Abstract
Purpose
Determine if abnormal autonomic nervous system (ANS) innervation of the bladder underlies interstitial cystitis/bladder pain syndrome (IC/BPS) differently than other chronic pelvic pain (CPP).
Material and Methods
IRB approved protocol, 134 subjects enrolled, 3 excluded, 39 healthy controls (HC), 36 IC/BPS, 14 myofascial pelvic pain (MPP), 42 IC/BPS plus MPP. ANS evaluations: deep breathing, Valsalva maneuver, tilt table test (TTT), and sudomotor test (evaluates for autonomic neuropathy (AN)). A modified validated composite autonomic laboratory score was applied.
Results
IC/BPS group 47.5 (21, 78) yrs was older than HC 34 (20, 75)yrs (p=0.006), MPP group 33 (22, 56)yrs (p=0.004), and IC/BPS plus MPP 38 (18, 64)yrs (p= 0.03). BMI was not significantly different among groups, but the MPP and IC/BPS plus MPP groups had a higher BMI than HC (p=0.03 and p= 0.05 respectively). Cardiovascular and adrenergic indexes did not differ among groups. TTT showed more orthostatic intolerance (OI) in all CPP groups. TTT diagnoses (orthostatic hypotension, postural tachycardia syndrome -POTS, reflex syncope) occurred rarely. Baseline heart rate (HR) was higher in all CPP groups (p=0.004). All MPP groups showed significantly more clearcut AN (defined as sweat score ≥ 3) compare to HC (HC vs IC/BPS plus MPP p= 0.007; HC vs MPP p = 0.03).
Conclusions
Some CPP types show an autonomic neuropathy, and some show vagal withdrawal. In all types, orthostatic intolerance likely reflects cenral sensitization and perhaps catastrophization. Some of these findings suggest novel therapeutic targets.
Keywords: Interstitial Cystitis/Bladder Pain Syndrome, pelvic pain, autonomic nervous system, autonomic neuropathy
Introduction
Autonomic abnormalities including postural tachycardia syndrome (POTS), reflex syncope or autonomic neuropathy may occur in functional disorders such as chronic fatigue syndrome1 functional gastrointestinal disorders2 and headaches3. The typical symptoms of interstitial cystitis/bladder pain syndrome (IC/BPS) (frequency, urgency, and CPP that depends on bladder fill state4) suggest a pathophysiologic role for a generalized cardiovascular and vasomotor autonomic nervous system (ANS) abnormality. We compared IC/BPS with another disorder, myofascial pelvic pain (MPP) with pain in the pelvis unrelated to bladder state, and hypothesized that it would harbor no cardiovascular or vasomotor autonomic dysfunction.
Preliminary findings5 showed no structural autonomic abnormalities in IC/BPS subjects, except higher baseline heart rate (HR) supporting the concept of functional rather than structural change in the ANS. Complicating that report, many of the subjects with IC/BPS had comorbid myofascial pelvic pain (MPP). In the current full follow-up report, we now have adequate power to determine if autonomic abnormalities differ between these two overlapping but clinically distinct pelvic pain disorders, IC/BPS and MPP. We sought to determine if the absence of structural abnormalities in the ANS persisted 1) when evaluating a larger cohort, and 2) in subjects with IC/BPS without comorbid MPP. In addition, recent findings of autonomic neuropathy in fibromyalgia6 led us to hypothesize similar findings in MPP without cardiovascular or vasomotor autonomic dysfunction, based on the concept of a “fibromyalgia of the pelvic musculature”.
Methods
Participants
This prospective IRB-approved study (University Hospitals Case Medical Center, Cleveland, OH) evaluated the structural integrity of the ANS of adult women diagnosed with IC/BPS, MPP, both or none (healthy control subjects) as a portion of the larger ICEPAC study (Interstitial Cystitis – Elucidation of Psychophysiologic and Autonomic Characteristics ClinicalTrials.gov Identifier: NCT01616992), with methods previously published7. All subjects were enrolled between 2/2011 and 12/2014 and provided informed consent prior to participating.
