Abstract
Over 230,000 new cases of breast cancer are expected to be diagnosed in the United States in 2015. Taxane-based chemotherapy is often an effective treatment, but can also cause adverse symptoms in patients due to neurotoxicity. These side effects can impair postural control in patients; however, this instability has scarcely been quantified. The purpose of this pilot study was to gain insight into the natural history of postural instability in breast cancer patients being treated with taxane-based chemotherapy. Thirty-two breast cancer patients (31 female/ 1 male; 47.6 ± 11.2 yr; 16 stage II/ 16 stage III) completed eyes open and eyes closed quiet standing trials in the oncology clinic where they were being treated. These trials were collected at five timepoints throughout their chemotherapy treatment: (1) before initiating chemotherapy to provide a baseline, (2–4) before starting subsequent chemotherapy cycles, and (5) 1–3 months after receiving their last taxane infusion. After the first chemotherapy cycle, patients demonstrated increases in 95% confidence ellipse area of center of pressure (CoP) [45.2%, p=0.01] and root mean squared CoP excursion [18%, p=0.006] compared to baseline values for the eyes closed condition. These balance deficiencies progressed with cumulative taxane exposure. Postural instability persisted 1–3 months after completing chemotherapy with increases in 95% CoP ellipse area [86.8%, p=0.002], root mean squared CoP excursion [32.6%, p=0.001], and mean CoP velocity [30.4%, p=0.024]. The balance impairments demonstrated by patients in this study appear to be clinically relevant when compared to balance impairments previously reported in other patient populations.
Keywords: Posturography, center of pressure, balance, quiet standing, cancer, chemotherapy
Introduction
Over 230,000 new cases of breast cancer are expected to be diagnosed in the United States alone in 2015 [1]. Fortunately, diagnostic and treatment advances including chemotherapy have yielded a current all-time high in survival rates for all stages of breast cancer [1]. As breast cancer survivors are living longer, there has been greater focus on understanding the side effects of treatment and taking steps to mitigate them during and after chemotherapy [2, 3]. In addition to improving the experiences of survivors during and after treatment, evidence is mounting that quality of life interventions can increase the amount of therapy received and may also improve survival [4].
While chemotherapy agents such as taxanes and platinum compounds have contributed to improved clinical outcomes, they can also cause many adverse symptoms including fatigue, pain, and numbness along with central and peripheral nervous system impairments [2, 3, 5–8]. Previous investigations have reported the detrimental effects that these toxicities can have on human postural control in other populations [9–14], but balance deficits in patients exposed to neurotoxic chemotherapy agents have scarcely been quantified. Similar to diabetic [15] and elderly populations [16, 17], patients treated with neurotoxic chemotherapy can be at an increased risk of falling [18, 19]. Additionally, a retrospective study identified balance deficits in breast cancer patients after completing taxane-based chemotherapy, which provides evidence for the presence of postural instability in patients undergoing neurotoxic chemotherapy [20]. However, the natural history of these balance deficits during repeat exposures to chemotherapy has yet to be elucidated. Reports on time-dependent changes in symptoms during taxane-based chemotherapy have, to-date, focused on patient-reported outcomes [21]. While patient reported symptoms are currently used to assess chemotherapy toxicities in the oncology clinic, they can be subjective and have coarse rating scales, making it difficult to discern early changes that may identify patients who are at risk for developing severe symptoms [2, 22].
We propose that objective, high-resolution, and clinically-oriented measures may improve clinicians’ ability to detect detrimental effects earlier during the course of treatment. Posturography offers these measure attributes by utilizing center of pressure (CoP) movement to gain insight into human postural control. This approach has been used to identify balance deficits in other balance impaired populations such as the elderly [23–25] and diabetic patients [9, 10]. The purpose of this pilot study was to establish the natural history of postural instability in breast cancer patients being treated with taxane-based chemotherapy. We hypothesized that patients would demonstrate balance deficits that would increase with cumulative chemotherapy exposure.
