the number of animals used.
2.2. Quantitative real-time PCR
qPCR was performed to determine relative SERT mRNA levels in brainstem, VB nuclei and PFC from P5 SERTCortexΔ mice and their SERTfl/fl littermates, as we have described previously in qPCR analyses of the corresponding brain samples in WT, SERTNull, SERTRapheΔ and SERTGluΔ mice (Chen et al., 2015). Briefly, brainstem, VB nuclei of the thalamus, and IL, PrL and Cg regions of the PFC were retrieved from the mice, and RNA from individual brain samples was extracted. One μg of RNA from each sample was used for reverse transcription to generate cDNA. The cDNA was analyzed by qPCR using the StepOnePlus machine (Applied Biosystems) and SYBR Green detection system (Applied Biosystems). The value of SERT was normalized against the reference gene β-actin for each sample, and the average of normalized SERTabundance in SERTCortexΔ samples of a given brain region relative to that of SERTfl/fl samples is presented.
2.3. Immunohistochemistry and histology
Mice were anesthetized by intraperitoneal injection of Avertin (400 mg/kg) prior to transcardial perfusion with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer. The brains were removed, post-fixed overnight in 4% PFA, and sectioned in the coronal plane at 40 μm thickness. To obtain tangential sections of sensory cortices, the neocortical sheets were flattened between two glass slides, post-fixed, and sectioned tangential to the pial surface at 50 μm thickness. Free-floating sections were used for immunostaining with the following antibodies: rabbit α-5-HT (1:50, Dr. W.M. Steinbusch, University of Limburg, the Netherlands), mouse α-Satb2 (1:1000, Abcam), rabbit α-SERT (1:1000, Millipore), and guinea pig α-Vglut2 (1:2000, Millipore). Immunostaining of the proteins and 5-HT was visualized by using Alexa Fluor 488 or 568 conjugated secondary antibodies. Sections were counterstained with DAPI to detect cell nuclei. Fluorescence images were captured using an Axiocam MR digital camera attached to an AxioImager Z1 microscope (Zeiss). For all the experiments, SERT mutant and control littermates were always analyzed in parallel.
2.4. Quantification of barrel map architecture
The patterning of TCAs and L4 neurons in the somatosensory barrel cortex in various genetic backgrounds was evaluated by quantifying barrel formation index (BFI) of immunohistochemistry for Vglut2 and Satb2 in the PMBSF, as previously described (Chen et al., 2015). Briefly, images of tangential sections immunostained with Vglut2 or doubled immunostained with Vglut2 and Satb2 were processed using the ImageJ (NIH), and the ratio of fluorescence signal intensities in the center of TCA patches to that in the septa regions between two adjacent TCA patches was calculated. BFI values are greater when a neuronal marker is more clearly segregated into barrel structures.
2.5. Statistical analysis
Data are presented as mean ± standard error of mean (SEM). The differences between two groups were evaluated by two-tailed, unpaired Student’s t-tests. For comparison of multiple groups, one-way ANOVA was performed followed if appropriate by a post hoc Bonferroni test. Differences were considered significant at p < 0.05.
3. Results
3.1. Generation of thalamic- and cortex-specific SERT knockout mice
In mice, temporal-specific topographic co-expression of multiple hcASD-associated genes occurs in the somatosensory cortex and PFC (Willsey et al., 2013), matching to the timing of SERT expression in the thalamic ventrobasal (VB) glutamatergic neurons projecting to the somatosensory barrel cortex and in PFC pyramidal neurons, raising our hypothesis that certain developmental processes in the somatosensory cortex and PFC may be coordinately regulated by SERT function in the 5-HT-absorbing neurons. While in the mature brain 5-HT is exclusively synthesized in raphe neurons, the developing brain has additional 5-HT sources, including maternal, gut and placental sources, and moreover, the raphe neuron axons release 5-HT acting as trophic 5-HT prior to the synapse formation (Lauder et al., 1981; Cote et al., 2007; Bonnin et al., 2011). The requirement of SERT for barrel map formation implies that the developing sensory cortex, by default, is exposed to excessive 5-HT, and a prompt removal of 5-HT from the extracellular space in a specific time window is key to proper cortical patterning and neural circuit formation. In order to determine whether SERT expressed in 5-HT-absorbing neurons provides temporal- and brain-region specific restriction of trophic 5-HT signaling, we utilized the Cre/LoxP recombinase system to knock out SERT in distinct brain areas.
Previously, we generated mice with selective SERT knockout in raphe 5-HT-producing neurons (SERTRapheΔ) or in thalamic glutamatergic neurons (SERTGluΔ). Analysis of postnatal day 7 (P7) SERTRapheΔ mice by quantitative reverse transcription PCR (qPCR), in situ hybridization and western blots showed that SERT mRNA and protein abundance in the brainstem raphe neurons was dramatically reduced, whereas SERT abundance in the thalamic VB nuclei that project to the primary somatosensory cortex was comparable to SERTfl/fl littermates as well as to age-matched WT mice, confirming that SERT presence in the thalamic neurons is independent of SERT expression in 5-HT-producing neurons (Chen et al., 2015) (Fig. 1B). SERTGluΔ mice were generated by crossing SERTfl/fl into mice expressing Cre driven by the vesicular glutamate transporter 2 (Vglut2) promoter (Vong et al., 2011). Vglut2 is expressed robustly in thalamic neurons, with lower levels in HPC neurons, as well as in some other brain areas that do not express SERT (Fremeau et al., 2001). P7 SERTGluΔ mice showed dramatically reduced SERT mRNA abundance in the VB nuclei, while SERT abundance in their raphe neurons was equivalent to control littermate mice (Chen et al., 2015) (Fig. 1B). In order to distinguish the role between SERT expressed in thalamic neurons and in PFC and HPC, we now specifically knocked out SERT in cortical neurons by crossing SERTfl/fl into mice expressing Cre driven by the Emx1 promoter, which is expressed in cortical excitatory neurons and commonly referred as Cortex-Cre (Iwasato et al., 2000; Gorski et al., 2002; Judson et al., 2010), to generate SERTCortexΔ mice. As an example, Fig. 1A shows that SERTCortexΔ mice displayed dramatically reduced SERT mRNA levels in the PFC, while SERT mRNA levels in the brainstem raphe nuclei and thalamic VB nuclei were undiminished, as compared to SERT levels in the corresponding brain regions in control littermate mice. These results indicate that SERTCortexΔ, SERTGluΔ and SERTRapheΔ mice can be utilized to explore how temporal-specific topographic SERT expression impacts on cortical map architecture in specific brain areas.
