Abstract
Purpose
To describe the efficacy of radical prostatectomy to achieve complete primary tumor excision while preserving erectile function in a cohort of patients with high risk features, in whom surgical resection was tailored according to clinical staging, biopsy data, preoperative imaging, and intraoperative findings.
Materials and Methods
In a retrospective review, we identified 584 patients with high-risk features (Prostate-specific antigen ≥ 20ng/mL; clinical stage ≥ T3; preoperative Gleason grade 8–10) who underwent radical prostatectomy between 2006 and 2012. The probability of neurovascular bundle preservation was estimated based on preoperative characteristics. Positive surgical margin rates and erectile function recovery were determined in patients who had some degree of neurovascular bundle preservation.
Results
The neurovascular bundles were resected bilaterally in 69/584 (12%), and unilaterally in 91/584 (16%) patients. The rest had some degree of bilateral neurovascular bundle preservation. Preoperative features associated with a lower probability of neurovascular bundle preservation were: biopsy primary Gleason grade 5, and clinical stage T3. Among patients who underwent some degree of neurovascular bundle preservation, 125/515(24%) had a positive surgical margin and 75/160(47%) men with preoperatively functional erections and available erectile function follow-up had recovered erectile function within 2 years.
Conclusions
High-risk features should not be considered an indication for complete bilateral neurovascular bundle resection. Some degree of neurovascular bundle preservation can be safely performed by high volume surgeons in the majority of these patients with an acceptable rate of positive surgical margins. Nearly half of high-risk patients with functional erections preoperatively recover erectile function after radical prostatectomy.
Keywords: Prostate Cancer, Prostatectomy, Erectile Dysfunction
INTRODUCTION
The optimal treatment of high-risk clinically localized prostate cancer is controversial due to a lack of randomized trials comparing different therapies, and retrospective studies showing disparate results.1 Current guidelines endorse radical prostatectomy (RP) as an acceptable treatment option for select high-risk patients: roughly one-half of these men can be cured with surgery alone and thus avoid additional treatment.2 When compared with radiotherapy alone or radiotherapy plus androgen deprivation therapy (ADT), studies reporting outcomes of institutional series3,4 and population based datasets5,6 suggest RP may be associated with a lower probability of developing distant metastasis as well as increased cancer-specific and overall survival.
Complete tumor excision is the primary goal of RP. However, a wider resection can compromise the neurovascular bundle (NVB) and hinder erectile function recovery. Data on the feasibility and efficacy of NVB preservation in high-risk patients has thus far been limited. While some authors suggest few patients with cT3 disease are candidates for NVB preservation,7 the incorporation of multi-parametric magnetic resonance imaging (MRI) to preoperative staging has challenged this paradigm, as recent studies have confirmed that MRI can identify extraprostatic extension (EPE) to a better extent than trans-rectal ultrasound and/or digital rectal examination.8,9
Positive surgical margins (PSM) have been consistently associated with an increased risk of biochemical recurrence (BCR), and are uniformly considered an adverse oncologic outcome.10 PSM may also generate anxiety among affected patients, often triggering additional therapy. In an effort to reduce PSM rates in patients at increased risk of EPE, surgeons may perform a wider resection including periprostatic structures. However, postoperative erectile dysfunction (ED) is related to damage of the NVB, so the expected oncologic advantage of a wider resection may come at the expense of an increased risk of postoperative ED if these nerves are compromised. Institutional and single surgeon series of high-risk prostate cancer patients show that surgeons use different techniques and have disparate results; reported NVB preservation rates in cohorts of high-risk prostate cancer patients range from 26% to 91%; PSM rates range from 31% to 56%, and postoperative erectile function recovery ranged from 25% to 60%.11–13
We sought to assess the efficacy of RP to remove the primary tumor with negative surgical margins while maintaining erectile function in a cohort of high-risk prostate cancer patients for whom the decision to perform NVB preservation was individualized, and the surgical resection plane was tailored on each side according to clinical staging, Gleason score, location of positive biopsies, preoperative MRI, and intraoperative findings. We also investigated preoperative characteristics associated with a higher probability of having both NVB resected.
