Abstract
The influenza PA-X protein is translated from the PA open reading frame from frameshifting and suppresses cellular gene expression due to its ribonuclease activity. We further defined the functional roles of PA-X by comparing PA-X proteins from two related viruses – equine influenza (EIV) and canine influenza (CIV) H3N8 – that differ in a C-terminal truncation and internal mutations. In vitro reporter gene assays revealed that both proteins were able to suppress gene expression. Interestingly, EIV PA-X demonstrated ~50% greater activity compared to CIV PA-X, and we identified the mutations that caused this difference. We used RNA-seq to evaluate the effects of PA-X on host gene expression after transfection into cultured cells. There were no significant differences in this property between EIV and CIV PA-X proteins, but expression of either resulted in the up-regulation of genes when compared to controls, most notably immunity-related proteins, trafficking proteins, and transcription factors.
Keywords: influenza, PA-X, equine H3N8, canine H3N8, gene suppression, RNA-seq
INTRODUCTION
The segmented influenza A virus (IAV) genome encodes 9 structural proteins and 3 nonstructural proteins, although it potentially encodes several other proteins (1). One non-structural protein, termed PA-X, was first identified in 2012 and is derived from the PA segment. It shares the first 191 amino acids with the PA protein, but has a unique C-terminal region derived from a +1 frameshift during translation (2, 3). Two natural variants of PA-X exist; the more common form has a length of 252 amino acids, while a C-terminally truncated 232 amino acid variant is found in H3N8 and H3N2 canine influenza viruses, the human H1N1/09 pandemic virus, and in some subtypes of swine influenza virus (3). Interestingly, it was recently shown that the PA-X Cterminal truncation in swine influenza may play a role in viral adaptation in pigs (4).
Several studies characterizing the infections of mutant viruses that expressed varying levels of PA-X showed that it modulated the host immune response, virus pathogenicity, and virus growth both in vitro and in vivo (5–9). Interestingly, the biological impact of the protein was dependent on the virus subtype and the host. For example, no difference in virus replication was observed comparing the wild type 1918 H1N1 and PA-X deficient virus infections in mice (2), while PA-X deficient HPAIV H5N1 reached higher titers in cell cultures and in mice compared to the wild type (6). Furthermore, it was shown that wild type 2009 human H1N1 virus replicated better in human respiratory cells compared to a mutant that expressed lower levels of PA-X (8). In addition to influencing virus replication, PA-X suppresses the host immune response by degrading host transcripts by its ribonuclease activity. For instance, loss of PA-X resulted in a stronger inflammatory response in mice, chickens, and ducks (2, 6, 7), an increased expression of IFN-β in mice (8), as well as greater virulence in the animal models. The ribonuclease activity of PA-X was demonstrated by its ability to suppress reporter gene expression and confirmed by incubating RNA substrates with purified PA-X protein (10). More recently it was shown that this ribonuclease activity was specific for host transcripts generated from Pol II while ignoring products from Pol I and Pol III (11). This ribonuclease domain has been attributed to the N terminal region, although several recent publications have shown that the C-terminal region may also be important in regulating ribonuclease activity (10, 12, 13). Interestingly, PA-X has also been shown to modulate other virus-host interactions such as preventing stress granule formation (and thereby preventing translational arrest), increasing the accumulation of poly(A)-binding proteins within the nucleus, and exhibiting anti-apoptotic activity (5, 6, 14).
The equine influenza (EIV) and canine influenza (CIV) H3N8 PA-X proteins differ in both length and sequence. We therefore sought to examine these proteins for functional differences by comparing gene suppression ability using reporter gene assays and by evaluating the details of their influence on host gene expression through RNA sequencing (RNA-seq). CIV emerged from EIV around 2000, and since then it has fixed a unique set of mutations in all its genes, including PA-X (15). By evaluating the biology of the two PA-X proteins we sought to further define PA-X function and to gain a better understanding of virus evolution and adaptation.
MATERIALS AND METHODS
Cells and cell culture
HEK293 human cells were grown in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C under 5% CO2.
PA-X protein sequence analysis
EIV and CIV PA-X protein sequences were compared using sequences of virus isolates deposited in GenBank (NCBI). In this context a “consensus” sequence is used to define the most common amino acid at each position for each EIV and CIV when comparing multiple protein sequences. We utilized EIV isolates sampled close to the ancestor CIV, starting from 1990 to 2013, and CIV isolates sampled soon after the emergence in dogs, from 2004 to 2013. Sequence alignments and the generation of consensus sequences were performed as described previously (15).
Plasmids and cloning
EIV and CIV PA-X genes were cloned into a mammalian expression plasmid, pcDNA3.1(−), and also into a T7 promoter plasmid, pT7CFE1-Chis (Thermo Fisher Scientific) as described previously (2). The EIV NP gene was also cloned into pcDNA3.1(−) as a control. The genes were derived from strains A/equine/NY/61191/2003 and A/canine/NY/dog23/2009 (15), and the sequences were checked to ensure they matched the consensus. A mammalian plasmid expressing green fluorescent protein (GFP) under the control of the CMV promoter (pCAGGS-GFP) was kindly provided by Dr. Luis Martinez-Sobrido, and a mammalian plasmid expressing β-galactosidase (pSV-β-Galactosidase) was purchased from Promega.