Fig. 1. SERTCortexΔ eliminates SERT expression in PFC but not in thalamic and raphe neurons.
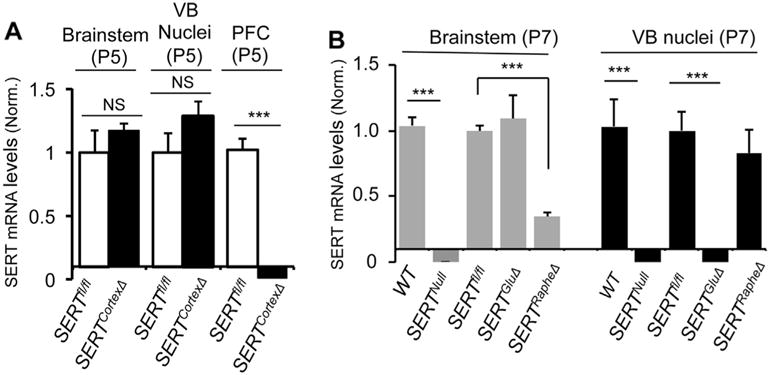
A. qPCR analysis of SERT mRNA in brainstem, thalamic VB nuclei and PFC from SERTCortexΔ and control SERTfl/fl littermate mice. P5 mice were analyzed, with 4–5 animals/genotype (mean ± SEM, ***, p < 0.001, Student’s t-test). NS, not statistically significantly different. B. qPCR analysis of SERT mRNA in brainstem and thalamic VB nuclei from WT, SERTNull, SERTGluΔ, SERTRapheΔ and control SERTfl/fl littermate mice. This graph is reproduced from our previous study (Chen et al., 2015) to facilitate comparison here of the efficiency and selectivity of neuron-specific Cre-mediated SERT knockouts.
3.2. SERT expression and 5-HT accumulation in sensory cortex are independent of SERT expression in cortical neurons
We assessed the impact of neuron-specific SERT knockout on SERT distribution in sensory cortex, by double immunostaining for SERT and Vglut2 of P7 mice. In control mice, tangential sections of the brain showed abundant SERT in primary sensory cortices, with the strongest SERT immunostaining seen as precisely arrayed patches in layer 4 (L4) in the somatosensory barrel cortex. SERT immunostaining in all primary sensory cortices was dramatically diminished in P7 SERTGluΔ mice (Chen et al., 2015). In contrast, SERT immunostaining in the sensory cortices in P7 SERTCortexΔ mice was indistinguishable from control littermate mice (Fig. 2A). On the other hand, SERT immunostaining of raphe 5-HT-producing neurons in SERTCortexΔ mice was comparable to SERTGluΔ and control littermates (Fig. 2B). These results indicate that the patterned SERT immunostaining in the sensory cortex was primarily attributed to SERT expressed in TCAs from thalamic neurons, and was abolished in SERTGluΔ but not in SERTCortexΔ mice.
Fig. 2. SERTGluΔ but not SERTCortexΔ or SERTRapheΔ abolishes SERT expression in primary sensory cortices.
