Abstract
Objective
In a sample of 368 postmenopausal women, we (1) determined within-cohort and between-cohort relationships between adjuvant systemic therapy for breast cancer and self-reported cognitive function during the first 18 months of therapy; and (2) evaluated the influence of co-occurring symptoms, neuropsychological function, and other covariates on relationships.
Methods
We evaluated self-reported cognitive function, using the Patient Assessment of Own Functioning Inventory (PAOFI), and potential covariates (e.g., co-occurring symptom scores, neuropsychological function z-scores) in 158 women receiving aromatase inhibitor (AI) therapy alone, 104 women receiving chemotherapy followed by AI therapy, and 106 non-cancer controls. Patients were assessed before systemic therapy and then every six months, for a total of four assessments over 18 months. Controls were assessed at matched time points. Mixed effects modeling was used to determine longitudinal relationships.
Results
Controlling for covariates, patients enrolled before chemotherapy reported poorer global cognitive function (p<0.001), memory (p<0.001), language and communication (p<0.001), and sensorimotor function (p=0.002) after chemotherapy. These patients reported poorer higher-level cognitive and intellectual functions from before chemotherapy to 12 months after initiation of AI therapy (p<0.001). Higher levels of depressive symptoms (p<0.001), anxiety (p<0.001), and fatigue (p=0.040) at enrollment were predictors of poorer cognitive function over time. PAOFI total score was a predictor of executive function (p=0.048) and visual working memory (p=0.005) z-scores, controlling for covariates.
Conclusions
Findings provide further evidence of poorer self-reported cognitive function after chemotherapy and of relationships between co-occurring symptoms and cognitive changes. AI therapy alone does not have an impact on self-reported cognitive function.
Keywords: cognition, aromatase inhibitors, postmenopause, breast neoplasms, cancer, oncology
Background
Women with breast cancer comprise the largest group of cancer survivors in the United States, with almost three million women alive today [1] due in part to advances in targeted therapies that prevent recurrence. Aromatase inhibitor (AI) therapy improves disease-free and overall survival for postmenopausal women with hormone-receptor positive breast cancer [2]. This oral anti-estrogen therapy is typically prescribed for at least a five-year course after mastectomy or breast conserving surgery [2]. Side effects of this therapy reported by some women include perceived changes in cognitive function, such as difficulty with concentration and recall [3]. These cognitive changes may negatively impact meaningful activities such as work, achievement of personal goals, and social interaction [4,5], as well as the ability to adhere to prescribed therapy [3]. Previous work by our group demonstrated that perceived cognitive changes negatively impacted long-term AI adherence more than a year after initiation of therapy [3]. Poorer adherence increases risk for recurrence and decreases long-term survival [6].
AI-associated cognitive changes may be mediated by estrogen deprivation [7,8]. Estrogen promotes synaptic and neural plasticity in the brain, which contributes to the growth and maintenance of neurons and white matter tracts [9,10]. Estrogen deprivation may result in brain alterations that manifest as variable phenotypes of objectively measured and self-reported cognitive changes. For women who receive chemotherapy followed by AI therapy, cognitive changes may initiate with chemotherapy [7], and may be potentiated or repaired more slowly because of reduced neural plasticity. Co-occurring symptoms may be associated with poorer cognitive function observed before systemic therapy and may influence cognitive decline over time [11].
Few studies have examined cognitive function associated with AI therapy in postmenopausal women with breast cancer. Self-report provides valuable information about patients’ perceptions of cognitive function, which may differ from results obtained using objective neuropsychological tests [12]. Objective measures were developed to assess cognitive disorders in patients with stroke, neurological trauma, or dementia [13]. Changes in cognitive function and underlying neural circuitry associated with breast cancer and its treatment may be more subtle than what occurs in these disorders. Therefore, inconsistencies between subjective and objective findings could be due to limited ecological validity and a lack of sensitivity of neuropsychological measures to the more subtle changes experienced by women with breast cancer [12]. It is also possible that patients do not notice or are not concerned about cognitive changes identified using neuropsychological measures.
A randomized controlled trial of anastrozole for chemoprevention in healthy women at high risk for breast cancer found no effect on perceived cognitive function during the first two years of therapy [14]. Studies of self-reported cognitive function in women with breast cancer found mixed relationships with anti-estrogen therapies (i.e., selective estrogen receptor modulators [SERMs], AIs). A recent study using the Patient Assessment of Own Functioning Inventory (PAOFI) found that these therapies were associated with poorer language and communication in the first six months of therapy [15]. A cross-sectional study conducted a mean of three years after the initiation of therapy using the Functional Assessment of Cancer Therapy Cognitive Scale and the Cognitive Symptom Checklist found associations among anti-estrogen therapies, poorer cognitive function, and worse mood [16]. A longitudinal study in which patients were assessed using the Cognitive Failures Questionnaire before and after cessation of anti-estrogen therapy found no change in self-reported cognitive function one year after completion of therapy [17], although neuropsychological performance improved during this period [18]. Because these studies included both premenopausal and postmenopausal women, it was not possible to determine the specific effect of AI therapy. No studies have evaluated self-reported cognitive functioning exclusively in postmenopausal women from before the initiation of adjuvant systemic therapy (i.e., a true pre-therapy assessment) through the first 18 months of therapy.
