Abstract
Introduction
Personalized, scheduled deep brain stimulation in Tourette syndrome (TS) may permit clinically meaningful tic reduction while reducing side effects and increasing battery life. Here, we evaluate scheduled DBS applied to TS at two-year follow-up.
Methods
Five patients underwent bilateral centromedian thalamic (CM) region DBS. A cranially contained constant-current device delivering stimulation on a scheduled duty cycle, as opposed to the standard continuous DBS paradigm was utilized. Baseline vs. 24-month outcomes were collected and analyzed, and a responder analysis was performed. A 40% improvement in the Modified Rush Tic Rating Scale (MRTRS) total score or Yale Global Tic Severity Scale (YGTSS) total score defined a full responder.
Results
Three of the 4 patients followed to 24 months reached full responder criteria and had a mean stimulation time of 1.85 hours per day. One patient lost to follow-up evaluated at the last time point (month 18) was a non-responder. Patients exhibited improvements in MRTRS score beyond the improvements previously reported for the 6 month endpoint; on average, MRTRS total score was 15.6% better at 24 months than at 6 months and YGTSS total score was 14.8% better. Combining the patients into a single cohort revealed significant improvements in the MRTRS total score (−7.6 [5.64]; p=.02).
Conclusion
Electrical stimulation of the centromedian thalamic region in a scheduled paradigm was effective in suppressing tics, particularly phonic tics. Full responders were able to achieve the positive DBS effect with a mean of 2.3 ± .9 (SEM) hours of DBS per day.
Introduction
Tourette syndrome (TS) is a childhood-onset disorder characterized by motor and vocal tics [1]. TS is a lifelong syndrome; however, in most cases, tics wane by the late teenage years[2]. Some patients with TS have symptoms resistant to medication and to behavioral intervention[3]. These individuals may develop severe complications, including strokes and cervical myelopathies[4–6]. Deep brain stimulation (DBS) has emerged as a highly efficacious treatment option for addressing tics in at least some of these cases; however, this technique should only be applied following appropriate multidisciplinary screening [7]. Several studies of thalamic DBS have previously demonstrated significant improvement in tic behavior [8]. A recent open-label study with a two-year follow-up which used continuous centromedian thalamic stimulation reported 52% and 54.2% mean tic reductions as measured by the Yale Global Tic Severity Scale (YGTSS) [9]. In addition, an open-label study of one-year outcomes following continuous centromedian thalamic stimulation in 6 patients by Ackermans and colleagues demonstrated a 49% improvement in YGTSS total score and a 35% improvement in the Modified Rush Tic Rating Scale (MRTRS) total score [10]. Though the results of the two studies were similar, the former group used a slightly more anterior target.
Based on the paroxysmal nature of tics in TS, we recently hypothesized that treatment via a scheduled as opposed to a continuous DBS approach [11] might be better suited for TS. Scheduled stimulation is a form of open loop DBS whereby stimulation is delivered in an a priori determined manner rather than from a responsive, or closed-loop approach. Still, it may be viewed on the continuum as moving closer to a responsive approach in that 1) it delivers less cumulative stimulation than continuous DBS and 2) it temporally limits the stimulation provided to more pathological states (i.e. periods of greater tic activity) and reduces duty cycles (e.g. turning off the device at night). One advantage to scheduled therapy is that the duty cycle can be personalized to an individual patient’s needs [12]. Other advantages include a potential decrease in stimulation-related side effects and an increased battery life [13,14].
We previously reported the six-month outcomes of 5 TS patients treated with bilateral centromedian thalamic region DBS in a scheduled stimulation paradigm[11]. In brief, there were significant improvements in several clinical measures of tic severity using this scheduled stimulation during the first six months of therapy. The goal of the scheduled stimulation paradigm was two-fold: 1) to tailor stimulation pulse trains to a stimulation ON period followed by a post-stimulation OFF interval (e.g., 2 seconds ON and 10 seconds OFF) and 2) to establish a 24-hour duty cycle for delivery of these pulse trains that targeted time periods when tic behavior posed the greatest burden to patient quality of life and interfered with daily activities important to the patient. The present study expanded the follow-up of scheduled stimulation to 24-month outcomes and presents a responder analysis.
Methods
Overview
The present study is a long-term continuation of a clinical trials planning grant (National Institutes of Health R34 Clinical Trials Planning Project), which explored the safety and preliminary effectiveness of bilateral simultaneous implantation of centromedian thalamic region deep brain stimulation (DBS). Details of this study, including surgical candidate selection, inclusion and exclusion criteria, and outcomes at 6-month follow-up, have been previously published [11]. In brief, the parent study included a cohort of 5 individuals with medication-refractory and severely disabling TS who underwent an approved DBS surgery protocol as part of the NIH study. Ethical approval to conduct the study was obtained by the institutional review board and all patients provided written informed consent to enroll in the study. Pre-surgical mean YGTSS total score and MRTRS total scores at baseline were 92.2 ±9.34 and 16.6 ±1.95, respectively. At baseline, information pertaining to general disease characteristics (age, disease duration, medication, tic subtypes) [11] was obtained along with the following scales: the 36-Item Short Form Health Survey Quality of Life Assessment [15], the modified Structured Clinical Interview for TS diagnosis [16], the Yale Global Tic Severity Scale (YGTSS) [17,18], the videotaped Modified Rush Tic Rating Scale (MRTRS) [18,19], the 17-item Hamilton Depression Rating Scale [20], the Yale-Brown Obsessive Compulsive Scale [20,21], and the Young Mania Rating Scale [22]. The scales were repeated at each six-month interval. Initial scheduled stimulation settings and revisions to these settings at 6-month follow-up appointments were also obtained.