A diagnosis of IC/BPS required ≥ 6 months of pain clearly linked to bladder fill state, and exclusion criteria aligned with the IC/BPS-NIDDK criteria8. In the absence of an accepted definition of MPP9, the diagnosis required (1) ≥ 3 months of non-cyclic CPP unrelated to bladder fill state, and (2) NRS pain score ≥ 4/10 in 2 of 5 pelvic floor muscles (bilateral puborectalis, obturator internus and midline perineal body) tested by applying 2 kg pressure with a gloved index finger to the relaxed muscle belly10. In addition to general subject exclusions (Appendix), healthy subjects were stringently screened to exclude any disorders commonly comorbid with pelvic pain (Appendix). Fibromyalgia diagnosis required a history of diffuse pain and ≥ 11/18 tender points on exam by a clinician11. By definition, healthy subjects could not have fibromyalgia.
Autonomic Testing
With rare exceptions explicitly discussed below, all autonomically active medications were stopped at least 5 half-lives prior to testing, performed in the morning or afternoon, with several components described in detail elsewhere12, 7: the cardiovascular response to deep breathing (DB, primarily testing cardiac parasympathetic integrity), the Valsalva maneuver (VM, testing cardiac sympathetic, parasympathetic and vasomotor sympathetic integrity), tilt table test (TTT, testing cardiac and vasomotor sympathetic integrity) and quantitative sudomotor axon reflex test (QSART, testing post-ganglionic sympathetic cholinergic integrity). TTT was performed at 70 degrees for 30 minutes. The VM (using 15 seconds and 40 mmHg) and DB (6 breaths per min) were performed at least 3 and 2 times, respectively, in each subject. Norms were utilized as described by Low and Sletten (2008)12 except for QSART values based on our own lab norms. Subjects ate a light meal 2 hours prior to the tests.
Data Analysis
As before5, we quantified ANS test results using a modified Composite Autonomic Severity Score (CASS)12 (table 1) with sudomotor, adrenergic and cardiovascular HR indices. TTT baseline utilized the mean of the last 4 minutes of the 10-minute supine recording prior to tilt-up. Vital sign outliers accompanying fidgeting and talking were excluded. Postural tachycardia syndrome (POTS) required a >30 bpm HR increase without hypotension in the first 10 min upright with associated orthostatic symptoms. Neurally mediated syncope required an abrupt drop in blood pressure13 and usually heart rate. Orthostatic hypotension (OH) required a >20/10 mmHg BP drop in the first 3 minutes upright, termed “delayed OH” when occurring later14. Orthostatic intolerance requires upright symptoms without POTS, OH or reflex syncope. The research coordinators queried subjects every minute for a 0–10 numeric score for each symptom with a ≥ 3 point increase considered clinically significant. Significant autonomic neuropathy was defined as a modified CASS sudomotor score of 3.
Table 1.
Modified CASS score
| 1 score point | 2 score points | 3 score points | 4 score points |
|
|---|---|---|---|---|
|
Sudomotor index |
Single QSART site ↓ |
Single QSART site <50% of lower limit |
≥ 2 QSART sites <50% of lower limit |
NA |
|
Adrenergic index |
Phase IIE ↓<40>25 mmHg, or ↓phase IIL or PP ↓to < 50% of baseline ↑PRT 4–5 sec Absent phase IV |
Phase IIL absent, or ↑PRT 6–9 sec |
Absent phase IIL and IV and ↑PRT ≥10 sec |
#3 plus OH defined as sBP↓≥30 mmHg; MBP ↓≥20 mmHg |
|
Cardiovascular index |
HRDB or VR ↓but >50% minimum |
HRDB or VR ↓but < 50% minimum |
HRDB AND VR ↓but < 50% minimum |
NA |
QSART: quantitative sudomotor axon reflex; Phase IIE; Phase II early; phase IIL: Phase II late; HRDB: Heart rate response to deep breathing; VR: Valsalva ratio; MBP: mean blood pressure; OH: orthostatic hypotension; PP: pulse pressure; PRT: pressure recovery time; sBP: systolic blood pressure
Chi-square tests compared categorical variables. Median and ranges characterize continuous variables. Due to data skewness and outliers we used non-parametric tests. Kruskal-Wallis and a Mann-Whitney test compared differences among groups. Logistic regression evaluated the frequency of clearcut autonomic neuropathy (sudomotor score of 3) across groups. The outcome was clearcut neuropathy, the considered dependent variables were: age, BMI, diagnosis group, the interaction terms of diagnosis group and age and BMI, as well as fibromyalgia. Forward model selection was used with criteria of stay. A Hosmer and Lemeshow test evaluated model fit. A general linear model (GLM) examined relationships to peak HR. The outcome was average peak HR, the considered dependent variables were: age, HR baseline, clearcut neuropathy, BMI and the interaction terms of clearcut neuropathy and (age, BMI), and the interaction term of diagnosis group and (age, BMI). The GLMselect procedure selected the significant predictors. The criterion of the stepwise selection for the entry and removal was 0.05 and a normal link was used. A QQ-plot diagnosed the optimal model fit. We used a P value <0.05 to indicate significance without adjustment for multiple testing.
