Abstract
Modulation of neurotransmission by the catecholamine dopamine (DA) is conserved across phylogeny. In the nematode C. elegans, excess DA signaling triggers Swimming-Induced Paralysis (Swip), a phenotype first described in animals with loss of function mutations in the presynaptic DA transporter (dat-1). Swip has proven to be a phenotype suitable for the identification of novel dat-1 mutations as well as the identification of novel genes that impact DA signaling. Pharmacological manipulations can also induce Swip, though the reagents employed to date lack specificity and potency, limiting their use in evaluation of dat-1 expression and function. Our lab previously established the mammalian norepinephrine transporter (NET) inhibitor nisoxetine to be a potent antagonist of DA uptake conferred by DAT-1 following heterologous expression. Here we demonstrate the ability of low (μM) concentrations of nisoxetine to trigger Swip within minutes of incubation, with paralysis dependent on DA release and signaling, and non-additive with Swip triggered by dat-1 deletion. Using nisoxetine in combination with genetic mutations that impact DA release, we further demonstrate the utility of the drug for demonstrating contributions of presynaptic DA receptors and ion channels to Swip. Together, these findings reveal nisoxetine as a powerful reagent for monitoring multiple dimensions of DA signaling in vivo, thus providing a new resource that can be used to evaluate contributions of DAT-1 and other genes linked to DA signaling without the potential for compensations that attend constitutive genetic mutations.
Keywords: dopamine, transporter, nematode, C. elegans, nisoxetine
2. Introduction
The modulation of behavior by the neurotransmitter dopamine (DA) is evolutionarily conserved, evident in animals that range by orders of magnitude in complexity, from humans to the soil-dwelling nematode Caenorhabditis elegans. As in humans, DA signaling in C. elegans regulates multiple behaviors including locomotion (Chase et al., 2004; Omura et al., 2012; Sawin et al., 2000) and associative learning (Voglis and Tavernarakis, 2008). The conservation of genes encoding proteins that support DA biosynthesis, vesicular packaging, release, and response, makes the worm a powerful tool to elucidate novel mechanisms that regulate DA signaling across phylogeny (McDonald et al., 2006). Particularly useful is the amenability of this organism to rapid genetic manipulation and behavioral characterization. Additionally, pharmacological agents have been utilized successfully to elicit behavioral responses through pathways shared with more complex vertebrates (Choy and Thomas, 1999; Dwyer et al., 2014; Miller et al., 1996; Weinshenker et al., 1995). As in vertebrates, the latter agents offer the opportunity to manipulate chemical signaling at specific time points in development and, when activity is evident with acute exposure, lessen concern for the compensations that arise from constitutive genetic manipulations.
A powerful example of a rapidly acting drug that has been successfully used in the worm to manipulate a specific chemical signaling pathway is the acetylcholinesterase inhibitor, aldicarb, which has been used extensively to evaluate the capacity for cholinergic signaling (Bany et al., 2003; Iwasaki et al., 1997; Miller et al., 1996; Mullen et al., 2007). Acetylcholine (ACh) is released at the neuromuscular junction in C. elegans to trigger muscle contraction. Aldicarb, by blocking the major determinant of extracellular ACh inactivation, acetylcholinesterase (AChE), induces rapid, hypercontracted, motor paralysis due to excessive activation of neuromuscular ACh receptors. Genetic and pharmacological modifiers of ACh signaling, such as proteins that regulate vesicular ACh release, can be studied via their ability to enhance or suppress aldicarb-induced paralysis (Jorgensen et al., 1995; Nonet et al., 1997). This enhancement or suppression can be used to determine how a particular genetic mutation or drug might alter ACh signaling, even if there is no obvious phenotype in the absence of drug. Dozens of genes have been identified or studied based on the presence of a Ric or Hic (Resistance or Hypersensitivity to Inhibitors of AChE) phenotype, and include genes that act both pre- and postsynaptically (For review, see (Rand, 2007)).
To date, examples of potent and selective agents that act like aldicarb at non-cholinergic synapses are limited, in part due to the high concentrations (typically mM) needed for many substances to afford penetration through the worm cuticle. Additionally, inactivation of small molecule neurotransmitters besides ACh is determined by transporter-mediated clearance, and many mammalian transporter antagonists lose potency as inhibitors of their C. elegans orthologs (Jayanthi et al., 1998; Ranganathan et al., 2001). Thus, the C. elegans DA transporter (DAT-1) is one to two orders of magnitude less sensitive in vitro to the mammalian DAT inhibitors GBR12909 and nomifensine, respectively (Jayanthi et al., 1998). Interestingly, the mammalian norepinephrine (NE) transporter (NET)-specific antagonist nisoxetine (NIS) exhibits low nanomolar potency for inhibition of DAT-1 mediated DA uptake (Jayanthi et al., 1998). Since the worm lacks a NET ortholog (worms do not make NE), we reasoned that NIS might prove a potent and selective antagonist of DAT-1 in vivo.
