SUMMARY
Telling time and anticipating when external events will happen is among the most important tasks the brain performs. Yet, the neural mechanisms underlying timing remain elusive. One theory proposes that timing is a general and intrinsic computation of cortical circuits. We tested this hypothesis using electrical and optogenetic stimulation to determine if brain slices could “learn” temporal intervals. Presentation of intervals between 100 to 500 ms altered the temporal profile of evoked network activity in an interval and pathway-specific manner—suggesting that the network learned to anticipate an expected stimulus. Recordings performed during training revealed a progressive increase in evoked network activity, followed by subsequent refinement of temporal dynamics, which was related to a time-window specific increase in the excitatory-inhibitory balance. These results support the hypothesis that subsecond timing is an intrinsic computation, and that timing emerges from network-wide, yet pathway-specific, changes in evoked neural dynamics.
INTRODUCTION
The ability to discriminate a quarter musical note from a half note, or produce the finely timed motor patterns necessary to tap a Morse code message, are but two examples of the brain’s sophisticated ability to tell time on the subsecond scale (Ivry and Spencer, 2004; Mauk and Buonomano, 2004; Buhusi and Meck, 2005). This ability allows animals to predict and anticipate when events will occur (Medina et al., 2000; Coull and Nobre, 2008; Merchant et al., 2013). For example, we can anticipate the onset of the next note of a song, and this ability is learned in an experience-dependent manner.
Despite the importance of timing and temporal information in sensori-motor processing and learning, we remain largely ignorant of how neural circuits encode the duration and intervals of temporal events. Based on psychophysical and pharmacological data, it is most likely the case that there are multiple neural mechanisms that code for temporal structure of sensory events since they are timed over a broad range of time scales, ranging from microseconds to days (Mauk and Buonomano, 2004; Buhusi and Meck, 2005). In this study we are focused on understanding the neural mechanisms in cortical networks that encode time in the subsecond range.
Traditionally, there have been two broadly defined opposing theories of how the brain tells time, namely, dedicated and intrinsic models (Ivry and Schlerf, 2008). Dedicated models propose that timing relies on specialized and centralized neural mechanisms, whereas intrinsic models suggest that timing is a general computation that most neural circuits can perform. A strong test of the intrinsic hypothesis is that even cortical circuits in vitro, might be able to tell time, and even “learn” simple temporal patterns. Several studies, have suggested that in vitro circuits can indeed, perform analogs of certain computations, including timing (Johnson et al., 2010; Chubykin et al., 2013) and pattern recognition (Hyde and Strowbridge, 2012; Dranias et al., 2013; Ju et al., 2015). Such in vitro approaches offer a powerful strategy to understand network-level computations in a reduced system—and thus unambiguously determining that the learning is occurring in, and constrained to, the local network. Here, using a novel protocol consisting of electrical and optogenetic stimulation, we examined how temporal interval learning emerges from local network dynamics and provide some of the first evidence for the network mechanisms involved.
Our results demonstrate that organotypic cortical slices exhibit temporally specific network plasticity wherein internal dynamics is modified to reflect the trained temporal interval. This plasticity is network wide and relies in part on temporally selective alterations in the balance of excitation-inhibition. Furthermore our results suggest that the effects of training are manifested in the development and establishment of reproducible neural trajectories. Our results provide strong support for the hypothesis that timing and temporal processing (on the subsecond scale), are computations that are intrinsic to local neural networks.
RESULTS
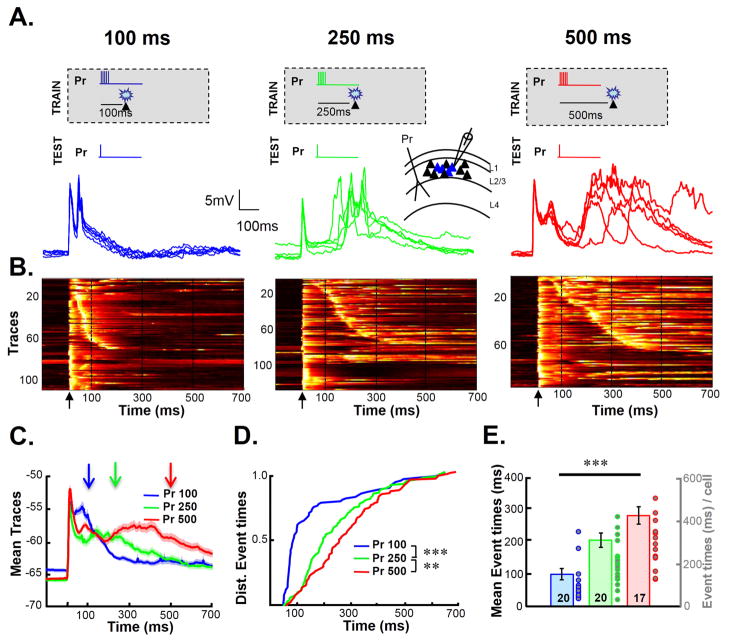