MATERIALS AND METHODS
Patient Cohort
After institutional review board approval, our prospectively-maintained clinical database was queried for men who underwent a RP at Memorial Sloan Kettering Cancer Center (MSKCC) between 2006–2012 as the primary treatment for prostate cancer and who had at least one high-risk criteria as defined by the NCCN: PSA 20 ng/mL or higher, clinical stage T3 or greater, or preoperative Gleason score 8–10.14 Patients were excluded if they had undergone previous radiotherapy or ADT.
Surgeon Experience
All of the 12 surgeons performing the procedures were high-volume surgeons and performed more than 50 radical prostatectomies per year during the study period. Previous experience ranged from 3 to 30 years.
Main Outcome Measures
Primary outcomes were NVB preservation status, PSM rate, and postoperative erectile function. NVB preservation status was graded and reported for each side at the time of RP by the attending surgeon using the 4-point scale previously described by Moskovic et al.15 NVB preservation grades 1–3 were considered “some degree of NVB preservation” and grade 4 was defined as “wide excision of the NVB.” Patients who underwent bilateral NVB resection were considered to have no NVB preservation; all other patients were considered to have some degree of NVB preservation.
Preoperative erectile function was assessed by the surgeon. Postoperative erectile function was assessed as a routine part of clinical follow-up practice at MSKCC through a system known as Webcore:16 patients were asked to complete the IIEF-6 questionnaire preoperatively and at each follow-up visit.17 Functional erections were defined as a score of ≥ 22 on the IIEF-6 questionnaire. If patient-reported IIEF-6 survey data was not available we used surgeon-reported quality of life (QOL). Appendix 1 describes the algorithm used to impute missing data.
We also assessed the proportion of patients with preoperative functional erections who received salvage treatments, as these may affect erectile function recovery. Adjuvant treatment was not routinely used. Only patients with a measurable PSA after surgery were offered early salvage treatment.
Statistical Analysis
We sought to investigate preoperative characteristics associated with a higher probability of undergoing bilateral NVB resection (non-NVB preservation procedure). The proportion of patients that underwent some degree of NVB preservation was estimated for the following preoperative characteristics: biopsy Gleason score 8 – 10; total PSA > 20 ng/ml; primary biopsy Gleason grade 5; and clinical stage T3 (by digital rectal examination). Preoperative characteristics and pathologic staging of patients undergoing some degree of NVB preservation were compared to those who underwent bilateral NVB resection, using the Kruskal-Wallis test for continuous variables and Fisher’s exact test for categorical variables. All analyses were conducted using Stata 12 (Stata Corp., College Station, Texas).
RESULTS
Surgical Approach
A total of 5104 men underwent RP at MSKCC during the study period. We identified 584 (11%) patients satisfying at least one high-risk criterion according to the NCCN guidelines, who underwent open retropubic (322), laparoscopic (154), or robot-assisted laparoscopic RP (108). Patient characteristics are displayed in Table 1.
Table 1.
Patient characteristics by NVB preservation status. Values are displayed as median (interquartile range) or frequency (percentage). (Total patients in study, N = 584)
| Characteristics | Bilateral NVB resection |
Some degree of NVB preservation |
p-value* |
|---|---|---|---|