Site-directed mutagenesis
Custom primers were constructed and used in Phusion (New England Biolabs) PCR mutagenesis following the commercial protocol. All mutagenesis of EIV and CIV PA-X were conducted in the pcDNA3.1(−) background. Single mutations in the EIV PA-X background included: D27N, D108A, S231F, and a 20 amino acid C-terminal truncation. Single mutations in the CIV PA-X background included: N27D, D108A, F231S, and a 20 amino acid C-terminal elongation. Combinations of mutations were made in the CIV PA-X background as well: N27D F231S, N27D with the C-terminal elongation, F231S with the C-terminal elongation, and N27D F231S with the C-terminal elongation. Based on the sequences, EIV PAX wild type and CIV PA-X N27D F231S with the C-terminal elongation were identical. Similarly, EIV PA-X with the C-terminal truncation and CIV PA-X N27D F231S also had the same sequences. Lastly, EIV PA-X D27N and EIV PA-X S231F had the same sequences as CIV PAX F231S with the C-terminal elongation and CIV PA-X N27D with the C-terminal elongation, respectively.
In vitro translation (IVT)
Both EIV and CIV PA-X were translated by in vitro translation using the 1-Step Human Coupled IVT Kit (Thermo Fisher Scientific). After translation samples were denatured and separated on a 10% SDS-PAGE gel by electrophoresis. The proteins were transferred onto a nitrocellulose membrane using the Trans-Blot Turbo Transfer System (Bio-Rad). After transfer, the membrane was blocked in 5% nonfat dried milk overnight on a shaker at 4°C. The next day the membrane was incubated with an affinity purified rabbit IgG anti-PA-X peptide antibody (Pacific Immunology) for 1 h on a shaker. This antibody was custom produced using the PA-X peptide sequence VSPREAKRQLKKDLKSQG (2). Next, the membrane was washed thoroughly using PBST and incubated with a goat IgG anti-rabbit HRP conjugated antibody (Jackson ImmunoResearch Laboratories, Inc) for 1 h on a shaker. The membrane was washed thoroughly using PBST and incubated with SuperSignal (Thermo Fisher Scientific) chemiluminescent substrate for 3 min. Protein bands were visualized using the ChemiDoc MP System (Bio-Rad).
β-galactosidase reporter assay
HEK293 cells were seeded in 24 well plates. Upon reaching 90% confluency cells were co-transfected with 400 ng of β-galactosidase and 4 – 400 ng of effector plasmid. The total concentration of DNA was 800 ng for each sample; empty pcDNA3.1(−) was used to standardize the concentration when necessary. Transfection cocktails were prepared in 100 μl of OPTI-MEM (Thermo Fisher Scientific) mixed with 3 μl of TransIT – 293T transfection reagent (Mirus Bio LLC) following the commercial protocol. After 48 h posttransfection cells were harvested and centrifuged at max speed for 5 min. The supernatant was decanted for each sample and cell pellets were lysed using 100 μl radioimmune precipitation assay (RIPA) buffer (Sigma-Aldrich) supplemented with protease inhibitors (Roche). Cell lysates were then centrifuged at max speed for 15 min to pellet down cell debris. The supernatant was collected and each sample was incubated with 100 μl of ortho-Nitrophenyl-β-galactoside (ONPG) substrate (Thermo Fisher Scientific) in Nunc MaxiSorp flat-bottom 96 well plates (eBioscience). A Tecan microplate reader was used to measure absorbance at 415 nm.
GFP reporter assay
HEK293 cells were seeded in 24 well plates. Upon reaching 90% confluency cells were co-transfected with 400 ng of GFP and 400 ng of effector plasmid. The transfection cocktails were prepared as described above. After 48 h post-transfection cells were viewed by a Nikon TE300 fluorescent microscope. Next, cells were resuspended in 0.5% BSA and assayed by flow cytometry following the commercial protocol using the Millipore Guava EasyCyte plus flow cytometer. The mean fluorescent intensity (MFI) was calculated using FlowJo (TreeStar) software.
RNA sequencing (RNA-seq
HEK293 cells were seeded in 24 well plates. Upon reaching 90% confluency the cells were transfected with 400 ng of effector plasmid. The transfection cocktails were prepared as described above. After 48 h cells were harvested and total RNA was extracted from each sample using an RNeasy Mini Kit (Qiagen). On-column DNase digestion was performed to remove genomic DNA contamination. Purified RNA samples were analyzed for quality at the Cornell University Institute of Biotechnology, and library construction and subsequent sequencing of RNA samples were performed by the Cornell University RNA Sequencing Core (RSC).
RNA-seq data analysis
RNA-seq reads were mapped to the human genome (hg19) using STAR aligner (version 2.4.2). FeatureCounts (version 1.5.0) was used to count the raw number of reads covering each gene, using the GTF file downloaded from the UCSC table browser (hg19 refGene). We then called differentially expressed genes from the EIV and CIV PA-X transfected samples against all other control samples (cells transfected with the pcDNA3.1 empty vector, or expressing GFP or NP) using the exact test in the edgeR software package, applying a FDR cutoff of 0.05, and a fold-change cutoff of at least 2. The resulting list of genes was plotted in a heatmap using the gplots software package.