Methods
Participants
Breast cancer patients (stages I–III) who were initiating taxane-based chemotherapy were recruited for this study. Patients unable to stand or walk without assistance, having a pre-existing diagnosis of neuropathy of any kind, or having previous exposure to taxane at any time were excluded from the study. Patients who had previous exposure to other chemotherapy or targeted therapy known to be associated with neuropathy (e.g., platinum therapy, bortezomib, vinblastine) within one year of starting in the study were also excluded. Prior exposure to other types of chemotherapy, such as doxorubicin and cyclophosphamide, were allowed. Patients that satisfied the inclusion criteria were enrolled in the study after providing institutional review board-approved informed consent (Table 1).
Table 1.
Patient demographics and treatment summary.
| General Characteristics | ||
|---|---|---|
| Enrolled (n) | 32 | |
| Completed Study | 27 (84%) | |
| Dropped Out | 4 (13%) | |
| Death | 1 (3%) | |
| Sex (f/m) | 31/1 | |
| Age (yr) | 47.6 ± 11.2 | |
| Mass (kg) | 74.6 ± 20.1 | |
| Height (m) | 1.64 ± 0.08 | |
| BMI (kg/m2) | 27.9 ± 7.8 | |
| Diabetes (n) | 0 | |
| Cancer Stage at Diagnosis (n) | ||
| I | 0 (0%) | |
| II | 16 (50%) | |
| III | 16 (50%) | |
| IV | 0 (0%) | |
| Chemotherapy Regimen | n (%) | |
| Doxorubicin /Cyclophosphamide prior to taxane therapy | 26 (81%) | |
| Taxane Therapy | ||
| Paclitaxel weekly | 18 (56%) | |
| Paclitaxel every 2 weeks | 6 (19%) | |
| Docetaxel every 3 weeks | 7 (22%) | |
| Weekly Paclitaxel → Docetaxel every 3 weeks (switched after cycle 1) | 1 (3%) | |
Age, Mass, Height, and BMI are presented as mean ± SD
Protocol
Patient balance was assessed at 5 timepoints: (1) prior to starting chemotherapy to provide a baseline, (2–4) before starting subsequent chemotherapy cycles, and (5) 1–3 months after receiving their last taxane infusion. As shown in Table 1, patients received different taxane-based treatments which caused the number of days between timepoints to vary on an individual basis. All balance measurements were collected by trained clinical research coordinators in the oncology clinic where the patients were being seen for regularly scheduled appointments. Balance tasks consisted of quiet standing while looking straight ahead with the eyes open as well as eyes closed. One trial was performed for each condition. The quiet standing trials were performed in a narrow stance (medial foot surfaces separated by 5 cm) with arms resting at the patients' sides for 30 seconds. Patients were also asked to complete functional reaching tasks and a limits-of-stability task; however, due to inconsistencies in how these tasks were administered and performed, the data could not be analyzed.
A custom LabVIEW program (Version 2011; National Instruments; Austin, TX) was developed to collect CoP data. The program also provided clinical personnel with step-by-step instructions on the administration of the balance tasks to ensure consistency in how the tests were administered between visits and operators. CoP data were calculated and recorded by the LabVIEW program using force and moment measurements from a BP5046 balance plate (Bertec Corporation; Worthington, OH). Over the course of the study, two versions of the LabVIEW program were utilized. The first version collected CoP data at 50 Hz while the second was increased to 1000 Hz. All CoP data were then 4th order low-pass Butterworth filtered at 20 Hz before being analyzed. While the use of two versions of the LabVIEW data collection program introduces a discontinuity in the methods, the balance parameters calculated from the two versions were checked to ensure consistency. Balance parameters calculated from trials collected at 1000 Hz were found to agree within 2% with those calculated from the same trials downsampled to 50 Hz before processing. Custom MATLAB (Version 2014b; MathWorks; Natick, MA) scripts were used to process all CoP data.