For the present follow-up study, the outcomes were examined at the 24-month endpoint. During the outcome assessments, all subjects were tested in the ON stimulation state at the parameters implemented during the prior programming session (i.e. no acute changes). While both subjects and raters were blinded to stimulation pulse train settings, patients were aware of the 24-hour duty cycle, i.e. the timing of stimulation ON hours during the 24-hour period, since this parameter was based on patient preference. Thus, the long-term evaluations were single-blinded.
Primary Outcome Measures
The two primary outcome measures were the Modified Rush Tic Rating Scale (MRTRS) [18,19] and the Yale Global Tic Severity Scale (YGTSS) [17,18]. The MRTRS assesses tic behavior using a structured short-term videotape protocol. This method can yield objective data on tic counts and anatomical distribution, but it remains vulnerable to sampling bias and bias due to TS patients’ ability to (unconsciously) suppress tics while being videotaped [23]. Thus, an MRTRS assessment performed at a random time in clinic may not validly approximate the usual degree of tic activity in the patient’s regular environment. In contrast, the YGTSS employs a clinician-rated scale based on information elicited during a semi-structured interview. This method affords a window into a longer time duration (the 1-week interval prior to clinical assessment) and the more subjective dimensions of tic symptoms such as interference and impairment; however, this method is vulnerable to recall and interviewer biases. Due to its relative simplicity, the YGTSS has been more widely used in research and clinical practice compared to the MRTRS. Given the relative advantages and disadvantages of the two scales described above, we elected to utilize both scales in our study to determine the merits of each scale in this population.
Stimulation Settings
Key terms are defined as follows: The pulse train was defined as the duration and spacing of stimulation delivery; it is given in a ratio of seconds of stimulation ON to seconds of stimulation OFF. The duty cycle was defined by one or more blocks of time of variable duration in which pulse trains were delivered. These blocks lasted between .5 and 24 hours, and occurred between 1 and 4 times per day. Total cycling time refers to the total number of scheduled hours within a 24-hour period that fixed pulse trains of stimulation were delivered. Total cycling time varied from 2 to 24 hours. Finally, total daily stimulation time was calculated as the amount of time within a 24-hour period that electrical current was actually emitted from the implanted electrode. For example, a pulse train of 4 seconds on, 30 seconds off in a duty cycle of 08:00–20:00 (12 hours total cycling time) would result in a total daily stimulation time of 1.6 hours. A schematic showing scheduled stimulation settings for a sample patient is shown in Figure 1.
Figure 1. Scheduled vs. Continuous Stimulation.
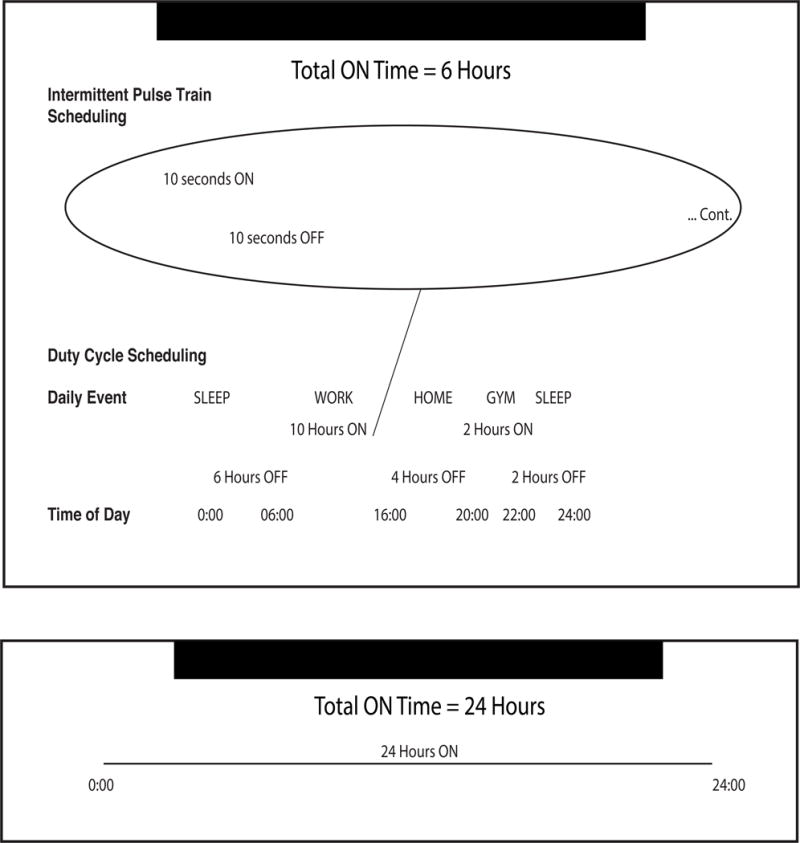
Sample scheduled stimulation settings (showing both pulse train scheduling and duty cycle scheduling) for a patient with a 10 seconds ON, 10 seconds OFF pulse train and 12 hours of total cycling time. This patient receives 6 hours of total daily stimulation (Top), as compared to the classic chronic stimulation paradigm delivering 24 hours of total daily stimulation (Bottom).