Here, we describe our efforts to demonstrate the utility of NIS as a potent pharmacological modulator of DA signaling-dependent behavior in the worm. The behavior monitored in our studies, Swimming-Induced Paralsis (Swip), was first described in worms bearing a dat-1 deletion (McDonald et al., 2007). We demonstrated that loss of DAT-1 expression leads to premature paralysis when worms are placed in water, with paralysis emerging in a few minutes versus the stable (>10 min) swimming evident in wildtype (N2) worms. Swip produced by dat-1 mutation can be rescued by mutation of DA biosynthetic and vesicular packaging genes (cat-2 and cat-1 respectively). Additionally, Swip in dat-1 animals is lost in a cross to animals with a loss of function mutation in the gene encoding the D2 receptor dop-3 (McDonald et al., 2007). These and other findings support the use of Swip as a behavioral readout of hyperdopaminergia. In this report, we demonstrate that Swip can be induced rapidly by incubation of worms with low concentrations of NIS, that Swip induction by NIS is DA-dependent, and that we can positively and negatively modify NIS-induced Swip by genetic manipulation of presynaptic regulators of DA release. We discuss the potential for NIS to serve as a useful tool for the identification and characterization of novel and potentially conserved regulators of DA signaling.
3. Materials and Methods
3.1 C. elegans strains
Strains were maintained at 15–20 °C using standard methods as described previously (Brenner, 1974). N2 (Bristol) served as our wild-type strain. Additional strains used in this work as follows: dat-1(ok157); dop-2(vs105); asic-1(ok415); cat-2(e1112); dop-3(vs106); dat-1(ok157), dop-2(vs105); dat-1(ok157), dop-2(vs105) vtEx58 (Pdat-1::dop-2).
dat-1(ok157), dop-2(vs105) vtEx58 (Pdat-1::dop-2) transgenic animals were generated by microinjection of dat-1(ok157); dop-2(vs106) worms with pdat-1::dop-2 transgene and co-injection markers punc-122::GFP and pdat-1::mCherry. Stable transgenic line was selected based on presence and high transmission of punc-122::GFP fluorescence, and pdat-1::mCherry fluorescence was used to pick animals for behavioral experiments.
3.2 Plasmid Construction
The pdat-1::dop-2 rescue construct was generated in the backbone pRB1106 which contains the dat-1 promoter and unique restriction enzymes for subcloning of open reading frames (ORFs). dop-2S cDNA was amplified from ceh-17::dop-2S plasmid (provided by Satoshi Suo, University of Tokyo, Tokyo, Japan) with AscI and KpnI engineered into the 5′ and 3′ ends, respectively. This PCR fragment was digested and subcloned into digested backbone. Successful cloning was verified by sequencing of the plasmid prior to microinjection.
3.3 Swip assays
Swip assays were performed as previously described (Hardaway et al., 2012; McDonald et al., 2007). Synchronous L4 worms were generated by hypochlorite treatment of adult animals, and plating of synchronized L1 animals. All manual assays were carried out by picking 10 L4 animals into a well of 100μL of water plus or minus nisoxetine hydrochloride (Sigma-Aldrich, St. Louis, MO) or methylphenidate (Sigma-Aldrich, St. Louis, MO) and scoring the number of animals paralyzed after 10 min. For osmosuppression studies, solutions of 100, 200, and 300 mOsm were generated with sucrose. Eight wells were scored per genotype/treatment, and were repeated on at least 3 separate days with 1–2 experimenters per day for an N=24–48 per condition. Experimenters were blind to genotype and/or drug. Automated analyses were performed using 10 min videos of individual worms captured from at least 25 worms per genotype/treatment using Tracker software and analyzed using SwimR software as previously described (Hardaway et al., 2015; Hardaway et al., 2014). Latency to paralysis was measured as the time until the thrashing frequency for each animal dropped below 20% of the maximum thrashing value for at least 20 seconds. Probability of reversion to swimming was measured as the percent of paralyzed animals that displayed bouts of thrashing above 20% of the maximum thrashing value after a bout of paralysis (defined as above).
For dop-2 rescue experiments, worms expressing the pdat-1::dop-2 transgene were selected based on the presence of the co-injection marker pdat-1::mCherry and were picked as L2–L3 animals the day before video acquisition.
3.4 Graphical and Statistical Methods
Data was analyzed and graphed using either SwimR software (described above), or using Prism 6.0 (GraphPad, Inc., La Jolla, CA). All statistical analyses and curve fits were performed in Prism 6.0. Descriptions of all statistical tests are noted in the figure legends.