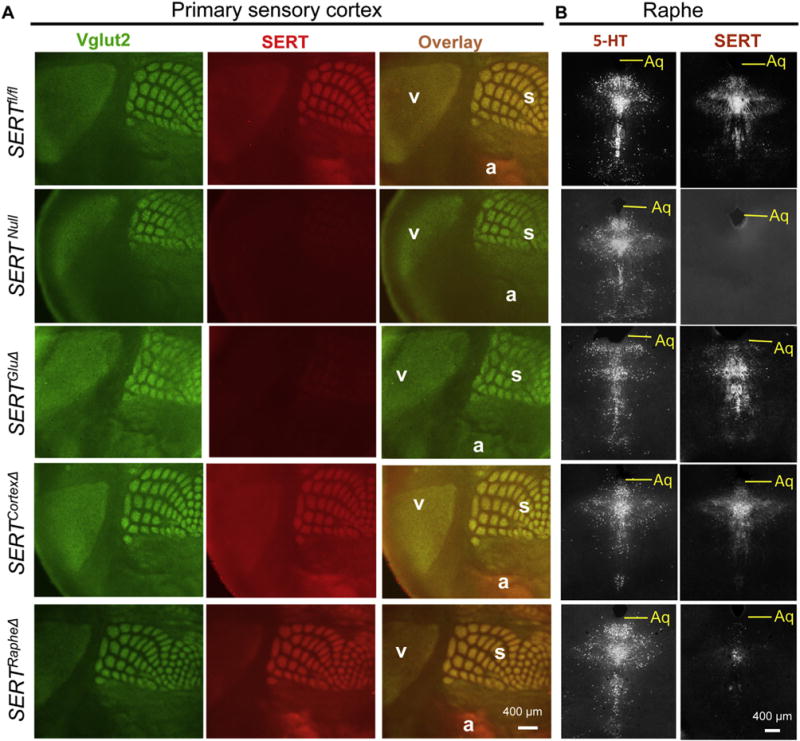
A. SERT (red) and Vglut2 (green) double immunostaining of tangential sections of primary visual (v), auditory (a) and somatosensory barrel (s) cortices in P7 mutant and control SERTfl/fl littermate mice. SERT staining in Vglut2+ patches in SERTCortexΔ mice was indistinguishable from the SERTfl/fl control or SERTRapheΔ mice, whereas the SERT staining in SERTGluΔ mice was diminished as in SERTNull mice. B. SERT and 5-HT immunostaining (both grayscale) of adjacent coronal sections of raphe nuclei from P7 mice. SERT staining was abolished in SERTNull, diminished in SERTRapheΔ, but unaffected in SERTGluΔ and SERTCortexΔ mice. Aq, aqueduct. Some images for SERTNull, SERTRapheΔ and SERTGluΔ brain sections are reproduced from a previous study (Chen et al., 2015) for comparison of SERT immunostaining patterns in SERTCortexΔ brain sections. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In addition to the temporal-specific patterned SERT expression in sensory cortex L4, we observed SERT immunostaining in another class of fibers throughout the cortices from coronal brain sections of the control mice. These fibers were thick and highly punctated and appeared to be randomly distributed (Fig. 3A, lower panels). We had confirmed the specificity of SERT immunostaining of these fibers, by determining that SERTNull mice abolished the staining of these fibers (Chen et al., 2015). However, we found no appreciable reduction in SERT immunostaining of these fibers in SERTGluΔ (Chen et al., 2015) or in SERTCortexΔ mice (Fig. 3A) as compared to the control littermates, indicating that these fibers were unlikely arising from thalamic neurons or cortical neurons. In line with the idea, SERT immunostaining of these fibers was dramatically diminished in SERTRapheΔ mice (Fig. 3A) (Chen et al., 2015). Combined, these data are consistent with the idea that these thick, punctated fibers primarily represent axons of raphe 5-HT-producing neurons projecting to the sensory cortex.
Fig. 3. Requirement of SERT expression in TCAs not SERT in cortical neurons for 5-HT uptake in the barrel cortex.
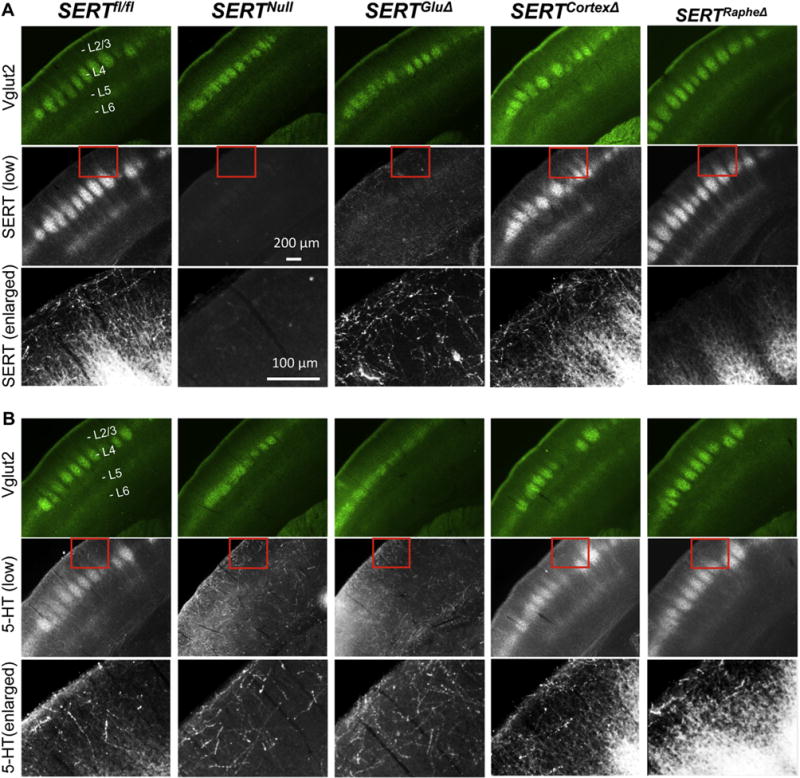
A. SERT (grayscale) and Vglut2 (green) double immunostaining of coronal sections of somatosensory cortex from P7 mice. Control SERTfl/fl mice displayed two distinct classes of SERT+ fibers: fibers that were relatively thin, smooth and co-labeled with Vglut2+ TCA patches at layer 4 (L4) (strongly) and layer 6 (L6) (weakly), and fibers that were thick, punctated, randomly distributed as shown in enlarged views in third row images. No SERT immunostaining was discernible in SERTNull mice. SERT immunostaining in Vglut2+ patches was not detectable in SERTGluΔ but undiminished in SERTCortexΔ and SERTRapheΔ mice. SERT immunostaining in the thick fibers was dramatically reduced in SERTRapheΔ, but undiminished in SERTGluΔ and SERTCortexΔ mice. B. 5-HT (grayscale) and Vglut2 (green) double immunolabeling of coronal sections of P7 somatosensory cortex. 5-HT immunostaining of Vglut2+ patches but not the thick fibers was dramatically reduced in SERTGluΔ. 5-HT immunostaining of both the thick fibers and Vglut2+ patches in SERTCortexΔ was indistinguishable from that in SERTRapheΔ and control littermate mice. For each brain sample, SERT and Vglut2 double immunostaining (A) and 5-HT and Vglut2 double immunostaining (B) represent adjacent sections. Third row images show an enlarged view of SERT immunohistochemistry (A) or 5-HT immunohistochemistry (B) in areas outlined by red boxes on images in respective second row. Images for SERTNull, SERTRapheΔ and SERTGluΔ cortices are reproduced from our previous study (Chen et al., 2015) for comparison of SERT and 5-HT immunostaining patterns in age-matched SERTCortexΔ mice. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
