Summary
Purkinje cells (PCs) provide the sole output from the cerebellar cortex. Although PCs are well characterized on many levels, surprisingly little is known about their axon collaterals and their target neurons within the cerebellar cortex. It has been proposed that PC collaterals transiently control circuit assembly in early development, but it is thought that PC to PC connections are subsequently pruned. Here, we find that all PCs have collaterals in young, juvenile and adult mice. Collaterals are restricted to the parasagittal plane, and most synapses are located in close proximity to PCs. Using optogenetics and electrophysiology we find that in juveniles and adults PCs make synapses onto other PCs, molecular layer interneurons and Lugaro cells, but not onto Golgi cells. These findings establish that PC output can feed back and regulate numerous circuit elements within the cerebellar cortex and is well suited to contribute to processing in parasagittal zones.
Introduction
The cerebellum is involved in diverse motor and non-motor behaviors (Ito, 2008; Wang et al., 2014), and consequently cerebellar dysfunction can contribute to disorders such as ataxia (Manto and Marmolino, 2009) and autism spectrum disorders (Baudouin et al., 2012; Piochon et al., 2014; Tsai et al., 2012). A large portion of cerebellar research has focused on a particular circuit element, the Purkinje cell (PC), whose axons provide the sole output of the cerebellar cortex. Although much is known about the intrinsic properties of PCs and their synaptic inputs from parallel fibers, climbing fibers, and inhibitory interneurons, remarkably little is known about the prominence and function of PC axon collaterals and their synaptic connections within the cerebellar cortex (Bernard and Axelrad, 1993; Bernard et al., 1993; Bornschein et al., 2013; Watt et al., 2009). Prominent collaterals would be a major deviation from the feedforward circuitry of the cerebellar cortex, with its main flow of signals from mossy fibers (MFs) through granule cells (grCs) to PCs (Eccles et al., 1967; Marr, 1969). PC collaterals would allow the output of the cerebellar cortex to influence processing within the cerebellar cortex. PC collaterals are known to inhibit Lugaro cells (LCs), which in turn inhibit Golgi cells (GoCs) (Crook et al., 2007; Hirono et al., 2012), PC inhibition of LCs would increase GoC inhibition of grCs and provide net negative feedback to the grC layer. In contrast, inhibition of GoCs, which inhibit grCs (Crowley et al., 2009; Palay and Chan-Palay, 1974), would make the grC input layer more excitable. PC to PC synapses could serve a number of roles in adults. Elevated PC activity could feed back and suppress the output of the cerebellar cortex and thereby control the gain of PCs. Theoretical studies suggest that mutual PC to PC inhibition could allow PCs to generate prolonged responses (Maex and Steuber, 2013). In addition, it has been proposed that PC collaterals could promote local synchronous firing (de Solages et al., 2008), which could be important in the ability of PCs to regulate the activity of their targets in the deep cerebellar nuclei (Gauck and Jaeger, 2000; Person and Raman, 2012). Inhibition of molecular layer interneurons (MLIs), which in turn inhibit PCs, could indirectly influence PC excitability and PC synchrony. It is therefore important to identify the targets of PC collaterals.
Despite the many potential roles of PC collaterals, we know very little about collaterals in adults. Anatomical characterization of PC collaterals has established that PCs contact other PCs in young animals (Chan-Palay, 1971; Watt et al., 2009), and suggested that they inhibit LCs, MLIs and GoCs (Crook et al., 2007; Hámori and Szentágothai, 1968). In p9 mice, PCs have prominent collaterals that provide the primary source of inhibition to other PCs and can mediate travelling waves of activity (Watt et al., 2009). Several studies reported that some PCs have collaterals in juveniles (p30) and adults (≥ p90) (Bernard et al., 1993; Bishop, 1982; Crook et al., 2007; Hawkes and Leclerc, 1989; Larramendi and Lemkey-Johnston, 1970; O’Donoghue and Bishop, 1990), but their prevalence and targets remain largely unknown. Recent work has found functional PC to LC connections in p18–25 animals (Hirono et al., 2012), but connections to other interneuron types or PCs have not been described, and it was hypothesized that PC to PC connections are only functional in young animals (Watt et al., 2009).
Here, using a combination of anatomical, optogenetic and electrophysiological approaches to examine PC collaterals, we find that all PCs have collaterals that are confined to narrow parasagittal zones. These collaterals synapse onto essentially all PCs and LCs, and onto about a third of MLIs. Our findings show that PC collaterals are prominent in the mature cerebellum where they allow the output of the cerebellar cortex to exert feedback control over processing in the cerebellar cortex.