Cortical organotypic slices were chronically “implanted” with a stimulating electrode (Johnson and Buonomano, 2009) and transfected with an adeno-associated virus (AAV) expressing ChR2-EYFP (Figure S1). Whole-cell recordings from L-II/III pyramidal neurons revealed a range of light evoked responses, including subthreshold depolarization and spikes (Figure S1D). Slices transfected with ChR2 underwent an interval training protocol in which electrical stimulation was paired with optogenetic stimulation. This novel training protocol allowed us to record from neurons that were directly optically stimulated during training. The interval between electrical stimulation (the paired pathway, Pr) and the light pulse was either 100, 250, or 500 ms (Figure 1A). Electrical stimulation consisted of 100 Hz burst of 5 pulses and optical stimulation was a 25 ms flash of light. After 4 hours of training (720 pairings of electrical and optical stimulation), whole-cell recordings were performed from layer-II/III ChR-positive (ChR+) pyramidal neurons (we are defining cells as ChR+ based on their direct responsivity to light). To determine if training shaped the timing of network activity, evoked responses to electrical stimulation alone was recorded. Consistent with previous studies in naive slices, electrical stimulation elicited an isolated monosynaptic EPSP, or a monosynaptic EPSP followed by polysynaptic activity (Johnson and Buonomano, 2007). Polysynaptic activity is the result of indirect activation of neurons in the network due to intrinsic network dynamics of recurrent cortical networks. This polysynaptic waveform obtained from whole-cell recordings, provides a natural readout of network activity (Buonomano, 2003). Figure 1B shows the normalized voltagegrams from all test trials across all cells (n=21/12, 25/14, and 18/10 cells/slices, in the 100, 250, and 500 ms groups, respectively). The differences in the slope of the diagonal band of the voltagegrams suggest that the timing of the network activity was dependent on the Pr-light interval the slice experienced during training. Thus indicating that the network activity evoked by the same stimulus differed across groups. Averaging across all the traces in which polysynaptic activity was present, revealed that despite the significant trial-by-trial variability of evoked polysynaptic activity (Buonomano, 2003; Luczak et al., 2007; Sadovsky and MacLean, 2014), there were differences in the averaged traces (Figure 1C). To quantify the differences in the temporal structure of the activity we examined the distribution of times of the polysynaptic events (Figure 1D). There was a significant difference between the three groups: the distribution was progressively right shifted (longer) with the increasing training intervals (100 × 250, P=10−23; 250 × 500; K-S test, P=0.007). The median time of the evoked polysynaptic events in each cell was also significantly different (Figure 1E): the mean median latencies were: 98±18, 203±22, and 279±27 ms for the 100, 250, and 500 ms groups, respectively (Kruskal-Wallis test, χ22,54=24.9, P=3.9*10−6; Mann-Whitney test between Pr 100 and Pr 250, P = 10−4; Pr 250 vs. Pr 500 P = 0.03). There was no difference in the intrinsic electrical properties of cells across groups (Supplementary Procedures).
Figure 1. The temporal profile of network activity is dependent on training interval.
(A) Slices were trained for 4 hours by pairing electrical stimulation of the paired (Pr) pathway followed and optical stimulation, at an interval of either 100 ms (top left panel), 250 ms (middle), or 500 ms (right). Inset: Schematic of implanted electrodes and recording site. Five traces from a cell in each group in response to the Pr pathway after training (bottom panels).
(B) Raw data across all cells and trials (5 traces from each cell) from the 100 (left panel), 250 (middle panel), and 500 ms (right panel) groups. Voltagegram traces are normalized to their own peak and sorted according to latency of the first polysynaptic peak (i.e, the first peak after the initial response) if present. Voltage is represented on a color scale where black is the minimum and white the maximum. Arrows represent the time of the test stimulus, and dashed lines are presented to provide a better comparison of the timing across different panels.
(C) Mean ± s.e.m. (shading) of all traces (that exhibited polysynaptic activity) from the three training-interval groups. Arrows indicate the time of light delivery during training. Note that the timing of the polysynaptic peak in each waveform increases with the training interval.
(D) Cumulative distributions of polysynaptic event times.
(E) The group means of median polysynaptic event times of each cell.
Training dependent changes in network activity is pathway sensitive
The robust difference in the evoked temporal dynamics as a function of the training interval demonstrates that the temporal profile of activity within a circuit adapts to the temporal structure of the stimuli it was exposed to. Such temporal learning effects could arise from either cellular or network mechanisms (Mauk and Buonomano, 2004). Cell autonomous mechanisms include those in which timing can be attributed to changes in cellular or synaptic time constants (Margoliash, 1983; Saitoh and Suga, 1995; Fiala et al., 1996; Hooper et al., 2002). A network mechanism would include one in which timing emerges from the intrinsic dynamics of recurrent circuits—e.g., a changing pattern of neural activity (Mauk and Buonomano, 2004; Buonomano and Laje, 2010).
