Abstract
Purpose
To develop diagnostic criteria for nonparaneoplastic autoimmune retinopathy (AIR) through expert panel consensus and to examine treatment patterns among clinical experts.
Design
Modified Delphi process.
Methods
A survey of uveitis specialists in the American Uveitis Society (AUS), a face-to-face meeting (AIR Workshop) held at the National Eye Institute (NEI), and two iterations of expert panel surveys were utilized in a modified Delphi process. The expert panel consisted of 17 experts including uveitis specialists and researchers with expertise in antiretinal antibody detection. Supermajority consensus was used and defined as 75% of experts in agreement.
Results
There was unanimous agreement among experts regarding the categorization of autoimmune retinopathies as nonparaneoplastic and paraneoplastic, including cancer-associated retinopathy (CAR) and melanoma-associated retinopathy (MAR). Diagnostic criteria and tests essential to the diagnosis of nonparaneoplastic AIR and multiple supportive criteria reached consensus.
For treatment, experts agreed that corticosteroids and conventional immunosuppressives should be used (prescribed) as 1st or 2nd line treatments, though a consensus agreed that biologics and intravenous immunoglobulin were considered appropriate in the treatment of nonparaneoplastic AIR patients regardless of the stage of disease. Experts agreed that more evidence is needed to treat nonparaneoplastic AIR patients with long-term immunomodulatory therapy and that there is enough equipoise to justify randomized, placebo-controlled trials to determine if nonparaneoplastic AIR patients should be treated with long-term immunomodulatory therapy.
Regarding antiretinal antibody detection, consensus agreed that a standardized assay system is needed to detect serum antiretinal antibodies. Consensus agreed that an ideal assay should have a two-tier design and that western blot (WB) and immunohistochemistry (IHC) should be the methods used to identify antiretinal antibodies.
Conclusions
Consensus was achieved using a modified Delphi process to develop diagnostic criteria for nonparaneoplastic AIR. There is enough equipoise to justify randomized, placebo-controlled trials to determine whether patients with nonparaneoplastic AIR should be treated with long-term immunomodulatory therapy. Efforts to develop a standardized two-tier assay system for the detection of antiretinal antibodies have been initiated as a result of this study.
Introduction
Autoimmune retinopathies are a group of inflammatory-mediated diseases characterized by the presence of antiretinal antibodies, visual field deficits, and photoreceptor dysfunction in the setting of progressive otherwise unexplained vision loss. Autoimmune retinopathies can be categorized as paraneoplastic AIR (pAIR), which includes cancer-associated retinopathy (CAR) and melanoma-associated retinopathy (MAR), or nonparaneoplastic autoimmune retinopathy in the absence of malignancy. As autoimmune retinopathy (AIR) is the preferred term for an acquired and presumed immune-mediated retinopathy due to antiretinal autoantibodies in the absence of a malignancy, we use AIR to indicate the nonparaneoplastic form of autoimmune retinopathy unless otherwise indicated.
Despite being described almost 20 years ago,1 AIR remains an ill-defined disease. The diagnosis of AIR is typically made based on the presence of antiretinal antibodies and a combination of certain clinical features, in the absence of another cause of symptoms. Although the prevalence of AIR is unknown, it is thought to be a rare entity. However, it is probable that AIR is more prevalent than thought and remains undiagnosed in many cases due to the lack of standardized diagnostic criteria and its protean clinical features that overlap with other retinal degenerative diseases. Nonetheless, it is important to rule out malignant etiologies and treatable conditions when considering the diagnosis of AIR to prevent morbidity and treatable vision loss.