Results
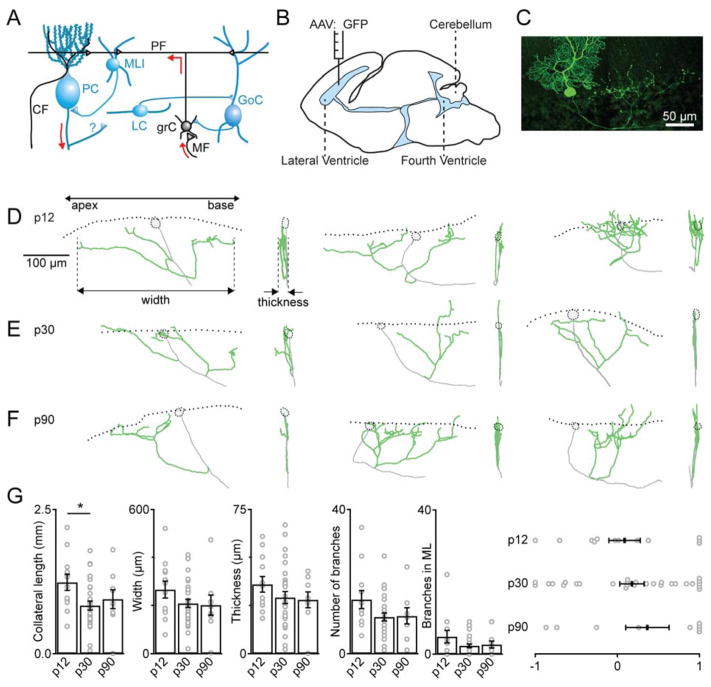
In order to evaluate the presence and properties of PC collaterals, an approach is warranted that allows visualization of the entire axonal arbor. A common approach is to fluorescently label neurons in an acute slice by whole-cell recording with a dye-filled electrode. Using this approach, however, collaterals are often severed, and axon health can be compromised. We instead labeled a small number of ineurons in the intact animal (Fig. 1C) by driving GFP expression with AAV vectors injected into the lateral ventricles of Pcp2-Cre mice at p0 (Fig. 1B). Intact PCs were identified within single parasagittal slices, allowing imaging of the entire PC collateral.
Figure 1. Purkinje cell collaterals are prominent in mice ranging from p12–p90.
A. Schematic of the circuitry of the cerebellar cortex. Mossy fiber (MF), granule cell (grC), Purkinje cell (PC), climbing fiber (CF), Parallel fiber (PF), Golgi cell (GoC), molecular layer interneuron (MLI) and Lugaro cell (LC).
B. Approach used to label PCs with GFP in p0 mice.
C. Example fluorescent image of a PC labeled with GFP from a p30 mouse.
D–F. Example reconstructions of PCs are shown for p12, p30 and p90 mice. Dotted lines are the lower edges of the molecular layer, cell bodies and main axons are indicated in gray, axon collateral is shown in green. For each cell a sagittal and transverse view is shown.
G. Summary of properties of PC collaterals. (* p < 0.05)
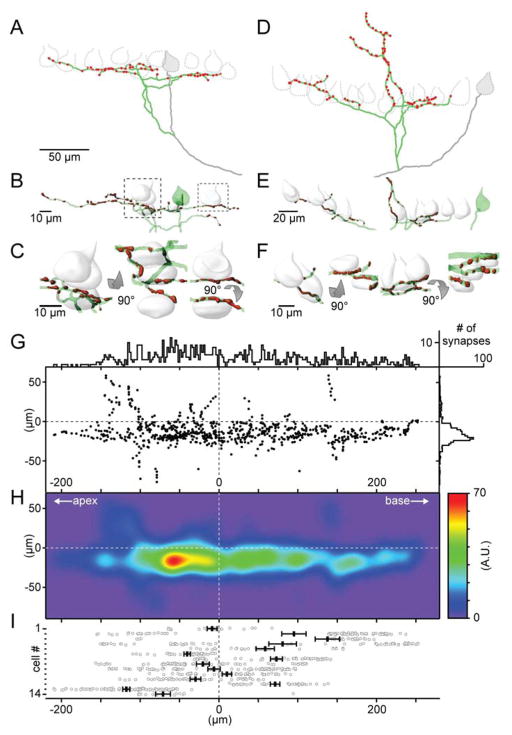
An example PC from a p30 animal shows a typical labeled PC with an axon that extends into the white matter after giving off several collateral branches (Fig. 1C). Collaterals from lobules II–IX of the cerebellar cortex were reconstructed from different age animals (p12, N=13; p30, N=28; and p90, N=9; Supplemental Figs. 1, 2). Extensive collaterals were observed in p12 animals (Fig. 1D). There were large differences in collateral properties within each age group. Collateral branches, and the total width of the arbor varied considerably, but all collaterals were confined to a narrow sagittal plane, as shown in the side views in Fig. 1D–F. Most branches reached the bottom of the PC layer, some extended to the top of the PC layer or into the molecular layer (Fig. 1D, right). In p30 and p90 animals PC collaterals showed considerable variability within each age group (Fig. 1D–G, Supplemental Figs. 1, 2), but collateral anatomy is similar between different ages, with only the total length of the collateral being significantly longer in p12 animals than in p30 and p90 animals (Fig. 1G, ANOVA p < 0.05). There was also a trend towards smaller width and thickness, and the number of branches in older animals (Fig. 1G). Collaterals were present towards both the apex and the base of the lobule (the direction the primary axon takes as it leaves the cortex and progresses towards the deep nuclei), but there was a slight directional bias such that more collaterals were present in the direction of the base that became somewhat more pronounced in older animals (Fig. 1G). Overall the qualitative impression is that collaterals are similar in p12, p30 and p90 animals, but they are slightly more complex in p12 animals than in juveniles and adults. In order to provide more insight into potential synapses made by PC collaterals, we combined single cell labelling with a transgenic approach to label PC synapses (Fig. 1). Pcp2-Cre animals crossed with conditional synaptophysin-tdTomato mice resulted in faint labeling of PC bodies, dendrites and axons, and intense labeling of presynaptic boutons (Supplemental Fig. 2A–F). By labeling individual PCs in Pcp2-Cre Ú synaptophysin-tdTomato mice, we were able to identify all presynaptic boutons of a given PC, as shown for 2 reconstructed cells (Fig. 2A–F). We reconstructed 14 cells from lobules III–VII (Supplemental Fig. 2G) in p30 mice. There is considerable variability in the location of synaptic contacts in the direction of the apex and base of the lobules (Fig. 2G–I). The total spatial range over which synaptic contacts were observed was comparable to the total width of the collateral (220 ± 35 μm vs. 210 ± 20 μm). Most synapses were near the PC layer (Fig. 2G,H). PC somata close to the GFP-labeled collateral were reconstructed and their distance to the nearest synaptic contact was determined. Our analysis shows that PC collaterals come within 1 μm of 7.1 ± 1.1 other PCs (Fig. 2C, F).