Therefore, the purposes of this study, in a multiple-cohort sample of 368 postmenopausal women, were to (1) determine within-cohort and between-cohort relationships between adjuvant systemic therapy for breast cancer and self-reported cognitive function during the first 18 months of therapy; and (2) evaluate the influence of co-occurring symptoms, neuropsychological function, and other covariates on these relationships.
Methods
Participants and settings
This analysis is part of a longitudinal study of cognitive function in postmenopausal women receiving the AI, anastrozole, for invasive breast cancer (CA107408) [19]. The University of Pittsburgh Institutional Review Board approved the study. Eligible women were postmenopausal and ≤75 years of age, were able to speak and read English, had completed a minimum of eight years of education, had no history of neurological illness or previous cancer (except non-melanoma skin cancer), were not receiving hormone replacement therapy, and had no hospitalizations for psychiatric illness within two years. Women with breast cancer were recruited 2005–2012 from the Magee-Women’s Breast Cancer Program of the University of Pittsburgh Cancer Institute. These women were newly diagnosed with stage I-IIIA breast cancer; had completed surgery; and were scheduled to receive anastrozole alone (n=158) or chemotherapy followed by anastrozole (n=104). We recruited a sample of postmenopausal controls without breast cancer (n=106), which was matched as closely as possible for age and education using frequency matching within a range of +/− three years of age. Controls were recruited during the same time period as patients through the University Center for Social and Urban Research via random digit dialing, response to an advertisement, or referral by patients in the study.
Instruments
Demographic and clinical characteristics were collected at enrollment, and clinical characteristics were verified using the medical record. The PAOFI was used to assess self-reported global cognitive function (i.e., total score) as well as higher-level cognitive and intellectual functions, memory, language and communication, and use of hands/sensory-perceptual function (i.e., sensorimotor function) using four subscales [20]. For each of 32 items, frequency of cognitive problems was rated on a six-point scale ranging from 0 (almost never) to 5 (almost always). Higher summed scores indicate poorer perceived functioning (range 0–160). The PAOFI was previously used in studies of women with breast cancer [15,21,12]. It has acceptable construct [20] and discriminant [22] validity. The PAOFI is unique among self-report cognitive measures in that it assesses sensorimotor function, which may be affected by neuropathies associated with chemotherapies (e.g., taxanes). Findings from factor analysis [20] support the combination of the original use of hands and sensory-perceptual subscales into one sensorimotor subscale. In the current study, internal consistency of the PAOFI as determined using Cronbach’s alpha was 0.92 for the total score, 0.88 for higher-level cognitive and intellectual functions, 0.82 for memory, 0.80 for language and communication, and 0.61 for sensorimotor function.
Potential covariates of self-reported cognitive function included age and well-validated measures of estimated verbal intelligence (National Adult Reading Test-Revised [23]), pain (Brief Pain Inventory [24]), depressive symptoms (Beck Depression Inventory-II [BDI-II] [25]), anxiety (Profile of Mood States [POMS] tension/anxiety subscale [26]), fatigue (POMS fatigue/inertia subscale [26]), and total symptom burden (Breast Cancer Prevention Trial [BCPT] symptom checklist [27]). Presence of neuropathic symptoms was evaluated by endorsement of the “numbness or tingling” item of the BCPT. As reported previously [19], neuropsychological function was assessed with a battery of measures evaluating multiple cognitive domains. See online supplemental table for a summary of the measures, outcome variables, and ranges of scores that comprised these domains.
Study procedures
Of the eligible women approached, 397 (49% response rate) provided written informed consent. All patients completed baseline assessments before initiating AI therapy. Patients receiving chemotherapy completed a baseline assessment before the first cycle and completed a pre-AI assessment after the last cycle. Patients were assessed every six months thereafter, for a total of four assessments over 18 months. The distribution of every-six month assessments was chosen to maximize the number of assessments while minimizing practice effects for neuropsychological tests. Controls were assessed at matched time points. Participants who completed at least the baseline assessment for self-reported cognitive and symptom measures (n=368) were included in this analysis.
Statistical methods
Descriptive statistics and frequency distributions were generated using SPSS Statistics 22 (IBM, New York) to characterize the three cohorts of participants and to identify data anomalies. Analysis of variance and the chi-square test were used to evaluate for differences among the cohorts at enrollment. Fisher’s exact test was used if unexpected cell counts existed. If data transformations did not correct for non-parametric distributions, the Kruskal-Wallis or Mann-Whitney U test was used. Baseline differences among the cohorts were considered statistically significant at p<0.05. For post hoc pairwise contrasts using the Bonferroni correction, p<0.017 (i.e., 0.05/3) was considered significant.