Quantifying Postural Control
Summary measures of CoP trajectory, consisting of 95% confidence ellipse area (EA), root mean squared CoP excursion (RMS), and mean CoP velocity (MVEL), were selected to characterize patients' postural stability. Detailed descriptions of the calculations for these balance parameters are provided elsewhere [23]. Briefly, EA provides a measure of spatial control of the CoP where it defines the area of an ellipse that contains approximately 95% of the data. A larger EA indicates greater dispersion of the CoP and a less tightly controlled CoP position. RMS characterizes the root mean squared displacement of the CoP from its mean position. A higher RMS suggests that, on average, the CoP is an increased distance away from the mean position and towards a boundary of the base of support [23, 26], which is indicative of a decrease in postural stability. MVEL is the total distance traveled of the CoP divided by the duration of the trial. MVEL provides insight into the extent of corrective CoP actions taken during a balance task, where an increased MVEL indicates less appropriate CoP adjustments (i.e., overcorrecting) were made to maintain stability. EA, RMS, and MVEL have previously been used to distinguish elderly fallers from non-fallers [24, 25]. These balance parameters were calculated for the 2-dimensional (planar) case as well as RMS and MVEL also being calculated for each 1-dimensional case (medial-lateral and anterior-posterior) to gain insight into whether changes were preferential to a given direction.
Statistics
Linear mixed models for repeated measures with patients as random effects and testing timepoint as the fixed effect were used to estimate the changes in the various balance parameters over time. The Mixed procedure in SAS (Version 9.4; SAS Institute Inc.; Cary, NC) was used to address the missing data problem observed in our study assuming data were missing at random. Least squares means and corresponding contrasts of the fixed time parameters from the mixed models were used to estimate the differences between timepoints. The significance level was set at α=0.05. Corrections for multiple comparisons were not made due to the exploratory nature of this pilot study.
Results
Thirty-two breast cancer patients satisfying the study inclusion criteria were enrolled. The demographic and treatment characteristics are summarized in Table 1. Five of the 32 accrued patients did not complete the study. Four patients dropped out of the study (n=3 after completing baseline testing, n=1 after completing the 2nd timepoint) and one patient passed away from complications of chemotherapy before completing the 2nd timepoint. The balance results presented here include partial datasets that were collected from these patients. The number of successful trials that were obtained during the study and included in data analyses were 30, 26, 24, 23 and 24 eyes open trials and 29, 26, 24, 23, and 24 eyes closed trials (timepoint 1 to timepoint 5, respectively).
All patients received taxane-based chemotherapy (e.g., paclitaxel and/or docetaxel). Patients were predominately treated with weekly paclitaxel infusions (n=18), but the frequency and/or chemotherapy agent varied based on individual patient needs. Six patients had holds placed on their chemotherapy treatment and five patients received dose reductions in their chemotherapy, with one of these as a result of neuropathy.
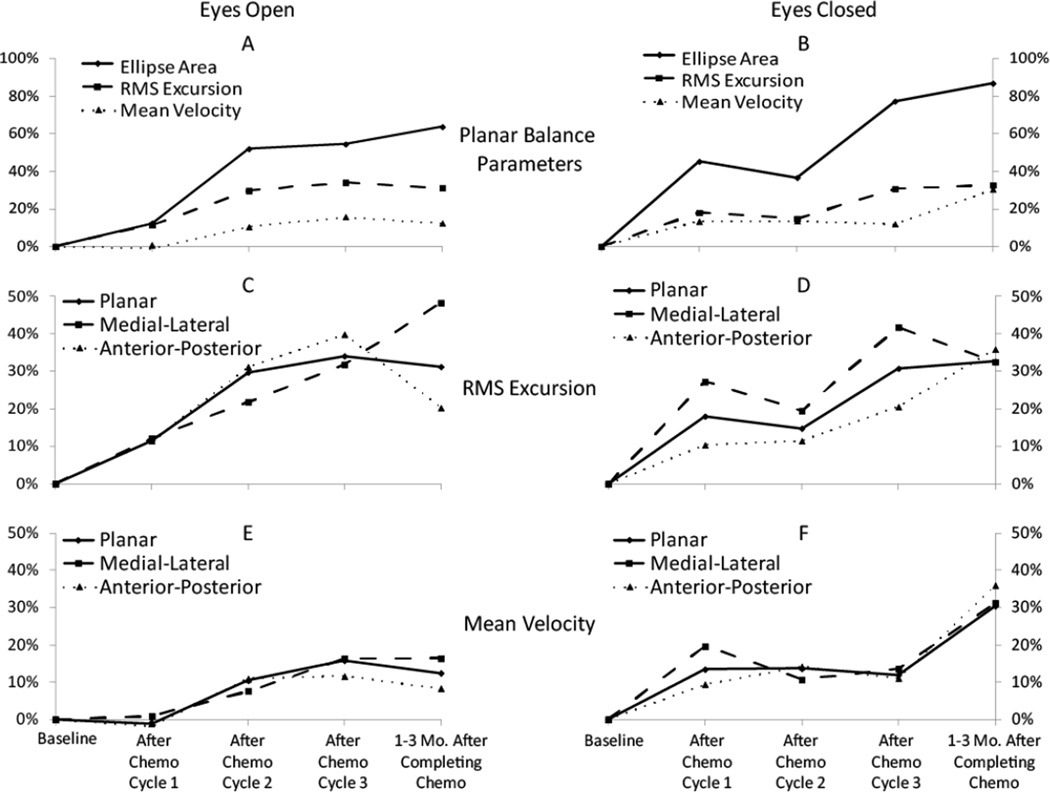
A group summary of the changes in balance parameters as patients progressed through their chemotherapy treatment is shown in Figure 1. In general, patients demonstrated increases in all balance parameters (i.e., patient balance degraded) as they progressed through chemotherapy. Increases in parameters were observed for both eyes open and eyes closed conditions. More pronounced effects for MVEL and EA were observed during the eyes closed task compared to the eyes open task.