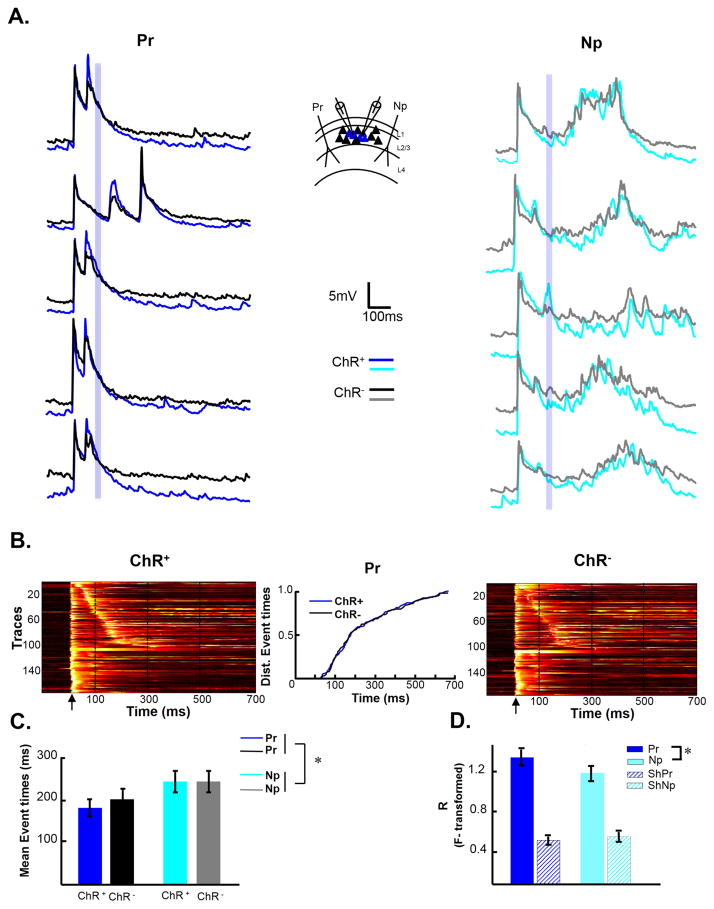
As a first step towards addressing whether this temporally-specific plasticity is most consistent with a cellular or network mechanism, we examined if the training-dependent changes in timing were pathway specific. In these experiments slices were implanted with two stimulating electrodes. One of the electrodes delivered electrical stimulation that was paired with optical stimulation at an interval of 100 ms (Pr pathway). The second electrode was activated 5–10 s after electrical stimulation from the Pr electrode and was not followed by optical stimulation (unpaired pathway, Np) (Figure S2A). Recordings made from ChR+ neurons following 2–4 hours of training, revealed significant pathway-sensitive differences. The voltagegrams of the activity elicited by the Pr and Np pathways appeared qualitatively different (Figure S2B). This was confirmed by multiple measures: differences in the distribution of polysynaptic event times (Figure S2C, K-S test, P=10−7), median time of evoked polysynaptic events (Figure S2D, Pr 100: 192±24 ms, Np 100: 288±35 ms, Mann-Whitney test, P=0.02), and the likelihood of eliciting network activity (Figure S2E)—specifically the mean number of polysynaptic events per cell elicited by the Pr and Np pathways was 1.72±0.3 and 0.95±0.2, respectively (Mann-Whitney test, P=0.002, n=40 cells). Thus training resulted in a form of in-vitro pathway-sensitive learning, in which the Pr pathway was more likely to elicit polysynaptic activity, and the timing of this activity was closer to the trained interval as compared to the Np pathway.
Temporally specific plasticity is expressed network wide
We reasoned that cell autonomous timing mechanisms would be more likely to be constrained to those cells directly stimulated by light. Whereas network mechanisms would likely alter the activity patterns of both ChR+ and ChR−. We thus examined the evoked responses in simultaneously recorded ChR+ and ChR− neurons after training with a 100 ms interval. Representative traces from ChR+ (blue traces) and ChR− (black traces) pairs from a single experiment indicate that the temporal profile of both populations of cells are similar—for example note the prominent polysynaptic peak around 100 ms in both the blue and black traces (Figure 2A). Group data reveal a similar distribution in evoked neural trajectories in both ChR+ and ChR− cells as quantified by the lack of significant difference in the temporal distribution of the evoked polysynaptic event times between ChR+ and ChR− neurons (K-S test, P=0.95) (Figure 2B), as well as of the mean polysynaptic event times across cells (two-way ANOVA, repeated measures on cell and pathway factors; cell factor: F1,94=0.16, P=0.69) (Figure 2C). But there was, again, a difference in the mean median times of events evoked by the Pr and Np pathways (Kruskal-Wallis test, χ21,96 = 6.07, P=0.01).
Figure 2. Simultaneous recording of ChR+ and ChR− cells suggests that temporally-specific plasticity of neural dynamics is network wide.
(A) Slices were trained with a 100 ms interval. Sample traces from the same simultaneously recorded ChR+ and ChR− cells in response to Pr (left) and Np (right) pathway. Inset: Schematic of implanted electrodes and recording site. Blue shading indicates the time window in which optical stimulation occurred during training.