Figure 2. Purkinje cell collaterals and the location of their synaptic boutons.
A. Reconstruction of a PC collateral labelled with GFP (green) and reconstructed presynaptic bouton locations (synaptophysin-tdTomato, red).
B. Detailed 3D isometric view of the same collateral as in A. Cell bodies of contacted PCs are shown in white.
C. Zoom-in and 90-degree rotations of two collateral locations in close apposition to PCs.
D–F. Same as A–C for a different collateral reconstruction.
G. Overview of all detected puncta in relation to their position to the soma. Puncta positions are summarized in histograms along the baso-apical (top) and across the layers of the cerebellar cortex (right). The zero point on the x-axis is aligned to the soma center, while the zero point on the y-axis is aligned to the bottom the molecular layer (top of the soma).
H. Puncta were convolved with a SD = 1 μm Gaussian to obtain a heat map of all detected puncta.
I. Line plot of synaptic contacts in the baso-apical direction for fourteen reconstructed collaterals.
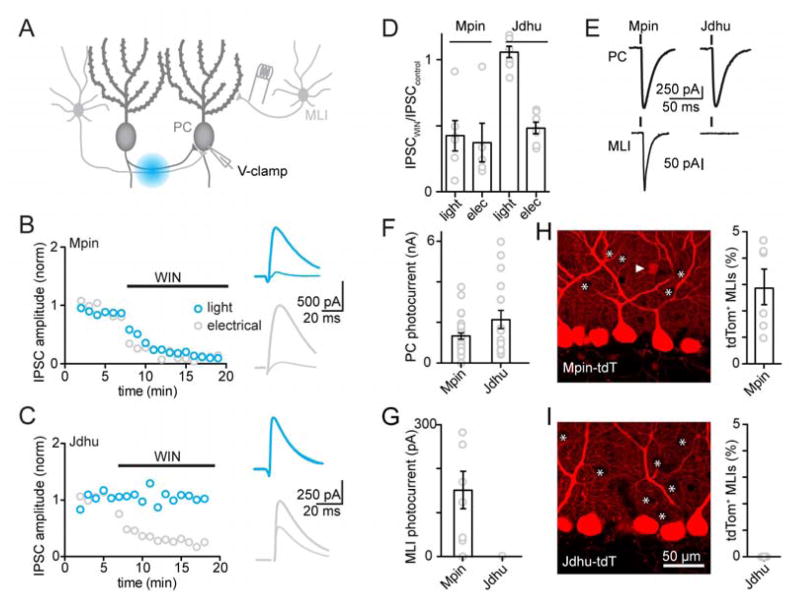
We next tested whether PC collaterals make functional synapses onto PCs, LCs, MLIs and GoCs. We used optogenetics to selectively activate PCs and record light-evoked inhibitory postsynaptic currents (IPSCs) in target neurons. This approach requires crossing a conditional Channelrhodopsin-2 (ChR2) mouse with a Cre line selective for PCs. We characterized two candidate Cre lines, the Pcp2-Cre Mpin (Barski et al., 2000) and the Pcp2-Cre Jdhu (Zhang et al., 2004) lines for expression selectivity. Our primary concern was possible nonspecific Cre expression(Lammel et al., 2015; Stuber et al., 2015).
Mice conditionally expressing ChR2 were crossed with either the Mpin or Jdhu Cre lines, and acute slices were cut. In both lines, large photocurrents were evoked in all PCs, indicating that PCs robustly express ChR2 (Fig. 3E, F). We also found that brief pulses (0.5 ms) of blue light evoked IPSCs in PCs of both lines, but it was unclear whether these IPSCs originated from PCs or from MLIs that non-specifically expressed ChR2. To distinguish between these possibilities, we used the cannabinoid type 1 receptor (CB1R) agonist WIN 55,212-2 (Fig. 3A–D). It is known that MLIs express CB1Rs and MLI to PC synapses are strongly attenuated by WIN (Kreitzer and Regehr, 2001), while PCs do not express CB1Rs. Therefore, synapses made by PCs should be insensitive to WIN. In the presence of inhibitors of excitatory and glycinergic synaptic transmission, we alternated optical stimulation just below the PC layer with electrical stimulation of the upper molecular layer, primarily activating MLI to PC synapses (Fig. 3A). We found that for the Mpin line, WIN strongly attenuated IPSCs evoked by either light or electrical stimulation (Fig. 3B, D). In contrast, WIN did not affect optically evoked IPSCs in the Jdhu line, but attenuated the electrically evoked responses (Fig. 3C, D). These experiments indicate that optically evoked IPSCs in the Jdhu line are the result of PC to PC connections, but in the Mpin line MLI to PC synapses also contribute.