Figure 1.
Percent change in balance parameters from baseline values for the group of breast cancer patients during the quiet standing task with eyes open (left column - A,C,E) and eyes closed (right column – B,D,F). Comparisons of the planar balance parameters for the eyes open and eyes closed tasks are provided in A and B, respectively. Planar along with anterior-posterior and medial-lateral cases are also presented for RMS (C,D) and MVEL (E,F) for the two different trial types.
The values of balance parameters at each timepoint are presented in Table 2. Significant detrimental changes were observed during both eyes open and eyes closed conditions in all planar balance parameters except for eyes open MVEL. Significant changes were observed in the eyes closed condition as early as after the first chemotherapy cycle (timepoint 2).
Table 2.
Least squares means estimates for balance parameters during quiet standing. Mean ± SE (p-value for contrast with timepoint 1)
| Eyes Open | Timepoint 1 | Timepoint 2 | Timepoint 3 | Timepoint 4 | Timepoint 5 |
|---|---|---|---|---|---|
| Planar | |||||
| EA | 211.3 ± 30.5 | 237.5 ± 23.5 (0.5) | 321.2 ± 41.0 (0.03)* | 326.4 ± 84.8 (0.2) | 345.9 ± 50.0 (0.02)* |
| RMS | 4.8 ± 0.3 | 5.3 ± 0.3 (0.1) | 6.2 ± 0.4 (0.005)* | 6.4 ± 0.6 (0.03)* | 6.3 ± 0.5 (0.009)* |
| MVEL | 10.5 ± 0.6 | 10.4 ± 0.7 (0.9) | 11.6 ± 0.8 (0.2) | 12.1 ± 1.1 (0.1) | 11.8 ± 1.0 (0.2) |
| Medial-Lateral | |||||
| RMS | 2.9 ± 0.2 | 3.2 ± 0.2 (0.2) | 3.5 ± 0.3 (0.08) | 3.8 ± 0.4 (0.02)* | 4.3 ± 0.4 (0.003)* |
| MVEL | 5.9 ± 0.4 | 6.0 ± 0.4 (0.9) | 6.4 ± 0.5 (0.4) | 6.9 ± 0.7 (0.1) | 6.9 ± 0.6 (0.08) |
| Anterior-Posterior | |||||
| RMS | 3.7 ± 0.3 | 4.1 ± 0.2 (0.2) | 4.9 ± 0.4 (0.01)* | 5.2 ± 0.6 (0.03)* | 4.5 ± 0.4 (0.1) |
| MVEL | 7.3 ± 0.4 | 7.2 ± 0.5 (0.8) | 8.1 ± 0.6 (0.1) | 8.1 ± 0.8 (0.3) | 7.9 ± 0.7 (0.4) |
| Eyes Closed | Timepoint 1 | Timepoint 2 | Timepoint 3 | Timepoint 4 | Timepoint 5 |
| Planar | |||||
| EA | 298.3 ± 31.1 | 433.3 ± 58.2 (0.01)* | 407.8 ± 63.4 (0.027)* | 529.0 ± 90.3 (0.007)* | 557.4 ± 88.7 (0.002)* |
| RMS | 5.8 ± 0.3 | 6.9 ± 0.5 (0.006)* | 6.7 ± 0.5 (0.005)* | 7.62 ± 0.6 (0.001)* | 7.7 ± 0.6 (<0.001)* |
| MVEL | 13.8 ± 0.9 | 15.7 ± 1.2 (0.07) | 15.7 ± 1.2 (0.06) | 15.5 ± 1.6 (0.3) | 18.1 ± 1.9 (0.02)* |
| Medial-Lateral | |||||
| RMS | 3.3 ± 0.2 | 4.2 ± 0.4 (0.03)* | 3.9 ± 0.4 (0.049)* | 4.7 ± 0.4 (<0.001)* | 4.4 ± 0.4 (0.007)* |
| MVEL | 7.3 ± 0.5 | 8.8 ± 0.8 (0.04)* | 8.1 ± 0.9 (0.3) | 8.3 ± 1.1 (0.3) | 9.6 ± 1.0 (0.01)* |
| Anterior-Posterior | |||||
| RMS | 4.7 ± 0.3 | 5.2 ± 0.3 (0.03)* | 5.2 ± 0.4 (0.008)* | 5.7 ± 0.5 (0.02)* | 6.4 ± 0.5 (<0.001)* |
| MVEL | 10.1 ± 0.6 | 11.0 ± 0.8 (0.2) | 11.5 ± 0.9 (0.07) | 11.2 ± 1.2 (0.3) | 13.7 ± 1.5 (0.02)* |
CoP: center of pressure. EA: 95% confidence CoP ellipse area (mm2). RMS: root mean squared excursion of CoP (mm). MVEL: mean velocity of CoP (mm/s).
p < 0.05 for contrast with baseline (timepoint 1)