While clinical features may vary considerably, commonly recognized manifestations have been identified.2,3,4,5 The presence of circulating antiretinal antibodies is considered essential to the diagnosis of AIR. While great strides have been made in the detection and measurement of antiretinal antibodies, there is no universally standardized assay for antiretinal antibody testing. Consequently, inconsistent diagnoses among institutions or physicians may result. One study compared the results of antiretinal antibody detection and measurement between two laboratories and found an overall concordance rate of any antiretinal antibodies detected to be 64% with a very poor interobserver agreement (kappa =−0.13). Further, the antiretinal antibody-specific concordance rate was a mere 36%.6 Currently, only one center in the United States provides antiretinal antibody testing commercially through a CLIA (clinical laboratory improvement amendments) certified laboratory (Ocular Immunology Laboratory, Casey Eye Institute, Oregon Health & Science University).7 To establish diagnostic criteria, promote collaboration, and advance our understanding of AIR, the development of a standardized assay to detect antiretinal antibodies is essential.8
Criteria have been proposed in the past2,3,9; however, efforts to establish comprehensive diagnostic criteria, including clinical criteria and a standardized assay system for antiretinal antibody detection, have not been documented until now. We believe the establishment of standardized diagnostic criteria and an assay system for antiretinal antibody detection is the first step towards understanding the pathogenesis of AIR. Ultimately, this will improve the management of patients with AIR. The purpose of this paper is to describe the consensus process and to report results of the consensus process among clinicians and researchers in establishing diagnostic criteria for AIR.
Methods
To develop consensus for the diagnosis of AIR, a modified Delphi process was utilized. The Delphi method is a structured communication method designed to elicit and collate the opinions of experts through anonymity, controlled feedback, statistical group response, and multiple iterations.10,11 The Delphi method was first developed by the RAND Corporation in the 1950s to forecast the impact of technology on warfare and has since been used throughout numerous healthcare fields including ophthalmology and in uveitis to build consensus among experts for the diagnosis and management of disease.10,11,12,13,14,15 In diseases where clinical evidence is lacking, this method is deemed suitable for the development of guidelines for diagnosis or management. The goal is to narrow the range of responses with each iteration to arrive at an expert consensus. Ultimately, the Delphi process allows experts to work together in a structured manner to gain a better understanding in areas where consensus is lacking. The modified Delphi process used in this study consisted of multiple rounds of surveys and a face-to-face meeting, after which structured feedback was given for each round. Experts were then encouraged to reconsider their opinion in light of the cumulative responses of other experts. This allowed experts to remain anonymous while considering the responses and opinions of the group and clarifying their opinions for others. To develop consensus, a survey of uveitis specialists in the American Uveitis Society (AUS), a face-to-face meeting (AIR Workshop) held at the National Eye Institute (NEI), and two iterations of an expert panel survey were utilized (FIGURE 1). This study was in adherence to the tenets of the Declaration of Helsinki.
FIGURE 1. Consensus Development for the Diagnosis and Management of Autoimmune Retinopathy (AIR).
A modified Delphi process was utilized to develop consensus for the diagnosis and management of AIR and consisted of multiple rounds of surveys and the AIR Workshop.
AUS: American Uveitis Society, AIR: Autoimmune retinopathy
AUS Member Survey
Consensus development began with a 10-question survey of AUS members to gauge the understanding of autoimmune retinopathies among uveitis specialists. The survey was developed electronically (Survey Monkey, Palo Alto, CA), and the survey link was posted in the AUS website forum. AUS is a subspecialty society for uveitis specialists with approximately 250 members. Only one response was allowed per computer in effort to limit multiple responses from one respondent.
AIR Workshop
Subsequently, a meeting of 40 clinicians and researchers in the field of uveitis and immunology was convened at the NIH on September 27, 2013. In the AIR Workshop meeting, the diagnosis, management, and pathophysiology of AIR were examined through presentations, expert panels, and group discussions. In addition, results of the initial AUS survey were reported. The most important questions in the field of AIR were identified through a group effort.
Expert Surveys
Based on discussions at the AIR Workshop meeting, two surveys, one clinical and one basic laboratory, were developed to further collate opinions of experts and define consensus for the diagnosis and management of AIR. The expert surveys mostly consisted of statements with 5-point Likert scales (1-strongly disagree, 2-disagree, 3-neither agree or disagree, 4-agree, 5-strongly agree). When appropriate, multiple choice, ranking, and multiple select questions were utilized in the modified Delphi process. For all items, experts were encouraged to provide comments and feedback. For the clinical survey, a summary of results from the initial AUS survey was included for experts to consider in the initial expert survey. In the subsequent expert surveys, a summary of results from the initial surveys was included for experts to consider as aligned with our modified Delphi process.