Because PAOFI sum scores were non-parametric, total and subscale scores were square-root transformed for longitudinal modeling. Multilevel regressions using STATA SE 13 (StataCorp, Texas) employed a backward stepwise approach to identify a parsimonious set of covariates from demographic, clinical, and symptom characteristics that significantly differed among the groups at baseline. Controlling for age and estimated verbal intelligence, significant covariates retained for final modeling included baseline levels of anxiety, depressive symptoms, and fatigue. Because performance in the domains of executive function, visual working memory, and concentration previously was found to vary significantly among the three cohorts [19], we evaluated for associations between PAOFI scores and these neuropsychological domains using a multi-level random intercepts model in which change over time was nested within individuals, which were nested within the cohorts.
Mixed effects modeling using SAS 9.4 (SAS Institute, North Carolina) was done to determine relationships between AI therapy and PAOFI scores within cohorts and between cohorts over time. Modeling proceeded in three steps: (1) an unadjusted model for the PAOFI total score was generated to determine how scores changed over time, (2) baseline differences in age and estimated verbal intelligence were controlled, and (3) covariate-adjusted final models were generated. Mean sum scores and standard errors reported in the figures were back-transformed from model-generated values. Where significant (i.e., p<0.05) overall cohort-by-time effects were found in the final models, within-cohort and between-cohort differences were evaluated using a more conservative alpha of 0.01 to reduce the risk of Type I error. Effect sizes were calculated using standardized mean difference (i.e., d).
Results
Differences among the cohorts
At enrollment, women scheduled to receive anastrozole alone were older (p’s<0.01), while controls without breast cancer had higher estimated verbal intelligence (p’s≤0.001), than other participants (Table 1). A greater proportion of women scheduled to receive chemotherapy followed by anastrozole had mastectomies and higher stage of disease. These patients reported greater average pain than controls (p=0.002) and greater anxiety than other participants (p’s≤0.001; Table 2). No differences were found between the patient cohorts in receipt of radiation therapy, weeks since diagnosis, or weeks since first surgery; and no differences were found among the three cohorts in other demographic or symptom characteristics. Participants who did not complete all time points of the study (n=177) reported poorer language and communication subscale scores (U=14,457.0; p=0.016) and greater total symptom burden (t=2.3, p=0.024) at enrollment than participants who completed the entire study (n=191). Although no differences in completion were found between the patient cohorts or between the women who received chemotherapy followed by anastrozole and controls, a smaller proportion of women who took anastrozole alone (46.2%) completed the entire study compared to controls (64.2%; p=0.006). See Online Supplemental Figure for CONSORT diagram.
Table 1.
Differences in demographic and clinical characteristics among the cohorts at enrollment (n=368)
| Characteristic | Controls n=106 (28.8%) | AI therapy alone n=158 (42.9%) | Chemotherapy followed by AI therapy n=104 (28.3%) | Statistic | p-value |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age (years) | 58.7 (5.91) | 61.7 (6.42) | 59.4 (5.49) | F(2,365)=9.1 | <0.001 |
| Education (years) | 14.7 (2.86) | 14.9 (2.85) | 14.8 (2.79) | KW=0.3 | 0.851 |
| NART-R score | 112.6 (9.14) | 108.7 (8.49) | 107.6 (8.66) | F(2,365)=9.7 | <0.001 |
| Weeks since diagnosis | 9.7 (4.15) | 9.6 (3.81) | t=0.3 | 0.788 | |
| Weeks since first surgery | 5.2 (3.00) | 5.2 (2.72) | t=0.1 | 0.901 | |
| Percent (n) | Percent (n) | Percent (n) | |||
| Married or partnered (yes) | 59.4 (63) | 63.3 (100) | 66.3 (69) | χ2=1.1 | 0.582 |
| White (yes) | 90.6 (96) | 97.5 (154) | 94.2 (98) | χ2=5.9 | 0.052 |
| Natural menopause (yes) | 84.9 (90) | 82.3 (130) | 80.8 (84) | χ2=1.3 | 0.535 |
| HRT-ever (yes) | 46.2 (49) | 52.5 (83) | 50.0 (52) | χ2=1.1 | 0.572 |
| Mastectomy (versus BCS) | 11.4 (18) | 20.2 (21) | FE | 0.048 | |
| Radiation therapy (yes) | 71.5 (113) | 69.2 (72) | FE | 0.348 | |
| Receipt of a taxane (yes) | 70.2 (73) | ||||
| Stage of disease: | |||||
| I | 86.1 (136) | 40.4 (42) | FE | <0.001 | |
| IIa | 11.4 (18) | 35.6 (37) | |||
| IIb | 2.5 (4) | 13.5 (14) | |||
| IIIa | 0.0 (0) | 10.6 (11) |
AI, aromatase inhibitor; SD, standard deviation; KW, Kruskal-Wallis Test; NART-R, North American Adult Reading Test (estimated verbal intelligence); HRT, hormone replacement therapy; BCS, breast conserving surgery; FE, Fisher’s Exact test.
Table 2.