(B) Voltagegrams of Pr evoked responses recorded simultaneously from ChR+ (left panel) and ChR− cells (right panel). Traces in both panels are sorted according to first polysynaptic event in the ChR+ cells. Middle panel. Cumulative distribution of event times from the Pr pathway between the ChR+ and ChR− cells.
(C) Average median event times of ChR+ and ChR− cells.
(D) Mean correlation coefficients of the activity between the ChR+ and ChR− cells in response to the Pr (blue) and Np (cyan) pathways. There was no significant difference in correlation between pathways when the data was shuffled (ShPr and ShNp; see Supplemental Procedures).
The simultaneous recording of ChR+ and ChR− neurons also allowed us to ask if there was a difference in the between neuron correlation of the PSP waveforms evoked by the Pr and Np pathways. One hypothesis is that training results in a differential “burning in” of the patterns of neural activity elicited by the Pr compared to the Np pathway—and thus that the activity between neurons would be more correlated when evoked by the Pr pathway. Indeed the mean cell correlations between ChR+ and ChR− cells were higher in response to activity evoked by Pr compared to the Np pathway (Fisher-transformed r values of 1.28±0.08 (Pr pathway) and 1.11±0.08 (Np pathway); paired t-test, t=2.6, P=0.02). A concern with this analysis is that the differences in correlations could be a result of overall differences in activity, e.g., the correlation in the Np pathway could be smaller because it elicited less polysynaptic activity. Thus, we also compared the correlations calculated by shuffling across cells. There was no significant difference in the mean shuffled ChR+/ChR− correlations between the Pr and Np pathways, indicating that the difference between pathways was not produced by differences in overall activity (Figure 2D). A cell autonomous timing mechanism would be more likely to generalize across pathways and less likely to generalize to the ChR− cells. Thus the pathway sensitivity together with the lack of cell specificity is suggestive of a network-based timing mechanism. Furthermore, it is important to note that the shallower angle of the diagonal band of the voltagegrams from the 100 to 500 ms groups (Figure 1B) is also expected from a network based mechanism: for a cell to exhibit a large polysynaptic response at 250 ms, a presynaptic neuron must fire shortly before, say at 240 ms, and for this cell to fire at 240 ms, another neuron would have to fire at 230 ms, and so forth. And finally, it would be difficult to account for the pathway-specific increase in the correlation of network activity in response to the Pr pathway, if learning was based on cell autonomous mechanisms. Nevertheless it was unexpected that there was no difference between the temporal profile of activity in ChR+ and ChR− neurons. It is possible that this is because within a recurrent network light will indirectly depolarize ChR− neurons.
Reproducible neural trajectories emerge over the course of training
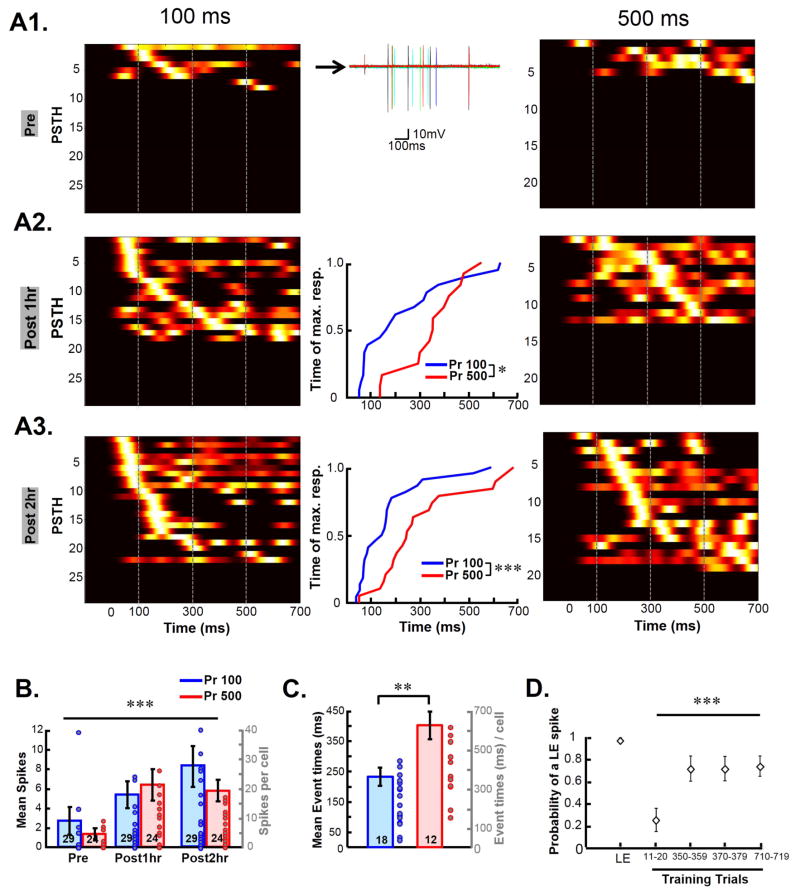
A timing mechanism based on the internal dynamics of a recurrent network requires that electrical stimulation elicit polysynaptic activity—i.e, neurons monosynaptically (or antidromically) activated by electrical stimulation must produce suprathreshold activity in other neurons in the network, leading to further self-perpetuating activity for a period of a few hundred milliseconds. We routinely observed that naïve slices were much less likely than trained slices to exhibit any evoked polysynaptic activity. Hence we next examined how network activity and timing emerged over the course of a training session. Training was performed on the rig while recording in cell-attached mode to monitor neurons over hours and avoid intracellular dialysis. Two groups of slices were trained on intervals of 100 and 500 ms (Figure 3A). In both groups the majority of neurons did not exhibit evoked spikes in response to the Pr pathway before training (Pre); but tests at one and two hours into training, revealed a progressive increase in polysynaptically evoked spikes (Figure 3B; training-time effect, Friedman test, F2,156 =24.8, P=10−6). The increase in polysynaptic activity was restricted to the first hour of training followed by a progressive refinement in the timing of the spikes over the second hour of training, as indicated by a significant difference in the median spike times between the 100 and 500 ms groups at 2-hr (Figure 3C; 100 ms Group: 231±34 ms, 500 ms Group: 359±59 ms, t-test, P=0.008), but not at 1-hr. This training dependent temporal refinement was absent in spikes elicited by the Np pathway (data not shown).
