Abstract
West Nile virus (WNV) is a flavivirus that swept rapidly across North America in 1999, declined in prevalence, and then resurged in 2012. To date, no vaccine is available to prevent infection in the human population. Herpes simplex virus (HSV) replication-defective vaccine vectors induce a durable immunity characterized by strong antibody and CD8+ T cell responses even in HSV-immune animals. In this study, a WNV protein expression cassette was optimized for virus-like particle (VLP) production in transfection studies, and the cassette was recombined into an HSV-1 d106-WNV virus vector, which produced extracellular VLPs, as confirmed by immunoelectron microscopy. Immunization of mice with the d106-WNV recombinant vector elicited a specific anti-WNV IgG response. This study highlights the flavivirus coding sequences needed for efficient assembly of virus-like particles. This information will facilitate generation of additional vaccine vectors against other flaviviruses including the recently emerged Zika virus.
Keywords: West Nile virus, vaccine vector, Flavivirus, Zika virus, HSV recombinant vector
Introduction
The North American distribution of the flavivirus West Nile virus (WNV) expanded dramatically after its introduction into New York in 1999. It is now found throughout the United States, southern Canada, Central and South America, and the Caribbean (Cruz et al., 2005; Dauphin et al., 2004; Estrada-Franco et al., 2003; Komar, 2003; Reisen et al., 2004). WNV, which is transmitted primarily through Culex mosquito bites (Kulasekera et al., 2001), is spread to new areas via infected birds. WNV has caused significant human disease in the United States with estimates of 780,000 illnesses from 1999–2010 (Petersen et al., 2013), and elderly individuals are at greatest risk of neuroinvasive disease (Carson et al., 2012; Hayes et al., 2005). Neuroinvasive disease cases peaked in 2002–2003 and then declined until 2012 when WNV re-emerged with over 2800 neuroinvasive cases in the United States (Centers for Disease Control and Prevention, 2013). With the wide distribution and significant human morbidity associated with WNV, it continues to be an important target for vaccine development.
Despite extensive efforts, no effective WNV vaccine is approved to protect the susceptible human population (Heinz and Stiasny, 2012; Volz et al., 2016); however, several vaccine approaches for animals or humans have been pursued, including inactivated virus (Samina et al., 2005); chimeric attenuated flavivirus viruses expressing WNV premembrane (prM) and envelope (E) proteins (Dayan et al., 2012; Monath et al., 2006); WNV virus-like particles (VLPs) (Qiao et al., 2004); pox vectors (Heinz and Stiasny, 2012; Siger et al., 2006); a lentivirus vector (Iglesias et al., 2006); a subunit vaccine (Chu, Chiang, and Ng, 2007; Watts et al., 2007); and DNA vaccines (Yang et al., 2001). The WNV proteins prM and E are necessary and sufficient for VLP production. When prM and E are expressed together within a cell, they self-assemble into VLPs that are released into the extracellular environment (Allison et al., 1995). VLPs derived from the co-expression of prM and E in cell culture systems independent of other viral factors are structurally and antigenically similar to genuine West Nile virions and have been shown to elicit neutralizing antibody titers in immunized mice. The protease required for cleaving E from prM is cellular, so E is released as a separate protein when expressed along with prM.
We have previously generated replication-defective HSV vaccine vectors expressing simian immunodeficiency virus (SIV) proteins that successfully protected non-human primates against mucosal challenge with virulent SIV (Kaur et al., 2007; Watanabe et al., 2007). Replication-defective HSV vectors are attractive because of their safety, as demonstrated in animal models (Hoshino et al., 2008), and ability to induce durable immune responses that include both B and T cell responses (Brehm et al., 1999; Brehm et al., 1997; Brockman and Knipe, 2002; Brubaker et al., 1996; Da Costa et al., 2000; Da Costa, Jones, and Knipe, 1999; Dudek and Knipe, 2006; Jones, Taylor, and Knipe, 2000; Kaur et al., 2007; Morrison, Da Costa, and Knipe, 1998; Murphy et al., 2000; Watanabe et al., 2007), and they are immunogenic even in the face of pre-existing HSV immunity (Brockman and Knipe, 2002). The replication-defective HSV-1 d106 virus contains multiple deletions that remove the coding sequences of the immediate-early proteins ICP4 and ICP27 and the promoter regions of the ICP22 and ICP47 genes (Samaniego, Neiderhiser, and DeLuca, 1998). The loss of ICP4, ICP27, and ICP22 results in a dramatic decrease in the number of HSV proteins expressed during infection on non-complementing cells; however, d106 expresses the immediate-early ICP0 protein, which stimulates heterologous protein expression from transgenes encoded within the viral genome through its effects on the chromatin associated with the HSV genome (Cliffe and Knipe, 2008; Lee, Raja, and Knipe, 2016). We have therefore applied the HSV vector technology to the design of a WNV vaccine, and in this study we describe a recombinant replication-defective herpes simplex virus (HSV) vector, d106-WNV, which expresses the WNV structural proteins prM and E and part of the capsid (C) protein.
Results
With the continued prevalence of WNV, there is renewed interest in developing a safe and effective human WNV vaccine. To determine if a replication-defective HSV vaccine vector could serve as a candidate for flavivirus vaccine development, we constructed a recombinant HSV vaccine vector that expresses the WNV structural proteins premembrane (prM) and envelope (E).
Design of WNV expression constructs
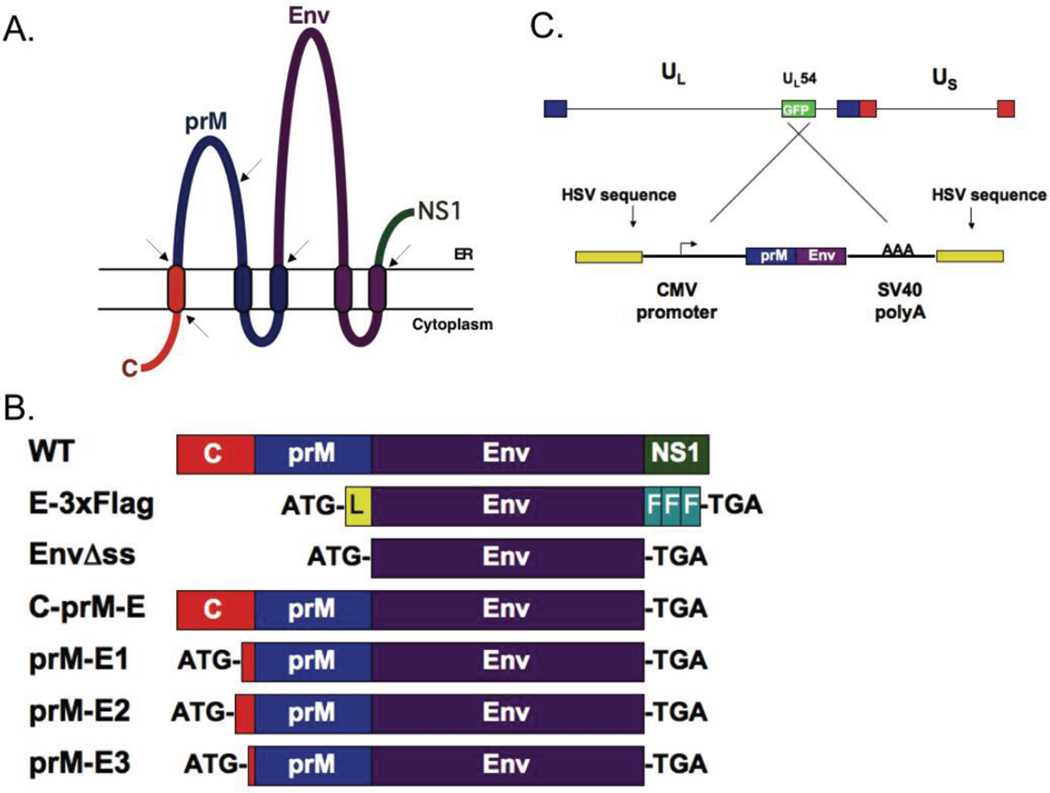
The WNV structural proteins prM and E require a signal sequence for proper orientation in the host cell membrane (Lindenbach, Thiel, and Rice, 2007). During natural infection, this signal sequence is derived from the C-terminal transmembrane region of the capsid (C) protein (Figure 1A). We first generated plasmid expression constructs expressing WNV E protein that lacked or contained a leader sequence for comparison with the WT C-prM-Env plasmid containing the coding sequences for the structural region starting with the methionine of the C protein (Figure 1B): 1. The EnvAss construct lacked a signal sequence. 2. The E-3XFlag construct expressed a modified WNV E protein containing the preprotrypsin leader sequence for proper membrane orientation and three consecutive FLAG epitope tags (Figure 1, Table 2).