As one way to explore the impact of neuron-specific SERT knockout on 5-HT distribution in the sensory cortex, we carried out 5-HT immunostaining of P7 mice. In coronal sections of the somatosensory cortex from control mice, 5-HT immunostaining recapitulated SERT immunostaining pattern, by labeling both the raphe neuron axons and Vglut2+ patches in L4 barrel cortex (Fig. 3B). In SERTNull mice, 5-HT immunostaining of the Vglut2+ patches was essentially not detectable, while 5-HT immunostaining of the raphe neuron axons was comparable to the control mice, showing that the absence of SERT did not grossly impair 5-HT neuron development, 5-HT synthesis or their axon projections to the sensory cortex but blocked 5-HT from entry into the Vglut2+ fibers in the patches (Chen et al., 2015). We previously observed that 5-HT immunostaining of the Vglut2+ patches was abolished in SERTGluΔ mice just like in SERTNull mice (Chen et al., 2015). In contrast, 5-HT staining of both the Vglut2+ patches and raphe neuron axons was not diminished in SERTCortexΔ mice (Fig. 3B). SERTRapheΔ mice also did not affect 5-HT immunostaining of raphe neuron axons or Vglut2+ patches (Chen et al., 2015). Together, these results support the hypothesis that raphe neuron projections release 5-HT into the developing cortices, whereas SERT expressed in the TCA terminal arbors take the primary role in clearing trophic 5-HT by transporting 5-HT from the extracellular space into the TCAs during a specific development period. However, we cannot exclude the possibility that SERT is expressed in certain PFC neurons projecting to the sensory cortex that cannot be detected at the level of the fluorescence microscopy.
3.3. SERT expressed in cortical neurons is not required for somatosensory map formation
The timing of SERT expression in the 5-HT-absorbing neurons coincides with the period of somatosensory barrel map formation. During the first postnatal week, TCAs from VB neurons segregate into distinct topographic maps in a sequential order: firstly, TCAs corresponding to sensory inputs from distinct body parts segregate into five distinct subfields, and then TCA terminal arbors within each subfield segregate into arrayed patches, with each TCA patch surrounded by a ring of densely packed L4 neurons with their dendrites oriented towards the TCA patch, forming barrel-like structures relaying specific sensory information (Woolsey et al., 1970). Our previous studies revealed that SERT expression in 5-HT-absorbing neurons is essential for the barrel map development (Chen et al., 2015). We reasoned that SERT expressed in 5-HT-absorbing neurons could regulate brain region-specific cortical architectural features. However, as those SERT takes up trophic 5-HT, which may diffuse throughout the brain, thus an alternative possibility could be that 5-HT-absorbing neurons of multiple brain areas set a 5-HT tone in the developing brain for coordinated cortical map development. Consequently, missing SERT expression in one set of 5-HT-absorbing neurons would alter the 5-HT tone in the entire brain leading to disrupted cortical architecture in all 5-HT-regulated brain areas. To distinguish between the two possibilities, we utilized Vglut2 immunostaining to assess TCA patterning in SERTGluΔ and SERTCortexΔ mice at P7. As previously observed (Chen et al., 2015), SERTGluΔ mice displayed no discernable TCA patterning in the subfields corresponding to the sinus hairs and digits, and TCA patterning in PMBSF corresponding to whiskers was blurred indicating for poor segregation as compared to control littermate mice. In contrast, TCA patterning in every subfield of SERTCortexΔ mice was indistinguishable from control littermate mice (Fig. 4A). We validated the intact TCA patterning in SERTCortexΔ mice, by determining barrel formation index (BFI), which is a quantitative measure of TCA segregation based on the ratio of Vglut2+ TCA abundance in the center of the patches to that in the septa region between adjacent patches, with BFI values greater when TCAs are more clearly segregated into barrel structures (Toda et al., 2013; Chen et al., 2015). BFI-Vglut2 values were significantly lower for SERTGluΔ mice even in the PMBSF subfield compared to control littermates, consistent with the poor TCA segregations (Chen et al., 2015). In contrast, we observed equivalent BFI-Vglut2 values between SERTCortexΔ and control littermate mice (Fig. 4B). Collectively, these results indicate that it is SERT expressed in TCAs alone that is necessary and sufficient for TCA terminal arborization patterning in the sensory cortex, while SERT expressed in PFC or HPC pyramidal neurons is not required for this regulation.
Fig. 4. SERT knockout in TCAs not in cortical neurons disrupts barrel map formation.