Figure 3. Emergence of interval specific network dynamics during the course of training.
(A) Slices were trained with either a 100 (left) or 500 (right) ms interval on the rig. Activity was monitored during training using cell-attached recordings. Each line of the plot represents the Pr-pathway evoked spikes of a cell as a PSTH (normalized and sorted within each panel) see Supplemental Procedures). (A1) Pr-evoked responses before the start of training (Pre). Middle panel shows sample traces from a single cell included in the PSTH (row 4) (A2) Pr-evoked responses after 1 hour of training (Post 1hr). Middle panel: Cumulative distribution of maximum peak of PSTH for the 100 vs. 500 ms trained group at the 1 hour time point (K-S test, P = 0.04). (A3) Evoked responses after 2 hours of training (Post 2hr). Inset: K-S test, P = 0.002.
(B) Number of polysynaptic spikes evoked by the Pr pathway in the 100 ms and 500 ms trained group.
(C) Group means of the median polysynaptic spike times of each cell.
(D) Increase in light evoked spike probability during training. The first point (LE) is the probability of light alone eliciting a spike before training. Subsequent points reflect the probability of a light evoked spike during training trials (i.e., light preceded by electrical stimulation). Note that between the 350–359 and 370–379 trials there was a testing block.
In a separate set of experiments slices were stimulated with electrical stimulation alone or similar to previous experiments electrical stimulation was paired with optical stimulation at 250 ms. In both groups evoked activity elicited similar levels of polysynaptic activity. However there was a significant difference in the timing of this activity (Figure S3C, S3D). Together these results suggest that over the course of training electrical stimulation increases network activity, which can be later refined by optical stimulation.
Mechanisms of Temporally specific plasticity
The progressive increase in polysynaptic activity over the course of training could arise from a number of mechanisms including decreased inhibition or increased excitation. Analysis of the light-evoked responses during training in our cell-attached data suggested that the increase in network activity was in part a result of a decrease in evoked inhibition. Specifically, in many cells the light pulse by itself elicited spikes in the cell-attached recordings; but at the beginning of training, there was a dramatic decrease in the probability of light evoked spikes (Figure 3D; Friedman test, Q3,39 = 9, P = 0.0006, n=16)—presumably as a result of electrically evoked inhibition. Over the course of training, however, the likelihood of light-evoked spikes increased, raising the hypothesis that an initial step in learning was a nonspecific decrease in inhibition.
A nonspecific decrease in inhibition is unlikely to account for the temporal specificity observed in Figure 1. Thus we examined the dynamic changes in the balance of excitation and inhibition by clamping cells at −53 mV or 10 mV, to estimate the excitatory and inhibitory currents, respectively. This approach however requires comparing the mean excitatory and inhibitory currents estimated on different trials, and thus benefits from trials with less variability and fewer failures (no polysynaptic activity elicited). Since the above results indicated that a decrease in inhibition is important for the generation of polysynaptic activity we trained slices on a 250 ms interval in the presence of a low dose (200 nM) of the GABAB antagonist CGP-55845. The use of a GABAB antagonist provided a means to increase network activity (Scanziani et al., 1994; Mann et al., 2009) without directly blocking GABAA receptors—which are critical to the standard measures of the balance of excitation and inhibition(Xue et al., 2014). CGP-55845 resulted in more consistent polysynaptic response, but as expected also prolonged the mean peak times. (Figure S4). The E/I balance was quantified in response to both the Pr and Np pathways during three time windows: before (50–150 ms), during (150–350 ms), and after (350–550 ms) the 250 ms training interval (Figure 4). A two-way ANOVA (repeated measures) revealed that the E/I ratio between the Pr and Np pathway was similar before and after the target interval, but enhanced around the training interval in response to the Pr pathway (interaction effect, F2,50=4.53, P=0.016). Thus it was not only that the training altered the E/I balance, but the E/I balance was dynamic, increasing around the trained window.