Figure 1. Diagrams of the constructs used in this study.
A. Graphical representation of the membrane orientation of the structural proteins in WNV including the initial portion of the nonstructural NS1 protein. Transmembrane regions are denoted by ovals passing through the lipid bilayer. Arrows indicate where cellular or viral proteases cleave the polyprotein to free the individual proteins: capsid (C), premembrane (prM), and envelope (Env). B. Comparison of the various plasmid constructs to the wildtype (WT) structural protein coding sequences. All plasmids used the CMV promoter/enhancer to drive transcription. The addition of start (ATG) and stop (TGA) codons are indicated. E-3xFlag contains the preprotrypsin leader sequence (L) and three sequential FLAG epitope tags (F). EnvΔss expresses the E protein lacking C and prM sequences. C-prM-E contains the WNV coding sequences for C-prM-Env-NS1. prM-E1-3 contain varying amino acid residues of C as described in Table 2. (C) Top: the d106 genome with the location of the UL54 (ICP27) region containing the GFP transgene indicated. Bottom: the pd27-WNV plasmid used to generate the recombinant d106-WNV vaccine vector. The crossed lines show the homologous recombination event between the reversed plasmid sequences with the d106 genome that generated the d106-WNV recombinant virus.
Table 2.
Comparison of WNV envelope construct properties in transfected Vero cells.
| Plasmid | Capsid Amino Acid Residues |
Expression in transfected cells |
VLP production |
|---|---|---|---|
| E-3xFlag | None | + | − |
| EnvΔss | None | + | − |
| C-prM-E | 1–123 | + | − |
| prM-E1 | 94–123 | ++ | +/− |
| prM-E2 | 78–123 | ++ | +/− |
| prM-E3 | 106–123 | +++ | ++ |
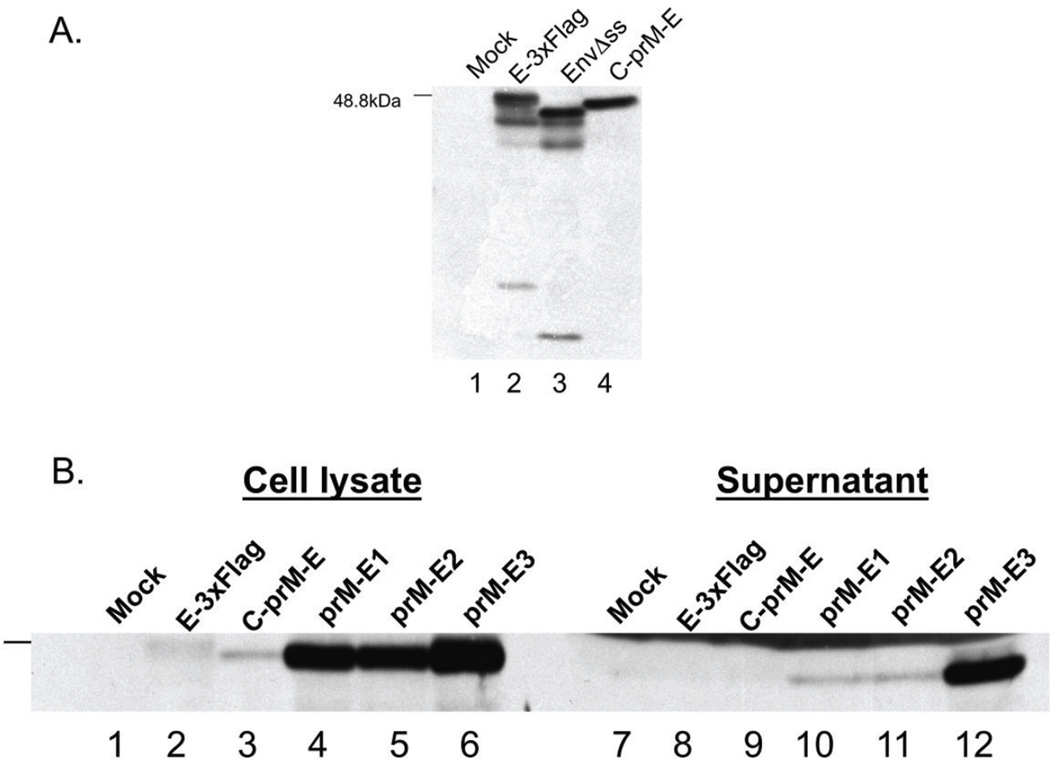
The constructs were evaluated for levels of protein expression and the ability to release virus-like particles (VLPs) into the supernatant in Vero cells transfected with the plasmids. The C-prM-E plasmid showed the highest levels of full-size E protein (Figure 2A, lane 4). The E-3XFlag and EnvΔss proteins showed a ladder of bands on Western blots, possibly due to proteolytic cleavage (Figure 2A, lanes 2 and 3, respectively), compared to the E protein produced by C-prM-E (Figure 2A, lane 4). Therefore, the natural signal sequence seemed to be the best for stable expression of full-size E protein.
Figure 2. Expression of WNV proteins and VLP release in transfected cells.
Vero cells mock-transfected or transfected with the plasmids expressing the indicated WNV construct were harvested at 72 hours post-transfection. An equal volume (4% of total sample) of either cell lysate or clarified supernatant was resolved by SDS-PAGE, transferred to a membrane, and the membrane was probed with an anti-E monoclonal antibody. The position of the 48.8 kDa ladder band is indicated. (A) Comparison of preprotrypsin leader (lane 2), leaderless (lane 3), and native E (lane 4) expressing constructs in transfected Vero cells. (B) Comparison of the VLP production by the indicated constructs as measured by E in the cells and supernatant.