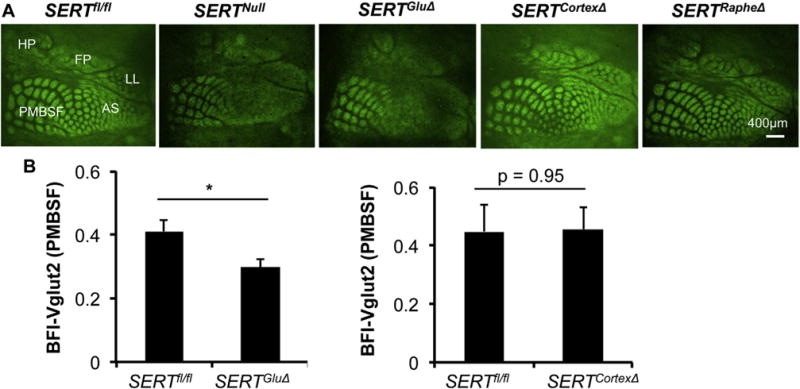
A. TCA patterning at L4 visualized by Vglut2 immunostaining of tangential sections through the somatosensory cortex of P7 mice. In control SERTfl/fl mice, Vglut2+ TCAs corresponding to distinct body parts display arrayed patches in five indicated reception subfields. In SERTGluΔ and SERTNull mice, the areas for the subfields were preserved, but no discernible Vglut2+ patterning in the subfields corresponding to anterior snout (AS), lower lip (LL), forepaw (FP) and hindpaw (HP), and Vglut2+ patches in PMBSF corresponding to whiskers were more blurred although the patches were preserved. Vglut2+ patterning in all the subfields in SERTCortexΔ mice was indistinguishable from that seen in SERTRapheΔ mice and control littermate mice. Images for SERTNull, SERTRapheΔ and SERTGluΔ cortices are reproduced from a previous study (Chen et al., 2015) for comparison of Vglut2 immunostaining and TCA patterning in SERTCortexΔ barrel cortex. B. Evaluation of Vglut2+ TCA patterning in P7 PMBSF, by calculating BFI-Vglut2. Mutant and control littermate mouse samples were stained in parallel. N = 5 each for SERTGluΔ and SERTfl/fl littermate mice; N = 3 each for SERTCortexΔ and SERTfl/fl littermate mice, mean ± SEM, *, p < 0.05, Student’s t-test.
3.4. Regulation of cortical cytoarchitectural patterning by area-specific SERT expression
It has been long established that mammalian neocortex is subdivided into distinct cytoarchitectural areas with specific clusters of neurons organized into stereotyped layers and columns and oriented connectivity (Goldman-Rakic, 1987; Mountcastle, 1995). A salient example is the mouse somatosensory barrel cortex, where L4 cortical neurons migrate during the first postnatal week away from TCA patches to form ring structures (or walls) surrounding individual TCA patches (Espinosa et al., 2009). We wished to learn how SERT knockout in TCAs versus cortex impact on spatiotemporal cortical neuron patterning. We addressed this question by using an established neuronal marker that is expressed in a class of cortical neurons present in both developing barrel cortex and PFC, and determined how SERTGluΔ and SERTCortexΔ impact on the neuronal organization in a specific cortical region — barrel cortex. The transcription factor Satb2 is specifically expressed in callosal projection neurons, which extend axons to contralateral cortex for interhemispheric integration (Alcamo et al., 2008; Britanova et al., 2008). Work from others showed that neonatal SSRI exposure (P1 to P7) caused abnormal callosal connectivity in rodents (Simpson et al., 2011). We found that in P7 control mice, immunolabeled Satb2+ cells exhibited distinct ring structures surrounding Vgtlu2+ TCA patches in the barrel cortex. We have shown that this Satb2+ neuronal patterning was disrupted in P7 SERTGluΔ mice (Chen et al., 2015). In contrast, we observed that P7 SERTCortexΔ mice displayed Satb2+ ring structures in the barrel cortex indistinguishable from that seen in the control mice (Fig. 5A). We further evaluated Satb2+ neuron patterning in SERTCortexΔ mice by determining BFI values of Satb2+ neuron abundance in barrel walls relative to that in the center of barrel patches. Previously, we showed that BFI-Satb2 values were significantly lower in P7 SERTGluΔ mice indicating for disrupted Satb2+ neuron patterning (Chen et al., 2015). In contrast, BFI-Satb2 values were comparable between P7 SERTCortexΔ and control littermate mice (Fig. 5B), showing that lacking SERT expression in the PFC does not perturb cortical neuron topographic patterning in the somatosensory cortex. Interestingly, it has been reported that SERTNull mice displayed altered Satb2+ neuron distribution in the PFC (Witteveen et al., 2013). We hypothesize that SERT expressed in TCAs and in PFC 5-HT-absorbing neurons provides regional instructions for cortical neuron patterning and SERT expressed in the TCAs dictates cortical neuron cytoarchitecture in the sensory cortex independently of SERT expression in the PFC.
Fig. 5. SERT knockout in TCAs not in cortical neurons disrupts cytoarchitectural organization of Satb2+ neurons in the primary somatosensory cortex.