| Number of patients | 69 (12%) | 515 (88%) | |
| NVB preservation Status | |||
| Non-NS | 69 (100%) | ||
| Unilateral NS | 91 (18%) | ||
| Bilateral NS | 424 (82%) | ||
| Functional erections at Baseline | |||
| Potent | 19 (28%) | 270 (52%) | <0.0001 |
| Unknown | 27 (39%) | 166 (32%) | |
| Pre-operative PSA (ng/mL) (N=583) | 10.0 (5.8, 15.8) | 6.7 (4.6, 12.8) | 0.006 |
| Clinical T Stage | |||
| T1 | 10 (14%) | 176 (34%) | 0.001 |
| T2 | 32 (46%) | 211 (41%) | |
| T3 | 27 (39%) | 126 (24%) | |
| Unknown | 0 (0%) | 2 (0.4%) | |
| Gleason Score at Biopsy | |||
| ≤6 | 1 (1.4%) | 19 (3.7%) | 0.6 |
| 7 | 14 (20%) | 91 (18%) | |
| ≥8 | 53 (77%) | 397 (77%) | |
| Unknown | 1 (1.4%) | 8 (1.6%) | |
| Age at Surgery | 64 (59, 71) | 62 (57, 67) | 0.018 |
| Gleason Score at RP | |||
| ≤6 | 0 (0%) | 6 (1.2%) | 0.053 |
| 7 | 27 (39%) | 274 (53%) | |
| ≥8 | 42 (61%) | 233 (45%) | |
| Unknown | 0 (0%) | 2 (0.4%) | |
| Seminal Vesical Invasion | |||
| Present | 35 (51%) | 122 (24%) | <0.0001 |
| Unknown | 1 (1.4%) | 2 (0.4%) | |
| Extracapsular Extension | |||
| Present | 65 (94%) | 375 (73%) | <0.0001 |
| Unknown | 0 (0%) | 2 (0.4%) |
p-values determined by Kruskal-Wallis for continuous variables and Fisher’s exact test for categorical variables
Preoperative Factors Associated with NVB Status
NVB preservation status was significantly associated with age and erectile function prior to surgery: bilateral NVB resection was more frequent in older patients and in those with preoperative ED. Of men with preoperative functional erections, 270/289 (93%) underwent some degree of NVB preservation. Men who underwent some degree of NVB preservation had a significantly lower clinical T stage and a lower preoperative total PSA than patients who underwent bilateral nerve resection (all p < 0.01). Table 2 displays the proportion of patients who underwent some degree of NVB preservation among all patients and among those who had functional erections prior to surgery, differentiated by various patient characteristics. Among patients with a Gleason score of 8 or higher and with a total PSA of 20 ng/mL or higher, 405/459 underwent some degree of NVB preservation. Men with primary biopsy Gleason grade 5 had the lowest rate of bilateral NVB preservation, followed by those with clinical stage T3. We did not find sufficient evidence to suggest that the number of high-risk features was associated with the probability of NVB resection among patients who were potent at baseline (Fisher’s exact test; p = 0.075).
Table 2.
The proportion of patients who underwent some degree of NVB preservation among all patients and among those who were potent prior to surgery, differentiated by various patient characteristics.
| All Patients | Functional erections at Baseline |
|||
|---|---|---|---|---|
| Condition | N | Some degree of NVB preservation (95% CI) |
N | Some degree of NVB preservation (95% CI) |
| All Patients | 584 | 88% (85%–91%) | 289 | 93% (90%–96%) |
| Biopsy Gleason 8–10 | 450 | 88% (85%–91%) | 222 | 94% (90%–97%) |
| Total PSA>20 ng/mL | 106 | 86% (78%–92%) | 49 | 92% (80%–98%) |
| Primary Biopsy Gleason 5 | 24 | 63% (41%–81%) | 11 | 82% (48%–98%) |
| Clinical T3 | 153 | 82% (75%–88%) | 77 | 88% (79%–95%) |
Surgical Outcomes
Bilateral NVB resection was performed in 69/584 (12%); while 515/584 (88%) underwent some degree of NVB preservation (95% CI, 86%–91%)(Fig. 1). The majority of patients (424/584; 73%) underwent some degree of bilateral NVB preservation. Overall, the PSM after RP in patients with high-risk features was 159/584 (27%); the PSM rate in patients that underwent some degree of NVB preservation was 125/515 (24%).