DBS programming sessions were performed at each 6-month follow-up interval. The stimulation settings were chosen empirically and were based on bedside observations of visible motor and phonic tic suppression. At follow-up visits, settings were revised empirically based on clinical observation of tic suppression, patient feedback about changes in symptoms, and the reported quality of life on the prior settings. Pulse train settings were initially approximated based on the frequency and duration of a patient’s tics, based on the hypothesis that patients with higher tic frequencies could benefit from more frequent pulses of stimulation and those with tics with longer duration could benefit from longer pulses. Ultimately, pulse train settings were refined empirically based on apparent bedside tic suppression as well as a desire to reduce side effects (e.g. for some patients, certain pulse train settings made them “feel the stimulator turn on/off,” which was described as uncomfortable). Settings were also chosen for battery life preservation since the cranially based neurostimulator (RNS300, Neuropace, Mountain View CA) had a limited battery capacity compared to conventional continuous neurostimulators. One patient (Subject 1) was lost to long-term follow-up as the patient declined to return for evaluation at 24 months.
Statistical Analysis
Means and standard deviations were calculated for all pre-surgical baseline scores and all scores up to month 24. Considering the small dataset, a Shapiro-Wilk normality test was performed for all outcomes data [24]. A paired t-test was used to distinguish significant change between baseline and subsequent months, provided that the data were normally distributed as defined by the Shapiro-Wilk test. The statistical test was two-sided and considered significant if p-values were less than 0.05.
Responder Analysis
A post hoc responder analysis was performed on the 5 subjects with 24-month follow-up. The subjects were categorized as responders, partial responders, or non-responders. Analyses were conducted separately for the two main outcomes, YGTSS total score and MRTRS total score. Since a 25% decrease on the YGTSS total score had been previously shown to predict clinically meaningful change in tic severity in TS (as correlated to the Clinical Global Impression-Improvement scale), we used this threshold [25]. We defined a 25% decrease in YGTSS total as the cutoff to distinguish responders from non-responders.
We further subdivided the responder classification into two categories to identify responders above a placebo threshold. Placebo response rate in TS DBS has not been directly measured, but in other treatments for tic disorders has been as high as 32.6% [26]. Full responders beyond a potential placebo threshold were therefore defined as having a >40% reduction in YGTSS total tic severity score, and partial responders were defined as having a 25–40% reduction in YGTSS total tic severity score (partial responders may have been biased by a placebo effect). It should be noted that the primary outcome measure in the parent 6-month outcomes study was a >50% improvement in the YGTSS. In the present study, the 40% cut-off was adopted to reflect a threshold for minimum meaningful clinical improvement above the estimated placebo effect. We implemented identical thresholds for the MRTRS total score based on the demonstrated correlations between MRTRS and YGTSS total scores previously reported in the literature [19].
Results
The five study subjects had a mean age of 34.4 (range, 28–39) years and a mean disease duration of 28.8 (range, 20–37) years. Three women and 2 men were included in the study. The specific disease characteristics, including the history of medication intake and pre- and post-DBS medications, were previously reported.
Due to variability in life circumstances, therapeutic goals, and the nature and thresholds for side effects, it was not possible to perform a well-controlled, cross-cohort investigation of the effects of stimulation parameters in this study. Therefore, we report summaries of each patient’s stimulation settings throughout the study (Figure 2).
Figure 2. Scheduled Stimulation Parameters and Clinical Outcomes.
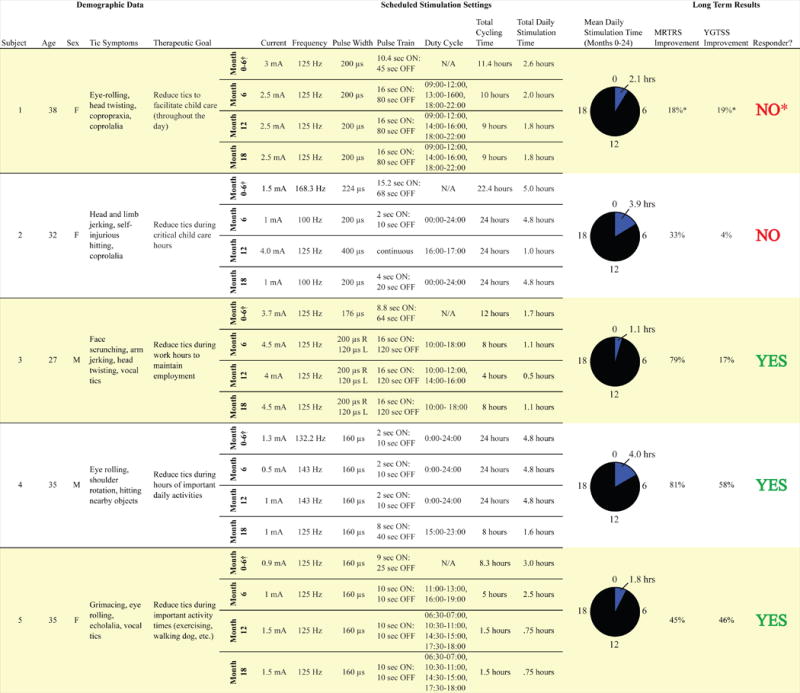
Demographic information, scheduled stimulation parameters, and long term outcomes. The duty cycle is based on times of the day when patients reported the greatest interference of tics with activities of daily living. Stimulation parameters reflect settings for leads in both left and right hemispheres, unless otherwise specified (L= Left, R=Right). Percent improvements in YGTSS and MRTRS total scores reflect change from pre-surgical baseline at 24-month follow-up. Responders achieved >40% improvement in either YGTSS or MRTRS total scores at month 24. *Subject 1 was lost to follow-up; long term results presented here reflect the final evaluation (month 18).
†Stimulation parameters were modified on a monthly basis for the first 6 months of the study. Here we present means for stimulation parameters in months 0–6. Parameters from individual months (including duty cycles) are available in Okun, et al, 2013. YGTSS= Yale Global Tic Severity Scale; MRTRS= Modified Rush Tic Rating Scale.