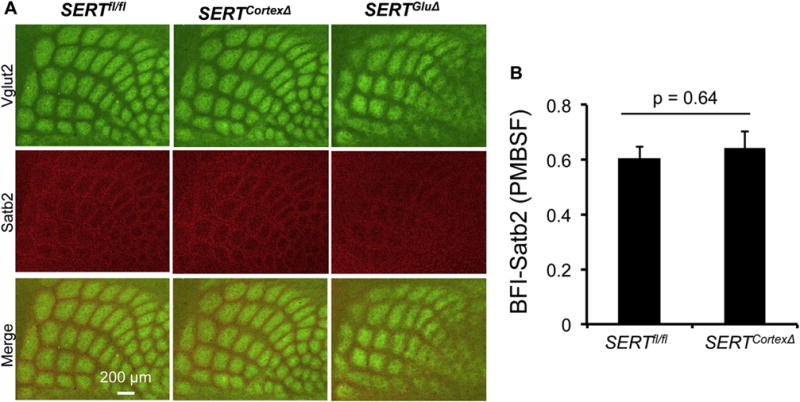
A. Tangential sections of P7 PMBSF of the somatosensory cortex labeled by double immunostaining of Vglut2+ TCAs (green) and Satb2+ cell nuclei (red). In control littermate mice, Satb2+ cells form a ring structure surrounding Vglut2+ TCA patches. This Satb2+ topographic patterning was preserved in SERTCortexΔ mice. B. Evaluation of Satb2+ neuron patterning in the barrel cortex based on BFI determination using Satb2 immunohistochemistry in P7 PMBSF. N = 4, mean ± SEM, Student’s t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Mapping hcASD genes has revealed temporal-specific coexpression networks shared between primary somatosensory cortex and PFC in developing human and mouse brain (Willsey et al., 2013). In the present study, we explored the functional relationship between SERT expression in 5-HT-absorbing neurons in TCAs and PFC and mouse somatosensory map formation. Our experimental data thus far support the idea that transient SERT expression in 5-HT-absorbing neurons represents an area-specific genetic determinant of cortical map architecture. We showed that SERTGluΔ mice have impaired TCAs and cortical neuron patterning in the barrel cortex just like SERTNull mice (Chen et al., 2015). We further demonstrated here that SERTCortexΔ eliminated the transient SERT expression in the PFC but did not cause an appreciable barrel architectural deficit. Based on these data, together with the finding that SERTRapheΔ abrogated SERT expression in raphe neuron projections to the sensory cortex but also did not perturb barrel cortex architecture (Chen et al., 2015), we propose that SERT expression in 5-HT-absorbing neurons provides certain spatiotemporal instructions for genetic programming of area-specific cortical maps, and that SERT expressed in the TCAs specifies the sensory reception field architecture in the somatosensory cortex.
It has long been proposed that the histogenic processes of mammalian brain development depend on regulated differential gene expression (Jessell and Sanes, 2000; Sur and Rubenstein, 2005; Geschwind and Rakic, 2013). Genetic programming essential for the initial phase of cortical patterning has been studied extensively. For example, secreted molecules such as sonic hedgehog (Shh), bone morphogenic proteins and Wnt family signaling factors specify cascades of transcription factors to orderly divide the brain into functional subdivisions, each with defined histological characteristics and function (Jessell and Sanes, 2000; Sur and Rubenstein, 2005). Our data, however, suggest that the role of SERT gene function differs from those signaling molecules, as SERT is not essential for cortical arealization or regional fate specification. For instance, the overall structures and relative location of the somatosensory, visual and auditory cortices, as well as PFC and HPC were established in the absence of SERT (Chen et al., 2015). Even within the barrel cortex, prospective sensory reception subfields corresponding to distinct body parts were preserved in the SERT mutant mice. TCAs attained L4 of the sensory cortices in SERT mutant mice as in control littermates. Characterization of Satb2+ neurons, coupled with previous studies of GABAergic neurons (Chen et al., 2015), showed that the number of the neurons and the neuronal identities in the barrel cortex are preserved in the absence of SERT gene function. Because SERT expression in the 5-HT-absorbing neurons is temporal specified, our data imply that the initial phase of cortical arealizations and generation of diverse neuronal types and subsequent elaboration of area-specific cortical cytoarchitectures (i.e. the relative neuronal positions and organizations within the area) involve distinct mechanisms and biochemical pathways, and that SERT expression in 5-HT-absorbing axons selectively dictates the cortical cytoarchitecture and synaptic patterning after the arealization.
While further experiments are required to define downstream effector pathways regulated by SERT expressed in the 5-HT-absorbing neurons, our data favor the scenario where SERT expressed in specific brain regions provides independent instructions for building cortex-specific architecture. Characterization of SERTCortexΔ mice confirmed that SERT is not expressed in the sensory cortical neurons and revealed that SERT expressed in PFC neurons is not required for the topographic organization of TCAs or the cortical neurons in the barrel cortex. Since innervation of cortical areas by the thalamic axons is the first step of the circuit formation in the cortex (Lopez-Bendito and Molnar, 2003), SERT expressed along the growing tangential TCA arbors could provide spatiotemporal guidance for establishing functional connectivity and cortical maps in the sensory reception subfield. In the PFC, SERT is expressed in L5/6 pyramidal neurons that project robustly to other brain areas, including the limbic system and brainstem. As noted above, SERTNull mice and human carriers of reduced SERT functionality alleles display altered dendritic morphology in the PFC and corticolimbic structures, and enhanced anxiety/depression behavior. The availability of SERTCortexΔ mice will provide a valuable tool for connecting spatiotemporal SERT expression to temporal-specific PFC topographic expression of ASD-associated genes, the synaptic architecture and activity within the PFC as well as PFC projections to the other brain areas, and PFC-regulation of behavior in the future.
Acknowledgments
This work was supported by grants from National Institute of Health to J.Y.S (MH098290, MH083982, MH105839) and Rose F. Kennedy Intellectual and Developmental Disabilities Research Center (P30 NIHHD71593).