Testing in the clinic was safely performed by the patients and did not interfere with normal clinic operations. The entire testing protocol took less than 10 minutes to administer on average and was conducted in-between normally scheduled appointments. In addition to the testing sessions missed due to patient dropout or death, ~10% of the collected balance trials were compromised due to patients improperly performing the task, operator error, or data communication error between the balance plate and LabVIEW data collection interface. This issue was improved with additional training and the implementation of the 2nd version of the data collection program.
Discussion
To our knowledge, this study is the first to longitudinally evaluate postural stability of cancer patients during chemotherapy. Our hypothesis was supported, as breast cancer patients demonstrated significant balance deficits as early as after the first chemotherapy cycle, which was 2–3 weeks after the first treatment. Increased MVEL suggests that patients tended to overcorrect in their CoP adjustments as they progressed through chemotherapy treatment. The concurrent increases in EA and RMS suggest that patients also demonstrated poorer control of their CoP position by allowing it to travel closer to their base of support boundaries. The significant changes that were observed in EA, RMS, and MVEL indicate that patients experienced deficits in their postural stability as they received taxane-based chemotherapy treatment. The results of this pilot study support the feasibility of implementing balance measures during routine oncology clinic flow to longitudinally quantify postural instability in breast cancer patients.
The effect of taxane-based chemotherapy on patient balance appears to be clinically relevant when compared to balance impairments previously observed in other patient populations (Table 3) [9, 23, 25]. The increases in balance parameters after three months of chemotherapy treatment exceed the effects resulting from 40 years of aging for EA and RMS, while not for MVEL [23].
Table 3.
Comparisons between balance impairments reported in select studies during eyes closed stance.
| Before vs. After Taxane Treatment (48 yr) |
Young (26 yr) vs. Elderly (68 yr) [23] |
Elderly Non-Faller vs. Elderly Faller [25] |
Control vs. Moderate- Severe Diabetic Peripheral Neuropathy [9] |
|
|---|---|---|---|---|
| 95% Ellipse Area | 87% Increase After Treatment* |
28% Increase for Elderly |
Not Reported | Not Reported |
| Mean Velocity (Planar) |
30% Increase* | 82% Increase* | Not Reported | 37% Increase for DPN* |
| RMS Excursion (Anterior-Posterior) |
36% Increase* | 16% Increase | 23% Increase for Fallers |
Not Reported |
| RMS Excursion (Medial-Lateral) |
33% Increase* | 17% Increase | 56% Increase* | Not Reported |
change was reported to be statistically significant
RMS: root mean square. DPN: diabetic peripheral neuropathy.