4. Results
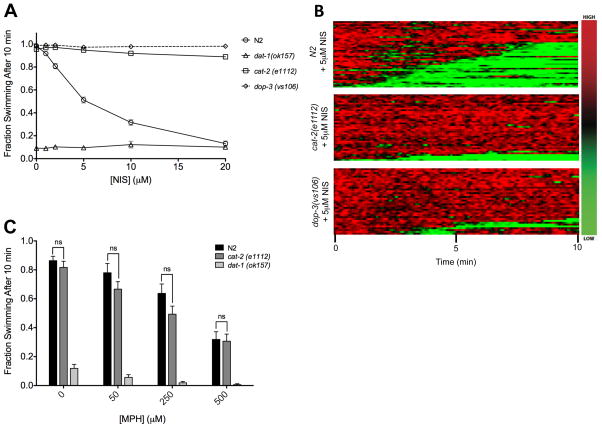
Wild-type worms placed in water supplemented with increasing concentrations of NIS demonstrated a dose-dependent increase in Swip, with diminished movement evident at 2 μM drug. At 20 μM, Swip levels matched those observed in dat-1 animals (Fig 1A). Heat maps that plot the population behavior of NIS (5 μM)-treated animals revealed a pronounced induction of Swip with a probability of reversion to a swimming state following paralysis that is comparable to dat-1(ok157) mutants (N2 + 5 μM NIS: 33.3%; dat-1(ok157): 33.6%). To demonstrate DA-dependence of NIS-induced Swip, we examined NIS effects in the background of genetic mutations in cat-2 (tyrosine hydroxylase) and dop-3 (D2-like DA receptor) genes that support DA synthesis and DA response, respectively. Importantly, we have previously shown that mutations in these genes suppress dat-1-dependent Swip (McDonald et al., 2007). Indeed, at all doses of NIS tested, cat-2(e1112) and dop-3(vs106) animals swam at rates comparable to wild-type animals in the absence of drug (Fig 1A). Heat map analysis demonstrated a dramatic reduction in penetrance of NIS-induced Swip in cat-2(e1112) and dop-3(vs106) mutants (Fig 1B). dat-1(ok157) animals exhibited paralysis at all doses of NIS tested, with no increase in paralysis seen as the drug dose increased, supporting a lack of additivity between NIS-induced and dat-1(ok157) Swip. These results are consistent with NIS acting in vivo to antagonize DAT-1 mediated DA clearance, thereby generating a excess extrasynaptic DA overflow and Swip.
Figure 1.
NIS induces DA-dependent Swip. (A) NIS-treated N2 animals show dose-dependent Swip with a curve fit yielding an EC50 = 5.80 ± 0.12 μM. Neither dop-3(vs106) nor cat-2(e1112) animals demonstrate Swip at any NIS doses whereas dat-1(ok157) animals paralyze similarly at all doses of NIS. (B) Heat maps display thrashing frequencies of individual animals over time, with red designating high frequency values, and green designating low values. This analysis shows a high degree of Swip in N2 animals treated with 5 μM NIS, with very little paralysis observed in cat-2(e1112) and dop-3(vs106) animals treated with the same dose of NIS. n ≥ 40 for each condition. (C) N2 animals treated with MPH show dose-dependent increases in paralysis, though cat-2(e1112) animals show paralysis comparable to N2 at all doses, with no significant difference (p>.05) between genotypes at any dose of MPH. dat-1(ok157) animals paralyzed in all doses of MPH. Significance was calculated using a two-way ANOVA with selected Bonferroni posttests comparing genotypes at each dose of MPH.
To evaluate the specificity of NIS over other drugs, we compared the paralysis observed by NIS treatment with the paralysis previously reported with methylphenidate (MPH), a human DAT-specific antagonist (Izquierdo et al., 2013). We observed that whereas MPH produced dose-dependent Swip in N2 animals, these effects occurred at much higher concentrations than seen with NIS. Moreover, MPH-treated cat-2(e1112) animals displayed Swip at levels comparable to N2 animals (Fig 1C), indicating that this drug induces paralysis through a DA-independent mechanism. Additionally, dat-1(ok157) animals displayed a trend towards increased paralysis in higher doses of MPH (Fig 1C). This finding supports the existence of other targets in the actions of MPH, though the effect did not reach significance, possibly due to floor effects.
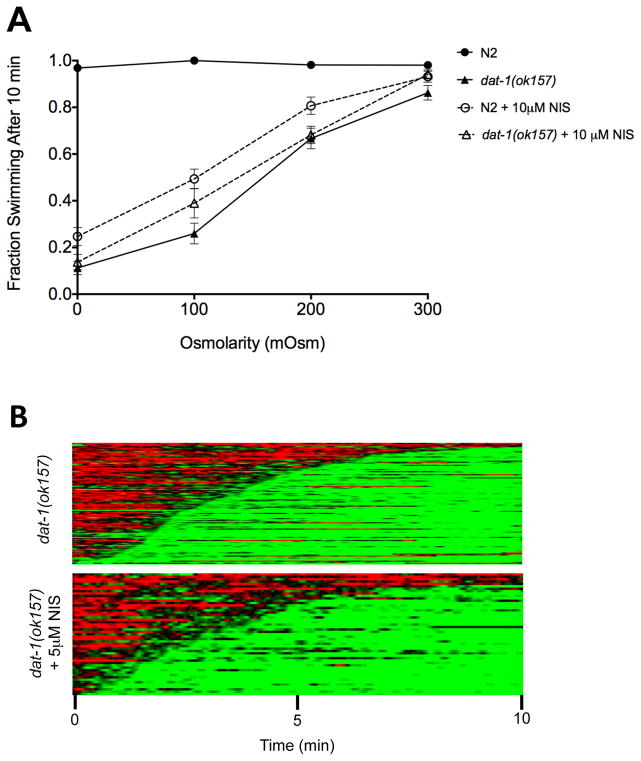
Evaluation of additivity for Swip produced by NIS and Swip produced by genetic perturbations can be difficult due to potential floor effects. Previously, we reported that the Swip phenotype observed in dat-1 mutants can be suppressed by increasing the osmolarity of the solution to which animals are exposed (Hardaway et al., 2010). Consistent with an action of NIS to bloc DAT-1, the paralysis induced by 10 μM NIS treatment of N2 animals was suppressed by increasing osmolarity of the assay medium (achieved with sucrose supplementation) (Fig 2A). Importantly, under osmosuppressed conditions, where an intermediate level of paralysis is present and potential drug additivity should be less sensitive to floor effects, dat-1(ok157) mutant animals still demonstrated no further increase in paralysis with NIS treatment, relative to untreated dat-1(ok157) animals. In heat map analyses (Fig 2B), dat-1(ok157) mutants treated with 5 μM NIS displayed nearly indistinguishable Swip onset and penetrance and no change in probability of reversion to swimming compared to untreated dat-1(ok157) animals. Along with the findings presented in Fig 1, these findings support the hypothesis that antagonism of DAT-1 is the basis of NIS actions to trigger Swip.