References
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Farinas I, Grosschedl R, McConnell SK. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC, Blakely RD, Deneris ES, Levitt P. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472:347–350. doi: 10.1038/nature09972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij L, Tremblay RE, Szyf M, Benkelfat C. Genetic and early environmental influences on the serotonin system: consequences for brain development and risk for psychopathology. J Psychiatry Neurosci. 2015;40:5–18. doi: 10.1503/jpn.140099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, Vogel T, Akopov S, Mitkovski M, Agoston D, Sestan N, Molnar Z, Tarabykin V. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–392. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Chen X, Ye R, Gargus JJ, Blakely RD, Dobrenis K, Sze JY. Disruption of transient serotonin accumulation by non-serotonin-producing neurons impairs cortical map development. Cell Rep. 2015;10:346–358. doi: 10.1016/j.celrep.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote F, Fligny C, Bayard E, Launay JM, Gershon MD, Mallet J, Vodjdani G. Maternal serotonin is crucial for murine embryonic development. Proc Natl Acad Sci U S A. 2007;104:329–334. doi: 10.1073/pnas.0606722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Suckling J, Deoni SC, Lombardo MV, Bullmore ET, Baron-Cohen S, Catani M, Jezzard P, Barnes A, Bailey AJ, Williams SC, Murphy DG, Consortium, M.A. Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch Gen Psychiatry. 2012;69:195–209. doi: 10.1001/archgenpsychiatry.2011.1251. [DOI] [PubMed] [Google Scholar]
- Espinosa JS, Wheeler DG, Tsien RW, Luo L. Uncoupling dendrite growth and patterning: single-cell knockout analysis of NMDA receptor 2B. Neuron. 2009;62:205–217. doi: 10.1016/j.neuron.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Jensen CL, French HT, Stein AR, Huang SJ, Tolliver TJ, Murphy DL. Neurochemical, behavioral, and physiological effects of pharmacologically enhanced serotonin levels in serotonin transporter (SERT)-deficient mice. Psychopharmacology. 2008;201:203–218. doi: 10.1007/s00213-008-1268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Rakic P. Cortical evolution: judge the brain by its cover. Neuron. 2013;80:633–647. doi: 10.1016/j.neuron.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Development of cortical circuitry and cognitive function. Child Dev. 1987;58:601–622. [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci Off J Soc Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson SR, Mezey E, Hoffman BJ. Serotonin transporter messenger RNA in the developing rat brain: early expression in serotonergic neurons and transient expression in non-serotonergic neurons. Neuroscience. 1998;83:1185–1201. doi: 10.1016/s0306-4522(97)00444-2. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Harrington RA, Lee LC, Crum RM, Zimmerman AW, Hertz-Picciotto I. Serotonin hypothesis of autism: implications for selective serotonin reuptake inhibitor use during pregnancy. Autism Res Off J Int Soc Autism Res. 2013;6:149–168. doi: 10.1002/aur.1288. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, Mann K, Buchel C. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Iwasato T, Datwani A, Wolf AM, Nishiyama H, Taguchi Y, Tonegawa S, Knopfel T, Erzurumlu RS, Itohara S. Cortex-restricted disruption of NMDAR1 impairs neuronal patterns in the barrel cortex. Nature. 2000;406:726–731. doi: 10.1038/35021059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari G, Xie Y, Kullyev A, Liang B, Sze JY. Regulation of extrasynaptic 5-HT by serotonin reuptake transporter function in 5-HT-absorbing neurons underscores adaptation behavior in Caenorhabditis elegans. J Neurosci. 2011;31:8948–8957. doi: 10.1523/JNEUROSCI.1692-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM, Sanes JR. Development. The decade of the developing brain. Curr Opin Neurobiol. 2000;10:599–611. doi: 10.1016/s0959-4388(00)00136-7. [DOI] [PubMed] [Google Scholar]
- Judson MC, Eagleson KL, Wang L, Levitt P. Evidence of cell-nonautonomous changes in dendrite and dendritic spine morphology in the met-signaling-deficient mouse forebrain. J Comp Neurol. 2010;518:4463–4478. doi: 10.1002/cne.22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, Sousa AM, Pletikos M, Meyer KA, Sedmak G, Guennel T, Shin Y, Johnson MB, Krsnik Z, Mayer S, Fertuzinhos S, Umlauf S, Lisgo SN, Vortmeyer A, Weinberger DR, Mane S, Hyde TM, Huttner A, Reimers M, Kleinman JE, Sestan N. Spatiotemporal transcriptome of the human brain. Nature. 2011;478:483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM, Sze PY, Krebs H. Maternal influences on tryptophan hydroxylase activity in embryonic rat brain. Dev Neurosci. 1981;4:291–295. doi: 10.1159/000112768. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Cases O, Wehrle R, Blakely RD, Edwards RH, Gaspar P. Transient developmental expression of monoamine transporters in the rodent forebrain. J Comp Neurol. 1998;401:506–524. [PubMed] [Google Scholar]