Results
Of 134 enrolled subjects, exclusions after testing included 2 for inability to stop hydroxazine or an anticholinergic, resulting in no sweat output and a third for prior cardiac ablations. Final analysis included HC 39, and the following CPP groups: IC/BPS 36, MPP 14, IC/BPS plus MPP 42. Significant artifact prevented calculation of the Valsalva maneuver adrenergic score in 4 subjects. TTT was not performed in 1 subject due to BMI above safety range of the tilt table. The analysis included 2 subjects with IC/BPS who continued low-dose chronic diphenhydramine or amitriptyline as their findings did not differ from other subjects.
The IC/BPS group (47.5 (21, 78)yrs) was significantly older than the other groups: healthy control (34 (20, 75)yrs, p=0.006), MPP (33 (22, 56)yrs, p=0.004), and IC/BPS plus MPP (38 (18, 64)yrs, p= 0.03). BMI did not differ among groups generally, but pairwise comparison revealed that the 2 groups with MPP (IC/BPS plus MPP and MPP alone) had higher BMI than the healthy controls (p=0.03 and p= 0.05 respectively) (table 2).
Table 2.
Median (min, max) age, BMI, and cardiac autonomic responses
| HC | IC/BPS | IC/BPS + MPP |
MPP | p | |
|---|---|---|---|---|---|
| Age (yrs) | 34 (20, 75) | 47.5 (21, 78) | 38 (18, 64) | 33 (22, 56) | 0.006 |
| BMI | 24.2 (18, 53.8) | 25.7 (15.1, 43.9) | 27.6 (17.3, 62.4) |
28.9 (17.6, 44.4) |
0.055 |
| DB | 23 (6, 43) | 16 (5, 38) | 21 (6, 44) | 24 (13, 32) | 0.006 |
| VM ratio | 2.0 (1.2, 2.9) | 1.9 (1.0, 2.9) | 1.7 (1.2, 3.2) | 1.7 (1.5, 2.9) | 0.18 |
HC: healthy controls; IC/BPS: interstitial cystitis/bladder pain syndrome; MPP: myofascial pelvic pain; DB: deep breathing (mean heart rate change between inspiration and expiration); VM: Valsalva maneuver (ratio between peak heart rate in phase II/lowest heart rate in phase IV). Values in () represent the range
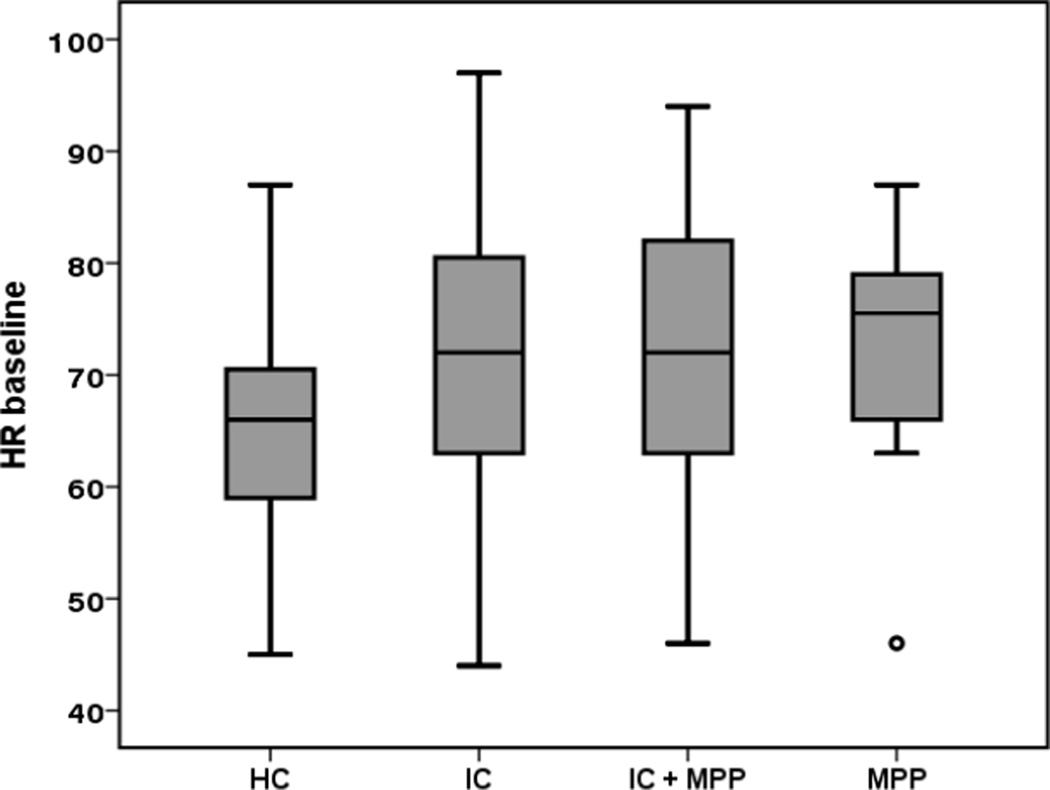
The cardiovascular and adrenergic indexes did not differ among groups. Physiologic TTT diagnoses such as OH, POTS and reflex syncope occurred rarely in all groups (Table 3). However, symptoms during TTT (without a physiologic diagnosis, indicating orthostatic intolerance) occurred more in subjects with pelvic pain (table 3). Heart rate at baseline (p<0.001) (Fig 1) and at peak in the first 10 minutes of TTT (p=0.009) was higher in all pelvic pain groups but not the change in HR from supine to upright. Predictors of peak HR included baseline HR (p<0.001), age (as expected p<0.0001) and BMI (p=0.007).