Because of the potential stability issue described above, we next determined the optimal sequences from the C coding sequences necessary for VLP production. We therefore constructed the prM-E1, prM-E2, and prM-E3 plasmids expressing prM and E fused to varying portions of C, residues 1–123, 94–123, 78–123, or 106–123 (Figure 1B and Table 2), to determine which region provided the best expression and protein maturation. The properties of the various constructs are shown in Figure 2B and summarized in Table 2. The prM-E1–3 constructs (Figure 2B, lanes 4–6) expressed more E protein than the E-3XFlag and C-prM-E constructs (Fig, 2B, lanes 2 and 3, respectively), as measured by Western blotting for E. While including most of the amino acid residues found at the end of the capsid sequence dramatically increased expression levels (Figure 2B), the highest production of VLPs from the cells was observed with only residues 106–123 of C fused to prM-E in prM-E3 (Figure 2B, lane 12), as measured by the presence of E in the extracellular media. We therefore used the prM-E3 construct to generate a recombinant HSV strain expressing WNV proteins.
Construction of an HSV-1 d106-WNV recombinant vector expressing WNV proteins
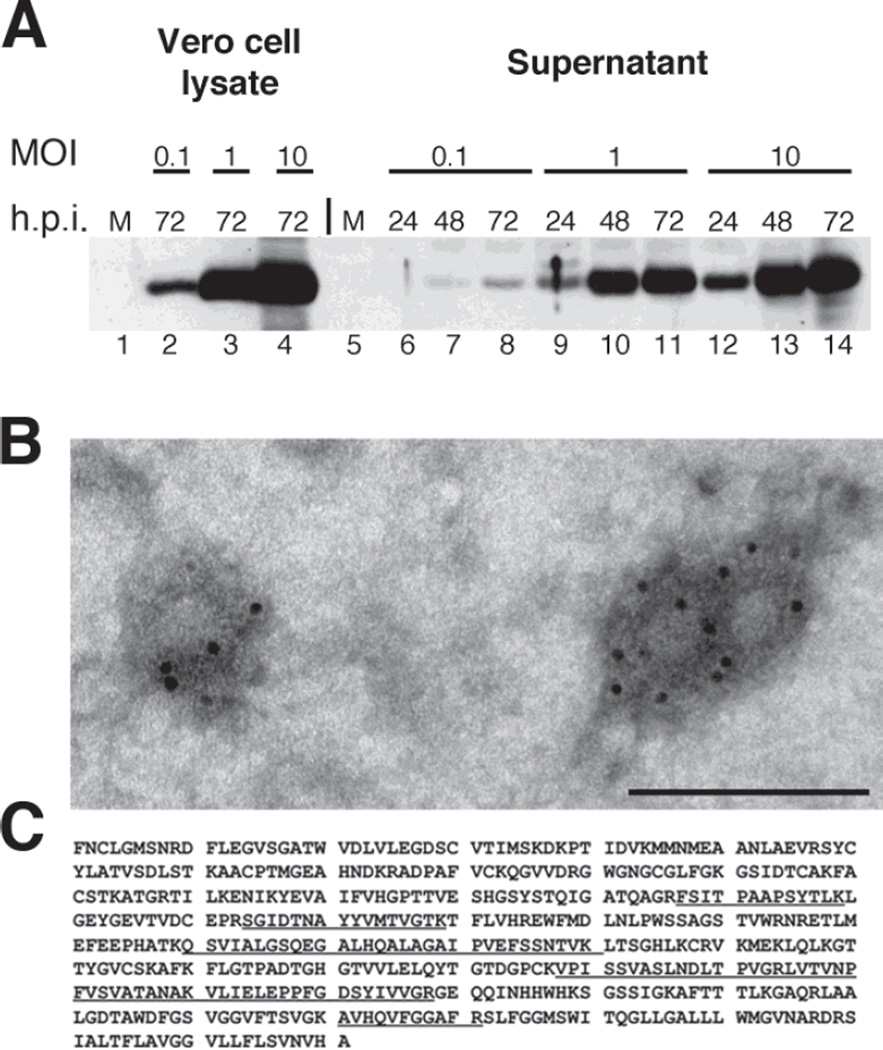
We next constructed a recombinant HSV strain expressing the prM and E proteins of WNV. Homologous recombination was used to introduce the prM-E3 expression cassette into the UL54 region of d106 virus to generate the d106-WNV virus, as described in Materials and Methods (Figure 1C). We examined the kinetics of E protein expression and VLP production by infecting Vero cells with the d106-WNV virus at various MOIs for 24–72 h. The highest level of E expression were observed in cell lysates at 72 h post-infection (hpi) in cells infected at an MOI of 10 (Figure 3, lanes 2–4). Even with this high MOI infection, most of the Vero cells were viable at 72 hpi, as detected by the lack of visible CPE (results not shown), likely due to the limited HSV gene expression of the d106 virus in non-complementing cells (Watanabe et al., 2007). Virus-like particles were measured by the presence of sedimentable E protein in the extracellular supernatant (Figure 3A, lanes 6–14). The highest level of VLP accumulation was observed at an MOI of 10 at 72 hpi (Figure 3A, lane 14). Therefore, the d106-WNV recombinant vector expressed high levels of WNV E protein both intra- and extracellularly.
Figure 3. Assembly and properties of virus-like particles in d106-WNV virus-infected cells.
A. Kinetics of WNV protein expression and VLP release. A. Vero cells were mock-infected or infected with d106-WNV virus at a range of MOIs (0.1, 1.0 or 10 PFU/cell) and harvested at 24, 48, or 72 hpi. Lysate (10% of sample total) and clarified supernatant (2% of sample total) were separated by SDS-PAGE, transferred to membrane, and probed with anti-E monoclonal antibody. The position of the 48.8 kDa ladder band is indicated. B. Electron micrograph of VLPs. WNV VLPs were diluted in Tris-buffered saline and adsorbed for 1 minute to a carbon coated grid that had been made hydrophilic by a 30 second exposure to a glow discharge. Samples were blocked with 1% BSA and incubated with a mouse anti West Nile Virus Envelope protein (Chemicon) for 30 minutes. After washing, samples were incubated with a rabbit anti mouse bridging antibody and protein A-gold (5nm) for 20 minutes. Samples were washed with PBS and water. Excess liquid was removed with a filter paper, and the samples were stained with 0.75% uranyl formate for 30 seconds. After removing the excess uranyl formate with a filter paper, the grids were examined in a JEOL 1200EX Transmission electron microscope, and images were recorded with an AMT 2k CCD camera. Scale bar: 100 nm. C. Identification of Peptides from E Protein in Extracellular Particles. Partially purified VLPs were resolved by SDS-PAGE, and the Coomassie blue-stained band corresponding to E was analyzed by mass spectrometry. The E-specific peptides identified by mass spectrometry are underlined.