Taxane therapy was also associated with similar or larger increases in balance parameters compared to fall status in an elderly population of one study [24], but only for RMS in the anterior-posterior direction in another study [25]. While the patients in our study demonstrated significant increases in MVEL, these changes were less substantial than the difference in MVEL between patients with moderate to severe diabetic peripheral neuropathy (DPN) versus controls [9]. Only one patient in our study required a dose reduction due to neuropathy. Cancer patients with more severe neuropathy symptoms or other sensory deficits may demonstrate balance deficits similar to this moderate to severe DPN group. These comparisons demonstrate the severity of the balance degrading effect observed by patients as they received taxane-based chemotherapy.
The balance parameter magnitudes that patients exhibited as they received taxane-based chemotherapy are also consistent with the observed increased risk of falling in patients treated with neurotoxic chemotherapy [18, 19]. The peak EA and RMS values demonstrated by the patients in this study exceeded those of a healthy elderly group [23]. The breast cancer patient group's peak medial-lateral MVEL was also comparable to an elderly faller group [25]. The presence of these deficits in spite of the 20 year difference in group age between the patients in our study and the participants in these previous studies attests to the balance deficits encountered by patients undergoing these treatments. While these patients demonstrated seemingly high-risk balance parameter magnitudes, no falls were reported to study personnel by patients during the study period. The patients in this study were relatively younger, not diabetic, and may have been biased to be relatively more fit due to self-selection into the study.
Further research is warranted to determine whether the balance deficits observed in this study are more pronounced in cancer patients who are older, have diabetes, or have pre-existing sensory deficiencies. Further comparison of the balance conditions and parameters provides additional insight into the effect of taxane-based chemotherapy on patient balance. The effect of chemotherapy on balance was more pronounced during the eyes closed task compared to the eyes open task, particularly for MVEL and EA. This result supports the idea that the driving factors for the balance deficiencies may be proprioceptive and/or vestibular deficits. This interpretation is also consistent with the toxicity to proprioceptive nerve fibers that has been associated with taxane therapy [3, 6, 27]. The increased sensitivity during the eyes closed task may also have been influenced in part by the superior reliability that we observed during this condition compared to the eyes open task. Additionally, the balance deficits observed in this study do not appear to be isolated to a given anatomical plane. Detrimental changes in the balance parameters were observed across their anterior-posterior, medial-lateral, and planar derivatives. Previous studies have reported that elderly fallers demonstrate medial-lateral, but not anterior-posterior instability compared to elderly non-fallers [24, 25, 28]. In comparison, both medial-lateral and anterior-posterior instability resulted from taxane therapy. The multidirectional deficits experienced by the breast cancer patients in this study may infer that these patients would be less stable in response to a perturbation regardless of the perturbation direction, which may contribute to an increased fall risk of cancer patients [18, 19].
The balance measures presented here provide an objective, high-resolution approach to quantify changes in patient function by assessing postural control. These methods have successfully detected balance deficiencies in elderly [23, 26] and DPN [9, 10] populations and identified fall-prone individuals [24, 25]. The results of this pilot study suggest that these methods may also be clinically useful in assessing balance deficiencies induced by chemotherapy treatment. Further exploration of posturography in this population may be able to reveal potential differences between various neurotoxic chemotherapy agents as well as the relationship between these balance parameters and other chemotherapy related side effects. The improved resolution and objective nature of posturography compared to patient-reported outcomes may also enhance the ability of researchers to evaluate candidate interventions for adverse side effects, such as chemotherapy-induced peripheral neuropathy, that have remained elusive to-date [2, 3, 6, 27, 29].