RESULTS
Genetic analysis and the expression of EIV and CIV PA-X
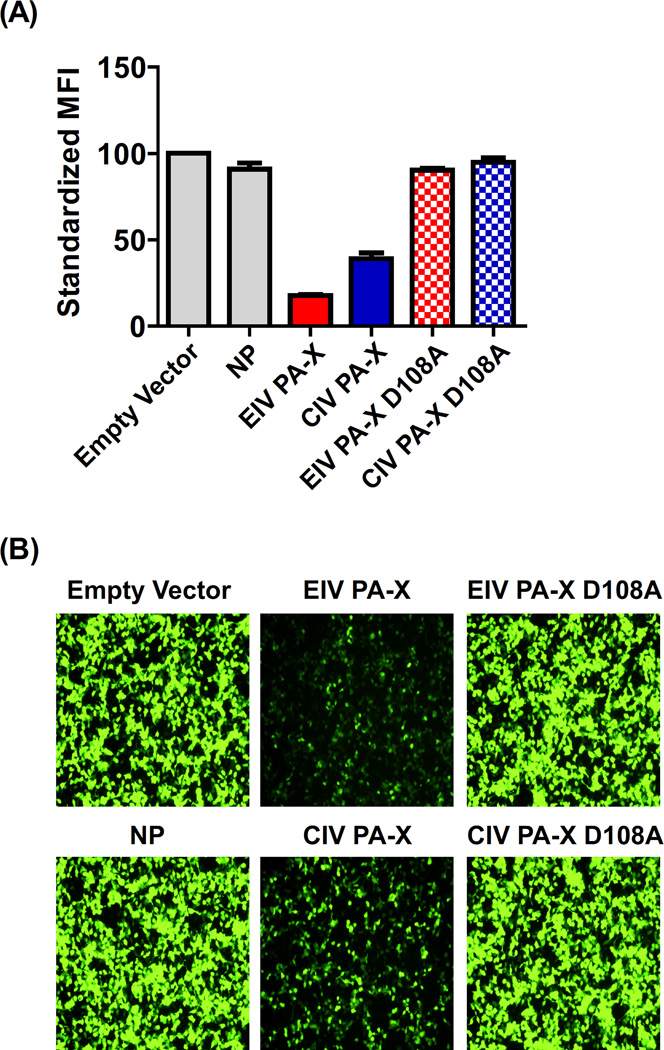
Aligning the EIV and CIV PA-X consensus sequences revealed three differences (Fig. 1A). Two of the differences were point mutations at amino acid positions 27 and 231. Specifically, EIV PA-X had an Asp and Ser at those positions, while CIV PA-X possessed an Asn and Phe, respectively. The third difference was that CIV PA-X had a 20 amino acid truncation at the C-terminus compared to the full-length variant of EIV PA-X. Expression of the PA-X genes by IVT yielded protein bands around 20 kDa; the CIV PA-X band ran slightly faster than the EIV PA-X band most likely due to its truncation (Fig. 1B). We used IVT because other methods of detection were not able to detect the protein (data not shown), in accordance with previous reports (2). Another study suggested that PA-X is difficult to detect due to strong self-suppression (13). Co-expression of β-galactosidase and PA-X in cell culture showed that both EIV and CIV PA-X were able to suppress gene expression in a dose dependent manner (Fig. 1C). At 400 ng there was no significant difference in β-galactosidase activity (p>0.05) comparing EIV and CIV PA-X transfected cells; however, as less PA-X DNA was used for transfections a consistent trend appeared: EIV PA-X had significantly (p<0.05) stronger suppression ability compared to CIV PA-X. The difference between the two grew larger as the concentration of DNA decreased from 40 – 4 ng (Fig. 1C). This difference was confirmed by flow cytometry (Fig. 2A) and microscopy (Fig. 2B) based on co-expression of PA-X and GFP. D108A mutants were tested as controls to ensure the ribonuclease domain resided at the N-terminus as previously described (2). Both mutants showed no gene suppression and thus, along with the protein bands shown by IVT (Fig. 1B), confirmed both EIV and CIV PA-X were properly expressed.
FIG 1.
Genetic comparison and expression of EIV and CIV PA-X in cell culture. (A) Comparison of EIV and CIV PA-X consensus protein sequences revealed three differences, two point mutations and one deletion. (B) Expression of PA-X was detected after IVT by Western blotting using a rabbit anti-PA-X peptide antibody. (C) EIV and CIV PA-X gene suppression ability was assayed by β-galactosidase reporter assay in a dose-dependent manner. Error bars represented the standard deviation of three independent experiments.
FIG 2.
EIV and CIV PA-X GFP reporter assay. Wild types and PA-X D108A mutants were assayed for their ability to suppress GFP expression in cell culture. Results were shown by comparing (A) fluorescent intensity and (B) by microscopy. In (A) the fluorescent intensity was standardized to the empty vector transfected control cells, and error bars represented the standard deviation of three independent experiments.
EIV and CIV PA-X site-directed mutagenesis
EIV and CIV PA-X mutants were created to identify the PA-X sequences associated with the difference in gene suppression activity. EIV PA-X D27N did not change its phenotype to resemble CIV PA-X. However, both S231F and its 20 amino acid C-terminal truncation mutants showed weaker suppression of GFP, similar to that seen for CIV PA-X based on flow cytometry (Fig. 3A) and microscopy (Fig. 3B). Next, reciprocal mutations were made in the CIV PA-X background: N27D, F231S, and a 20 amino acid C-terminal elongation mutant to match EIV PA-X. Interestingly, none of the three mutants resulted in a stronger GFP gene suppression phenotype compared to EIV PA-X based on flow cytometry (Fig. 4A) and microscopy (Fig. 4B). This suggested a combination of mutations must be required in the CIV PA-X background to change its phenotype to match EIV PA-X’s. All possible combinations were created and results showed two mutants had phenotypes like EIV PA-X’s based on flow cytometry (Fig. 5A) and microscopy (Fig. 5B): one had all three EIV PA-X mutations and the other construct carried two out of three mutations, F231S and the elongated C-terminus.
FIG 3.