Differences in self-reported symptom scores among the cohorts at enrollment (n=368)
| Score | Controls n=106 (28.8%) | AI therapy alone n=158 (42.9%) | Chemotherapy followed by AI therapy n=104 (28.3%) | Statistic | p-value |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| BPI average pain | 1.4 (2.30) | 2.0 (2.25) | 2.2 (2.15) | KW=10.5 | 0.005 |
| POMS tension/anxietya | 6.9 (6.11) | 6.8 (5.00) | 9.6 (6.18) | F(2,365)=9.7 | <0.001 |
| POMS fatigue/inertia | 5.6 (5.69) | 5.4 (6.04) | 5.6 (5.32) | KW=0.7 | 0.691 |
| BDI-II | 5.5 (6.38) | 5.0 (5.28) | 6.3 (6.43) | KW=3.4 | 0.183 |
| BCPT symptom checklista | 18.7 (14.65) | 18.8 (13.36) | 21.0 (13.51) | F(2,365)=1.7 | 0.181 |
AI, aromatase inhibitor; SD, standard deviation; BPI, Brief Pain Inventory; KW, Kruskal-Wallis Test; POMS, Profile of Mood States; BDI-II, Beck Depression Inventory II; BCPT, Breast Cancer Prevent Trial symptom checklist.
Although actual means and SDs are reported for each group, analysis of variance was performed on the square-root transformed variables for POMS tension-anxiety and BCPT symptom checklist scores.
Longitudinal findings
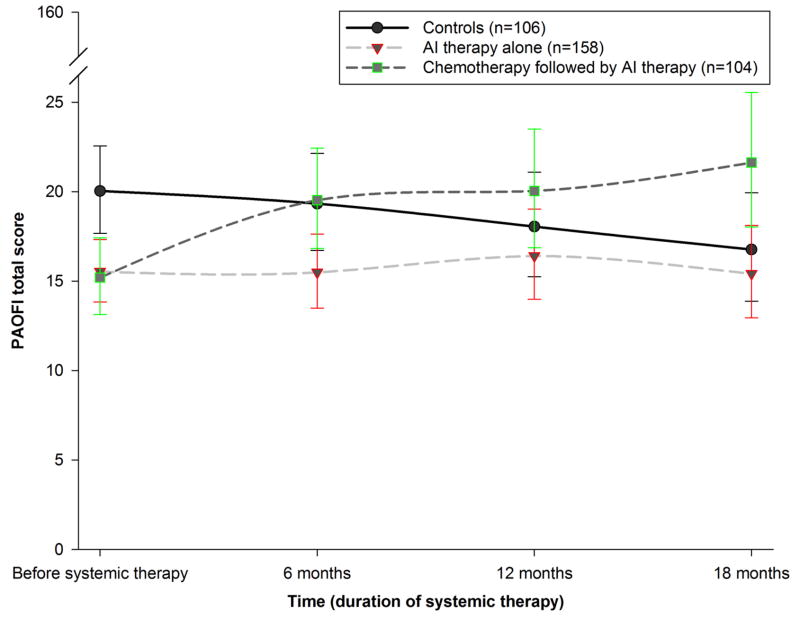
Figure 1 shows model-predicted, unadjusted trajectories for PAOFI total scores for the three cohorts. Controlling for age, estimated verbal intelligence, and symptom covariates (i.e., baseline BDI-II, POMS tension/anxiety, and POMS fatigue/inertia scores), an overall cohort-by-time effect was found (p<0.001). Women reported deterioration in global cognitive function from before the initiation of chemotherapy to after the last cycle of chemotherapy (p<0.001, d=0.23), which persisted after one year of anastrozole (p<0.001, d=0.29). Although controls reported poorer cognitive function than both patient cohorts at enrollment (p<0.01, d=0.15), no within-cohort or between-cohort differences were found after the initiation of anastrozole. Controls reported no change over time in global cognitive function.
Figure 1.
Unadjusted Patient Assessment of Own Functioning Inventory (PAOFI) total score trajectories for the three cohorts from before systemic therapy to 18 months after starting therapy, with standard errors of the model-predicted means. The cohort that received chemotherapy was assessed before chemotherapy and, at its second time point, after chemotherapy and before aromatase inhibitor therapy. Higher scores indicate poorer perceived global cognitive function (range of 0–160)
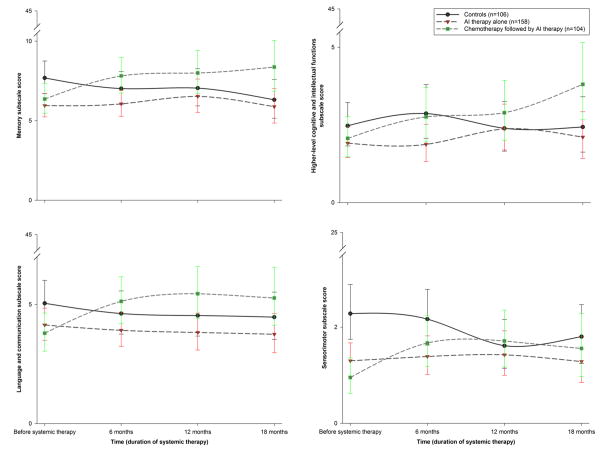
Figure 2a–d shows unadjusted cohort trajectories for PAOFI subscale scores. Controlling for covariates, overall cohort-by-time effects were found for all subscales (p’s<0.05). At enrollment, controls reported poorer memory than patients who would receive anastrozole alone (p=0.008, d=0.14), and poorer sensorimotor function than patients who would receive anastrozole alone (p=0.003, d=0.11) or chemotherapy followed by anastrozole (p<0.001, d=0.15). Patients who received chemotherapy reported poorer memory (p<0.001, d=0.15), language and communication (p<0.001, d=0.15), and sensorimotor function (p=0.002, d=0.13) from before to after chemotherapy. These changes did not improve until after six months of anastrozole for sensorimotor function (p=0.004, d=0.11) and persisted after one year of anastrozole for memory (p=0.005, d=0.18) and language and communication (p=0.005, d=0.15). Before the initiation of anastrozole, patients who received chemotherapy reported poorer memory than the women who would receive anastrozole alone (p=0.006, d=0.13). The poorer higher-level cognitive and intellectual functions subscale scores observed after chemotherapy were not significant, but these poorer scores became significant by 12 months after the initiation of AI therapy (p<0.001, d=0.23). No within-cohort or between-cohort differences in subscale scores were found during any six-month period after the initiation of anastrozole. Controls reported no significant change over time in subscale scores.