Supermajority consensus was used and defined as 75% of experts who selected either 4 (agree) or 5 (strongly agree) OR 1 (disagree) or 2 (strongly disagree) for items with Likert scales. For questions in which Likert scale was not utilized, supermajority consensus was defined as 75% of respondents who selected a given answer choice. Below in results, we use “consensus” to indicate supermajority consensus unless otherwise indicated. Due to the low number (n=6) of experts to whom the basic laboratory survey was sent (experts with basic science and laboratory expertise in antiretinal antibody detection), we also report a simple majority consensus (>50%) and indicate when doing so.
In the subsequent iterations, only questions where at least 75% of responses fell within a range of three consecutive values (i.e. 3,4,5) were included in order to obtain further consideration and consensus. Certain questions were reformulated and reiterated in the subsequent surveys based on comments and feedback from experts. Items reaching supermajority consensus were not reiterated. For example, when considering “response to treatment” as a Supportive Diagnostic Criteria in the diagnosis of AIR, four experts selected 3 (neither agree or disagree), three experts selected 4 (agree), and three experts selected 5 (strongly agree). This item did not meet supermajority consensus, but as the response rate was ≥ 75% for consecutive values of 3,4,5, the item was reiterated to be considered as Supportive Diagnostic Criteria in the subsequent iteration.
To disseminate clinical and basic laboratory survey iterations and results, a password-protected site was developed using the NIH Clinical Trial Survey System (Bethesda, MD). The clinical and basic laboratory surveys were developed using the NICHD/NIH Clinical Trials Database (CTDB) (Bethesda, MD). Unique identifiers and passwords were distributed to experts via individual emails.
Definitions
Essential Diagnostic Criteria: essential to the diagnosis of AIR and must be present to make the diagnosis of AIR.
Supportive Diagnostic Criteria: supports the diagnosis of AIR but is not necessary to make the diagnosis of AIR.
Core Diagnostic Test: essential to the diagnosis of AIR and should be performed at the initial or first diagnostic evaluation when AIR is suspected.
Results
Fifty-four uveitis specialists participated in the initial AUS survey. Seventeen experts participated in the expert surveys (one person had expertise in both clinical and basic fields). Twelve uveitis specialists participated in the initial clinical expert survey, and eleven participated in the subsequent expert survey. Six researchers participated in the initial and subsequent basic laboratory surveys. Here, we report the results of the final rounds of consensus development.
Clinical Survey
To gauge the experience of those surveyed in the final two iterations, experts (clinicians) were asked to approximate the number of patients with the diagnosis of paraneoplastic or nonparaneoplastic AIR seen in one years’ time: seven see 3–7 patients, three see >7 patients, and two see 1–3 patients.
As anticipated, there was unanimous agreement among all clinicians regarding the categorization of autoimmune retinopathies as nonparaneoplastic and paraneoplastic, including CAR and MAR.
Diagnostic Criteria
All five items considered as Essential Diagnostic Criteria in the diagnosis of AIR achieved consensus, including: 1) no apparent cause responsible for visual function abnormality such as malignancy, inflammation, infection, surgery, drug toxicity, trauma, hereditary retinal degeneration; 2) ERG abnormality with or without visual field abnormality; 3) presence of serum antiretinal antibodies; 4) absence of fundus lesions and retinal degeneration or dystrophy that may explain visual function loss; 5) absence of overt intraocular inflammation (TABLE 1).
TABLE 1.