EIV PA-X mutants GFP reporter assay. EIV PA-X mutants were made based on the differences in CIV PA-X. The mutants were assayed for their ability to suppress GFP expression in cell culture. Results were shown by comparing (A) fluorescent intensity and (B) by microscopy (B). In (A) the fluorescent intensity was standardized to the empty vector transfected control cells, and error bars represented the standard deviation of three independent experiments.
FIG 4.
CIV PA-X mutants GFP reporter assay. Reciprocal CIV PA-X mutants were made based on the EIV PA-X mutants. The mutants were assayed for their ability to suppress GFP expression in cell culture. Results were shown by comparing (A) fluorescent intensity and (B) by microscopy. In (A) the fluorescent intensity was standardized to the empty vector transfected control cells, and error bars represented the standard deviation of three independent experiments
FIG 5.
Multiple CIV PA-X mutants GFP reporter assay. All possible CIV PA-X mutant combinations were made to identify the key changes that resulted in the gene suppression phenotype difference comparing EIV and CIV PA-X. The mutants were assayed for their ability to suppress GFP expression in cell culture. Results were shown by comparing (A) fluorescent intensity and (B) by microscopy (B). In (A) the fluorescent intensity was standardized to the empty vector transfected control cells, and error bars represented the standard deviation of three independent experiments.
EIV and CIV PA-X’s effects on host gene expression
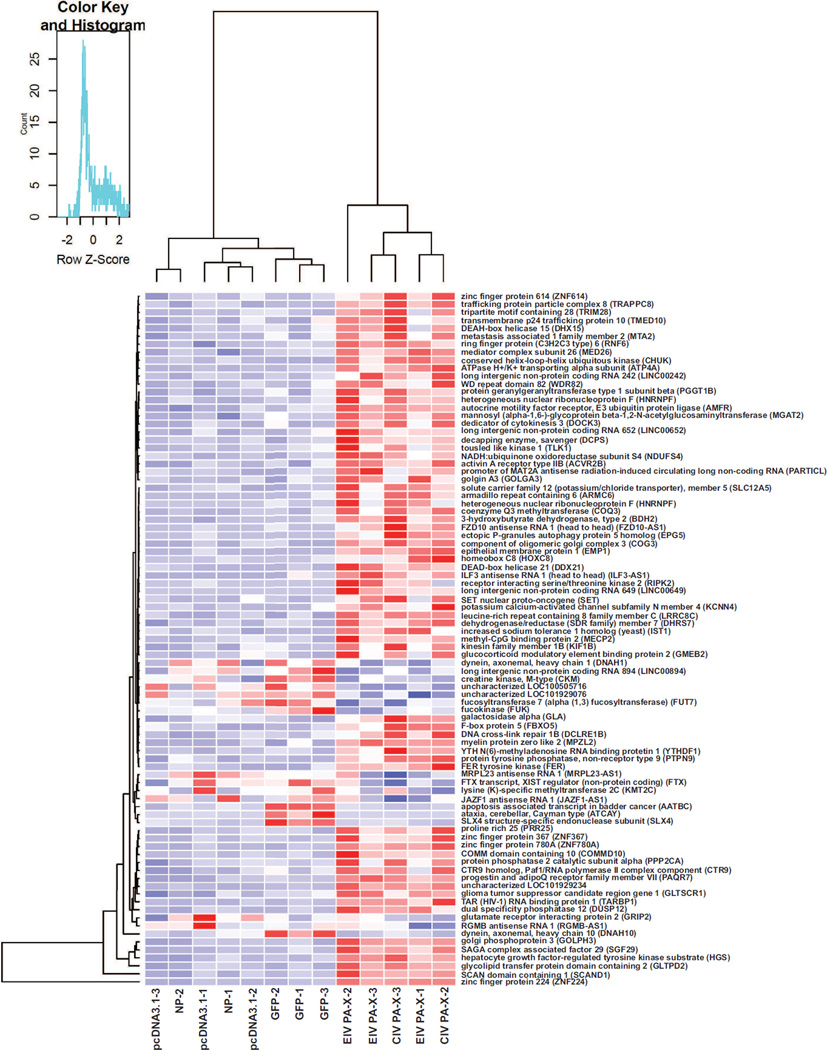
RNA-seq analysis identified genes that were differentially expressed (p<0.001) comparing the controls (pcDNA3.1, GFP, NP) to the EIV and CIV PA-X samples, and this was organized into a heatmap (Fig. 6). Each gene was further analyzed using the NCBI database and notable/related genes were extracted from the heatmap and tabulated (Table 1). These genes showed higher levels compared to the controls and encoded proteins related to the immune response, ER-golgi trafficking, transcription. All down-regulated genes relative to the controls were not included in the table because of inconsistent levels of expression and variation within the same sample category, and because several of these gene products have not been characterized (Fig. 6). When comparing the EIV and CIV PA-X samples alone, there were no statistically significant differences (p>0.05) in detected gene expression.
FIG 6.
Heatmap generated after stringent statistical analysis showing differentially expressed genes comparing EIV and CIV PA-X samples to controls. Controls (pcDNA, NP, GFP) and PA-X samples (EIV and CIV) were labeled at the bottom of the heatmap while gene names (full and abbreviated) were labeled on the right side. The heatmap shows three independent replicates for each sample except for one NP and one CIV sample which represent extreme outliers. Blue shading indicated down-regulation of gene expression while red shading indicated up-regulation of gene expression.
Table 1.
