Abstract
Development of effective treatments for alcohol use disorder (AUD) represents an important public health goal. This review provides a summary of completed preclinical and clinical studies testing pharmacotherapies for treatment of AUD. We discuss opportunities for improving the translation from preclinical findings to clinical trial outcomes, focusing on the validity and predictive value of animal and human laboratory models of AUD. Specifically, while preclinical studies of medications development have offered important insights into the neurobiology of the disorder and alcohol's molecular targets, limitations include the lack of standardized methods and streamlined processes whereby animal studies can readily inform human studies. Behavioral pharmacology studies provide a less expensive and valuable opportunity to assess the feasibility of a pharmacotherapy prior to initiating larger scale clinical trials by providing insights into the mechanism of the drug, which can then inform recruitment, analyses, and assessments. Summary tables are provided to illustrate the wide range of preclinical, human laboratory, and clinical studies of medications development for alcoholism. Taken together, this review highlights the challenges associated with animal paradigms, human laboratory studies and clinical trials with the overarching goal of advancing treatment development and highlighting opportunities to bridge the gap between preclinical and clinical research.
Keywords: addiction, valley of death, novel therapeutics
Introduction
Alcohol use disorder (AUD) has a major public health impact in the United States affecting nearly 18 million people and causing over 100,000 deaths annually (Bouchery et al., 2011; Grant et al., 2004; Harwood, 2000). Worldwide, alcohol abuse and misuse is the third leading risk factor for premature death and disabilities and is responsible for 4% of all deaths (2011). Although treatments for AUD have improved in past decades (Miller et al., 2011), there is still a great need to develop more effective interventions. Pharmacotherapies for AUD are used less often than psychosocial interventions (Fuller and Hiller-Sturmhofel, 1999), yet without a pharmacological adjunct to psychosocial therapy nearly three quarters of patients resume drinking within 1 year (Johnson, 2008). The limited use of pharmacotherapy for AUD is due, in part, to the relative lack of pharmacological options to successfully treat these disorders (Edlund et al., 2012). As such, development of effective treatments for AUD represents an important public health goal (Bouchery et al., 2011; Heilig and Egli, 2006; Johnson, 2010; Johnson et al., 2007; Steensland et al., 2007).
Litten and colleagues (2012) have argued that there are three overarching aims for ensuring the successful development of novel therapeutics for AUD: 1) improve the drug development process, 2) identify more effective therapeutics and/or use personalized medicine, and 3) enable the use of these novel medications in clinical practice (Litten et al., 2012). In order to achieve these goals, Litten and colleagues emphasize the importance of bridging the gap between preclinical and clinical research. In this paper, we will provide a perspective on medications development and a review of the pharmacotherapies for AUD that have been tested using animal paradigms, human laboratory paradigms and clinical trials focusing on the validity and predictive value of animal and human laboratory models of AUD. To do so, we will first discuss the neural targets of alcohol in relation to medications development including both the traditional targets such as ligand-gated ion channels and the endogenous opioid system, and novel targets such as ghrelin and neuropeptide Y (NPY). We will then delve into a review of the literature focused on identifying the challenges associated with animal paradigms, human laboratory studies and clinical trials with the overarching goal of advancing treatment development and highlighting opportunities to bridge the gap between preclinical and clinical research.
Neural Targets of Alcohol
One of the major obstacles for developing effective drugs for the treatment of AUD is that alcohol does not have a single molecular target but instead acts on a variety of different neurotransmitter receptors, ion channels, transporters and pathways in the central nervous system (CNS) to exert its behavioral effects [for review see (Gilpin and Koob, 2008; Koob and Volkow, 2010; Soderpalm and Ericson, 2013; Spanagel, 2009; Weiss and Porrino, 2002)]. Although not the focus of this review, we will briefly introduce some of the more prominent targets as they relate to medications development for AUD.
A long-standing belief is that alcohol interacts with the mesolimbic dopamine (DA) pathway to produce its behavioral effects [for review see (Gonzales et al., 2004; Pierce and Kumaresan, 2006)]. Specifically, DA release in the nucleus accumbens (NAc) is thought to be central in the motivation and positive reinforcement associated with acute alcohol administration. Alcohol causes an increase in synaptic DA concentration in the NAc similar to other drugs of abuse (Di Chiara and Imperato, 1988; Gessa et al., 1985). Importantly, many of the targets described below do indirectly affect DA neurotransmission.
Ligand-gated ion channels are widely held to play an important role in ethanol-induced behaviors [for review see (Dopico and Lovinger, 2009; Harris et al., 1995; Spanagel, 2009)]. Research in this area has focused on investigating the effects of ethanol on two large superfamilies of ligand-gated ion channels. The first is the Cys-loop superfamily including nicotinic acetylcholine receptors (nAChRs), 5-hydroxytryptamine type 3 receptor (5-HT3Rs), GABAARs and glycine receptors. Varenicline, an FDA-approved smoking cessation aid, is a full and partial agonist at several nAChR subtypes and has been shown to attenuate the reinforcing effects associated with alcohol in both mice (Blomqvist et al., 1996; Steensland et al., 2007) and humans (Fucito et al., 2011; Litten et al., 2013; Mitchell et al., 2012c), while others suggest it might be effective in reducing alcohol consumption by exacerbating the negative effects of alcohol (Childs et al., 2012; Kamens et al., 2010). Ondansetron, a 5-HT3R antagonist has been shown to decrease alcohol intake in preclinical studies (Tomkins et al., 1995) and decrease alcohol intake in early onset alcoholics in several clinical trials (Johnson et al., 2000; Kranzler et al., 2003) possibly through decreasing alcohol craving and diminishing the pleasurable effects associated with alcohol [for review see (Ye et al., 2001)]. The second superfamily of ligand-gated ion channels that are targets for alcohol action is the glutamate superfamily with members including α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), kainate receptors and N-methyl-d-aspartate receptors (NMDARs) [for review see (Dodd et al., 2000; Moykkynen and Korpi, 2012; Tsai and Coyle, 1998)]. Acamprosate, one of three FDA approved medications for AUD, is an NMDAR antagonist and has been shown to prevent relapse in alcohol dependent individuals acting as an anti-craving medication [for review see (Littleton, 1995; Witkiewitz et al., 2012)]. Additionally, memantine, another NMDAR antagonist, currently used in the treatment of moderate to severe dementia, has shown great promise in preclinical studies (Piasecki et al., 1998; Sabino et al., 2013), yet the sole clinical study conducted on memantine for AUD yielded negative results (Evans et al., 2007).
P2X receptors (P2XRs) constitute a third superfamily of ligand-gated ion channels that are becoming a focus of investigation in neuroscience and alcohol studies [for review see (Asatryan et al., 2011)]. Preclinical studies suggest that ivermectin, a selective, positive allosteric modulator of P2X4R, is able to decrease alcohol self-administration in wildtype mice using multiple models of alcohol intake but to a lesser extent in P2X4R knock out mice (Wyatt et al., 2014; Yardley et al., 2012).
Another well-known target of alcohol in the CNS is the endogenous opioid system [for review see (Gianoulakis et al., 1996; Herz, 1997)]. There are 3 known opioid receptor subtypes: μ, δ, and κ. In addition to endogenous opioid peptides: β-endorphins, enkephalins, and dynorphins, exogenous ligands, such as morphine, also act on the opioid receptors. Naltrexone, one of the three drugs approved by the FDA for the treatment of AUD, blocks opioid receptors and is believed to decrease the reinforcing effects of alcohol [for review see (Johnson, 2008)]. Nalmefene, another opioid receptor antagonist with a mechanism of action similar to naltrexone, is currently being developed as a medication for AUD in the United States but has already received European marketing authorization [for review see (Paille and Martini, 2014)].
Novel targets are being actively explored. One such novel targets is the ghrelin receptor. Ghrelinis known to stimulate food consumption through indirect interaction with the hypothalamus; however, there is evidence that it also plays an important role in alcohol consumption [for review see (Vadnie et al., 2014)]. Additional studies suggest ghrelin might also play a role in alcohol craving (Leggio et al., 2012; Leggio et al., 2014), reward (Jerlhag et al., 2009), withdrawal and relapse (Suchankova et al., 2013), but the exact role of ghrelin in mediating the behavioral effects of alcohol remains unknown.
The endocannabinoid (EC) system and its involvement in alcohol dependence have received much attention since the identification of the cannabinoid 1 receptor (CB1) [for review see (Ciccocioppo et al., 2009; Hungund and Yaragudri, 2009; Pacher et al., 2006; Pava and Woodward, 2012)]. Due to the comorbidity of cannabis use and AUD, it has been suggested that cannabis and alcohol may act on similar targets in the CNS. Rimonabant, a cannabinoid receptor 1 blocker, appears to be effective in reducing consumption in multiple preclinical models of alcohol self-administration (Arnone et al., 1997; Cippitelli et al., 2005; Gessa et al., 2004), clinical studies conducted thus far do not support the use of rimonabant for treatment of AUD (George et al., 2010; Soyka et al., 2008).
There are a number of stress-related neuropeptides that have been implicated as important targets for alcohol such as NPY, corticotropin-releasing factor (CRF) and nociceptin/orphanin FQ (N/OFQ) signaling [for review see (Ciccocioppo et al., 2009; Heilig and Egli, 2006)]. NPY is believed to play a role in alcohol intake, dependence and withdrawal via interruption of NPY signaling by alcohol [for review see (Thiele and Badia-Elder, 2003; Thorsell, 2007; Vadnie et al., 2014)]. NPY is an endogenous ligand shown to have anxiolytic and anti-depressant properties that might contribute to its ability to attenuate alcohol consumption. Corticotropin-releasing factor is another stress-related neuropeptide and appears to be involved in excessive alcohol consumption in post-dependent animals, stress-induced reinstatement of alcohol seeking, and anxiety associated with alcohol withdrawal [for review see (Heilig and Koob, 2007)]. Lastly, N/OFQ, an endogenous ligand for the nociception receptor (NOP), has been shown to block drug-induced increases in extracellular DA in the NAc [for review see (Heilig and Egli, 2006)].
Neurotrophic factor signaling represents an important target for medications development for AUD [for review see (Janak et al., 2006; Russo et al., 2009)]. Multiple neurotrophins such as brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3) and neurotrophin 4 (NT4) have been implicated in drug addiction [for review see (Janak et al., 2006)]. In more recent years, the neuroimmune signaling pathway has garnered attention as a probable target for alcohol action, specifically in regards to its role in intoxication, negative affect, and craving [for review see (Coller and Hutchison, 2012; Mayfield et al., 2013). Both human and animal studies provide support for the role of alcohol-induced neuroimmune signaling [for review see (Coller and Hutchison, 2012)]. Pioglitazone, a peroxisome proliferator-activated receptor agonist, has generated positive results in preclinical studies but results from clinical studies have not yet been published [for review see (Robinson et al., 2014)].
Despite the long list of implicated targets of alcohol action, demonstrations in humans are still lacking and the specific contributions of these targets are only recently beginning to be explored (Mitchell et al., 2012b). Molecular targets such as the cys loop and glutamate superfamily of ligand-gated ion channels and the mesolimbic dopamine pathway are widely accepted as for alcohol action (Johnson, 2008). Others, such as P2X4Rs, ghrelin receptors (Vadnie et al., 2014), the EC system (Johnson, 2008), and neuroimmune signaling [for review see (Coller and Hutchison, 2012; Mayfield et al., 2013) have been clinically investigated as possible targets of alcohol action more recently. These targets have been the focus of medications development for AUD. Table 1 details medications that have previously undergone or are currently undergoing testing that were identified from clinicaltrials.gov. The primary indication and mechanism of action is listed for each. In the following sections, using the medications included in Table 1, we will discuss 3 different stages of medications development for AUD: preclinical, human laboratory and clinical research. For each stage, we will briefly discuss commonly used paradigms, limitations associated with these models, and recommendations to increase the successful translation of a drug from preclinical to clinical research. Not all medications in Table 1 have been tested in each stage of drug development and as a result, these medications are excluded from subsequent tables as no results are yet published.
Table 1. Identified from actively studied medications and completed trials for the treatment of AUD (registered to Clinicaltrials.gov).
| Name | Primary Indication | Primary Mechanism of Action |
|---|---|---|
| Disulfiram | Alcohol dependence | Blocks ethanol metabolism |
| Naltrexone | Alcohol dependence | Opioid antagonist |
| Acamprosate | Alcohol dependence | Glutamatergic activity modulator* |
| Nalmefene | Opioid dependence | Opioid receptor antagonist |
| Ondansetron | Antiemetic | 5-HT3 receptor antagonist |
| LY686017 | Antiemetic ** | Neurokinin-1 (NK-1) antagonist |
| Topiramate | Anticonvulsant | Glutamate & GABAA receptor modulator |
| Zonisamide | Anticonvulsant | Sodium channel blocker and calcium channel modulator * |
| Levetiracetam | Anticonvulsant | Interaction with synaptic vesicle protein SV2A * |
| Gabapentin | Analgesic/ anticonvulsant | Modulation of GABA synthesis and glutamate synthesis * |
| Pregabalin | Neuropathic pain/ anticonvulsant | Binds with high affinity to the α2-delta site on voltage-gated calcium channels |
| Baclofen | Anti-spasmodic | GABAB receptor agonist |
| Ivermectin | Antiparasitic | Glutamate-gated chloride channels |
| Minocycline | Antibiotic – acne/ infections | Inhibition of protein synthesis |
| Ibudilast | Bronchodilator/ vasodilator | Phosphodiesterase inhibitor |
| Varenicline | Smoking cessation | nACH receptor partial agonist |
| Mifepristone | Antiprogestational activity | Progesterone receptor antagonist |
| Oxytocin | Labor induction | Oxytocin receptors |
| ABT-436 | Anxiety/ Major depressive disorder ** | HPA axis normalization via pituitary V1B antagonism |
| Memantine | Moderate- severe dementia | NMDA receptor antagonist |
| Pioglitazone | Antidiabetic | PPARγ agonist |
| Mecamylamine | Antihypertensive | Non competitive nACh receptor antagonist |
| Prazosin | Antihypertensive | Relaxant action on vascular smooth muscle; Postsynaptic alpha-adrenoceptors blocker * |
| Psilocybin | Psychomimetic | 5HT2A serotonin receptor |
| Olanzapine | Antipsychotic | D2 receptor antagonist and 5HT2 receptor antagonist |
| Doxazosin | Benign prostatic hyperplasia | Selective inhibitor of the α1-subtype of α adrenergic receptors |
| Dutasteride | Benign prostatic hyperplasia | 5α-reductase inhibitor |
| Mirtazapine | Antidepressant | α2 adrenergic receptor antagonist * |
| Rimonabant | Obesity** | CB1 endocannabinoid antagonist |
Note:
Current beliefs presented as the exact mechanism remains unknown;
Not FDA approved for this indication; Not all trials are registered to Clinicaltrials.gov
Animal Paradigms
After considering the molecular targets of alcohol itself, we turn our attention to medications development for AUD at the preclinical level. Table 2 provides a detailed summary of preclinical studies using multiple animal paradigms thought to model different facets of alcoholism with the ultimate goal of testing medications that can be advanced from preclinical to clinical testing. To that end, one of the most common and important phenotypes studied using animal models is alcohol intake. There are numerous paradigms used to model social drinking, excessive alcohol consumption, and operant self-administration of alcohol in animals. The two-bottle choice paradigm is a frequently used model of social drinking because animals do not generally achieve clinically relevant blood alcohol contents [BACs; for review see (Crabbe et al., 2011; Tabakoff and Hoffman, 2000)]. In the two-bottle choice paradigm, animals have continuous access to one bottle of alcohol and one bottle of water and are able to choose freely between the two. Chronic intermittent access, scheduled high alcohol consumption, drinking in the dark, and chronic intermittent vapor exposure are some of the more commonly employed animal models of excessive alcohol consumption [for review see (Becker and Ron, 2014; Crabbe et al., 2011)]. There are numerous variations to each paradigm; however, in each case, the animals reach intoxicating BACs. Operant self-administration is unique in that it allows for evaluation of the animal's motivation to consume alcohol [for review see (Cunningham et al., 2000; Tabakoff and Hoffman, 2000)]. In this paradigm, animals are trained to press a lever to receive alcohol, however, the frequency of access to alcohol, amount of alcohol available, and number of lever presses required to gain access to alcohol can be adapted.
Table 2.