For all patients, the parameters for the left and right hemispheres were commonly the same with differences occurring only in the programming of the pulse width for Subject 3 at months 6, 12, and 18. Wide variations in the employed currents were observed with a range of 1.0 to 4.5 mA. Stimulation frequencies across subjects were 125 Hz with two exceptions: a frequency of 83.3 Hz at month 6 for subject 2, and 143 Hz at months 6 and 12 for Subject 4. The pulse widths were variable across subjects and ranged from 80 to 320 microseconds. Mean daily stimulation times and clinical outcomes for all patients are shown in Figure 2.
Primary Outcomes
Baseline vs. 24-month data revealed the YGTSS total score was improved by 10%, 46%, 58%, and 17% for the 4 active study subjects. The mean YGTSS total score improvement across the cohort was 30% (range, 10–58%). The subject lost to follow-up exhibited an 18% improvement in YGTSS score at month 18 (final measure). The MRTRS total score was improved by 21%, 79%, 81%, and 44% respectively at 24 months. The mean MRTRS total score improvement across the cohort was 56% (range, 21–81%). The subject lost to follow-up exhibited a 19% improvement in MRTRS score at month 18 (final measure). In addition, patients followed to 24 months exhibited improvements in MRTRS score beyond the improvements previously reported for the 6 month endpoint; on average, MRTRS score was 15.6% better at 24 months than at 6 months and YGTSS total score was 14.8% better. MRTRS and YGTSS total scores for all patients at 6 month intervals are shown in Figure 3.
Figure 3. Change in YGTSS and MRTRS Total Scores.
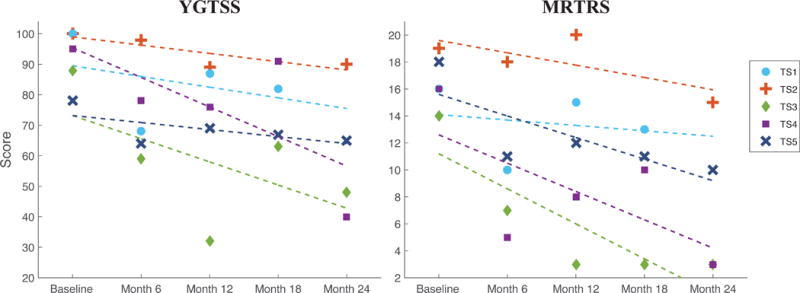
Changes in YGTSS and MRTRS total scores for all subjects at 6 month intervals. The dotted lines signify trends of improvement via linear regression. Responders achieved >40% improvement in YGTSS or MRTRS total scores at month 24 compared to pre-surgical baseline; partial responders achieved 25%–40% improvement; non-responders exhibited <25% improvement.
Change from baseline at each 6-month interval is shown in Table 1. When final outcome measure data (month 18 for subject 1 and month 24 for all others) were analyzed, there were statistically significant improvements in the YGTSS total score, MRTRS total score, and MRTRS phonic tic severity score.
Table 1.
Baseline and 6 Month Interval Outcome Scores
| Scale | Domain | Month 6 |
Month 12 |
Month 18 |
Month 24 |
Final Outcome |
|---|---|---|---|---|---|---|
| YGTSS | Motor severity | −16 | X | −19 | −32 | −29 |
| Phonic severity | −22 | −20 | −23 | −44 | −38 | |
| Total severity | −19 | −16 | −21 | −38 | −33 | |
| Impairment | −22 | X | X | X | X | |
| Total | −20 | X | −16 | −33 | −29.5* | |
|
| ||||||
| HAM-D 17 | Total | 5.26 | 28.95 | 19.35 | −70.83 | −48.39 |
|
| ||||||
| MRTRS | No. of body areas | −36.84 | −21.05 | X | −40 | −31.58 |
| Motor tics/min | −38.46 | X | −33.33 | −63.64 | −53.85 | |
| Phonic tics/min | −33.33 | −33.33 | X | X | X | |
| Motor tic severity | −25 | −25 | −31.25 | −50 | −40 | |
| Phonic tic severity | −57.89 | −52.63 | −73.33** | −60 | −57.89* | |
| Total | −38.55* | −30.12 | −42.19* | −53.73* | −46.99* | |
|
| ||||||
| YMRS | Total | −7.14 | 0 | −8.33 | X | −25 |
|
| ||||||
| YBOCS | Obsessions | −4.44 | −4.44 | 18.18 | X | −12.12 |
| Compulsions | −12.28 | −15.79 | 14.29 | 3.33 | 4.76 | |
| Total | −8.82 | −10.78 | 16 | −13.21 | −2.67 | |
Abbreviations: DBS, deep brain simulation; HAM-D, Hamilton Depression Rating Scale; MRTRS, Modified Rush Tic Rating Scale; SD, Standard Deviation; TS, Tourette syndrome, YBOCS, Yale-Brown Obsessive Compulsive Scale; YGTSS, Tale Global Tic Severity Scale; YMRS, Young Mania Rating Scale.
X: No value computed; data not normally distributed (Shapiro-Wilk test)
Outcomes significant at the .05 level are highlighted in bold.
Other Scales
There were no statistically significant changes in the Short Form 36, The Hamilton Depression Rating Scale (HAM-D), Young Mania Rating Scale (YMRS), and Yale-Brown Obsessive Compulsive Scale (YBOCS), and there were only trends toward improvement in the HAM-D-17 and YMRS.
Responder Analysis
A responder analysis was conducted using baseline versus 24-month values in the YGTSS and MRTRS total scores.