Table 3.
Frequency of autonomic diagnoses in each group
| HC (%) | IC/BPS (%) | IC/BPS plus MPP (%) |
MPP (%) |
p All CPP vs HC |
|
|---|---|---|---|---|---|
| # of subjects | 39 | 36 | 41* | 14 | |
| Syncope | 3 (8%) | 2 (6%) | 5 (12%) | 0 (0%) | 0.62 |
| OH | 0 (0%) | 1(3%) | 0 (0%) | 0 (0%) | 0.38 |
| POTS | 0 (0%) | 1 (3%) | 2 (5%) | 1 (7%) | 0.39 |
| OI | 0 (0%) | 7 (19%) | 15 (37%) | 4 (29%) | < 0.0001 |
| p vs HC | 0.004 | < 0.0001 | 0.003 | ||
1 excluded due to BMI too high above safety range of tilt table
HC: healthy control; MPP myofascial pelvic pain; IC/BPS: interstitial cystitis/ Bladder pain syndrome
Fig 1.
Baseline heart rate during tilt table test per groups
HC: healthy control; MPP myofascial pelvic pain; IC/BPS: interstitial cystitis/ Bladder pain syndrome
Subjects with MPP (alone or with IC/BPS) had more frequent clearcut autonomic neuropathy (sweat score ≥ 3; table 5), and the groups differed in their sudomotor index assessed as a categorical variable (merging all MPP groups together, “1” or “2” vs “3” p=0.012). Except in the forearm that was low in all groups, subjects with MPP (alone or with IC/BPS) also showed diffusely decreased sweat output (Table 4 and fig 2). Though clearcut neuropathy occurred more frequently in patients with fibromyalgia (assessed in 107 subjects; odds ratio= 3.441 and 95% C.I. 1.364, 8.684), MPP was still significantly related to clearcut autonomic neuropathy considering fibromyalgia by logistic regression (p=0.016).
Table 5.
Frequency of Cass sudomotor score per group (Chi-square test p-value = 0.042)
| CASS sudomotor score | HC (%) | IC/BPS (%) | IC/BPS + MPP & MPP (%) |
|---|---|---|---|
| 0 | 5 (13%) | 9 (25%) | 11 (20%) |
| 1 | 12 (31%) | 6 (17%) | 12 (21%) |
| 2 | 18 (46%) | 13 (36%) | 13 (23%) |
| 3 | 4 (10%) | 8 (22%) | 20 (36%) |
HC: healthy control; MPP myofascial pelvic pain; IC/BPS: interstitial cystitis/ Bladder pain syndrome
Table 4.