Figure 2.
a–d. Unadjusted Patient Assessment of Own Functioning Inventory subscale score trajectories for the three cohorts from before systemic therapy to 18 months after starting therapy, with standard errors of the model-predicted means. The cohort that received chemotherapy was assessed before chemotherapy and, at its second time point, after chemotherapy and before aromatase inhibitor therapy. Higher scores indicate poorer perceived function for each of the subscales (ranges of 0–45 for memory, language and communication, and higher-level cognitive and intellectual functions; 0–25 for sensorimotor)
Relationships with mood and neuropsychological performance
Controlling for age, estimated verbal intelligence, and cohort membership, we found that poorer baseline BDI-II (p<0.001, d=0.22), POMS tension/anxiety (p<0.001, d=0.20), and POMS fatigue/inertia (p=0.040, d=0.11) scores were significant predictors of poorer PAOFI total scores over time. Controlling for all other variables, lower estimated verbal intelligence was associated with poorer PAOFI total scores (p<0.001, d=0.22), but age was not associated with these scores.
Neuropsychological performance in three cognitive domains (i.e., executive function, visual working memory, concentration) previously was reported to differ significantly among the cohorts [19]. When controlling for PAOFI total score at baseline, time-varying total score was a significant predictor of executive function z-scores (p=0.048). This longitudinal association held for the higher-level cognitive and intellectual functions subscale score (p=0.015). PAOFI total score at baseline was a significant predictor of visual working memory z-scores (p=0.005), although the time-varying total score was not significant when controlling for baseline scores. This baseline association held for the higher-level cognitive and intellectual functions (p=0.003), language and communication (p=0.001), and sensorimotor (p=0.011) subscale scores. For all these associations, as PAOFI scores increased, indicating poorer self-reported cognitive function, z-scores decreased, indicating worse performance in these cognitive domains. The relationships did not change when controlling for symptom covariates. PAOFI scores were not related to concentration z-scores.
Conclusions
These findings provide further evidence of poorer self-reported cognitive function after chemotherapy but do not support perceived cognitive changes in the first 18 months of AI therapy alone. Because poor mood and fatigue are common predictors of poor self-reported cognitive function, these symptoms could account for associations between treatment and perceived cognitive function. However, controlling for age, estimated verbal intelligence, and these co-occurring symptoms did not remove the significant impact of chemotherapy on cognitive function, which persisted throughout the study. The effect sizes for these cognitive changes were small and may not have been clinically meaningful (i.e., d<.5), since mean sum scores corresponded to an average report of experiencing cognitive problems very infrequently. While on average women in the study did not report clinically meaningful cognitive changes, future studies should evaluate for subgroups of women at increased risk.
Clinically, the patient groups differed because treatment is based on disease characteristics. However, disease and treatment characteristics were not associated with cognitive changes in this study, which is consistent with findings from previous reports [28]. It is interesting that patients did not report worse cognitive function over time compared to postmenopausal controls. We explored whether differences in comorbidities experienced by the controls at baseline could explain this finding. The most common comorbidities for all participants were hypertension (42.1%, n=155), osteoarthritis (34.2%, n=126), and hyperlipidemia (27.4%, n=101). The frequency of type 1 (n=6) and type 2 (n=37) diabetes was 11.7%. Of these comorbidities, the cohorts differed only in the frequency of osteoarthritis, with a significantly lower proportion of the control group (22.6%, n=24) experiencing it than patients receiving chemotherapy followed by anastrozole (42.3%, n=44; FE p=0.002). Although this difference does not explain our finding, comorbidities may play an important role in perceived cognitive function. It is also possible that stress associated with either a diagnosis of cancer or comorbid conditions influences self-reported cognitive function during cancer therapy [29].