Criteria and Tests for the Diagnosis of Autoimmune Retinopathy (AIR)
| Diagnostic Criteria for AIRa | |
| Essential Diagnostic Criteria | Supportive Diagnostic Criteria |
| No apparent cause responsible for visual function abnormalityb |
Symptoms: Photopsias or scotomas or dychromatopsia or nyctalopia or photoaversion |
| ERG abnormality (with or without visual field abnormality) |
Systemic autoimmune disease: Personal or Family History |
| Presence of serum antiretinal antibodies | Rapidity of onset of vision changee |
| Absence of fundus lesions and retinal degeneration or dystrophy that may explain visual function lossc |
|
| Absence of overt intraocular inflammationd | |
| Core Diagnostic Testsf | |
| Malignancy workup by appropriate physician | Fundus Autofluorescence (FAF) |
| Electroretinogram (ERG) | Optical Coherence Tomography (OCT) |
| Serum antiretinal antibody testing | Fluorescein Angiogram (FA) |
. All Essential Diagnostic Criteria must be present and Supportive Diagnostic Criteria are not necessary to make the diagnosis of AIR
. Including no evidence of malignancy
. Absence of chorioretinal lesions (other than incidental/small peripheral benign degenerations such as pavingstone, lattice, etc., or old toxoplasmosis scar) or absence of retinal dystrophy, retinitis pigmentosa, or other hereditary retina vitreal disorders
. Less than 1+ intraocular cells or haze present
. Acute (0–3 months) or Subacute (3–6 months)
. Essential to the diagnosis of AIR and should be performed at the initial or first diagnostic evaluation when AIR is suspected
Absence of fundus lesions and retinal degeneration or dystrophy was defined through consensus as the absence of chorioretinal lesions (other than incidental or small peripheral benign degenerations such as pavingstone, lattice, etc., or old toxoplasmosis scar) and the absence of retinal dystrophy, retinitis pigmentosa, or other hereditary retina vitreal disorders. Absence of overt intraocular inflammation was defined through consensus as less than 1+ intraocular cells (anterior chamber or vitreous) or vitreous haze.
Supportive Diagnostic Criteria for the diagnosis of AIR reaching consensus included: 1) a personal or family history of systemic autoimmune disease; 2) the presence of photopsias, scotomas, nyctalopia or photoaversion, dyschromatopsia, and 3) rapidity of onset of vision change (TABLE 1). Though rapidity of onset of vision change reached consensus as supportive criteria, defining rapidity of onset of vision change failed to reach consensus: six of eleven clinician experts selected subacute (3–6 months) and five selected acute (0–3 months) to define rapidity of onset of vision change in the subsequent expert survey. Age and response to treatment did not reach consensus to be included as Supportive Diagnostic Criteria in the diagnosis of AIR.
Experts agreed that in order to make the diagnosis of AIR, all Essential Diagnostic Criteria needed to be present. The significance of supportive criteria in making the diagnosis was not explored.
Consensus was reached on the following Core Diagnostic Tests to be performed at the initial or first diagnostic examination: malignancy workup by an appropriate physician, serum antiretinal antibody testing, electroretinogram (ERG), fluorescein angiogram (FA), fundus autofluorescence (FAF), and optical coherence tomography (OCT) (TABLE 1). Consensus could not be achieved for including the following as Core Diagnostic Tests: Goldmann visual field (GVF), Humphrey visual field (HVF), dark adaptation testing (DA), or color vision testing.
Treatment and management
To assess preferred treatment practices among experts, the clinicians were surveyed regarding specific treatments in the management of AIR patients. Clinicians unanimously agreed that steroids (local or systemic) and conventional immunosuppressives (antimetabolites or T-cell inhibitors) should be used as 1st or 2nd line treatments in the management of AIR patients. Consensus was achieved for the following treatment types to be considered appropriate regardless of the stage of disease: steroids (local or systemic), conventional immunosuppressives (such as antimetabolites or T-cell inhibitors), biologics (such as monoclonal antibodies), and intravenous immunoglobulin (IVIG) (TABLE 2). Consensus was not reached for considering plasmapheresis in the management of AIR patients.
TABLE 2.