Effect of drugs on animal models of AUD.
| Medication | Model | Effect | References |
|---|---|---|---|
| Naltrexone | 24-h access two-bottle choice voluntary intake | Decreased alcohol intake | (Froehlich et al., 1990) |
| Decreased alcohol intake in h/mOPRM1-118GG mice only (no effect in 118AA mice) | (Bilbao et al., 2015) | ||
| Operant self-administration | Decreased operant self-administration of alcohol | (Bilbao et al., 2015; Gonzales and Weiss, 1998; Le et al., 1999; Steensland et al., 2007; Tanchuck et al., 2011; Walker and Koob, 2008) | |
| Scheduled high alcohol consumption | Decreased alcohol intake | (Tanchuck et al., 2011) | |
| Drinking in the dark | Decreased alcohol intake | (Kamdar et al., 2007) | |
| Limited access two-bottle choice voluntary intake | Decreased alcohol intake | (Ji et al., 2008) | |
| Operant binge drinking | Decreased alcohol intake | (Ji et al., 2008) | |
| Alcohol- induced locomotion | Suppressed alcohol-induced locomotion (higher dose needed for C57BL/6 mice compared to BALB/c and DBA/2 mice | (Kiianmaa et al., 1983) | |
| Alcohol discrimination | Failed to alter discrimination of alcohol | (Middaugh et al., 1999) | |
| Alcohol-induced mesolimbic dopamine release | Prevented alcohol-induced mesolimbic dopamine release | (Gonzales and Weiss, 1998) | |
| Alcohol deprivation effect | Diminished alcohol deprivation effect (naltrexone + acamprosate also reduced ADE) | (Heyser et al., 2003) | |
| Alcohol-induced reinstatement of alcohol-seeking behavior | Diminished alcohol-induced reinstatement | (Le et al., 1999) | |
| Stress-induced reinstatement of alcohol-seeking behavior | No effect | (Le et al., 1999) | |
| Intravenous self-administration | Dose dependently decrease self-administration in rhesus monkeys | (Altshuler et al., 1980) | |
|
| |||
| Acamprosate | 24-h access two-bottle choice voluntary intake | Decreased alcohol intake in high-preference rats; No effect on low-preference rats | (Oka et al., 2013) |
| Limited access two-bottle choice voluntary intake | Decreased alcohol intake | (Olive et al., 2002) | |
| Alcohol-induced mesolimbic dopamine release | Suppressed alcohol-induced mesolimbic dopamine release | (Olive et al., 2002) | |
| Drinking in the dark | Decreased alcohol intake | (Gupta et al., 2008) | |
| Alcohol discrimination | Failed to alter discrimination of alcohol | (Spanagel et al., 1996c) | |
| Operant self-administration | No effect in alcohol preferring rats | (Spanagel et al., 2014) | |
| Alcohol deprivation effect | Diminished alcohol deprivation effect | (Heyser et al., 1998; Oka et al., 2013; Spanagel et al., 1996a) | |
| No effect | (Spanagel et al., 2014) | ||
| Alcohol withdrawal | Reduced some withdrawal signs | (Spanagel et al., 1996b) | |
| Cue-induced reinstatement of alcohol-seeking behavior | Reduced ethanol-paired cue effects | (Bachteler et al., 2005) | |
| No effect | (Spanagel et al., 2014) | ||
|
| |||
| Nalmefene | Operant self-administration | Decreased operant self-administration of alcohol | (Bilbao et al., 2015; Nealey et al., 2011; Walker and Koob, 2008) |
| Fluid deprivation + Limited access two-bottle choice voluntary intake | Decreased alcohol intake | (Hubbell et al., 1991) | |
|
| |||
| Ondansetron | Limited access two-bottle choice voluntary intake | Decreased alcohol intake | (Tomkins et al., 1995) |
| Alcohol withdrawal | Reduced withdrawal signs | (Costall et al., 1990) | |
| Operant self-administration | No effect | (Beardsley et al., 1994) | |
| Stress-induced reinstatement of alcohol-seeking behavior | Diminished stress-induced reinstatement | (Le et al., 2006) | |
|
| |||
| LY686017 | Insufficient affinity for the mouse or rat NK1R | (George et al., 2008) | |
|
| |||
| Topiramate | 24-h access two-bottle choice voluntary intake | Decreased alcohol intake at 2-h time point (50 mg/kg dose) and increased alcohol intake at 23-h time point (25 mg/kg dose) in C57BL/6J | (Gabriel and Cunningham, 2005) |
| Decreased alcohol intake at 2-h time point but not at 21-h time point in C57BL/6J | (Ngyuen et al., 2007) | ||
| Decreased alcohol intake in P rats; No effect in Wistar rats | (Breslin et al., 2010) | ||
| Three-bottle choice voluntary intake | No effect | (Breslin et al., 2010) | |
| Limited access alcohol only | Decreased alcohol intake | (Knapp et al., 2007a) | |
| Alcohol-induced motor locomotion | No effect | (Ngyuen et al., 2007) | |
| Alcohol withdrawal | Reduced alcohol withdrawal signs | (Farook et al., 2007) | |
|
| |||
| Zonisamide | Limited access alcohol only | Decreased alcohol intake | (Knapp et al., 2007a) |
|
| |||
| Levetiracetam | 24-h access two-bottle choice voluntary intake | Decreased in alcohol intake | (Zalewska-Kaszubska et al., 2011) |
| Alcohol-induced motor locomotion | Decreased alcohol-induced motor locomotion | (Robinson et al., 2013) | |
| Drinking in the dark | Increased alcohol intake | (Fish et al., 2014) | |
| Intermittent access two-bottle choice | Decreased alcohol intake | (Fish et al., 2014) | |
|
| |||
| Gabapentin | Operant self-administration | Decreased operant self-administration of alcohol in dependent rats; No effect in non-dependent rats | (Roberto et al., 2008) |
| Alcohol-induced anxiety | Increased % time spent in open arms in plus-maze in ethanol-injected rats only | (Roberto et al., 2008) | |
|
| |||
| Pregabalin | 24-h access two-bottle choice voluntary intake | Decreased alcohol intake | (Stopponi et al., 2012) |
| Operant self-administration | Decreased operant self-administration of alcohol; No effect on operant responding for food | (Stopponi et al., 2012) | |
| Stress-induced reinstatement of alcohol-seeking behavior | Inhibited reinstatement | (Stopponi et al., 2012) | |
| Cue-induced reinstatement of alcohols-seeking behavior | Diminished cue-induced reinstatement | (Stopponi et al., 2012) | |
|
| |||
| Baclofen | Alcohol withdrawal | Decrease in total score of intensity of ethanol withdrawal in dependent rats | (Colombo et al., 2000) |
| Reduced withdrawal signs in ethanol-withdrawn rats | (Knapp et al., 2007b) | ||
| 24-h access two-bottle choice voluntary intake | Decreased alcohol intake | (Colombo et al., 2000) | |
| Scheduled high alcohol consumption | Decreased alcohol intake | (Tanchuck et al., 2011) | |
| Operant self-administration | No effect | (Tanchuck et al., 2011) | |
| Decreased operant self-administration of alcohol in dependent and non-dependent rats | (Walker and Koob, 2007) | ||
| Decreased alcohol-reinforced responding | (Besheer et al., 2004) | ||
| Alcohol-induced locomotion | Suppressed alcohol-induced locomotion | (Besheer et al., 2004; Broadbent and Harless, 1999; Chester and Cunningham, 1999) | |
| Alcohol deprivation effect | Diminished alcohol deprivation effect | (Colombo et al., 2003) | |
| Cue-induced reinstatement of alcohol-seeking behavior | Diminished cue-induced reinstatement | (Maccioni et al., 2008) | |
|
| |||
| Ivermectin | 24-h access two-bottle choice voluntary intake | Decreased alcohol intake | (Asatryan et al., 2014; Yardley et al., 2012; Yardley et al., 2014) |
| Intermittent limited access | Decreased alcohol intake | (Yardley et al., 2012) | |
|
| |||
| Minocycline | 24-h access two-bottle choice voluntary intake | Decreased alcohol intake | (Agrawal et al., 2011) |
|
| |||
| Ibudilast | Limited access two-bottle choice voluntary intake | Decreased alcohol intake | (Bell et al., 2013) |
|
| |||
| Varenicline | Operant self-administration | Decreased operant self-administration of alcohol | (Steensland et al., 2007; Wouda et al., 2011) |
| 24-h access two-bottle choice voluntary intake | Decreased alcohol intake | (Steensland et al., 2007) | |
| Intermittent access two-bottle choice | Decreased alcohol intake | (Steensland et al., 2007) | |
| Cue-induced reinstatement of alcohol-seeking behavior | Diminished cue-induced reinstatement | (Wouda et al., 2011) | |
|
| |||
| Mifepristone | Limited access two-bottle choice voluntary intake | Decreased alcohol intake | (Koenig and Olive, 2004) |
| Alcohol withdrawal | Reduced withdrawal signs | (Jacquot et al., 2008; Sharrett-Field et al., 2013) | |
| Operant self-administration | Decreased operant self-administration of alcohol in dependent rats | (Vendruscolo et al., 2012) | |
| Stress-induced reinstatement of alcohol-seeking behavior | Diminished stress-induced reinstatement | (Simms et al., 2012) | |
|
| |||
| Oxytocin | Alcohol withdrawal | Reduced withdrawal signs | (Szabo et al., 1987) |
| Operant self-administration | Decreased preference for alcohol relative to sucrose | (McGregor and Bowen, 2012) | |
|
| |||
| Memantine | Limited access two-bottle choice voluntary intake | Decreased alcohol intake | (Piasecki et al., 1998) |
| Operant self-administration | No effect | (Piasecki et al., 1998) | |
| Decreased operant self-administration of alcohol | (Sabino et al., 2013) | ||
| Alcohol withdrawal | Reduced withdrawal signs | (Lukoyanov and Paula-Barbosa, 2001) | |
| Alcohol deprivation effect | Diminished alcohol deprivation effect | (Holter et al., 1996) | |
|
| |||
| Pioglitazone | 24-h access two-bottle choice voluntary intake | Decreased alcohol intake | (Stopponi et al., 2011) |
| Operant self-administration | Decreased operant self-administration of alcohol | (Stopponi et al., 2011) | |
| Stress-induced reinstatement of alcohol-seeking behavior | Diminished stress-induced reinstatement | (Stopponi et al., 2011) | |
| Cue-induced reinstatement of alcohol-seeking behavior | No effect | (Stopponi et al., 2011) | |
| Alcohol withdrawal | Reduced withdrawal signs | (Stopponi et al., 2011) | |
|
| |||
| Mecamylamine | 24-h access two-bottle choice voluntary intake | Decreased alcohol intake | (Farook et al., 2009) |
| Alcohol-induced dopamine release | Prevented alcohol-induced dopamine release | (Blomqvist et al., 1997; Ericson et al., 1998; Larsson et al., 2002) | |
| Limited access two-bottle choice voluntary intake | Decreased alcohol intake | (Ericson et al., 1998; Ford et al., 2009; Le et al., 2000) | |
| Alcohol-induced locomotion | Suppressed alcohol-induced locomotion | (Bhutada et al., 2010; Blomqvist et al., 1992; Kamens and Phillips, 2008; Larsson et al., 2002) | |
| Conditioned place preference (CPP) | Prevented development, expression, and reinstatement of ethanol-induced CPP | (Bhutada et al., 2012) | |
| Stress-induced reinstatement of CPP | Blocked stress-induced reinstatement of ethanol-induced CPP | (Bhutada et al., 2012) | |
| Operant self-administration | Decreased operant self-administration of alcohol | (Ford et al., 2008; Kuzmin et al., 2009; Nadal et al., 1998) | |
| Alcohol deprivation effect | Diminished alcohol deprivation effect | (Kuzmin et al., 2009) | |
| Drinking in the dark | Decreased alcohol intake | (Hendrickson et al., 2009) | |
|
| |||
| Prazosin | Intermittent access two-bottle choice | Decreased alcohol intake | (Skelly and Weiner, 2014) |
| Limited access two-bottle choice voluntary intake | Decreased alcohol intake | (Froehlich et al., 2013; Rasmussen et al., 2009) | |
| Operant self-administration | Decreased operant self-administration of alcohol | (Verplaetse et al., 2012) | |
| Stress-induced reinstatement of alcohol-seeking behavior | Diminished stress-induced reinstatement | (Le et al., 2011) | |
|
| |||
| Olanzapine | Limited access two-bottle choice voluntary intake | Decreased alcohol intake | (Ingman and Korpi, 2006) |
| Alcohol withdrawal | Reduced some withdrawal signs | (Unsalan et al., 2008) | |
|
| |||
| Doxazosin | Limited access two-bottle choice voluntary intake | Decreased alcohol intake | (O'Neil et al., 2013) |
|
| |||
| Rimonabant | Limited access two-bottle choice voluntary intake | Decreased alcohol intake | (Arnone et al., 1997; Colombo et al., 1998; Dyr et al., 2008; Gessa et al., 2004) |
| Operant self-administration | Decreased operant self-administration of alcohol | (Cippitelli et al., 2005; Economidou et al., 2005; Freeland et al., 2001; Maccioni et al., 2009) | |
| Decreased extinction responding | (Colombo et al., 2004) | ||
| Stress-induced reinstatement of alcohol-seeking behavior | No effect | (Economidou et al., 2005) | |
| Cue-induced reinstatement of alcohol-seeking behavior | Diminished cue-induced reinstatement | (Cippitelli et al., 2005; Economidou et al., 2005) | |
| Alcohol deprivation effect | Diminished alcohol deprivation effect | (Gessa et al., 2004; Serra et al., 2002) | |
| 24-h access two-bottle choice voluntary intake | Decreased alcohol intake | (Gessa et al., 2004; Lallemand et al., 2001) | |
Although preclinical research represents a crucial step in the drug development process, several factors must be considered when using animals to model human behavior. Results from preclinical studies can vary depending upon the strain and species used. For example, the study conducted by Breslin and colleagues (2010) found that treatment with topiramate decreased alcohol consumption in alcohol-preferring (P) rats but had no effect on alcohol consumption in Wistar rats (Breslin et al., 2010). Furthermore, studies reported differences in response to medication between alcohol dependent and non-alcohol dependent rats (Roberto et al., 2008) and high-preference and low-preference rats (Oka et al., 2013). A similar phenomenon is observed in clinical studies, whereby treatment response appears to be dependent on treatment population. Nevertheless, a deeper understanding of why a drug is effective in one strain or one species and not another is often elusive. Delving into these differences may ultimately inform precision medicine efforts. In addition to strain, alcohol intake can also fluctuate depending on the concentration of the alcohol solution and the addition of a sweetener (Yoneyama et al., 2008).
Another important issue to consider is that drugs are rarely compared against each other at a preclinical level but rather, are tested against a placebo. Using the field standard, such as naltrexone, in models where the drug has already shown efficacy, as a comparison may help to identify the animal paradigms that are predictive of human behavior through reverse translation. Perhaps equally important, reverse translation could prove informative for promising medications that do not show clinical efficacy as a means of identifying responders via animal and human laboratory studies. Unfortunately, reverse translation is uncommon as many compounds that progress to advanced stages of clinical drug development rarely endure additional testing at the preclinical level to validate the animal paradigms. Furthermore, unlike in human testing, animals are not susceptible to the “placebo effect” (van der Worp et al., 2010), which likely leads to an overestimation of the medication effects in animal models In other words, the signal-to-noise ratio is clearly higher in animal studies, yet the “signal” often fades and is no longer detectable or clinically relevant when tested in clinical samples.
It is also important to consider that FDA approved drugs that are being investigated for other indications often do not follow linear progression from preclinical to clinical stages of drug development. For example, dutasteride, approved for the treatment of benign prostatic hyperplasia, has been tested in human laboratory studies for the treatment of AUD (see Table 3), but no animal studies have been published for this indication thus far (see Table 2). In other cases such as nalmefene, topiramate and gabapentin, there are relatively fewer reported preclinical studies (see Table 2) as compared to clinical studies (see Table 4).
Table 3.
Effect of drugs on human laboratory models of AUD.