Full responder criterion (>40% reduction in symptoms) was attained with respect to the YGTSS total score for 2 out of 4 patients (50%, Subjects 3 and 4) (Table 2, esupp), and in 3 out of 4 patients (75%, Subjects 3, 4, and 5) with respect to the MRTRS total score (Table 3, esupp).
Table 2.
Responder Analysis for Yale Global Tic Severity Scale (YGTSS)
| Responder Category | Patient | YGTSS Domain | Baseline | 24 Months |
Change (%) |
|
|---|---|---|---|---|---|---|
| Subject | ||||||
| Non-Responder‡ | 5 | Motor severity | 20 | 16 | −20.00 | ‡ |
| Phonic severity | 18 | 19 | 5.56 | ‡ | ||
| Total severity | 38 | 35 | −7.90 | ‡ | ||
| Impairment | 40 | 30 | −25.00 | † | ||
| Total | 78 | 65 | −16.67 | ‡ | ||
|
| ||||||
| Subject | ||||||
| Full Responder* | 4 | Motor severity | 25 | 10 | −60.00 | ★ |
| Phonic severity | 20 | 0 | −100.0 | ★ | ||
| Total severity | 45 | 10 | −77.78 | ★ | ||
| Impairment | 50 | 30 | −40.00 | ★ | ||
| Total | 95 | 40 | −57.90 | ★ | ||
|
| ||||||
| Subject | ||||||
| Full Responder* | 3 | Motor severity | 24 | 13 | −45.84 | ★ |
| Phonic severity | 24 | 5 | −79.17 | ★ | ||
| Total severity | 48 | 18 | −62.50 | ★ | ||
| Impairment | 40 | 30 | −25.00 | † | ||
| Total | 88 | 48 | −45.46 | ★ | ||
|
| ||||||
| Subject | ||||||
| Non-Responder‡ | 2 | Motor severity | 25 | 25 | 0.00 | ‡ |
| Phonic severity | 25 | 25 | 0.00 | ‡ | ||
| Total severity | 50 | 50 | 0.00 | ‡ | ||
| Impairment | 50 | 40 | −20.00 | ‡ | ||
| Total | 100 | 90 | −10.00 | ‡ | ||
|
| ||||||
| 18 Month | ||||||
|
| ||||||
| Subject | ||||||
| Non-Responder‡ | 1 | Motor severity | 25 | 21 | −16.00 | ‡ |
| Phonic severity | 25 | 21 | −16.00 | ‡ | ||
| Total severity | 50 | 42 | −16.00 | ‡ | ||
| Impairment | 50 | 40 | −20.00 | ‡ | ||
| Total | 100 | 82 | −18.00 | ‡ | ||
|
| ||||||
| Mean Responder Outcomes
|
Motor severity | 23 | 13 | −41.95 | ★ | |
| Phonic severity | 20.67 | 8 | −57.87 | ★ | ||
| Total severity | 43.67 | 21 | −49.40 | ★ | ||
| Impairment | 43.34 | 30 | −30.00 | † | ||
| Total | 87 | 51 | −40.01 | ★ | ||
Full Responder:≥40% improvement in YGTSS total score
Partial Responder=40%–25% improvement in YGTSS total score
Non-Responder: < 25%improvement in YGTSS total score
Table 3.
Responder Analysis for Modified Rush Tic Rating Scale (MRTRS)
| Category | Patient | MRTRS Domain | Baseline | 24 Months | Change (%) | |
|---|---|---|---|---|---|---|
| Full Responder* | Subject 5 | No. of body areas | 4 | 3 | −25.00 | † |
| Motor tics/min | 2 | 1 | −50.00 | ★ | ||
| Phonic tics/min | 4 | 2 | −50.00 | ★ | ||
| Motor tic severity | 4 | 2 | −50.00 | ★ | ||
| Phonic tic severity | 4 | 2 | −50.00 | ★ | ||
| Total | 18 | 10 | −44.45 | ★ | ||
|
| ||||||
| Full Responder* | Subject 4 | No. of body areas | 4 | 1 | −75.00 | ★ |
| Motor tics/min | 4 | 1 | −75.00 | ★ | ||
| Phonic tics/min | 1 | 0 | −100.0 | ★ | ||
| Motor tic severity | 4 | 1 | −75.00 | ★ | ||
| Phonic tic severity | 3 | 0 | −100.0 | ★ | ||
| Total | 16 | 3 | −81.25 | ★ | ||
|
| ||||||
| Full Responder* | Subject 3 | No. of body areas | 3 | 1 | −66.67 | ★ |
| Motor tics/min | 1 | 1 | 0.00 | ‡ | ||
| Phonic tics/min | 2 | 0 | −100.0 | ★ | ||
| Motor tic severity | 4 | 1 | −75.00 | ★ | ||
| Phonic tic severity | 4 | 0 | −100.0 | ★ | ||
| Total | 14 | 3 | −78.58 | ★ | ||
|
| ||||||
| Non-Responder‡ | Subject 2 | No. of body areas | 4 | 4 | 0.00 | ‡ |
| Motor tics/min | 4 | 1 | −75.00 | ★ | ||
| Phonic tics/min | 3 | 2 | −33.34 | † | ||
| Motor tic severity | 4 | 4 | 0.00 | ‡ | ||
| Phonic tic severity | 4 | 4 | 0.00 | ‡ | ||
| Total | 19 | 15 | −21.06 | ‡ | ||
| 18 Month | ||||||
|
| ||||||
| Non-Responder‡ | Subject 1 | No. of body areas | 4 | 4 | 0.00 | ‡ |
| Motor tics/min | 2 | 2 | 0.00 | ‡ | ||
| Phonic tics/min | 2 | 1 | −50.00 | ★ | ||
| Motor tic severity | 4 | 4 | 0.00 | ‡ | ||
| Phonic tic severity | 4 | 2 | −50.00 | ★ | ||
| Total | 16 | 13 | −18.75 | ‡ | ||
|
| ||||||
| Mean Responder Outcomes
|
No. of body areas | 3.67 | 1.67 | −55.56 | ★ | |
| Motor tics/min | 2.34 | 1 | −41.67 | ★ | ||
| Phonic tics/min | 2.34 | 0.67 | −83.34 | ★ | ||
| Motor tic severity | 4 | 1.34 | −66.67 | ★ | ||
| Phonic tic severity | 3.67 | 0.67 | −83.34 | ★ | ||
| Total | 16 | 5.34 | −68.10 | ★ | ||
Full Responder: ≥40% improvement in MRTRS total score
Partial Responder=40%–25% improvement in MRTRS total score
Non-Responder: < 25%improvement in MRTRS total score
Ultimately, 3 out of 4 patients (Subjects 3, 4, and 5) fulfilled the full responder criterion of >40% reduction in either YGTSS or MRTRS total scores. It should be noted that with regard to the patient lost to follow-up, applying responder analysis criteria to the last available time point (month 18) revealed that the subject was a non-responder for both the YGTSS and MRTRS.
