Abstract
In the mammalian brain, a family of sodium-dependent transporters maintains low extracellular glutamate and shapes excitatory signaling. The bulk of this activity is mediated by the astroglial glutamate transporters GLT-1 and GLAST (also called EAAT2 and EAAT1). In this review, we will discuss evidence that these transporters co-localize with, form physical (co-immunoprecipitable) interactions with, and functionally couple to various ‘energy-generating’ systems, including the Na+/K+-ATPase, the Na+/Ca2+ exchanger, glycogen metabolizing enzymes, glycolytic enzymes, and mitochondria/mitochondrial proteins. This functional coupling is bidirectional with many of these systems both being regulated by glutamate transport and providing the ‘fuel’ to support glutamate uptake. Given the importance of glutamate uptake to maintaining synaptic signaling and preventing excitotoxicity, it should not be surprising that some of these systems appear to ‘redundantly’ support the energetic costs of glutamate uptake. Although the glutamate-glutamine cycle contributes to recycling of neurotransmitter pools of glutamate, this is an over-simplification. The ramifications of co-compartmentalization of glutamate transporters with mitochondria for glutamate metabolism are discussed. Energy consumption in the brain accounts for ~20% of the basal metabolic rate and relies almost exclusively on glucose for the production of ATP. However, the brain does not possess substantial reserves of glucose or other fuels. To ensure adequate energetic supply, increases in neuronal activity are matched by increases in cerebral blood flow via a process known as ‘neurovascular coupling’. While the mechanisms for this coupling are not completely resolved, it is generally agreed that astrocytes, with processes that extend to synapses and endfeet that surround blood vessels, mediate at least some of the signal that causes vasodilation. Several studies have shown that either genetic deletion or pharmacologic inhibition of glutamate transport impairs neurovascular coupling. Together these studies strongly suggest that glutamate transport not only coordinates excitatory signaling, but also plays a pivotal role in regulating brain energetics.
Keywords: Glutamate transport, GLT-1, GLAST, EAAT, mitochondria, glycolysis, glycogen
1. Introduction to Glutamate Transporters
Glutamate is arguably the most important neurotransmitter in mammals; it mediates the fast excitatory signaling that is needed for essentially all motor, sensory, and autonomic processing (for reviews, see McDonald and Johnston, 1990; Robinson and Coyle, 1987). The plasticity of excitatory signaling also underlies memory formation (for review, see Ho et al., 2011). ‘Appropriate’ excitatory signaling is required for normal brain development and abnormal excitatory signaling contributes to many neurodevelopmental and neurological disorders (for reviews, see Choi, 1992; Coyle and Puttfarcken, 1993; Faden et al., 1989; Greene and Greenamyre, 1996; McDonald and Johnston, 1990). The levels of glutamate are about 1000- to 10,000-fold higher than the biogenic amines and approach 10 mmol/Kg. After release, glutamate is cleared from the extracellular space by a family of Na+-dependent transporters that are primarily enriched in astrocytes; there is no evidence of extracellular metabolism (for review, see Schousboe, 1981).
In mammals, there are five distinct gene products that encode Na+-dependent glutamate transporters. It can be a bit confusing because there are several names associated with these transporters. The proteins have been called GLAST, GLT-1, EAAC1, EAAT4, and EAAT5 (note: the first three are also called EAAT1, EAAT2, and EAAT3, respectively). The gene names are also different; the human genes are called SLC1A3, 2, 1, 6 & 7, respectively. There are several review articles that discuss various aspects of glutamate transporter localization, function, structure, regulation, role in control of excitatory signaling, and associations with diseases processes (for reviews, see Anderson and Swanson, 2000; Beart and O’Shea, 2007; Danbolt, 2001; Dunlop, 2006; Gegelashvili and Schousboe, 1997; Kanner, 2006; Kim et al., 2011; Robinson and Dowd, 1997; Robinson, 1999; Ryan and Vandenberg, 2005; Sattler and Rothstein, 2006; Seal and Amara, 1999; Sheldon and Robinson, 2007; Shigeri et al., 2004; Sims and Robinson, 1999; Tanaka, 2000; Trotti et al., 1998; Vandenberg and Ryan, 2013; Yi and Hazell, 2006).
These transporters maintain low concentrations of glutamate near receptors to avoid chronic receptor activation/desensitization, to ensure an appropriate signal-to-noise ratio, and to limit excitotoxic activation of glutamate receptors. At some synapses, the levels of these transporters are sufficient to buffer the amount of free extracellular glutamate that is available for receptor activation and thereby limit excitatory signaling. Although the transport cycle itself is slow relative to the fastest forms of excitatory signaling (i.e. depolarization that starts and finishes within 1–2 msec of presynaptic activation) slower forms of excitatory signaling are shaped by the transport activity. The transporters also control spillover of glutamate between synapses, sometimes referred to as volume transmission (for reviews, see Conti and Weinberg, 1999; Huang and Bergles, 2004; Marcaggi and Attwell, 2004; Otis et al., 2004; Tzingounis and Wadiche, 2007).
These transporters stoichiometrically co-transport one molecule of glutamate, three molecules of Na+ and 1 proton; 1 molecule of K+ is also counter-transported with each cycle. From the Nernst equation, one can calculate that these transporters are capable of generating a 1-million fold concentration gradient of glutamate across the membrane at a reasonable resting membrane potential (Levy et al., 1998; Owe et al., 2006; Zerangue and Kavanaugh, 1996). As indicated above, total brain concentrations of glutamate are estimated at 10 mmol/Kg; this would translate into 10 mM concentrations if it were homogeneously distributed. It has been suggested that cytosolic levels of glutamate are likely higher in neurons than astrocytes based on the fact that glutamine synthetase is selectively expressed in astrocytes, providing a pathway to inactivate glutamate (Rossi et al., 2000). In fact immunocytochemical analyses suggest that the highest levels of glutamate are found in presynaptic vesicles (Storm-Mathisen et al., 1983; Taxt and Storm-Mathisen, 1984). Although microdialysis studies suggest that extracellular glutamate may be in the micromolar range, basal synaptic concentrations have been estimated to be as low as 25 nM (Herman and Jahr, 2007 for recent discussion, see Sun et al., 2014).
With a relatively large gene family, one might wonder if these transporters are functionally redundant to prevent a toxic accumulation of glutamate; for the most part this seems unlikely. EAAT4 expression is enriched in Purkinje cells of the cerebellum, but is also found at lower levels in other cells in the brain (Dehnes et al., 1998; Furuta et al., 1997; Massie et al., 2001; Massie et al., 2008; Nagao et al., 1997; Tanaka et al., 1997a). EAAT5 is found on the presynaptic termini of retinal bipolar cells (Pow and Barnett, 2000; Schneider et al., 2014; Wersinger et al., 2006). Both function as very slow transporters with turnover times of 300 msec or longer (this is the length of time it takes to move a single molecule of glutamate across the membrane), but gate fairly large Cl− -currents (Gameiro et al., 2011; Leary et al., 2007; Mim et al., 2005; Schneider et al., 2014). Given the slow transporter rates and the relatively large Cl− currents, EAAT4 and EAAT5 likely function as inhibitory receptors rather than as mechanisms to clear glutamate. The other three transporters all transport glutamate with cycle times of 10–50 msec (Mim et al., 2005). GLT-1 and GLAST are not associated with a significant Cl− current, whereas EAAC1 has a moderate Cl− conductance (Koch et al., 2007; Torres-Salazar and Fahlke, 2007; Wadiche et al., 1995). EAAC1 (EAAT3) is found on both excitatory and inhibitory neurons throughout the brain, but is also found in other cells (e.g. oligodendroglia) (Conti et al., 1998; Kugler and Schmitt, 1999; Rothstein et al., 1994). This transporter is also somewhat unique in that it is relatively rapidly recycled between the plasma membrane and the cell surface (Fournier et al., 2004; Gonzalez et al., 2007). In vivo, it seems that most EAAC1 protein is found on intracellular vesicles, and the levels of EAAC1 are ~1% of that observed for GLT-1 (Holmseth et al., 2012). Finally, deletion of EAAC1 results in depletion of glutathione and neuronal cell loss (Aoyama et al., 2006), suggesting that EAAC1 is mostly responsible for providing cysteine for the synthesis of glutathione.
This leaves two transporters to clear the bulk of glutamate in brain, GLT-1 (or EAAT2) and GLAST (or EAAT1). GLAST is expressed by astrocytes and oligodendrocytes in the nervous system (Domercq et al., 2005; Rothstein et al., 1994). GLT-1 expression is much higher in astrocytes than in other cells in the brain (Rothstein et al., 1994; Sutherland et al., 1996). When mice are engineered to express enhanced green fluorescent protein (eGFP) using the human GLT-1 (SLC1A2) gene from a bacterial artificial chromosome, eGFP expression is essentially restricted to astrocytes (Regan et al., 2007). Several lines of evidence suggest that GLT-1 mediates the bulk of glutamate uptake in forebrain. Genetic deletion of GLT-1 uniquely results in seizures early in development and premature death of the animals (Tanaka et al., 1997b). By comparison, mice deleted of GLAST have relatively minor abnormalities in gait (Watase et al., 1998). Based on the fold-enrichment required to purify it to homogeneity, GLT-1 is thought to represent up to 1% of brain protein (Danbolt et al., 1990). Reconstitution studies of glutamate transporters immune-isolated from brain also suggest that GLT-1 mediates the bulk of uptake (Danbolt et al., 1992; Huageto et al., 1996, for reviews see Danbolt, 2001; Robinson, 1999). Dihydrokainate has been used as a tool to examine the relative contributions of the transporters to uptake in various preparations because it relatively selectively inhibits GLT-1 with an IC50 of ~50 μM, but this compound should be used with some caution because it also inhibits EAAC1-mediated uptake at roughly 20 times higher concentrations (Arriza et al., 1994; Ferkany and Coyle, 1986; Robinson et al., 1991 for review, see Robinson and Dowd, 1997). Recordings of transporter evoked currents from astrocytes in brain slices reveal that around 30–50% of the current is blocked by concentrations of dihydrokainate that should selectively reduce GLT-1-mediated activity by 80 to 90% (Bergles and Jahr, 1997; Kojima et al., 1999; Otis and Kavanaugh, 2000). This suggests that GLAST makes a fairly substantial contribution to glutamate clearance even in the forebrain. Together, these studies strongly suggest that the bulk of glutamate clearance in brain is mediated by the astroglial pools of GLT-1 and GLAST.
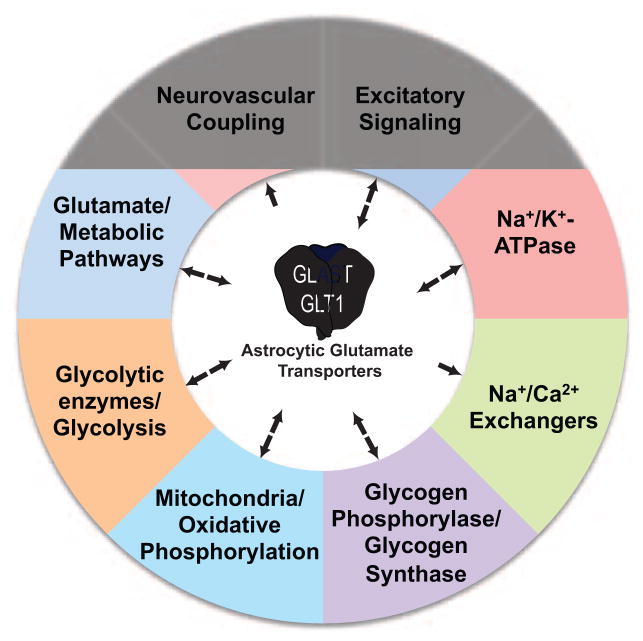
These and other studies clearly demonstrate that glutamate transporters shape excitatory/glutamatergic signaling in the brain. In this review, we will discuss evidence that GLAST and GLT-1 also coordinate the activities of several different sources of energy, including the Na+/K+-ATPases, the Na+/Ca2+ exchangers, glycogen metabolizing enzymes, glycolytic enzymes, mitochondria, and glutamate metabolism (Figure 1). We will describe many different studies that have demonstrated functional coupling between these transporters and these processes/organelles. This functional coupling is supported by co-compartmentalization with the transporters in fine astroglial processes, and there is evidence that this co-compartmentalization is supported by protein-protein interactions. This coordination of brain energetics may extend to the delivery of fuel to the brain by coupling increases in brain activity to increased blood flow.
Figure 1.
Summary schematic of functional coupling between the astroglial glutamate transporters, GLT-1 and GLAST, and various processes. Bidirectional arrows imply that glutamate uptake regulates the process and the process also regulates uptake. Arrows with a single direction imply the glutamate uptake activates/controls these processes.
2. Model systems to Study GLT-1- or GLAST-mediated uptake
Although astroglial GLT-1 arguably represents the predominant route for glutamate clearance, it is not clear that there is a system or technique that can be readily used to assay this pool of transporter for biochemical analyses. Compared to brain tissue, astrocytes in culture express very little or no GLT-1 and there is essentially no dihydrokainate-sensitive (GLT-1-mediated) uptake. In contrast, cultured astrocytes express high levels of GLAST protein and the pharmacology of uptake is consistent with that expected for GLAST-mediated activity (Garlin et al., 1995; Schlag et al., 1998; Swanson et al., 1997). Even when they are induced to express GLT-1 with dbcAMP or epidermal growth factor (EGF), the amount of GLT-1-mediated uptake is less than 10% of the total uptake (Ghosh et al., 2011; Schlag et al., 1998; Swanson et al., 1997). While this makes cultured astrocytes a potentially useful system to study GLAST-mediated uptake and possible functional coupling to sources of energy, this system is not particularly useful for analyses of GLT-1.
The pharmacology of synaptosomal uptake of glutamate is identical to that observed for GLT-1 (Arriza et al., 1994; Ferkany and Coyle, 1986; Robinson et al., 1991) and genetic deletion of GLT-1 essentially abolishes synaptosomal glutamate uptake (Tanaka et al., 1997b). Since synaptosomes (or crude synaptosomes) are only enriched in resealed synaptic terminals and they clearly can contain glial membranes (Danbolt et al., 1992; Henn et al., 1976), we and others suggested that synaptosomal uptake was mediated by glial pools of GLT-1 (for review, see Robinson and Dowd, 1997). GLT-1 is such an abundant protein in astrocytes that it is difficult to detect small pools of GLT-1 in other cells. Over the last decade, several studies have identified GLT-1 immunoreactivity in presynaptic termini (Chen et al., 2002; Chen et al., 2004; Furness et al., 2008; Melone et al., 2009). Recently, Paul Rosenberg and his colleagues used selective genetic deletion of GLT-1 from neurons or astrocytes to explore this issue. They show that deletion of astrocytic GLT-1 decreases GLT-1 protein levels to ~20% of control, but only has a small (not statistically significant) effect on uptake activity into synaptosomal membrane preparations. Deletion of neuronal GLT-1 has no significant effect on GLT-1 protein levels and reduces synaptosomal uptake activity to about 40% of control (Petr et al., 2015). Thus, although there is good evidence that GLT-1 mediates glutamate uptake into synaptosomes, it seems likely that this represents the smaller neuronal pools of GLT-1, not the astrocytic pools of GLT-1.
3. Glutamate transporter interactions (co-immunoprecipitating proteins)
A variety of hypothesis-driven or yeast two-hybrid approaches were used to identify proteins that co-immunoprecipitate with one or more of the Na+-dependent glutamate transporters, including Ajuba (Marie et al., 2002), glutamate transporter associated proteins (GTRAPs) (Jackson et al., 2001; Lin et al., 2001), protein kinase Cα (González et al., 2003; González et al., 2005), a septin GTPase (Sept 2) (Kinoshita et al., 2004), syntaxin 1A (Yu et al., 2006), PSD-95 (Gonzalez-Gonzalez et al., 2008; Gonzalez-Gonzalez et al., 2009), the actin binding protein α-adducin (Bianchi et al., 2010), Na+/H+ exchanger regulatory proteins (Lee et al., 2007; Sato et al., 2013), protein interacting with C kinase (PICK1) (Bassan et al., 2008), membrane-associated guanylate kinase with inverted orientation protein-1 (MAGI-1) (Zou et al., 2011), a glutamine transporter (SNAT3) (Martinez-Lozada et al., 2013), and the sodium/calcium exchanger (NCX1) (Magi et al., 2012; Magi et al., 2013). Many of these proteins directly or indirectly regulate various aspects of transporter function, including maturation, targeting, trafficking, or functional coupling of these transporters. While this approach has led to the identification of many interesting proteins, it is limited by having to first discover (or guess) proteins that may be functionally linked to glutamate transporters or by having to choose a domain as bait that may not be the domain of the transporter required for an interaction with another protein.