| Medication | Model | Population | Effect | References |
|---|---|---|---|---|
| Naltrexone | Self-administration in a naturalistic setting | AD | Decreased number of drinks consumed | (Drobes et al., 2003) |
| Social drinkers | No effect | (Drobes et al., 2003) | ||
| Non-treatment seeking AD | No effect | (Anton et al., 2004a; Krishnan-Sarin et al., 2007; O'Malley et al., 2002) | ||
| Heavy beer drinkers | No effect on number of drinking days or amount of drinks per drinking days | (Davidson et al., 1999) | ||
| Self-administration alcohol in a bar-lab setting | AD | Decreased number of drinks consumed (priming dose) | (Drobes et al., 2003) | |
| Social drinkers | No effect (priming dose) | (Drobes et al., 2003) | ||
| Non-treatment seeking AD | Decreased number of drinks consumed (delayed access group; priming dose); No effect on immediate access group | (Anton et al., 2004a) | ||
| Heavy beer drinkers | Decreased number of beers consumed and subjective positive affect; No effect on subjective negative affect | (Davidson et al., 1999) | ||
| Alcohol self-administration following priming drink | Non-treatment seeking AD | Decreased number drinks consumed in FH+ only | (Krishnan-Sarin et al., 2007) | |
| Non-treatment seeking AD | Decreased number of drinks consumed | (O'Malley et al., 2002) | ||
| Alcohol-induced craving | AD | Decreased craving | (Drobes et al., 2004) | |
| Non-treatment seeking AD | No effect during delayed access | (Anton et al., 2004a) | ||
| Non-treatment seeking AD | Decreased craving during ad lib drinking period; No effect during the priming dose | (O'Malley et al., 2002) | ||
| Heavy beer drinkers | Decreased craving before and after alcohol consumption | (Davidson et al., 1999) | ||
| Alcohol-induced stimulation | AD | Decreased stimulation (in alcoholics only) | (Drobes et al., 2004) | |
| Non-treatment seeking AD | No effect during delayed access | (Anton et al., 2004a) | ||
| Heavy beer drinkers | Decreased stimulation | (Davidson et al., 1999) | ||
| Non AD male social high risk drinkers | Decreased stimulation | (King et al., 1997) | ||
| Non AD male social low risk drinkers | No effect | (King et al., 1997) | ||
| Non AD social drinking African Americans | No effect | (Plebani et al., 2011) | ||
| Alcohol-induced sedation | AD | No effect | (Drobes et al., 2004) | |
| Non-treatment seeking AD | No effect during delayed access | (Anton et al., 2004a) | ||
| Heavy beer drinkers | No effect | (Davidson et al., 1999) | ||
| Non AD male social high and low risk drinkers | No effect | (King et al., 1997) | ||
| Non AD social drinking African Americans | No effect | (Plebani et al., 2011) | ||
| Moderate-heavy drinkers | Increased alcohol-induced sedation | (McCaul et al., 2000) | ||
| Alcohol-induced intoxication | Non-treatment seeking AD | No effect during delayed access | (Anton et al., 2004a) | |
| Non AD social drinking African Americans | No effect | (Plebani et al., 2011) | ||
| Moderate-heavy drinkers | No effect | (McCaul et al., 2000) | ||
| Alcohol cue exposure | Non-treatment seeking AD | Naltrexone alone: Decreased alcohol cue-induced activation of the ventral striatum; No effect in self-reported craving | (Myrick et al., 2008) | |
| Naltrexone + Ondansetron: Decreased alcohol cue-induced activation of the ventral striatum and self-reported craving | (Myrick et al., 2008) | |||
| Treatment seeking AD | Decreased percent reporting urge to drink; No effect on degree of urge to drink | (Monti et al., 1999) | ||
| Experimenter administered alcohol (IV) | Non treatment seeking heavy drinkers of East Asian ethnicity | Compared to Asn40 homozygotes: Increased alcohol-induced sedation and subjective intoxication in Asp40 carriers; Decreased alcohol-induced craving in Asp40 carriers; No effect on alcohol-induced stimulation | (Ray et al., 2012) | |
| Subjective measures | Moderate-heavy drinkers | Post alcohol challenge session: Decreased baseline desire to drink, alcohol-induced desire to drink, best and like effects; Increased sick/unpleasant effects | (McCaul et al., 2000) | |
|
| ||||
| Acamprosate | Challenge-induced craving: yohimbine and mCPP | Treatment seeking AD in early abstinence | No effect on PACS scores or anxiety during the challenge treatments | (Umhau et al., 2011) |
| Alcohol cue exposure | Treatment seeking AD | No effect | (Hammarberg et al., 2009) | |
| Alcohol-induced craving | Treatment seeking AD | Prevented increase in short-DAQ score | (Hammarberg et al., 2009) | |
| Alcohol choice paradigm after priming dose | Treatment seeking AD | No effect on alcohol consumed, positive or negative subscale | (Hammarberg et al., 2009) | |
| Self-administration in a naturalistic setting | Treatment seeking AD | No effect on number of drinking days or HDD | (Hammarberg et al., 2009) | |
| Alcohol-induced stimulation | Heavy social drinkers | No effect | (Brasser et al., 2004) | |
| Alcohol-induced sedation | Heavy social drinkers | No effect | (Brasser et al., 2004) | |
| Alcohol-induced intoxication | Heavy social drinkers | No effect | (Brasser et al., 2004) | |
|
| ||||
| Nalmefene | Self-administration in a naturalistic setting | AD | Decreased number of drinks consumed | (Drobes et al., 2003) |
| Social drinkers | No effect | (Drobes et al., 2003) | ||
| Self-administration in a bar-lab alcohol setting | AD | Decreased number of drinks consumed (priming dose) | (Drobes et al., 2003) | |
| Social drinkers | No effect (priming dose) | (Drobes et al., 2003) | ||
| Alcohol-induced craving | AD | Decreased craving | (Drobes et al., 2004) | |
| Alcohol-induced stimulation | AD | Decreased stimulation (in alcoholics only) | (Drobes et al., 2004) | |
| Alcohol-induced sedation | AD | No effect | (Drobes et al., 2004) | |
|
| ||||
| Ondansetron | Alcohol cue exposure | Non-treatment seeking AD | No effect on alcohol cue-induced activation of the ventral striatum or self-reported craving | (Myrick et al., 2008) |
|
| ||||
| Topiramate | Self-administration in a naturalistic setting | Heavy drinkers | During titration period: Reduced % HDD and drinks/week | (Miranda Jr. et al., 2008) |
| Alcohol cue exposure | Heavy drinkers | No effect | (Miranda Jr. et al., 2008) | |
| Subjective measures | Heavy drinkers | No effect on positive or negative affect post alcohol challenge session | (Miranda Jr. et al., 2008) | |
| Alcohol-induced sedation | Heavy drinkers | No effect | (Miranda Jr. et al., 2008) | |
| Alcohol-induced stimulation | Heavy drinkers | Decreased alcohol-induced stimulation | (Miranda Jr. et al., 2008) | |
| Alcohol-induced craving | Heavy drinkers | No effect | (Miranda Jr. et al., 2008) | |
|
| ||||
| Zonisamide | Alcohol self-administration following priming drink | Non treatment seeking risky drinkers | Decreased number of drinks consumed in second SA session only | (Sarid-Segal et al., 2009) |
| Alcohol-induced craving | Non treatment seeking risky drinkers | Decreased alcohol-induced craving | (Sarid-Segal et al., 2009) | |
| Alcohol-induced stimulation | Non treatment seeking risky drinkers | No effect | (Sarid-Segal et al., 2009) | |
| Alcohol-induced sedation | Non treatment seeking risky drinkers | No effect | (Sarid-Segal et al., 2009) | |
|
| ||||
| Gabapentin | Self-administration in a bar-lab alcohol setting | Non-treatment seeking AD | No effect (after priming dose) | (Myrick et al., 2007) |
| Self-administration in a naturalistic setting | Non-treatment seeking AD | No effect | (Myrick et al., 2007) | |
| Alcohol-induced craving | Non-treatment seeking AD | No effect on craving after initial drink and during free-choice drinking period | (Myrick et al., 2007) | |
| Non AD heavy drinkers | No effect | (Bisaga and Evans, 2006) | ||
| Alcohol-induced stimulation | Non-treatment seeking AD | No effect (after priming dose) | (Myrick et al., 2007) | |
| Non AD heavy drinkers | No effect | (Bisaga and Evans, 2006) | ||
| Alcohol-induced sedation | Non-treatment seeking AD | No effect (after priming dose) | (Myrick et al., 2007) | |
| Non AD heavy drinkers | No effect | (Bisaga and Evans, 2006) | ||
| Alcohol-induced intoxication | Non-treatment seeking AD | No effect (after priming dose) | (Myrick et al., 2007) | |
| Alcohol cue exposure | Non-treatment seeking, cue-reactive AD | Decreased alcohol cue-induced craving | (Mason et al., 2009) | |
| Affective cue reactivity | Non-treatment seeking, cue-reactive AD | Decreased affectively-evoked craving | (Mason et al., 2009) | |
| Subjective measures | Non AD heavy drinkers | Post alcohol challenge session: No effect on BVAS measures, ratings of drink taste, CADSS scores or DEQ ratings | (Bisaga and Evans, 2006) | |
|
| ||||
| Baclofen | Self-administration in a naturalistic setting | Non-treatment seeking AD heavy drinkers | No effect | (Leggio et al., 2013) |
| Self-administration in a bar-lab alcohol setting | Non-treatment seeking AD heavy drinkers | No statistically significant effect (robust medication effect d=0.76) | (Leggio et al., 2013) | |
| Alcohol cue exposure | Non-treatment seeking AD heavy drinkers | No effect | (Leggio et al., 2013) | |
| Alcohol-induced stimulation | Non-treatment seeking AD heavy drinkers | Increased stimulation during pre ad-libitum period | (Leggio et al., 2013) | |
| Non treatment seeking heavy social drinkers | No effect | (Evans and Bisaga, 2009) | ||
| Alcohol-induced sedation | Non-treatment seeking AD heavy drinkers | Increased sedation during ad-libitum period | (Leggio et al., 2013) | |
| Non treatment seeking heavy social drinkers | No effect | (Evans and Bisaga, 2009) | ||
| Alcohol-induced craving | Non treatment seeking heavy social drinkers | No effect | (Evans and Bisaga, 2009) | |
| Subjective measures | Non treatment seeking heavy social drinkers | Post alcohol challenge session: No effect on VAS score, DEQ score; Increased ratings of High on BVAS scale | (Evans and Bisaga, 2009) | |
|
| ||||
| Varenicline | Alcohol-induced craving | Non AD heavy drinkers and daily smokers | Decreased craving following priming drink; No effect during SA period | (McKee et al., 2009) |
| Alcohol self-administration following priming drink | Non AD heavy drinkers and daily smokers | Decreased number of drinks consumed and subjective effects of alcohol; Increased likelihood of remaining abstinent during SA period | (McKee et al., 2009) | |
| Subjective measures | Moderate-to-heavy social drinkers | Increased ratings of dysphoria; Decreased ratings of drug liking | (Childs et al., 2012) | |
|
| ||||
| Memantine | Alcohol-induced craving | Non AD moderate drinkers | No effect (decreased craving prior to alcohol administration) | (Bisaga and Evans, 2004) |
| Alcohol-induced stimulation | Non AD moderate drinkers | No effect | (Bisaga and Evans, 2004) | |
| Alcohol-induced sedation | Non AD moderate drinkers | No effect | (Bisaga and Evans, 2004) | |
| Subjective measures | Non AD moderate drinkers | Post alcohol challenge session: No effect on BVAS measures, POMS scores or performance tasks; Increased CADSS score; Decreased DEQ ratings of “drug strength” | (Bisaga and Evans, 2004) | |
| Alcohol cue exposure | AD males | Decreased alcohol cue-induced craving; No effect on craving prior to alcohol exposure | (Krupitsky et al., 2007) | |
|
| ||||
| Mecamylamine | Subjective measures | Healthy volunteers | Decreased DEQ and Alcohol Sensation Scale stimulant subscale scores | (Blomqvist et al., 2002) |
| Alcohol-induced stimulation | Social drinkers | Decreased alcohol-induced stimulation | (Chi and de Wit, 2003; Young et al., 2005) | |
| Alcohol-induced sedation | Social drinkers | No effect | (Chi and de Wit, 2003) | |
| Subjective effects | Social drinkers | Decreased ratings of ‘want more’ and euphoric effects | (Chi and de Wit, 2003) | |
| Alcohol choice paradigm | Social drinkers | No effect | (Young et al., 2005) | |
|
| ||||
| Prazosin | Stress imagery exposure | Early abstinent, treatment seeking AD | Decreased stress-induced craving | (Fox et al., 2012) |
| Alcohol cue exposure | Early abstinent, treatment seeking AD | Blocked increase in alcohol cue-induced craving | (Fox et al., 2012) | |
|
| ||||
| Olanzapine | Alcohol cue exposure | Heavy social drinkers | Decreased urge to drink and positive affect after exposure to water and alcohol; No effect on negative affect | (Hutchison et al., 2001) |
| Compared to control medication (cyproheptadine, 4 mg): Decreased craving in DRD4-L patients; No effect in DRD4-S patients | (Hutchison et al., 2003) | |||
| AD | In DRD4-L Patients: Decreased alcohol cue-induced craving and alcohol cue-induced increases in depression and anxiety | (Hutchison et al., 2006) | ||
| Alcohol-induced intoxication | Heavy social drinkers | No effect | (Hutchison et al., 2001) | |
| Compared to control medication (cyproheptadine, 4 mg): No effect | (Hutchison et al., 2003) | |||
| Alcohol-induced stimulation | Heavy social drinkers | No effect | (Hutchison et al., 2001) | |
| Compared to control medication (cyproheptadine, 4 mg): No effect | (Hutchison et al., 2003) | |||
| Alcohol-induced sedation | Heavy social drinkers | Compared to control medication (cyproheptadine, 4 mg): No effect | (Hutchison et al., 2003) | |
| Alcohol-induced craving | Heavy social drinkers | In alcohol group only: Decreased alcohol-induced craving and subjective want | (Hutchison et al., 2001) | |
| Compared to control medication (cyproheptadine, 4 mg): Decreased alcohol-induced craving in DRD4-L patients; No effect in DRD4-S patients | (Hutchison et al., 2003) | |||
| Subjective measures | Heavy social drinkers | Post alcohol challenge session: No effect on subjective liking | (Hutchison et al., 2001) | |
| Self-administration in a naturalistic setting | AD | In DRD4-L Patients: Decreased drinks per drinking day and total number of drinks; No effect on % days abstinent | (Hutchison et al., 2006) | |
|
| ||||
| Dutasteride | Alcohol-induced stimulation | Male light and heavy drinkers | No effect | (Covault et al.) |
| Alcohol-induced sedation | Male light and heavy drinkers | Decreased alcohol-induced sedation | (Covault et al.) | |
| Self-administration in a naturalistic setting | Male light drinkers | No effect | (Covault et al.) | |
| Male heavy drinkers | Decreased HDD and total number of drinks consumed | (Covault et al.) | ||
|
| ||||
| Rimonabant | Self-administration in a naturalistic setting | Heavy drinkers | No effect | (George et al., 2010) |
| Alcohol self-administration following priming drink | Heavy drinkers | No effect | (George et al., 2010) | |
Table 4.
Primary outcomes of clinical trials testing drugs for the treatment of AUD.
| Medication | Time Abstinent | Treatment Duration/Target Dose | Primary Outcome | References |
|---|---|---|---|---|
| Disulfiram | 0 days | 119 weeks (12 week supervised medication, up to 52 week targeted medication, 67 week follow-up period); 100-200 mg q.d. or 2 × 400 mg twice a week | Compared to naltrexone (50 mg q.d.) and acamprosate (2 × 333 mg t.i.d. for people ≥ 60 kg body weight; 1332 mg for people < 60 kg body weight): Increased time to first HDD and time to first drink during the first 12 weeks | (Laaksonen et al., 2008) |
| Men; abstinent 19 ± 5 days on average for DSF group; 20 ± 11 for TPM group | 9 months/ 250 mg q.d. | Compared to TPM (50 mg t.i.d.): Increased days to first relapse; No effect on days of abstinence, discontinuation of treatment, or drop out rate; Decreased craving severity and GGT | (De Sousa et al., 2008) | |
|
| ||||
| Naltrexone | 5 days | 12 weeks/ 50 mg q.d. | Decreased drinks per drinking day; Increased time to first relapse, and % days abstinent | (Anton et al., 1999) |
| AD or Alcohol abusers, 5-30 days abstinent | 12 weeks/ 50 mg q.d. | No effect on time to first episode of heavy drinking | (Chick et al., 2000a) | |
| 0 days | 24 weeks/ 380 mg or 190 mg long-acting injectable naltrexone administered monthly | 380 mg dose decreased event rate of HDD; Treatment effects were greater in subpopulation that were abstinent for 7 days prior to treatment | (Garbutt et al., 2005) | |
| 5- 30 days (19.5 ± 9.4 days on average) | 12 weeks/ 50 mg q.d. | No effect on time to first heavy drinking episode | (Gastpar et al., 2002) | |
| 12-15 days | 12 weeks/ 50 mg q.d. | Increased time to first relapse and time to first drink | (Kiefer et al., 2003) | |
| 12-15 days | 12 weeks/ 50 mg naltrexone q.d.+ 2 × 333 mg t.i.d. | Increased time to first relapse and time to first drink (compared to both placebo and acamprosate alone) | (Kiefer et al., 2003) | |
| Predominantly male, 5 days abstinent | 12 months/ 50 mg q.d. for 12 months; 50 mg q.d. for 3 months + placebo for 9 months | No effect on time to relapse during the first 3 months, % drinking days over the 12 month period or number of drinks per drinking day over the 12 month period | (Krystal et al., 2001) | |
| 3-21 days | 12 weeks/ 50 mg q.d. | Compared to both placebo and acamprosate: No effect on number of days to first lapse, days to first relapse, cumulative days abstinent, or drinks per drinking day | (Morley et al., 2006) | |
| Males; 3-30 days abstinent (8 ± 5 days on average for NTX group; 9 ± 6 for placebo) | 12 weeks/ 50 mg q.d. | Decreased relapse to drinking; No effect on maintenance of abstinence | (Morris et al., 2001) | |
| Non treatment seeking heavy drinkers (63% AD); 0 days abstinent | 3 weeks/ 50 mg q.d. (in addition to a 1-week placebo lead-in) | Decreased % drinking days; No effect on drinks per day, drinks per drinking day, % HDD or any subjective effects of alcohol | (Tidey et al., 2008) | |
| 4-21 days | 16 weeks/ 50 mg b.i.d. | Increased % days abstinent; Decreased risk of HDD | (Anton et al., 2006) | |
| Males; 3-30 days abstinent | 12 weeks/ 50 mg q.d. | Decreased relapse to heavy drinking | (Ahmadi and Ahmadi, 2002) | |
| 14-28 days | 12 weeks/ 50 mg q.d. (in addition to a 1 week placebo run-in and therapy every 4th week from week 12-24) | Decreased HDD | (Balldin et al., 2003) | |
| Non AD heavy drinkers; 0 days | 6 weeks/ 25 mg q.d.; 50 mg q.d. (in addition to a one month post treatment follow-up) | Compared to pre-treatment measures: Decreased number of standard drinks consumed, HDD, and drinks per drinking days; increased number of days abstinent | (Bohn et al., 1994) | |
| 5-30 days | 12 weeks/ 50 mg q.d. | Decreased relapse to heavy drinking | (Guardia et al., 2002) | |
| 0 days | 12 weeks/ 50 mg q.d. (in addition to a 1 week placebo run-in and 20 week post treatment targeted medication) | Naltrexone + cognitive coping skills decreased relapse to heavy drinking | (Heinala et al., 2001) | |
| 0 days | 12 weeks/ 50 mg q.d. | Compared to placebo + treatment as usual and treatment as usual alone: No effect on % days drinking, average drinks per day, average drinks per drinking day, HDD, or time to first heavy drink | (Killeen et al., 2004) | |
| 3 days | 8 weeks/ 50 mg PO daily for 2 weeks, followed by a 2-week, no-medication wash out period, a 4-week 206 mg injection (single) period, and a 4-week follow-up period | Compared to placebo injection: Decreased % HDD during injection period; No effect on average drinks per drinking day during injection period; Decreased % HDD and average drinks per day during follow-up period | (Kranzler et al., 1998) | |
| 7-51 days (11.7 day on average) | 12 weeks/ 50 mg q.d. | Decreased relapse rate; Increased time to first relapse; No effect on reported side effects | (Latt et al., 2002) | |
| 3 days | 12 weeks/ 50 mg b.i.d. (in addition to a one week placebo lead-in) | Decreased number of heavy drinking days | (Monterosso et al., 2001) | |
|
| ||||
| Acamprosate | 12-15 days | 12 weeks/ 2 × 333 mg t.i.d. | Increased time to first relapse and time to first drink | (Kiefer et al., 2003) |
| <10 days (must have reduced drinking to no more than 2 (F) or 3 (M) drinks in the 2-10 days pre randomization) | 24 weeks/ 2 × 500 mg b.i.d.; 3 × 500 mg b.i.d. | No effect on % days abstinent | (Mason et al., 2006) | |
| 3-21 days | 12 weeks/ 2 × 333 mg t.i.d. | Compared to both placebo and NTX: No effect on number of days to first lapse, days to first relapse, cumulative days abstinent, or drinks per drinking day | (Morley et al., 2006) | |
| Predominantly male; 1 day abstinent | 8 weeks/ 1998 mg for people ≥ 60 kg body weight or 1332 mg for people < 60 kg body weight (dosing schedule not specified) | No effect on time to first drink, time to relapse, or % days abstinent | (Namkoong et al., 2003) | |
| 7-28 days (18 days on average) | 12 months/ 1332 mg per day (4 × 333 mg per day); 1998 mg per day (6 × 333 mg per day) (in addition to a single-blind 6 month follow-up on placebo) | Dose dependently increased continuous abstinence at 6 months; No effect on continuous abstinence at 12 months | (Paille et al., 1995) | |
| 5 days | 24 weeks/ 2 × 333 mg t.i.d. (in addition to a 12 week medication-free follow-up) | Increased abstinence rate, cumulative abstinence duration, period of continued abstinence | (Tempesta et al., 2000) | |
| 4-21 days | 16 weeks/ 2 × 500 mg t.i.d. | No effect on mean % days abstinent or time to first HDD | (Anton et al., 2006) | |
| 5 days | 360 days/ 2 × 333 mg t.i.d. for people ≥ 60 kg body weight; 1332 mg (2+1+1) for people < 60 kg body weight (in addition to a 360 day follow up period) | Increased cumulative abstinence duration; Decreased relapse rate through assessment day 270 | (Besson et al., 1998) | |
| 5 days | 24 weeks/ 2 × 333 mg t.i.d. | No effect on continuous abstinence or cumulative abstinence duration | (Chick et al., 2000b) | |