Figure 2.
NIS does not enhance Swip in dat-1 mutants. (A) N2 animals treated with 10 μM NIS show robust Swip that is suppressed by increasing osmolarity. dat-1(ok157) animals display Swip suppression in higher osmolarity solutions, though dat-1(ok157) animals treated with 10 μM NIS exhibit no significant increase in Swip compared to untreated dat-1(ok157) animals at any osmolarity (p>.05). Data were analyzed using a two-way ANOVA with selected Bonferroni posttests comparing genotypes/treatments at each osmolarity. (B) Heat map analysis shows no significant difference between untreated dat-1(ok157) animals and those treated with 5 μM NIS. This similarity between treatments was reflected in percent paralysis (96.6% untreated vs. 90.2% treated), latency to paralyze (205 ± 11.5 sec untreated vs. 197 ± 16.8 sec treated, p<.0001 two-tailed Student’s t-test), and percent reversions to swimming (33.6% untreated vs. 34.8% treated). n ≥ 50 for each condition
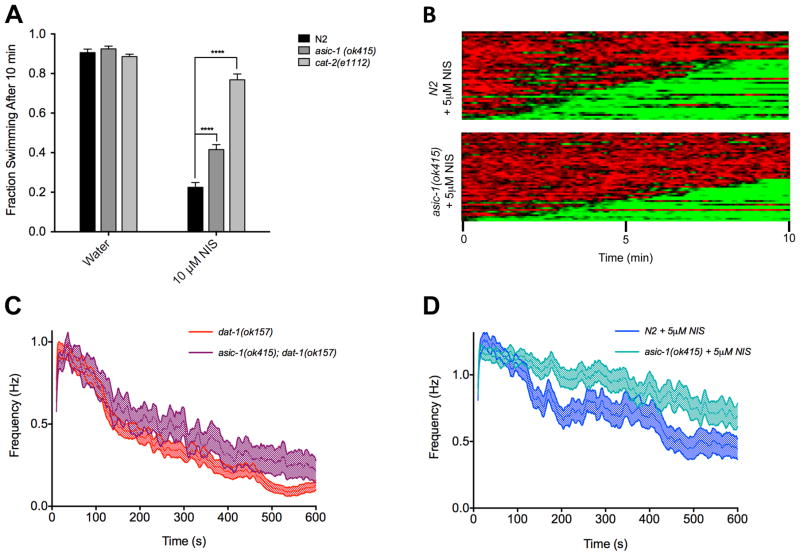
Having demonstrated that the actions of NIS likely derive from DAT-1 blockade and the resulting hyperdopaminergia, we wished to determine whether the drug could be useful as a tool to evaluate other elements of DA signaling. To explore this issue, we evaluated animals with a loss of function mutation in asic-1, a gene that encodes an acid-sensing cation channel expressed by DA terminals and that is activated by protons released with the fusion of acidic, DA-containing synaptic vesicles. ASIC-1 activation enhances presynaptic depolarization and rates of DA release (Voglis and Tavernarakis, 2008). Consistent with this model, fluorescence recovery after photobleaching (FRAP) experiments have demonstrated that animals with a loss of function asic-1 mutation diminishes DA release rates, paralleled by defects in associative learning (Voglis and Tavernarakis, 2008). However, as asic-1 mutations have little to no effect on locomotion, effects on DA signaling are difficult to observe by assessment of worm movement assays, including Swip (Fig 3A). However, NIS-induced Swip can be significantly suppressed in asic-1(ok415) animals, effects that are observed in both manual assays at 10 μM NIS (Fig 3A) and in population heat maps generated with 5 μM NIS (Fig 3B). Mutation of asic-1 is also able to significantly suppress dat-1(ok157) paralysis, as demonstrated by comparison of thrashing frequency over time between dat-1(ok157) and asic-1(ok157); dat-1(ok157) animals (Fig 3C). This suppression was less dramatic than the suppression we observe with 5 μM NIS treatment, however, where we see both significant genotype and time x genotype interaction effects when comparing asic-1(ok157) to N2 animals. We hypothesize that the constitutive nature of full loss of dat-1 produces a level of synaptic DA overflow and robust Swip which loss of asic-1 can suppress only to a small degree. The acute, titratable nature of DAT-1 blockade by NIS application may thus provide a more sensitive paradigm for evaluation of the contributions of genes like asic-1 to the regulation of DA release.