Using a combination of hypothesis-driven and discovery-based approaches, David Hampson’s group demonstrated co-localization and co-immunoprecipitable interactions between the subunits of the Na+/K+ ATPase and GLAST or GLT-1. They also subjected semi-purified GLAST/ATPase subunit-containing fractions isolated from cerebellum to mass spectrometry and identified a number putative interacting proteins (Rose et al., 2009). Around the same time, we used anti-GLT-1 antibodies to immunoisolate GLT-1 from rat cortex and performed mass spectrometry on fractions isolated from SDS-PAGE gels (Genda et al., 2011). Three slightly different independent experiments were performed; 23 ‘putative’ interacting proteins were identified in all three analyses. Seventy-three putative interacting proteins were identified in at least two of the analyses. Of the 23 proteins, six were glycolytic enzymes and 8 were mitochondrial proteins. In fact, mitochondrial proteins were significantly enriched relative to other protein categories. We relatively randomly chose putative interacting proteins for further validation based on cellular localization, subcellular localization, functional implications, and availability of antibodies (Genda et al., 2011). In the immunoprecipitations in which the antibodies pulled down the putative interacting protein, GLT-1 was observed 100% of the time. This included two different mitochondrial proteins. In the same study, we used biolistically transduced astrocytes in organotypic slices to show that mitochondria overlap clusters of GLT-1 ~70% of the time and more often than occurs by chance in Monte Carlo simulations. In a subsequent study, we showed that subunits of the Na+/K+ ATPase, two different glycolytic enzymes, and two different mitochondrial proteins co-immunoprecipitate with GLAST (Bauer et al., 2012). We also showed that GLAST and mitochondria substantially co-localize in astrocytic processes. About 30% of the proteins identified by Hampson and his colleagues were identified in our analyses of the GLT-1 proteome. The simplest explanation of these data is that GLT-1 (or GLAST) is part of a multiprotein complex that also includes mitochondria. It should be noted that there is no evidence for co-immunoprecipitable interactions between GLAST and GLT-1, so these two transporters would be part of different complexes (Huageto et al., 1996).
It is not unprecedented for plasma membrane transporters to interact with glycolytic enzymes. For example, there is evidence that glycolytic enzymes interact with the membrane anion transport protein, band 3, in erythrocytes and functionally couple to the Na+/K+-ATPase and Ca2+ pumps (for recent discussions, see Campanella et al., 2005; Chu et al., 2012). Furthermore, hexokinase, the first enzyme and the rate limiting step in glycolysis, is known to interact with the glucose transporter (GLUT4) (Zaid et al., 2009) as well as the voltage-dependent anion channel (VDAC) in the outer mitochondrial membrane (Linden et al., 1982; Nakashima et al., 1986; Sui and Wilson, 1997). VDAC interacts with the adenine nucleotide translocase (ANT) that is located in the inner mitochondrial membrane (Beutner et al., 1998; Crompton et al., 1998a; Crompton et al., 1998b). VDAC and ANT are thought to work together to increase the rate at which mitochondrially generated ATP is provided to hexokinase to generate glucose-6-phosphate. Originally, we hypothesized that hexokinase might form a scaffold between GLT-1 and mitochondrial proteins because both VDAC and ANT were identified in the proteomic analysis (Genda et al., 2011). The domain of hexokinase required for interaction with VDAC has been mapped and membrane permeant peptides that disrupt this interaction have been identified (for discussion, see Jackson et al., 2015). We used these peptides to demonstrate that disruption of the hexokinase-VDAC interaction in crude synaptosomes does not reduce the co-immunoprecipitable interactions between GLT-1 and two different mitochondrial proteins, ANT or ubiquinol-cytochrome-c reductase complex (UQCRC2) (Jackson et al., 2015). In these same studies, disruption of this interaction reduced GLT-1-dependent uptake suggesting that this interaction is either required to fuel uptake or supports co-compartmentalization.
At present, we are left with two possible explanations for these studies that identify several different proteins, including mitochondrial proteins, that co-immunoprecipitate with GLT-1 and GLAST. First, it is possible that GLT-1 or GLAST exist as part of a large complex that consists of several different proteins, one or more of which ultimately tether to mitochondria. The reader is reminded that many scientists accept that the post-synaptic density is composed of up to ~300 proteins based on immunoprecipitations (Cheng et al., 2006 for reviews, see Feng and Zhang, 2009; Sheng and Hoogenraad, 2007). The second possibility is that these proteins aggregate after solubilization. Many of the proteins identified contain hydrophobic domains that could interact. It seems unlikely that one can prove that these interactions are simply caused by aggregation after solubilization. If these are indeed representative of direct protein interactions, one expects that somebody will either be lucky enough to guess which proteins might be directly interacting or devise a screening tool to disrupt subsets of the interactions. Another strategy will be to develop Forster resonance energy transfer (FRET)-based tools to demonstrate that subsets of proteins are within 1–10 nm, but this strategy also requires guessing which proteins might directly interact (Jares-Erijman and Jovin, 2003). For example, it is possible that these proteins could interact directly with GLT-1 (or GLAST) or these proteins could interact with the Na+/K+ ATPase that in turn could interact with GLT-1 (or GLAST). Although it is not possible to prove that these co-immunoprecipitable proteins are part of a true protein complex, it is clear that GLT-1 and/or GLAST co-compartmentalize with many of these proteins and mitochondria, that GLT-1 and/or GLAST functionally couple to many of these proteins and mitochondria, and that some of these events are regulated.
4. Coupling to Na+/K+-ATPase
Of all of the proteins known/thought to interact with the glial glutamate transporters GLT-1 and GLAST, the interactions with the Na+/K+-ATPase are the best characterized and conceptually the most straight-forward. Glutamate entry through GLT-1/GLAST is steeply dependent upon the Na+ gradient, with each glutamate molecule accompanied by the influx of 3 Na+ ions (and a proton) and the efflux of a K+ ion (Zerangue and Kavanaugh, 1996). Glutamate uptake is associated with a sharp increase in the intracellular Na+ concentration within the processes of the astrocyte (Kirischuk et al., 1997; Langer and Rose, 2009). An interdependence of the glutamate transporters and the Na+/K+-ATPase pumps is thus not surprising.
The Na+/K+-ATPase is a heteromeric transmembrane enzyme consisting of a non-covalently linked α and β subunit (for review, see Kaplan, 2002). The α-subunits are responsible for ATP hydrolysis, while the β subunits appear to be important for correct trafficking of the α subunit. There are three α and two β subunits encoded by different genes expressed in the brain. Of these α1 and β1 appear to be ubiquitous, while α2 and β2 are selectively enriched in astrocytes (McGrail et al., 1991; Watts et al., 1991). Interestingly β2 was originally identified as a glial adhesion molecule (AMOG) that contributes to granule neuron migration in the cerebellum (Antonicek and Schachner, 1988; Kleene et al., 2007). Additional subunits (e.g. NKAIN, gamma/FKYD subunits) are found in a developmental and tissue dependent manner (Jorgensen et al., 2003).
Given the strong dependence on Na+, it is not surprising that inhibitors of the Na+/K+-ATPase inhibit glutamate uptake and that uptake stimulates the ATPase. In fact, the cardiac glycoside, ouabain, was identified as one of the first non-competitive inhibitors of glutamate uptake into synaptosomes (Logan and Snyder, 1972). This effect of inhibitors of the Na+/K+-ATPase has been replicated in several systems (Abe and Saito, 2000; Pellerin and Magistretti, 1994; Rose et al., 2009). Acute energy depletion causes a reversal of the glutamate transporters, and increases extracellular glutamate, a result thought to be secondary to collapse of ionic gradients (Na+, K+) (Jabaudon et al., 2000). However, it should be noted that glutamate uptake displays incomplete dependence upon the Na+/K+-ATPase, as inhibition of 100% of the Na+/K+-ATPase activity with ouabain only occludes ~50–60% of glutamate uptake (Illarionova et al., 2014; Rose et al., 2009). Glutamate (and D-Aspartate) also activate Na+/K+-ATPase activity, but remarkably it preferentially stimulates the Na+/K+-ATPase activity that is most sensitive to ouabain inhibition (generally thought to be mediated by the α2-containing ATPase) (Pellerin and Magistretti, 1997).
Based on the functional evidence, it was hypothesized that co-compartmentalization and physical interactions of transporter proteins with the Na+/K+-ATPase isoforms might facilitate this bi-directional regulation. The glutamate transporters were found to co-localize with the Na+/K+-ATPase within the fine processes of astrocytes (Cholet et al., 2002; Roberts et al., 2014). Several groups have now demonstrated co-immunoprecipitable interactions between Na+/K+-ATPase isoforms and GLT1 and/or GLAST (Bauer et al., 2012; Genda et al., 2011; Illarionova et al., 2014; Matos et al., 2012; Matos et al., 2013; Rose et al., 2009; Shan et al., 2014).
Why do the glutamate transporters interact with different Na+/K+-ATPase isoforms? From the first observation that astrocytes (and neurons) express multiple alpha and beta isoforms, it was suggested that they might play different roles, be differentially regulated, or be differentially trafficked. The α2 isoform has a lower affinity for Na+ than does α1 (22mM vs and 12mM, respectively; Zahler et al., 1997). Thus α1 will be half-maximally active at resting [Na+] (10mM), while α2 will reach Vmax at higher [Na+] concentrations (Illarionova et al., 2014; Zahler et al., 1997). Na+ transients during uptake might exceed the Vmax for α1 while α2 will continue to efficiently clear intracellular Na+. Several studies have shown that glutamate uptake is more sensitive to inhibition of the α2 subunit than the α1 subunit (Illarionova et al., 2014; Rose et al., 2009) and conversely that glutamate transport selectively activates the α2-containing ATPase (Pellerin and Magistretti, 1997). Using a GST-pulldown assay, Eli Gunnarson’s group demonstrated relatively more GST-α2 was co-immunoprecipitated with anti-GLT1 or anti-GLAST antibodies than GST-α1, implying a stronger interaction between GLT1/GLAST and α2-containing ATPase (Illarionova et al., 2014). Conversely, inhibition of α2 does not alter basal [Na+] while inhibition of α1 and α2 results in an increase in Na+ to ~35 mM. These results suggest that one reason for multiple interactions is to support glutamate uptake across a range of glutamate and Na+ concentrations.
Another possible explanation for the diversity of interactions between transporters and ATPase isoforms concerns the role that the Na+/K+-ATPases play as organizers of cellular signaling. The Na+/K+-ATPase isoforms physically interact with ankyrin, caveolin1, phosphoinositide 3-kinase, phospholipase C gamma, cofilin, and Src (Devarajan et al., 1994; Tian et al., 2006; Xie and Cai, 2003; Yuan et al., 2005), amongst others, suggesting that the Na+/K+-ATPase might play a role in scaffolding various signaling molecules into signaling microdomains. In particular, the α2 subunit has been implicated in organizing signaling between the plasma membrane and Ca2+ stores in endoplasmic reticulum (Golovina et al., 2003). Similarly, the Na+/K+-ATPase may play a role in coordinating interactions of GLT-1/GLAST with other protein complexes and signaling modules.
The appropriate positioning and trafficking of glutamate transporters within astrocytes is controlled by synaptic activity (Benediktsson et al., 2012; Murphy-Royal et al., 2015) and in turn controls synaptic activation (Murphy-Royal et al., 2015; Oliet et al., 2001). There is evidence that interactions between the glial glutamate transporters and the Na+/K+ ATPase subunits reciprocally regulate each other’s trafficking and membrane localization, potentially controlling local glutamate uptake. Interleukin-1 stimulates glutamate uptake secondary to increasing surface availability of the Na+/K+-ATPase (Namekata et al., 2008). Glutamate stimulation of Na+/K+-ATPase activity depends on increased membrane insertion of the FKYD protein/gamma subunit of the Na+/K+-ATPase (Gegelashvili et al., 2007). Conversely, inhibition of the Na+/K+-ATPase with ouabain results in transporter clustering and redistribution (Nakagawa et al., 2008; Nguyen et al., 2010). Given that the Na+ pump isoforms are differently distributed in the membranes of astrocytes (Juhaszova and Blaustein, 1997) and form specialized plasma membrane microdomains in other cells (Golovina et al., 2003; Song et al., 2006), it seems reasonable to suggest that the differential association of transporters with the ATPase isoforms might also control the localization of the transporters. The coordinate regulation of transporter/ATPase complexes at the plasma membrane would provide a mechanism to tune the localization and activity of these proteins.
How are the interactions between transporter and ATPase isoforms regulated? Several pieces of evidence provide clues as to the regulation of these interactions. Glutamate uptake is negatively regulated by interactions between the Na+/K+-ATPase and acetylated tubulin (Casale et al., 2003; Casale et al., 2005). There is evidence that rottlerin (originally identified as an inhibitor of the delta subtype of protein kinase C) inhibits GLAST (and possibly GLT-1 mediated uptake) by inhibiting Na+/K+-ATPase activity (Sheean et al., 2013; Susarla and Robinson, 2003). Adenosine A2A receptors physically interact with both GLT-1 and the Na+/K+-ATPase α2 isoform in astrocytes and acute activation of A2A receptors decreases glutamate uptake and Na+/K+-ATPase activation. Conversely, deletion of astrocytic A2A receptors increases glutamate uptake (Matos et al., 2012; Matos et al., 2013). Perhaps other modulators of synaptic transmission target this interaction to fine tune glutamate availability in the synaptic cleft.
5. Coupling to the Na+/Ca2+ Exchanger
The Na+/Ca2+ exchanger (NCX) is a bidirectional exchanger that, in its so-called ‘forward mode’, couples the inward movement of three Na+ ions to the outward movement of one Ca2+ ion (Blaustein and Lederer, 1999). There are three isoforms (NCX1–3) that are the product of distinct genes. All three isoforms are expressed in astrocytes (Minelli et al., 2007; Pappalardo et al., 2014; Zhang et al., 2014).
There are multiple lines of evidence suggesting physical and functional interactions between the Na+-dependent transporters (Glutamate and GABA), Na+/K+-ATPase isoforms, and Na+/Ca2+ exchanger isoforms. All three NCX isoforms are enriched at perisynaptic distal astrocyte processes (Minelli et al., 2007) along with glutamate transporters and Na+/K+-ATPase isoforms (Cholet et al., 2002). In cardiac myocytes and in brain, NCX1 co-localizes with and co-immunoprecipitates with the α2 (astrocytic) and α3 (neuronal) isoforms of the Na+/K+-ATPase (Dostanic et al., 2004; Juhaszova and Blaustein, 1997; Lencesova et al., 2004; Moore et al., 1993). NCX is a bidirectional exchanger whose orientation depends on the membrane potential and ionic gradients of Na+ and Ca2+. The reversal potential of the NCX in astrocytes is close to the resting membrane potential (Kirischuk et al., 1997; Reyes et al., 2012), such that NCX can rapidly operate in the so-called reverse mode (Na+ out/Ca2+ in) in response to small Na+ loads such as occur with glutamate transport (Kirischuk et al., 1997; Langer and Rose, 2009; Reyes et al., 2012; Rojas et al., 2013). In astrocyte cultures or in organotypic hippocampal slice cultures, NCX appears to operate predominately in the reversed mode even under resting conditions (Jackson and Robinson, 2015; Magi et al., 2013; Reyes et al., 2012; Rojas et al., 2013) and thus appears to control basal [Ca2+] in astrocytes. This control of [Ca2+]i may be direct (via NCX) or indirect via Ca2+-induced Ca2+-release from intracellular Ca2+stores (Lencesova et al., 2004).