The structure of the extracellular particles containing E protein was determined by electron microscopy of VLP preparations stained with anti-E mAb (Figure 3B). Most of the particles were stained by anti-E antibody and were approximately 28 nm inner diameter surrounded by glycoproteins and bound antibodies (Figure 3B). The presence of E protein in VLPs was further confirmed by mass spectrometry analysis. Proteins in a VLP preparation were resolved by SDS-PAGE, and a Coomassie blue-stained band in the estimated size range of E (not shown) was excised from the gel and subjected to mass spectroscopic analysis. Multiple peptides specific to E protein were identified (Figure 3C), thus confirming the presence of E in extracellular particles in the medium.
Elicitation of anti-WNV antibodies in immunized mice
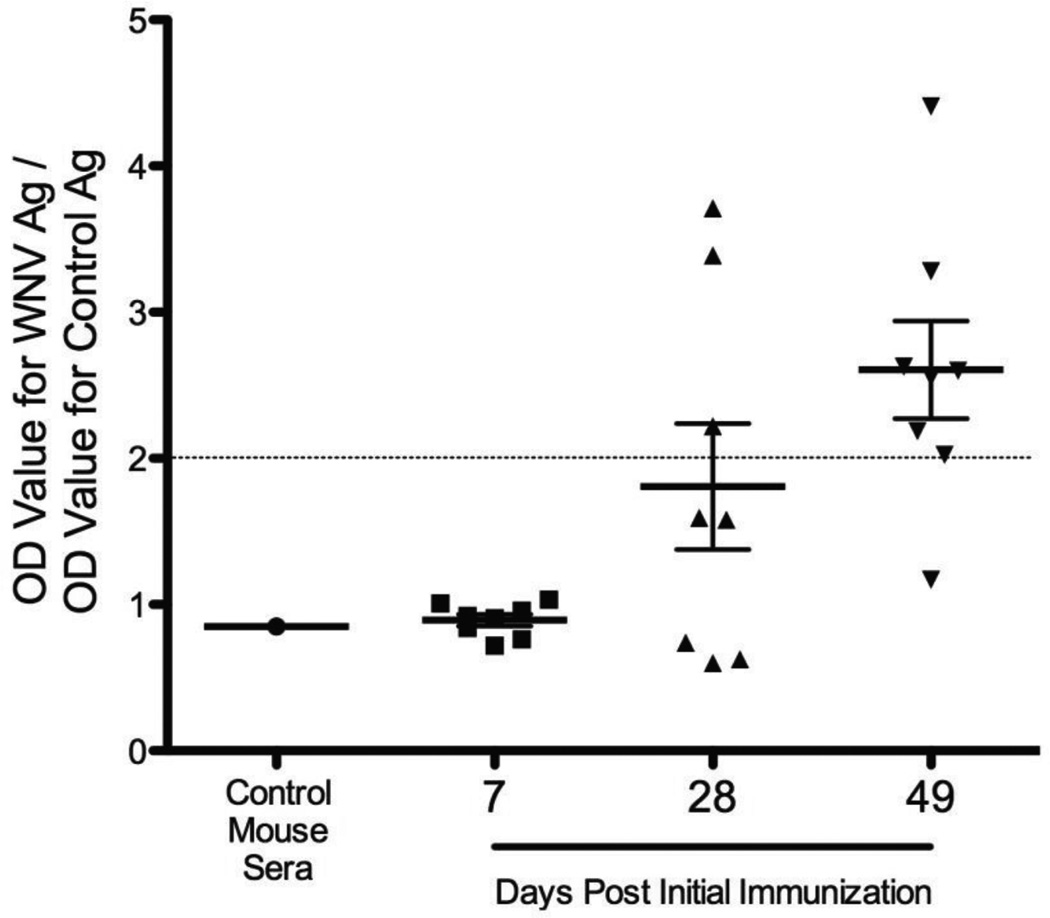
We examined the ability of the d106-WNV recombinant vector to elicit a specific anti-WNV response in immunized Balb/c mice. Mice were immunized subcutaneously with doses of 2 × 106 PFU of the d106-WNV recombinant vaccine vector at days 0, 21, and 42. At the times shown, serum samples were drawn to measure antibody responses. While control mice and mice immunized with d106-WNV showed no WNV-specific IgG at 7 days, 3 of 8 mice immunized with d106-WNV showed significant titers at 28 days and 7 of 8 showed significant titers at 49 days (Figure 4). Qualitative neutralization assays showed the presence of WNV neutralizing antibodies in the sera from the immunized mice (results not shown). Therefore, the d106-WNV recombinant vector elicited a specific anti-WNV IgG antibody response in the immunized mice.
Figure 4. WNV-specific IgG responses in d106-WNV immunized mice.
Sera were taken from 8 immunized mice at 7, 28, and 49 days post-initial immunization, and IgG levels were determined by indirect ELISA using a 1:100 dilution of serum. The dashed line represents the OD ratio threshold that is considered positive for this assay. The values shown are mean and standard error of the mean. The value for control mice was from a pool of sera from unimmunized mice.
Discussion
In this study, we used a transfection assay to define and optimize a WNV expression cassette that efficiently expresses WNV E protein and forms VLPs. We then constructed and characterized a replication-defective HSV recombinant virus, d106-WNV, which expresses the WNV prM and E proteins fused to C-terminal residues of the C protein. Vero cells infected with d106-WNV virus secreted VLPs that contained E, and immunization of mice with d106-WNV virus induced a specific anti-WNV IgG response. We will discuss each of these points individually and how these results will provide important information for future vaccine vectors Optimization of WNV Expression Cassettes for VLP Production. We used a transfection approach to define the sequences from the C protein needed to serve as a secretion signal for the prM and E proteins and to optimize WNV protein expression and VLP release from infected cells. Previous studies of the flavivirus proteins (Allison et al., 1999; Chang, Hunt, and Davis, 2000; Fonseca et al., 1994; Ishikawa et al., 2007; Kojima et al., 2003; Konishi et al., 2000; Takahashi et al., 2009) have found that a sequence from the C-terminus of the C protein is needed to serve as a signal sequence for PrM and for efficient E protein accumulation and assembly into VLPs, but the optimal sequence had not been defined. Our analysis showed that the 18 C-terminal residues of C protein were the most efficient for E protein accumulation and VLP formation. This expression cassette also generated VLPs in an HSV d106 recombinant virus. As in cells transfected with plasmids encoding the WNV proteins, cellular proteases cleave E from prM so E is released as a separate protein, and the HSV vector did not inhibit this process.
Long-term Expression of WNV proteins by the d106 vector. We observed that d106-WNV-infected Vero cells accumulated high levels of E protein through at least 72 hpi. The d106 virus does not express several major immediate-early regulatory proteins, ICP4, ICP27, and ICP22, resulting in a limited HSV gene expression profile with few HSV proteins detected post-infection. Although the d106 virion contains the virion host shut off (UL41) protein, vhs, ICP27 is also needed for shutoff (Song et al., 2001). The limited HSV expression profile also decreases the cytopathogenicity of the vector allowing.prolonged expression of the transgene.. Because Vero cells do not express type I interferons (Desmyter, Melnick, and Rawls, 1968; Mosca and Pitha, 1986), it is conceivable that type I interferon production in normal cells would limit d106S viral gene expression. However, ICP0 expressed by d106 virus can inhibit interferon-β by several mechanisms (Melroe, DeLuca, and Knipe, 2004; Melroe et al., 2007; Orzalli, DeLuca, and Knipe, 2012). Therefore, this is unlikely to be a factor affecting d106-encoded transgene expression in normal cells.