Figure 3. Identification of a Cre line that allows expression restricted to Purkinje cells.
A. Schematic of the experimental approach to evaluate conditional expression of ChR2 in two different Cre lines. A Purkinje cell was recorded in whole cell configuration while synaptic inputs were either evoked with light or by electrical stimulation.
B,C. Representative experiments showing the effects of the CB1R agonist WIN on optically and electrically evoked IPSCs. B, Pcp2-Cre Mpin and C, Pcp2-Cre Jdhu line.
D. Summary of the experiments in B and C.
E. Example photocurrents measured in PCs (top traces) and MLIs (bottom traces) for the Mpin line (left) and the Jdhu line (right).
F.G. Summary of photocurrents measured in PCs and MLIs.
H, I. Confocal images of the cerebellar cortex from the Pcp2-Cre Mpin x tdTomato (top, left) and Pcp2-Cre Jdhu x tdTomato mice (bottom,left) and summary of the percentage of MLIs expressing tdTomato in the two different Cre lines (right, also see Supplementary Figure 3).
We further also tested for photocurrents in MLIs and never recorded photocurrents in the Jdhu line (0 of 55), but 21% of the MLIs (6 of 29) had photocurrents in the Mpin line (Fig. 3E, G). A cross of each Cre line with a conditional tdTomato line labelled PCs in both lines (Fig 3H, I), but some cell bodies in the molecular layer were tdTomato positive in the Mpin line (Fig. 3H, white arrow head) but not in the Jdhu line (Fig. 3I). tdTomato expression was apparent in 3 ± 0.2 % (N=3 animals, 738 neurons) of MLIs in Mpin-tdTomato mice but not by MLIs in Jdhu-tdTomato mice (N=3 animals, 705 neurons, Supplemental Fig. 3, Fig. 3HI). In Jdhu-mice photocurrents were never observed in GoCs (N=19), LCs (N=12) or grCs (N=69). To sample from a larger population of grCs we recorded from MLIs with only inhibitory transmission blocked while stimulating with light and never observed any excitatory inputs (N=28).
These experiments highlight the importance of assessing the specificity of Cre lines. The observation that for the Mpin line a fluorescent reporter was detected in 3% of MLIs, whereas a photocurrent was recorded in 21% of MLIs, suggests that functional testing is important for optogenetic studies that rely on a Cre line. We conclude the Jdhu line is suitable for our studies but the Mpin line is not.
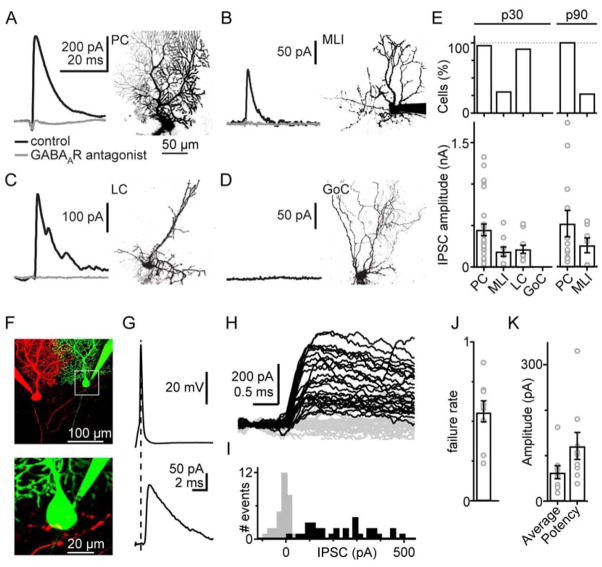
We examined synaptic contacts onto different potential PC targets using P30 Pcp2-Cre Jdhu × ChR2-EYFP mice. Optically evoked synaptic currents were observed in PCs (Fig. 4A, 28 of 29), MLIs (Fig. 4B, 9 of 29) and LCs (Fig. 4C, 11 of 12), but not in GoCs (Fig. 4D 0 of 19). Optically evoked IPSCs were blocked by the GABAAR antagonist SR95531 (5 μM, Fig. 4A, B, C, grey traces) and were reduced to 7 ± 2.6% (N=14, PCs), 7 ± 3.4% (N=5, MLIs) and 5 ± 1.1% (N=9, LCs) of the initial IPSC values. There was considerable variability in the amplitude of optically evoked IPSCs observed in the different cell types, and the average amplitude was 454 ± 71 pA in PCs (N=28), 192 ± 55 pA in MLIs (N=9) and 221 ± 48 pA in LCs (N=11; Fig. 4E). In p90 animals we found that all PCs (12 of 12) received optically evoked IPSCs (530 ±160 pA, N=12) and a fraction of MLIs (7 of 25) received inputs (266 ± 90 pA, N=7, Fig. 4E).
Figure 4. Functional identification of the synaptic targets of Purkinje cell collaterals.
A–D. Example recordings and cell identification using two-photon imaging. Responses were evoked with a brief pulse of light in the grC layer. Experiments for p30 mice are shown for: A. Purkinje cells, B. molecular layer interneurons, C. Lugaro cells, and D. Golgi cells.