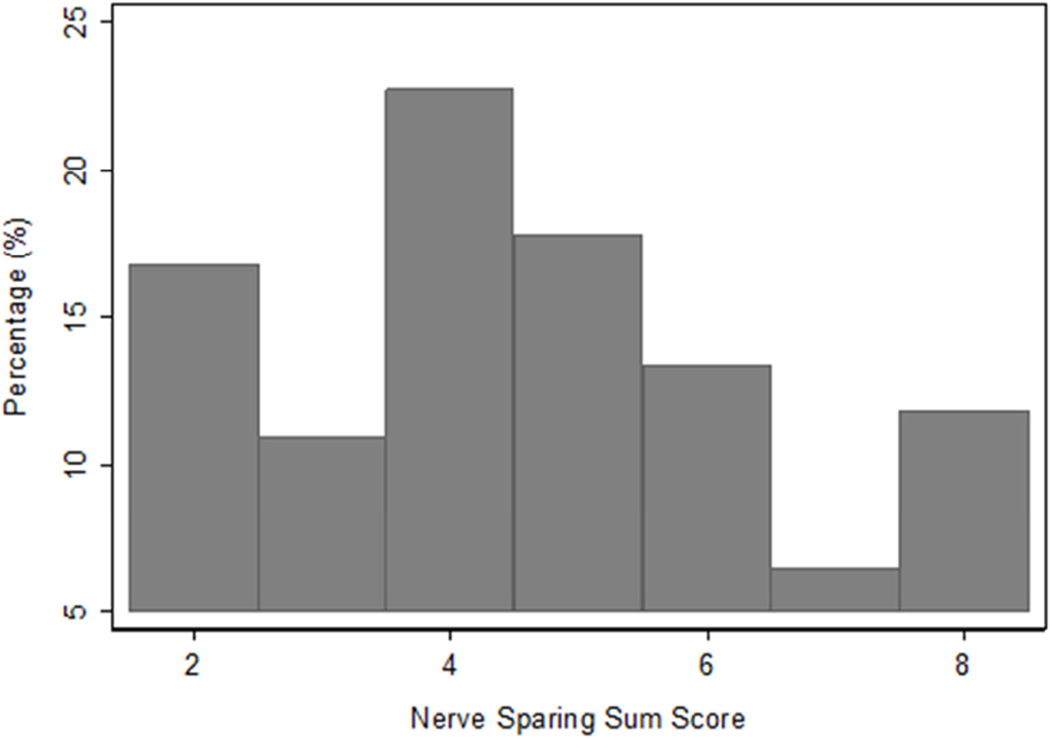
Figure 1.
Distribution of NVB preservation score sums. The score is recorded at each side. Complete Preservation denotes score 1 on both sides (sum = 2), while complete resection denotes score 4 in both sides (sum =8). Some preservation includes all the other possible score combinations (1-2; 1-3; 1-4; 2-2; 2-3; 2-4; 3-3; 3-4).
Salvage treatments
Overall, among the 289 patients who had functional erections preoperatively, the Kaplan-Meier estimated probability of undergoing salvage radiotherapy within 2 years of RP was 24% (95% CI, 19%–30%), the probability of receiving ADT within 2 years of RP was 28% (95% CI, 23%–34%), and the probability of undergoing either salvage radiotherapy or ADT within 2 years of RP was 34% (95% CI, 28%–40%).
Erectile Function Recovery in Men with Some Degree of NVB Preservation
Among men who had functional erections prior to surgery, we identified 166 with 24-month erectile function follow-up data available; this data was patient-reported IIEF-6 scores in 86% (143/166), and surgeon-reported in 14% (23/166).
Of the 166 patients who had functional erections prior to surgery and 2-year follow-up available, 160 patients had received some degree of NVB preservation: in this group the Kaplan-Meier estimated probability of salvage radiotherapy, ADT, or either treatment within 2 years of RP was 21% (95% CI, 16%–29%), 20% (95% CI, 14%–27%) and 27% (95% CI, 21%–35%), respectively. Of these patients, 75/160 (47%; 95% CI, 39%–55%) had regained erectile function at 24 months (Table 3).
Table 3.
Outcomes of patients who underwent some degree of NVB preservation (N = 515). Functional erections were defined as IIEF score ≥22, or if surgeon reported full erections in case of missing patient reported data. Patients with functional erections prior to surgery were analyzed for the outcome of erectile function.
| Outcome Rate | (95% CI) |
|---|---|
| Positive Surgical Margins (N=515) | 24% (21%–28%) |
| Functional erections at baseline | |
| Functional erections at 12 months (N=181) | 32% (25%–39%) |
| Functional erections at 24 months (N=160) | 47% (39%–55%) |
DISCUSSION
In this cohort of patients with NCCN-defined high-risk features, only 12% underwent bilateral NVB resection, 27% had PSM, and 47% of patients with preoperatively functional erections had regained erectile function, defined as an IIEF-6 score ≥ 22, within 2 years. These results show that bilateral NVB resection is rarely indicated: some degree of NVB preservation can be safely performed in the vast majority of high-risk patients undergoing RP. The PSM rate (27%) falls within an acceptable range considering this cohort represents the 10% highest risk of all patients undergoing RP at our institution, and more than 75% of these patients had EPE on final pathology. Moreover, PSM rate was slightly lower in patients that received NVB preservation (24%), reflecting that this procedure is safe in carefully selected patients.