Immunogenicity of the HSV-1 d106 Vector. The HSV-1 d106S-WNV virus was immunogenic in that mice immunized with three doses of the d106S-WNV recombinant virus nearly all seroconverted. Although this could appear to be less efficacious than other immunogens that used less viral vector or fewer immunizations to achieve complete seroconversion (Dayan et al., 2012; Iglesias et al., 2006; Monath et al., 2006), it is hard to compare these results because the sensitivities of the ELISA assays may be different. Furthermore, some of the vaccine constructs, such as the ChimeriVax viruses, are replication-competent, so they will spread and therefore be more immunogenic than a replication-defective virus. Complete head-to-head comparisons of the immunogenicity and safety of the various WNV vaccine candidates are needed.
Protective Immune Responses Against WNV. It is known that a neutralizing antibody response is required to protect against WNV infection and that CTL activity is critical for WNV clearance of infected animals (Chu, Chiang, and Ng, 2007; Diamond et al., 2003a; Diamond et al., 2003b; Mehlhop et al., 2005; Shrestha and Diamond, 2004; Wang et al., 2003). Replication-defective HSV strains induce durable immune responses with induction of both antibody and CD8+ CTL responses (Brehm et al., 1999; Brehm et al., 1997; Brubaker et al., 1996; Da Costa et al., 1997; Kaur et al., 2007; Morrison, Da Costa, and Knipe, 1998; Morrison and Knipe, 1997; Murphy et al., 2000). Also, antigens expressed by replicative-defective HSV recombinants are acquired by dendritic cells at the site of inoculation that then traffic to the lymph nodes for antigen presentation (Watanabe et al., 2007; Zhao et al., 2003). We observed that expression of the E protein without prM or a signal sequence resulted in proteolytic cleavage of the expressed E protein. The internal expression of E protein may enhance presentation of E epitopes on class I MHC; therefore, HSV vectors that express E protein that assembles into VLPs or E protein that accumulates intracellularly should be tested for induction of E-specific CD8+ CTLs. Therefore, one or both of these vectors should induce a broader immune response than just administering VLPs alone. In this study, d106-WNV-immunized mice produced anti-WNV antibody, and further studies will be needed to determine the breadth of immune responses and protection of animals against lethal WNV challenge induced by different vectors.
Because HSV vectors induce a Th1 response in immunized animals (Brubaker et al., 1996; Nguyen, Knipe, and Finberg, 1994), they may provide a useful prime immunization in prime-boost protocols. We have observed that optimal protection against SIV infection was observed with d106 vectors providing the priming immunization followed by a heterologous boost (Kaur et al., 2007; Kaur et al., in preparation). Therefore the HSV-1 d106 WNV vector might be a useful and safe prime for the more well-developed, but replication-competent chimeric flavivirus WNV vaccine (Dayan et al., 2012) or vesicular stomatitis virus vector (Marzi et al., 2015).
In conclusion, this study provides the blueprint for the design of expression cassettes expressing flavivirus proteins that efficiently form virus-like particles. Inclusion of the 18 C-terminal residues of the C protein provides an effective signal sequence that promotes membrane insertion and formation of VLPs. This strategy should be readily adaptable to other flaviviruses including Zika virus. This study also demonstrates that a replication-defective HSV vector can express WNV structural proteins that form extracellular virus-like particles. This vector can induce a specific anti-WNV IgG antibody response in immunized mice. Further research is required to optimize the dose and route of inoculation to determine what combination is required to optimally protect against lethal challenge with WNV or other flaviviruses.
Materials and Methods
Cells and viruses
African green monkey kidney (Vero) cells were grown and maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) with 5% fetal bovine serum-5% newborn calf serum. The E11 cell line, which expresses ICP27 and ICP4 and complements replication of HSV-1 d106 virus (Samaniego, Neiderhiser, and DeLuca, 1998), was maintained in DMEM 5% fetal bovine serum-5% bovine calf serum + G418 (400 (µg/ml).
Plasmids
To construct the d106-WNV recombinant vaccine vector, we first cloned WNV coding sequences into the CMV expression vector pCIΔAflII (Murphy et al., 2000). DNA copies of WNV coding sequences were derived from RNA preparations of infected cells provided by Sharon Isern and Scott Michael (Florida Gulf Coast University), using the One-Step RT-PCR process (Qiagen) with the primers shown in Table 1, and were cloned into the pCR2.1 TA TOPO vector (Invitrogen). Plasmids pCR2.1prM-E1, prM-E2, and prM-E3 were generated using the indicated forward primers (Table 1) and the reverse primer R-Sal. Subcloning of the EcoRI fragments from the pCR2.1-based plasmids into the pCIΔAflII plasmid generated the plasmids pCMVprM-E1, prM-E2, and prM-E3. pCMVEnvΔss and pCMV-C-prM-E were constructed in a similar fashion using primer pairs F2-Eco/R-Sal and CF/R-Sal, respectively. The plasmid used to generate the recombinant d106-WNV virus via homologous recombination was derived from the pPs27pd1 plasmid (Rice, Su, and Knipe, 1989), which contains the HSV genomic sequences surrounding the UL54 (ICP27) gene. The SalI site in pPs27pd1 was converted to a BglII site by inserting a linker (New England Biolabs). The BamHI/BglII fragment from pCMVprM-E3 was then subcloned into the BglII site to generate the pd27-WNV plasmid, which contained the prM-E3 expression cassette in the opposite orientation of UL54. The plasmid pCMV-E-3XFlag expressing WNV E with a preprotrypsin leader was the kind gift of K.G. Kousoulas (Louisiana State University).
Table 1.
Primers used in this study.
| prM-E1: 5’ ATGGCTATCAATCGGCGGAGCTC |
| prM-E2: 5’ ATGAAACACCTTCTGAGT |
| prM-E3: 5’ ATGGCAGCAGGAGGCAAGACCGGAATTGC |
| F2-Eco: 5’ GAATTCATGTTCAACTGCCTTGGAATGAGC |
| CF: 5’ATGTCTAAGAAACCAGGAGG |
| R-Sal: 5’ GTCGACTCAAGCGTGCACGTTCACGGAGAG |
Isolation of the d106-WNV recombinant virus vector
Infectious d106 viral DNA purified from infected E11 cell lysates by sodium iodide gradient centrifugation (Walboomers and ter Schegget, 1976) was co-transfected with varying amounts of linearized pd27-WNV into E11 cells using Lipofectamine 2000 (Invitrogen). The resulting progeny viruses were expanded before plaque purification. Potential recombinants were screened for by the loss of the green fluorescent protein (GFP) signal in individual plaques using an inverted fluorescence microscope (Nikon). Recombinants were confirmed by the detection of WNV E protein in cell lysates by Western blot analysis. d106-WNV was triple-plaque purified, and a stock was grown on E11 cells.