In addition to helping to clear intracellular [Na+], NCX also fills a signaling role by coupling increases in intracellular [Na+] to increases in [Ca2+]. In organotypic hippocampal cultures, reversed-mode NCX-mediated Ca2+ controls the arrest of mitochondria in astrocyte processes and may thus underlie the accumulation of mitochondria near glutamate transporters in this system (Jackson et al., 2014). Using brain- and heart-derived clonal cell lines, Magi et al. developed evidence that increased glutamate uptake and a consequent increase in NCX reversal increases ATP production in the presence of glucose (Magi et al., 2013). Others have found that Ca2+ influx into mitochondria increases ATP production via activation of Ca2+-dependent dehydrogenases in mitochondria (Denton et al., 1972; Denton, 2009). Using the glutamate transport-dependent increase in Na+ to stimulate an increase in intracellular Ca2+ via NCX reversal may represent a mechanism to match energy production to metabolic demand by positioning mitochondria at domains of elevated activity and enable mitochondria to tune ATP production to the local energy requirements of the astrocyte (and neuron).
6. Coupling of glutamate transport to glycogen phosphorylase
Glycogen phosphorylase was also identified in co-immunoprecipitates with GLT-1 (Genda et al., 2011). In the nervous system, glycogen and glycogen phosphorylase are almost exclusively found in astrocytes (Pfeiffer et al., 1992; Pfeiffer-Guglielmi et al., 2003; Richter et al., 1996). There are two forms of glycogen phosphorylase, a brain form and a muscle form (for recent discussions, see Brown and Ransom, 2015; Dienel and Cruz, 2015; DiNuzzo et al., 2013; Hertz et al., 2015). These two forms are differentially activated by Ca2+ and adenosine monophosphate (AMP) (Muller et al., 2015). We identified the brain form of glycogen phosphorylase in GLT-1 immunoprecipitates (Genda et al., 2011); this isoform is more sensitive to allosteric regulation by AMP. It may only be a coincidence, but adenylate kinase, an enzyme that generates AMP and ATP from two molecules of ADP was also identified in GLT-1 immunoprecipitates. Functional coupling of glutamate transport to either of these enzymes have not been described to date.
Glutamate or glutamate uptake have been linked to glycogen in three ways. First, glutamate or L-aspartate increases glycogen pools in astrocytes (Swanson et al., 1990). This effect is not blocked by the glutamate receptor antagonist, kynurenic acid, but is blocked by threo-hydroxy-aspartate or by removal of Na+. These studies are consistent with a glutamate uptake-dependent phenomenon. In a subsequent study, Hamai and colleagues demonstrated that glutamate or D-aspartate increase the rate of incorporation of labeled glucose into glycogen, and they attributed this effect to glutamate-uptake-dependent increases in glucose uptake (Hamai et al., 1999). Second, an inhibitor of glycogen phosphorylase reduces D-aspartate uptake in astrocyte cultures, suggesting that glycogen provides fuel for glutamate uptake (Sickmann et al., 2009). Finally, glycogenolysis contributes to glutamate synthesis perhaps as a mechanism to stimulate pyruvate carboxylase (for recent discussions, see Hertz et al., 2013; Schousboe et al., 2010). Together these studies support the notion that there is bi-directional functional coupling between glutamate uptake and the enzymes that synthesize or degrade glycogen. It should be noted that several studies have linked glycogenolysis to various forms of learning and memory (Boury-Jamot et al., 2015; Gibbs et al., 2006; Hertz et al., 2013).
7. Coupling of glutamate transport to glycolysis
We identified five glycolytic enzymes in the proteomic analysis of GLT-1 immunoprecipitates; this would seemingly support functional coupling between GLT-1 and glycolysis (Genda et al., 2011). In fact almost 20 years earlier, Pellerin and Magistretti demonstrated that glutamate stimulates glucose uptake and increases extracellular lactate in astrocyte cultures. They showed that the effects of glutamate were mimicked by a non-metabolizable transporter substrate (D-aspartate) and blocked by an inhibitor of glutamate uptake (threo-hydroxyaspartate) (Pellerin and Magistretti, 1994). They also showed that the effects of glutamate are blocked by an inhibitor of the Na+/K+-ATPase, ouabain. Several other non-metabolizable glutamate transporter substrates also increase astrocytic glucose uptake (Debernardi et al., 1999). These observations strongly suggest that glutamate transport and/or the Na+/K+-ATPase can be functionally coupled to increased glycolysis. Although there are examples of studies in which glutamate does not activate glycolysis in various preparations including astrocyte cultures (Gramsbergen et al., 2003; Hertz et al., 1998; Peng et al., 2001; Swanson et al., 1990 for review, see Dienel, 2012), the effects of glutamate and D-aspartate on glycolysis have been replicated by other groups using a variety of technologies (Bittner et al., 2011; Takahashi et al., 1995). It seems highly unlikely that either set of observations is ‘wrong’ given the fact these studies have been performed by several different investigators and over long periods of time. If one assumes that both sets of observations reflect in vivo biology, then this implies that the coupling of glutamate transport to increased glycolysis is regulated. Identifying the basis for this regulation and determining if regulation occurs by differential positioning of transporters to glycolytic enzymes or mitochondria might be an interesting direction for the field.
Glutamate also increases astrocytic glucose uptake in mixed cultures of neurons and astrocytes; these effects were remarkably fast, occurring within 10 seconds (Loaiza et al., 2003). Although the glucose transporter that is likely involved, GLUT1, was not identified in the proteomic analysis of GLT-1, this effect of glutamate is mimicked by D-aspartate, blocked by an inhibitor of glutamate uptake (L-threo-beta-benzyloxyasparate), or by ouabain (Porras et al., 2008). Activation of the Na+/K+-ATPase was not sufficient to stimulate glucose uptake, but a combination of both Ca2+ and Na+ were required to stimulate glucose transport. By a process of elimination, it was suggested that the Na+/Ca2+ exchanger might mediate the increase in Ca2+. Together these two sets of studies demonstrate functional coupling of glutamate transport, the Na+/K+ ATPase, and possibly the Na+/Ca2+ exchanger to both increased glucose uptake and glycolysis.
8. Coupling of glutamate transport to mitochondria
Of the 73 proteins that co-immunoprecipitate with GLT-1, 25 were categorized as mitochondrial (Genda et al., 2011). Several of the proteins are found on the outer mitochondrial membrane, including voltage dependent anion channel isoforms 2 and 3 (VDAC2, VDAC3), mitochondrial glutamate carrier 1 (GC1, Slc25a22), the glutamate-aspartate exchanger (Aralar, Slc25a12), and the α-ketoglutarate/malate exchanger (Slc25a11). Several other proteins found on the inner mitochondrial membrane or in the matrix were also identified, including ANT1 (Slc25a4), ATP synthase, isocitrate dehydrogenase, and UQCRC2.
Astrocytes, particularly the so-called protoplasmic astrocytes, are morphologically complex, with many highly branched processes. The smallest of these processes, sometimes called the peripheral astrocytic processes or fine processes may be only 20–200 nm in diameter and possess very little cytoplasm (for review, see Benjamin Kacerovsky and Murai, 2015). While GLT-1 (GLAST, glutamine synthetase, Na+/K+-ATPase, etc) have been localized to the fine processes of astrocytes (Chaudhry et al., 1995; Cholet et al., 2002; Norenberg and Martinez-Hernandez, 1979), there was a general belief that mitochondria were too large to fit into these fine processes (in many other cells mitochondria are 1 μm in diameter or larger). In fact, several studies have demonstrated the presence of mitochondria in fine astrocytic processes (Aoki et al., 1987; Derouiche et al., 2015; Fernandez et al., 1983; Genda et al., 2011; Jackson et al., 2014; Lovatt et al., 2007; Mathiisen et al., 2010; Motori et al., 2013; Mugnaini, 1964; Oberheim et al., 2009; Stephen et al., 2015; Xu et al., 2003 for review, see Stephen et al., 2014). In organotypic hippocampal slice cultures, mitochondria occupy 45% of the average astrocytic process and overlap with GLT-1 puncta occurs more often than would occur by chance (~70% of the time) (Genda et al., 2011). Similarly, ~50% of GLAST puncta are overlapped by mitochondria (Bauer et al., 2012). In this same system, we have monitored the movement of tips of astrocytic processes and mitochondria over a 24 hour period of time using confocal microscopy and noted that mitochondria are frequently found at the tips of mobile processes (O’Donnell and Robinson, unpublished observations). Using electroporation to sparsely label astrocytic mitochondria in vivo, mitochondria are observed throughout the astrocyte including distal processes and vascular endfeet (Benjamin Kacerovsky and Murai, 2015). Together these studies provide compelling evidence that mitochondria are found throughout the astrocyte processes. It remains to be determined if subtypes of astrocytes differentially control mitochondrial distribution, and it is not clear if mitochondria localize to the smallest peripheral astrocyte processes.
Together these studies show that mitochondrial proteins both co-immunoprecipitate with GLT-1 or GLAST and that mitochondria co-compartmentalize with these transporters. Several different approaches have been used to demonstrate that mitochondrial function is coupled to glutamate uptake and that mitochondria generate energy to support glutamate uptake. Mitochondrial uncoupling agents, such as dinitrophenol, or inhibitors of oxidative metabolism (e.g. fluorocitrate or fluoroacetate) partially block Na+-dependent glutamate uptake (Balcar and Johnston, 1972; Logan and Snyder, 1972; Roberts and Watkins, 1975; Schousboe et al., 2011; Swanson and Benington, 1996). Displacement of hexokinase from VDAC with a peptide reduces GLT-1-mediated glutamate uptake to ~70% of control in crude synaptosomes (Jackson et al., 2015). Three different inhibitors of the mitochondrial enzyme, glutamate dehydrogenase, non-competitively reduce uptake of L-glutamate or D-aspartate (Whitelaw and Robinson, 2013 for review, see McKenna, 2013). Inhibition of ANT1 with carboxyatractyloside or genetic deletion of ANT1 reduce glutamate uptake. Conversely up-regulation of ANT1 in reactive astrocytes is associated with increased glutamate uptake. This effect seemed rather selective, as deletion of ANT1 did not alter other energy using processes, such as proliferation (Buck et al., 2003). In some studies, inhibition of mitochondrial function alone with inhibitors or by removing oxygen has no effect on glutamate uptake. Similarly, inhibition of glycolysis alone has no effect on glutamate uptake, but inhibition of both glycolysis and oxidative phosphorylation together reduces glutamate uptake (Genda et al., 2011; Swanson, 1992; Swanson et al., 1994). It should be noted that others have found that inhibition of mitochondrial function or glycolysis separately reduces D-aspartate uptake by about 15 to 20% (Schousboe et al., 2011). This suggests that mitochondria/oxidative phosphorylation can serve as redundant energy sources for glutamate uptake in some preparations.
While glutamate transport can be dependent upon mitochondrial function, it also regulates mitochondrial function. For example, Eriksson and colleagues demonstrated that glutamate uptake in astrocytes increases oxygen consumption (e.g. stimulates oxidative metabolism) (Eriksson et al., 1995). However, others have observed the opposite effect with glutamate uptake decreasing oxygen consumption and causing mitochondrial acidification (Azarias et al., 2011; Perreten Lambert et al., 2014). Glutamate uptake is also coupled to increases in mitochondrial Na+ (Bernardinelli et al., 2006). These results suggest a role for mitochondria in sensing and regulating metabolic responses secondary to glutamate uptake.
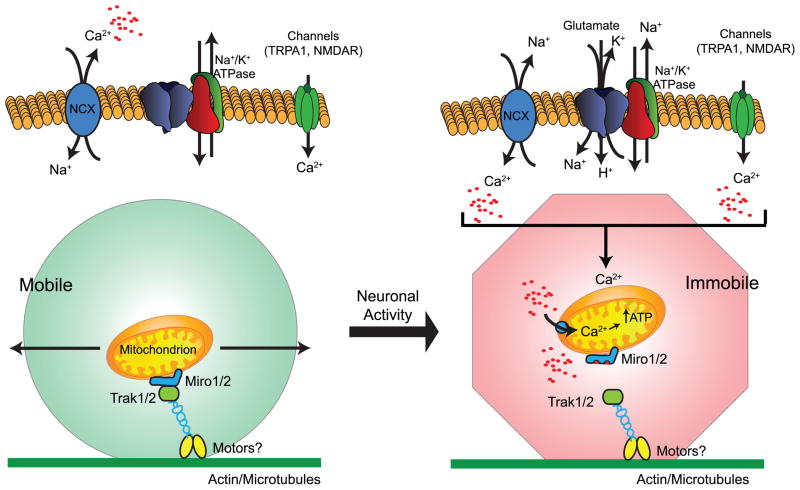
Mitochondria are mobile organelles known to redistribute under a number of different circumstances. In fact, neurons with their highly elongated processes have provided an ideal system to define the mechanisms involved (Hollenbeck and Saxton, 2005; Rintoul et al., 2003 for reviews, see Chang and Reynolds, 2006; MacAskill and Kittler, 2009; Schwarz, 2013; Sheng and Cai, 2012). Although some details are still unresolved, mitochondrial movement is dependent upon tethering to the cell’s motor apparatus through Ca2+-sensitive Miro (RhoT) and Trak1/2 (Milton) (for reviews, see Birsa et al., 2013; Schwarz, 2013). Local elevations in Ca2+ result in disengagement of the Miro proteins and an arrest of mitochondria. Thus at this point it appears that mitochondria are not formally recruited to a particular location, but rather they accumulate in response to an elevation in local Ca2+ concentration that causes Miro to disengage from motor proteins. In fact, mitochondria have been shown to accumulate at nodes of Ranvier, presynaptic termini, or post-synaptic spines in response to activity (Li et al., 2004; Macaskill et al., 2009; Ohno et al., 2011; Sun et al., 2013; Zhang et al., 2010). It is assumed that mitochondria redistribute to provide a local source of ATP; and this is certainly a reasonable assumption. In addition mitochondria can either increase or decrease cyctoplasmic Ca2+ signals by releasing Ca2+ or by accumulating/buffering Ca2+ (Demaurex et al., 2009; Kristian et al., 2007; Parnis et al., 2013; Chouhan et al., 2010).
Mitochondria in primary cultures of astrocytes do not approach the membrane and do not colocalize with GLT-1 particles (Ugbode et al., 2014). Neuronal activity increases co-localization of GLT1 with mitochondria and increases TRAK expression (Ugbode et al., 2014). In organotypic cultures of rat hippocampus, astrocytes maintain their highly branched morphology (Benediktsson et al., 2005). Using this system and biolistically introduced fluorescent reporter proteins, we and others have recently demonstrated that between 15 and 30% of mitochondria are mobile in astrocytic processes (Jackson et al., 2014; Stephen et al., 2015). In some of our analyses, the percentage of mobile mitochondria was as low as 15%. Although we had hypothesized that neuronal activity would result in the accumulation of mitochondria near glutamate transporters and synapses, we were unable to detect decreases in the percentage of mobile mitochondria with high K+ that should increase neuronal activity (Jackson, O’Donnell, and Robinson unpublished observations). Therefore, we examined the effects of inhibiting activity with tetrodotoxin or a cocktail of ionotropic receptor antagonists. Both of these treatments significantly increase the percentage of mobile mitochondria (Jackson et al., 2014). We also found that inhibition of glutamate uptake or inhibition of reversed operation of the NCX increases the percentage of mobile mitochondria and decrease cytoplasmic Ca2+ levels. Based on these analyses, we proposed that neuronal activity and release of glutamate is followed by astrocytic glutamate uptake, reversed operation of the NCX with an increase in Ca2+, and arrest of mitochondrial movement (Jackson et al., 2014; Jackson and Robinson, 2015). In a similar analysis, Stephen and colleagues observed a much higher percentage of mobile mitochondria under baseline conditions (~30%) (Stephen et al., 2015). They demonstrated that glutamate in combination with glycine results in a decrease in mitochondrial mobility. This effect of glutamate and glycine on mitochondrial mobility was blocked by two different antagonists of the NMDA subtype of glutamate receptor, MK-801 or D-2-amino-5-phosphonopentanoic acid. This analysis shows that NMDA receptor activation mediates this effect. Thus there are some differences in the proposed mechanisms that need to be resolved in future studies.
In spite of the difference in mechanisms discussed in the previous paragraph, both studies provide evidence that neuronal activity decreases the distance between synapses (identified with antibodies against a vesicular glutamate transporter) and mitochondria in these astrocytic processes that surround synapses (Jackson et al., 2014; Stephen et al., 2015). Both groups also demonstrated that miro proteins contribute to the regulation of mitochondrial mobility and that mitochondria shape Ca2+ signals in astrocytic processes and prevent the spread of Ca2+ waves (Jackson and Robinson, 2015; Stephen et al., 2015) (see Figure 2 for schematic model).