Median (min, max) Sweat output in the different locations according to groups. Values in () represent the range
| HC | IC/BPS | IC/BPS plus MPP |
MPP | p | |
|---|---|---|---|---|---|
| Foot | 22 (0, 119) | 17 (0, 85) | 12 (0, 78) | 12 (0, 57) | 0.02 |
| Calf | 50 (0, 182) | 27 (0, 156) | 30 (0, 134) | 19 (0, 68) | 0.01 |
| Hand | 44 (0, 164) | 55 (0,197) | 25 (0, 115) | 20 (0, 117) | 0.01 |
| Forearm | 9 (0, 86) | 8 (0, 121) | 9 (0, 67) | 12 (0, 64) | 0.93 |
HC: healthy control; MPP myofascial pelvic pain; IC/BPS: interstitial cystitis/ Bladder pain syndrome
Fig 2.
Sweat output per location and group
HC: healthy control; MPP myofascial pelvic pain; IC/BPS: interstitial cystitis/ Bladder pain syndrome. *, ○, represent extremevalues and outliers respectively.
Discussion
In summary, our study demonstrates that: 1) in contrast to other functional disorders such as IBS15 and fibromyalgia16, POTS, reflex syncope and OH are uncommon in IC/BPS or MPP; 2) however, orthostatic complaints while upright termed “orthostatic intolerance” occurred commonly in all forms of CPP compatible with central hypervigilance; 3) higher baseline heart rate in subjects with pelvic pain suggests higher sympathetic drive or vagal withdrawal related to deconditioning, or both; 4) MPP with or without IC/BPS more frequently harbors a clearcut autonomic neuropathy, occurring in 36% of this population, compared to 22% in those with IC/BPS alone, and 10% of healthy subjects.
The low frequency of POTS in subjects with IC/BPS, seen previously5 and now, is surprising, since POTS is commonly associated with functional gastrointestinal symptoms in pediatrics15 as well as adults17, with chronic fatigue18, and fibromyalgia16, all of which occur commonly in women with IC/BPS19,20. Several explanations appear plausible. The physiologic findings of POTS lessen with increasing chronicity though symptoms may not change. Thus some of our subjects might in fact have had POTS at one time, and now only show residual orthostatic intolerance (upright symptoms without findings, see below). Perhaps the age of our subjects rendered this diagnosis less likely, as POTS peaks in teens and twenties, though it clearly occurs in the 30’s, the age of our population. The most intriguing hypothesis states that the reduced autonomic outflow (both sympathetic and parasympathetic) in IC/BPS21 impairs autonomic reactivity to the point that POTS cannot be manifested. This hypothesis would not explain the low frequency of POTS in MPP, but the low sample size might be explanatory.
In contrast, 19% of IC/BPS subjects, 29% of MPP subjects, and 37% of subjects with both, against 0% of healthy subjects, showed orthostatic intolerance (upright symptoms without changes in vital signs). Also described in pediatric subjects with dizziness without POTS on autonomic testing22, this finding likely suggests central hypervigilance or hypersensitivity, known to occur in various chronic pain states23, and probably associated with catastrophization. Hypervigilance simply reflects amplification of stimuli, painful or painless24. Indeed, orthostatic intolerance is restricted to symptoms, not reflecting any currently measurable autonomic physiologic abnormality. This may be why orthostatic intolerance does not parse by specific CPP disorder, but only becomes more prominent with higher disease burden, in contrast to autonomic neuropathy that specifically parses with MPP, and low heart rate variability that specifically parses with IC/BPS.
The increased heart rate at baseline may reflect overactive sympathetic drive or reduction in vagal outflow, both associated with chronic pain of any type. For example, increasing vagal tone through biofeedback decreases functional abdominal pain symptoms in children25. Heart rate variability analysis in our patient sample confirms the presence of reduced cardiac vagal tone in all CPP groups, greatest in the IC/BPS group. In addition, sympathetic tone also seems to be reduced (albeit to a lesser extent than vagal tone), particularly in the IC/BPS group21. Thus, it appears that people with IC/BPS experience a state of reduced overall autonomic outflow affecting both parasympathetic and sympathetic functions. This is reminiscent of another pain disorder, complex regional pain syndrome (CRPS), where withdrawal of sympathetic function occurs in the affected limb (the parasympathetic system does not innervate limbs)26. Perhaps autonomic withdrawal characterizes certain chronic pain disorders like IC/BPS or CRPS, but not others like MPP or fibromyalgia.