Western blot analysis
For analysis of transfected cells, confluent Vero cells in 6-well plates were transfected with 2 µg of plasmid DNA using Lipofectamine 2000 (Invitrogen). For analysis of infected cells, Vero cells in 6-well plates were infected with d106-WNV at the indicated multiplicity of infection (MOI). Supernatants from 6-well plates were clarified by centrifugation at 15,000 rpm for 30 minutes at 4°C. Proteins were resolved in 12% polyacrylamide gels and analyzed by western blotting as described previously (Watanabe et al., 2007), using an anti-E monoclonal antibody (Chemicon) as the primary antibody.
Mice and immunizations
Animal studies were performed in accordance with Harvard University and National Institutes of Health guidelines. BALB/c mice (Jackson Laboratories) were immunized with 2 × 106 PFU of d106-WNV (8 mice) subcutaneously followed by two booster immunizations at days 21 and 42. Immunizations consisted of virus stock diluted into a volume of 20 µl of sterile 0.9% NaCl solution (Sigma). At 0, 7, 28, and 49 days, 100 µl of blood was drawn from each mouse via the tail vein. Sera were prepared using microtainer serum separators (Beckton Dickinson) and stored at −70 °C until use. Enzyme-linked immunosorbent assays (ELISA) were conducted to measure WNV-specific IgG as described previously (Brown et al., 2007) using WNV-infected Vero cell lysates (WNV antigen) or mock-inoculated Vero cell lysates (control antigen) for coating ELISA plates. Serum samples were tested at a dilution of 1:100 in the same assay. The relative OD values were calculated by dividing the mean optical density (OD) value from the WNV antigen-coated wells by the OD value from control antigen-coated well. Samples were considered positive if the relative OD value was greater than 2.
Purification and analysis of WNV virus-like particles
Vero cells in 150-cm2 flasks were infected with the d106-WNV virus at an MOI of 10. At three days post-infection, the supernatant was clarified by centrifugation in a Sorvall SA-600 rotor at 4 °C for 30 minutes at 15,000 rpm. The VLPs were pelleted from the clarified supernatant by centrifugation in a Beckman 50Ti rotor at 4 °C overnight at 50,000 rpm. The pellet was resuspended in 1 ml of phosphate-buffered saline. VLPs were stored at −70 °C until use. The structure of VLPs was determined by visualization in a JEOL 1200EX transmission electron microscope following incubation with anti-E mAb and goat anti-mouse antibody complexed with protein A-gold and negative staining. VLP proteins were analyzed by western blotting for E protein and by mass spectrometry at the Taplin Biological Mass Spectrometry Facility, Harvard Medical School by microcapillary liquid chromatography-tandem mass spec with an LCQ DECA ion-trap mass spectrometer (Thermo Finnigan).
Research Highlights.
Defined optimal conditions for West Nile virus virus-like particle formation.
Optimal signal sequence was 18 C-terminal residues of capsid (C) protein for E protein expression.
Expression cassette of signal sequence-PrM-E was recombined into an HSV-1 vector.
HSV-1 recombinant vector expressed E protein that was assembled into VLPs and was immunogenic.
Acknowledgments
We thank Kim Appler for her technical assistance with the antibody analysis, Maria Ericsson for her assistance with electron microscopy, Gus Kousoulis for plasmids, and Patrick T. Waters for assistance with preparation of the manuscript. This research was supported by NIAID grants U54-AI057159 (New England Regional Center for Excellence in Biodefense and Emerging Infections) and U54-AI057158 (Northeast Biodefense Center) and R01 AI057552 to DMK and U19-AI109740 to SPJW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Allison SL, Stadler K, Mandl CW, Kunz C, Heinz FX. Synthesis and secretion of recombinant tick-borne encephalitis virus protein E in soluble and particulate form. J Virol. 1995;69:5816–5820. doi: 10.1128/jvi.69.9.5816-5820.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison SL, Stiasny K, Stadler K, Mandl CW, Heinz FX. Mapping of functional elements in the stem-anchor region of tick-borne encephalitis virus envelope protein E. J Virol. 1999;73:5605–5612. doi: 10.1128/jvi.73.7.5605-5612.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm M, Samaniego LA, Bonneau RH, DeLuca NA, Tevethia SS. Immunogenicity of herpes simplex virus type 1 mutants containing deletions in one or more alpha-genes: ICP4, ICP27, ICP22, and ICP0. Virology. 1999;256:258–269. doi: 10.1006/viro.1999.9653. [DOI] [PubMed] [Google Scholar]
- Brehm MA, Bonneau RH, Knipe DM, Tevethia SS. Immunization with a replication deficient mutant of herpes simplex virus type 1 (HSV-1) induces a CD8+ cytotoxic T- lymphocyte (CTL) response and confers a level of protection comparable to wild-type HSV-1. J Virol. 1997;71:3534–3544. doi: 10.1128/jvi.71.5.3534-3544.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman M, Knipe DM. Herpes simplex virus vectors elicit a durable antibody response in mice despite the presence of preexisting host immunity. J Virol. 2002;76:3678–3687. doi: 10.1128/JVI.76.8.3678-3687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AN, Kent KA, Bennett CJ, Bernard KA. Tissue tropism and neuroinvasion of West Nile virus do not differ for two mouse strains with different survival rates. Virology. 2007;368:422–430. doi: 10.1016/j.virol.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker JO, Thompson CM, Morrison LA, Knipe DM, Siber GR, Finberg RW. Th1-associated immune responses to beta-galactosidase expressed by a replication-defective herpes simplex virus. J Immunol. 1996;157:1598–1604. [PubMed] [Google Scholar]
- Carson PJ, Borchardt SM, Custer B, Prince HE, Dunn-Williams J, Winkelman V, Tobler L, Biggerstaff BJ, Lanciotti R, Petersen LR, Busch MP. Neuroinvasive disease and West Nile virus infection, North Dakota, USA, 1999–2008. Emerg Infect Dis. 2012;18:684–686. doi: 10.3201/eid1804.111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. West Nile virus and other arboviral diseases--United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:513–517. [PMC free article] [PubMed] [Google Scholar]
- Chang GJ, Hunt AR, Davis B. A single intramuscular injection of recombinant plasmid DNA induces protective immunity and prevents Japanese encephalitis in mice. J Virol. 2000;74:4244–4252. doi: 10.1128/jvi.74.9.4244-4252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu JH, Chiang CC, Ng ML. Immunization of flavivirus West Nile recombinant envelope domain III protein induced specific immune response and protection against West Nile virus infection. J Immunol. 2007;178:2699–2705. doi: 10.4049/jimmunol.178.5.2699. [DOI] [PubMed] [Google Scholar]