Treatments and Follow-up Tests to consider in Autoimmune Retinopathy (AIR)
| Treatment & Management of AIR | Follow up Tests |
|---|---|
| Steroids (systemic or local)a | Electroretinography (ERG) |
| Conventional immunosuppressivesa (such as antimetabolites and T-cell inhibitors) |
Humphrey visual fields (HVF) Goldmann visual fields (GVF) |
| Biologics (such as monoclonal antibodies) | Visual acuity (VA) |
| Intravenous immunoglobulin (IVIG) | Optical Coherence Tomography(OCT) |
| Color vision testing |
. All experts agreed that steroids and conventional immunosuppressives should be considered as 1st or 2nd line in the management of AIR patients
Experts agreed that more evidence is needed when asked whether AIR patients should be treated with long-term immunomodulatory therapy. Subsequently, consensus agreed that there is enough equipoise to justify randomized, placebo-controlled trials to determine if AIR patients should be treated with long-term immunomodulatory therapy. Lastly, the follow-up testing and monitoring of AIR patients was surveyed. Consensus was reached for the following tests to be utilized to regularly follow AIR patients: ERG, HVF, GVF, visual acuity (VA), OCT, and color vision testing (TABLE 2). Experts agreed that follow-up time intervals (for these tests) for AIR patients should be three-month intervals in general; however, it was acknowledged that monitoring may differ among patients depending on the treatment types and clinical characteristics of AIR.
Consensus was not reached for repeated serum antiretinal antibodies testing or DA testing in the follow-up of AIR patients. Utility of other potentially useful tests, such as automated kinetic visual fields, was not investigated in this survey, and while ERG was considered essential to the diagnosis and follow-up of AIR, consensus was not sought on the specific type of ERG to use for the diagnosis of AIR.
Basic Laboratory Survey
Experts agreed that a diagnostic assay system to detect antiretinal antibodies should have a two-tier design. For example, experts suggested that western blot (WB) or immunohistochemistry (IHC) can be performed initially and subsequently followed by a different diagnostic method, including WB, IHC, or enzyme-linked immunosorbent assay (ELISA), to maximize sensitivity and specificity. Consensus was also reached on the methods to identify antiretinal antibodies; a consensus of experts agreed that IHC and WB should be used to detect retinal proteins in the diagnosis of AIR, while a simple majority consensus agreed that ELISA should be used.
A simple majority consensus agreed that the number of positive antiretinal antibody subtypes should have more weight towards the diagnosis of AIR. When asked whether antiretinal antibody subtypes should have differential weight towards the diagnosis of AIR, a simple majority consensus agreed that there is NOT enough evidence for or against defining this as a criterion. For example, while the presence of 3 antibodies to different antigens would be more meaningful than detecting an antibody to one antigen, we do not have enough evidence to determine whether the presence of one type of antibody (enolase) would be more meaningful than another (carbonic anhydrase) for the diagnosis of AIR. Consensus was not reached for whether an assay system should provide weight to presence of antibodies against “yet unknown” antigens. Though multiple items regarding a diagnostic assay system met consensus (simple or supermajority) (TABLE 3), multiple experts stated that more evidence and studies are needed before developing a more complex, weighted assay system.
TABLE 3.
Basic Laboratory Survey Summary: Detection of Antiretinal Antibodies for Diagnosis of Autoimmune Retinopathy (AIR)
| Basic Laboratory Survey Consensus Itemsa |
|---|
| Standardization of a diagnostic assay system is essential to understanding the role of antiretinal antibodies in the pathogenesis of AIR |
| A two-tier diagnostic assay system should be used to detect antiretinal antibodies |
| Methods to identify antiretinal antibodies should include: western blot (WB), immunohistochemistry (IHC), or enzyme-linked immunosorbent assay (ELISA)b |
| Number of positive antiretinal antibody subtypes identified should have more weight towards the diagnosis of AIR b |
| There is not enough evidence for or against determining whether antiretinal antibody subtypes should have differential weight towards the diagnosis of AIR b |
. Includes items meeting simple or supermajority consensus
. Items meeting simple majority consensus
Consensus was not reached on the ideal tissue type to use for fixation and detection of serum antiretinal antibodies: three experts selected human tissue, and three selected monkey tissue as the ideal tissue type. Experts suggested that fresh normal human tissue would be ideal, but the limited availability of human tissue may restrict its use in a standardized assay system. In addition, it was suggested that monkey tissue would allow for standardized fixation conditions and reproducible dissection for a standardized assay system. Consensus was also not reached regarding optimal dilution of patient serum; however, it was suggested that assays should be conducted for a range of dilutions due to multiple variables affecting antiretinal antibody titers. Experts acknowledged that standardization of a diagnostic assay system to detect antiretinal antibodies is essential to better understand the role of antiretinal antibodies in the pathogenesis of AIR.