E. Summary of the fraction of cells with optically evoked IPSCs (top), and IPSC size (bottom) in each cell type both for juvenile (p30) and for adult animals (p90).
F. Example of a connected PC pair (top). The presynaptic neuron was loaded with Alexa 594 (red), and the postsynaptic neuron with Alexa 488 (green). Magnified image (bottom) shows red boutons in close proximity to the green PC body.
G. Example average response from one pair. Presynaptic spike (top), postsynaptic current (bottom).
H. Example successes (black) and failures (grey).
I. Amplitude histogram of postsynaptic currents. Successes are shown in black, failures in grey.
J–K. Summary of failure rate, average synaptic current and potency of the nine connected pairs
Our findings contrast to a previous study where PC to PC synapses were not found using paired recordings in animals older than p22 (Watt et al., 2009). To resolve this discrepancy we performed recordings from pairs of PCs in juvenile animals to directly test for functional synapses between PCs. Based on our anatomical findings, we hypothesized that connections between two random PCs would be rare and that severing collaterals during the slicing procedure would further impede finding connected pairs. We therefore fluorescently labeled individual PCs (see supplemental experimental procedures) and used fluorescence to identify target PCs. We recorded from 9 pairs of synaptically connected PCs of p31–p39 animals. A typical paired recording is shown in Fig. 4F, G. The presynaptic cell was hyperpolarized to prevent spontaneous firing, action potentials were evoked with brief current steps and IPSCs were measured from the postsynaptic PC. Repeated stimulation of the presynaptic cell resulted in responses that varied in size, with clear successes and failures (Fig. 4H, I). The average IPSC amplitude was 65 ± 14 pA (N=9), the potency was 122 ± 29 pA (Fig. 4K), the success rate was 44 ± 7%, (Fig. 4J) and the latency was 0.94 ± 0.07 ms.
PC collaterals may help to synchronize activity. A recent in vivo study observed high-frequency oscillations that reflect synchronous PC firing (de Solages et al., 2008). Our observation that collaterals are prominent and functional in the adult is consistent with PC to PC connections contributing to synchrony in vivo (see Discussion and Supplemental Fig. 4). We therefore studied PC synchrony in brain slices. Although the firing of nearby PCs was rarely correlated (Supplemental Fig. 4C, D), we found that most healthy PCs had intact dendrites, but severed axons (Supplemental Fig. 4A). However, short latency correlated activity was often observed when the slice orientation preserved PC collaterals (Supplemental Fig. 4B–D). Spike triggered average synaptic currents revealed that inhibition decreases at the time of synchronous firing (Supplemental Fig. 4G, H). In many cases large spike triggered averages and correlated activity were observed even when no direct connection between the cells was apparent (although we only tested in one direction, Supplemental Fig. 4E, F). Correlated activity and spike triggered averages were both suppressed by blocking GABAA receptors. Thus, when the slice orientation preserves PC collaterals, we observed correlated activity that appears to be a network phenomenon that likely involves many PCs and possibly MLIs
Discussion
Our major finding is that PCs have prominent axon collaterals that contact neighboring PCs and local interneurons in adult mice. These findings indicate that PC collaterals are well suited to route inhibitory feedback to interneurons and PCs in the cerebellar cortex to regulate cerebellar processing, which is counter to the view that cerebellar processing is strictly feedforward.
It was surprising that all PCs, even in adults, have collaterals and receive collateral input. Moreover, we observed only a mild reduction in the complexity of axon collaterals over time (p12 vs. p30 & p90), which was much less than the overall variation within age groups. Collaterals were confined to a narrow sagittal plane, but extended hundreds of micrometers within that plane. The prominence of collaterals in adults suggests that they are functional in adults and they do not only serve a developmental role. The location of synaptic contacts in and near the PC layer, and to a lesser extent in the molecular layer, suggested that apart from the known contacts to LCs in juvenile animals (Dieudonné and Dumoulin, 2000; Hirono et al., 2012), PC collaterals might contact PCs and perhaps MLIs (Fig. 2). Functional studies established that essentially all PCs are inhibited by other PCs, both in juveniles (p30) and adults (p90). Paired recordings allowed us to measure the properties of synapses between 2 PCs. By combining the average amplitude of PC to PC connections (65 ± 14 pA) with the amplitude of light-evoked IPSCs (454 ± 71 pA) we estimate that each PC receives input from 5 – 10 other PCs (Fig. 4). This is in good agreement with our estimates of convergence from our synaptic labeling experiments in which each PC forms synaptic contacts near approximately 6–8 PCs (Fig. 2).
PC axon collateral synapses onto PCs and MLIs could regulate activity in narrow parasagittal strips, which are likely contained within broader zebrin bands that constitute functional units (Apps and Hawkes, 2009). Both PC collaterals and MLI axons are restricted to narrow parasagittal planes (Gao et al., 2006; Hawkes and Leclerc, 1989). Therefore, PC feedback regulates cerebellar activity at the output stage in these functionally delimited zones, and could potentially act to regulate the rate or timing of firing of PCs and MLIs.