Figure 3.
NIS-induced Swip is suppressed by loss of asic-1. (A) asic-1(ok415) animals show no basal Swip in water, but display a significant reduction in Swip in 10 μM NIS compared to N2 animals. Data were analyzed using a two-way ANOVA with selected Bonferroni post-tests comparing genotypes at each dose of NIS. **** p<.0001 (B) Heat map analysis shows asic-1(ok157) animals treated with 5 μM NIS have a suppression of Swip compared to N2 animals, with fewer asic-1(ok157) animals paralyzing (48.0% vs. 68.2% for N2). (C) Graphing of average thrashing behavior over time (mean ± SE) reveals a significant genotype difference in thrashing rates between asic-1(ok415); dat-1(ok157) double mutants and dat-1(ok157) animals (p<.0001). (D) Thrashing plots show asic-1(ok415) animals treated with 5 μM NIS have increased average thrashing rates compared to N2 animals treated with 5 μM NIS, with both significant genotype (p<.0001) and time x genotype interaction effects (p<.0001). n ≥ 34 for each condition
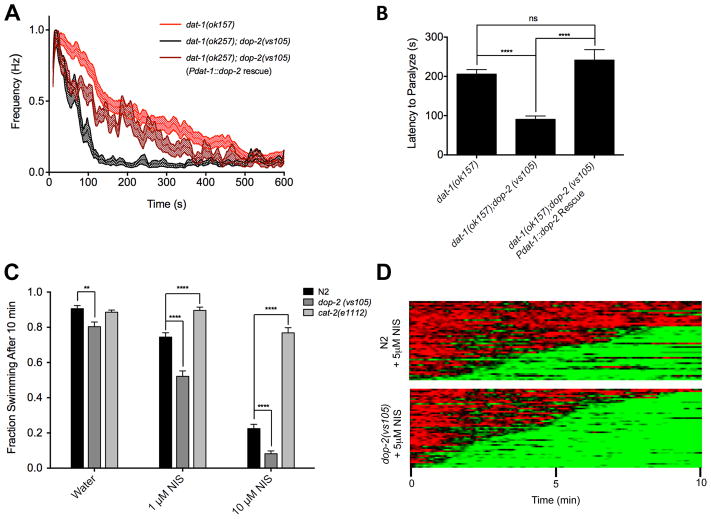
As a second test of NIS utility for the study of DA release-modulatory genes, we sought to determine whether sensitivity to NIS treatments could reveal functional contributions to DA release exerted by negative modulators. For this evaluation, we examined drug sensitivity of animals with a loss of function mutation in the D2-like dopamine receptor dop-2, a gene expressed, among other places, in DA neurons (Suo et al., 2003). Although the function of dop-2 has received little attention, in mammalian DA neurons, D2 receptors function as inhibitory autoreceptors, providing for feedback reductions in cell firing at the somatodendritic level as well inducing diminished DA release at presynaptic terminals (De Mei et al., 2009; Ford, 2014; Mercuri et al., 1997). In keeping with these mechanisms, we observed that dat-1(ok157);dop-2(vs105) double mutants displayed a greater degree of Swip as compared to dat-1(ok157) mutants (Fig 4A), consistent with a reduction in DOP2-mediated inhibitory feedback on DA neuron firing and/or DA release. This hypothesis is supported by our finding of a reversal of the effects of dop-2 mutation on Swip levels in the double mutant by DA neuron-specific expression of dop-2, evident in comparison of average thrashing rates over time (Fig 4A) as well as the average latency to paralysis (Fig 4B). Having provided evidence for a cell autonomous action of dop-2 in suppressing DA signaling, we sought to determine whether loss of the receptor would enhance NIS-induced Swip, similar to effects of the dop-2 mutation when combined with genetic loss of dat-1. Indeed, we observed that dop-2(vs105) mutants treated with various doses of NIS displayed significantly increased Swip as compared to N2 animals (Fig 4C). Heat map analyses further demonstrate this enhancement, where we again observed a higher penetrance of Swip in dop-2(vs105) vs. N2 animals (Fig 4D). Interestingly, we also found that the percent of animals reverting to swimming and the average number of reversion events were reduced in dop-2(vs105) animals (Fig 4D). These observations may be a reflection of elevated synaptic DA levels that, in the absence of autoreceptor feedback, induce a more irreversible paralysis. These findings of a hypersensitivity to NIS demonstrate that the drug can be used to interrogate mechanisms that afford inhibitory control of DA excitability of release and that the drug can reveal states of increased DA signaling that are too subtle to reliably observe via mutations alone.
Figure 4.