Genes that showed significant differential expression from the RNA-seq analysis heatmap comparing PA-X samples to the controls were individually analyzed and tabulated. Genes (abbreviated) are displayed in the first column followed by a brief description in the second column. All genes shown here were up-regulated in expression relative to the controls with RNAseq cut-offs of 0.05 FDR and > 2 fold change.
| Gene | Key Words / Functions |
|---|---|
| AMFR | E3 ubiquitin ligase, ubiquitination pathway, takes part in ER-associated protein degradation (ERAD) |
| ATP4A | ion channel, hydrogen / potassium exchange |
| CHUK | kinase, phosphorylates NF-κB inhibitory proteins for ubiquitination and subsequent degradation, promotes NF-κB response |
| COG3 | regulates ER-golgi transport, defines golgi morphology, protein glycosylation |
| COMMD10 | modulates activity of E3 ubiquitin ligase, may down-regulate NF-κB response |
| COQ3 | methyltransferase, functions in mitochondria, takes part in the electron transport chain (ETC) |
| CTR9 | binds to RNA polymerase II, takes part in histone methylation, regulates transcription |
| DCLRE1B | DNA interstrand cross-link repair, promotes transcription |
| DCPS | takes part in mRNA degradation pathway, up-regulated during virus infections |
| DDX21 | putative RNA helicase, may regulate transcription |
| DHX15 | putative pre-mRNA splicing function, may regulate gene expression |
| DUSP12 | phosphatase, negatively regulates mitogen-activated protein (MAP) kinase superfamily |
| FER | kinase, plays role in activating NF-κB response |
| GOLGA3 | regulates golgi transport |
| GOLPH3 | regulates golgi transport, vesicle budding, takes part in secretion pathway |
| HGS | endosomal sorting, sorts ubiquitinated proteins for degradation |
| HNRNPF* | associates with pre-mRNA, splicing and transport, plays role in mRNA maturation |
| HOXC8 | transcription factor for cell development |
| IST1 | vesicle budding, interacts with endosomal sorting complexes required for transport (ESCRT) |
| KCNN4 | ion channel, calcium activated, promotes potassium influx into cells |
| MECP2 | suppresses transcription by binding to methylated promoters |
| MED26 | subunit of cofactor required for SP1 activation (CRSP) transcription factor |
| MGAT2 | functions in golgi, converts N-glycans for protein glycosylation |
| MTA2 | takes part in chromatin remodeling to facilitate transcription |
| NDUFS4 | oxidoreductase, functions in mitochondria, takes part in the electron transport chain (ETC) |
| PPP2CA | phosphatase, negative control of cell growth |
| PTPN9 | phosphatase, functions in golgi, takes part in transferring hydrophobic ligands |
| RIPK2 | kinase, ubiquitination pathway, plays role in activating NF-κB response |
| RNF6 | E3 ubiquitin ligase, ubiquitination pathway, may bind DNA to regulate transcription |
| SCAND1 | binds to and regulates transcription factor myeloid zinc factor 1B |
| SET | prevents histone acetylation, inhibits transcription |
| SGF29 | promotes transcription through association with histone acetyltransferases |
| SLC12A5 | ion channel, lowers intracellular chloride concentration |
| TMED10 | cis-golgi network, ER, takes part in early secretory pathway, cargo loading |
| TRAPPC8 | may be involved in ER to golgi trafficking early on |
| TRIM28 | represses transcription, binds to E3 ubiquitin ligase (MDM2), inhibits apoptosis by marking p53 for degradation |
| WDR82 | promotes transcription by histone H3 methylation |
| ZNF367 | promotes transcription |
| ZNF614 | may be involved in transcription regulation |
| ZNF780A | may be involved in transcription regulation |
Two variants of HNRNPF were identified
DISCUSSION
Genetic differences between EIV and CIV PA-X resulted in a phenotypic difference comparing gene suppression
We have previously shown that the numerous genetic changes between EIV and CIV did not result in easily detected significant differences in comparisons of virus growth and infectivity in different host cells, HA cleavage, or receptor specificity (15). In contrast, we show here that genetic differences between the PA-X proteins of the two viruses resulted in a biological difference, such that EIV PA-X had a greater (p<0.05) gene suppression ability compared with CIV PA-X based on β-galactosidase and GFP reporter assays (Fig. 1 – 5), although it is not clear how this difference would influence the evolution and adaptation of EIV to dogs. We used HEK293 cells for these reporter assays because dog (MDCK and A72) and horse cells (EQKD) could not be efficiently transfected after various attempts (data not shown). Furthermore, previous studies that analyzed PA-X’s reporter gene suppression ability also used HEK293 cells for both human-and avian-origin PA-X proteins (12, 16).
The results showed the mutation S231F and the C-terminal truncation in EIV PA-X changed its gene suppression phenotype to resemble CIV PA-X’s, however; the reciprocal mutations in CIV PA-X (F231S and elongating the C-terminus) did not convert its phenotype to resemble EIV PA-X’s (Fig. 3 – 4). Interestingly, a complete conversion to EIV PA-X’s level of GFP suppression required both mutations in the CIV PA-X backbone (Fig. 5). This indicated that both position 231 and the C-terminal elongated tail defined EIV PA-X’s stronger phenotype, thereby providing evidence that amino acids near the truncation site of PA-X, such as position 231, can influence function. These results are consistent with previously published reports that showed the 252 amino acid long (full-length) PA-X protein from different IAV subtypes had greater ribonuclease activity compared to the 232 amino acids truncated variant (10, 12). Taken together this indicates that the C-terminus most likely interacts with or regulates the N-terminal ribonuclease domain (Fig. 2). Understanding the mechanism clearly requires additional studies, such as solving the structures of the PA-X in full-length and truncated variants.