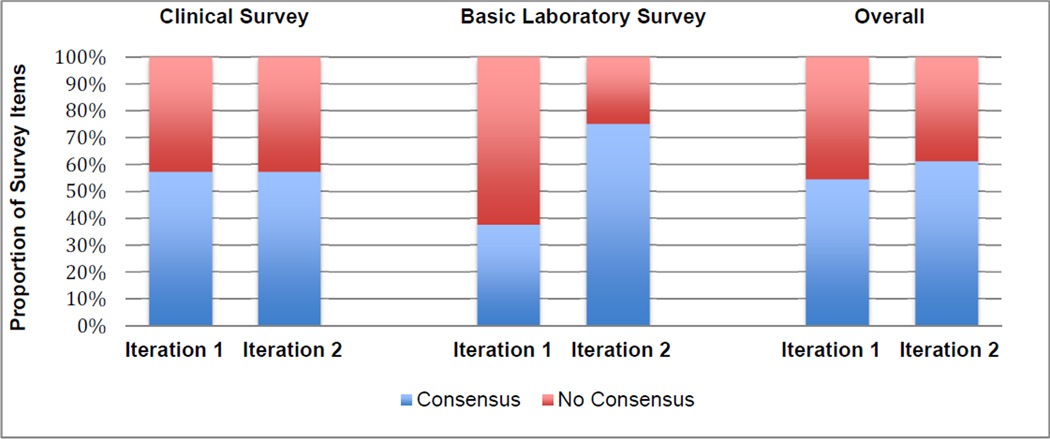
The percent of items achieving consensus between the 1st and 2nd iterations of the expert surveys remained stable and marginally increased overall (6.7%). (FIGURE 2). Percent of agreement and the median including interquartile range are included for Likert scale items used in our study and can be found in supplemental material (Supplemental Material at AJO.com).
FIGURE 2. Percent of Expert Survey Items Achieving Consensus for the Diagnosis and Management of Autoimmune Retinopathy (AIR).
Percent of items achieving consensusa between iteration 1 and 2 of the expert surveys remained stable and marginally increased overall (6.7%).
a. Supermajority consensus is used for the clinical survey, while simple majority consensus was used for the basic laboratory survey due to the smaller number of items surveyed.
Discussion
As AIR is a rare, complex disease with protean clinical features and eventual vision loss, the natural history and response to treatment is variable. There are no practice guidelines for diagnosis or management of AIR. In addition, the detection of antiretinal antibodies, whose presence is considered essential to the diagnosis by most, lacks standardization. Both the diagnosis and management rely heavily on the individual physician’s opinion. Here, we used a modified Delphi process to facilitate communication among experts to develop consensus and establish criteria for the diagnosis of AIR. The lack of standardized criteria for the diagnosis of AIR hinders our progress to understanding the pathophysiology of AIR, and developing such standardized criteria is an imperative step towards gaining a better understanding of the pathophysiology, diagnosis, and management of AIR.