| 5 days | 24 weeks/ 2 × 333 mg t.i.d. for people ≥ 60 kg body weight; 1332 mg (333 mg, 2+1+1) for people < 60 kg body weight (in addition to a medication free 6-month follow-up period) | Increased cumulative duration of abstinence, time to first relapse, % abstinent on assessment day 135 | (Geerlings et al., 1997) | |
| 0 days | 180 days/ 2 × 333 mg t.i.d. | Increased cumulative abstinence duration | (Gual and Lehert, 2001) | |
| Within 48 h following hospitalization for alcohol withdrawal; 5-30 days abstinent | 90 days; 1332 mg (333 mg, 2+1+1) | Decreased GGT | (Lhuintre et al., 1990) | |
| 14 day inpatient detoxification program | 90 days/ 1332 mg (333 mg, 2+1+1); 2 × 333 mg t.i.d. | Increased cumulative abstinence duration; Decreased relapse rate | (Pelc et al., 1997) | |
| 5 days | 24 weeks/ 2 × 333 mg t.i.d. for people ≥ 60 kg body weight; 1332 mg (333 mg, 2+1+1) for people < 60 kg body weight (in addition to a 24 week follow-up period) | Increased abstinence at month 1, 6, and 12; No effect on abstinence at month 3 and 9 | (Poldrugo, 1997) | |
| 5 days | 360 days/ 2 × 333 mg t.i.d. for people > 60 kg body weight; 1332 mg (333 mg, 2+1+1) for people ≤ 60 kg body weight (in addition to a 360 day follow-up period) | Increased time to first treatment failure | (Whitworth et al., 1996) | |
|
| ||||
| Nalmefene | 3 days | 12 weeks/ 2 × 2.5 mg q.d.; 2 × 10 mg q.d.; 2 × 20 mg q.d. | No effect of treatment on number of HDD per month | (Anton et al., 2004b) |
| 0 days | 24 weeks/ up to 18 mg per day prn (in addition to a 1-2 week screening period and 4-week double-blind run-out period) | Decreased HDD; No effect on monthly total alcohol consumption | (Gual et al., 2013) | |
| 0 days | 24 weeks/ up to 18 mg per day prn (in addition to a 1-2 week screening period and 4-week double-blind run-out period) | Decreased number of HDD and total alcohol consumption | (Mann et al., 2013) | |
| 2 weeks on average | 12 weeks/ 10 mg b.i.d.; 40 mg b.i.d. (in addition to a 2-week single-blind placebo period) | Decreased relapse to heavy drinking; No effect on drinks per drinking day or % days abstinent | (Mason et al.) | |
| 0 days | 12 weeks/ 20 mg b.i.d; 5 mg b.i.d. (in addition to a 2-week single-blind placebo lead-in) | 40 mg dose compared to 10 mg and placebo: Decreased relapse to heavy drinking; Increased change mean abstinence days/week from single-blind placebo phase to treatment phase | (Mason et al., 1994) | |
| Both doses compared to placebo: Decreased change in number of drinks per drinking day from single-blind placebo phase to treatment phase; No effect on craving or retention in treatment | (Mason et al., 1994) | |||
|
| ||||
| Ondansetron | 0 days | 11 weeks/ 1 μg/kg b.i.d.; 4 μg/kg b.i.d.; 16 μg/kg b.i.d. (in addition to a 1 week placebo lead-in) | All doses in early onset alcoholics: Decreased drinks per day and drinks per drinking day | (Johnson et al., 2000) |
| 4 μg/kg b.i.d. in early onset alcoholics: Increased % days abstinent and total day abstinent per study week | (Johnson et al., 2000) | |||
| 0 days | 8 weeks/ 4 μg/kg/ml b.i.d. | In early onset alcoholics: Decreased drinks per day and drinks per drinking day compared to late onset alcoholics; No effect on % days abstinent or number of HDD between groups | (Kranzler et al., 2003) | |
| Non severely AD males; 0 days | 6 weeks/ 0.25 mg b.i.d.; 2 mg b.i.d. (in addition to a 2 week baseline period) | In all patients: No effects on number of standard drinks per drinking day between baseline and treatment | (Sellers et al., 1994) | |
| In light drinkers: Decreased number of drinks per drinking day compared to baseline | (Sellers et al., 1994) | |||
|
| ||||
| Topiramate | 0 days | 12 weeks/ escalating dose of 25-300 mg per day (weeks 8-12 100 mg + 2 × 25 mg b.i.d.) | Decreased drinks per day, drinks per drinking day, % HDD and plasma GGT; Increased % days abstinent | (Johnson et al., 2003) |
| 0 days | 14 weeks/ 300 mg per day (100 q.a.m. + 2 × 100 mg q.p.m.) | Decreased % HDD | (Johnson et al., 2007) | |
| Men; abstinent 19 ± 5 days on average for DSF group; 20 ± 11 for TPM group | 9 months/ 50 mg t.i.d. | Compared to DSF (250 mg q.d.): Decreased days to first relapse; No effect on days of abstinence, discontinuation of treatment, or drop out rate; Increased craving severity and GGT | (De Sousa et al., 2008) | |
|
| ||||
| Zonisamide | 0 days | 12 weeks/ 100-500 mg q.d. (increased 100 mg every 2 weeks for 8 weeks) | Medications × Treatment week interaction: Decreased HDD per week and drinks per week; No effect on abstinent days per week | (Arias et al., 2010) |
| Detoxified or present mild symptoms of abstinence (scores on the CIWA for Alcohol-Revised of <6) | 12 weeks/ 50-300 mg per day (flexible-dose schedule with average of 220 mg per day ± 50) | Compared to baseline: Decreased number of drinks per week, craving severity and GGT levels | (Rubio et al., 2010) | |
|
| ||||
| Levetiracetam | Heavy social drinkers; 0 days abstinent | 2, 14 day treatment periods (one cycle with placebo and the other with low or high dose Levetiracetam)/ 250-500 g b.i.d.; 500-1000 g b.i.d. (in addition to a 3-day drug taper and 7 day washout period) | No effect on number of drinks consumed | (Mitchell et al., 2012a) |
| 0 days | 6 days/ fixed dose schedule (days: 1-3: 1000-0-1000 mg; 4: 500-0-1000 mg; 5: 500-0-500 mg; 6: 0-0-500 mg) | No effect on dose of diazepam as a rescue medication or the severity of withdrawal symptoms | (Richter et al., 2010) | |
| 0 days | 10 weeks/ titrated up to 1000 mg b.i.d. over the first 3 weeks to a total of 2000 mg (in addition to 1 week of screening and 2 weeks taper) | Decreased standard drinks per day | (Sarid-Segal et al., 2008) | |
| 0 days | 16 weeks/ titrated for the first 4 weeks from 500 to 2000 mg/day week 5-14 followed by a 2 week taper (in addition to a follow-up interview week 19) | No effect on percent HDD and percent subjects with no HDD | (Fertig et al., 2012) | |
|
| ||||
| Gabapentin | 3 days | 12 weeks/ 2 × 150 mg t.i.d.; 2 × 300 mg t.i.d. | Dose dependently increased rates of complete abstinence and no heavy drinking | (Mason et al., 2014) |
| Patients with moderate-severe AWS; 0 days | 2 days/ 400 mg q.i.d. (data on safety and tolerability continued to be measured until day 7) | No effect on amount of CLO required in the first 24 hours (no psychosocial component specified) | (Bonnet et al., 2003) | |
|
| ||||
| Pregabalin | 5-10 days | 16 weeks/ flexible dose of 150-450 mg per day (mean 262.5 mg per day ± 117.9) | Half (n=10) were completely abstinent for duration of the study; One quarter (n=5) relapsed | (Martinotti et al., 2008) |
| 0 days | 14 days; up to 450 mg per day | Compared to both tiapride and lorazepam: Increased abstinence; Decreased CIWA-Ar scores on items regarding headache and orientation Compared to tiapride only: Increased time to dropout |
(Martinotti et al., 2010) | |
|
| ||||
| Baclofen | 12-24 h | 30 days/ 10 mg t.i.d. | Increased % abstinent and number of cumulative abstinent days | (Addolorato et al., 2002) |
| 3 days | 12 weeks/ 10 mg t.i.d.; 20 mg t.i.d. | Compared to baseline: Decreased number of drinks per day | (Addolorato et al., 2011) | |
| AD with liver cirrhosis, 3-4 days abstinent | 12 weeks/ 10 mg t.i.d. | Increased % abstinent and cumulative abstinent duration | (Addolorato et al., 2007) | |
| 3 days | 12 weeks/ 30 mg per day (dosing schedule not specified) | No effect on % HDD | (Garbutt et al., 2010) | |
| 3 days | 12 weeks/ 10 mg t.i.d. | Compared to baseline measures: Decreased number of drinks per drinking day and HDD; Increased number of abstinent days | (Flannery et al., 2004) | |
|
| ||||
| Varenicline | 0 days | 13 weeks/ 1 mg b.i.d. | Decreased weekly % HDD | (Litten et al., 2013) |
| Heavy drinking smokers seeking treatment for smoking only; 0 days abstinent | 12 weeks/ 1 mg b.i.d. (in addition to 2 follow-up visits at week 14 and 16) | Decreased drinks and cigarettes per week from weeks 3-11; No effect on craving per week | (Mitchell et al., 2012c) | |
| 0 days | 12 weeks/ 1 mg b.i.d. | No effect on alcohol use | (Plebani et al., 2013) | |
|
| ||||
| Oxytocin | 0 days | 3 days/ 24 IU/dose b.i.d. | Required less total lorazepam to complete detoxification | (Pedersen et al., 2013) |
|
| ||||
| Memantine | 0 days | 12 weeks/ 20 mg b.i.d. (in addition to a 2 week placebo lead-in and a 2 week placebo lead-out) | Increased % HDD; Decreased % days abstinent; No effect on average drinks per day or drinks per drinking day | (Evans et al., 2007) |
|
| ||||
| Prazosin | 0 days | 6 weeks/ 4 mg q.a.m. + 4 mg q.p.m. + 8 mg q.h.s. | No effect on mean drinks per week or mean drinking days per week; Decreased drinking days per week in the final 3 weeks | (Simpson et al., 2009) |
| In men only in the final 3 weeks: Decreased drinking days per week, average total number of drinking days, drinks per week, average number of total drinks | (Simpson et al., 2009) | |||
|
| ||||
| Doxazosin | 0 days | 10 weeks/ titrated during the first 4 weeks up to 16 mg per day and a 1-week downward titration at week 10 (in addition to a follow-up week 12) | In AD patients with high family history density of alcoholism (FHDA): Reduced drinks per week and HDD per week In AD patients with low FHDA: Increased drinks per week, No effect on HDD per week |
(Kenna et al., 2015) |
|
| ||||
| Rimonabant | 7-28 days | 12 weeks/ 20 mg q.d. | No effect on time to first drink or time to first HDD | (Soyka et al., 2008) |
Note: All results are compared to placebo unless otherwise stated; Population was AD males and females unless otherwise stated. All treatment included a psychosocial/medical management component.
Although preclinical development represents an important part of the drug development pathway, there are many factors that limit the usefulness of these models in their current format. One such obstacle may be publications bias. For example, one study analyzed over 4600 published papers across disciplines in 2007 and found that 85.9% of papers reported a positive result (Fanelli, 2012). This strong bias towards positive publications makes it extremely difficult to draw conclusions between the predictive validity of animal data to clinical outcomes. Furthermore, despite the misconception that negative results are not as valuable as positive results, reporting of negative results can allow for refinement of theories or methods, encourage discussion within the field, improve quality control and ultimately help to advance science by filling gaps in knowledge (Lehrer et al., 2007; Matosin et al., 2014). Data repositories may be also be helpful in increasing access to preclinical findings and mitigating the issue of publication bias.
In summary, preclinical studies of medications development for AUD have offered important insights into the neurobiology of the disorder and alcohol's molecular targets. Current limitation of this approach include the lack of standardized methods and streamlined processes whereby animal studies can readily inform human studies, which in turn would start at the point of safety and initial efficacy (described below).
Human Laboratory Paradigms
Human laboratory studies offer unique opportunities to gain insight into the safety, efficacy and most importantly, the mechanism of action of the drug being tested serving as a less expensive alternative compared to full-scale clinical trials. Table 3 summarizes the results of human laboratory studies investigating the mechanism by which drugs being developed for the treatment of AUD exert their effect. As exemplified in Table 3, there are numerous laboratory paradigms used to model facets of AUD (Ray et al., 2010). Commonly used paradigms include alcohol self-administration, experimenter administered alcohol (i.e., alcohol challenge), alcohol cue-reactivity, and stress induction. For example, in one iteration of the alcohol self-administration paradigm, participants complete 2 1-h self-administration (SA) periods having the option of consuming up to 4 alcoholic drinks (0.015 g/dl each) or receiving a monetary compensation of $3 per beverage not consumed (O'Malley et al., 2007). Typically, total number of drinks consumed during the SA sessions is considered the primary outcome variable and rate of drinking (i.e., time to first drink, inter-drink interval) is often used as a secondary outcome. Regarding the ethics of alcohol administration to clinical samples, it is important to note that many studies have assessed the effect of laboratory self-administration of alcohol on future alcohol use and found that alcohol use does not increase in subjects following participation in an alcohol administration study (Pratt and Davidson, 2005; Sommer et al., 2015). Importantly, the National Advisory Council on Alcohol Abuse and Alcoholism's recommended council guidelines on ethyl alcohol administration in human experimentation encourages experiments involving alcohol administration to be conducted in non-treatment seeking subjects (Enoch et al., 2009). Yet, because of the distinct differences between non-treatment seeking and treatment seeking populations and given the lack of successful medications to treat this disorder, the benefits to society oftentimes outweigh the risks to the individual. Additional human laboratory paradigms include stress and cue-reactivity. The cue-reactivity paradigm measures alcohol craving (Bohn et al., 1995; MacKillop, 2006). In this paradigm, participants are asked to hold and smell a glass of water for 3 minutes to control for the effects of simple exposure to any potable liquid. Next, participants hold and smell a glass of their preferred alcoholic beverage for three 3-minute trials (Monti et al., 1987; Monti et al., 2001). After every 3 minutes of exposure, craving for alcohol is assessed. Given the number of studies that suggest an association between stress and alcohol use, stress-induction in the laboratory has been used to understand the relationship between stress- and cue-induced craving in relation to alcohol use (Plebani et al., 2012). Two paradigms are often used to induce stress in the laboratory: 1) the Trier Social Stress Test [TSST; (Kirschbaum et al., 1993)] and 2) guided imagery exposure to a stressful event (Sinha et al., 1999).
In addition to behavioral assessments, brain imaging techniques can provide additional insight into the mechanism of the pharmacotherapies being tested. Although beyond the scope of this review, brain imaging studies have become increasingly popular in clinical and therapeutic developments in addictive disorders (Fowler et al., 2007), with a particular focus on the neural bases of cue-reactivity (Jasinska et al., 2014). A review by Borsook and colleagues (2011) highlights the importance of brain imagining in bridging preclinical and clinical CNS drug discovery (Borsook et al., 2011). Specifically, they emphasize that this technique may be able to help better identify pharmacodynamics markers, improve paradigms to predict efficacy, evaluate safety, elucidate dose-response relationships, and more accurately define symptom response. As noted in a recent review by our group, neural markers, in particular those during cue reactivity, appear to be promising predictors of relapse in clinical contexts (Courtney et al., 2015). Taken together these paradigms and techniques used in behavioral pharmacology studies provide insight into the mechanism of action of the drug; however, certain precautions, such as sample size and consideration of inclusion and exclusion criteria due to known variations in response associated with certain clinical characteristics, need to be taken to ensure the conclusions reached are valid.
As discussed for animal studies, different populations respond differently to each drug therefore, Table 3 is organized according to the lab paradigm and sample tested. In the study by Drobes and colleagues (2003), naltrexone decreased alcohol self-administration in a naturalistic setting in non-treatment seeking AD individuals but had no effect on social drinkers in the same study (Drobes et al., 2003), non-treatment seeking AD individuals (Anton et al., 2004a; O'Malley et al., 2002) or heavy beer drinkers (Davidson et al., 1999) suggesting that the results of each study should be interpreted carefully and the population tested must be taken into consideration. Interestingly, human laboratory studies are more often conducted in non-treatment seeking AD individuals whereas clinical trials employ treatment seeking AD individuals, which likely accounts for the at least part of the discrepancy between results from human laboratory studies and clinical trials. It remains unclear what variables differentiate treatment seekers from non-treatment seekers for alcoholism, whether it be severity of the disorder or the act of treatment seeking itself. Importantly, epidemiological data suggest that there is an average lag of 8 years between AUD onset and treatment seeking (Hasin et al., 2007). Ongoing studies in our laboratory suggest that treatment-seekers are older and have a more severe AD presentation, as compared to non-treatment seekers. Additional attention to discrepancies in sample characteristics between human laboratory and clinical trials is likely to promote greater consilience across approaches.
In addition to the variance regarding drinking status and treatment-seeking efforts, sample size is another significant factor contributing to the lack of predictability between human laboratory studies and clinical trials. Human laboratory studies tend to have a much smaller sample size compared to clinical trials and therefore, may affect the reliability of the estimates. The average samples size for the human laboratory studies included in Table 3 is 47 ± 48 participants whereas the average sample size for the clinical trials listed in Table 4 is 207 ± 235 participants. Unlike the p-value, effect size is independent of sample size and indicates the magnitude of the effect (Sullivan and Feinn, 2012). Therefore, both effect size and p-value should be considered when interpreting and comparing results from human laboratory studies and clinical trials.
Similar to the preclinical models, human laboratory studies could be strengthened if the drugs of interest were tested against a field standard pharmacotherapy instead of, or in addition to, a placebo treatment (Rothman and Michels, 1994). Arguments can be made that placebos offer a more suitable reference for determining efficacy, provide a more straightforward comparison, and increase the likelihood of achieving statistical significance; however, the use of active medication as a comparison can be beneficial to establish whether the new treatment is superior to the currently available/approved treatment. It is important to acknowledge that comparison to a placebo may be important in earlier stages of development to establish initial efficacy. However, in the later in development, it might be more informative to include both a placebo arm and a gold standard arm although this introduces additional challenges as it requires a larger sample. Comparing multiple doses of the drug could also provide a strategic method for conducting dose-finding studies prior to proceeding to relatively expensive clinical trials.
Another important issue to consider is the monetary compensation of research subjects, which provides an incentive for non-treatment seeking subjects and can strongly influence participation in the research study (Grady, 2005). As these subjects are not seeking medical benefit from the treatment, their primary motivation to participate in the research study is the monetary compensation, investigators should guard against the compensation becoming coercive or an excessive inducement. Further, there are concerns that the motivation for monetary compensation itself could lead to a general disinterest in the study and low level of concern about data accuracy. A recent commentary by Resnik and McCann (2015) highlights this complex issue (Resnik and McCann, 2015). The authors cite a recent study reporting that a quarter of respondents admitted to exaggerating their symptoms and 14% pretended to have a health problem to qualify for a study. While these concerns are often mitigated by an effective consent process and by forming a strong alliance with research participants as they are helping others with similar conditions through their participation in research studies, Resnik and McCann suggest that additional strategies can be used to address this concern including the use of laboratory tests to confirm self-reported information, the use of reinforcements to promote truthfulness, and increased utilization of available clinical trial registries (Resnik and McCann, 2015).
In sum, considering clinical costs associated with drug development are estimated to be more than $500 million, it is crucial to find novel ways to improve the translational predictability between relatively less expensive human laboratory studies and clinical trials (Paul et al., 2010). Specifically, phase I studies can provide a less expensive and extremely valuable opportunity to assess the feasibility of an approach prior to initiating larger scale clinical trials such as identifying a specific population more likely to respond to the medication and issues concerning retention, analyses, assessments, etc. (Leon et al., 2011). These studies can be then be used to establish standardized procedures in regards to environment, treatment goals and drinking severity of the population as well as sample size. Limitations not with standing, before the FDA will approve a drug, clinical trials must be conducted.