Poorer perceived cognitive function over time was associated with greater baseline levels of depressive symptoms, anxiety, and fatigue. However, most participants (89.9%, n=331) reported minimal depressive symptoms at baseline. Only 3.8% of participants (n=14) reported moderate to severe levels of depressive symptoms, which did not vary significantly among the cohorts. Moreover, participants on average reported significantly lower anxiety (p<.001) and fatigue (p<.001) than an adult female normative sample [26], which may in part be due to the fact that the normative sample included younger adults aged 18–65. Jenkins et al. [14] found that worse mood, but not receipt of anastrozole, was associated with poorer perceived cognitive function during chemoprevention in women at high risk for breast cancer. Biglia et al. [30] found that worse mood during chemotherapy was associated with poorer perceived cognitive function before anti-estrogen therapy in a mixed sample of pre- and postmenopausal women with breast cancer. Breckenridge et al. [16] found that, while mood was not associated with anti-estrogen therapy, worse mood was associated with poorer perceived cognitive function in women a mean of three years post primary therapy for breast cancer. Clinicians should evaluate women for poor mood before systemic therapy, because addressing these symptoms early may improve perceived cognitive function. Adherence to therapy may also improve [3]. However, poor mood does not explain all self-reported cognitive changes during cancer therapy [31], which suggests that interventions should be chosen depending on whether mood disturbance is present [32].
A recent study found that, while perceived cognitive function did not change during the first six months of AI therapy, cerebral metabolic activity did change compared to controls [33]. This finding suggests that AI therapy alters brain function but that this alteration is not noticeable by patients early in AI therapy. Although we similarly found no differences in self-reported cognitive function either within or between cohorts after initiation of anastrozole, we previously reported that differences were found during this time in three domains of neuropsychological function (i.e., executive function, visual working memory, concentration) [19]. The associations we found between self-reported cognitive function and the domains of executive function and visual working memory were significant, but the percent residual variance in domain z-scores explained by PAOFI scores was very small (i.e., <1%). It is possible that these neuropsychological changes were too subtle to be perceived or that women were not concerned about these changes in the first 18 months of therapy. However, Ganz et al. [12] found that poorer memory subscale scores on the PAOFI were associated with worse verbal memory performance and that, similar to our findings, poorer higher-level cognitive and intellectual functions subscale scores were associated with worse visual memory performance approximately seven months after breast cancer diagnosis compared to controls. In a cross-sectional study of women an average of 19 months after initiation of SERMs or AI therapy, Bender et al. [31] found that poorer PAOFI scores were associated with worse performance on individual neuropsychological tests of verbal learning and memory. Neuropsychological changes may become more noticeable with longer duration of AI therapy, and longitudinal neuroimaging assessments may help determine if AI therapy impacts underlying neural circuitry over time.
Receipt of taxanes by 70% of the women who received chemotherapy may have influenced our findings of poorer sensorimotor function and poorer PAOFI total scores after chemotherapy. Indeed, a greater proportion of women who received chemotherapy reported neuropathic symptoms (i.e., tingling and numbness; 50.6%; p<0.001) before beginning AI therapy compared to women who would receive anastrozole alone (25.9%) or controls (25.5%). Because the PAOFI is unique in its incorporation of sensorimotor function into its total score, our finding of perceived changes in cognitive function after chemotherapy might be explained in part by the occurrence of neuropathy in this cohort. However, significant worsening was found from before to after chemotherapy for memory, language and communication, and sensorimotor functioning. Therefore, it is unlikely that the sensorimotor subscale score alone is driving the finding of poorer overall cognitive function after chemotherapy for the total score.
Unlike a common finding in other studies, age was not a significant predictor of self-reported cognitive function. Younger patients generally report greater severity for symptoms than older patients [34]. Therefore, the lack of a relationship between age and perceived cognitive function may be due the inclusion of only postmenopausal women in our sample. The Critical Window Theory suggests that estrogen replacement is most protective of cognitive function during the peri-menopausal period [35]. Therefore, estrogen deprivation in the postmenopausal period after this critical window may not have a noticeable effect on cognitive function [36]. Accelerated aging may explain the effect of adjuvant therapies on cognitive function [37,7]. In a recent cross-sectional study conducted after mixed adjuvant therapies, poorer PAOFI language and communication scores were associated with electroencephalograph slowing, similar to what is found in aging [38]. Since postmenopausal women in our sample may already have experienced some aging effects, aging effects attributable to adjuvant systemic therapy may have been less noticeable to them.
Limitations
This study was limited by a sample of mostly white and well-educated women. Although we controlled for baseline differences in participant characteristics that remained in final models, differences among the cohorts may have influenced our findings. All patients received general anesthesia for breast cancer surgery prior to enrollment, which may have influenced their cognitive functioning. While we found no differences in the receipt of radiation therapy among the patient cohorts, radiation therapy has been associated with multiple symptoms [39], including long-term changes in cognitive function [40]. Although cognitive changes occurred primarily during the period of chemotherapy, without comparison to a cohort receiving chemotherapy alone it is not possible to evaluate additive effects of AI therapy after chemotherapy versus long-term effects of chemotherapy.
We did not evaluate the effect of specific comorbidities on trajectories of cognitive function. Attrition was greatest in the cohort that received AI therapy alone, which was due in part to the fact that 57.8% (n=48) of patients who dropped out were switched from anastrozole to other AI therapies, which excluded them from further participation in the study. Because these patients stopped taking anastrozole due to side effects, the women who continued in the study may have noticed less impact on cognitive function.