Discussion
In this study, the long-term effects of scheduled stimulation of the centromedian thalamus were analyzed in five patients suffering from severe refractory Tourette syndrome.
A key finding of this study was that TS patients on a scheduled regimen of thalamic stimulation continue to improve beyond 6 months of therapy. It should be noted that this improvement was not uniform across each 6-month follow up; in fact, an increase in tics as measured by the MRTRS was observed in most (4 of 5) patients at 12 months. However, at the 24-month mark patients experienced, on average, a 15.6% improvement in MRTRS score and a 14.8% improvement in YGTSS score compared to 6-month outcomes. Changes in stimulation parameters could explain this unexpected increase in tics at the 12-month follow-up for subjects 1, 2, 4, and 5 (Figure 2). For example, stimulation current was decreased for subjects 2 and 4; interestingly, these patients continued to improve in the YGTSS score, despite poorer MRTRS outcomes. For subject 1, the pulse train interval was lengthened, and total duty cycle time was decreased for subject 5; for both subjects 1 and 5 the total daily stimulation time was decreased. These changes in scheduling parameters were undertaken to reduce side effects and/or improve battery longevity. In light of this unusual reversal of response, it is also worth considering that scheduled stimulation may function differently than continuous stimulation and that responses to scheduled stimulation may not match the pattern observed in continuous paradigms. Variability at 6 months intervals in this cohort suggests the importance of long-term follow-up and that more research is needed to confirm this trend in a larger cohort and, if confirmed, to investigate potential underlying causes.
We observed a significant beneficial effect of the scheduled stimulation paradigm when the 24-month follow-up assessment was compared to the preoperative baseline assessment (30% mean improvement in YGTSS total score and 56% mean improvement in MRTRS total score). Three of four patients followed to 24 months met the stringent full responder criterion (>40% improvement in either MRTRS or YGTSS total score). Two patients achieved >40% improvement in total score for both primary outcome measures, while one patient achieved >40% improvement for the MRTRS only. We thus observed a relative inconsistency between YGTSS and MRTRS outcomes in this cohort. Discrepancies between these outcomes have been observed in other similarly-sized studies of TS DBS, but almost all point to larger improvements in YGTSS total scores than in MRTRS [10] [27]. Here we present the first study where the relative improvement in MRTRS exceeded that of the YGTSS. These findings make sense in light of the variability in duty cycle scheduling across the cohort. For example, subject 5, who met responder criteria for the MRTRS but not for the YGTSS, received the fewest hours of total cycling time throughout the study [1.6 ± .5 (SEM) hours/day]. During months 12–24, Subject 5 was programmed for 4 intervals of 30 minutes per day—a total of 2 hours of total cycling time compared to a mean of 14.6±2.4 (SEM) hours of total cycling time during months 12–24 for the rest of the cohort.
The YGTSS utilizes patient reporting to measure tic behavior during the week prior to the assessment, while the MRTRS utilizes video recording to measure tic behavior in short durations (10 minutes). Since the MRTRS evaluation occurred during the stimulation ON state (when pulse trains were delivered), the MRTRS examined patients only in the treated state whereas the YGTSS combined patient feedback about the treated (i.e. stimulation ON) state with the non-treated state (stimulation OFF, no pulse trains delivered). In addition, since all MRTRS assessments were performed in the ON state, patients and raters were not blinded to the stimulation state during these assessments. These factors may have contributed to the observed discrepancy in MRTRS and YGTSS scores.
Our findings suggest that scheduled stimulation can reduce tic behavior on a level comparable to continuous stimulation paradigms reported by other studies [9,10,27–29]. However, scheduled stimulation may not be as effective for certain patients as continuous stimulation, particularly in settings where patients opt for duty cycles that may result in sub-optimal total stimulation time. We hypothesize that Subject 5’s classification as a responder by the MRTRS criterion and a non-responder by the YGTSS criterion may be explained by the delivery of clinically effective stimulation parameters for sub-optimal durations (at the patient’s request) and it is possible that tic behavior was inadequately controlled across the 24–hour period.