Enhancement of NIS-induced Swip by dop-2 mutation supports negative presynaptic regulation of DA signaling (A) Average thrashing plots show enhanced paralysis in dat-1(ok157); dop-2(vs105) double mutants compared to dat-1(ok157) mutants. DA neuron expression of dop-2 with the plasmid Pdat-1::dop-2 suppressed this enhancement. (B) dat-1(ok157); dop-2(vs105) double mutants have a significantly decreased average latency to paralysis compared to dat-1(ok157) mutants, and DA neuron expression of dop-2 rescues this effect up to dat-1(ok157) mutant levels. No significant difference was observed between dat-1(ok157) and dat-1(ok157); dop-2(vs105) (pdat-1::dop-2) transgenic animals. Data was analyzed using individual two-tailed Student’s t-tests. ****p<.0001. n ≥ 25 for each condition. (C) dop-2(vs105) animals have subtle, yet significant increase in Swip compared to N2 in water, and a greater enhancement in 1 μM and 10 μM NIS. cat-2(e1112) animals have significantly less paralysis than N2 in both doses of NIS. Data was analyzed by two-way ANOVA with Bonferroni posttests comparing genotypes at each dose of NIS. **p<.01, ****p<.0001. (D) Heat map analysis of animals in 5 μM NIS further demonstrates the increase in Swip in dop-2(vs105) mutants vs. N2. dop-2(vs105) showed a higher percentage of paralyzed animals (96.0% vs. 68.2% for N2), lower percentage of animals reverting to swimming (14.6% vs. 33.3% for N2) and significantly fewer average reversion events per paralyzed animal (2.14 ± 0.15 events vs. 4.40 ± 0.33 events for N2, p<.0001 two-tailed Student’s t-test). n ≥ 44 for each condition.
5. Discussion
The nematode C. elegans has long been a favorite model for geneticists interested in the molecular basis of neural development and function due to its ease of husbandry and genetic manipulation, and for the spectrum of simple behaviors that can afford insights into nervous system function. The model is also amenable to use of pharmacological agents, though the specificity and potency of these drugs does not always directly translate from studies of vertebrate targets. The AChE inhibitor aldicarb has proved the utility of agents that can act quickly to interfere with specific chemical signaling pathways, contributing to paradigms that can elucidate mechanisms by which mutations impact synaptic transmission. The findings presented in the current study make a case that NIS may provide a comparable tool for the dissection of components of DA signaling. Though a target of NET in mammalian systems, previous in vitro studies demonstrated NIS to be a potent inhibitor of DAT-1 mediated DA uptake (Jayanthi et al., 1998). Here, we show that low μM concentrations of NIS trigger Swip upon acute application, effects that are lost in animals that cannot produce DA (cat-2) and in animals that cannot support inhibitory DA signaling to motor neurons (dop-3). Furthermore, additivity experiments indicate that the behavioral effects of NIS are mediated through dat-1. The latter findings stand in contrast to those obtained with MPH, which appears to exert its Swip effects in a DA-independent manner. Our findings with MPH are reminiscent of the effects we reported with high dose imipramine, a tricyclic antidepressant that can induce DA-dependent Swip at low concentrations but also has potent actions at the serotonin transporter MOD-5 (Ranganathan et al., 2001). We suspect that imipramine blockade of MOD-5 also supports to the suppression of Swip observed at higher concentrations of imipramine detected in dat-1(ok157) animals (Hardaway et al., 2014). Cocaine, a nonspecific biogenic amine antagonist has also been used in C. elegans. We previously reported an inhibition of DAT-1 in transfected mammalian cells by cocaine, though at a potency orders of magnitude lower than observed with nisoxetine (Jayanthi et al., 1998). Consistent with the lack of specificity of cocaine in vertebrate preparations, cocaine also blocks MOD-5 (Ranganathan et al., 2001). This action can induce activation of ionotropic serotonin receptors, leading to movement inhibition that is independent of DA signaling (Ward et al., 2009). Nonetheless, Musselman and colleagues detected an ability of cocaine to support chemosensory cue conditioning, with effects lost in mutants impacting DA synthesis and release (Musselman et al., 2012). Overall, these findings suggest that the actions and specificity of cocaine for augmenting DA signaling are likely to be dose-limited and must be carefully evaluated through the use of complementary genetic studies. D-amphetamine can also trigger Swip upon acute application, though some of these actions are mediated by targets besides DAT-1 (Carvelli et al., 2010; Safratowich et al., 2013). Altogether, NIS appears to be the most DAT-1 specific drug studied to date, where use induces robust and specific DA-dependent behavioral responses.