Clinical Trials
A relatively small percentage of drugs successfully make the transition from preclinical studies to clinical development and even fewer make it all the way through phase 3 clinical trials (Paul et al., 2010). Table 4 summarizes the results from clinical trials on drugs being developed as treatments for AUD. As evident in Table 4, clinical trials usually employ multiple primary efficacy outcomes such as time to first heavy drinking day (HDD), time to first lapse, days abstinent, maintenance of abstinence, drinks per drinking day, and percent drinking days. In addition to the outcomes measured, duration of trial, time abstinent prior to the clinical trial and dosing regimen are also variable across trials of different drugs and different trials of the same drug, as illustrated in Table 4. Once again, the lack of standardized methods among clinical trials and between human laboratory studies and clinical trials hinders the translation from human laboratory findings to clinical outcomes. First, although there tends to be less heterogeneity regarding drinking status and treatment seeking status in clinical trial participants, there are marked differences in AD phenotype and treatment goals that have been shown to alter the effect of medication (Bujarski et al., 2013; DeMartini et al., 2014). For instance, analyses of the COMBINE Study found that a goal of complete abstinence was associated with an increase in percent days abstinent, days to relapse to heavy drinking and global clinical outcome compared to a goal of conditional abstinence or controlled drinking (Bujarski et al., 2013). Therefore, it is important to acknowledge the known, clinically significant differences between human laboratory and clinical trial participants when drawing associations between human laboratory results to clinical trial outcomes. Similarly, it is important to recognize there are differences not only between but within each population as well and these should be considered when interpreting data.
In clinical trials, the FDA requires investigators to commit to an a priori hypothesis as stated in the Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims making the selection of an appropriate endpoint imperative (2009). This guidance requires investigators to thoroughly consider the aims of the clinical trial prior to execution by having them declare the hypothesis and primary outcomes ahead of time allowing investigators to test for statistical significance (Furberg and Furberg, 2007). The analyses are focused specifically on the predetermined outcome(s) and represent an important safeguard to eliminate coincidental findings. Therefore, selection of an appropriate hypothesis and outcome measures becomes extremely vital for the proper evaluation of a drug in clinical trials.
The FDA recommends that percent subjects with no heavy drinking days (PSNHDDs) be the primary endpoint measure for phase III clinical trials evaluating pharmacotherapy for AUD (FDA, 2006). Further examination of the utility and validity of this particular outcome measure was pursued by Falk and colleagues (2010) (Falk et al., 2010) who concluded that not only was this endpoint clinically relevant and as sensitive as other endpoints such as percent subjects abstinent, percent days abstinent, drinks per day, drinks per drinking day or drinks per drinking week, but that a grace period should be used where appropriate. For example, studies involving medications that require titration to reach the target dose should allow a grace period to ensure subjects are receiving the full effect of the medication prior to evaluation. Additionally, studies might include a grace period to confirm that participation in the clinical trial, itself, is not the only factor affecting changes in drinking habits. Allowing the novelty of participating in a clinical trial to diminish prior to evaluation could be especially important in preventing false negatives that can arise with the use of a placebo.
Importantly, as many clinical trials compare the treatment under investigation to placebo, there are ethical issues that arise from administering placebo to a treatment seeking population of individuals with AUD when there is a known, effective treatment. Furthermore, given that Weiss and colleagues (2008) found that administration of placebo medication in the COMBINE study lead to a significant “placebo effect” (Weiss et al., 2008), it is important to consider that the use of a placebo could potentially lead to false negatives. A possible avenue to addressing the placebo effect in AUD is to provide less robust behavioral interventions within the treatment protocol and to provide longer duration of trial and follow-up, which could unmask “real” medication versus placebo differences emerging over time.
In brief, clinical development (phase I-III studies) represents the most expensive part of drug development, making up just over 60% of the total cost, highlighting the need for a streamlined process (Paul et al., 2010) and utilization of alternative methods to reduce costs. One possible solution for alleviating the financial burden associated with clinical trials is through the use of interim analyses as it allows for the investigator to halt the study when there is enough data available to reach a conclusion (Todd et al., 2001). Not only is this beneficial in terms of financial obligations but it also carries significant ethical implications.
Moving from the Human Laboratory to the Clinic
The potential translational value of animal, human laboratory and clinical studies can be better achieved through refinements of the drug development process to ensure the successful development of novel therapeutics for AUD (Litten et al., 2012). To more fully appreciate the predictive value of preclinical and human laboratory results to clinical outcomes, we have classified each study, including drugs with at least 3 or more reported clinical trials, as either positive or negative (Figure 1; Supplementary Table 1). For the purposes of this summary figure, if the human laboratory study or clinical trial showed a statistically significant positive effect for any one of the outcomes tested, it was considered positive. As previously stated, there appears to be a bias towards positive findings in the studies reported, particularly with the animal and human laboratory studies. Interestingly, with the exception of naltrexone, there have been more clinical trials, compared to human laboratory studies, conducted on all the drugs included in Figure 1 and Supplementary Table 1. This suggests that there is less information being obtained concerning mechanism of action and dosing and more of an emphasis on efficacy outcomes. Understanding the mechanism of action can provide insight that can be advantageous when designing a clinical trial such as by helping to determine the patient population most likely to respond to the drug, identifying the most suitable drinking endpoint, establishing a more accurate dosing regimen, or predicting common side effects associated with the drug (2010). The central questions remaining are: what specific animal paradigms are predictive of human laboratory and clinical trial success and which human laboratory paradigms are predictive of clinical trial success. Further, it remains crucial to identify which experimental paradigms (in animal and in humans) can meaningfully inform our understanding of mechanisms of action of AUD pharmacotherapies and can in turn help target medications to patient populations on the basis of these mechanisms.
Figure 1.
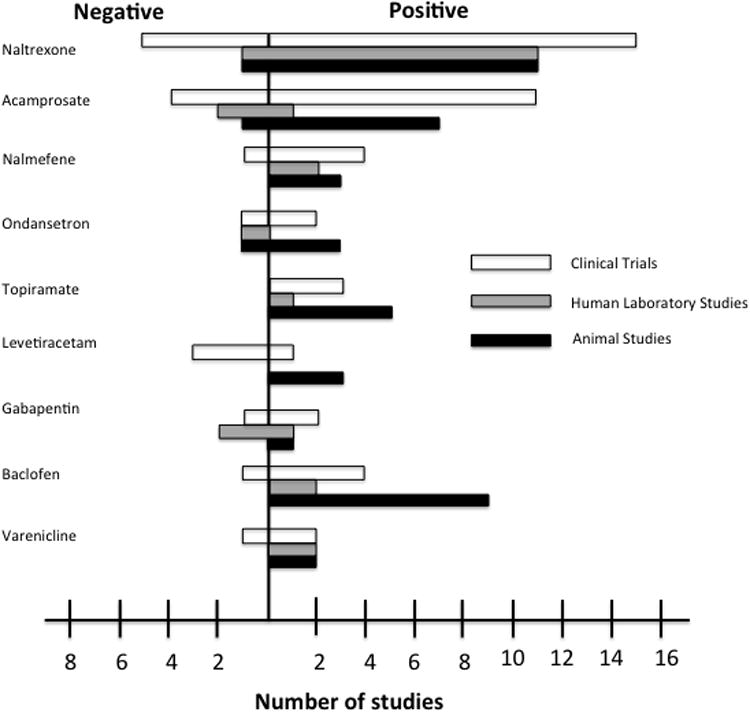
Translational research outcomes figure with depicting the number of positive (right side) and negative (left side) outcomes for each clinical trial (white bars), human laboratory study (gray bar) and animal study (black bar).
Note: Only pharmacotherapies with three or more reported clinical trials were included.
Conclusions
While only four pharmacotherapies are currently approved for the indication of AUD and their efficacy is small-to-moderate, the past two decades has seen extensive research on medications development for AUD. The neuropharmacology of alcohol is such that it targets multiple brain systems, thus offering unique challenges and opportunities. Research to date has focused primarily on medications targeting endogenous opioids and associated dopamine release in the ventral striatum, a brain region often implicated in the rewarding properties of alcohol and drugs. More recently however, increased attention has been paid to novel targets, such as CRF, ligand-gated ion channels, and the neuroimmune system. Medications in these novel drug classes are still early in their development and their potential efficacy remains unclear. The primary goal of this manuscript was to provide a perspective on medications development for AUD along with an illustrative review of the literature encompassing preclinical, human laboratory, and clinical trials. In order to provide an up-to-date survey of the field, medications undergoing testing were identified from clinicaltrials.gov and extensive literature searches were conducted. Tables were developed to characterize the medications and their purported mechanisms of action (Table 1), preclinical studies including animal models selected and results obtained (Table 2), human laboratory studies including experimental paradigms, population studied, and results (Table 3), and clinical trials, including abstinence period at study entry, treatment and dosing protocol, and results from primary outcomes (Table 4). Finally, a comparison across animal, human laboratory, and clinical trial findings was provided for pharmacotherapies for which three or more clinical trials were completed to date (Figure 1; Supplementary Table 1).
This extensive effort towards covering a large body of research has allowed us to derive some important conclusions and recommendations for the field. While a critical interpretation of the studies summarized in the tables is provided at each level of analysis (i.e., preclinical, human lab, and clinical trials), some general conclusions can also be drawn. Specifically, there is a marked need for standardization of testing procedures at each level of medications development, including standard protocols for experimental paradigms, population characteristics (in both animal and human studies), and analyses of predefined primary and secondary outcomes. Such standardization would allow us to more effectively integrate results from various studies using both critical reviews of the literature as well as quantitative studies (i.e., meta-analysis). In addition, opportunities for studies that can more effectively detect ideal dosing and mechanisms of action were highlighted throughout the review. Finally, it is important to recognize that this review ends at the efficacy testing stage, namely clinical trials. The dissemination of these findings at the level of effectiveness studies and public health efforts represents an important next frontier from the development of efficacious medications. In the current health care context, only a very small minority of patients ever receive a medication for the treatment of AUD (Bates, 2005) and that dissemination of research findings to the clinical community represents a crucial step towards the ultimate goal of alleviating suffering from this prevalent and debilitating disorder.
Supplementary Material
Acknowledgments
Funding: MMY was supported as a postdoctoral trainee from the National Institutes of Alcohol Abuse and Alcoholism (F32 AA023449). This work was further supported by R01 AA021744 (LAR), R21 AA022214 (LAR) and R21 AA022752 (LAR).
Footnotes
Disclosures: None of the authors have conflicts of interest to disclose.
Author Contributions: MMY wrote the first draft of the manuscript. LAR provided critical revisions of the manuscript. Both authors critically reviewed content and approved final version for publication.
References
- DHHS US, FDA, CDER, CBER, CDRH, editor. Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, Agabio R, Colombo G, Gessa GL, Gasbarrini G. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37:504–508. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Bedogni G, Caputo F, Gasbarrini G, Landolfi R Group BS. Dose–Response Effect of Baclofen in Reducing Daily Alcohol Intake in Alcohol Dependence: Secondary Analysis of a Randomized, Double-Blind, Placebo-Controlled Trial. Alcohol Alcohol. 2011;46:312–317. doi: 10.1093/alcalc/agr017. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Ferrulli A, Cardone S, Vonghia L, Mirijello A, Abenavoli L, D'Angelo C, Caputo F, Zambon A, Haber PS, Gasbarrini G. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370:1915–1922. doi: 10.1016/S0140-6736(07)61814-5. [DOI] [PubMed] [Google Scholar]
- Agrawal RG, Hewetson A, George CM, Syapin PJ, Bergeson SE. Minocycline reduces ethanol drinking. Brain Beh Immun. 2011;25:S165–S169. doi: 10.1016/j.bbi.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi J, Ahmadi N. A Double Blind, Placebo-Controlled Study of Naltrexone in the Treatment of Alcohol Dependence. Ger J Psychiat. 2002;5:85–89. [Google Scholar]
- Altshuler HL, Phillips PE, Feinhandler DA. Alteration of ethanol self-administration by naltrexone. Life Sci. 1980;26:679–688. doi: 10.1016/0024-3205(80)90257-x. [DOI] [PubMed] [Google Scholar]
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology (Berl) 2004a;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiat. 1999;156:1758–1764. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Anton RF, Pettinati H, Zweben A, Kranzler HR, Johnson B, Bohn MJ, McCaul ME, Anthenelli R, Salloum I, Galloway G, Garbutt J, Swift R, Gastfriend D, Kallio A, Karhuvaara S. A mult-site dose ranging study of nalmefene in the treatment of alcohol dependence. J Clin Psychopharm. 2004b;24:421–428. doi: 10.1097/01.jcp.0000130555.63254.73. [DOI] [PubMed] [Google Scholar]
- Arias A, Feinn R, Oncken C, Covault J, Kranzler HR. Placebo-controlled trial of zonisamide for the treatment of alcohol dependence. J Clin Psychopharm. 2010;30:318–322. doi: 10.1097/JCP.0b013e3181db38bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, Le Fur G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- Asatryan L, Nam HW, Lee MR, Thakkar MM, Dar MS, Davies DL, Choi DS. Implication of the purinergic system in alcohol use disorders. Alcohol Clin Exp Res. 2011;35:584–594. doi: 10.1111/j.1530-0277.2010.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asatryan L, Yardley MM, Khoja S, Trudell JR, Huynh N, Louie SG, Petasis NA, Alkana RL, Davies DL. Avermectins differentially affect ethanol intake and receptor function: Implications for developing new therapeutics for alcohol use disorders. Int J Neuropsychopharmacol. 2014;17:907–916. doi: 10.1017/S1461145713001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachteler D, Economidou D, Danysz W, Ciccocioppo R, Spanagel R. The effects of acamprosate and neramexane on cue-induced reinstatement of ethanol-seeking behavior in rat. Neuropsychopharmacol. 2005;30:1104–1110. doi: 10.1038/sj.npp.1300657. [DOI] [PubMed] [Google Scholar]
- Balldin J, Berglund M, Borg S, Mansson M, Bendtsen P, Franck J, Gustafsson L, Halldin J, Nilsson LH, Stolt G, Willander A. A 6-month controlled naltrexone study: combined effect with cognitive behavioral therapy in outpatient treatment of alcohol dependence. Alcohol Clin Exp Res. 2003;27:1142–1149. doi: 10.1097/01.ALC.0000075548.83053.A9. [DOI] [PubMed] [Google Scholar]
- Bates B. Physicians reluctant to prescribe for alcoholism. Internal Medicine News. 2005:42. [Google Scholar]
- Beardsley PM, Lopez OT, Gullikson G, Flynn D. Serotonin 5-HT3 antagonists fail to affect ethanol self-administration of rats. Alcohol. 1994;11:389–395. doi: 10.1016/0741-8329(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Ron D. Animal models of excessive alcohol consumption: Recent advances and future challenges. Alcohol. 2014;48:205–208. doi: 10.1016/j.alcohol.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Lopez MF, Cui C, Egli M, Johnson KW, Frankline KM, Becker HC. Ibudilast reduces alcohol drinking in multiple animal models of alcohol dependence. Addict Biol. 2013 doi: 10.1111/adb.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Lepoutre V, Hodge CW. GABAB receptor agonists reduce operant ethanol self-administration and enhance ethanol sedation in C57BL/6J mice. Psychopharmacology. 2004;174:358–366. doi: 10.1007/s00213-003-1769-3. [DOI] [PubMed] [Google Scholar]
- Besson J, Aeby F, Kasas A, Lehert P, Potgieter A. Combined Efficacy of Acamprosate and Disulfiram in the Treatment of Alcoholism: A Controlled Study. Alcohol Clin Exp Res. 1998;22:573–579. doi: 10.1111/j.1530-0277.1998.tb04295.x. [DOI] [PubMed] [Google Scholar]
- Bhutada P, Mundhada Y, Ghodki Y, Dixit P, Umathe S, Jain K. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of exposure to stress and modulation by mecamylamine. J Psychopharmacol. 2012;26:315–323. doi: 10.1177/0269881111431749. [DOI] [PubMed] [Google Scholar]
- Bhutada PS, Mundhada YR, Bansod KU, Dixit PV, Umathe SN, Mundhada DR. Inhibitory influence of mecamylamine on the development and the expression of ethanol-induced locomotor sensitization in mice. Pharmacol Biochem Be. 2010;96:266–273. doi: 10.1016/j.pbb.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Bilbao A, Robinson JE, Heilig M, Malanga CJ, Spanagel R, Sommer WH, Thorsell A. A pharmacogenetic determinant of mu-opioid receptor antagonist effects on alcohol reward and consumption: Evidence from humanized mice. Biol Psychiat. 2015;77:850–858. doi: 10.1016/j.biopsych.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Evans SM. Acute effects of memantine in combination with alcohol in moderate drinkers. Psychopharmacology. 2004;172:16–24. doi: 10.1007/s00213-003-1617-5. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Evans SM. The acute effects of gabapentin in combination with alcohol in heavy drinkers. Drug Alcohol Depen. 2006;83:25–32. doi: 10.1016/j.drugalcdep.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Engel JA, Soderpalm B. Accumbal dopamine overflow after ethanol: Localization of the antagonizing effect of mecamylamine. Eur J Pharmacol. 1997;334:149–156. doi: 10.1016/s0014-2999(97)01220-x. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Hernandez-Avila CA, Van Kirk J, Rose JE, Kranzler HR. Mecamylamine Modifies the Pharmacokinetics and Reinforcing Effects of Alcohol. Alcohol Clin Exp Res. 2002;26:326–331. [PubMed] [Google Scholar]
- Blomqvist O, Soderpalm B, Engel JA. Ethanol-Induced Locomotor Activity: Involvement of Central Nicotinic Acetylcholine Receptors? Brain Res Bull. 1992;29:173–178. doi: 10.1016/0361-9230(92)90023-q. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Kranzler HR, Beazoglou D, Staehler BA. Naltrexone and Brief Counseling to Reduce Heavy Drinking. Am J Addiction. 1994;3:91–99. [Google Scholar]