The main limitation of this study was the statistical power for the small number of enrolled patients. In addition, given this small sample size it was not feasible to conduct a randomized, prospective comparison of scheduled and continuous stimulation. Furthermore, the non-standardized nature of the scheduled settings in each patient—necessitated by battery constraints—in some sense limits the ability to compare across patients. However, this assessment of a personalized stimulation schedule remains valuable, given the heterogeneity of tic presentation and the hypothesis that intermittent stimulation during hours of greatest tic severity (which varied across patients) more closely resembles a responsive approach. Another limitation of this study was the use of a neurostimulator designed for the treatment of epilepsy. Since stimulation for epilepsy can be achieved via a responsive approach that does not entail continuous stimulation, battery life for these stimulators in practice can be quite short [30]. Therefore, the duty cycle had to be programmed based on both patient preferences (benefits, side effects, and most useful time for activation) and clinical judgment of optimal settings based on the confines of projected battery life.
Conclusion
Electrical stimulation of the centromedian thalamic region with scheduled pulse trains and duty cycles was effective in suppressing tics at the 24-month follow-up. Full responders (patients with >40% improvement in YGTSS or MRTRS) were able to achieve the positive effect of scheduled DBS with a mean of 2.3 ± .9 (SEM) hours of total daily stimulation.
Supplementary Material
Highlights.
We tested personalized, scheduled DBS in Tourette at 24 month time point.
75% of patients followed to 24 months had >40% reduction in MRTRS (responders).
Responders improved with only 1.85 hours of DBS per day on average.
MRTRS score at 24 months improved beyond the 6 month endpoint.
On average, MRTRS was 15.6% better at 24 mo. than at 6 mo.
Acknowledgments
A. Gunduz has on-going grants from NIH/NINDS, the Michael J. Fox Foundation for Parkinson’s Research, NSF and DARPA. She has served as a consultant to Medtronic, LLC.
D. Bowers has on-going grants from NIH/NINDS.
M. Okun serves as a consultant for the National Parkinson Foundation, and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. Dr. Okun has previously received honoraria, but in the past >36 months has received no support from industry. Dr. Okun has received royalties for publications with Demos, Manson, Amazon, and Cambridge (movement disorders books). Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. Dr. Okun has participated in CME activities on movement disorders in the last 36 months sponsored by PeerView, Prime, and by Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic and ANS/St. Jude, and the PI has no financial interest in these grants. Dr. Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
P. J. Rossi reports no conflict of interest.
H. Ward reports no conflict of interest.
K. Foote reports no conflict of interest.
Contributor Information
P. Justin Rossi, Email: pjrossi@ufl.edu.
Enrico Opri, Email: enrico.opri@ufl.edu.
Jonathan B. Shute, Email: jbshute@ufl.edu.
Rene Molina, Email: phys.molina@gmail.com.
Dawn Bowers, Email: dawnbowers@phhp.ufl.edu.
Herbert Ward, Email: hward@ufl.edu.
Kelly D. Foote, Email: foote@neurosurgery.ufl.edu.
Aysegul Gunduz, Email: gunduz@bme.ufl.edu.
Michael S. Okun, Email: okun@neurology.ufl.edu.
References
- 1.Jankovic J. Tourette’s Syndrome. N Engl J Med. 2001;345:1184–1192. doi: 10.1056/NEJMra010032. [DOI] [PubMed] [Google Scholar]
- 2.McNaught KSP, Mink JW. Advances in understanding and treatment of Tourette syndrome. Nat Rev Neurol. 2011;7:667–676. doi: 10.1038/nrneurol.2011.167. [DOI] [PubMed] [Google Scholar]
- 3.Cheung MY, Shahed J, Jankovic J. Malignant Tourette syndrome. Mov Disord Off J Mov Disord Soc. 2007;22:1743–1750. doi: 10.1002/mds.21599. [DOI] [PubMed] [Google Scholar]
- 4.Isaacs JD, Adams M, Lees AJ. Noncompressive myelopathy associated with violent axial tics of tourette syndrome. Neurology. 2010;74:697–698. doi: 10.1212/WNL.0b013e3181d0cc77. [DOI] [PubMed] [Google Scholar]
- 5.Lehman LL, Gilbert DL, Leach JL, Wu SW, Standridge SM. Vertebral artery dissection leading to stroke caused by violent neck tics of Tourette syndrome. Neurology. 2011;77:1706–1708. doi: 10.1212/WNL.0b013e318238253c. [DOI] [PubMed] [Google Scholar]
- 6.Lin JJ, Wang HS, Wong MC, Wu CT, Lin KL. Tourette’s syndrome with cervical disc herniation. Brain Dev. 2007;29:61–63. doi: 10.1016/j.braindev.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Mink JW. Clinical review of DBS for Tourette Syndrome. Front Biosci Elite Ed. 2009;1:72–76. doi: 10.2741/E8. [DOI] [PubMed] [Google Scholar]
- 8.Ackermans L, Neuner I, Temel Y, Duits A, Kuhn J, Visser-Vandewalle V. Thalamic deep brain stimulation for Tourette syndrome. Behav Neurol. 2013;27:133–138. doi: 10.3233/BEN-120301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porta M, Brambilla A, Cavanna AE, Servello D, Sassi M, Rickards H, Robertson MM. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: two-year outcome. Neurology. 2009;73:1375–1380. doi: 10.1212/WNL.0b013e3181bd809b. [DOI] [PubMed] [Google Scholar]