This study indicates that consensus among experts can be used to establish clinical criteria for the diagnosis of AIR (TABLE 1). We recommend these criteria be used for the diagnosis of AIR to allow for meaningful comparisons of data between institutions, for creating a registry, and for future prospective multicenter trials. As previously recognized, a developed standardized assay is important to understanding the role of antiretinal antibodies in the pathogenesis of AIR and correlation with clinical outcomes.7,8 Such a standardized assay system for the detection of antiretinal antibodies has not yet been established; however, efforts to develop a standardized two-tier assay system for the detection of antiretinal antibodies have been initiated as a result of this study. Consensus was reached for core tests needed to make the diagnosis, treatment options, and follow-up guidelines. It is important to note that the panel agreed that steroids, biologic and non-biologic immunosuppressives, and IVIG should be considered in the treatment of AIR. However, the supermajority agreed that more evidence was needed for considering long-term immunomodulatory therapy and that there is currently enough equipoise to justify randomized, placebo-controlled trials to determine if AIR patients should be treated with long-term immunomodulatory therapy. As more cases of AIR are identified, future studies and collaborations will be needed to validate the proposed diagnostic criteria. Further, the diagnostic criteria and the assay systems for detection of antiretinal antibodies should be improved as the pathogenesis and natural history of AIR are further characterized in the future.
Though the Delphi process has great utility to develop guidelines for diseases where clinical evidence is lacking, there are limitations to such a study. It is a possibility that experts erroneously reinforce incorrect concepts. For this reason, it is essential to select qualified experts with utmost experience and to conduct the process in a rigorous manner. We selected experts from diverse institutions who we considered highly-qualified in uveitis and experienced in AIR. For the panel, an invitation was initially extended to over 50 clinicians and researchers from over 20 different institutions, and only those with significant exposure to this rare disease who attended the AIR Workshop were considered. In addition, to limit the potential to reinforce incorrect concepts throughout the process, great care was taken to conduct the Delphi process in a rigorous manner. Anonymity, a core feature of the Delphi method, was maintained throughout the process to limit interaction and potential biases. In addition, to ensure that the structure and content of the survey did not impose preconceptions, experts were encouraged to comment and provide feedback at every survey item. An additional limitation in the Delphi process is the limit of time and low response rates. As we could not exhaustively survey all aspects in the diagnosis and management AIR, we carefully formulated each iteration to include the most necessary items to gauge consensus while maximizing the response rate. To do so, we initially conducted the AUS survey of 54 uveitis specialists and held the AIR Workshop to identify the most necessary issues, and as a result, our response rate for all surveys was very high (97%), allowing us to maximize the Delphi process.
Though steps may be taken to conduct a rigorous and efficient Delphi study, we recognize that incorrect concepts may inevitably be propagated. We acknowledge that the criteria for the diagnosis of AIR is not definitive but encourage all to utilize this criteria as a standard to allow collaboration and gain a better understanding of AIR. It is our hope that a larger and more diverse group of experts will participate in similar consensus studies in the future to ultimately improve the diagnosis and management of AIR.
Supplementary Material
Acknowledgments
Funding/Support: This work is supported by the NEI Intramural Research Program, the NIH Office of Rare Diseases Research (ORDR), and the NIH Medical Research Scholars Program. The NIH Medical Research Scholars Program is a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Newport Foundation, The American Association for Dental Research, The Howard Hughes Medical Institute, and the Colgate-Palmolive Company, as well as other private donors. For a complete list, please visit the Foundation website at: http://fnih.org/work/education-training-0/medical-research-scholars-program.
Janet L. Davis, Consultant: AbbVie Inc (Chicago, IL); Debra A. Goldstein, Consultant: Bausch & Lomb (Bridgewater, NJ), Xoma (Berkeley, CA), Clearside Biomedical (Alpharetta, GA), Independent Data Monitoring Committee: AbbVie Inc (Chicago, IL); Careen Y. Lowder, Data and safety monitoring committee: Allergan (Dublin, Ireland); Richard W. Lee, Consultant: Roche-Genentech (South San Francisco, CA), EMD Serono (Darmstadt, Germany); Barbara Detrick, Consultant: Siemens Healthcare Diagnostics Inc (Tarrytown, NY) and Abbott Laboratories (Chicago, IL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Material available at AJO.com.