Expression of EIV and CIV PA-X alone resulted in significant up-regulated expression of host genes
Several previous reports have examined the effects of PA-X on host response by comparing wild type and mutant virus infections, and shown that PA-X was important for modulating various immune responses (2, 6–8, 12, 14). To further elucidate PA-X’s effects on the host, we transfected plasmids expressing only PA-X into cell cultures and used RNA-seq to analyze the transcripts. While artificial, this allowed us to study PA-X’s influence on the cell, as there would not be other expressed viral proteins that might change PA-X’s interactions with host gene expression (Fig. 6). Notably, many genes were up-regulated (Table 1), a number of which were related to specific aspects of the innate immunity response. For example, we found genes related to the modulation of the NF-κB transcription factor: a well described response that leads to the transcription of several anti-viral cytokines, such as IFN-β (17). It has been previously noted that productive IAV infections required the activation of NF-κB (18, 19), and influenza HA and vRNA have been shown to trigger the pathway while influenza NS1 inhibited it (20–22). Our results suggest that influenza PA-X may also be directly involved in up-regulating genes involved in both activating (CHUK, FER, RIPK2 – all kinases) and repressing (COMMD10) the pathway, and thus playing an important role in virus replication. Another immunity related gene (TRIM28) that was up-regulated by EIV and CIV PA-X modulated apoptosis, a well described antiviral response (23–25). TRIM28 interacts with ubiquitin E3 ligase, MDM2, to drive ubiquitination and subsequent degradation of tumor suppressor protein p53, resulting in inhibition of apoptosis (26, 27). Our finding was consistent with studies that described PA-X (from H1N1 and H5N1 subtypes) as having anti-apoptotic activity (6, 7). Lastly, we also found two phosphatases (DUSP12, PPP2CA) that negatively regulated the MAP kinase cascades; these pathways have been shown to drive expression of antiviral cytokines such as TNF-α which causes an inflammatory response in cells (28). This finding further suggests that PA-X is able to function as a negative controller of the immune response.
Interestingly, our results revealed the up-regulation of two E3 ubiquitin ligases (AMFR, RNF6). Ubiquitination can either promote or restrict influenza virus infections. For example, while E3 ubiquitin ligase TRIM32 restricts influenza by marking PB1 for proteasomal degradation (29), there is also evidence that NEDD4 facilitates infections by accelerating the turnover rate of the antiviral factor IFITM3 (30). Additionally, disrupting the ubiquitination pathway by treating cells with proteasome inhibitors results in a decrease in infection (31). Thus, our results suggest that PA-X may control the expression of some E3 ubiquitin ligases to potentially modulate infections.
It was previously suggested that PA-X might be accumulated in the late phase of a virus infection due to the inefficiency of frame-shifting during translation and hence might exert more influence during this period (9). Our over-expression of PA-X in cells (no frame-shifting) may therefore have simulated a late phase infection environment and caused up-regulation of genes that reflect this time window. For example, several genes involved in vesicle transport and budding (COG3, GOLGA3, GOLPH3, IST1, TMED10, TRAPPC8) and related to protein posttranslational modification in the Golgi (COG3, MGAT2, PTPN9) were up-regulated. The upregulation of these genes makes sense in the context of a late virus infection phase – when viral components need to go through the secretory pathway (HA, NA, M2) and traverse the endocytic system via recycling endosomes (viral RNPs) in order to congregate at the cellular membrane in preparation for assembly and egress (32–36).
The RNA-seq results revealed that many genes involved with transcription and/or protein expression were up-regulated, although these were more generic as opposed to targeting a specific pathway or transcription factor. These genes can be sorted into two categories: (1) genes that, when expressed, produced proteins that modulated transcription and (2) those that regulated gene expression after transcription. The first category included genes related to histone modification (CTR9, SET, SGF29, WDR82), DNA template binding (DCLRE1B, MECP2, MTA2), and other putative transcription factors (HOXC8, ZNF367, ZNF614, ZNF780A). The second category included genes associated with pre-mRNA splicing and maturation (DHX15, HNRNPF – two variants) and an mRNA de-capping enzyme, DCPS. Of note, viruses that carry their own de-capping enzymes, such as influenza and vaccinia viruses, use them to facilitate virus gene expression while inhibiting host translation (37, 38). Consequently, PA-X’s ability to up-regulate a host de-capping enzyme may provide an additional method for IAV to inhibit host translation.
More in-depth studies will be required in the future to fully understand the mechanisms underlying these apparent changes in host gene expression, particularly whether PA-X interacts directly with the described genes or through binding partners. Additionally, it will be important to use RNA-seq to analyze the consequences of live virus infections, such as wild type and PA-X negative viruses, and compare their gene expression profiles with transfected PA-X’s. Comparing these profiles would help to reveal a clearer picture of the effects of influenza PA-X on host gene expression. Although the RNA-seq analysis did not reveal any differences in gene expression when comparing EIV and CIV PA-X samples, this was not surprising given that we previously observed that CIV and EIV exhibited few phenotypic differences in assays that considered virus growth in cell culture, infections in different host cells, receptor (sialic acid) binding with purified HAs, and HA cleavage efficiency (15). For the future it may be possible to use RNA-seq to compare EIV and CIV PA-X effects when expressed in dog and horse cells. As stated previously, we have tried transfections using dog (MDCK and A72) and horse (EQKD) cells but the efficiencies were very poor and inconsistent based on GFP reporter; we would like to further explore this comparison at a later date.
Highlights.
Compare the variant PA-X proteins from two related viruses – equine influenza (EIV) and canine influenza (CIV) H3N8 – which differ in sequence.