- Cliffe AR, Knipe DM. Herpes simplex virus ICP0 promotes both histone removal and acetylation on viral DNA during lytic infection. J Virol. 2008;82:12030–12038. doi: 10.1128/JVI.01575-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz L, Cardenas VM, Abarca M, Rodriguez T, Reyna RF, Serpas MV, Fontaine RE, Beasley DW, Da Rosa AP, Weaver SC, Tesh RB, Powers AM, Suarez-Rangel G. Short report: serological evidence of West Nile virus activity in El Salvador. Am J Trop Med Hyg. 2005;72:612–615. [PubMed] [Google Scholar]
- Da Costa X, Kramer MF, Zhu J, Brockman MA, Knipe DM. Construction, phenotypic analysis, and immunogenicity of a UL5/UL29 double deletion mutant of herpes simplex virus 2. J Virol. 2000;74:7963–7971. doi: 10.1128/jvi.74.17.7963-7971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa XJ, Bourne N, Stanberry LR, Knipe DM. Construction and characterization of a replication-defective herpes simplex virus 2 ICP8 mutant strain and its use in immunization studies in a guinea pig model of genital herpes. Virology. 1997;232:1–12. doi: 10.1006/viro.1997.8564. [DOI] [PubMed] [Google Scholar]
- Da Costa XJ, Jones CA, Knipe DM. Immunization against genital herpes with a vaccine virus that has defects in productive and latent infection. Proc Natl Acad Sci U S A. 1999;96:6994–6998. doi: 10.1073/pnas.96.12.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphin G, Zientara S, Zeller H, Murgue B. West Nile: worldwide current situation in animals and humans. Comp Immunol Microbiol Infect Dis. 2004;27:343–355. doi: 10.1016/j.cimid.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Dayan GH, Bevilacqua J, Coleman D, Buldo A, Risi G. Phase II, dose ranging study of the safety and immunogenicity of single dose West Nile vaccine in healthy adults ≥50 years of age. Vaccine. 2012;30:6656–6664. doi: 10.1016/j.vaccine.2012.08.063. [DOI] [PubMed] [Google Scholar]
- Desmyter J, Melnick JL, Rawls WE. Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero) J Virol. 1968;2:955–961. doi: 10.1128/jvi.2.10.955-961.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Shrestha B, Marri A, Mahan D, Engle M. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol. 2003a;77:2578–2586. doi: 10.1128/JVI.77.4.2578-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Shrestha B, Mehlhop E, Sitati E, Engle M. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol. 2003b;16:259–278. doi: 10.1089/088282403322396082. [DOI] [PubMed] [Google Scholar]
- Dudek T, Knipe DM. Replication-defective viruses as vaccines and vaccine vectors. Virology. 2006;344:230–239. doi: 10.1016/j.virol.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Estrada-Franco JG, Navarro-Lopez R, Beasley DW, Coffey L, Carrara AS, Travassos da Rosa A, Clements T, Wang E, Ludwig GV, Cortes AC, Ramirez PP, Tesh RB, Barrett AD, Weaver SC. West Nile virus in Mexico: evidence of widespread circulation since July 2002. Emerg Infect Dis. 2003;9:1604–1607. doi: 10.3201/eid0912.030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca BA, Pincus S, Shope RE, Paoletti E, Mason PW. Recombinant vaccinia viruses co-expressing dengue-1 glycoproteins prM and E induce neutralizing antibodies in mice. Vaccine. 1994;12:279–285. doi: 10.1016/0264-410x(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Hayes EB, Komar N, Nasci RS, Montgomery SP, O’Leary DR, Campbell GL. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz FX, Stiasny K. Flaviviruses and flavivirus vaccines. Vaccine. 2012;30:4301–4306. doi: 10.1016/j.vaccine.2011.09.114. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Pesnicak L, Dowdell KC, Lacayo J, Dudek T, Knipe DM, Straus SE, Cohen JI. Comparison of immunogenicity and protective efficacy of genital herpes vaccine candidates herpes simplex virus 2 dl5-29 and dl5-29-41L in mice and guinea pigs. Vaccine. 2008;26:4034–4040. doi: 10.1016/j.vaccine.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias MC, Frenkiel MP, Mollier K, Souque P, Despres P, Charneau P. A single immunization with a minute dose of a lentiviral vector-based vaccine is highly effective at eliciting protective humoral immunity against West Nile virus. J Gene Med. 2006;8:265–274. doi: 10.1002/jgm.837. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Takasaki T, Kurane I, Nukuzuma S, Kondo T, Konishi E. Co-immunization with West Nile DNA and inactivated vaccines provides synergistic increases in their immunogenicities in mice. Microbes Infect. 2007;9:1089–1095. doi: 10.1016/j.micinf.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Jones CA, Taylor TJ, Knipe DM. Biological properties of herpes simplex virus 2 replication-defective mutant strains in a murine nasal infection model. Virology. 2000;278:137–150. doi: 10.1006/viro.2000.0628. [DOI] [PubMed] [Google Scholar]
- Kaur A, Sanford HB, Garry D, Lang S, Klumpp SA, Watanabe D, Bronson RT, Lifson JD, Rosati M, Pavlakis GN, Felber BK, Knipe DM, Desrosiers RC. Ability of herpes simplex virus vectors to boost immune responses to DNA vectors and to protect against challenge by simian immunodeficiency virus. Virology. 2007;357:199–214. doi: 10.1016/j.virol.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A, et al. manuscript in preparation [Google Scholar]
- Kojima A, Yasuda A, Asanuma H, Ishikawa T, Takamizawa A, Yasui K, Kurata T. Stable high-producer cell clone expressing viruslike particles of the Japanese encephalitis virus e protein for a second-generation subunit vaccine. J Virol. 2003;77:8745–8755. doi: 10.1128/JVI.77.16.8745-8755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N. West Nile virus: epidemiology and ecology in North America. Adv Virus Res. 2003;61:185–234. doi: 10.1016/s0065-3527(03)61005-5. [DOI] [PubMed] [Google Scholar]
- Konishi E, Yamaoka M, Kurane I, Mason PW. A DNA vaccine expressing dengue type 2 virus premembrane and envelope genes induces neutralizing antibody and memory B cells in mice. Vaccine. 2000;18:1133–1139. doi: 10.1016/s0264-410x(99)00376-x. [DOI] [PubMed] [Google Scholar]
- Kulasekera VL, Kramer L, Nasci RS, Mostashari F, Cherry B, Trock SC, Glaser C, Miller JR. West Nile virus infection in mosquitoes, birds, horses, and humans, Staten Island, New York, 2000. Emerg Infect Dis. 2001;7:722–725. doi: 10.3201/eid0704.010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Raja P, Knipe DM. Herpesviral ICP0 Protein Promotes Two Waves of Heterochromatin Removal on an Early Viral Promoter During Lytic Infection. mBio. 2016;7:e02007–e02015. doi: 10.1128/mBio.02007-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach BD, Murray CL, Thiel H-J, Rice CM. Flaviviridae. In: Knipe DM, Howley PM, editors. Fields Virology. 6th. Vol. 1. Philadelphia: Lippincott, Williams and Wilkins; 2013. pp. 712–746. 2 vols. [Google Scholar]