DISCLOSURES
Financial disclosures:
The following authors have no financial disclosures: Austin R. Fox, Robert B. Nussenblatt, Lynn K. Gordon, John R. Heckenlively, Nicholas J. Butler, Monica Dalal, Thiran Jayasundera, Wendy M. Smith, Grazyna Adamus, Chi-Chao Chan, John J. Hooks, Catherine W. Morgans, and H. Nida Sen.
REFERENCES
- 1.Mizener JB, Kimura AE, Adamus G, Thirkill CE, Goeken JA, Kardon RH. Autoimmune retinopathy in the absence of cancer. Am J Ophthalmol. 1997;123(5):607–618. doi: 10.1016/s0002-9394(14)71073-6. [DOI] [PubMed] [Google Scholar]
- 2.Ferreyra HA, Jayasundera T, Khan NW, He S, Lu Y, Heckenlively JR. Management of autoimmune retinopathies with immunosuppression. Arch Ophthalmol. 2009;127(4):390–397. doi: 10.1001/archophthalmol.2009.24. [DOI] [PubMed] [Google Scholar]
- 3.Sen HN, Nussenblatt RB. Autoimmune retinopathies. In: Ryan, editor. Retina. 5th. London: Elsevier; 2013. pp. 1381–1389. [Google Scholar]
- 4.Braithwaite T, Holder GE, Lee RW, Plant GT, Tufail A. Diagnostic features of the autoimmune retinopathies. Autoimmun Rev. 2014;13(4–5):534–538. doi: 10.1016/j.autrev.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 5.Larson TA, Gottlieb CC, Zein WM, et al. Autoimmune retinopathy: prognosis and treatment. Invest Ophthalmol Vis Sci. 2010;51 ARVO E-Abstract 6375. [Google Scholar]
- 6.Faez S, Loewenstein J, Sobrin L. Concordance of antiretinal antibody testing results between laboratories in autoimmune retinopathy. JAMA Ophthalmol. 2013;131(1):113–115. doi: 10.1001/jamaophthalmol.2013.574. [DOI] [PubMed] [Google Scholar]
- 7.Adamus G, Wilson DJ. The need for standardization of antiretinal antibody detection and measurement. Am J Ophthalmol. 2009;147(3):557. doi: 10.1016/j.ajo.2008.11.005. author reply 557–558. [DOI] [PubMed] [Google Scholar]
- 8.Forooghian F, Macdonald IM, Heckenlively JR, et al. The need for standardization of antiretinal antibody detection and measurement. Am J Ophthalmol. 2008;146(4):489–495. doi: 10.1016/j.ajo.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grange L, Dalal M, Nussenblatt RB, Sen HN. Autoimmune retinopathy. Am J Ophthalmol. 2014;157(2):266.e1–272.e1. doi: 10.1016/j.ajo.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376–380. doi: 10.1136/bmj.311.7001.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalkey N. The Delphi Method: an experimental study of group opinion. Rand Corporation 1969. RM-5888-PR. [Accessed March 21, 2015]; Available at http://www.rand.org/content/dam/rand/pubs/research_memoranda/2005/RM5888.pdf. [Google Scholar]
- 12.Lee PP, Sultan MB, Grunden JW, Cioffi GA IOP Consensus Panel. Assessing the Importance of IOP Variables in Glaucoma Using a Modified Delphi Process. J Glaucoma. 2010;19(5):281–287. doi: 10.1097/IJG.0b013e3181b4ca8d. [DOI] [PubMed] [Google Scholar]
- 13.Trusko B, Thorne J, Jabs D, et al. The Standardization of Uveitis Nomenclature (SUN) Project. Development of a clinical evidence base utilizing informatics tools and techniques. Methods Inf Med. 2013;52(3):259–265. S1–S6. doi: 10.3414/ME12-01-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferris FL, 3rd, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25(8):900–907. doi: 10.1097/01.ico.0000214802.40313.fa. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.