This study provides new insight into the manner in which balance deficiencies develop in cancer patients being treated with taxane-based chemotherapy; however, several limitations exist. One limitation is that while quiet stance is commonly used to evaluate postural control, performance during quiet stance may not directly correspond to performance during more dynamic tasks. Our pilot study attempted to also investigate postural control during more dynamic tasks (i.e., functional reaching and limits of stability), but our initial training of clinical research coordinators and the instructional script used with patients were insufficient to ensure consistency in the data for these activities. These inconsistencies were largely resolved after administering additional training and improving the script, though ensuring a consistent level of effort remains a challenge. While 32 patients were recruited into the study, four patients voluntarily dropped out, citing the hassle of having additional testing on top of their other appointments and treatments. However, the results of this study should alleviate some of these concerns as the balance protocol was administered while patients were waiting for their next appointment and did not typically add any delay in patients’ treatments. We speculate that cancer patients may be more willing to participate in these tasks if they are incorporated as a quality of life aspect of their treatment rather than as an experiment aimed at improving treatments in the future. Our group analysis of balance changes may mask the effects of variations in chemotherapy regimen and alterations to patient treatment, but this preliminary study was not powered to stratify by these characteristics or to correct for multiple comparisons. Additionally, the last testing visit conducted as part of this study was 1–3 months after finishing chemotherapy treatment. Future studies of the same nature could be strengthened by extending follow-up visits beyond this time frame to gain insight into the extent to which patients spontaneously recover from the balance deficits presented here. Finally, this study focused on one cancer type being treated with a single class of neurotoxic chemotherapy drug. Additional research is needed to understand the extent to which these results translate to patients of other cancer types being treated with different chemotherapy agents. Further research is also needed to validate the methods described here for clinical use.
Conclusions
Objective, quantifiable balance measures were successfully and safely implemented in an oncology clinic by trained clinical research coordinators. The balance data that were collected from these measures demonstrated progressive deterioration of postural control in a group of breast cancer patients that was detected early in chemotherapy treatment. This postural instability appears to be clinically relevant when compared to balance impairments previously observed in other patient populations. The clinical utility of balance measures to quantify side effects of chemotherapy treatment on patient stability were supported by this study and warrant further investigation.
Highlights.
Balance data were collected in the oncology clinic without disrupting productivity.
Balance deficits were observed after the 1st chemotherapy cycle.
Balance deficits experienced by patients were clinically relevant.
Acknowledgments
This research was supported by the National Cancer Institute (Grant No. R03 CA182165-01) and the National Science Foundation Graduate Research Fellowship Program (Grant No. DGE-1343012).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no conflict of interest to disclose
References
- 1.Howlander N, Noone A, Krapacho M, Garshell J, Miller D, Altekruse S, et al. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute; 2014. [Google Scholar]
- 2.Cavaletti G. Chemotherapy-Induced Peripheral Nuerotoxicity (CIPN): What We Need and What We Know. J Peripher Nerv Syst. 2014;19:66–76. doi: 10.1111/jns5.12073. [DOI] [PubMed] [Google Scholar]
- 3.Ocean AJ, Vahdat LT. Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Support Care Cancer. 2004;12:619–625. doi: 10.1007/s00520-004-0657-7. [DOI] [PubMed] [Google Scholar]
- 4.Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res. 2008;27:32. doi: 10.1186/1756-9966-27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avan A, Postma TJ, Ceresa C, Cavaletti G, Giovannetti E, Peters GJ. Platinum-induced neurotoxicity and preventive strategies: past, present, and future. Oncologist. 2015;20:411–432. doi: 10.1634/theoncologist.2014-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–1967. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 7.Raffa RB, Tallarida RJ. Chemo fog: cancer chemotherapy-related cognitive impairment: Springer Science & Business Media. 2011. [PubMed] [Google Scholar]