Figure 2.
Schematic model summarizing our current understanding of the signals and proteins that may control the mobility of mitochondria in astrocytic processes (adapted from Jackson and Robinson, 2015, also see Jackson et al., 2014; Stephen et al., 2015)
9. Implications for Glutamate Metabolism
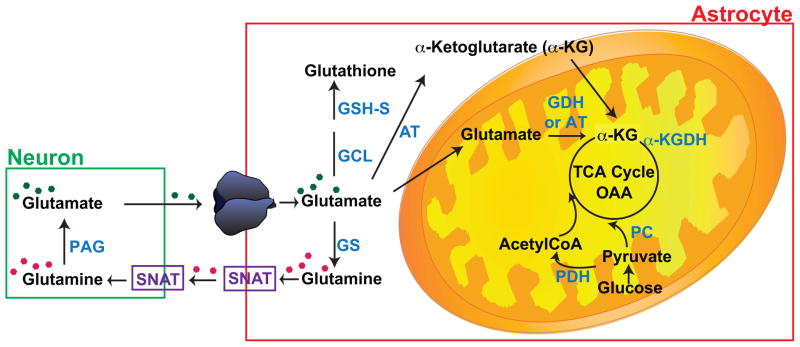
Generally, recycling of transmitter pools of glutamate is simplified into a glutamate-glutamine cycle (Figure 3). In this cycle, glutamate is cleared by astroglial transporters. After transport, glutamate is converted to glutamine by glutamine synthetase, an enzyme that is almost exclusively expressed in astrocytes (Norenberg and Martinez-Hernandez, 1979). This glutamine is then exported from astrocytes by transporters (SNATs) and the extracellular glutamine is then imported into neurons for conversion back to glutamate by phosphate activated glutaminase (for reviews, see Chaudhry et al., 2002; Hertz, 1979; Schousboe, 1981), but this is not stoichiometric (for discussion, see McKenna, 2007 and see next paragraph). In addition, it is relatively hard to demonstrate a role for glutamine in maintaining glutamatergic signaling (Kam and Nicoll, 2007, but also see Tani et al., 2014). Glutamate is also the only direct precursor for the inhibitory neurotransmitter, γ-aminobutyric acid (GABA) and is a component of glutathione.
Figure 3.
Simplified schematic model of glutamate shuttling between neurons and astrocytes. For more detailed schematics and descriptions of the metabolic pathways, including stoichiometry of ATP generation or consumption see there are several recent reviews (Dienel, 2013; Hertz, 2013; McKenna, 2013; Sonnewald, 2014). Abbreviations: PAG, phosphate activate glutaminase; GS, glutamine synthetase; GCL, glutamate-cysteine ligase; GSH-S, glutathione synthetase; AT, amino transferase; GDH, glutamate dehydrogenase; α-KGDH, α-ketoglutarate dehydrogenase; PC, pyruvate carboxylase; PDH, pyruvate dehydrogenase; and SNAT, sodium-coupled amino acid transporters. Note there are multiple members of the SNAT gene family (for reviews, see Chaudhry et al., 2002; Pochini et al., 2014).
It is somewhat underappreciated that a fairly large percentage of glutamate is oxidized in astrocytes, presumably to generate ATP. Glutamate can be converted to α-ketoglutarate by amino transferases that are found in the cytosol or mitochondria (Spanaki and Plaitakis, 2012) or by glutamate dehydrogenase that is restricted to mitochondria (Aoki et al., 1987). Although modeling studies suggest that cytosolic conversion of glutamate to α-ketoglutarate is not likely (Calvetti and Somersalo, 2012), the mitochondrial transporter that exchanges cytosolic α-ketoglutarate for mitochondrial malate (Slc25a11) was identified in the GLT-1 proteome (Genda et al., 2011). In 1982, Yu and his colleagues found that the flux of glutamate into the tricarboxylic acid cycle was about 50% greater than the flux into glutamine in astrocyte cultures (Yu et al., 1982). Other studies have observed between ~15 and 70% oxidation of glutamate using astrocytes, brain slices, or in vivo analyses (Kanamori et al., 2002; McKenna et al., 1996a; McKenna et al., 1996b; Oz et al., 2004; Rothman et al., 2011; Sonnewald et al., 1993; Sonnewald et al., 1997; Zielke et al., 1998). A variety of isotopic approaches have been used to estimate the percentage of glutamate that is oxidized in vivo, and ~30% of glutamate is oxidized on a continuous basis (for reviews, see Dienel, 2013; Hertz, 2013; McKenna, 2013; Sonnewald, 2014). The importance of this process is underscored by the fact that deletion of glutamate dehydrogenase specifically from brain increases peripheral glucose mobilization (Karaca et al., 2015). This suggests that oxidation of glutamate is a sufficiently important source of fuel in the brain that peripheral metabolism is adjusted in its absence to provide alternate sources of ATP.
As glutamate is not thought to cross the blood brain barrier, this means that glutamate needs to be continuously replenished. This is accomplished by the astrocyte specific enzyme pyruvate carboxylase which, combined with pyruvate dehydrogenase, can convert two molecules of pyruvate generated from glucose into a new molecule of glutamate (for reviews, see Dienel, 2013; McKenna, 2013; Schousboe et al., 2014; Sonnewald, 2014). Interestingly many of the proteins involved in this cycling, including pyruvate carboxylase, aralar/slc25a12, the mitochondrial glutamate carrier/slc25a22/GC1, and one of the subunits of pyruvate dehydrogenase were also identified in co-immunoprecipitates of GLT-1 (Genda et al., 2011). Thus, the very high levels of glutamate that are found in brain provide a readily accessible source of fuel for the generation of ATP in addition to the other roles that are generally ascribed to glutamate (e.g. neurotransmitter and precursor for the inhibitory neurotransmitter GABA) (for reviews, see Dienel, 2013; McKenna, 2013; Schousboe et al., 2014; Sonnewald, 2014). Given the variability in the extent of glutamate oxidation observed in different studies, it seems likely that this process is regulated. In fact, increasing extracellular glutamate not only increases glutamate oxidation, but also increases the percentage of glutamate that is oxidized, suggesting that glutamate itself may regulate the extent of glutamate oxidation (McKenna et al., 1996a; Torres et al., 2013). It seems likely that the proximity of glutamate transporters to mitochondria or to glutamine synthetase will govern the extent of glutamate oxidation, but the field still needs a few additional tools to test this hypothesis.
10. Glutamate transport and the neurovascular response
The human brain, which represents ~2% of body weight, consumes a disproportionate amount of the body’s basal energy budget (roughly 20%) (for discussions, see Harris et al., 2012; Hertz et al., 2007; Stobart and Anderson, 2013; Weber and Barros, 2015). Unlike other biological systems that can use fats, amino acids, or carbohydrates/glucose for fuel, the brain is essentially dependent upon glucose for fuel (Stanley et al., 2014; Weber and Barros, 2015). While glycogen and glutamate provide local reserves for generation of ATP (see discussions above), neuronal activity relatively rapidly (within ~1 sec) results in an increase blood flow and delivery of glucose to the brain. This increase in blood flow provides the signal analyzed in functional MRI analyses (for review, see Hillman, 2014; Raichle and Mintun, 2006). The mechanisms underlying this neurovascular coupling have been the subject of intense scrutiny and several different signals have been implicated (for reviews, see Attwell et al., 2010; Figley and Stroman, 2011; Howarth, 2014; Iadecola and Nedergaard, 2007; Koehler et al., 2009; Munoz et al., 2015; Petzold and Murthy, 2011; Tran and Gordon, 2015). The signals and cells that mediate this response are likely different for arterioles and capillaries (Attwell et al., 2010; Hall et al., 2014).
For arterioles, most in the field agree that astrocytes have a major role for coupling neuronal activity to increased blood flow (for reviews, see Attwell et al., 2010; Figley and Stroman, 2011; Filosa et al., 2015; Howarth, 2014; Koehler et al., 2009; Petzold and Murthy, 2011). Several different studies have implicated glutamate transporters in this response. For example, genetic deletion of either GLT-1 or GLAST reduces the whisker stimulation-evoked increase in accumulation of radioactive 2-deoxyglucose in somatosensory cortex in young animals (P10) (Voutsinos-Porche et al., 2003). The glutamate uptake inhibitor, DL-threo-β-benzyloxyaspartate (DL-TBOA), attenuates visual system activation-evoked changes in blood flow in the visual cortex in ferrets ~40 days of age (Schummers et al., 2008). Similarly, DL-TBOA attenuates the odor-evoked increases in intrinsic optical signals in the olfactory bulb (Gurden et al., 2006) or the odor-evoked increases in blood flow in the olfactory bulb (Petzold et al., 2008). In these two studies, the investigators also examined the effects of inhibitors of ionotropic glutamate receptors antagonists. In the former study, these antagonists had no effect on the intrinsic optical signals (Gurden et al., 2006). In the latter study, antagonists for ionotropic and metabotropic receptors were combined with a GABAB receptor antagonist. This cocktail reduced the increase in blood flow, and dihydrokainate or TBOA further attenuated the response (Petzold et al., 2008). These later studies suggest that the effects of inhibition of glutamate transport are not likely due to receptor desensitization. Instead they suggest that glutamate transport itself somehow couples to the increase in blood flow. The mechanism underlying this effect of transport inhibition has not been identified.
11. Summary and Future directions
In summary, several studies have demonstrated that the astroglial glutamate transporters, GLT-1 and GLAST, co-compartmentalize with and functionally couple to diverse sources of ‘energy’, including the Na+/K+-ATPase, Na+/Ca2+ exchanger, glycogen, glycolysis, and mitochondria. Many of these sources of ‘energy’ either directly or indirectly support glutamate uptake by maintaining the Na+ gradient required for active transport or by generating ATP. All of these processes are also functionally coupled to glutamate transport. In order to effectively meet the energetic costs of neuronal activity, it seems reasonable to consider the possibility that these events also support the astrocyte-dependent coupling of neuronal activity to increases in blood flow. In this review, we have chosen to remain relatively focused, but there are many others aspects that may be worth pursuing, including:
In a recent study, a collaborator found that nitric oxide modifies and regulates that activity of many of these processes including GLT-1-mediated uptake and glutamate oxidation (Raju et al., 2015). These observations suggest that, in addition to the reciprocal regulation discussed above, the overall coupling of glutamate transporters to various sources of energy may be regulated. This has not been explored.
There is some evidence similar signals regulate expression of the components of this ‘complex’. For example, the developmental expression of glutamate dehydrogenase parallels that observed for GLT-1 (Kugler and Schleyer, 2004). Neurons induce astrocytic expression of GLT-1 (Gegelashvili et al., 1997; Schlag et al., 1998; Swanson et al., 1997) and many of the glycolytic enzymes and glycogen metabolizing enzymes (Mamczur et al., 2015). Sleep deprivation also affects expression of many of these enzymes in a parallel fashion (Petit et al., 2013). This raises the possibility that these processes might be under coordinate transcriptional regulation.
If this functional coupling is mediated by formation of complexes, there is likely significant heterogeneity. For example, GLAST and GLT-1 do not co-immunoprecipitate so these transporters would have to be parts of different complexes. In the initial mass spectrometric analysis of GLT-1 immunoprecipitates, we identified the α3 subunit of the Na+/K+-ATPase (Genda et al., 2011). As this subunit is neuronal, this might indicate that GLT-1 in neurons forms a different complex. There is evidence that α-ketoglutarate dehydrogenase distribution defines different populations of mitochondria in astrocytes (Waagepetersen et al., 2006). One wonders if subtypes of mitochondria are differentially coupled to glutamate transporters.
We have not discussed the astrocyte-neuron lactate shuttle. When it was thought that there are no mitochondria in fine astrocytic processes, it meant that these processes might primarily derive their ATP from glycolysis. This meant that lactate or pyruvate could be exported for oxidation by neurons (for reviews, see Belanger et al., 2011; Magistretti and Allaman, 2015). This shuttle is still an active area for debate (for summary, see Dienel, 2012), but it seems fairly clear that import of lactate (or pyruvate or ketones) into neurons is important for neuronal functioning including long-term potentiation (Suzuki et al., 2011) or learning (Yang et al., 2014 Boury-Jamot et al., 2015). One wonders if the source of lactate might be glutamate itself (see Dienel, 2013; McKenna et al., 1996a; McKenna, 2013; Sonnewald et al., 1993; Sonnewald, 2014 for metabolic pathway).
Robert McCullumsmith and his colleagues have begun to examine this complex in schizophrenia (Shan et al., 2014, also see Regenold et al., 2012). Using mass spectrometry, they have identified many of the same proteins in a complex with GLT-1 in human tissue and alterations in the interactions between hexokinase and mitochondria in autopsy material from patients with schizophrenia. It will be interesting to both learn if these ‘complexes’ are altered in various neurologic or psychiatric diseases. As the field learns how these complexes are regulated by extrinsic signals, it may interesting to determine if there are opportunities for therapeutic interventions.
The interaction of glutamate transporters with mitochondria is supported by several different lines of evidence. The fact that the enzyme that metabolizes GABA, GABA-transaminase, is a mitochondrial enzyme (Hyde and Robinson, 1976; Schousboe et al., 1977) suggests that plasma membrane GABA transporters are likely coupled to mitochondria. It not known if mitochondria assemble near GABA transporters. Although an Arabidopsis ‘mitochondrial’ GABA transporter has been identified (Michaeli et al., 2011), the mammalian mitochondrial transporter does not appear to have been identified based on literature searches.
Highlights.
In this article, we review evidence that the glutamate transporters, GLT-1 and GLAST, co-compartmentalize with, interact with, and functionally couple to the Na+/K+-ATPases, Na+/Ca2+ exchangers, glycogen metabolizing enzymes, glycolytic enzymes, and mitochondria
We discuss our current understanding of the regulation of these interactions
We discuss the implications of this co-compartmentalization for glutamate metabolism
We conclude by suggesting some opportunities to further examine these interactions.
Acknowledgments
The authors are supported by the National Institutes of Neurologic Disease and Stroke (R01 NS077773). Joshua Jackson is also partially supported by a Foerderer award from Children’s Hospital of Philadelphia. The authors would like to thank John O’Donnell and Zila Martinez-Lozado for their comments that were used to improve the manuscript. The authors would also like to thank Elizabeth Krizman who helped with the preparation of the figures.