Both proteins suppress gene expression, EIV PA-X slightly more active than CIV PA-X
Mutations that caused this difference.
RNA-seq showed no significant differences in this property between EIV and CIV PA-X proteins.
Effects on some genes when compared to controls, including immunity-related proteins, rafficking proteins, and transcription factors.
Acknowledgments
KHF was supported by an NSF Graduate Research Fellowship. ECH is supported by an NHMRC Australian Fellowship (AF30). The research was supported by National Institutes of Health (NIH) grant R01 GM8496821 to CRP and ECH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Vasin AV, Temkina OA, Egorov VV, Klotchenko SA, Plotnikova MA, Kiselev OI. Molecular mechanisms enhancing the proteome of influenza A viruses: an overview of recently discovered proteins. Virus research. 2014;185:53–63. doi: 10.1016/j.virusres.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM, Xiao YL, Dunfee RL, Schwartzman LM, Ozinsky A, Bell GL, Dalton RM, Lo A, Efstathiou S, Atkins JF, Firth AE, Taubenberger JK, Digard P. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science. 2012;337:199–204. doi: 10.1126/science.1222213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi M, Jagger BW, Wise HM, Digard P, Holmes EC, Taubenberger JK. Evolutionary conservation of the PA-X open reading frame in segment 3 of influenza A virus. Journal of virology. 2012;86:12411–12413. doi: 10.1128/JVI.01677-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu G, Zhang X, Sun Y, Liu Q, Sun H, Xiong X, Jiang M, He Q, Wang Y, Pu J, Guo X, Yang H, Liu J. Truncation of C-terminal 20 amino acids in PA-X contributes to adaptation of swine influenza virus in pigs. Scientific reports. 2016;6:21845. doi: 10.1038/srep21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khaperskyy DA, Emara MM, Johnston BP, Anderson P, Hatchette TF, McCormick C. Influenza a virus host shutoff disables antiviral stress-induced translation arrest. PLoS pathogens. 2014;10:e1004217. doi: 10.1371/journal.ppat.1004217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu J, Mo Y, Wang X, Gu M, Hu Z, Zhong L, Wu Q, Hao X, Hu S, Liu W, Liu H, Liu X, Liu X. PA-X Decreases the Pathogenicity of Highly Pathogenic H5N1 Influenza A Virus in Avian Species by Inhibiting Virus Replication and Host Response. Journal of virology. 2015;89:4126–4142. doi: 10.1128/JVI.02132-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao H, Sun Y, Hu J, Qi L, Wang J, Xiong X, Wang Y, He Q, Lin Y, Kong W, Seng LG, Sun H, Pu J, Chang KC, Liu X, Liu J. The contribution of PA-X to the virulence of pandemic 2009 H1N1 and highly pathogenic H5N1 avian influenza viruses. Scientific reports. 2015;5:8262. doi: 10.1038/srep08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi T, MacDonald LA, Takimoto T. Influenza A virus protein PA-X contributes to viral growth and suppression of the host antiviral and immune responses. Journal of virology. 2015 doi: 10.1128/JVI.00319-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khaperskyy DA, McCormick C. Timing Is Everything: Coordinated Control of Host Shutoff by Influenza A Virus NS1 and PA-X Proteins. Journal of virology. 2015 doi: 10.1128/JVI.00386-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bavagnoli L, Cucuzza S, Campanini G, Rovida F, Paolucci S, Baldanti F, Maga G. The novel influenza A virus protein PA-X and its naturally deleted variant show different enzymatic properties in comparison to the viral endonuclease PA. Nucleic acids research. 2015;43:9405–9417. doi: 10.1093/nar/gkv926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khaperskyy DA, Schmaling S, Larkins-Ford J, McCormick C, Gaglia MM. Selective Degradation of Host RNA Polymerase II Transcripts by Influenza A Virus PA-X Host Shutoff Protein. PLoS pathogens. 2016;12:e1005427. doi: 10.1371/journal.ppat.1005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao H, Sun H, Hu J, Qi L, Wang J, Xiong X, Wang Y, He Q, Lin Y, Kong W, Seng LG, Pu J, Chang KC, Liu X, Liu J, Sun Y. Twenty amino acids at the C-terminus of PA-X are associated with increased influenza A virus replication and pathogenicity. The Journal of general virology. 2015;96:2036–2049. doi: 10.1099/vir.0.000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oishi K, Yamayoshi S, Kawaoka Y. Mapping of a Region of the PA-X Protein of Influenza A Virus That Is Important for Its Shutoff Activity. Journal of virology. 2015;89:8661–8665. doi: 10.1128/JVI.01132-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao H, Xu G, Sun Y, Qi L, Wang J, Kong W, Sun H, Pu J, Chang KC, Liu J. PA-X is a virulence factor in avian H9N2 influenza virus. The Journal of general virology. 2015;96:2587–2594. doi: 10.1099/jgv.0.000232. [DOI] [PubMed] [Google Scholar]