- Bonnet U, Banger M, Leweke M, Specka M, Muller BW, Hashemi T, Nyhuis PW, Kutscher S, Burtscheidt W, Gastpar M. Treatment of Acute Alcohol Withdrawal With Gabapentin: Results From a Controlled Two-Center Trial. J Clin Psychopharm. 2003;23:514–519. doi: 10.1097/01.jcp.0000088905.24613.ad. [DOI] [PubMed] [Google Scholar]
- Borsook D, Hargreaves R, Becerra L. Can functional magnetic resonance imaging improve success rates in CNS drug discovery? Expert Opin Drug Discov. 2011;6:597–617. doi: 10.1517/17460441.2011.584529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Brasser SM, McCaul ME, Houtsmuller EJ. Alcohol Effects During Acamprosate Treatment: A Dose-Response Study in Humans. Alcohol Clin Exp Res. 2004;28:1074–1083. doi: 10.1097/01.alc.0000130802.07692.29. [DOI] [PubMed] [Google Scholar]
- Breslin FJ, Johnson BA, Lynch WJ. Effect of topiramate treatment on ethanol consumption in rats. Psychopharmacology. 2010;207:529–534. doi: 10.1007/s00213-009-1683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent J, Harless WE. Differential effects of GABAA and GABAB agonists on sensitization to the locomotor stimulant effects on ethanol in DBA/2 J mice. Psychopharmacology. 1999;141:197–205. doi: 10.1007/s002130050825. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Myra Kim H, Strobbe S, Karam-Hage MA, Consens F, Zucker RA. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32:1429–1438. doi: 10.1111/j.1530-0277.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, O'Malley SS, Lunny K, Ray LA. The effects of drinking goal on treatment outcome for alcoholism. J Consult Clin Psych. 2013;81:13–22. doi: 10.1037/a0030886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. Baclofen alters ethanol-stimulated activity but not conditioned place preference or taste aversion in mice. Pharmacol Biochem Be. 1999;63:325–331. doi: 10.1016/s0091-3057(98)00253-6. [DOI] [PubMed] [Google Scholar]
- Chi H, de Wit H. Mecamylamine attenuates the subjective stimulant-like effects of alcohol in social drinkers. Alcohol Clin Exp Res. 2003;27:780–786. doi: 10.1097/01.ALC.0000065435.12068.24. [DOI] [PubMed] [Google Scholar]
- Chick J, Anton R, Checinski K, Croop R, Drummond DC, Farmer R, Labriola D, Marshall J, Moncrieff J, Morgan MY, Peters T, Ritson B. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcoholism. 2000a;35:587–593. doi: 10.1093/alcalc/35.6.587. [DOI] [PubMed] [Google Scholar]
- Chick J, Howlett H, Morgan MY, Ritson B Investigators U. United Kingdom multicentre acamprosate study (UKMAS): A 6-month prospective study of acamprosate versus placebo in preventing relapse after withdrawal from alcohol. Alcohol Alcoholism. 2000b;35:176–187. doi: 10.1093/alcalc/35.2.176. [DOI] [PubMed] [Google Scholar]
- Childs E, Roche DJ, King AC, de Wit H. Varenicline potentiates alcohol-induced negative subjective responses and offsets impaired eye movements. Alcohol Clin Exp Res. 2012;36:906–914. doi: 10.1111/j.1530-0277.2011.01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Gehlert DR, Ryabinin A, Kaur S, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Economidou D, Stopponi S, Cannella N, Braconi S, Kallupi M, de Guglielmo G, Massi M, George DT, Gilman J, Hersh J, Tauscher JT, Hunt SP, Hommer D, Heilig M. Stress-related neuropeptides and alcoholism: CRH, NPY, and beyond. Alcohol. 2009;43:491–498. doi: 10.1016/j.alcohol.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Hansson AC, del Arco I, Sommer W, Heilig M, Massi M, Bermúdez-Silva FJ, Navarro M, Ciccocioppo R, de Fonseca FR, Consortium ET. Cannabinoid CB1 receptor antagonism reduces conditioned reinstatement of ethanol-seeking behavior in rats. Eur J Neurosci. 2005;21:2243–2251. doi: 10.1111/j.1460-9568.2005.04056.x. [DOI] [PubMed] [Google Scholar]
- Coller JK, Hutchison MR. Implications of central immune signaling caused by drugs of abuse: Mechanisms, mediators and new therapeutic approaches for prediction and treatment of drug dependence. Pharmacol Therapeut. 2012;134:219–245. doi: 10.1016/j.pharmthera.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Carai MA, Lobina C, Pani M, Reali R, Addolorato G, Gessa GL. Ability of baclofen in reducing alcohol intake and withdrawal severity: I--Preclinical evidence. Alcohol Clin Exp Res. 2000;24:58–66. [PubMed] [Google Scholar]
- Colombo G, Agabio R, Fa M, Guano L, Lobina C, Loche A, Reali R, Gessa GL. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcoholism. 1998;33:126–130. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Brunetti G, Vacca G, Carai MA, Gessa GL. Suppression by baclofen of alcohol deprivation effect in Sardinian alcohol-preferring (sP) rats. Drug Alcohol Depen. 2003;70:105–108. doi: 10.1016/s0376-8716(02)00333-2. [DOI] [PubMed] [Google Scholar]
- Colombo G, Vacca G, Serra S, Carai MAM, Gessa GL. Suppressing effect of the cannabinoid CB1 receptor antagonist, SR 141716, on alcohol's motivational properties in alcohol-preferring rats. Eur J Pharmacol. 2004;498:119–123. doi: 10.1016/j.ejphar.2004.07.069. [DOI] [PubMed] [Google Scholar]
- Costall B, Jones BJ, Kelly ME, Naylor RJ, Onaivi ES, Tyers MB. Ondansetron inhibits a behavioural consequence of withdrawing from drugs of abuse. Pharmacol Biochem Be. 1990;36:339–344. doi: 10.1016/0091-3057(90)90414-d. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Schacht JP, Hutchinson K, Roche DJ, Ray LA. Neural substrates of cue reactivity: Association with treatment outcomes and relapse. Addict Biol. 2015 doi: 10.1111/adb.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J, Pond T, Feinn R, Arias AJ, Oncken C, Kranzler HR. Dutasteride reduces alcohol's sedative effects in men in a human laboratory setting and reduces drinking in the natural environment. Psychopharmacology. 2014;231:3609–3618. doi: 10.1007/s00213-014-3487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA, Koob GF. Preclinical studies of alcohol binge drinking. Ann NY Acad Sci. 2011;1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Fidler TL, Hill KG. Animal models of alcohol's motivational effects. Alcohol Res Health. 2000;24:85–92. [PMC free article] [PubMed] [Google Scholar]
- Davidson D, Palfai T, Bird C, Swift R. Naltrexone on alcohol self-administration in heavy drinkers. Alcohol Clin Exp Res. 1999;23:195–203. [PubMed] [Google Scholar]
- De Sousa AA, De Sousa JA, Kapoor H. An open randomized trial comparing disulfiram and topiramate in the treatment of alcohol dependence. J Subst Abuse Treat. 2008;34:460–463. doi: 10.1016/j.jsat.2007.05.012. [DOI] [PubMed] [Google Scholar]
- DeMartini KS, Devine EG, DiClemente CC, Martin DJ, Ray LA, O'Malley SS. Predictors of pretreatment commitment to abstinence: Results from the COMBINE study. J Stud Alcohol Drugs. 2014;75:438–446. doi: 10.15288/jsad.2014.75.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. P Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd PR, Beckmann AM, Davidson MS, Wilce PA. Glutamate-mediated transmission, alcohol, and alcoholism. Neurochem Int. 2000;37:509–533. doi: 10.1016/s0197-0186(00)00061-9. [DOI] [PubMed] [Google Scholar]
- Dopico AM, Lovinger DM. Acute Alcohol Action and Desensitization of Ligand-Gated Ion Channels. Pharmacol Rev. 2009;61:98–114. doi: 10.1124/pr.108.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K. A Clinical Laboratory Paradigm for Evaluating Medication Effects on Alcohol Consumption: Naltrexone and Nalmefene. Neuropsychopharmacol. 2003;28:755–764. doi: 10.1038/sj.npp.1300101. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K. Effects of naltrexone and nalmefene on subjective response to alcohol among non-treatment-seeking alcoholics and social drinkers. Alcohol Clin Exp Res. 2004;28:1362–1370. doi: 10.1097/01.alc.0000139704.88862.01. [DOI] [PubMed] [Google Scholar]
- Dyr W, Ligieza J, Kostowski W. The effect of cannabinoid CB1 receptor antagonist rimonabant (SR-141716) on ethanol drinking in high-preferring rats. Alcohol. 2008;42:509–512. doi: 10.1016/j.alcohol.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, Trabace L, Ciccocioppo R. Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology. 2005;183:394–403. doi: 10.1007/s00213-005-0199-9. [DOI] [PubMed] [Google Scholar]
- Editorial. Mechanism matters. Nat Med. 2010;16:347. doi: 10.1038/nm0410-347. [DOI] [PubMed] [Google Scholar]
- Edlund MJ, Booth BM, Han X. Who seeks care where? Utilization of mental health and substance use disorder treatment in two national samples of indviduals with alcohol use disorders. J Stud Alcohol Drugs. 2012;73:12. doi: 10.15288/jsad.2012.73.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Johnson K, George DT, Schumann G, Moss HB, Kranzler HR, Goldman D. Ethical considerations for administering alcohol or alcohol cues to treatment-seeking alcoholics in a research setting: can the benefits to society outweigh the risks to the individual? A commentary in the context of the National Advisory Council on Alcohol Abuse and Alcoholism -- Recommended Council Guidelines on Ethyl Alcohol Administration in Human Experimentation (2005) Alcohol Clin Exp Res. 2009;33:1508–1512. doi: 10.1111/j.1530-0277.2009.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M, Blomqvist O, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol. 1998;358:189–196. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- Evans SM, Bisaga A. Acute Interaction of Baclofen in Combination With Alcohol in Heavy Social Drinkers. Alcohol Clin Exp Res. 2009;33:19–30. doi: 10.1111/j.1530-0277.2008.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Levin FR, Brooks DJ, Garawi F. A Pilot Double-Blind Treatment Trial of Memantine for Alcohol Dependence. Alcohol Clin Exp Res. 2007;31:775–782. doi: 10.1111/j.1530-0277.2007.00360.x. [DOI] [PubMed] [Google Scholar]
- Falk D, Wang XQ, Liu L, Fertig J, Mattson M, Ryan M, Johnson B, Stout R, Litten RZ. Percentage of subjects with no heavy drinking days: Evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol Clin Exp Res. 2010;34:2022–2034. doi: 10.1111/j.1530-0277.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- Fanelli D. Negative results are disappearing from most disciplines and countries. Scientometrics. 2012;90:891–904. [Google Scholar]
- Farook JM, Lewis B, Gaddis JG, Littleton JM, Barron S. Effects of Mecamylamine on Alcohol Consumption and Preference in Male C57BL/6J Mice. Pharmacology. 2009;83:379–384. doi: 10.1159/000219488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farook JM, Morrell DJ, Lewis B, Littleton JM, Barron S. Topiramate (Topamax) reduces conditioned abstinence behaviours and handling-induced convulsions (HIC) after chronic administration of alcohol in Swiss-Webster mice. Alcohol Alcoholism. 2007;42:296–300. doi: 10.1093/alcalc/agm047. [DOI] [PubMed] [Google Scholar]
- Government US, editor. FDA. Medical Review of Vivitrol. Rockville, Maryland: 2006. [Google Scholar]
- Fertig JB, Ryan ML, Falk DE, Litten RZ, Mattson ME, Ransom J, Rickman WJ, Scott C, Ciraulo D, Green AI, Tiouririne NA, Johnson B, Pattinati H, Strain EC, Devine E, Brunette MF, Kampman K, Tompkins A, Stout R Group N-S. A double-blind, placebo-controlled trial assessing the efficacy of levetiracetam extended release in very heavy drinking alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36:1421–1430. doi: 10.1111/j.1530-0277.2011.01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Agoglia AE, Krouse MC, Muller RG, Robinson JE, Malanga CJ. Levetiracetam results in increased and decreased alcohol drinking with different access procedures in C57BL/6J mice. Behav Pharmacol. 2014;25:61–70. doi: 10.1097/FBP.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery BA, Garbutt JC, Cody MW, Renn W, Grace K, Osborne M, Crosby K, Morreale M, Trivette A. Baclofen for alcohol dependence: A preliminary open-label study. Alcohol Clin Exp Res. 2004;28:1517–1523. doi: 10.1097/01.alc.0000141640.48924.14. [DOI] [PubMed] [Google Scholar]
- Ford MM, Beckley EH, Nickel JD, Eddy S, Finn DA. Ethanol intake patterns in female mice: influence of allopregnanolone and the inhibition of its synthesis. Drug Alcohol Depen. 2008;97:73–85. doi: 10.1016/j.drugalcdep.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Fretwell AM, Nickel JD, Mark GP, Strong MN, Yoneyama N, Finn DA. The influence of mecamylamine on ethanol and sucrose self-administration. Neuropharmacology. 2009;57:250–258. doi: 10.1016/j.neuropharm.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Kassed CA, Chang L. Imaging the addicted human brain. Sci Pract Perspect. 2007;3:4–16. doi: 10.1151/spp07324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Morgan PT, Sinha R. Prazosin Effects on Stress- and Cue-Induced Craving and Stress Response in Alcohol-Dependent Individuals: Preliminary Findings. Alcohol Clin Exp Res. 2012;36:351–360. doi: 10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeland CS, Sharpe AL, Samson HH, Porrino LJ. Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 2001;25:277–282. [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lumeng L, Li TK. Naloxone attenuates voluntary ethanol intake in rats selectively bred for high ethanol preference. Pharmacol Biochem Be. 1990;35:385–390. doi: 10.1016/0091-3057(90)90174-g. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Hausauer BJ, Federoff DL, Fischer SM, Rasmussen DD. Prazosin reduces alcohol drinking throughout prolonged treatment and blocks the initiation of drinking in rats selectively bred for high alcohol intake. Alcohol Clin Exp Res. 2013;37:1552–1560. doi: 10.1111/acer.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O'Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl) 2011;215:655–663. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RK, Hiller-Sturmhofel S. Alcoholism treatment in the United States. An overview. Alcohol Res Health. 1999;23:69–77. [PMC free article] [PubMed] [Google Scholar]
- Furberg BD, Furberg CD. Evaluating Clinical Research: All that glitters is not gold. 2. Springer Science and Business Media; 2007. [Google Scholar]
- Gabriel KI, Cunningham CL. Effects of topiramate on ethanol and saccharin consumption and preferences in C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:75–80. doi: 10.1097/01.alc.0000150014.79657.64. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kampov-Polevoi AB, Gallop R, Kalka-Juhl L, Flannery BA. Efficacy and Safety of Baclofen for Alcohol Dependence: A Randomized, Double-Blind, Placebo-Controlled Trial. Alcohol Clin Exp Res. 2010;34:1849–1857. doi: 10.1111/j.1530-0277.2010.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O'Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Gastpar M, Bonnet U, Boning J, Mann K, Schmidt LG, Soyka M, Wetterling T, Kielstein V, Labriola D, Croop R. Lack of efficacy of naltrexone in the prevention of alcohol relapse: Results from a German multicenter study. J Clin Psychopharm. 2002;22:592–598. doi: 10.1097/00004714-200212000-00009. [DOI] [PubMed] [Google Scholar]
- Geerlings PJ, Ansoms C, van den Brink W. Acamprosate and prevention of relapse in alcoholics. Eur Addict Res. 1997;3:129–137. [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Helig M. Neurokinin 1 Receptor Antagonism as a Possible Therapy for Alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- George DT, Herion DW, Jones CL, Phillips MJ, Hersh J, Hill D, Helig M, Ramchandani VA, Geyer C, Spero DE, Singley E, O'Malley SS, Bishai R, Rawlings RR, Kunos G. Rimonabant (SR141716) has no effect on alcohol self-administration or endocrine measures in nontreatment-seeking heavy alcohol drinkers. Psychopharmacology. 2010;208:37–44. doi: 10.1007/s00213-009-1704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessa GL, Muntoni G, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Serra S, Vacca G, Carai MA, Colombo G. Suppressing effect of the cannabinoid CB1 receptor antagonist, SR147778, on alcohol intake and motivational properties of alcohol in alcohol-preferring sP rats. Alcohol Alcoholism. 2004;40:46–53. doi: 10.1093/alcalc/agh114. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, de Waele J, Thavundayil J. Implication of the endogenous opioid system in excessive ethanol consumption. Alcohol. 1996;13:19–23. doi: 10.1016/0741-8329(95)02035-7. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Neurobiology of alcohol dependence. Alcohol Res Health. 2008;31:185–195. [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Therapeut. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of Ethanol-Reinforced Behavior by Naltrexone Is Associated with Attenuation of the Ethanol-Induced Increase in Dialysate Dopamine Levels in the Nucleus Accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C. Payment of clinical research subjects. J Clin Invest. 2005;115:1681–1687. doi: 10.1172/JCI25694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou P, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depen. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Gual A, He Y, Torup L, van den Brink W, Mann K Group ES. A randomised, double-blind, placebo-controlled, efficacy study of nalmefene, as-needed use, in patients with alcohol dependence. Eur Neuropsychopharmacol. 2013;23:1432–1442. doi: 10.1016/j.euroneuro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Gual A, Lehert P. Acamprosate during and after acute alcohol withdrawal: A double-blind placebo-controlled study in Spain. Alcohol Alcoholism. 2001;36:413–418. doi: 10.1093/alcalc/36.5.413. [DOI] [PubMed] [Google Scholar]
- Guardia J, Caso C, Arias F, Gual A, Sanahuja J, Ramirez M, Mengual I, Gonzalvo B, Segura L, Trujols J, Casas M. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol-dependence disorder: results from a multicenter clinical trial. Alcohol Clin Exp Res. 2002;26:1381–1387. doi: 10.1097/01.ALC.0000030561.15921.A9. [DOI] [PubMed] [Google Scholar]
- Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, Demeyer MR, Patel KY, Brzezinkska WJ, Rhodes JS. Acute Effects of Acamprosate and MPEP on Ethanol Drinking-in-the-Dark in Male C57BL/6J Mice. Alcohol Clin Exp Res. 2008;32:1992–1998. doi: 10.1111/j.1530-0277.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Hammarberg A, Jayaram-Lindstrom N, Beck O, Franck J, Reid MS. The effects of acamprosate on alcohol-cue reactivity and alcohol priming in dependent patients: a randomized controlled trial. Psychopharmacology. 2009;205:53–62. doi: 10.1007/s00213-009-1515-6. [DOI] [PubMed] [Google Scholar]
- Harris RA, Mihic SJ, Dildy-Mayfield JE, Machu TK. Actions of anesthetics on ligand-gated ion channels: role of receptor subunit composition. FASEB J. 1995;9:1454–1462. doi: 10.1096/fasebj.9.14.7589987. [DOI] [PubMed] [Google Scholar]
- Harwood H. Updating estimates of the economic costs of alcohol abuse in the United States: Estimates, update methods, and data. NIAAA Newsletter. 2000 Available at : http://www.niaaa.nih.gov.