- Marzi A, Robertson SJ, Haddock E, Feldmann F, Hanley PW, Scott DP, Strong JE, Kobinger G, Best SM, Feldmann H. EBOLA VACCINE. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science. 2015;349:739–742. doi: 10.1126/science.aab3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlhop E, Whitby K, Oliphant T, Marri A, Engle M, Diamond MS. Complement activation is required for induction of a protective antibody response against West Nile virus infection. J Virol. 2005;79:7466–7477. doi: 10.1128/JVI.79.12.7466-7477.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melroe GT, DeLuca NA, Knipe DM. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. J Virol. 2004;78:8411–8420. doi: 10.1128/JVI.78.16.8411-8420.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melroe GT, Silva L, Schaffer PA, Knipe DM. Recruitment of activated IRF-3 and CBP/p300 to herpes simplex virus ICP0 nuclear foci: Potential role in blocking IFN-beta induction. Virology. 2007;360:305–321. doi: 10.1016/j.virol.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monath TP, Liu J, Kanesa-Thasan N, Myers GA, Nichols R, Deary A, McCarthy K, Johnson C, Ermak T, Shin S, Arroyo J, Guirakhoo F, Kennedy JS, Ennis FA, Green S, Bedford P. A live, attenuated recombinant West Nile virus vaccine. Proc Natl Acad Sci U S A. 2006;103:6694–6699. doi: 10.1073/pnas.0601932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison LA, Da Costa XJ, Knipe DM. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology. 1998;243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- Morrison LA, Knipe DM. Contributions of antibody and T cell subsets to protection elicited by immunization with a replication-defective mutant of herpes simplex virus type 1. Virology. 1997;239:315–326. doi: 10.1006/viro.1997.8884. [DOI] [PubMed] [Google Scholar]
- Mosca JD, Pitha PM. Transcriptional and posttranscriptional regulation of exogenous human beta interferon gene in simian cells defective in interferon synthesis. Mol Cell Biol. 1986;6:2279–2283. doi: 10.1128/mcb.6.6.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CG, Lucas WT, Means R, Czajak S, Hale CL, Lifson JD, Kauer A, Johnson RP, Knipe DM, Desrosiers RC. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J Virol. 2000;74:7745–7754. doi: 10.1128/jvi.74.17.7745-7754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Knipe DM, Finberg RW. Mechanism of virus-induced Ig subclass shifts. J Immunol. 1994;152:478–484. [PubMed] [Google Scholar]
- Orzalli MH, DeLuca NA, Knipe DM. Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection and degradation of IFI16 by the viral ICP0 protein. Proc Natl Acad Sci U S A. 2012;109:E3008–E3017. doi: 10.1073/pnas.1211302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen LR, Carson PJ, Biggerstaff BJ, Custer B, Borchardt SM, Busch MP. Estimated cumulative incidence of West Nile virus infection in US adults, 1999–2010. Epidemiol Infect. 2013;141:591–595. doi: 10.1017/S0950268812001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao M, Ashok M, Bernard KA, Palacios G, Zhou ZH, Lipkin WI, Liang TJ. Induction of sterilizing immunity against West Nile Virus (WNV), by immunization with WNV-like particles produced in insect cells. J Infect Dis. 2004;190:2104–2108. doi: 10.1086/425933. [DOI] [PubMed] [Google Scholar]
- Reisen W, Lothrop H, Chiles R, Madon M, Cossen C, Woods L, Husted S, Kramer V, Edman J. West Nile virus in California. Emerg Infect Dis. 2004;10:1369–1378. doi: 10.3201/eid1008.040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice SA, Su LS, Knipe DM. Herpes simplex virus alpha protein ICP27 possesses separable positive and negative regulatory activities. J Virol. 1989;63:3399–3407. doi: 10.1128/jvi.63.8.3399-3407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaniego LA, Neiderhiser L, DeLuca NA. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J Virol. 1998;72:3307–3320. doi: 10.1128/jvi.72.4.3307-3320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samina I, Khinich Y, Simanov M, Malkinson M. An inactivated West Nile virus vaccine for domestic geese-efficacy study and a summary of 4 years of field application. Vaccine. 2005;23:4955–4958. doi: 10.1016/j.vaccine.2005.03.052. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Diamond MS. Role of CD8+ T cells in control of West Nile virus infection. J Virol. 2004;78:8312–8321. doi: 10.1128/JVI.78.15.8312-8321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siger L, Bowen R, Karaca K, Murray M, Jagannatha S, Echols B, Nordgren R, Minke JM. Evaluation of the efficacy provided by a Recombinant Canarypox-Vectored Equine West Nile Virus vaccine against an experimental West Nile Virus intrathecal challenge in horses. Vet Ther. 2006;7:249–256. [PubMed] [Google Scholar]
- Song B, Yeh KC, Liu JJ, Knipe DM. Herpes simplex virus gene products required for viral inhibition of expression of G1-phase functions. Virology. 2001;290:320–328. doi: 10.1006/viro.2001.1175. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Ohtaki N, Maeda-Sato M, Tanaka M, Tanaka K, Sawa H, Ishikawa T, Takamizawa A, Takasaki T, Hasegawa H, Sata T, Hall WW, Kurata T, Kojima A. Effects of the number of amino acid residues in the signal segment upstream or downstream of the NS2B-3 cleavage site on production and secretion of prM/M-E virus-like particles of West Nile virus. Microbes Infect. 2009;11:1019–1028. doi: 10.1016/j.micinf.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Volz A, Lim S, Kaserer M, Lulf A, Marr L, Jany S, Deeg CA, Pijlman GP, Koraka P, Osterhaus AD, Martina BE, Sutter G. Immunogenicity and protective efficacy of recombinant Modified Vaccinia virus Ankara candidate vaccines delivering West Nile virus envelope antigens. Vaccine. 2016;34:1915–1926. doi: 10.1016/j.vaccine.2016.02.042. [DOI] [PubMed] [Google Scholar]
- Walboomers JM, ter Schegget J. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976;74:256–258. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lobigs M, Lee E, Mullbacher A. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J Virol. 2003;77:13323–13334. doi: 10.1128/JVI.77.24.13323-13334.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Brockman MA, Ndung’u T, Mathews L, Lucas WT, Murphy CG, Felber BK, Pavlakis GN, Deluca NA, Knipe DM. Properties of a herpes simplex virus multiple immediate-early gene-deleted recombinant as a vaccine vector. Virology. 2007;357:186–198. doi: 10.1016/j.virol.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Watts DM, Tesh RB, Siirin M, Rosa AT, Newman PC, Clements DE, Ogata S, Coller BA, Weeks-Levy C, Lieberman MM. Efficacy and durability of a recombinant subunit West Nile vaccine candidate in protecting hamsters from West Nile encephalitis. Vaccine. 2007;25:2913–2918. doi: 10.1016/j.vaccine.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JS, Kim JJ, Hwang D, Choo AY, Dang K, Maguire H, Kudchodkar S, Ramanathan MP, Weiner DB. Induction of potent Th1-type immune responses from a novel DNA vaccine for West Nile virus New York isolate (WNV-NY1999) J Infect Dis. 2001;184:809–816. doi: 10.1086/323395. [DOI] [PubMed] [Google Scholar]
- Zhao X, Deak E, Soderberg K, Linchen M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhan’s cells, induce protective TH1 responses to herpes simplex virus 2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]