Abbreviations
- ANT
adenine nucleotide translocase
- DL-TBOA
DL-threo-β-benzyloxyaspartate
- EAAT
excitatory amino acid transporter
- eGFP
enhanced green fluorescent protein
- GABA
γ-aminobutryic acid
- GLAST
glutamate aspartate transporter
- GLT-1
glutamate transporter 1
- NCX
Na+/Ca2+ exchanger, UQCRC2, ubiquinol-cytochrome-c reductase complex
- VDAC
voltage-dependent anion channel
Footnotes
Conflicts-of-Interests
Michael B. Robinson is the Editor-in-Chief for Neurochemistry International and is financially reimbursed for his work as Editor. The workflow for the review process was modified so that M.B.R. did not have editorial access and was kept blinded to the reviewers’ identities.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe K, Saito H. Involvement of Na+-K+ pump in L-glutamate clearance by cultured rat cortical astrocytes. Biol Pharm Bull. 2000;23:1051–4. doi: 10.1248/bpb.23.1051. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Antonicek H, Schachner M. The adhesion molecule on glia (AMOG) incorporated into lipid vesicles binds to subpopulations of neurons. J Neurosci. 1988;8:2961–6. doi: 10.1523/JNEUROSCI.08-08-02961.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Milner TA, Berger SB, Sheu KF, Blass JP, Pickel VM. Glial glutamate dehydrogenase: ultrastructural localization and regional distribution in relation to the mitochondrial enzyme, cytochrome oxidase. J Neurosci Res. 1987;18:305–18. doi: 10.1002/jnr.490180207. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–26. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. Journal of Neuroscience. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–43. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarias G, Perreten H, Lengacher S, Poburko D, Demaurex N, Magistretti PJ, Chatton JY. Glutamate Transport Decreases Mitochondrial pH and Modulates Oxidative Metabolism in Astrocytes. J Neurosci. 2011;31:3550–9. doi: 10.1523/JNEUROSCI.4378-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcar VJ, Johnston GAR. The structural specificity of the high affinity uptake of L-glutamate and L-aspartate by rat brain slices. Journal of Neurochemistry. 1972;19:2657–2666. doi: 10.1111/j.1471-4159.1972.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Bassan M, Liu H, Madsen KL, Armsen W, Zhou J, Desilva T, Chen W, Paradise A, Brasch MA, Staudinger J, Gether U, Irwin N, Rosenberg PA. Interaction between the glutamate transporter GLT1b and the synaptic PDZ domain protein PICK1. Eur J Neurosci. 2008;27:66–82. doi: 10.1111/j.1460-9568.2007.05986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DE, Jackson JG, Genda EN, Montoya MM, Yudkoff M, Robinson MB. The glutamate transporter, GLAST, participates in a macromolecular complex that supports glutamate metabolism. Neurochem Int. 2012;61:566–74. doi: 10.1016/j.neuint.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beart PM, O’Shea RD. Transporters for L-glutamate: an update on their molecular pharmacology and pathological involvement. Br J Pharmacol. 2007;150:5–17. doi: 10.1038/sj.bjp.0706949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–38. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Benediktsson AM, Schachtele SJ, Green SH, Dailey ME. Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. J Neurosci Methods. 2005;141:41–53. doi: 10.1016/j.jneumeth.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Benediktsson AM, Marrs GS, Tu JC, Worley PF, Rothstein JD, Bergles DE, Dailey ME. Neuronal activity regulates glutamate transporter dynamics in developing astrocytes. Glia. 2012;60:175–88. doi: 10.1002/glia.21249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin Kacerovsky J, Murai KK. Stargazing: Monitoring subcellular dynamics of brain astrocytes. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Bernardinelli Y, Azarias G, Chatton JY. In situ fluorescence imaging of glutamate-evoked mitochondrial Na+ responses in astrocytes. Glia. 2006;54:460–70. doi: 10.1002/glia.20387. [DOI] [PubMed] [Google Scholar]
- Beutner G, Ruck A, Riede B, Brdiczka D. Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biochim Biophys Acta. 1998;1368:7–18. doi: 10.1016/s0005-2736(97)00175-2. [DOI] [PubMed] [Google Scholar]
- Bianchi MG, Gatti R, Torielli L, Padoani G, Gazzola GC, Bussolati O. The glutamate transporter excitatory amino acid carrier 1 associates with the actin-binding protein alpha-adducin. Neuroscience. 2010;169:584–95. doi: 10.1016/j.neuroscience.2010.05.029. [DOI] [PubMed] [Google Scholar]
- Birsa N, Norkett R, Higgs N, Lopez-Domenech G, Kittler JT. Mitochondrial trafficking in neurons and the role of the Miro family of GTPase proteins. Biochem Soc Trans. 2013;41:1525–31. doi: 10.1042/BST20130234. [DOI] [PubMed] [Google Scholar]
- Bittner CX, Valdebenito R, Ruminot I, Loaiza A, Larenas V, Sotelo-Hitschfeld T, Moldenhauer H, San Martin A, Gutierrez R, Zambrano M, Barros LF. Fast and reversible stimulation of astrocytic glycolysis by k+ and a delayed and persistent effect of glutamate. J Neurosci. 2011;31:4709–13. doi: 10.1523/JNEUROSCI.5311-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Boury-Jamot B, Carrard A, Martin JL, Halfon O, Magistretti PJ, Boutrel B. Disrupting astrocyte-neuron lactate transfer persistently reduces conditioned responses to cocaine. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Ransom BR. Astrocyte glycogen as an emergency fuel under conditions of glucose deprivation or intense neural activity. Metab Brain Dis. 2015;30:233–9. doi: 10.1007/s11011-014-9588-2. [DOI] [PubMed] [Google Scholar]
- Buck CR, Jurynec MJ, Gupta DK, Law AK, Bilger J, Wallace DC, McKeon RJ. Increased adenine nucleotide translocator 1 in reactive astrocytes facilitates glutamate transport. Exp Neurol. 2003;181:149–58. doi: 10.1016/s0014-4886(03)00043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvetti D, Somersalo E. Menage a trois: the role of neurotransmitters in the energy metabolism of astrocytes, glutamatergic, and GABAergic neurons. J Cereb Blood Flow Metab. 2012;32:1472–83. doi: 10.1038/jcbfm.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella ME, Chu H, Low PS. Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. Proc Natl Acad Sci U S A. 2005;102:2402–7. doi: 10.1073/pnas.0409741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casale CH, Previtali G, Barra HS. Involvement of acetylated tubulin in the regulation of Na+,K+-ATPase activity in cultured astrocytes. FEBS Lett. 2003;534:115–8. doi: 10.1016/s0014-5793(02)03802-4. [DOI] [PubMed] [Google Scholar]
- Casale CH, Previtali G, Serafino JJ, Arce CA, Barra HS. Regulation of acetylated tubulin/Na+,K+-ATPase interaction by L-glutamate in non-neural cells: involvement of microtubules. Biochim Biophys Acta. 2005;1721:185–92. doi: 10.1016/j.bbagen.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Chang DT, Reynolds IJ. Mitochondrial trafficking and morphology in healthy and injured neurons. Prog Neurobiol. 2006;80:241–68. doi: 10.1016/j.pneurobio.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Lehre KP, Campagne MVL, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: Highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Edwards RH. The glutamine commute: take the N line and transfer to the A. Journal of Cell Biology. 2002;157:349–55. doi: 10.1083/jcb.200201070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Aoki C, Mahadomrongkul V, Gruber CE, Wang GJ, Blitzblau R, Irwin N, Rosenberg PA. Expression of a variant form of the glutamate transporter GLT1 in neuronal cultures and in neurons and astrocytes in the rat brain. Journal of Neuroscience. 2002;22:2142–2152. doi: 10.1523/JNEUROSCI.22-06-02142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Mahadomrongkul V, Berger UV, Bassan M, DeSilva T, Tanaka K, Irwin N, Aoki C, Rosenberg PA. The glutamate transporter GLT1a is expressed in excitatory terminals of mature hippocampal neurons. Journal of Neuroscience. 2004;24:1136–1148. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Hoogenraad CC, Rush J, Ramm E, Schlager MA, Duong DM, Xu P, Wijayawardana SR, Hanfelt J, Nakagawa T, Sheng M, Peng J. Relative and absolute quantification of postsynaptic density proteome isolated from rat forebrain and cerebellum. Mol Cell Proteomics. 2006;5:1158–70. doi: 10.1074/mcp.D500009-MCP200. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. Journal of Neurobiology. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Cholet N, Pellerin L, Magistretti PJ, Hamel E. Similar perisynaptic glial localization for the Na+,K+-ATPase a2 subunit and the glutamate transporters GLAST and GLT-1 in the rat somatosensory cortex. Cerebral Cortex. 2002;12:515–525. doi: 10.1093/cercor/12.5.515. [DOI] [PubMed] [Google Scholar]
- Chouhan AK, Zhang J, Zinsmaier KE, Macleod GT. Presynaptic mitochondria in functionally different motor neurons exhibit similar affinities for Ca2+ but exert little influence as Ca2+ buffers at nerve firing rates in situ. J Neurosci. 2010;30:1869–81. doi: 10.1523/JNEUROSCI.4701-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Puchulu-Campanella E, Galan JA, Tao WA, Low PS, Hoffman JF. Identification of cytoskeletal elements enclosing the ATP pools that fuel human red blood cell membrane cation pumps. Proc Natl Acad Sci U S A. 2012;109:12794–9. doi: 10.1073/pnas.1209014109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F, DiBiasi S, Minelli A, Rothstein JD, Melone M. EAAC1, a high-affinity glutamate transporter, is localized to astrocytes and gabaergic neurons besides pyramidal cells in the rat cerebral cortex. Cerebral Cortex. 1998;8:108–116. doi: 10.1093/cercor/8.2.108. [DOI] [PubMed] [Google Scholar]
- Conti F, Weinberg RJ. Shaping excitation at glutamatergic synapses. Trends in Neurosciences. 1999;22:451–458. doi: 10.1016/s0166-2236(99)01445-9. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Crompton M, Virji S, Ward JM. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur J Biochem. 1998a;258:729–35. doi: 10.1046/j.1432-1327.1998.2580729.x. [DOI] [PubMed] [Google Scholar]
- Crompton M, Virji S, Ward JM. Cyclophilin-D binding proteins. Biochem Soc Trans. 1998b;26:S330. doi: 10.1042/bst026s330. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, Pines G, Kanner BI. Purification and reconstitution of the sodium- and potassium-coupled glutamate transport glycoprotein from rat brain. Biochemistry. 1990;29:6734–6740. doi: 10.1021/bi00480a025. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, Storm-Mathisen J, Kanner BI. A [Na+ + K+] coupled L-glutamate transporter purified from rat brain is located in glial cell processes. Neuroscience. 1992;51:295–310. doi: 10.1016/0306-4522(92)90316-t. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in Neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Debernardi R, Magistretti PJ, Pellerin L. Trans-inhibition of glutamate transport prevents excitatory amino acid-induced glycolysis in astrocytes. Brain Res. 1999;850:39–46. doi: 10.1016/s0006-8993(99)02022-3. [DOI] [PubMed] [Google Scholar]
- Dehnes Y, Chaudhry FA, Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in the parts of the dendritic membrane facing astroglia. Journal of Neuroscience. 1998;18:3606–3610. doi: 10.1523/JNEUROSCI.18-10-03606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N, Poburko D, Frieden M. Regulation of plasma membrane calcium fluxes by mitochondria. Biochim Biophys Acta. 2009;1787:1383–94. doi: 10.1016/j.bbabio.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Denton RM, Randle PJ, Martin BR. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem J. 1972;128:161–3. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta. 2009;1787:1309–16. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Derouiche A, Haseleu J, Korf HW. Fine Astrocyte Processes Contain Very Small Mitochondria: Glial Oxidative Capability May Fuel Transmitter Metabolism. Neurochem Res. 2015;40:2402–13. doi: 10.1007/s11064-015-1563-8. [DOI] [PubMed] [Google Scholar]
- Devarajan P, Scaramuzzino DA, Morrow JS. Ankyrin binds to two distinct cytoplasmic domains of Na,K-ATPase alpha subunit. Proc Natl Acad Sci U S A. 1994;91:2965–9. doi: 10.1073/pnas.91.8.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2012;32:1107–38. doi: 10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA. Astrocytic energetics during excitatory neurotransmission: What are contributions of glutamate oxidation and glycolysis? Neurochem Int. 2013;63:244–58. doi: 10.1016/j.neuint.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Contributions of glycogen to astrocytic energetics during brain activation. Metab Brain Dis. 2015;30:281–98. doi: 10.1007/s11011-014-9493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNuzzo M, Mangia S, Maraviglia B, Giove F. Regulatory mechanisms for glycogenolysis and K+ uptake in brain astrocytes. Neurochem Int. 2013;63:458–64. doi: 10.1016/j.neuint.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domercq M, Etxebarria E, Perez-Samartin A, Matute C. Excitotoxic oligodendrocyte death and axonal damage induced by glutamate transporter inhibition. Glia. 2005;52:36–46. doi: 10.1002/glia.20221. [DOI] [PubMed] [Google Scholar]
- Dostanic I, Schultz Jel J, Lorenz JN, Lingrel JB. The alpha 1 isoform of Na,K-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na/Ca exchanger in heart. J Biol Chem. 2004;279:54053–61. doi: 10.1074/jbc.M410737200. [DOI] [PubMed] [Google Scholar]
- Dunlop J. Glutamate-based therapeutic approaches: targeting the glutamate transport system. Curr Opin Pharmacol. 2006;6:103–7. doi: 10.1016/j.coph.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Eriksson G, Peterson A, Iverfeldt K, Walum E. Sodium-dependent glutamate uptake as an activator of oxidative metabolism in primary astrocyte cultures from newborn rat. Glia. 1995;15:152–6. doi: 10.1002/glia.440150207. [DOI] [PubMed] [Google Scholar]
- Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nat Rev Neurosci. 2009;10:87–99. doi: 10.1038/nrn2540. [DOI] [PubMed] [Google Scholar]
- Ferkany J, Coyle JT. Heterogeneity of sodium-dependent excitatory amino acid uptake mechanisms in rat brain. Journal of Neuroscience Research. 1986;16:491–503. doi: 10.1002/jnr.490160305. [DOI] [PubMed] [Google Scholar]
- Fernandez B, Suarez I, Gianonatti C. Fine structure of astrocytic mitochondria in the hypothalamus of the hamster. J Anat. 1983;137(Pt 3):483–8. [PMC free article] [PubMed] [Google Scholar]
- Figley CR, Stroman PW. The role(s) of astrocytes and astrocyte activity in neurometabolism, neurovascular coupling, and the production of functional neuroimaging signals. Eur J Neurosci. 2011;33:577–88. doi: 10.1111/j.1460-9568.2010.07584.x. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Morrison HW, Iddings JA, Du W, Kim KJ. Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier KM, González MI, Robinson MB. Rapid trafficking of the neuronal glutamate transporter, EAAC1: Evidence for distinct trafficking pathways differentially regulated by protein kinase C and platelet-derived growth factor. Journal of Biological Chemistry. 2004;279:34505–34513. doi: 10.1074/jbc.M404032200. [DOI] [PubMed] [Google Scholar]
- Furness DN, Dehnes Y, Akhtar AQ, Rossi DJ, Hamann M, Grutle NJ, Gundersen V, Holmseth S, Lehre KP, Ullensvang K, Wojewodzic M, Zhou Y, Attwell D, Danbolt NC. A quantitative assessment of glutamate uptake into hippocampal synaptic terminals and astrocytes: new insights into a neuronal role for excitatory amino acid transporter 2 (EAAT2) Neuroscience. 2008;157:80–94. doi: 10.1016/j.neuroscience.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta A, Martin LJ, Lin C-LG, Dykes-Hoberg M, Rothstein JD. Cellular and synaptic localization of the neuronal glutamate transporters EAAT3 and EAAT4. Neuroscience. 1997;81:1031–1042. doi: 10.1016/s0306-4522(97)00252-2. [DOI] [PubMed] [Google Scholar]
- Gameiro A, Braams S, Rauen T, Grewer C. The discovery of slowness: low-capacity transport and slow anion channel gating by the glutamate transporter EAAT5. Biophys J. 2011;100:2623–32. doi: 10.1016/j.bpj.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlin AB, Sinor AD, Sinor JD, Jee SH, Grinspan JB, Robinson MB. Pharmacology of sodium-dependent high-affinity L-[3H]glutamate transport in glial cultures. Journal of Neurochemistry. 1995;64:2572–2580. doi: 10.1046/j.1471-4159.1995.64062572.x. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Danbolt NC, Schousboe A. Neuronal soluble factors differentially regulate the expression of the GLT1 and GLAST glutamate transporters in cultured astroglia. J Neurochem. 1997;69:2612–5. doi: 10.1046/j.1471-4159.1997.69062612.x. [DOI] [PubMed] [Google Scholar]