Figure 4. Interval specific shifts in the balance of excitation-inhibition.
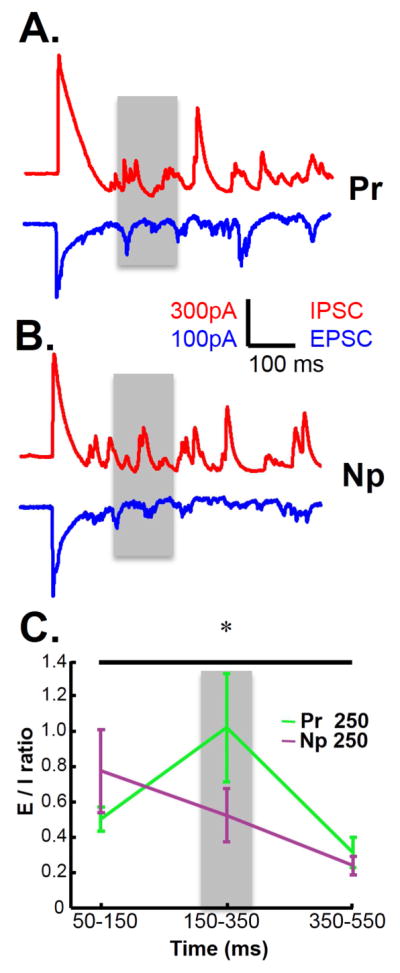
(A–B) Slices were trained with a 250 ms interval for 4 hours and whole-cell recordings were obtained in voltage clamp mode. Sample traces of Pr→ChR+ (A) and Np→ChR+ (B) evoked responses to a single test pulse following training. IPSCs are shown in red and EPSCs in blue. Gray shading indicates the time window (150 – 350 ms) around the trained interval of 250 ms. (C) E/I ratio at 3 different time windows (50 – 150 ms, 150 – 350 ms, 350 – 550 ms) for the Pr→ChR and Np→ChR responses.
DISCUSSION
Consistent with two previous in vitro studies, these results demonstrate that the activity profiles of neurons adapt to the temporal structure of previously presented stimuli (Johnson et al., 2010; Chubykin et al., 2013). By using an optogenetic approach we were able to examine how this learning emerges over training, and show that the learning generalized to ChR− cells—establishing that temporal learning is a network wide phenomenon, even though the timing is pathway specific. Previous computational results suggest that changes in the dynamics of recurrent neural networks require orchestrated plasticity at multiple loci, such as plasticity at excitatory and inhibitory synapses governed by different rules (Lazar et al., 2009; Liu and Buonomano, 2009; Vogels et al., 2011). With this caveat in mind, we provide evidence that the temporally-specific plasticity of network dynamics engages changes in inhibition, including a window specific increase in the E/I balance. And furthermore, that nonspecific decreases in inhibition can enhance temporal learning. These results provide evidence that networks have the ability to not only modulate E/I balance over long time frames (Froemke et al., 2007; Sun et al., 2010; Xue et al., 2014), but to “learn” to dynamically shift the E/I balance within specific windows over the course of hundreds of milliseconds.
Theories of Timing
As mentioned above, efforts to understand the neural mechanisms of timing, have led to two general classes of timing models: dedicated and intrinsic. The prototypical example of a dedicated model, the internal clock (Creelman, 1962; Gibbon, 1977), proposes that timing relies on a centralized (Ivry and Schlerf, 2008) neural integrator that counts the events of a neural pacemaker. In contrast, intrinsic models propose that timing is a local and general computation. Intrinsic models argue that because order, interval, duration, and temporal structure, are fundamental to most forms of neural computation, cortical circuits evolved in part to process temporal information on the subsecond scale. This view has received support from psychophysical (Johnston et al., 2006; Burr et al., 2007; Karmarkar and Buonomano, 2007; Bueti et al., 2012) and in vivo (Shuler and Bear, 2006; Sumbre et al., 2008; Chubykin et al., 2013) studies, suggesting that timing is a general and local, i.e. intrinsic, computation.
We suggest that the temporally-specific plasticity of neural dynamics described here, can indeed, be considered an example of in vitro learning because the previous “experience” of the circuit does not simply alter the subsequent “behavior” of the circuit, but does so in a computationally relevant manner: in a sense, the activity predicts the expected time of the optical stimulus. Such, in vitro learning approaches will be critical to elucidate how computations emerge from local neural circuits. First, in vitro networks provide absolute control over the external sensory experience of the circuits. Second, in contrast to timed neural responses observed in vivo, we can be sure that the observed timing is produced within the circuit being studied, as opposed to a reflection of computations performed in other brain areas. Thus allowing us to provide strong support for the intrinsic model of timing, and the first insights into the underlying mechanisms. A potential mechanistic explanation of our results is that temporal pattern learning develops in two stages. First, electrical stimulation results in the emergence of evoked but nonspecific time-varying neuronal activity. Second, optical stimulation acts as a “teacher” further depolarizing neurons and potentiating inputs active during that window—thus refining the temporal PSP profile of some neurons.