Implications
In summary, we found that chemotherapy followed by anastrozole, but not AI therapy alone, was associated with poorer self-reported cognitive function during adjuvant systemic therapy. The effects of other treatments, such as surgery and radiation therapy, may impact these relationships, although in this sample no differences were found in self-reported cognitive function compared to postmenopausal controls without breast cancer. Regardless of cohort membership, poorer self-reported cognitive function was associated with poorer mood and fatigue, as well as worse neuropsychological performance, which suggests that baseline characteristics of participants at higher risk may predict cognitive changes more so than treatment. Although women in our study did not report clinically meaningful cognitive changes on average, future studies should evaluate for subgroups of participants at higher risk for cognitive changes over the full course of AI therapy.
Supplementary Material
Summary of neuropsychological tests and outcome variables
Acknowledgments
This study was funded by a National Cancer Institute R01 (CA107408). Drs. Merriman and Myers were supported by a National Institute of Nursing Research (NINR) T32, Interdisciplinary Training of Nurse Scientists in Cancer Survivorship Research (NR011972). Dr. Merriman received additional support from the NINR T32, Targeted Research and Academic Training Program for Nurses in Genomics (NR009759). Dr. Phillips was supported as the Pittsburgh Foundation-Emmerling Endowed Chair of Psychotic Disorders.
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest.
Compliance with Ethical Standards
Ethical statement: All procedures involving human participants were done in accordance with the ethical standards of the University of Pittsburgh Institutional Review Board, and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all participants before enrollment in the study.
References
- 1.American Cancer Society. [Accessed August 7, 2015];Breast cancer facts & figures 2013–2014. http://www.cancer.org/research/cancerfactsstatistics/breast-cancer-facts-figures.
- 2.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: Update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28(23):3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender CM, Gentry AL, Brufsky AM, et al. Influence of patient and treatment factors on adherence to adjuvant endocrine therapy in breast cancer. Oncol Nurs Forum. 2014;41(3):274–285. doi: 10.1188/14.ONF.274-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cimprich B, Visovatti M, Ronis DL. The Attentional Function Index—A self-report cognitive measure. Psychooncology. 2011;20(2):194–202. doi: 10.1002/pon.1729. [DOI] [PubMed] [Google Scholar]
- 5.Myers JS. Chemotherapy-related cognitive impairment: The breast cancer experience. Oncol Nurs Forum. 2012;39(1):E31–40. doi: 10.1188/12.ONF.E31-E40. [DOI] [PubMed] [Google Scholar]
- 6.Makubate B, Donnan PT, Dewar JA, et al. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer. 2013;108(7):1515–1524. doi: 10.1038/bjc.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. J Clin Oncol. 2012;30(30):3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamniansawat S, Chongthammakun S. A priming role of local estrogen on exogenous estrogen-mediated synaptic plasticity and neuroprotection. Exp Mol Med. 2012;44(6):403–411. doi: 10.3858/emm.2012.44.6.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu F, Day M, Muniz LC, et al. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11(3):334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 10.Gibbs RB. Estrogen therapy and cognition: A review of the cholinergic hypothesis. Endocr Rev. 2010;31(2):224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berman MG, Askren MK, Jung M, et al. Pretreatment worry and neurocognitive responses in women with breast cancer. Health Psychol. 2014;33(3):222–231. doi: 10.1037/a0033425. [DOI] [PubMed] [Google Scholar]
- 12.Ganz PA, Kwan L, Castellon SA, et al. Cognitive complaints after breast cancer treatments: Examining the relationship with neuropsychological test performance. J Natl Cancer Inst. 2013;105(11):791–801. doi: 10.1093/jnci/djt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wefel JS, Vardy J, Ahles T, et al. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins VA, Ambroisine LM, Atkins L, et al. Effects of anastrozole on cognitive performance in postmenopausal women: A randomised, double-blind chemoprevention trial (IBIS II) Lancet Oncol. 2008;9(10):953–961. doi: 10.1016/S1470-2045(08)70207-9. [DOI] [PubMed] [Google Scholar]
- 15.Ganz PA, Petersen L, Castellon SA, et al. Cognitive function after the initiation of adjuvant endocrine therapy in early-stage breast cancer: An observational cohort study. J Clin Oncol. 2014;32(31):3559–3567. doi: 10.1200/JCO.2014.56.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breckenridge LM, Bruns GL, Todd BL, et al. Cognitive limitations associated with tamoxifen and aromatase inhibitors in employed breast cancer survivors. Psychooncology. 2012;21(1):43–53. doi: 10.1002/pon.1860. [DOI] [PubMed] [Google Scholar]