PC collaterals could allow the output of the cerebellar cortex to feed back and control the gain of the cerebellar cortex. Gain control by inhibitory feedback is a common mechanism to maintain the dynamic range of neural circuits. When principal output neurons are excitatory, inhibitory feedback requires interneurons as in the cerebral cortex (Olsen et al., 2012) and hippocampus (Freund and Buzsáki, 1996). When output neurons are GABAergic as in the basal ganglia and as described here for the cerebellum, gain control can be achieved by connections between the output cells (Brown et al., 2014). If PC collaterals control the firing rate of their targets, then PC to PC connections allow PC activity to suppress the output of the cerebellar cortex. In contrast, PC to MLI synapses would have the opposite effect and would suppress inhibition of MLIs to PCs, thereby providing positive feedback. The time course and extent of feedback on PC firing rates will thus depend on collateral connectivity and the balance of direct inhibition and indirect disinhibition.
Another possibility suggested by a recent modeling study is that reciprocal inhibition between populations of PCs would allow PCs to generate firing-rate changes lasting tens of seconds (Maex and Steuber, 2013). Here we find that PC to PC connections are made towards both the apex and the base, and they could provide the requisite reciprocal connections. Thus, the anatomy of PC collaterals is compatible with a role in generating long-lasting signals.
PC collaterals could also control spike timing and synchrony. Loosely connected inhibitory networks, such as the one formed by PCs and MLIs, have also been shown to promote synchrony in other brain areas (Diba et al., 2014; Hu et al., 2011; Lagier et al., 2004). Indeed, a previous study described synchronous firing of nearby PCs and high frequency oscillations in the cerebellar cortex in vivo (de Solages et al., 2008). A mechanism that relied on PC to PC collaterals was advanced to explain these observations, but the apparent absence of PC to PC connections in adults called this mechanism into question (Watt et al., 2009). Our findings suggest that the mechanism to explain in vivo synchronous and oscillatory activity advanced by de Solonges et al. (2008) is viable. In our in vitro recordings, where even in optimal conditions the PC collateral network is not completely intact, we observed synchronous PC activity that relied on inhibitory neurotransmission and was more prominent when the network of PC collaterals was preserved (Supplemental Fig. 4). The issue of PC synchrony is important because it has been proposed that synchronously firing PCs could entrain firing in the deep cerebellar nuclei if their main axons converged onto common cerebellar nuclei neurons (De Zeeuw et al., 2011; Gauck and Jaeger, 2000; Person and Raman, 2012).
PC collaterals could also be important when climbing fibers (CFs) are activated. CFs evoke characteristic complex spikes followed by a brief pause in target PCs, whereas neighboring PCs are inhibited (Bosman et al., 2010; Schwarz and Welsh, 2001). This inhibition is thought to arise from spillover activation of MLIs (Szapiro and Barbour, 2007), but our findings suggest that PC collaterals could also contribute to surround inhibition. CF activation could also help to reset the phase of synchronously firing PCs.
The cerebellum has long been considered primarily a feed forward circuit where inputs are processed sequentially without much feedback (Eccles et al., 1967; Marr, 1969). The ubiquitous presence of PC collateral synapses within the cerebellar cortex indicates that this is not the case. Our findings establish that feedback is prominent in the adult cerebellum. Until recently, the only feedback described in the cerebellar system was that of GoC inhibition of local grCs. More recently it was shown that some neurons of the cerebellar nuclei send collaterals back up to the cerebellar cortex contacting grCs or GoCs (Ankri et al., 2015; Gao et al., 2016; Houck and Person, 2015). Here we establish that feedback is prominent and important in the cerebellum, and establish that the cerebellum is not exclusively a feedforward circuit.
Supplementary Material
Witter et al. show that in the juvenile and adult cerebellum Purkinje cells have collaterals that provide inhibitory feedback to neighboring Purkinje cells and interneurons. These collaterals could allow cerebellar output to feed back and regulate firing in parasagittal zones.
In adults all Purkinje cells have axon collaterals confined to a parasagittal plane
Collaterals contact Purkinje cells, Lugaro cells and molecular layer interneurons
Collaterals allow cerebellar output to regulate processing in parasagittal zones
Collaterals could regulate firing rate and synchrony in the cerebellum
Acknowledgments
This work was supported by NIH R01NS032405 and R01NS092707, the Nancy Lurie Marks, Goldenson, Lefler and Khodadad Foundations (W.G.R), a Goldenson and a Mahoney Fellowship to L.W., a Brooks fellowship and an NIH F32NS087708 to S.R. and NEI F32EY020718 to R.T.P. We thank the Image and Data Analysis Core (IDAC), and H.L. Elliott, for help with image analysis, the Neurobiology Imaging Facility (NINDS P30 Core Center Grant NS072030), C. Hull and the Regehr lab for comments on the manuscript, and K. McDaniels for mouse colony maintenance.
Footnotes
Experimental procedures
In all figures bars are average ± S.E.M.. Methods are described in detail in the Supplemental Experimental Procedures.
Author Contributions
L.W. and S.R. contributed equally. R.T.P. and W.G.R. conceived the experiments. L.W., S.R. and R.T.P. conducted electrophysiological experiments. L.W., S.R. and S.L. did anatomical studies. L.W., S.R., R.T.P. and S.L. conducted analysis. L.W., S.R. and W.G.R. wrote the manuscript, with contributions from all authors.