Of course, this study does not provide direct evidence that the forms of network plasticity observed here contribute to behavioral temporal learning. However the use of a training paradigm that parallels behavioral experiments and addresses timing specificity demonstrates that the computational machinery necessary to capture the temporal structure of external stimuli is in place in local cortical circuits. Furthermore, we provide the first mechanistic insights as to the network mechanisms underlying this ability. But our results also highlight the challenges that lie ahead in terms of understanding the plasticity of network dynamics within cortical microcircuits. Specifically, it seems unlikely that the learning can be attributed to any specific synapse class, and rather that the dynamic changes in E/I balance rely on plasticity at select subgroups of different classes of synapses embedded within complex recurrent circuits.
EXPERIMENTAL PROCEDURES
All animal procedures followed the National Institutes of Health (NIH) guidelines, and were approved by the UCLA Institutional Animal Care and Use Committee (IACUC). Experimental procedures and details of statistical tests performed are available in Supplemental Experimental Procedures.
Supplementary Material
HIGHLIGHTS.
Timing is fundamental to behavior and sensorimotor processing.
Temporal profile of network activity adapts to the interval used during training.
In vitro temporal learning was network-wide, generalizing from ChR+ to ChR− neurons.
Learning induced evoked network dynamics supports the intrinsic model of timing.
IN BRIEF.
Goel and Buonomano show that in response to chronic presentation of specific temporal intervals in the range of hundreds of milliseconds, cortical organotypic slices learn the presented interval. This in vitro learning is a network-level phenomenon that relies on changes in the balance of excitation and inhibition.
Acknowledgments
We thank Jack Feldman, Nick Hardy, Helen Motanis, Carlos Portera-Cailliau, and Alcino Silva for helpful comments on this manuscript; Kayla Gurley, Daisy Vallesfino, and John Tsiang for technical assistance. This work was supported by the National Institute of Mental Health (MH60163) and NIH training grant (T32 NS058280).
Footnotes
AUTHOR CONTRIBUTIONS
A.G. and D.V.B. conceived and designed the experiments; A.G. performed experiments; A.G. and D.V.B. analyzed data; A.G. and D.V.B. interpreted results of experiments; A.G. prepared figures; A.G. and D.V.B. drafted manuscript and revised the manuscript; A.G. and D.V.B. approved the final version of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bueti D, Lasaponara S, Cercignani M, Macaluso E. Learning about Time: Plastic Changes and Interindividual Brain Differences. Neuron. 2012;75:725–737. doi: 10.1016/j.neuron.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Buonomano DV. Timing of Neural Responses in Cortical Organotypic Slices. Proc Natl Acad Sci USA. 2003;100:4897–4902. doi: 10.1073/pnas.0736909100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Laje R. Population clocks: motor timing with neural dynamics. Trends in Cognitive Sciences. 2010;14:520–527. doi: 10.1016/j.tics.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr D, Tozzi A, Morrone MC. Neural mechanisms for timing visual events are spatially selective in real-world coordinates. Nat Neurosci. 2007;10:423–425. doi: 10.1038/nn1874. [DOI] [PubMed] [Google Scholar]
- Chubykin Alexander A, Roach Emma B, Bear Mark F, Shuler Marshall GH. A Cholinergic Mechanism for Reward Timing within Primary Visual Cortex. Neuron. 2013;77:723–735. doi: 10.1016/j.neuron.2012.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull J, Nobre A. Dissociating explicit timing from temporal expectation with fMRI. Curr Opin Neurobiol. 2008;18:137–144. doi: 10.1016/j.conb.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Creelman CD. Human discrimination of auditory duration. J Acoust Soc Am. 1962;34:582–593. [Google Scholar]
- Dranias MR, Ju H, Rajaram E, VanDongen AMJ. Short-Term Memory in Networks of Dissociated Cortical Neurons. The Journal of Neuroscience. 2013;33:1940–1953. doi: 10.1523/JNEUROSCI.2718-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC, Grossberg S, Bullock D. Metabotropic glutamate receptor activation in cerebellar Purkinje Cells as substrate for adaptive timing of the classically conditioned eye-blink response. J Neurosci. 1996;16:3760–3734. doi: 10.1523/JNEUROSCI.16-11-03760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Gibbon J. Scalar expectancy theory and Weber’s law in animal timing. Psychological Review. 1977;84:279–325. [Google Scholar]
- Hooper SL, Buchman E, Hobbs KH. A computational role for slow conductances: single-neuron models that measure duration. Nat Neurosci. 2002;5:551–556. doi: 10.1038/nn0602-838. [DOI] [PubMed] [Google Scholar]