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiat. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Therapeut. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinala P, Alho H, Kiianmaa K, Lonnqvist J, Kuoppasalmi K, Sinclair JD. Targeted use of naltrexone without prior detoxification in the treatment of alcohol dependence: a factorial double-blind, placebo-controlled trial. J Clin Psychopharm. 2001;21:287–292. doi: 10.1097/00004714-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Tapper AR. Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6J mice. Psychopharmacology. 2009;204:563–572. doi: 10.1007/s00213-009-1488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Moc K, Koob GF. Effects of naltrexone alone and in combination with acamprosate on the alcohol deprivation effect in rats. Neuropsychopharmacology. 2003;28:1463–1471. doi: 10.1038/sj.npp.1300175. [DOI] [PubMed] [Google Scholar]
- Heyser CJ, Schulteis G, Durbin P, Koob GF. Chronic acamprosate eliminates the alcohol deprivation effect while having limited effects on baseline responding for ethanol in rats. Neuropsychopharmacology. 1998;18:125–133. doi: 10.1016/S0893-133X(97)00130-9. [DOI] [PubMed] [Google Scholar]
- Holter SM, Danysz W, Spanagel R. Evidence for alcohol anti-craving properties of memantine. Eur J Pharmacol. 1996;314:R1–R2. doi: 10.1016/s0014-2999(96)00670-x. [DOI] [PubMed] [Google Scholar]
- Hubbell CL, Marglin SH, Spitalnic SJ, Abelson ML, Wild KD, Reid LD. Opioidergic, Serotonergic, and Dopaminergic Manipulations and Rats' Intake of a Sweetened Alcoholic Beverage. Alcohol. 1991;8:355–367. doi: 10.1016/0741-8329(91)90573-f. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Yaragudri KV. Role of the Endocannabinoid System in Alcohol-Related Behaviors. The Open Neuropharmcology Journal. 2009;2:31–39. [Google Scholar]
- Hutchison KE, Ray L, Sandman E, Rutter MC, Peters A, Davidson D, Swift R. The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacology. 2006;31:1310–1317. doi: 10.1038/sj.npp.1300917. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Swift R, Rohsenow DJ, Monti PM, Davidson D, Almeida A. Olanzapine reduces urge to drink after drinking cues and a priming dose of alcohol. Psychopharmacology. 2001;155:27–34. doi: 10.1007/s002130000629. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Wooden A, Swift RM, Smolen A, McGeary J, Adler L, Paris L. Olanzapine Reduces Craving for Alcohol: A DRD4 VNTR Polymorphism by Pharmacotherapy Interaction. Neuropsychopharmacology. 2003;28:1882–1888. doi: 10.1038/sj.npp.1300264. [DOI] [PubMed] [Google Scholar]
- Ingman K, Korpi ER. Alcohol drinking of alcohol-preferring AA rats is differentially affected by clozapine and olanzapine. Eur J Pharmacol. 2006;534:133–140. doi: 10.1016/j.ejphar.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Jacquot C, Croft AP, Prendergast MA, Mulholland P, Shaw SG, Little HJ. Effects of the Glucocorticoid Antagonist, Mifepristone, on the Consequences of Withdrawal From Long Term Alcohol Consumption. Alcohol Clin Exp Res. 2008;32:2107–2116. doi: 10.1111/j.1530-0277.2008.00799.x. [DOI] [PubMed] [Google Scholar]
- Janak PH, Wolf FW, Heberlein U, Pandey SC, Logrip ML, Ron D. BIG News in Alcohol Addiction: New Findings on Growth Factor Pathways BDNF, Insulin, and GDNF. Alcohol Clin Exp Res. 2006;30:214–221. doi: 10.1111/j.1530-0277.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav R. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, Engel JA. Requirement of central ghrelin signaling for alcohol reward. P Natl Acad Sci USA. 2009;106:11318–11323. doi: 10.1073/pnas.0812809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier C, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. Medication treatment of different types of alcoholism. Am J Psychiat. 2010;167:630–639. doi: 10.1176/appi.ajp.2010.08101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Javors MA, DiClemente CC, Cloninger CR, Prihoda TJ, Bordnick PS, Ait-Daoud N, Hensler J. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: A randomized controlled trial. JAMA. 2000;284:963–971. doi: 10.1001/jama.284.8.963. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O'Malley SS, Swift R. Topiramate for treating alcohol dependence: A randomized controlled trial. JAMA. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of Naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology. 2007;192:207–217. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Anderson J, Picciotto MR. The Nicotinic Acetylcholine Receptor Partial Agonist Varenicline Increases the Ataxic and Sedative-Hypnotic Effects of Acute Ethanol Administration in C57BL/6J Mice. Alcohol Clin Exp Res. 2010;34:2053–2060. doi: 10.1111/j.1530-0277.2010.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Phillips TJ. A role for neuronal nicotinic acetylcholine receptors in ethanol-induced stimulation, but not cocaine-or methamphetamine-induced stimulation. Psychopharmacology. 2008;196:377–387. doi: 10.1007/s00213-007-0969-7. [DOI] [PubMed] [Google Scholar]
- Kenna GA, Haass-Koffler CL, Zywiak WH, Edwards SM, Brickley MB, Swift RM, Leggio L. Role of the α1 blocker doxazosin in alcoholism: a proof-of-concept randomized controlled trial. Addict Biol. 2015 doi: 10.1111/adb.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Tarnaske T, Helwig H, Briken P, Holzbach R, Kampf P, Stracke R, Baehr M, Naber D, Wiedemann K. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiat. 2003;60:92–99. doi: 10.1001/archpsyc.60.1.92. [DOI] [PubMed] [Google Scholar]
- Kiianmaa K, Hoffman PL, Tabakoff B. Antagonism of the behavioral effects of ethanol by naltrexone in BALB/c, C57BL/6, and DBA/2 mice. Psychopharmacology. 1983;79:291–294. doi: 10.1007/BF00433403. [DOI] [PubMed] [Google Scholar]
- Killeen TK, Brady KT, Gold PB, Simpson KT, Faldowski RA, Tyson C, Anton RF. Effectiveness of Naltrexone in a Community Treatment Program. Alcohol Clin Exp Res. 2004;28:1710–1717. doi: 10.1097/01.alc.0000145688.30448.2c. [DOI] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O'Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology (Berl) 1997;129:15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Knapp CM, Mercado M, Markley T, Crosby S, Ciraulo DA, Kornetsky C. Zonisamide decreases ethanol intake in rats and mice. Pharmacol Biochem Be. 2007a;87:65–72. doi: 10.1016/j.pbb.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Baclofen blocks expression and sensitization of anxiety-like behavior in an animal model of repeated stress and ethanol withdrawal. Alcohol Clin Exp Res. 2007b;31:582–595. doi: 10.1111/j.1530-0277.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig HN, Olive MF. The glucocorticoid receptor antagonist mifepristone reduces ethanol intake in rats under limited access conditions. Psychoneuroendocrino. 2004;29:999–1003. doi: 10.1016/j.psyneuen.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Modesto-Lowe V, Nuwayser ES. Sustained-Release Naltrexone for Alcoholism Treatment: A Preliminary Study. Alcohol Clin Exp Res. 1998;22:1074–1079. [PubMed] [Google Scholar]
- Kranzler HR, Pierucci-Lagha A, Feinn R, Hernandez-Avila C. Effects of ondansetron in early- versus late-onset alcoholics: a prospective, open-label study. Alcohol Clin Exp Res. 2003;27:1150–1155. doi: 10.1097/01.ALC.0000075547.77464.76. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, O'Malley SS. Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biol Psychiat. 2007;62:694–697. doi: 10.1016/j.biopsych.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Neznanova O, Masalov D, Burakov AM, Didenko T, Romanova T, Tsoy M, Bespalov A, Slavina TY, Grineko AA, Petrakis IL, Pittman B, Gueorguieva R, Zvartau EE, Krystal JH. Effect of Memantine on Cue-Induced Alcohol Craving in Recovering Alcohol-Dependent Patients. Am J Psychiat. 2007;164:519–523. doi: 10.1176/ajp.2007.164.3.519. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Jerlhag E, Liljequist S, Engel J. Effects of subunit selective nACh receptors on operant ethanol self-administration and relapse-like ethanol-drinking behavior. Psychopharmacology. 2009;203:99–108. doi: 10.1007/s00213-008-1375-5. [DOI] [PubMed] [Google Scholar]
- Laaksonen E, Koski-Jannes A, Salaspuro M, Ahtinen H, Alho H. A randomized, multicentre, open-label, comparative trial of disulfiram, naltrexone, and acamprosate in the treatment of alcohol dependence. Alcohol Alcoholism. 2008;43:53–61. doi: 10.1093/alcalc/agm136. [DOI] [PubMed] [Google Scholar]
- Lallemand F, Soubrie PH, De Witte PH. Effects of CB1 Cannabinoid Receptor Blockade on Ethanol Preference After Chronic Ethanol Administration. Alcohol Clin Exp Res. 2001;25:1317–1323. [PubMed] [Google Scholar]
- Larsson A, Svensson L, Soderpalm B, Engel JA. Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol. 2002;28:157–167. doi: 10.1016/s0741-8329(02)00244-6. [DOI] [PubMed] [Google Scholar]
- Latt NC, Jurd S, Houseman J, Wutzke SE. Naltrexone in alcohol dependence: a randomised controlled trial of effectiveness in a standard clinical setting. Med J Australia. 2002;176:530–534. doi: 10.5694/j.1326-5377.2002.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Le AD, Corrigall WA, Watchus J, Harding S, Juzytsch W, Li TK. Involvement of Nicotinic Receptors in Alcohol Self-Administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. Effects of dexfenfluramine and 5-HT3 receptor antagonists on stress-induced reinstatement of alcohol seeking in rats. Psychopharmacology. 2006;186:82–92. doi: 10.1007/s00213-006-0346-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology. 2011;218:89–99. doi: 10.1007/s00213-011-2178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, Nesci A, Miceli A, Malandrino N, Capristo E, Canestrelli B, Monteleone P, Kenna GA, Swift RM, Addolorato G. Ghrelin system in alcohol-dependent subjects: role of plasma ghrelin levels in alcohol drinking and craving. Addict Biol. 2012;17:452–464. doi: 10.1111/j.1369-1600.2010.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, Fricchione SR, Edwards SM, de la Monte SM, Swift RM, Kenna GA. Intravenous Ghrelin Administration Increases Alcohol Craving in Alcohol-Dependent Heavy Drinkers: A Preliminary Investigation. Biol Psychiat. 2014;76:734–741. doi: 10.1016/j.biopsych.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, McGeary JE, Edwards S, Fricchione SR, Shoaff JR, Addolorato G, Swift RM, Kenna GA. A human laboratory pilot study with baclofen in alcoholic individuals. Pharmacol Bichem Be. 2013;103:784–791. doi: 10.1016/j.pbb.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer D, Leschke J, Lhachimi S, Vasiliu A, Weiffen B. Negative results in social science. Eur Polit Sci. 2007;6:51–68. [Google Scholar]
- Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiat Res. 2011;45:626–629. doi: 10.1016/j.jpsychires.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhuintre JP, Moore N, Tran G, Steru L, Langrenon S, Daoust M, Parot P, Ladure P, Libert C, Boismare F, Hillemand B. Acamprosate appears to decrease alcohol intake in weaned alcoholics. Alcohol Alcoholism. 1990;25:613–622. doi: 10.1093/oxfordjournals.alcalc.a045057. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, Cui C, Fertig JB, Ryan ML, Falk DE, Moss H, Huebner R, Noronha A. Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol. 2012;17:513–527. doi: 10.1111/j.1369-1600.2012.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R NCIG. A Double-Blind, Placebo-Controlled Trial Assessing the Efficacy of Varenicline Tartrate for Alcohol Dependence. J Addict Med. 2013;7:277–286. doi: 10.1097/ADM.0b013e31829623f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton J. Acamprosate in alcohol dependence: how does it work? Addiction. 1995;90:1179–1188. doi: 10.1046/j.1360-0443.1995.90911793.x. [DOI] [PubMed] [Google Scholar]
- Lukoyanov NV, Paula-Barbosa MM. Memantine, but not dizocilpine, ameliorates cognitive deficits in adult rats withdrawn from chronic ingestion of alcohol. Neurosci Lett. 2001;309:45–48. doi: 10.1016/s0304-3940(01)02037-7. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Bienkowski P, Carai MA, Gessa GL, Colombo G. Baclofen attenuates cue-induced reinstatement of alcohol-seeking behavior in Sardinian alcohol-preferring (sP) rats. Drug Alcohol Depen. 2008;95:284–287. doi: 10.1016/j.drugalcdep.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Fantini N, Carai MAM, Gessa GL, Colombo G. Suppressing Effect of the Cannabinoid CB1 Receptor Antagonist, Rimonabant, on Alcohol Self-Administration in Alcohol-Preferring Rats. The Open Neuropharmcology Journal. 2009;2:40–44. [Google Scholar]
- MacKillop J. Factor structure of the alcohol urge questionnaire under neutral conditions and during a cue-elicited urge state. Alcohol Clin Exp Res. 2006;30:1315–1321. doi: 10.1111/j.1530-0277.2006.00159.x. [DOI] [PubMed] [Google Scholar]
- Mann K, Bladstrom A, Torup L, Gual A, van den Brink W. Extending the Treatment Options in Alcohol Dependence: A Randomized Controlled Study of As-Needed Nalmefene. Biol Psychiat. 2013;73:706–713. doi: 10.1016/j.biopsych.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Martinotti G, di Nicola M, Frustaci A, Romanelli R, Tedeschi D, Guglielmo R, Guerriero L, Bruschi A, De Filippis R, Pozzi G, Di Giannantonio M, Bria P, Janiri L. Pregabalin, tiapride and lorazepam in alcohol withdrawal syndrome: a multi-centre, randomized, single-blind comparison trial. Addiction. 2010;105:288–299. doi: 10.1111/j.1360-0443.2009.02792.x. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Di Nicola M, Tedeschi D, Mazza M, Janiri L, Bria P. Efficacy and safety of pregabalin in alcohol dependence. Adv Ther. 2008;25:608–618. doi: 10.1007/s12325-008-0066-2. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Goodman AM, Chabac S, Lehert P. Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double-blind, placebo-controlled trial: The role of patient motivation. J Psychiat Res. 2006;40:383–393. doi: 10.1016/j.jpsychires.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Light JM, Williams LD, Drobes DJ. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: effects of gabapentin. Addict Biol. 2009;14:73–83. doi: 10.1111/j.1369-1600.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: A randomized clinical trial. JAMA. 2014;174:70–77. doi: 10.1001/jamainternmed.2013.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Ritvo EC, Morgan RO, Salvato FR, Goldberg G, Welch B, Mantero-Atienza E. A Double-Blind, Placebo-Controlled Pilot Study to Evaluate the Efficacy and Safety of Oral Nalmefene HCl for Alcohol Dependence. Alcohol Clin Exp Res. 1994;18:1162–1167. doi: 10.1111/j.1530-0277.1994.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Salvato FR, Williams LD, Ritvo EC, Cutler RB. A double-blind, placebo-controlled study of oral nalmefene for alcohol dependence. Arch Gen Psychiat. 1999;56:719–724. doi: 10.1001/archpsyc.56.8.719. [DOI] [PubMed] [Google Scholar]
- Matosin N, Frank E, Engel M, Lum JS, Newell KA. Negativity towards negative results: a discussion of the disconnect between scientific worth and scientific culture. Dis Model Mech. 2014;7:171–173. doi: 10.1242/dmm.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J, Ferguson L, Harris RA. Neuroimmune signaling: a key component of alcohol abuse. Curr Opin Neurobiol. 2013;23:513–520. doi: 10.1016/j.conb.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology. 2000;22:480–492. doi: 10.1016/S0893-133X(99)00147-5. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT. Breaking the loop: Oxytocin as a potential treatment for drug addiction. Horm Behav. 2012;61:331–339. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiat. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Cuison ER, Groseclose CH. Naltrexone effects on ethanol reward and discrimination in C57BL/6 mice. Alcohol Clin Exp Res. 1999;23:456–464. [PubMed] [Google Scholar]
- Miller PM, Book SW, Stewart SH. Medical Treatment of Alcohol Dependence: A Systematic Review. Int J Psychiat Med. 2011;42:227–266. doi: 10.2190/PM.42.3.b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Jr, MacKillop J, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, Swift R, Ray L, McGeary J. Effects of Topiramate on Urge to Drink and the Subjective Effects of Alcohol: A Preliminary Laboratory Study. Alcohol Clin Exp Res. 2008;32:489–497. doi: 10.1111/j.1530-0277.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Grossman LE, Coker AR, Messing RO. The Anticonvulsant Levetiracetam Potentiates Alcohol Consumption in Non-Treatment Seeking Alcohol Abusers. J Clin Psychopharm. 2012a;32:269–272. doi: 10.1097/JCP.0b013e318248ba69. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, O'Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med. 2012b;4:116ra116. doi: 10.1126/scitranslmed.3002902. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl) 2012c;223:299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O'Brien CP, Volpicelli JR. Predicting treatment response to naltrexone: the influence of craving and family history. Am J Addiction. 2001;10:258–268. doi: 10.1080/105504901750532148. [DOI] [PubMed] [Google Scholar]
- Monti PM, Binkoff JA, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. J Abnorm Psychol. 1987;96:122–126. doi: 10.1037//0021-843x.96.2.122. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, Brown RA, Gulliver SB, Gordon A, Abrams DB. Naltrexone's effects on cue-elicited craving among alcoholics in treatment. Alcohol Clin Exp Res. 1999;23:1386–1394. [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Swift RM, Gulliver SB, Colby SM, Mueller TI, Brown RA, Gordon A, Abrams DB, Niaura RS, Asher MK. Naltrexone and cue exposure with coping and communication skills training for alcoholics: treatment process and 1-year outcomes. Alcohol Clin Exp Res. 2001;25:1634–1647. [PubMed] [Google Scholar]
- Morley KC, Teesson M, Reid SC, Sannibale C, Thomson C, Phung N, Weltman M, Bell JR, Richardson K, Haber PS. Naltrexone versus acamprosate in the treatment of alcohol dependence: a multi-centre, randomized, double-blind, placebo-controlled trial. Addiction. 2006;101:1451–1462. doi: 10.1111/j.1360-0443.2006.01555.x. [DOI] [PubMed] [Google Scholar]
- Morris PL, Hopwood M, Whelan G, Gardiner J, Drummond E. Naltrexone for alcohol dependence: a randomized controlled trial. Addiction. 2001;96:1565–1573. doi: 10.1046/j.1360-0443.2001.961115654.x. [DOI] [PubMed] [Google Scholar]
- Moykkynen T, Korpi ER. Acute Effects of Ethanol on Glutamate Receptors. Basic Clin Pharmacol Toxicol. 2012;111:4–13. doi: 10.1111/j.1742-7843.2012.00879.x. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton R, Voronin K, Wang W, Henderson S. A Double-Blind Evaluation of Gabapentin on Alcohol Effects and Drinking in a Clinical Laboratory Paradigm. Alcohol Clin Exp Res. 2007;31:221–227. doi: 10.1111/j.1530-0277.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of Naltrexone and Ondansetron on Alcohol Cue–Induced Activation of the Ventral Striatum in Alcohol-Dependent People. Arch Gen Psychiat. 2008;63:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal R, Chappell AM, Samson HH. Effects of Nicotine and Mecamylamine Microinjections into the Nucleus Accumbens on Ethanol and Sucrose Self-Adminis tration. Alcohol Clin Exp Res. 1998;22:1190–1198. [PubMed] [Google Scholar]