- 8.Overcash J. Prediction of falls in older adults with cancer: a preliminary study. Oncol Nurs Forum. 2007;34:341–346. doi: 10.1188/07.ONF.341-346. [DOI] [PubMed] [Google Scholar]
- 9.Boucher P, Teasdale N, Courtemanche R, Bard C, Fleury M. Postural stability in diabetic polyneuropathy. Diabetes Care. 1995;18:638–645. doi: 10.2337/diacare.18.5.638. [DOI] [PubMed] [Google Scholar]
- 10.Simoneau GG, Ulbrecht JS, Derr JA, Becker MB, Cavanagh PR. Postural instability in patients with diabetic sensory neuropathy. Diabetes Care. 1994;17:1411–1421. doi: 10.2337/diacare.17.12.1411. [DOI] [PubMed] [Google Scholar]
- 11.Horak FB, Hlavacka F. Somatosensory loss increases vestibulospinal sensitivity. J Neurophysiol. 2001;86:575–585. doi: 10.1152/jn.2001.86.2.575. [DOI] [PubMed] [Google Scholar]
- 12.Simoneau GG, Ulbrecht JS, Derr JA, Cavanagh PR. Role of somatosensory input in the control of human posture. Gait Posture. 1995;3:115–122. [Google Scholar]
- 13.Lafond D, Corrveau H, Prince F. Postural Control Mechanisms During Quiet Standing in Patients with Diabetic Sensory Neuropathy. Diabetes Care. 2004;27:173–178. doi: 10.2337/diacare.27.1.173. [DOI] [PubMed] [Google Scholar]
- 14.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 15.Richardson JK, Hurvitz EA. Peripheral neuropathy: a true risk factor for falls. J Gerontol A Biol Sci Med Sci. 1995;50:M211–M215. doi: 10.1093/gerona/50a.4.m211. [DOI] [PubMed] [Google Scholar]
- 16.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 17.O'Loughlin JL, Robitaille Y, Boivin JF, Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol. 1993;137:342–354. doi: 10.1093/oxfordjournals.aje.a116681. [DOI] [PubMed] [Google Scholar]
- 18.Tofthagen C, Overcash J, Kip K. Falls in Persons with Chemotherapy-Induced Peripheral Neuropathy. Support Care Cancer. 2012;20:583–589. doi: 10.1007/s00520-011-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wildes TM, Dua P, Fowler SA, Miller JP, Carpenter CR, Avidan MS, et al. Systematic review of falls in older adults with cancer. J Geriatr Oncol. 2015;6:70–83. doi: 10.1016/j.jgo.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wampler MA, Topp KS, Maiskowski C, Byl NN, Rugo HS, Hamel K. Quantitative and Clinical Description of Postural Instability in Women With Breast Cancer Treated With Taxane Chemotherapy. Arch Phys Med Rehabil. 2007;88:1002–1008. doi: 10.1016/j.apmr.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Loprinzi CL, Reeves BN, Dakhil SR, Sloan JA, Wolf SL, Burger KN, et al. Natural History of Paclitaxel-Associated Acute Pain Syndrome: Prospective Cohort Study NCCTG N08C1. J Clin Oncol. 2011;29:1472–1478. doi: 10.1200/JCO.2010.33.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith EML, Beck SL, Cohen J. The total neuropathy score: a tool for measuring chemotherapy-induced peripheral neuropathy. Oncol Nurs Forum: ONCOLOGY NURSING SOCIETY. 2008:96. doi: 10.1188/08.ONF.96-102. [DOI] [PubMed] [Google Scholar]
- 23.Prieto TE, Myklebust JB, Hoffmann RG, Lovett EG, Myklebust BM. Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Trans Biomed Eng. 1996;43:956–966. doi: 10.1109/10.532130. [DOI] [PubMed] [Google Scholar]
- 24.Melzer I, Benjuya N, Kaplanski J. Postural stability in the elderly: a comparison between fallers and non-fallers. Age Ageing. 2004;33:602–607. doi: 10.1093/ageing/afh218. [DOI] [PubMed] [Google Scholar]
- 25.Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994;49:M72–M84. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- 26.Maki B, Holliday P, Fernie G. Aging and postural control. J Am Geriatr Soc. 1990;38:1–9. doi: 10.1111/j.1532-5415.1990.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 27.Pachman DR, Barton DL, Watson JC, Loprinzi CL. Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin Pharmacol Ther. 2011;90:377–387. doi: 10.1038/clpt.2011.115. [DOI] [PubMed] [Google Scholar]
- 28.Lord SR, Rogers MW, Howland A, Fitzpatrick R. Lateral stability, sensorimotor function and falls in older people. J Am Geriatr Soc. 1999;47:1077–1081. doi: 10.1111/j.1532-5415.1999.tb05230.x. [DOI] [PubMed] [Google Scholar]
- 29.Tofthagen C, Visovsky C, Berry DL. Strength and balance training for adults with peripheral neuropathy and high risk of fall: current evidence and implications for future research. Oncol Nurs Forum: Onc Nurs Society. 2012:E416–E424. doi: 10.1188/12.ONF.E416-E424. [DOI] [PMC free article] [PubMed] [Google Scholar]