- Gegelashvili G, Schousboe A. High affinity glutamate transporters: regulation of expression and activity. Mol Pharmacol. 1997;52:6–15. doi: 10.1124/mol.52.1.6. [DOI] [PubMed] [Google Scholar]
- Gegelashvili M, Rodriguez-Kern A, Sung L, Shimamoto K, Gegelashvili G. Glutamate transporter GLAST/EAAT1 directs cell surface expression of FXYD2/gamma subunit of Na, K-ATPase in human fetal astrocytes. Neurochem Int. 2007;50:916–20. doi: 10.1016/j.neuint.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Genda EN, Jackson JG, Sheldon AL, Locke SF, Greco TM, O’Donnell JC, Spruce LA, Xiao R, Guo W, Putt M, Seeholzer S, Ischiropoulos H, Robinson MB. Co-compartmentalization of the astroglial glutamate transporter, GLT-1, with glycolytic enzymes and mitochondria. Journal of Neuroscience. 2011;31:18275–18288. doi: 10.1523/JNEUROSCI.3305-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Yang Y, Rothstein JD, Robinson MB. Nuclear Factor-kB contributes to neuron-dependent induction of GLT-1 expression in astrocytes. Journal of Neuroscience. 2011;31:9159–9169. doi: 10.1523/JNEUROSCI.0302-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs ME, Anderson DG, Hertz L. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia. 2006;54:214–22. doi: 10.1002/glia.20377. [DOI] [PubMed] [Google Scholar]
- Golovina V, Song H, James P, Lingrel J, Blaustein M. Regulation of Ca2+ signaling by Na+ pump alpha-2 subunit expression. Ann N Y Acad Sci. 2003;986:509–13. doi: 10.1111/j.1749-6632.2003.tb07236.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, Susarla BT, Fournier KM, Sheldon AL, Robinson MB. Constitutive endocytosis and recycling of the neuronal glutamate transporter, excitatory amino acid carrier 1. J Neurochem. 2007;103:1917–31. doi: 10.1111/j.1471-4159.2007.04881.x. [DOI] [PubMed] [Google Scholar]
- González MI, Bannerman PG, Robinson MB. Phorbol myristate acetate-dependent interaction of protein kinase Ca and the neuronal glutamate transporter EAAC1. Journal of Neuroscience. 2003;23:5589–5593. doi: 10.1523/JNEUROSCI.23-13-05589.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González MI, Susarla BTS, Robinson MB. Evidence that protein kinase Ca interacts with an regulates the glial glutamate transporter GLT-1. Journal of Neurochemistry. 2005;94:1180–1188. doi: 10.1111/j.1471-4159.2005.03330.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gonzalez IM, Garcia-Tardon N, Cubelos B, Gimenez C, Zafra F. The glutamate transporter GLT1b interacts with the scaffold protein PSD-95. J Neurochem. 2008;105:1834–48. doi: 10.1111/j.1471-4159.2008.05281.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gonzalez IM, Garcia-Tardon N, Gimenez C, Zafra F. Splice variants of the glutamate transporter GLT1 form hetero-oligomers that interact with PSD-95 and NMDA receptors. J Neurochem. 2009;110:264–74. doi: 10.1111/j.1471-4159.2009.06125.x. [DOI] [PubMed] [Google Scholar]
- Gramsbergen JB, Leegsma-Vogt G, Venema K, Noraberg J, Korf J. Quantitative on-line monitoring of hippocampus glucose and lactate metabolism in organotypic cultures using biosensor technology. J Neurochem. 2003;85:399–408. doi: 10.1046/j.1471-4159.2003.01673.x. [DOI] [PubMed] [Google Scholar]
- Greene JG, Greenamyre JT. Bioenergetics and glutamate excitotoxicity. Progress in Neurobiology. 1996;48:613–634. doi: 10.1016/0301-0082(96)00006-8. [DOI] [PubMed] [Google Scholar]
- Gurden H, Uchida N, Mainen ZF. Sensory-evoked intrinsic optical signals in the olfactory bulb are coupled to glutamate release and uptake. Neuron. 2006;52:335–45. doi: 10.1016/j.neuron.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamai M, Minokoshi Y, Shimazu T. L-Glutamate and insulin enhance glycogen synthesis in cultured astrocytes from the rat brain through different intracellular mechanisms. J Neurochem. 1999;73:400–7. doi: 10.1046/j.1471-4159.1999.0730400.x. [DOI] [PubMed] [Google Scholar]
- Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–77. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Henn FA, Anderson DJ, Rustad DG. Glial contamination of synaptosomal fractions. Brain Research. 1976;101:341–344. doi: 10.1016/0006-8993(76)90274-2. [DOI] [PubMed] [Google Scholar]
- Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice. J Neurosci. 2007;27:9736–41. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L. Functional interactions between neurons and astrocytes I. Turnover and metabolism of putative amino acid transmitters. Progress in Neurobiology. 1979;13:277–323. doi: 10.1016/0301-0082(79)90018-2. [DOI] [PubMed] [Google Scholar]
- Hertz L, Swanson RA, Newman GC, Marrif H, Juurlink BH, Peng L. Can experimental conditions explain the discrepancy over glutamate stimulation of aerobic glycolysis? Dev Neurosci. 1998;20:339–47. doi: 10.1159/000017329. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–49. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Hertz L. The Glutamate-Glutamine (GABA) Cycle: Importance of Late Postnatal Development and Potential Reciprocal Interactions between Biosynthesis and Degradation. Front Endocrinol (Lausanne) 2013;4:59. doi: 10.3389/fendo.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Xu J, Song D, Du T, Yan E, Peng L. Brain glycogenolysis, adrenoceptors, pyruvate carboxylase, Na(+),K(+)-ATPase and Marie E. Gibbs’ pioneering learning studies. Front Integr Neurosci. 2013;7:20. doi: 10.3389/fnint.2013.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Xu J, Song D, Du T, Li B, Yan E, Peng L. Astrocytic glycogenolysis: mechanisms and functions. Metab Brain Dis. 2015;30:317–33. doi: 10.1007/s11011-014-9536-1. [DOI] [PubMed] [Google Scholar]
- Hillman EM. Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci. 2014;37:161–81. doi: 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho VM, Lee JA, Martin KC. The cell biology of synaptic plasticity. Science. 2011;334:623–8. doi: 10.1126/science.1209236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–9. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmseth S, Dehnes Y, Huang YH, Follin-Arbelet VV, Grutle NJ, Mylonakou MN, Plachez C, Zhou Y, Furness DN, Bergles DE, Lehre KP, Danbolt NC. The density of EAAC1 (EAAT3) glutamate transporters expressed by neurons in the mammalian CNS. J Neurosci. 2012;32:6000–13. doi: 10.1523/JNEUROSCI.5347-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth C. The contribution of astrocytes to the regulation of cerebral blood flow. Front Neurosci. 2014;8:103. doi: 10.3389/fnins.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huageto O, Ullensveng K, Levy LM, Chaudhry FA, Honore T, Neilsen M, Lehre KP, Danbolt NC. Brain glutamate transporter proteins form homomultimers. Journal of Biological Chemistry. 1996;271:27715–27722. doi: 10.1074/jbc.271.44.27715. [DOI] [PubMed] [Google Scholar]
- Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Current Opinion in Neurobiology. 2004;14:346–352. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Hyde JC, Robinson N. Electron cytochemical localization of gamma-aminobutyric acid catabolism in rat cerebellar cortex. Histochemistry. 1976;49:51–65. doi: 10.1007/BF00490126. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–76. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Illarionova NB, Brismar H, Aperia A, Gunnarson E. Role of Na,K-ATPase alpha1 and alpha2 isoforms in the support of astrocyte glutamate uptake. PLoS One. 2014;9:e98469. doi: 10.1371/journal.pone.0098469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabaudon D, Scanziani M, Gähwiler BH, Gerber U. Acute decrease in net glutamate upatke during energy failure. Proceedings of the National Academy of Sciences USA. 2000;97:5610–5615. doi: 10.1073/pnas.97.10.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JG, O’Donnell JC, Takano H, Coulter DA, Robinson MB. Neuronal activity and glutamate uptake decrease mitochondrial mobility in astrocytes and position mitochondria near glutamate transporters. J Neurosci. 2014;34:1613–24. doi: 10.1523/JNEUROSCI.3510-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JG, O’Donnell JC, Krizman E, Robinson MB. Displacing hexokinase from mitochondrial voltage-dependent anion channel impairs GLT-1-mediated glutamate uptake but does not disrupt interactions between GLT-1 and mitochondrial proteins. J Neurosci Res. 2015;93:999–1008. doi: 10.1002/jnr.23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JG, Robinson MB. Reciprocal Regulation of Mitochondrial Dynamics and Calcium Signaling in Astrocyte Processes. J Neurosci. 2015;35:15199–213. doi: 10.1523/JNEUROSCI.2049-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M, Song W, Liu M-Y, Jin L, Dykes-Hoberg M, Lin C-LG, Bowers WJ, Federoff HJ, Sternwels PC, Rothstein JD. Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature. 2001;410:89–93. doi: 10.1038/35065091. [DOI] [PubMed] [Google Scholar]
- Jares-Erijman EA, Jovin TM. FRET imaging. Nat Biotechnol. 2003;21:1387–95. doi: 10.1038/nbt896. [DOI] [PubMed] [Google Scholar]
- Jorgensen PL, Hakansson KO, Karlish SJ. Structure and mechanism of Na,K-ATPase: functional sites and their interactions. Annu Rev Physiol. 2003;65:817–49. doi: 10.1146/annurev.physiol.65.092101.142558. [DOI] [PubMed] [Google Scholar]
- Juhaszova M, Blaustein MP. Distinct distribution of different Na+ pump alpha subunit isoforms in plasmalemma. Physiological implications. Ann N Y Acad Sci. 1997;834:524–36. doi: 10.1111/j.1749-6632.1997.tb52310.x. [DOI] [PubMed] [Google Scholar]
- Kam K, Nicoll R. Excitatory synaptic transmission persists independently of the glutamate-glutamine cycle. J Neurosci. 2007;27:9192–200. doi: 10.1523/JNEUROSCI.1198-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori K, Ross BD, Kondrat RW. Glial uptake of neurotransmitter glutamate from the extracellular fluid studied in vivo by microdialysis and (13)C NMR. J Neurochem. 2002;83:682–95. doi: 10.1046/j.1471-4159.2002.01161.x. [DOI] [PubMed] [Google Scholar]
- Kanner BI. Structure and function of sodium-coupled GABA and glutamate transporters. J Membr Biol. 2006;213:89–100. doi: 10.1007/s00232-006-0877-5. [DOI] [PubMed] [Google Scholar]
- Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–35. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- Karaca M, Frigerio F, Migrenne S, Martin-Levilain J, Skytt DM, Pajecka K, Martin-Del-Rio R, Gruetter R, Tamarit-Rodriguez J, Waagepetersen HS, Magnan C, Maechler P. GDH-Dependent Glutamate Oxidation in the Brain Dictates Peripheral Energy Substrate Distribution. Cell Rep. 2015;13:365–75. doi: 10.1016/j.celrep.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Kim K, Lee SG, Kegelman TP, Su ZZ, Das SK, Dash R, Dasgupta S, Barral PM, Hedvat M, Diaz P, Reed JC, Stebbins JL, Pellecchia M, Sarkar D, Fisher PB. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J Cell Physiol. 2011;226:2484–93. doi: 10.1002/jcp.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita N, Kimura K, Matsumoto N, Watanabe M, Fukaya M, Ide C. Mammalian septin Sept2 modulates the activity of GLAST, a glutamate transporter in astrocytes. Genes Cells. 2004;9:1–14. doi: 10.1111/j.1356-9597.2004.00696.x. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Kettenmann H, Verkhratsky A. Na+/Ca2+ exchanger modulates kainate-triggered Ca2+ signaling in Bergmann glial cells in situ. FASEB J. 1997;11:566–72. doi: 10.1096/fasebj.11.7.9212080. [DOI] [PubMed] [Google Scholar]
- Kleene R, Loers G, Langer J, Frobert Y, Buck F, Schachner M. Prion protein regulates glutamate-dependent lactate transport of astrocytes. J Neurosci. 2007;27:12331–40. doi: 10.1523/JNEUROSCI.1358-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HP, Brown RL, Larsson HP. The glutamate-activated anion conductance in excitatory amino acid transporters is gated independently by the individual subunits. J Neurosci. 2007;27:2943–7. doi: 10.1523/JNEUROSCI.0118-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–9. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Kojima S, Nakamura T, Nidaira T, Nakamura K, Ooashi N, Ito E, Watase K, Tanaka K, Wada K, Kudo Y, Miyakawa H. Optical detection of synaptically induced glutamate transport in hippocampal slices. J Neurosci. 1999;19:2580–8. doi: 10.1523/JNEUROSCI.19-07-02580.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristian T, Pivovarova NB, Fiskum G, Andrews SB. Calcium-induced precipitate formation in brain mitochondria: composition, calcium capacity, and retention. J Neurochem. 2007;102:1346–56. doi: 10.1111/j.1471-4159.2007.04626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler P, Schmitt A. Glutamate transporter EAAC! is expressed in neurons and glial cells in the rat nervous system. Glia. 1999;27:129–142. doi: 10.1002/(sici)1098-1136(199908)27:2<129::aid-glia3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Kugler P, Schleyer V. Developmental expression of glutamate transporters and glutamate dehydrogenase in astrocytes of the postnatal rat hippocampus. Hippocampus. 2004;14:975–85. doi: 10.1002/hipo.20015. [DOI] [PubMed] [Google Scholar]
- Langer J, Rose CR. Synaptically induced sodium signals in hippocampal astrocytes in situ. J Physiol. 2009;587:5859–77. doi: 10.1113/jphysiol.2009.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary GP, Stone EF, Holley DC, Kavanaugh MP. The glutamate and chloride permeation pathways are colocalized in individual neuronal glutamate transporter subunits. J Neurosci. 2007;27:2938–42. doi: 10.1523/JNEUROSCI.4851-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Rayfield A, Hryciw DH, Ma TA, Wang D, Pow D, Broer S, Yun C, Poronnik P. Na+-H+ exchanger regulatory factor 1 is a PDZ scaffold for the astroglial glutamate transporter GLAST. Glia. 2007;55:119–29. doi: 10.1002/glia.20439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencesova L, O’Neill A, Resneck WG, Bloch RJ, Blaustein MP. Plasma membrane-cytoskeleton-endoplasmic reticulum complexes in neurons and astrocytes. J Biol Chem. 2004;279:2885–93. doi: 10.1074/jbc.M310365200. [DOI] [PubMed] [Google Scholar]
- Levy LM, Warr O, Attwell D. Stoichiometry of the glial glutamte transporter GLT-1 expressed inducibly in a chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. Journal of Neuroscience. 1998;18:9620–9628. doi: 10.1523/JNEUROSCI.18-23-09620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Okamoto K, Hayashi Y, Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119:873–87. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lin C-LG, Orlov I, Ruggiero AM, Dykes-Hoberg M, Lee A, Jackson M, Rothstein JD. Modulation of the neuronal glutamate transporter EAAC1 by the interacting protein GTRAP3–18. Nature. 2001;410:84–88. doi: 10.1038/35065084. [DOI] [PubMed] [Google Scholar]
- Linden M, Gellerfors P, Nelson BD. Pore protein and the hexokinase-binding protein from the outer membrane of rat liver mitochondria are identical. FEBS Lett. 1982;141:189–92. doi: 10.1016/0014-5793(82)80044-6. [DOI] [PubMed] [Google Scholar]
- Loaiza A, Porras OH, Barros LF. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J Neurosci. 2003;23:7337–42. doi: 10.1523/JNEUROSCI.23-19-07337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan WJ, Snyder SH. High affinity uptake systems for glycine, glutamic and aspartic acids in synaptosomes of rat central nervous tissues. Brain Research. 1972;42:413–431. doi: 10.1016/0006-8993(72)90540-9. [DOI] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–66. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAskill AF, Kittler JT. Control of mitochondrial transport and localization in neurons. Trends Cell Biol. 2009;20:102–12. doi: 10.1016/j.tcb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, Fransson A, Aspenstrom P, Attwell D, Kittler JT. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61:541–55. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magi S, Lariccia V, Castaldo P, Arcangeli S, Nasti AA, Giordano A, Amoroso S. Physical and functional interaction of NCX1 and EAAC1 transporters leading to glutamate-enhanced ATP production in brain mitochondria. PLoS One. 2012;7:e34015. doi: 10.1371/journal.pone.0034015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magi S, Arcangeli S, Castaldo P, Nasti AA, Berrino L, Piegari E, Bernardini R, Amoroso S, Lariccia V. Glutamate-induced ATP synthesis: relationship between plasma membrane Na+/Ca2+ exchanger and excitatory amino acid transporters in brain and heart cell models. Mol Pharmacol. 2013;84:603–14. doi: 10.1124/mol.113.087775. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86:883–901. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Mamczur P, Borsuk B, Paszko J, Sas Z, Mozrzymas J, Wisniewski JR, Gizak A, Rakus D. Astrocyte-neuron crosstalk regulates the expression and subcellular localization of carbohydrate metabolism enzymes. Glia. 2015;63:328–40. doi: 10.1002/glia.22753. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Role of glial amino acid transporters in synaptic transmission and brain energetics. Glia. 2004;47:217–25. doi: 10.1002/glia.20027. [DOI] [PubMed] [Google Scholar]