- Namkoong K, Lee BO, Lee PG, Choi MJ, Lee E Investigators KACT. Acamprosate in Korean alcohol-dependent patients: A multi-centre, randomized, double-blind, placebo-controlled study. Alcoholism. 2003;38:135–141. doi: 10.1093/alcalc/agg038. [DOI] [PubMed] [Google Scholar]
- Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM. κ-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology. 2011;61:35–42. doi: 10.1016/j.neuropharm.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngyuen SA, Malcom R, Middaugh LD. Topiramate reduces alcohol consumption by C57BL/6 mice. Synapse. 2007;61:150–156. doi: 10.1002/syn.20350. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Garbutt JC, Gastfriend DR, Dong Q, Kranzler HR. Efficacy of extended-release naltrexone in alcohol-dependent patients who are abstinent before treatment. J Clin Psychopharm. 2007;27:507–512. doi: 10.1097/jcp.0b013e31814ce50d. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo–pituitary–adrenocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- O'Neil ML, Beckwith LE, Kincaid CL, Rasmussen DD. The α1-adrenergic receptor antagonist, doxazosin, reduces alcohol drinking in alcohol-preferring (P) Rats. Alcohol Clin Exp Res. 2013;37:202–212. doi: 10.1111/j.1530-0277.2012.01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka M, Hirouchi M, Tamura M, Sugahara S, Oyama T. Acamprosate {monocalcium bis(3-acetamidopropane-1-sulfonate)} reduces ethanol-drinking behavior in rats and glutamate-induced toxicity in ethanol-exposed primary rat cortical neuronal cultures. Eur J Pharmacol. 2013;718:323–331. doi: 10.1016/j.ejphar.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Olive MF, Nannini MA, Ou CJ, Koenig HN, Hodge CW. Effects of acute acamprosate and homotaurine on ethanol intake and ethanol-stimulated mesolimbic dopamine release. Eur J Pharmacol. 2002;437:55–61. doi: 10.1016/s0014-2999(02)01272-4. [DOI] [PubMed] [Google Scholar]
- Gogek J, Hopkins D, editors. World Health Organization. Global status report on alcohol and health. Switzerland: 2011. pp. 1–286. [Google Scholar]
- Pacher P, Batkai S, Kunos G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paille F, Martini H. Nalmefene: a new approach to the treatment of alcohol dependence. Subst Abuse Rehab. 2014;5:87–94. doi: 10.2147/SAR.S45666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paille FM, Guelfi JD, Perkins AC, Royer RJ, Steru L, Parot P. Double-blind randomized multicentre trial of acamprosate in maintaining abstinence from alcohol. Alcohol Alcoholism. 1995;30:239–247. [PubMed] [Google Scholar]
- Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, Schacht AL. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- Pava MJ, Woodward JJ. A Review of the Interactions between Alcohol and the Endocannabinoid System: Implications for Alcohol Dependence and Future Directions for Research. Alcohol. 2012;46:185–204. doi: 10.1016/j.alcohol.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Casey RL, Fender T, Garbutt JC. Intranasal Oxytocin Blocks Alcohol Withdrawal in Human Subjects. Alcohol Clin Exp Res. 2013;37:484–489. doi: 10.1111/j.1530-0277.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelc I, Verbanck P, Le Bon O, Garvrilovic M, Lion K, Lehert P. Efficacy and safety of acamprosate in the treatment of detoxified alcohol-dependent patients. A 90-day placebo-controlled dose-finding study. Br J Psychiat. 1997;171:73–77. doi: 10.1192/bjp.171.1.73. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Rounsaville B Group VNEVIMS. Naltrexone and Disulfiram in Patients with Alcohol Dependence and Comorbid Psychiatric Disorders. Biol Psychiat. 2005;57:1128–1137. doi: 10.1016/j.biopsych.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Piasecki J, Koros E, Dyr W, Kostowski W, Danysz W, Bienkowski P. Ethanol-reinforced behaviour in the rat: effects of uncompetitive NMDA receptor antagonist, memantine. Eur J Pharmacol. 1998;354:135–143. doi: 10.1016/s0014-2999(98)00442-7. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav R. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Plebani JG, Lynch KG, Rennert L, Pettinati HM, O'Brien CP, Kampman KM. Results from a pilot clinical trial of varenicline for the treatment of alcohol dependence. Drug Alcohol Depen. 2013;133:754–758. doi: 10.1016/j.drugalcdep.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebani JG, Oslin DW, Lynch KG. Examining naltrexone and alcohol effects in a minority population: results from an initial human laboratory study. Am J Addiction. 2011;20:330–336. doi: 10.1111/j.1521-0391.2011.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebani JG, Ray LA, Morean ME, Corbin WR, MacKillop J, Amlung M, King AC. Human laboratory paradigms in alcohol research. Alcohol Clin Exp Res. 2012;36:972–983. doi: 10.1111/j.1530-0277.2011.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrugo F. Acamprosate treatment in a long-term community-based alcohol rehabilitation programme. Addiction. 1997;92:1537–1546. [PubMed] [Google Scholar]
- Pratt WM, Davidson D. Does participation in an alcohol administration study increase risk for excessive drinking? Alcohol. 2005;37:135–141. doi: 10.1016/j.alcohol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2009;33:264–272. doi: 10.1111/j.1530-0277.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Chin PF, Miotto K. Pharmacogenetics of naltrexone in asian americans: a randomized placebo-controlled laboratory study. Neuropsychopharmacology. 2012;37:445–455. doi: 10.1038/npp.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE, Tartter M. Application of human laboratory models to pharmacotherapy development for alcohol dependence. Curr Pharm Des. 2010;16:2149–2158. doi: 10.2174/138161210791516422. [DOI] [PubMed] [Google Scholar]
- Resnik DB, McCann DJ. Deception by research participants. N Engl J Med. 2015;373:1192–1193. doi: 10.1056/NEJMp1506985. [DOI] [PubMed] [Google Scholar]
- Richter C, Hinzpeter A, Schmidt F, Kienast T, Preuss UW, Plenge T, Heinz A, Schaefer M. Levetiracetam for the treatment of alcohol withdrawal syndrome. J Clin Psychopharm. 2010;30:720–725. doi: 10.1097/jcp.0b013e3181faf53e. [DOI] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, O'Dell LE, Cruz MT, Morse AC, Siggins GR, Koob GF. Cellular and Behavioral Interactions of Gabapentin with Alcohol Dependence. J Neurosci. 2008;28:5762–5771. doi: 10.1523/JNEUROSCI.0575-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G, Most D, Ferguson LB, Mayfield J, Harris RA, Blednov YA. Neuroimmune Pathways in Alcohol Consumption: Evidence from Behavioral and Genetic Studies in Rodents and Humans. Int Rev Neurobiol. 2014;118:13–39. doi: 10.1016/B978-0-12-801284-0.00002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JE, Chen M, Stamatakis AM, Krouse MC, Howard EC, Faccidomo S, Hodge CW, Fish EW, Malanga CJ. Levetiracetam Has Opposite Effects on Alcohol- and Cocaine-Related Behaviors in C57BL/6J Mice. Neuropsychopharmacology. 2013;38:1322–1333. doi: 10.1038/npp.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Michels KB. The continuing unethical use of placebo controls. N Engl J Med. 1994;331:394–398. doi: 10.1056/NEJM199408113310611. [DOI] [PubMed] [Google Scholar]
- Rubio G, Lopez-Munoz F, Ferre F, Martinez-Gras I, Ponce G, Pascual JM, Jimenez-Arriero MA, Alamo C. Effects of Zonisamide in the Treatment of Alcohol Dependence. Clin Neuropharmacol. 2010;33:250–253. doi: 10.1097/WNF.0b013e3181f0ed9a. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Albes JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56:73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Narayan AR, Zeric T, Steardo L, Cottone P. mTOR activation is required for the anti-alcohol effect of ketamine, but not memantine, in alcohol-preferring rats. Behav Brain Res. 2013;247:9–16. doi: 10.1016/j.bbr.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarid-Segal O, Knapp CM, Burch W, Richardson MA, Bahtia S, DeQuattro K, Afshar M, Richambault C, Sickels L, Devine E, Ciraulo DA. The Anticonvulsant Zonisamide Reduces Ethanol Self-Administration by Risky Drinkers. Am J Drug Alcohol Use. 2009;35:316–319. doi: 10.1080/00952990903060150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarid-Segal O, Piechniczek-Buczek J, Knapp C, Afshar M, Devine E, Sickels L, Uwodukunda E, Richambault C, Koplow J, Ciraulo D. The effects of levetiracetam on alcohol consumption in alcohol-dependent subjects: An open label study. Am J Drug Alcohol Ab. 2008;34:441–447. doi: 10.1080/00952990802082180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers EM, Toneatto T, Romach MK, Somer GR, Sobell LC, Sobell MB. Clinical efficacy of the 5-HT3 antagonist ondansetron in alcohol abuse and dependence. Alcohol Clin Exp Res. 1994;18:879–885. doi: 10.1111/j.1530-0277.1994.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Serra S, Brunetti G, Pani M, Vacca G, Carai MA, Gessa GL, Colombo G. Blockade by the cannabinoid CB(1) receptor antagonist, SR 141716, of alcohol deprivation effect in alcohol-preferring rats. Eur J Pharmacol. 2002;443:95–97. doi: 10.1016/s0014-2999(02)01594-7. [DOI] [PubMed] [Google Scholar]
- Sharrett-Field L, Butler TR, Berry JN, Reynolds AR, Prendergast MA. Mifepristone Pretreatment Reduces Ethanol Withdrawal Severity In Vivo. Alcohol Clin Exp Res. 2013;37:1417–1422. doi: 10.1111/acer.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Haass-Koffler CL, Bito-Onon J, Li R, Bartlett SE. Mifepristone in the Central Nucleus of the Amygdala Reduces Yohimbine Stress-Induced Reinstatement of Ethanol-Seeking. Neuropsychopharmacol. 2012;37:906–918. doi: 10.1038/npp.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M. A Pilot Trial of the Alpha-1 Adrenergic Antagonist, Prazosin, for Alcohol Dependence. Alcohol Clin Exp Res. 2009;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Catapano D, OM SS. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology. 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Weiner JL. Chronic treatment with prazosin or duloxetine lessens concurrent anxiety-like behavior and alcohol intake: evidence of disrupted noradrenergic signaling in anxiety-related alcohol use. Brain Beh. 2014;4:468–483. doi: 10.1002/brb3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderpalm B, Ericson M. Neurocircuitry involved in the development of alcohol addiction: the dopamine system and its access points. Curr Top Behav Neurosci. 2013;13:127–161. doi: 10.1007/7854_2011_170. [DOI] [PubMed] [Google Scholar]
- Sommer C, Seipt C, Spreer M, Blumke T, Markovic A, Junger E, Plawecki MH, Zimmerman US. Laboratory alcohol self-administration experiments do not increase subsequent real-life drinking in young adult social drinkers. Alcohol Clin Exp Res. 2015;39:1057–1063. doi: 10.1111/acer.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, Koller G, Schmidt P, Lesch OM, Leweke M, Fehr C, Gann H, Mann KF Investigators AS. Cannabinoid Receptor 1 Blocker Rimonabant (SR 141716) for Treatment of Alcohol Dependence: Results From a Placebo-Controlled, Double-Blind Trial. J Clin Psychopharm. 2008;28:317–324. doi: 10.1097/JCP.0b013e318172b8bc. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Holter SM, Allingham K, Landgraf R. Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharmacol. 1996a;305:39–44. doi: 10.1016/0014-2999(96)00174-4. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Putzke J, Stefferi A, Schobitz B, Zieglgansberger W. Acamprosate and alcohol: II. Effects on alcohol withdrawal in the rat. Eur J Pharmacol. 1996b;305:45–50. doi: 10.1016/0014-2999(96)00175-6. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Vengeliene V, Jandeleit B, Fischer WN, Grindstaff K, Zhang X, Gallop MA, Krstew EV, Lawrence AJ, Kiefer F. Acamprosate produces its anti-relapse effects via calcium. Neuropsychopharmacology. 2014;39:783–791. doi: 10.1038/npp.2013.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Zieglgansberger W, Hundt W. Acamprosate and alcohol: III. Effects on alcohol discrimination in the rat. Eur J Pharmacol. 1996c;305:51–56. doi: 10.1016/0014-2999(96)00176-8. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. P Natl Acad Sci USA. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, Ruggeri B, Helig M, Demopulos G, Gaitanaris G, Massi M, Ciccocioppo R. Activation of Nuclear PPARγ Receptors by the Antidiabetic Agent Pioglitazone Suppresses Alcohol Drinking and Relapse to Alcohol Seeking. Biol Psychiat. 2011;69:642–649. doi: 10.1016/j.biopsych.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Stopponi S, Somaini L, Cippitelli A, de Guglielmo G, Kallupi M, Cannella N, Gerra G, Massi M, Ciccocioppo R. Pregabalin reduces alcohol drinking and relapse to alcohol seeking in the rat. Psychopharmacology. 2012;220:87–96. doi: 10.1007/s00213-011-2457-3. [DOI] [PubMed] [Google Scholar]
- Suchankova P, Steensland P, Fredriksson I, Engel JA, Jerlhag E. Ghrelin receptor (GHS-R1A) antagonism suppresses both alcohol consumption and the alcohol deprivation effect in rats following long-term voluntary alcohol consumption. PLoS ONE. 2013;8:e71284. doi: 10.1371/journal.pone.0071284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Feinn R. Using effect size- or why the p value is not enough. J Grad Med Educ. 2012;4:279–282. doi: 10.4300/JGME-D-12-00156.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Kovacs GL, Telegdy G. Effects of neurohypophyseal peptide hormones on alcohol dependence and withdrawal. Alcohol Alcoholism. 1987;22:71–74. [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. Animal models in alcohol research. Alcohol Res Health. 2000;24:77–84. [PMC free article] [PubMed] [Google Scholar]
- Tanchuck MA, Yoneyama N, Ford MM, Fretwell AM, Finn DA. Assessment of GABA-B, metabotropic glutamate, and opioid receptor involvement in an animal model of binge drinking. Alcohol. 2011;45:33–44. doi: 10.1016/j.alcohol.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempesta E, Janiri L, Bignamini A, Chabac S, Potgieter A. Acamprosate and relapse prevention in the treatment of alcohol dependence: A placebo-controlled study. Alcohol Alcoholism. 2000;35:202–209. doi: 10.1093/alcalc/35.2.202. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Badia-Elder NE. A role for neuropeptide Y in alcohol intake control: evidence from human and animal research. Physiol Behav. 2003;79:95–101. doi: 10.1016/s0031-9384(03)00109-4. [DOI] [PubMed] [Google Scholar]
- Thorsell A. Neuropeptdie Y (NPY) in alcohol intake and dependence. Peptides. 2007;28:480–483. doi: 10.1016/j.peptides.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Monti PM, Rohsenow DJ, Gwaltney CJ, Miranda R, Jr, McGeary JE, MacKillop J, Swift RM, Abrams DB, Shiffman S, Paty JA. Moderators of Naltrexone's Effects on Drinking, Urge, and Alcohol Effects in Non-Treatment-Seeking Heavy Drinkers in the Natural Environment. Alcohol Clin Exp Res. 2008;32:58–66. doi: 10.1111/j.1530-0277.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd S, Whitehead A, Stallard N, Whitehead J. Interim analyses and sequential designs in phase III studies. Brit J Clin Pharmaco. 2001;51:394–399. doi: 10.1046/j.1365-2125.2001.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins DM, Le AD, Sellers EM. Effect of the 5-HT3 antagonist ondansetron on voluntary ethanol intake in rats and mice maintained on a limited access procedure. Psychopharmacology. 1995;117:479–485. doi: 10.1007/BF02246222. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med. 1998;49:173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT, Helig M. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropharmacology. 2011;36:1178–1186. doi: 10.1038/npp.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsalan N, Saglam E, Kayir H, Uzbay TI. Effects of olanzapine on ethanol withdrawal syndrome in rats. Eur J Pharmacol. 2008;579:208–214. doi: 10.1016/j.ejphar.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Vadnie CA, Park JH, Gawad NA, Ho AMC, Hinton DJ, Choi DS. Gut-brain peptides in corticostriatal-limbic circuitry and alcohol use disorders. Front Neurosci. 2014;8:1–25. doi: 10.3389/fnins.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O'Collins V, Macleod MR. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Helig M, Koob GF. Corticosteroid-Dependent Plasticity Mediates Compulsive Alcohol Drinking in Rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Rasmussen DD, Froehlich JC, Czachowski CL. Effects of prazosin, an α1-adrenergic receptor antagonist, on the seeking and intake of alcohol and sucrose in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2012;36:881–886. doi: 10.1111/j.1530-0277.2011.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological Evidence for a Motivational Role of κ-Opioid Systems in Ethanol Dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Porrino LJ. Behavioral neurobiology of alcohol addiction: recent advances and challenges. J Neurosci. 2002;22:3332–3337. doi: 10.1523/JNEUROSCI.22-09-03332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, OM SS, Hosking JD, LoCastro JS, Swift R Group ES. Do patients with alcohol dependence respond to placebo? Results from the COMBINE Study. J Stud Alcohol Drugs. 2008;69:878–884. doi: 10.15288/jsad.2008.69.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth AB, Fischer F, Lesch OM, Nimmerrichter A, Oberbauer H, Platz T, Potgieter A, Walter H, Fleischhacker WW. Comparison of acamprosate and placebo in long-term treatment of alcohol dependence. Lancet. 1996;347 doi: 10.1016/s0140-6736(96)91682-7. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Saville K, Hamreusm K. Acamprosate for treatment of alcohol dependence: mechanisms, efficacy, and clinical utility. Ther Clin Risk Manag. 2012;8:45–53. doi: 10.2147/TCRM.S23184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D, Schoffelmeer ANM, Pattij T, De Vries TJ. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology. 2011;216:267–277. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt LR, Finn DA, Yardley MM, Khoja S, Asatryan L, Alkana RL, Davies DL. Contribution of P2X4 receptors to ethanol intake in male C57BL/6 mice. Neurochem Res. 2014;39:1127–1139. doi: 10.1007/s11064-014-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley M, Wyatt L, Khoja S, Asatryan L, Ramaker MJ, Finn DA, Alkana RL, Huynh N, Louie SG, Petasis NA, Bortolato M, Davies DL. Ivermectin reduces alcohol intake and preference in mice. Neuropharmacology. 2012;63:190–201. doi: 10.1016/j.neuropharm.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley MM, Neely M, Huynh N, Asatryan L, Louie SG, Alkana RL, Davies DL. Multiday administration of ivermectin is effective in reducing alcohol intake in mice at doses shown to be safe in humans. NeuroReport. 2014;25:1018–1023. doi: 10.1097/WNR.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Ponnudurai R, Schaefer R. Ondansetron: A Selective 5-HT3 Receptor Antagonist and Its Applications in CNS-Related Disorders. CNS Drug Rev. 2001;7:199–213. doi: 10.1111/j.1527-3458.2001.tb00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EM, Mahler S, Chi H, de Wit H. Mecamylamine and ethanol preference in healthy volunteers. Alcohol Clin Exp Res. 2005;29:58–65. doi: 10.1097/01.alc.0000150007.34702.16. [DOI] [PubMed] [Google Scholar]
- Zalewska-Kaszubska J, Bajer B, Czarnecka E, Dyr W, Gorska D. Voluntary alcohol consumption and plasma beta-endorphin levels in alcohol preferring rats chronically treated with levetiracetam: A preliminary study. Physiol Behav. 2011;102:538–541. doi: 10.1016/j.physbeh.2010.12.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