The use of a mammalian NET-specific drug to study DA signaling in the worm may seem surprising. However, pharmacological sensitivities are expected to diverge more across phylogeny than a protein’s primary function due to a lack of selection pressure. Importantly, nematodes do not synthesize NE, nor do they express another catecholamine transporter in the SLC6 family besides DAT-1, which our prior in vitro work demonstrated to be highly NIS sensitive (Jayanthi et al., 1998). Together these findings suggested to us that NIS could be a useful agent to modulate nematode DA signaling in vivo. Supporting this possibility, we showed that the Swip behavior generated by NIS treatment can be bidirectionally modified by genetic ablation of either positive or negative, presynaptic regulators of DA signaling. The acid-sensing channel ASIC-1 has been identified as a positive presynaptic regulator of DA release, and we found that mutation of asic-1 could suppress Swip induced by NIS, classifying asic-1(ok415) animals as a RID (Resistant to Inhibitors of DAT-1) mutant. Because loss of asic-1 results in a hypodopaminerigic state, we did not observe a Swip effect in asic-1 mutants in the absence of NIS, highlighting the opportunity to reveal the effects of DA signaling mutants that may not have detectible behavioral effects on their own. We also demonstrated that NIS can reveal the actions of a negative regulator of DA release, exemplified by dop-2. The DOP-2 receptor is expressed in DA neurons, and we show here for the first time, to our knowledge, that restoration of expression of dop-2 in DA neurons significantly rescues a DA-dependent behavior, consistent with inhibitory autoreceptor action. Moreover, we showed that whereas loss of DOP-2 results in a barely detectable Swip phenotype on its own, a robust enhancement of paralysis is induced when a dop-2 mutation is tested in combination with NIS application. These observations are reminiscent of the effects that loss of dop-2 has on Swip in dat-1 mutants. Importantly, however, the use of NIS to induce paralysis obviates the need for time-consuming genetic crosses and rescue experiments to provide evidence for DA signaling-dependent effects. Given that asic-1 and dop-2 have orthologs in mammals that appear to play conserved roles in the regulation of DA signaling (Ford, 2014; Pidoplichko and Dani, 2006), we suggest that the use of NIS may be a significant aid to efforts to exploit the worm model for the identification of novel and conserved regulators of DA signaling.
6. Conclusions
We demonstrate that the mammalian NET-specific antagonist NIS induces Swip in C. elegans, a phenotype that our studies indicate as arising from high-affinity DAT-1 antagonism, leading to excess DA signaling at extrasynaptic D2-type DA receptors. The NIS-induced Swip phenotype can be used to probe the state of DA signaling in various genetic backgrounds. Unlike other drugs used by the field to induce this behavior, NIS effects are DA-specific, and its use can reveal states of both hyper- and hypodopaminergia based on enhancement or suppression of the Swip phenotype. By analogy with the AChE aldicarb, NIS can be used to probe the molecular mechanics of synaptic transmission, as well as for more targeted analysis of candidate genes that support DA signaling.
Acknowledgments
This work was supported by NIH Awards DA035559 (DPB), MH064913 (JAH), MH093102 (JAH), and MH090544 (RDB). We thank J. Rand (Oklahoma Medical Research Foundation), the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440), and Dan Chase for providing the strains used in this work. We gratefully acknowledge excellent laboratory support provided by Sarah Sturgeon, Chris Svitek, Jane Wright, Tracy Moore-Jarrett and Angela Steele.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bany IA, Dong MQ, Koelle MR. Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior. J Neurosci. 2003;23:8060–8069. doi: 10.1523/JNEUROSCI.23-22-08060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, Matthies DS, Galli A. Molecular mechanisms of amphetamine actions in Caenorhabditis elegans. Mol Pharmacol. 2010;78:151–156. doi: 10.1124/mol.109.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase DL, Pepper JS, Koelle MR. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci. 2004;7:1096–1103. doi: 10.1038/nn1316. [DOI] [PubMed] [Google Scholar]
- Choy RK, Thomas JH. Fluoxetine-resistant mutants in C. elegans define a novel family of transmembrane proteins. Mol Cell. 1999;4:143–152. doi: 10.1016/s1097-2765(00)80362-7. [DOI] [PubMed] [Google Scholar]
- De Mei C, Ramos M, Iitaka C, Borrelli E. Getting specialized: presynaptic and postsynaptic dopamine D2 receptors. Curr Opin Pharmacol. 2009;9:53–58. doi: 10.1016/j.coph.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer DS, Aamodt E, Cohen B, Buttner EA. Drug elucidation: invertebrate genetics sheds new light on the molecular targets of CNS drugs. Front Pharmacol. 2014;5:177. doi: 10.3389/fphar.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282C:13–22. doi: 10.1016/j.neuroscience.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway JA, Hardie SL, Whitaker SM, Baas SR, Zhang B, Bermingham DP, Lichtenstein AJ, Blakely RD. Forward genetic analysis to identify determinants of dopamine signaling in Caenorhabditis elegans using swimming-induced paralysis. G3 (Bethesda) 2012;2:961–975. doi: 10.1534/g3.112.003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway JA, Sturgeon SM, Snarrenberg CL, Li Z, Xu XZ, Bermingham DP, Odiase P, Spencer WC, Miller DM, 3rd, Carvelli L, Hardie SL, Blakely RD. Glial Expression of the Caenorhabditis elegans Gene swip-10 Supports Glutamate Dependent Control of Extrasynaptic Dopamine Signaling. J Neurosci. 