- Marie H, Billups D, Bedford FK, Dumoulin A, Goyal RK, Longmore GD, Moss SJ, Attwell D. The amino terminus of the glial glutamate transporter GLT-1 interacts with the LIM protein Ajuba. Molecular and Cellular Neuroscience. 2002;19:152–164. doi: 10.1006/mcne.2001.1066. [DOI] [PubMed] [Google Scholar]
- Martinez-Lozada Z, Guillem AM, Flores-Mendez M, Hernandez-Kelly LC, Vela C, Meza E, Zepeda RC, Caba M, Rodriguez A, Ortega A. GLAST/EAAT1-induced glutamine release via SNAT3 in Bergmann glial cells: evidence of a functional and physical coupling. J Neurochem. 2013;125:545–54. doi: 10.1111/jnc.12211. [DOI] [PubMed] [Google Scholar]
- Massie A, Vandesande F, Arckens L. Expression of the high-affinity glutamate transporter EAAT4 in mammalian cerebral cortex. Neuroreport. 2001;12:393–7. doi: 10.1097/00001756-200102120-00041. [DOI] [PubMed] [Google Scholar]
- Massie A, Cnops L, Smolders I, McCullumsmith R, Kooijman R, Kwak S, Arckens L, Michotte Y. High-affinity Na+/K+-dependent glutamate transporter EAAT4 is expressed throughout the rat fore- and midbrain. J Comp Neurol. 2008;511:155–72. doi: 10.1002/cne.21823. [DOI] [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- Matos M, Augusto E, Santos-Rodrigues AD, Schwarzschild MA, Chen JF, Cunha RA, Agostinho P. Adenosine A2A receptors modulate glutamate uptake in cultured astrocytes and gliosomes. Glia. 2012;60:702–16. doi: 10.1002/glia.22290. [DOI] [PubMed] [Google Scholar]
- Matos M, Augusto E, Agostinho P, Cunha RA, Chen JF. Antagonistic interaction between adenosine A2A receptors and Na+/K+-ATPase-alpha2 controlling glutamate uptake in astrocytes. J Neurosci. 2013;33:18492–502. doi: 10.1523/JNEUROSCI.1828-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Research Reviews. 1990;15:41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- McGrail KM, Phillips JM, Sweadner KJ. Immunofluorescent localization of three Na,K-ATPase isozymes in the rat central nervous system: both neurons and glia can express more than one Na,K-ATPase. J Neurosci. 1991;11:381–91. doi: 10.1523/JNEUROSCI.11-02-00381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR. Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J Neurochem. 1996a;66:386–93. doi: 10.1046/j.1471-4159.1996.66010386.x. [DOI] [PubMed] [Google Scholar]
- McKenna MC, Tildon JT, Stevenson JH, Huang X. New insights into the compartmentation of glutamate and glutamine in cultured rat brain astrocytes. Dev Neurosci. 1996b;18:380–90. doi: 10.1159/000111431. [DOI] [PubMed] [Google Scholar]
- McKenna MC. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J Neurosci Res. 2007;85:3347–58. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- McKenna MC. Glutamate pays its own way in astrocytes. Front Endocrinol (Lausanne) 2013;4:191. doi: 10.3389/fendo.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melone M, Bellesi M, Conti F. Synaptic localization of GLT-1a in the rat somatic sensory cortex. Glia. 2009;57:108–17. doi: 10.1002/glia.20744. [DOI] [PubMed] [Google Scholar]
- Michaeli S, Fait A, Lagor K, Nunes-Nesi A, Grillich N, Yellin A, Bar D, Khan M, Fernie AR, Turano FJ, Fromm H. A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J. 2011;67:485–98. doi: 10.1111/j.1365-313X.2011.04612.x. [DOI] [PubMed] [Google Scholar]
- Mim C, Balani P, Rauen T, Grewer C. The glutamate transporter subtypes EAAT4 and EAATs 1–3 transport glutamate with dramatically different kinetics and voltage dependence but share a common uptake mechanism. J Gen Physiol. 2005;126:571–89. doi: 10.1085/jgp.200509365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, Castaldo P, Gobbi P, Salucci S, Magi S, Amoroso S. Cellular and subcellular localization of Na+-Ca2+ exchanger protein isoforms, NCX1, NCX2, and NCX3 in cerebral cortex and hippocampus of adult rat. Cell Calcium. 2007;41:221–34. doi: 10.1016/j.ceca.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Moore ED, Etter EF, Philipson KD, Carrington WA, Fogarty KE, Lifshitz LM, Fay FS. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature. 1993;365:657–60. doi: 10.1038/365657a0. [DOI] [PubMed] [Google Scholar]
- Motori E, Puyal J, Toni N, Ghanem A, Angeloni C, Malaguti M, Cantelli-Forti G, Berninger B, Conzelmann KK, Gotz M, Winklhofer KF, Hrelia S, Bergami M. Inflammation-induced alteration of astrocyte mitochondrial dynamics requires autophagy for mitochondrial network maintenance. Cell Metab. 2013;18:844–59. doi: 10.1016/j.cmet.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Mugnaini E. Helical Filaments in Astrocytic Mitochondria of the Corpus Striatum in the Rat. J Cell Biol. 1964;23:173–82. doi: 10.1083/jcb.23.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MS, Pedersen SE, Walls AB, Waagepetersen HS, Bak LK. Isoform-selective regulation of glycogen phosphorylase by energy deprivation and phosphorylation in astrocytes. Glia. 2015;63:154–62. doi: 10.1002/glia.22741. [DOI] [PubMed] [Google Scholar]
- Munoz MF, Puebla M, Figueroa XF. Control of the neurovascular coupling by nitric oxide-dependent regulation of astrocytic Ca(2+) signaling. Front Cell Neurosci. 2015;9:59. doi: 10.3389/fncel.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Royal C, Dupuis JP, Varela JA, Panatier A, Pinson B, Baufreton J, Groc L, Oliet SH. Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat Neurosci. 2015;18:219–26. doi: 10.1038/nn.3901. [DOI] [PubMed] [Google Scholar]
- Nagao S, Kwak S, Kanaza I. EAAT4, a glutamate transporter with properties of a chloride channel, is predominantly localized in purkinje cell dendrites, and forms parasagittal compartments in rat cerebellum. Neuroscience. 1997;78:929–933. doi: 10.1016/s0306-4522(97)00021-3. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Otsubo Y, Yatani Y, Shirakawa H, Kaneko S. Mechanisms of substrate transport-induced clustering of a glial glutamate transporter GLT-1 in astroglial-neuronal cultures. Eur J Neurosci. 2008;28:1719–30. doi: 10.1111/j.1460-9568.2008.06494.x. [DOI] [PubMed] [Google Scholar]
- Nakashima RA, Mangan PS, Colombini M, Pedersen PL. Hexokinase receptor complex in hepatoma mitochondria: evidence from N,N′-dicyclohexylcarbodiimide-labeling studies for the involvement of the pore-forming protein VDAC. Biochemistry. 1986;25:1015–21. doi: 10.1021/bi00353a010. [DOI] [PubMed] [Google Scholar]
- Namekata K, Harada C, Kohyama K, Matsumoto Y, Harada T. Interleukin-1 stimulates glutamate uptake in glial cells by accelerating membrane trafficking of Na+/K+-ATPase via actin depolymerization. Mol Cell Biol. 2008;28:3273–80. doi: 10.1128/MCB.02159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Buljan V, Else PL, Pow DV, Balcar VJ. Cardiac glycosides ouabain and digoxin interfere with the regulation of glutamate transporter GLAST in astrocytes cultured from neonatal rat brain. Neurochem Res. 2010;35:2062–9. doi: 10.1007/s11064-010-0274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Research. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–87. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno N, Kidd GJ, Mahad D, Kiryu-Seo S, Avishai A, Komuro H, Trapp BD. Myelination and axonal electrical activity modulate the distribution and motility of mitochondria at CNS nodes of Ranvier. J Neurosci. 2011;31:7249–58. doi: 10.1523/JNEUROSCI.0095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SHR, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- Otis TS, Kavanaugh MP. Isolation of current components and partial reaction cycles in the glial glutamate transporter EAAT2. J Neurosci. 2000;20:2749–57. doi: 10.1523/JNEUROSCI.20-08-02749.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Brasnjo G, Dzubay JA, Pratap M. Interactions between glutamate transporters and metabotropic glutamate receptors at excitatory synapses in the cerebellar cortex. Neurochemistry International. 2004;45:537–544. doi: 10.1016/j.neuint.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Owe SG, Marcaggi P, Attwell D. The ionic stoichiometry of the GLAST glutamate transporter in salamander retinal glia. J Physiol. 2006;577:591–9. doi: 10.1113/jphysiol.2006.116830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci. 2004;24:11273–9. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappalardo LW, Samad OA, Black JA, Waxman SG. Voltage-gated sodium channel Nav 1.5 contributes to astrogliosis in an in vitro model of glial injury via reverse Na+/Ca2+ exchange. Glia. 2014;62:1162–75. doi: 10.1002/glia.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnis J, Montana V, Delgado-Martinez I, Matyash V, Parpura V, Kettenmann H, Sekler I, Nolte C. Mitochondrial exchanger NCLX plays a major role in the intracellular Ca2+ signaling, gliotransmission, and proliferation of astrocytes. J Neurosci. 2013;33:7206–19. doi: 10.1523/JNEUROSCI.5721-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–9. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake stimulates Na+,K+-ATPase activity in astrocytes via activation of a distinct subunit highly sensitive to ouabain. J Neurochem. 1997;69:2132–7. doi: 10.1046/j.1471-4159.1997.69052132.x. [DOI] [PubMed] [Google Scholar]
- Peng L, Swanson RA, Hertz L. Effects of L-glutamate, D-aspartate, and monensin on glycolytic and oxidative glucose metabolism in mouse astrocyte cultures: further evidence that glutamate uptake is metabolically driven by oxidative metabolism. Neurochem Int. 2001;38:437–43. doi: 10.1016/s0197-0186(00)00104-2. [DOI] [PubMed] [Google Scholar]
- Perreten Lambert H, Zenger M, Azarias G, Chatton JY, Magistretti PJ, Lengacher S. Control of mitochondrial pH by uncoupling protein 4 in astrocytes promotes neuronal survival. J Biol Chem. 2014;289:31014–28. doi: 10.1074/jbc.M114.570879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit JM, Gyger J, Burlet-Godinot S, Fiumelli H, Martin JL, Magistretti PJ. Genes involved in the astrocyte-neuron lactate shuttle (ANLS) are specifically regulated in cortical astrocytes following sleep deprivation in mice. Sleep. 2013;36:1445–58. doi: 10.5665/sleep.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petr GT, Sun Y, Frederick NM, Zhou Y, Dhamne SC, Hameed MQ, Miranda C, Bedoya EA, Fischer KD, Armsen W, Wang J, Danbolt NC, Rotenberg A, Aoki CJ, Rosenberg PA. Conditional deletion of the glutamate transporter GLT-1 reveals that astrocytic GLT-1 protects against fatal epilepsy while neuronal GLT-1 contributes significantly to glutamate uptake into synaptosomes. J Neurosci. 2015;35:5187–201. doi: 10.1523/JNEUROSCI.4255-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold GC, Albeanu DF, Sato TF, Murthy VN. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron. 2008;58:897–910. doi: 10.1016/j.neuron.2008.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71:782–97. doi: 10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Pfeiffer B, Meyermann R, Hamprecht B. Immunohistochemical co-localization of glycogen phosphorylase with the astroglial markers glial fibrillary acidic protein and S-100 protein in rat brain sections. Histochemistry. 1992;97:405–12. doi: 10.1007/BF00270387. [DOI] [PubMed] [Google Scholar]
- Pfeiffer-Guglielmi B, Fleckenstein B, Jung G, Hamprecht B. Immunocytochemical localization of glycogen phosphorylase isozymes in rat nervous tissues by using isozyme-specific antibodies. J Neurochem. 2003;85:73–81. doi: 10.1046/j.1471-4159.2003.01644.x. [DOI] [PubMed] [Google Scholar]
- Pochini L, Scalise M, Galluccio M, Indiveri C. Membrane transporters for the special amino acid glutamine: structure/function relationships and relevance to human health. Front Chem. 2014;2:61. doi: 10.3389/fchem.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras OH, Ruminot I, Loaiza A, Barros LF. Na(+)-Ca(2+) cosignaling in the stimulation of the glucose transporter GLUT1 in cultured astrocytes. Glia. 2008;56:59–68. doi: 10.1002/glia.20589. [DOI] [PubMed] [Google Scholar]
- Pow DV, Barnett NL. Developmental expression of excitatory amino acid transporter 5: a photoreceptor and bipolar cell glutamate transporter in rat retina. Neuroscience Letters. 2000;280:21–24. doi: 10.1016/s0304-3940(99)00988-x. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–76. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Raju K, Doulias PT, Evans P, Krizman EN, Jackson JG, Horyn O, Daikhin Y, Nissim I, Yudkoff M, Nissim I, Sharp KA, Robinson MB, Ischiropoulos H. Regulation of brain glutamate metabolism by nitric oxide and S-nitrosylation. Sci Signal. 2015;8:ra68. doi: 10.1126/scisignal.aaa4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci. 2007;27:6607–19. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenold WT, Pratt M, Nekkalapu S, Shapiro PS, Kristian T, Fiskum G. Mitochondrial detachment of hexokinase 1 in mood and psychotic disorders: implications for brain energy metabolism and neurotrophic signaling. J Psychiatr Res. 2012;46:95–104. doi: 10.1016/j.jpsychires.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Reyes RC, Verkhratsky A, Parpura V. Plasmalemmal Na+/Ca2+ exchanger modulates Ca2+-dependent exocytotic release of glutamate from rat cortical astrocytes. ASN Neuro. 2012;4 doi: 10.1042/AN20110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Hamprecht B, Scheich H. Ultrastructural localization of glycogen phosphorylase predominantly in astrocytes of the gerbil brain. Glia. 1996;17:263–73. doi: 10.1002/(SICI)1098-1136(199608)17:4<263::AID-GLIA1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J Neurosci. 2003;23:7881–8. doi: 10.1523/JNEUROSCI.23-21-07881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts PJ, Watkins JC. Structural requirements for the inhibition for L-glutamate uptake by glia and nerve endings. Brain Research. 1975;85:120–125. doi: 10.1016/0006-8993(75)91016-1. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Roche JK, McCullumsmith RE. Localization of excitatory amino acid transporters EAAT1 and EAAT2 in human postmortem cortex: a light and electron microscopic study. Neuroscience. 2014;277:522–40. doi: 10.1016/j.neuroscience.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MB, Coyle JT. Glutamate and related acidic excitatory neurotransmitters: from basic science to clinical application. Faseb J. 1987;1:446–55. doi: 10.1096/fasebj.1.6.2890549. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Hunter-Ensor M, Sinor J. Pharmacologically distinct sodiumdependent L-[3H]glutamate transport processes in rat brain. Brain Research. 1991;544:196–202. doi: 10.1016/0006-8993(91)90054-y. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Dowd LA. Heterogeneity and functional properties of subtypes of sodium-dependent glutamate transporters in the mammalian central nervous system. Advances in Pharmacology. 1997;37:69–115. doi: 10.1016/s1054-3589(08)60948-5. [DOI] [PubMed] [Google Scholar]
- Robinson MB. The family of sodium-dependent glutamate transporters: A focus on the GLT-1/EAAT2 subtype. Neurochemistry International. 1999;33:479–491. doi: 10.1016/s0197-0186(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Rojas H, Colina C, Ramos M, Benaim G, Jaffe E, Caputo C, Di Polo R. Sodium-calcium exchanger modulates the L-glutamate Ca(i) (2+) signalling in type-1 cerebellar astrocytes. Adv Exp Med Biol. 2013;961:267–74. doi: 10.1007/978-1-4614-4756-6_22. [DOI] [PubMed] [Google Scholar]
- Rose EM, Koo JC, Antflick JE, Ahmed SM, Angers S, Hampson DR. Glutamate transporter coupling to Na,K-ATPase. J Neurosci. 2009;29:8143–55. doi: 10.1523/JNEUROSCI.1081-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011;24:943–57. doi: 10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Vandenberg RJ. A channel in a transporter. Clin Exp Pharmacol Physiol. 2005;32:1–6. doi: 10.1111/j.1440-1681.2005.04164.x. [DOI] [PubMed] [Google Scholar]