- Hyde RA, Strowbridge BW. Mnemonic representations of transient stimuli and temporal sequences in the rodent hippocampus in vitro. Nat Neurosci. 2012;15:1430–1438. doi: 10.1038/nn.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB, Spencer RMC. The neural representation of time. Curr Opinion Neurobiol. 2004;14:225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Schlerf JE. Dedicated and intrinsic models of time perception. Trends in Cognitive Sciences. 2008;12:273–280. doi: 10.1016/j.tics.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HA, Buonomano DV. Development and Plasticity of Spontaneous Activity and Up States in Cortical Organotypic Slices. J Neurosci. 2007;27:5915–5925. doi: 10.1523/JNEUROSCI.0447-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HA, Buonomano DV. A method for chronic stimulation of cortical organotypic cultures using implanted electrodes. Journal of Neuroscience. 2009;176:136–143. doi: 10.1016/j.jneumeth.2008.08.037. [DOI] [PubMed] [Google Scholar]
- Johnson HA, Goel A, Buonomano DV. Neural dynamics of in vitro cortical networks reflects experienced temporal patterns. Nat Neurosci. 2010;13:917–919. doi: 10.1038/nn.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A, Arnold DH, Nishida S. Spatially localized distortions of event time. Curr Biol. 2006;16:472–479. doi: 10.1016/j.cub.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Ju H, Dranias MR, Banumurthy G, VanDongen AMJ. Spatiotemporal Memory Is an Intrinsic Property of Networks of Dissociated Cortical Neurons. The Journal of Neuroscience. 2015;35:4040–4051. doi: 10.1523/JNEUROSCI.3793-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmarkar UR, Buonomano DV. Timing in the absence of clocks: encoding time in neural network states. Neuron. 2007;53:427–438. doi: 10.1016/j.neuron.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar A, Pipa G, Triesch J. SORN: a self-organizing recurrent neural network. Front Comput Neurosci. 2009;3:23. doi: 10.3389/neuro.10.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JK, Buonomano DV. Embedding Multiple Trajectories in Simulated Recurrent Neural Networks in a Self-Organizing Manner. J Neurosci. 2009;29:13172–13181. doi: 10.1523/JNEUROSCI.2358-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak A, Bartho P, Marguet SL, Buzsaki G, Harris KD. Sequential structure of neocortical spontaneous activity in vivo. PNAS. 2007 doi: 10.1073/pnas.0605643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Kohl MM, Paulsen O. Distinct Roles of GABAA and GABAB Receptors in Balancing and Terminating Persistent Cortical Activity. The Journal of Neuroscience. 2009;29:7513–7518. doi: 10.1523/JNEUROSCI.6162-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D. Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J Neurosci. 1983;3:133–143. doi: 10.1523/JNEUROSCI.03-05-01039.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk MD, Buonomano DV. The Neural Basis of Temporal Processing. Ann Rev Neurosci. 2004;27:307–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- Medina JF, Garcia KS, Nores WL, Taylor NM, Mauk MD. Timing mechanisms in the cerebellum: testing predictions of a large-scale computer simulation. J Neurosci. 2000;20:5516–5525. doi: 10.1523/JNEUROSCI.20-14-05516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant H, Harrington DL, Meck WH. Neural Basis of the Perception and Estimation of Time. Annual Review of Neuroscience. 2013;36:313–336. doi: 10.1146/annurev-neuro-062012-170349. [DOI] [PubMed] [Google Scholar]
- Sadovsky AJ, MacLean JN. Mouse Visual Neocortex Supports Multiple Stereotyped Patterns of Microcircuit Activity. The Journal of Neuroscience. 2014;34:7769–7777. doi: 10.1523/JNEUROSCI.0169-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh I, Suga N. Long delay lines for ranging are created by inhibition in the inferior colliculus of the mustached bat. J Neurophysiol. 1995;74:1–11. doi: 10.1152/jn.1995.74.1.1. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Debanne D, Muller M, Gahwiler BH, Thompson SM. Role of excitatory amino acid and GABAB receptors in the generation of epileptiform activity in disinhibited hippocampal slice cultures. Neuroscience. 1994;61:823–832. doi: 10.1016/0306-4522(94)90405-7. [DOI] [PubMed] [Google Scholar]
- Shuler MG, Bear MF. Reward timing in the primary visual cortex. Science. 2006;311:1606–1609. doi: 10.1126/science.1123513. [DOI] [PubMed] [Google Scholar]
- Sumbre G, Muto A, Baier H, Poo MM. Entrained rhythmic activities of neuronal ensembles as perceptual memory of time interval. Nature. 2008;456:102–106. doi: 10.1038/nature07351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YJ, Wu GK, Liu B-h, Li P, Zhou M, Xiao Z, Tao HW, Zhang LI. Fine-tuning of prebalanced excitation and inhibition during auditory cortical development. Nature. 2010;465:927–931. doi: 10.1038/nature09079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels TP, Sprekeler H, Zenke F, Clopath C, Gerstner W. Inhibitory Plasticity Balances Excitation and Inhibition in Sensory Pathways and Memory Networks. Science. 2011;334:1569–1573. doi: 10.1126/science.1211095. [DOI] [PubMed] [Google Scholar]
- Xue M, Atallah BV, Scanziani M. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature. 2014;511:596–600. doi: 10.1038/nature13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.