- Lewis AJ, Galbally M, Gannon T, Symeonides C. Early life programming as a target for prevention of child and adolescent mental disorders. BMC Med. 2014;12:33. doi: 10.1186/1741-7015-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bendito G, Molnar Z. Thalamocortical development: how are we going to get there? Nat Rev Neurosci. 2003;4:276–289. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, Szafer A, Ebbert A, Riley ZL, Royall JJ, Aiona K, Arnold JM, Bennet C, Bertagnolli D, Brouner K, Butler S, Caldejon S, Carey A, Cuhaciyan C, Dalley RA, Dee N, Dolbeare TA, Facer BA, Feng D, Fliss TP, Gee G, Goldy J, Gourley L, Gregor BW, Gu G, Howard RE, Jochim JM, Kuan CL, Lau C, Lee CK, Lee F, Lemon TA, Lesnar P, McMurray B, Mastan N, Mosqueda N, Naluai-Cecchini T, Ngo NK, Nyhus J, Oldre A, Olson E, Parente J, Parker PD, Parry SE, Stevens A, Pletikos M, Reding M, Roll K, Sandman D, Sarreal M, Shapouri S, Shapovalova NV, Shen EH, Sjoquist N, Slaughterbeck CR, Smith M, Sodt AJ, Williams D, Zollei L, Fischl B, Gerstein MB, Geschwind DH, Glass IA, Hawrylycz MJ, Hevner RF, Huang H, Jones AR, Knowles JA, Levitt P, Phillips JW, Sestan N, Wohnoutka P, Dang C, Bernard A, Hohmann JG, Lein ES. Transcriptional landscape of the prenatal human brain. Nature. 2014;508:199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle V. The evolution of ideas concerning the function of the neocortex. Cereb Cortex. 1995;5:289–295. doi: 10.1093/cercor/5.4.289. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Sahara S. Genetic regulation of arealization of the neocortex. Curr Opin Neurobiol. 2008;18:90–100. doi: 10.1016/j.conb.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin Pharmacol Ther. 2009;86:672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikshak NN, Gandal MJ, Geschwind DH. Systems biology and gene networks in neurodevelopmental and neurodegenerative disorders. Nat Rev Genet. 2015;16:441–458. doi: 10.1038/nrg3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikshak NN, Luo R, Zhang A, Won H, Lowe JK, Chandran V, Horvath S, Geschwind DH. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell. 2013;155:1008–1021. doi: 10.1016/j.cell.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I, Arellano J, DeFelipe J, Gimenez-Amaya JM, Conciatori M, Marino R, Baldi A, Cabib S, Pascucci T, Uhl GR, Murphy DL, Lesch KP, Keller F. Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J Neurosci Off J Soc Neurosci. 2001;21:6862–6873. doi: 10.1523/JNEUROSCI.21-17-06862.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pletikos M, Sousa AM, Sedmak G, Meyer KA, Zhu Y, Cheng F, Li M, Kawasawa YI, Sestan N. Temporal specification and bilaterality of human neocortical topographic gene expression. Neuron. 2014;81:321–332. doi: 10.1016/j.neuron.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rash BG, Grove EA. Area and layer patterning in the developing cerebral cortex. Curr Opin Neurobiol. 2006;16:25–34. doi: 10.1016/j.conb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Rebello TJ, Yu Q, Goodfellow NM, Caffrey Cagliostro MK, Teissier A, Morelli E, Demireva EY, Chemiakine A, Rosoklija GB, Dwork AJ, Lambe EK, Gingrich JA, Ansorge MS. Postnatal day 2 to 11 constitutes a 5-HT-sensitive period impacting adult mPFC function. J Neurosci. 2014;34:12379–12393. doi: 10.1523/JNEUROSCI.1020-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum Dev. 1991;26:61–67. doi: 10.1016/0378-3782(91)90044-4. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Kramer R, Blakely RD, Murphy DL, Verrey F. The SLC6 transporters: perspectives on structure, functions, regulation, and models for transporter dysfunction. Pflugers Arch. 2014;466:25–42. doi: 10.1007/s00424-013-1410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci U S A. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson KL, Weaver KJ, de Villers-Sidani E, Lu JY, Cai Z, Pang Y, Rodriguez-Porcel F, Paul IA, Merzenich M, Lin RC. Perinatal antidepressant exposure alters cortical network function in rodents. Proc Natl Acad Sci U S A. 2011;108:18465–18470. doi: 10.1073/pnas.1109353108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- State MW, Levitt P. The conundrums of understanding genetic risks for autism spectrum disorders. Nat Neurosci. 2011;14:1499–1506. doi: 10.1038/nn.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Toda T, Homma D, Tokuoka H, Hayakawa I, Sugimoto Y, Ichinose H, Kawasaki H. Birth regulates the initiation of sensory map formation through serotonin signaling. Dev Cell. 2013;27:32–46. doi: 10.1016/j.devcel.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Upton AL, Salichon N, Lebrand C, Ravary A, Blakely R, Seif I, Gaspar P. Excess of serotonin (5-HT) alters the segregation of ispilateral and contralateral retinal projections in monoamine oxidase A knock-out mice: possible role of 5-HT uptake in retinal ganglion cells during development. J Neurosci Off J Soc Neurosci. 1999;19:7007–7024. doi: 10.1523/JNEUROSCI.19-16-07007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ, Geschwind DH. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci Off J Soc Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsey AJ, Sanders SJ, Li M, Dong S, Tebbenkamp AT, Muhle RA, Reilly SK, Lin L, Fertuzinhos S, Miller JA, Murtha MT, Bichsel C, Niu W, Cotney J, Ercan-Sencicek AG, Gockley J, Gupta AR, Han W, He X, Hoffman EJ, Klei L, Lei J, Liu W, Liu L, Lu C, Xu X, Zhu Y, Mane SM, Lein ES, Wei L, Noonan JP, Roeder K, Devlin B, Sestan N, State MW. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell. 2013;155:997–1007. doi: 10.1016/j.cell.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witteveen JS, Middelman A, van Hulten JA, Martens GJ, Homberg JR, Kolk SM. Lack of serotonin reuptake during brain development alters rostral raphe-prefrontal network formation. Front Cell Neurosci. 2013;7:143. doi: 10.3389/fncel.2013.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]