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ankri L, Husson Z, Pietrajtis K, Proville R, Léna C, Yarom Y, Dieudonné S, Uusisaari MY. A novel inhibitory nucleo-cortical circuit controls cerebellar Golgi cell activity. eLife. 2015;4 doi: 10.7554/eLife.06262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. 2009;10:670–681. doi: 10.1038/nrn2698. [DOI] [PubMed] [Google Scholar]
- Barski JJ, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis. 2000;28:93–98. [PubMed] [Google Scholar]
- Baudouin SJ, Gaudias J, Gerharz S, Hatstatt L, Zhou K, Punnakkal P, Tanaka KF, Spooren W, Hen R, De Zeeuw CI, et al. Shared Synaptic Pathophysiology in Syndromic and Nonsyndromic Rodent Models of Autism. Science. 2012;338:128–132. doi: 10.1126/science.1224159. [DOI] [PubMed] [Google Scholar]
- Bernard C, Axelrad H. Effects of recurrent collateral inhibition on Purkinje cell activity in the immature rat cerebellar cortex--an in vivo electrophysiological study. Brain Res. 1993;626:234–258. doi: 10.1016/0006-8993(93)90584-a. [DOI] [PubMed] [Google Scholar]
- Bernard C, Axelrad H, Giraud BG. Effects of collateral inhibition in a model of the immature rat cerebellar cortex: multineuron correlations. Brain Res Cogn Brain Res. 1993;1:100–122. doi: 10.1016/0926-6410(93)90016-x. [DOI] [PubMed] [Google Scholar]
- Bishop GA. The pattern of distribution of the local axonal collaterals of Purkinje cells in the intermediate cortex of the anterior lobe and paramedian lobule of the cat cerebellum. J Comp Neurol. 1982;210:1–9. doi: 10.1002/cne.902100102. [DOI] [PubMed] [Google Scholar]
- Bornschein G, Arendt O, Hallermann S, Brachtendorf S, Eilers J, Schmidt H. Paired-pulse facilitation at recurrent Purkinje neuron synapses is independent of calbindin and parvalbumin during high-frequency activation. J Physiol (Lond) 2013;591:3355–3370. doi: 10.1113/jphysiol.2013.254128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman LWJ, Koekkoek SKE, Shapiro J, Rijken BFM, Zandstra F, Van Der Ende B, Owens CB, Potters JW, De Gruijl JR, Ruigrok TJH, et al. Encoding of whisker input by cerebellar Purkinje cells. J Physiol (Lond) 2010;588:3757–3783. doi: 10.1113/jphysiol.2010.195180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Pan WX, Dudman JT. The inhibitory microcircuit of the substantia nigra provides feedback gain control of the basal ganglia output. eLife. 2014;3:e02397. doi: 10.7554/eLife.02397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V. The recurrent collaterals of Purkinje cell axons: A correlated study of the rat’s cerebellar cortex with electron microscopy and the Golgi method. Z Anat Entwicklungsgesch. 1971;134:200–234. doi: 10.1007/BF00519300. [DOI] [PubMed] [Google Scholar]
- Crook JD, Hendrickson A, Erickson A, Possin D, Robinson FR. Purkinje cell axon collaterals terminate on Cat-301+ neurons in Macaca monkey cerebellum. Neuroscience. 2007;149:834–844. doi: 10.1016/j.neuroscience.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JJ, Fioravante D, Regehr WG. Dynamics of Fast and Slow Inhibition from Cerebellar Golgi Cells Allow Flexible Control of Synaptic Integration. Neuron. 2009;63:843–853. doi: 10.1016/j.neuron.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Solages C, Szapiro G, Brunel N, Hakim V, Isope P, Buisseret P, Rousseau C, Barbour B, Léna C. High-Frequency Organization and Synchrony of Activity in the Purkinje Cell Layer of the Cerebellum. Neuron. 2008;58:775–788. doi: 10.1016/j.neuron.2008.05.008. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Hoebeek FE, Bosman LWJ, Schonewille M, Witter L, Koekkoek SK. Spatiotemporal firing patterns in the cerebellum. Nat Rev Neurosci. 2011;12:327–344. doi: 10.1038/nrn3011. [DOI] [PubMed] [Google Scholar]
- Diba K, Amarasingham A, Mizuseki K, Buzsaki G. Millisecond Timescale Synchrony among Hippocampal Neurons. J Neurosci. 2014;34:14984–14994. doi: 10.1523/JNEUROSCI.1091-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudonné S, Dumoulin A. Serotonin-Driven Long-Range Inhibitory Connections in the Cerebellar Cortex. J Neurosci. 2000;20:1837–1848. doi: 10.1523/JNEUROSCI.20-05-01837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J, Ito M, Szentagothai J. The Cerebellum as a Neuronal Machine. Springer; Berlin Heidelberg: 1967. [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gao W, Chen G, Reinert KC, Ebner TJ. Cerebellar Cortical Molecular Layer Inhibition Is Organized in Parasagittal Zones. J Neurosci. 2006;26:8377–8387. doi: 10.1523/JNEUROSCI.2434-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Proietti-Onori M, Lin Z, ten Brinke Michiel M, Boele HJ, Potters JW, Ruigrok Tom JH, Hoebeek Freek E, De Zeeuw Chris I. Excitatory Cerebellar Nucleocortical Circuit Provides Internal Amplification during Associative Conditioning. Neuron. 2016;89:645–657. doi: 10.1016/j.neuron.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauck V, Jaeger D. The Control of Rate and Timing of Spikes in the Deep Cerebellar Nuclei by Inhibition. J Neurosci. 2000;20:3006–3016. doi: 10.1523/JNEUROSCI.20-08-03006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamori J, Szentagothai J. Identification of synapses formed in the cerebellar cortex by purkinje axon collaterals: an electron microscope study. Exp Brain Res. 1968;5:118–128. doi: 10.1007/BF00238701. [DOI] [PubMed] [Google Scholar]