- 10.Ackermans L, Duits A, van der Linden C, Tijssen M, Schruers K, Temel Y, Kleijer M, Nederveen P, Bruggeman R, Tromp S, van Kranen-Mastenbroek V, Kingma H, Cath D, Visser-Vandewalle V. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain J Neurol. 2011;134:832–844. doi: 10.1093/brain/awq380. [DOI] [PubMed] [Google Scholar]
- 11.Okun MS, Foote KD, Wu SS, Ward HE, Bowers D, Rodriguez RL, Malaty IA, Goodman WK, Gilbert DM, Walker HC, Mink JW, Merritt S, Morishita T, Sanchez JC. A trial of scheduled deep brain stimulation for tourette syndrome: Moving away from continuous deep brain stimulation paradigms. JAMA Neurol. 2013;70:85–94. doi: 10.1001/jamaneurol.2013.580. [DOI] [PubMed] [Google Scholar]
- 12.Morrell M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol. 2006;19:164–168. doi: 10.1097/01.wco.0000218233.60217.84. [DOI] [PubMed] [Google Scholar]
- 13.Montuno MA, Kohner AB, Foote KD, Okun MS. An algorithm for management of deep brain stimulation battery replacements: devising a web-based battery estimator and clinical symptom approach. Neuromodulation J Int Neuromodulation Soc. 2013;16:147–153. doi: 10.1111/j.1525-1403.2012.00457.x. [DOI] [PubMed] [Google Scholar]
- 14.Fakhar K, Hastings E, Butson CR, Foote KD, Zeilman P, Okun MS. Management of deep brain stimulator battery failure: battery estimators, charge density, and importance of clinical symptoms. PloS One. 2013;8:e58665. doi: 10.1371/journal.pone.0058665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratzke R, Moreno DH, Gorenstein C, Moreno RA. Validity and reliability of the Structured Clinical Interview for Mood Spectrum: Brazilian version (SCIMOODS-VB) Rev Bras Psiquiatr São Paulo Braz. 2011;1999(33):64–67. doi: 10.1590/s1516-44462011000100013. [DOI] [PubMed] [Google Scholar]
- 17.Storch EA, Murphy TK, Geffken GR, Sajid M, Allen P, Roberti JW, Goodman WK. Reliability and validity of the Yale Global Tic Severity Scale. Psychol Assess. 2005;17:486–491. doi: 10.1037/1040-3590.17.4.486. [DOI] [PubMed] [Google Scholar]
- 18.Storch EA, Merlo LJ, Lehmkuhl H, Grabill KM, Geffken GR, Goodman WK, Murphy TK. Further psychometric examination of the Tourette’s Disorder Scales. Child Psychiatry Hum Dev. 2007;38:89–98. doi: 10.1007/s10578-006-0043-4. [DOI] [PubMed] [Google Scholar]
- 19.Goetz CG, Pappert EJ, Louis ED, Raman R, Leurgans S. Advantages of a modified scoring method for the Rush Video-Based Tic Rating Scale. Mov Disord Off J Mov Disord Soc. 1999;14:502–506. doi: 10.1002/1531-8257(199905)14:3<502::aid-mds1020>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 21.Storch EA, Kaufman DAS, Bagner D, Merlo LJ, Shapira NA, Geffken GR, Murphy TK, Goodman WK. Florida Obsessive-Compulsive Inventory: development, reliability, and validity. J Clin Psychol. 2007;63:851–859. doi: 10.1002/jclp.20382. [DOI] [PubMed] [Google Scholar]
- 22.Marchand WR, Clark SC, Wirth L, Simon C. Validity of the parent young mania rating scale in a community mental health setting. Psychiatry Edgmont Pa Townsh. 2005;2:31–35. [PMC free article] [PubMed] [Google Scholar]
- 23.Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen DJ. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Rochon J, Gondan M, Kieser M. To test or not to test: Preliminary assessment of normality when comparing two independent samples. BMC Med Res Methodol. 2012;12:81. doi: 10.1186/1471-2288-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon S, Walkup JT, Woods DW, Peterson A, Piacentini J, Wilhelm S, Katsovich L, McGuire JF, Dziura J, Scahill L. Detecting a clinically meaningful change in tic severity in Tourette syndrome: A comparison of three methods, Contemp. Clin Trials. 2013;36:414–420. doi: 10.1016/j.cct.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo HK, Joung YS, Lee JS, Song DH, Lee YS, Kim JW, Kim BN, Cho SC. A multicenter, randomized, double-blind, placebo-controlled study of aripiprazole in children and adolescents with Tourette’s disorder. J Clin Psychiatry. 2013;74:e772–780. doi: 10.4088/JCP.12m08189. [DOI] [PubMed] [Google Scholar]
- 27.Maciunas RJ, Maddux BN, Riley DE, Whitney CM, Schoenberg MR, Ogrocki PJ, Albert JM, Gould DJ. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007;107:1004–1014. doi: 10.3171/JNS-07/11/1004. [DOI] [PubMed] [Google Scholar]
- 28.Visser-Vandewalle V, Ackermans L, van der Linden C, Temel Y, Tijssen MA, Schruers KRJ, Nederveen P, Kleijer M, Boon P, Weber W, Cath D. Deep brain stimulation in Gilles de la Tourette’s syndrome. Neurosurgery. 2006;58:E590. doi: 10.1227/01.NEU.0000207959.53198.D6. [DOI] [PubMed] [Google Scholar]
- 29.Servello D, Sassi M, Brambilla A, Defendi S, Porta M. Long-term, post-deep brain stimulation management of a series of 36 patients affected with refractory gilles de la tourette syndrome. Neuromodulation J Int Neuromodulation Soc. 2010;13:187–194. doi: 10.1111/j.1525-1403.2009.00253.x. [DOI] [PubMed] [Google Scholar]
- 30.Fountas KN, Smith JR, Murro AM, Politsky J, Park YD, Jenkins PD. Implantation of a closed-loop stimulation in the management of medically refractory focal epilepsy: a technical note. Stereotact Funct Neurosurg. 2005;83:153–158. doi: 10.1159/000088656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