NVB preservation is appropriately approached more cautiously in patients thought to be at higher risk for EPE. Our results indicate that NVB preservation is feasible even in patients with EPE, and the rate of PSM compares favorably with previously published outcomes of high risk patients. Ward et al reported a series of 841 patients staged cT3 that underwent RP: bilateral NVB resection was performed in 74% and PSM were present in 56%, while surgeon reported potency in preoperatively potent patients was 25%.11 In another series of 123 high-risk patients that underwent robotic RP, Lavery et al reported that bilateral NVB resection was performed in 27%, and PSM were present in 31%; potency (defined as a SHIM score > 16) was reported by 58% of patients.12 In contrast, in a single surgeon series of 288 patients staged ≥ cT2c who underwent RP by Catalona, bilateral NVB resection was performed in 9%; PSM were present in 41%, and surgeon reported potency was 60%.13 Novara et al reported outcomes of a series of patients undergoing roboticassisted laparoscopic RP in which all patients underwent some degree of NVB preservation: PSM were present in 64% of pT3 patients, and among patients satisfying the D’Amico criteria for high-risk, 33% were potent after surgery.18 Jayram published an institutional series of 148 patients satisfying the D’Amico criteria for high risk.19 In this cohort, bilateral nerve preservation was feasible in 28%, and PSM were present in 21%. 2 years following surgery, total impotence (inability to masturbate) was 48%.19
The decision to perform NVB preservation is a complex algorithm. Table 1 indicates some of the selection criteria: younger patients with better erectile function at baseline and fewer high-risk features were more likely to have NVB preservation. Previous attempts to predict EPE have sometimes been used as a guide to determine when a surgeon should widely resect one or both NVB.20,21 However, these tools do not incorporate MRI findings in the decision-making process. At MSKCC, the dissection plane is tailored according to all available preoperative information estimating the location, grade and volume of the tumor, including clinical biopsy data, number, location, and grade of positive cores, digital rectal examination, MRI findings, and intraoperative cues. The main objective is always the complete resection of the primary tumor; surgeons attempt to preserve as much of the NVB as feasible while observing the basic principles of surgical oncology. If an MRI shows convincing evidence of tumor invasion of the NVB, a wider resection may be required on that side to prevent a positive surgical margin, and it might not be possible to preserve that NVB. However, tumor invasion of the NVB is uncommon and, as demonstrated by these results, we believe that some degree of NVB preservation can be performed safely even in the presence of high-risk features. Nerve preservation should not be considered an all-or-none phenomenon, as several planes can be entertained while dissecting the prostate from its surrounding tissues. In our study, the plane along which prostatic dissection from the NVB was performed was chosen individually at the surgeons’ discretion. While no standard tool was used to decide whether to preserve or resect the NVB, our results show that experienced surgeons can make these decisions without an increase in the likelihood of a PSM.
Our study has some limitations. First, selection bias is present; patients undergoing NVB preservation had less advanced disease. We didn’t find any evidence of increased risk of PSM in those who were selected for either unilateral or bilateral NVB preservation surgery; however, we cannot evaluate this association reliably with our dataset. A randomized trial would be required to determine if NVB preservation increases the risk of PSM. Second, while the use of patient-reported outcomes is an advantage of the study, missing patient-reported QOL data was replaced by surgeon-reported erectile function, which has been shown to overestimate recovery. However, the criteria we used to define functional erections (IIEF-6 ≥ 22) exclude patients with mild to moderate ED. This cutoff was chosen based on data from a sensitivity analysis by Cappelleri et al, showing that the optimal cutoff is related to the prevalence of ED in the study population, with an optimal cutoff ≥ 22 for populations with a low ED prevalence such as our study population (only patients with preoperative functional erections).22 Third, we report PSM rates instead of BCR or clinical recurrence. While PSM is a surrogate oncologic outcome, we believe it represents a better marker of surgical quality, as many biochemical recurrences in this group are due to distant failure. Fourth, although an association between NVB preservation and urinary function recovery has been suggested, the relationship between NVB preservation and erectile function preservation is clearly established; therefore, we chose to limit the scope of this paper to erectile function recovery.