- Háwkes R, Leclerc N. Purkinje cell axon collateral distributions reflect the chemical compartmentation of the rat cerebellar cortex. Brain Res. 1989;476:279–290. doi: 10.1016/0006-8993(89)91248-1. [DOI] [PubMed] [Google Scholar]
- Hirono M, Saitow F, Kudo M, Suzuki H, Yanagawa Y, Yamada M, Nagao S, Konishi S, Obata K. Cerebellar globular cells receive monoaminergic excitation and monosynaptic inhibition from Purkinje cells. PLoS One. 2012;7:e29663. doi: 10.1371/journal.pone.0029663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck BD, Person AL. Cerebellar premotor output neurons collateralize to innervate the cerebellar cortex. J Comp Neurol. 2015;523:2254–2271. doi: 10.1002/cne.23787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Ma Y, Agmon A. Submillisecond Firing Synchrony between Different Subtypes of Cortical Interneurons Connected Chemically But Not Electrically. J Neurosci. 2011;31:3351–3361. doi: 10.1523/JNEUROSCI.4881-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001;21:RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier S, Carleton A, Lledo PM. Interplay between Local GABAergic Interneurons and Relay Neurons Generates γ Oscillations in the Rat Olfactory Bulb. J Neurosci. 2004;24:4382–4392. doi: 10.1523/JNEUROSCI.5570-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Steinberg Elizabeth E, Foldy C, Wall Nicholas R, Beier K, Luo L, Malenka Robert C. Diversity of Transgenic Mouse Models for Selective Targeting of Midbrain Dopamine Neurons. Neuron. 2015;85:429–438. doi: 10.1016/j.neuron.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larramendi LM, Lemkey-Johnston N. The distribution of recurrent Purkinje collateral synapses in the mouse cerebellar cortex: an electron microscopic study. J Comp Neurol. 1970;138:451–459. doi: 10.1002/cne.901380405. [DOI] [PubMed] [Google Scholar]
- Maex R, Steuber V. An integrator circuit in cerebellar cortex. Eur J Neurosci. 2013;38:2917–2932. doi: 10.1111/ejn.12272. [DOI] [PubMed] [Google Scholar]
- Manto M, Marmolino D. Cerebellar ataxias. Curr Opin Neurol. 2009;22:419–429. doi: 10.1097/WCO.0b013e32832b9897. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol (Lond) 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donoghue DL, Bishop GA. A quantitative analysis of the distribution of Purkinje cell axonal collaterals in different zones of the cat’s cerebellum: an intracellular HRP study. Exp Brain Res. 1990;80:63–71. doi: 10.1007/BF00228848. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Bortone DS, Adesnik H, Scanziani M. Gain control by layer six in cortical circuits of vision. Nature. 2012;483:47–52. doi: 10.1038/nature10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cerebellar cortex:cytology and organization. springer; 1974. [Google Scholar]
- Person AL, Raman IM. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature. 2012;481:502–505. doi: 10.1038/nature10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piochon C, Kloth AD, Grasselli G, Titley HK, Nakayama H, Hashimoto K, Wan V, Simmons DH, Eissa T, Nakatani J, et al. Cerebellar plasticity and motor learning deficits in a copy-number variation mouse model of autism. Nat Commun. 2014:5. doi: 10.1038/ncomms6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz C, Welsh JP. Dynamic Modulation of Mossy Fiber System Throughput by Inferior Olive Synchrony: A Multielectrode Study of Cerebellar Cortex Activated by Motor Cortex. J Neurophysiol. 2001;86:2489–2504. doi: 10.1152/jn.2001.86.5.2489. [DOI] [PubMed] [Google Scholar]
- Stuber Garret D, Stamatakis Alice M, Kantak Pranish A. Considerations When Using Cre-Driver Rodent Lines for Studying Ventral Tegmental Area Circuitry. Neuron. 2015;85:439–445. doi: 10.1016/j.neuron.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapiro G, Barbour B. Multiple climbing fibers signal to molecular layer interneurons exclusively via glutamate spillover. Nat Neurosci. 2007;10:735–742. doi: 10.1038/nn1907. [DOI] [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–651. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Samuel SH, Kloth Alexander D, Badura A. The Cerebellum, Sensitive Periods, and Autism. Neuron. 2014;83:518–532. doi: 10.1016/j.neuron.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AJ, Cuntz H, Mori M, Nusser Z, Sjostrom PJ, Hausser M. Traveling waves in developing cerebellar cortex mediated by asymmetrical Purkinje cell connectivity. Nat Neurosci. 2009;12:463–473. doi: 10.1038/nn.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Ng AHL, Tanner JA, Wu WT, Copeland NG, Jenkins NA, Huang JD. Highly restricted expression of Cre recombinase in cerebellar Purkinje cells. Genesis. 2004;40:45–51. doi: 10.1002/gene.20062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.