- 15.Feng KH, Gonzalez G, Deng L, Yu H, Tse VL, Huang L, Huang K, Wasik BR, Zhou B, Wentworth DE, Holmes EC, Chen X, Varki A, Murcia PR, Parrish CR. Equine and Canine Influenza H3N8 Viruses Show Minimal Biological Differences Despite Phylogenetic Divergence. Journal of virology. 2015;89:6860–6873. doi: 10.1128/JVI.00521-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desmet EA, Bussey KA, Stone R, Takimoto T. Identification of the N-terminal domain of the influenza virus PA responsible for the suppression of host protein synthesis. Journal of virology. 2013;87:3108–3118. doi: 10.1128/JVI.02826-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 18.Nimmerjahn F, Dudziak D, Dirmeier U, Hobom G, Riedel A, Schlee M, Staudt LM, Rosenwald A, Behrends U, Bornkamm GW, Mautner J. Active NF-kappaB signalling is a prerequisite for influenza virus infection. The Journal of general virology. 2004;85:2347–2356. doi: 10.1099/vir.0.79958-0. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig S, Planz O. Influenza viruses and the NF-kappaB signaling pathway - towards a novel concept of antiviral therapy. Biological chemistry. 2008;389:1307–1312. doi: 10.1515/BC.2008.148. [DOI] [PubMed] [Google Scholar]
- 20.Pahl HL, Baeuerle PA. Expression of influenza virus hemagglutinin activates transcription factor NF-kappa. B Journal of virology. 1995;69:1480–1484. doi: 10.1128/jvi.69.3.1480-1484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar N, Xin ZT, Liang Y, Ly H, Liang Y. NF-kappaB signaling differentially regulates influenza virus RNA synthesis. Journal of virology. 2008;82:9880–9889. doi: 10.1128/JVI.00909-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Li M, Zheng H, Muster T, Palese P, Beg AA, Garcia-Sastre A. Influenza A virus NS1 protein prevents activation of NF-kappaB and induction of alpha/beta interferon. Journal of virology. 2000;74:11566–11573. doi: 10.1128/jvi.74.24.11566-11573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowy RJ. Influenza virus induction of apoptosis by intrinsic and extrinsic mechanisms. International reviews of immunology. 2003;22:425–449. doi: 10.1080/08830180305216. [DOI] [PubMed] [Google Scholar]
- 24.Herold S, Ludwig S, Pleschka S, Wolff T. Apoptosis signaling in influenza virus propagation, innate host defense, and lung injury. Journal of leukocyte biology. 2012;92:75–82. doi: 10.1189/jlb.1011530. [DOI] [PubMed] [Google Scholar]
- 25.Tripathi S, Batra J, Cao W, Sharma K, Patel JR, Ranjan P, Kumar A, Katz JM, Cox NJ, Lal RB, Sambhara S, Lal SK. Influenza A virus nucleoprotein induces apoptosis in human airway epithelial cells: implications of a novel interaction between nucleoprotein and host protein Clusterin. Cell death & disease. 2013;4:e562. doi: 10.1038/cddis.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatakeyama S. TRIM proteins and cancer. Nature reviews. Cancer. 2011;11:792–804. doi: 10.1038/nrc3139. [DOI] [PubMed] [Google Scholar]
- 27.Nailwal H, Sharma S, Mayank AK, Lal SK. The nucleoprotein of influenza A virus induces p53 signaling and apoptosis via attenuation of host ubiquitin ligase RNF43. Cell death & disease. 2015;6:e1768. doi: 10.1038/cddis.2015.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabio G, Davis RJ. TNF and MAP kinase signalling pathways. Seminars in immunology. 2014;26:237–245. doi: 10.1016/j.smim.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu B, Wang L, Ding H, Schwamborn JC, Li S, Dorf ME. TRIM32 Senses and Restricts Influenza A Virus by Ubiquitination of PB1 Polymerase. PLoS pathogens. 2015;11:e1004960. doi: 10.1371/journal.ppat.1004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chesarino NM, McMichael TM, Yount JS. E3 Ubiquitin Ligase NEDD4 Promotes Influenza Virus Infection by Decreasing Levels of the Antiviral Protein IFITM3. PLoS pathogens. 2015;11:e1005095. doi: 10.1371/journal.ppat.1005095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widjaja I, de Vries E, Tscherne DM, Garcia-Sastre A, Rottier PJ, de Haan CA. Inhibition of the ubiquitin-proteasome system affects influenza A virus infection at a postfusion step. Journal of virology. 2010;84:9625–9631. doi: 10.1128/JVI.01048-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohkura T, Momose F, Ichikawa R, Takeuchi K, Morikawa Y. Influenza A virus hemagglutinin and neuraminidase mutually accelerate their apical targeting through clustering of lipid rafts. Journal of virology. 2014;88:10039–10055. doi: 10.1128/JVI.00586-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Li H, Chen Y, Wei H, Gao GF, Liu H, Huang S, Chen JL. Transport of influenza virus neuraminidase (NA) to host cell surface is regulated by ARHGAP21 and Cdc42 proteins. The Journal of biological chemistry. 2012;287:9804–9816. doi: 10.1074/jbc.M111.312959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veit M, Thaa B. Association of influenza virus proteins with membrane rafts. Advances in virology. 2011;2011:370606. doi: 10.1155/2011/370606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutchinson EC, Fodor E. Transport of the influenza virus genome from nucleus to nucleus. Viruses. 2013;5:2424–2446. doi: 10.3390/v5102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossman JS, Lamb RA. Influenza virus assembly and budding. Virology. 2011;411:229–236. doi: 10.1016/j.virol.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narayanan K, Makino S. Interplay between viruses and host mRNA degradation. Biochimica et biophysica acta. 2013;1829:732–741. doi: 10.1016/j.bbagrm.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu SW, Wyatt LS, Orandle MS, Minai M, Moss B. The D10 decapping enzyme of vaccinia virus contributes to decay of cellular and viral mRNAs and to virulence in mice. Journal of virology. 2014;88:202–211. doi: 10.1128/JVI.02426-13. [DOI] [PMC free article] [PubMed] [Google Scholar]