The main limitation to the generalizability of these results is that this data represents a cohort from single a high-volume academic institution where RP is performed by high volume surgeons. Also, the routine use of adjuvant radiotherapy for pT3 disease or PSM may have a negative impact on postoperative potency. At our institution, adjuvant radiotherapy or ADT are not routinely employed. The benefit of NVB preservation as observed in our data may be attenuated if adjuvant radiotherapy or ADT are routinely used.
We believe this data supports that some degree of NVB preservation can be safely performed in most high-risk patients undergoing RP, and it’s effective in preserving erectile function when performed by high volume surgeons in an academic setting. Further research should aim to integrate clinical, biopsy and imaging data with intraoperative cues in a standardized fashion to identify patients that actually require NVB resection to achieve negative surgical margins at RP.
CONCLUSIONS
High-risk features should not be considered an indication for complete bilateral neurovascular bundle resection. Some degree of neurovascular bundle preservation can be safely performed by high volume surgeons in the majority of these patients with an acceptable rate of positive surgical margins. Nearly half of high-risk patients with functional erections preoperatively recover erectile function after radical prostatectomy.
Acknowledgments
Supported by: the Sidney Kimmel Center for Prostate and Urologic Cancers; funds provided by David H. Koch through the Prostate Cancer Foundation; and NIH/NCI Cancer Center Support Grant to MSKCC under award number P30 CA008748.
ABBREVIATIONS AND ACRONYMS
- ADT
Androgen Deprivation Therapy
- BCR
Biochemical Recurrence
- EPE
Extraprostatic Extension
- ED
Erectile Dysfunction
- IIEF
International Index of Erectile Function
- MRI
Magnetic Resonance Imaging
- MSKCC
Memorial Sloan Kettering Cancer Center
- NCCN
National Comprehensive Cancer Network
- NVB
Neurovascular Bundle
- PSA
Prostate-Specific Antigen
- PSM
Positive Surgical Margins
- RP
Radical Prostatectomy
- QOL
Quality Of Life
APPENDIX 1
For patients who did not provide QOL data within a 2 month window around 24 months, the following algorithm was used to imputed missing data: patients reporting erectile function before 24 months post-surgery were assumed to be functional at 24 months; patients reporting erectile dysfunction after 24 months post-surgery were assumed not to be functional at 24 months; patients reporting no function before 24 months with missing data after 24 months were excluded from analysis.
In all patients, erectile function was assessed by the surgeon before the operation. 23/160 (14%) patient records were missing patient-reported erectile function status at 24 months: these patients were considered potent if the surgeon reported “normal erectile function with full erections” or “full erections but recently diminished.”
Footnotes
DISCLAIMER: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our subscribers we are providing this early version of the article. The paper will be copy edited and typeset, and proof will be reviewed before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to The Journal pertain.
REFERENCES
- 1.Boorjian SA, Eastham JA, Graefen M, et al. A critical analysis of the long-term impact of radical prostatectomy on cancer control and function outcomes. Eur Urol. 2012;61(4):664–675. doi: 10.1016/j.eururo.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 2.Carver BS, Bianco FJ, Jr, Scardino PT, et al. Long-term outcome following radical prostatectomy in men with clinical stage T3 prostate cancer. J Urol. 2006;176(2):564–568. doi: 10.1016/j.juro.2006.03.093. [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky MJ, Eastham JA, Cronin AM, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol. 2010;28(9):1508–1513. doi: 10.1200/JCO.2009.22.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nepple KG, Stephenson AJ, Kallogjeri D, et al. Mortality after prostate cancer treatment with radical prostatectomy, external-beam radiation therapy, or brachytherapy in men without comorbidity. Eur Urol. 2013;64(3):372–378. doi: 10.1016/j.eururo.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdollah F, Schmitges J, Sun M, et al. Comparison of mortality outcomes after radical prostatectomy versus radiotherapy in patients with localized prostate cancer: a population-based analysis. Int J Urol. 2012;19(9):836–844. doi: 10.1111/j.1442-2042.2012.03052.x. [DOI] [PubMed] [Google Scholar]