2015;35:9409–9423. doi: 10.1523/JNEUROSCI.0800-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway JA, Wang J, Fleming PA, Fleming KA, Whitaker SM, Nackenoff A, Snarrenberg CL, Hardie SL, Zhang B, Blakely RD. An open-source analytical platform for analysis of C. elegans swimming-induced paralysis. J Neurosci Methods. 2014;232:58–62. doi: 10.1016/j.jneumeth.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway JA, Whitaker SM, Blakely RD. Media osmolarity modulates dopamine-dependent, swimming induced paralysis (SWIP) The Worm Breeder’s Gazette. 2010:18. [Google Scholar]
- Iwasaki K, Staunton J, Saifee O, Nonet M, Thomas JH. aex-3 encodes a novel regulator of presynaptic activity in C.elegans. Neuron. 1997;18:613–622. doi: 10.1016/s0896-6273(00)80302-5. [DOI] [PubMed] [Google Scholar]
- Izquierdo PG, Calahorro F, Ruiz-Rubio M. The dopamine reuptake inhibitor methylphenidate causes swimming- induced paralysis in C. elegans. The Worm Breeder’s Gazette. 2013:19. [Google Scholar]
- Jayanthi LD, Apparsundaram S, Malone MD, Ward E, Miller DM, Eppler M, Blakely RD. The Caenorhabditis elegans gene T23G5.5 encodes an antidepressant- and cocaine-sensitive dopamine transporter. Mol Pharmacol. 1998;54:601–609. [PubMed] [Google Scholar]
- Jorgensen EM, Hartwieg E, Schuske K, Nonet ML, Jin Y, Horvitz HR. Defective recycling of synaptic vesicles in synaptotagmin mutants of Caenorhabditis elegans. Nature. 1995;378:196–199. doi: 10.1038/378196a0. [DOI] [PubMed] [Google Scholar]
- McDonald PW, Hardie SL, Jessen TN, Carvelli L, Matthies DS, Blakely RD. Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J Neurosci. 2007;27:14216–14227. doi: 10.1523/JNEUROSCI.2992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald PW, Jessen T, Field JR, Blakely RD. Dopamine Signaling Architecture in Caenorhabditis elegans. Cell Mol Neurobiol. 2006;26:591–616. doi: 10.1007/s10571-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri NB, Saiardi A, Bonci A, Picetti R, Calabresi P, Bernardi G, Borrelli E. Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience. 1997;79:323–327. doi: 10.1016/s0306-4522(97)00135-8. [DOI] [PubMed] [Google Scholar]
- Miller KG, Alfonso A, Nguyen M, Crowell JA, Johnson CD, Rand JB. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc Natl Acad Sci. 1996;93:12593–12598. doi: 10.1073/pnas.93.22.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen GP, Mathews EA, Vu MH, Hunter JW, Frisby DL, Duke A, Grundahl K, Osborne JD, Crowell JA, Rand JB. Choline transport and de novo choline synthesis support acetylcholine biosynthesis in Caenorhabditis elegans cholinergic neurons. Genetics. 2007;177:195–204. doi: 10.1534/genetics.107.074120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman HN, Neal-Beliveau B, Nass R, Engleman EA. Chemosensory cue conditioning with stimulants in a Caenorhabditis elegans animal model of addiction. Behav Neurosci. 2012;126:445–456. doi: 10.1037/a0028303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet ML, Staunton JE, Kilgard MP, Fergestad T, Hartwieg E, Horvitz HR, Jorgensen EM, Meyer BJ. Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J Neurosci. 1997;17:8061–8073. doi: 10.1523/JNEUROSCI.17-21-08061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura DT, Clark DA, Samuel AD, Horvitz HR. Dopamine signaling is essential for precise rates of locomotion by C. elegans. PLoS One. 2012;7:e38649. doi: 10.1371/journal.pone.0038649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoplichko VI, Dani JA. Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage. Proc Natl Acad Sci U S A. 2006;103:11376–11380. doi: 10.1073/pnas.0600768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand JB. Acetylcholine. WormBook,WormBook; 2007. [Google Scholar]
- Ranganathan R, Sawin ER, Trent C, Horvitz HR. Mutations in the Caenorhabditis elegans serotonin reuptake transporter MOD-5 reveal serotonin-dependent and -independent activities of fluoxetine. J Neurosci. 2001;21:5871–5884. doi: 10.1523/JNEUROSCI.21-16-05871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safratowich BD, Lor C, Bianchi L, Carvelli L. Amphetamine activates an amine-gated chloride channel to generate behavioral effects in Caenorhabditis elegans. J Biol Chem. 2013;288:21630–21637. doi: 10.1074/jbc.M113.484139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Suo S, Sasagawa N, Ishiura S. Cloning and characterization of a Caenorhabditis elegans D2-like dopamine receptor. J Neurochem. 2003;86:869–878. doi: 10.1046/j.1471-4159.2003.01896.x. [DOI] [PubMed] [Google Scholar]
- Voglis G, Tavernarakis N. A synaptic DEG/ENaC ion channel mediates learning in C. elegans by facilitating dopamine signalling. Embo J. 2008;27:3288–3299. doi: 10.1038/emboj.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A, Walker VJ, Feng Z, Xu XZ. Cocaine modulates locomotion behavior in C. elegans. PLoS One. 2009;4:e5946. doi: 10.1371/journal.pone.0005946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Garriga G, Thomas JH. Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J Neurosci. 1995;15:6975–6985. doi: 10.1523/JNEUROSCI.15-10-06975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]