- Sato K, Otsu W, Otsuka Y, Inaba M. Modulatory roles of NHERF1 and NHERF2 in cell surface expression of the glutamate transporter GLAST. Biochem Biophys Res Commun. 2013;430:839–45. doi: 10.1016/j.bbrc.2012.11.059. [DOI] [PubMed] [Google Scholar]
- Sattler R, Rothstein JD. Regulation and dysregulation of glutamate transporters. Handb Exp Pharmacol. 2006:277–303. doi: 10.1007/3-540-29784-7_14. [DOI] [PubMed] [Google Scholar]
- Schlag BD, Vondrasek JR, Munir M, Kalandadze A, Zelenaia OA, Rothstein JD, Robinson MB. Regulation of the glial Na+-dependent glutamate transporters by cyclic AMP analogs and neurons. Molecular Pharmacology. 1998;53:355–369. doi: 10.1124/mol.53.3.355. [DOI] [PubMed] [Google Scholar]
- Schneider N, Cordeiro S, Machtens JP, Braams S, Rauen T, Fahlke C. Functional properties of the retinal glutamate transporters GLT-1c and EAAT5. J Biol Chem. 2014;289:1815–24. doi: 10.1074/jbc.M113.517177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe A. Transport and metabolism of glutamate and GABA in neurons are glial cells. Int Rev Neurobiol. 1981;22:1–45. doi: 10.1016/s0074-7742(08)60289-5. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sickmann HM, Walls AB, Bak LK, Waagepetersen HS. Functional importance of the astrocytic glycogen-shunt and glycolysis for maintenance of an intact intra/extracellular glutamate gradient. Neurotox Res. 2010;18:94–9. doi: 10.1007/s12640-010-9171-5. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sickmann HM, Bak LK, Schousboe I, Jajo FS, Faek SA, Waagepetersen HS. Neuron-glia interactions in glutamatergic neurotransmission: roles of oxidative and glycolytic adenosine triphosphate as energy source. J Neurosci Res. 2011;89:1926–34. doi: 10.1002/jnr.22746. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Scafidi S, Bak LK, Waagepetersen HS, McKenna MC. Glutamate metabolism in the brain focusing on astrocytes. Adv Neurobiol. 2014;11:13–30. doi: 10.1007/978-3-319-08894-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe I, Bro B, Schousboe A. Intramitochondrial localization of the 4- aminobutyrate-2-oxoglutarate transaminase from ox brain. Biochem J. 1977;162:303–7. doi: 10.1042/bj1620303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–43. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- Schwarz TL. Mitochondrial trafficking in neurons. Cold Spring Harb Perspect Biol. 2013;5:a011304. doi: 10.1101/cshperspect.a011304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Amara SG. Excitatory amino acid transporters: A family in flux. Annual Reviews in Pharmacology and Toxicology. 1999;39:431–456. doi: 10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- Shan D, Mount D, Moore S, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal partitioning of hexokinase 1 suggests disruption of a glutamate transport protein complex in schizophrenia. Schizophr Res. 2014;154:1–13. doi: 10.1016/j.schres.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheean RK, Lau CL, Shin YS, O’Shea RD, Beart PM. Links between L-glutamate transporters, Na+/K+-ATPase and cytoskeleton in astrocytes: evidence following inhibition with rottlerin. Neuroscience. 2013;254:335–46. doi: 10.1016/j.neuroscience.2013.09.043. [DOI] [PubMed] [Google Scholar]
- Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int. 2007;51:333–55. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–47. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Sheng ZH, Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci. 2012;13:77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Research Reviews. 2004;45:250–265. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Sickmann HM, Walls AB, Schousboe A, Bouman SD, Waagepetersen HS. Functional significance of brain glycogen in sustaining glutamatergic neurotransmission. J Neurochem. 2009;109(Suppl 1):80–6. doi: 10.1111/j.1471-4159.2009.05915.x. [DOI] [PubMed] [Google Scholar]
- Sims KD, Robinson MB. Expression patterns and regulation of glutamate transporters in the developing and adult nervous system. Critical Reviews in Neurobiology. 1999;13:169–197. doi: 10.1615/critrevneurobiol.v13.i2.30. [DOI] [PubMed] [Google Scholar]
- Song H, Lee MY, Kinsey SP, Weber DJ, Blaustein MP. An N-terminal sequence targets and tethers Na+ pump alpha2 subunits to specialized plasma membrane microdomains. J Biol Chem. 2006;281:12929–40. doi: 10.1074/jbc.M507450200. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Westergaard N, Petersen SB, Unsgard G, Schousboe A. Metabolism of [U-13C]glutamate in astrocytes studied by 13C NMR spectroscopy: incorporation of more label into lactate than into glutamine demonstrates the importance of the tricarboxylic acid cycle. J Neurochem. 1993;61:1179–82. doi: 10.1111/j.1471-4159.1993.tb03641.x. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Westergaard N, Schousboe A. Glutamate transport and metabolism in astrocytes. Glia. 1997;21:56–63. doi: 10.1002/(sici)1098-1136(199709)21:1<56::aid-glia6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Sonnewald U. Glutamate synthesis has to be matched by its degradation - where do all the carbons go? J Neurochem. 2014;131:399–406. doi: 10.1111/jnc.12812. [DOI] [PubMed] [Google Scholar]
- Spanaki C, Plaitakis A. The role of glutamate dehydrogenase in mammalian ammonia metabolism. Neurotox Res. 2012;21:117–27. doi: 10.1007/s12640-011-9285-4. [DOI] [PubMed] [Google Scholar]
- Stanley IA, Ribeiro SM, Gimenez-Cassina A, Norberg E, Danial NN. Changing appetites: the adaptive advantages of fuel choice. Trends Cell Biol. 2014;24:118–27. doi: 10.1016/j.tcb.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen TL, Gupta-Agarwal S, Kittler JT. Mitochondrial dynamics in astrocytes. Biochem Soc Trans. 2014;42:1302–10. doi: 10.1042/BST20140195. [DOI] [PubMed] [Google Scholar]
- Stephen TL, Higgs NF, Sheehan DF, Al Awabdh S, Lopez-Domenech G, Arancibia-Carcamo IL, Kittler JT. Miro1 Regulates Activity-Driven Positioning of Mitochondria within Astrocytic Processes Apposed to Synapses to Regulate Intracellular Calcium Signaling. J Neurosci. 2015;35:15996–6011. doi: 10.1523/JNEUROSCI.2068-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobart JL, Anderson CM. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Front Cell Neurosci. 2013;7:38. doi: 10.3389/fncel.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm-Mathisen J, Leknes AK, Bore AT, Vaaland JL, Edminson P, Haug FM, Ottersen OP. First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature. 1983;301:517–20. doi: 10.1038/301517a0. [DOI] [PubMed] [Google Scholar]
- Sui D, Wilson JE. Structural determinants for the intracellular localization of the isozymes of mammalian hexokinase: intracellular localization of fusion constructs incorporating structural elements from the hexokinase isozymes and the green fluorescent protein. Arch Biochem Biophys. 1997;345:111–25. doi: 10.1006/abbi.1997.0241. [DOI] [PubMed] [Google Scholar]
- Sun T, Qiao H, Pan PY, Chen Y, Sheng ZH. Motile axonal mitochondria contribute to the variability of presynaptic strength. Cell Rep. 2013;4:413–9. doi: 10.1016/j.celrep.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Shchepakin D, Kalachev LV, Kavanaugh MP. Glutamate transporter control of ambient glutamate levels. Neurochem Int. 2014;73:146–51. doi: 10.1016/j.neuint.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Susarla BTS, Robinson MB. Rottlerin, an inhibitor of protein kinase Cdelta (PKCδ), inhibits astrocytic glutamate transport activity and reduces GLAST immunoreactivity by a mechanism that appears to be PKCdelta-independent. Journal of Neurochemistry. 2003;86:635–645. doi: 10.1046/j.1471-4159.2003.01886.x. [DOI] [PubMed] [Google Scholar]
- Sutherland ML, Delaney TA, Noebels JL. Glutamate transporter mRNA expression in proliferative zones of the developing and adult murine CNS. Journal of Neuroscience. 1996;16:2191–2207. doi: 10.1523/JNEUROSCI.16-07-02191.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–23. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Yu AC, Chan PH, Sharp FR. Glutamate increases glycogen content and reduces glucose utilization in primary astrocyte culture. J Neurochem. 1990;54:490–6. doi: 10.1111/j.1471-4159.1990.tb01898.x. [DOI] [PubMed] [Google Scholar]
- Swanson RA. Astrocyte glutamate uptake during chemical hypoxia in vitro. Neurosci Lett. 1992;147:143–6. doi: 10.1016/0304-3940(92)90580-z. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Chen J, Graham SH. Glucose can fuel glutamate uptake in ischemic brain. J Cereb Blood Flow Metab. 1994;14:1–6. doi: 10.1038/jcbfm.1994.1. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Benington JH. Astrocyte glucose metabolism under normal and pathological conditions in vitro. Dev Neurosci. 1996;18:515–21. doi: 10.1159/000111448. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Liu J, Miller JW, Rothstein JD, Farrell K, Stein BA, Longuemare MC. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J Neurosci. 1997;17:932–40. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Driscoll BF, Law MJ, Sokoloff L. Role of sodium and potassium ions in regulation of glucose metabolism in cultured astroglia. Proc Natl Acad Sci U S A. 1995;92:4616–20. doi: 10.1073/pnas.92.10.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Ichikawa R, Watanabe M, Tanaka K, Inoue Y. Extra-junctional localization of glutamate transporter EAAT4 at excitatory Purkinje cell synapses. NeuroReport. 1997a;8:2461–2464. doi: 10.1097/00001756-199707280-00010. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997b;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Functions of glutamate transporters in the brain. Neurosci Res. 2000;37:15–9. doi: 10.1016/s0168-0102(00)00104-8. [DOI] [PubMed] [Google Scholar]
- Tani H, Dulla CG, Farzampour Z, Taylor-Weiner A, Huguenard JR, Reimer RJ. A local glutamate-glutamine cycle sustains synaptic excitatory transmitter release. Neuron. 2014;81:888–900. doi: 10.1016/j.neuron.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxt T, Storm-Mathisen J. Uptake of D-aspartate and L-glutamate in excitatory axon terminals in hippocampus: autoradiographic and biochemical comparisons with gamma-aminobutyrate and other amino acids in normal rats and rats with lesions. Neuroscience. 1984;11:79–100. doi: 10.1016/0306-4522(84)90215-x. [DOI] [PubMed] [Google Scholar]
- Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang XY, Xie ZJ. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell. 2006;17:317–26. doi: 10.1091/mbc.E05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres FV, Hansen F, Locks-Coelho LD. Increase of extracellular glutamate concentration increases its oxidation and diminishes glucose oxidation in isolated mouse hippocampus: reversible by TFB-TBOA. J Neurosci Res. 2013;91:1059–65. doi: 10.1002/jnr.23187. [DOI] [PubMed] [Google Scholar]
- Torres-Salazar D, Fahlke C. Neuronal glutamate transporters vary in substrate transport rate but not in unitary anion channel conductance. J Biol Chem. 2007;282:34719–26. doi: 10.1074/jbc.M704118200. [DOI] [PubMed] [Google Scholar]
- Tran CH, Gordon GR. Acute two-photon imaging of the neurovascular unit in the cortex of active mice. Front Cell Neurosci. 2015;9:11. doi: 10.3389/fncel.2015.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration. Trends in Pharmacological Sciences. 1998;19:328–334. doi: 10.1016/s0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–47. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- Ugbode CI, Hirst WD, Rattray M. Neuronal influences are necessary to produce mitochondrial co-localization with glutamate transporters in astrocytes. J Neurochem. 2014;130:668–77. doi: 10.1111/jnc.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg RJ, Ryan RM. Mechanisms of glutamate transport. Physiol Rev. 2013;93:1621–57. doi: 10.1152/physrev.00007.2013. [DOI] [PubMed] [Google Scholar]
- Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton JY, Magistretti PJ, Pellerin L. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron. 2003;37:275–86. doi: 10.1016/s0896-6273(02)01170-4. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Hansen GH, Fenger K, Lindsay JG, Gibson G, Schousboe A. Cellular mitochondrial heterogeneity in cultured astrocytes as demonstrated by immunogold labeling of alpha-ketoglutarate dehydrogenase. Glia. 2006;53:225–31. doi: 10.1002/glia.20276. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Amara SG, Kavanaugh MP. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S-i, Kawachima N, Hori S, Takimoto M, Wada K, Tanaka K. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. European Journal of Neuroscience. 1998;10:976–988. doi: 10.1046/j.1460-9568.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G, Emanuel JR, Levenson R. Cell-specific expression of mRNAs encoding Na+,K(+)-ATPase alpha- and beta-subunit isoforms within the rat central nervous system. Proc Natl Acad Sci U S A. 1991;88:7425–9. doi: 10.1073/pnas.88.16.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber B, Barros LF. The Astrocyte: Powerhouse and Recycling Center. Cold Spring Harb Perspect Biol. 2015;7 doi: 10.1101/cshperspect.a020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger E, Schwab Y, Sahel JA, Rendon A, Pow DV, Picaud S, Roux MJ. The glutamate transporter EAAT5 works as a presynaptic receptor in mouse rod bipolar cells. J Physiol. 2006;577:221–34. doi: 10.1113/jphysiol.2006.118281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw BS, Robinson MB. Inhibitors of glutamate dehydrogenase block sodium-dependent glutamate uptake in rat brain membranes. Front Endocrinol (Lausanne) 2013;4:123. doi: 10.3389/fendo.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Cai T. Na+-K+--ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv. 2003;3:157–68. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- Xu NJ, Bao L, Fan HP, Bao GB, Pu L, Lu YJ, Wu CF, Zhang X, Pei G. Morphine withdrawal increases glutamate uptake and surface expression of glutamate transporter GLT1 at hippocampal synapses. Journal of Neuroscience. 2003;23:4775–4784. doi: 10.1523/JNEUROSCI.23-11-04775.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ruchti E, Petit JM, Jourdain P, Grenningloh G, Allaman I, Magistretti PJ. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci U S A. 2014;111:12228–33. doi: 10.1073/pnas.1322912111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Yu AC, Schousboe A, Hertz L. Metabolic fate of 14C-labeled glutamate in astrocytes in primary cultures. J Neurochem. 1982;39:954–60. doi: 10.1111/j.1471-4159.1982.tb11482.x. [DOI] [PubMed] [Google Scholar]
- Yu YX, Shen L, Xia P, Tang YW, Bao L, Pei G. Syntaxin 1A promotes the endocytic sorting of EAAC1 leading to inhibition of glutamate transport. J Cell Sci. 2006;119:3776–87. doi: 10.1242/jcs.03151. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie Z. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell. 2005;16:4034–45. doi: 10.1091/mbc.E05-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler R, Zhang ZT, Manor M, Boron WF. Sodium kinetics of Na,K-ATPase alpha isoforms in intact transfected HeLa cells. J Gen Physiol. 1997;110:201–13. doi: 10.1085/jgp.110.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid H, Talior-Volodarsky I, Antonescu C, Liu Z, Klip A. GAPDH binds GLUT4 reciprocally to hexokinase-II and regulates glucose transport activity. Biochem J. 2009;419:475–84. doi: 10.1042/BJ20081319. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Ho PL, Kintner DB, Sun D, Chiu SY. Activity-dependent regulation of mitochondrial motility by calcium and Na/K-ATPase at nodes of Ranvier of myelinated nerves. J Neurosci. 2010;30:3555–66. doi: 10.1523/JNEUROSCI.4551-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–47. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke HR, Collins RM, Jr, Baab PJ, Huang Y, Zielke CL, Tildon JT. Compartmentation of [14C]glutamate and [14C]glutamine oxidative metabolism in the rat hippocampus as determined by microdialysis. J Neurochem. 1998;71:1315–20. doi: 10.1046/j.1471-4159.1998.71031315.x. [DOI] [PubMed] [Google Scholar]
- Zou S, Pita-Almenar JD, Eskin A. Regulation of glutamate transporter GLT-1 by MAGI-1. J Neurochem. 2011;117:833–40. doi: 10.1111/j.1471-4159.2011.07250.x. [DOI] [PubMed] [Google Scholar]