- 6.Sooriakumaran P, Nyberg T, Akre O, et al. Comparative effectiveness of radical prostatectomy and radiotherapy in prostate cancer: observational study of mortality outcomes. BMJ. 2014;348:g1502. doi: 10.1136/bmj.g1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skinner EC, Glode LM. High-risk localized prostate cancer: primary surgery and adjuvant therapy. Urol Oncol. 2003;21:219. doi: 10.1016/s1078-1439(03)00018-8. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence EM, Gallagher FA, Barrett T, et al. Preoperative 3-T diffusion-weighted MRI for the qualitative and quantitative assessment of extracapsular extension in patients with intermediate- or high-risk prostate cancer. AJR. 2014 Sep;203(3):W280–W286. doi: 10.2214/AJR.13.11754. [DOI] [PubMed] [Google Scholar]
- 9.Boesen L, Chabanova E, Logager V, et al. Prostate cancer staging with extracapsular extension risk scoring using multiparametric MRI: a correlation with histopathology. Eur Radiol. 2015;25(6):1776–1785. doi: 10.1007/s00330-014-3543-9. [DOI] [PubMed] [Google Scholar]
- 10.Yossepowitch O, Briganti A, Eastham JA, et al. Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol. 2014;65(2):303–313. doi: 10.1016/j.eururo.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 11.Ward JF, Slezak JM, Blute ML, et al. Radical prostatectomy for clinically advanced (cT3) prostate cancer since the advent of prostate-specific antigen testing: 15-year outcome. BJU Int. 2005;95:751. doi: 10.1111/j.1464-410X.2005.05394.x. [DOI] [PubMed] [Google Scholar]
- 12.Lavery HJ, Nabizada-Pace F, Carlucci JR, et al. Nerve-sparing robotic prostatectomy in preoperatively high-risk patients is safe and efficacious. Urol Oncol. 2012;30:26. doi: 10.1016/j.urolonc.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Loeb S, Smith ND, Roehl KA, et al. Intermediate-term potency, continence, and survival outcomes of radical prostatectomy for clinically high-risk or locally advanced prostate cancer. Urology. 2007;69:1170. doi: 10.1016/j.urology.2007.02.054. [DOI] [PubMed] [Google Scholar]
- 14.Mohler J, Armstrong AJ, Bahnson RR, et al. NCCN clinical practice guidelines in oncology: prostate cancer. Version. 1.2015. [Accessed April 20, 2015];National Comprehensive Cancer Network. 2014 http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Published October 24. [Google Scholar]
- 15.Moskovic DJ, Alphs H, Nelson CJ, et al. Subjective characterization of NVB preservation predicts recovery of erectile function after radical prostatectomy: defining the utility of a NVB preservation grading system. J Sex Med. 2011;8:255. doi: 10.1111/j.1743-6109.2010.01972.x. [DOI] [PubMed] [Google Scholar]
- 16.Vickers AJ, Savage CJ, Shouery M, et al. Validation study of a web-based assessment of functional recovery after radical prostatectomy. Health Qual Life Outcomes. 2010;8:82. doi: 10.1186/1477-7525-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11(6):319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 18.Novara G, Ficarra V, D'Elia C, et al. Preoperative criteria to select patients for bilateral nerve-sparing robotic-assisted radical prostatectomy. J Sex Med. 2010;7:839. doi: 10.1111/j.1743-6109.2009.01589.x. [DOI] [PubMed] [Google Scholar]
- 19.Jayram G, Decastro GJ, Large MC, et al. Robotic radical prostatectomy in patients with high-risk disease: a review of short-term outcomes from a high-volume center. J Endourol. 2011;25:455. doi: 10.1089/end.2010.0349. [DOI] [PubMed] [Google Scholar]
- 20.Graefen M, Haese A, Pichlmeier U, et al. A validated strategy for side specific prediction of organ confined prostate cancer: a tool to select for NVB preservation radical prostatectomy. J Urol. 2001;165:857. [PubMed] [Google Scholar]
- 21.Zorn KC, Gofrit ON, Steinberg GP, et al. Planned nerve preservation to reduce positive surgical margins during robot-assisted laparoscopic radical prostatectomy. J Endourol. 2008;22:1303. doi: 10.1089/end.2008.0009. [DOI] [PubMed] [Google Scholar]
- 22.Cappelleri JC, Rosen RC, Smith MD, et al. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology. 1999 Aug;54(2):346–351. doi: 10.1016/s0090-4295(99)00099-0. [DOI] [PubMed] [Google Scholar]