- 17.Ribi K, Aldridge J, Phillips KA, et al. Subjective cognitive complaints one year after ceasing adjuvant endocrine treatment for early-stage breast cancer. Br J Cancer. 2012;106(10):1618–1625. doi: 10.1038/bjc.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips KA, Aldridge J, Ribi K, et al. Cognitive function in postmenopausal breast cancer patients one year after completing adjuvant endocrine therapy with letrozole and/or tamoxifen in the BIG 1–98 trial. Breast Cancer Res Treat. 2011;126(1):221–226. doi: 10.1007/s10549-010-1235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bender CM, Merriman JD, Gentry AL, et al. Patterns of change in cognitive function with anastrozole therapy. Cancer. 2015;121(15):2627–2636. doi: 10.1002/cncr.29393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell MJ, Terhorst L, Bender CM. Psychometric analysis of the Patient Assessment of Own Functioning Inventory in women with breast cancer. J Nurs Meas. 2013;21(2):320–334. doi: 10.1891/1061-3749.21.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pullens MJ, De Vries J, Roukema JA. Subjective cognitive dysfunction in breast cancer patients: A systematic review. Psychooncology. 2010;19(11):1127–1138. doi: 10.1002/pon.1673. [DOI] [PubMed] [Google Scholar]
- 22.Richardson-Vejlgaard R, Dawes S, Heaton RK, et al. Validity of cognitive complaints in substance-abusing patients and non-clinical controls: The Patient's Assessment of Own Functioning Inventory (PAOFI) Psychiatry Res. 2009;169(1):70–74. doi: 10.1016/j.psychres.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bright P, Jaldow E, Kopelman MD. The National Adult Reading Test as a measure of premorbid intelligence: A comparison with estimates derived from demographic variables. J Int Neuropsychol Soc. 2002;8(6):847–854. doi: 10.1017/s1355617702860131. [DOI] [PubMed] [Google Scholar]
- 24.Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 25.Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II. The Psychological Corporation; San Antonio: 1996. [Google Scholar]
- 26.McNair D, Lorr M, Droppleman LF. Edits manual for the Profile of Mood States. EdITS/Educational and Industrial Testing Service; San Diego: 1992. [Google Scholar]
- 27.Terhorst L, Blair-Belansky H, Moore PJ, et al. Evaluation of the psychometric properties of the BCPT symptom checklist with a sample of breast cancer patients before and after adjuvant therapy. Psychooncology. 2011;20(9):961–968. doi: 10.1002/pon.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miaskowski C, Cooper BA, Melisko M, et al. Disease and treatment characteristics do not predict symptom occurrence profiles in oncology outpatients receiving chemotherapy. Cancer. 2014;120(15):2371–2378. doi: 10.1002/cncr.28699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreotti C, Root JC, Ahles TA, et al. Cancer, coping, and cognition: A model for the role of stress reactivity in cancer-related cognitive decline. Psychooncology. 2015;24(6):617–623. doi: 10.1002/pon.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biglia N, Bounous VE, Malabaila A, et al. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: A prospective study. Eur J Cancer Care. 2012;21(4):485–492. doi: 10.1111/j.1365-2354.2011.01320.x. [DOI] [PubMed] [Google Scholar]
- 31.Bender CM, Pacella ML, Sereika SM, et al. What do perceived cognitive problems reflect? J Support Oncol. 2008;6(5):238–242. [PMC free article] [PubMed] [Google Scholar]
- 32.Wefel JS, Kesler SR, Noll KR, et al. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65(2):123–138. doi: 10.3322/caac.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurria A, Patel SK, Mortimer J, et al. The effect of aromatase inhibition on the cognitive function of older patients with breast cancer. Clin Breast Cancer. 2014;14(2):132–140. doi: 10.1016/j.clbc.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cataldo JK, Paul S, Cooper B, et al. Differences in the symptom experience of older versus younger oncology outpatients: A cross-sectional study. BMC Cancer. 2013;13:6. doi: 10.1186/1471-2407-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherwin BB. Estrogen and cognitive functioning in women: Lessons we have learned. Behav Neurosci. 2012;126(1):123–127. doi: 10.1037/a0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berga SL. Anastrozole: Brain draining or sparing? Lancet Oncol. 2008;9(10):913–914. doi: 10.1016/S1470-2045(08)70240-7. [DOI] [PubMed] [Google Scholar]
- 37.Mandelblatt JS, Hurria A, McDonald BC, et al. Cognitive effects of cancer and its treatments at the intersection of aging: What do we know; what do we need to know? Semin Oncol. 2013;40(6):709–725. doi: 10.1053/j.seminoncol.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunter AM, Kwan L, Ercoli LM, et al. Quantitative electroencephalography biomarkers of cognitive complaints after adjuvant therapy in breast cancer survivors: A pilot study. Psychooncology. 2014;23(6):713–715. doi: 10.1002/pon.3487. [DOI] [PubMed] [Google Scholar]
- 39.Shibayama O, Yoshiuchi K, Inagaki M, et al. Association between adjuvant regional radiotherapy and cognitive function in breast cancer patients treated with conservation therapy. Cancer Med. 2014;3(3):702–709. doi: 10.1002/cam4.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stouten-Kemperman MM, de Ruiter MB, Koppelmans V, et al. Neurotoxicity in breast cancer survivors >/=10 years post-treatment is dependent on treatment type. Brain Imaging Behav. 2015;9(2):275–284. doi: 10.1007/s11682-014-9305-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of neuropsychological tests and outcome variables