The finding of more frequent clearcut autonomic neuropathy in subjects with a specific type of chronic pelvic pain, MPP (with or without IC/BPS) is novel to the best of our knowledge. Since almost no subjects demonstrated cardiac vagal dysfunction (seen in the deep breathing response, table 2), autonomic neuropathies seen in our population were at most mild to moderate. Since fibromyalgia is linked with small fiber neuropathy involving the autonomic fibers6, as well as with MPP (about 20% in IC/BPS alone, vs 50% in MPP with or without IC/BPS)21, fibromyalgia alone might have accounted entirely for this finding, but in fact does not. The presence or absence of MPP still accounts for the majority of the variance in autonomic neuropathy in this population. The neuropathy might occur first and predispose to the development of a myofascial syndrome such as MPP or fibromyalgia. Further studies will elucidate this pathophysiology.
This study has some limitations: 1) Our pure MPP group is smaller than the other groups due to difficulty enrolling pure MPP subjects, perhaps biasing some of the results and underestimating some of the differences across groups since the study is underpowered in this group; 2) The diagnosis of an autonomic neuropathy is based only on sudomotor function, and should ideally be confirmed using other methodologies, such as skin biopsy, which we did not perform.
CONCLUSION
In summary, taking the current study together with our previous publication on heart rate variability in subjects with CPP21, three major findings characterize this patient population. First, like subjects with fibromyalgia6, subjects with MPP appear to suffer from a mild to moderate autonomic neuropathy, not seen in subjects with IC/BPS alone. Second and previously reported21, like subjects with complex regional pain syndrome26, subjects with IC/BPS appear to demonstrate major withdrawal of autonomic innervation, with vagal withdrawal exceeding sympathetic withdrawal, resulting in sympathetic predominance. Third, irrespective of CPP type, all CPP subjects experience orthostatic intolerance, probably reflecting central sensitization and a change in homeostatic perception, not an autonomic efferent abnormality, based on current understanding, and perhaps associated with catastrophization.
Each of these findings broadens our fundamental conceptual framework for chronic pelvic pain and potential therapeutic strategies. First, both the presence of an autonomic neuropathy and withdrawal of cardiac vagal tone imply broad systemic neural changes, rather than abnormalities restricted to an end-organ, consistent with the conclusion drawn from the ever-increasing panoply of disorders co-morbid with different types of chronic pelvic pain. Second, autonomic neuropathies often occur in the setting of systemic inflammatory illnesses such as diabetes, Sjogren’s, amyloidosis etc. or other immunologic illnesses. By increasing systemic inflammation, vagal withdrawal27, could constitute a core pathophysiologic component of both chronic pelvic pain and autonomic neuropathy. Vagal withdrawal could thus provide a novel therapeutic target in the management of CPP. In addition to direct vagal nerve stimulation, for which one study found promising evidence in CPP28, the main tools available to increase vagal tone clinically are exercise training29 and cognitive behavior training30. Finally, orthostatic intolerance implies central sensitization and possibly catastrophization and may reflect the central correlate and driver of reduced vagal tone in the first place.
Supplementary Material
Acknowledgments
The ICEPAC Study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01DK083538) and grant from Advancing Healthier Wisconsin 5520298. The following individuals are members of the ICEPAC Study Advisory Board and have helped shape the design and methodologies describe herein: Debra Erickson, M.D. (Dept. of Surgery, University of Kentucky College of Medicine, Lexington, KY, USA), Kathleen Pajer, M.D., M.P.H. (IWK Health Centre, Dalhousie University, Halifax, Nova Scotia, Canada), Julian Thayer, Ph.D. (Dept. of Psychology, The Ohio State University, Columbus, OH, USA), Ursula Wesselmann, M.D., Ph.D. (Dept. of Anesthesiology, UAB School of Medicine, Birmingham, AL, USA), Phyllis Zee, M.D. (Center for Sleep & Circadian Biology, Northwestern University, Evanston, IL, USA), and Denniz Zolnoun, M.D., M.P.H. (Dept. of Obstetrics and Gynecology, UNC School of Medicine, Chapel Hill, NC, USA). We also thank Daniel Wayer for excellent technical assistance.
Disclosures: Thomas Chelimsky is advisory board member for Lundbeck (2015) and Ironwood (ongoing) Pharmaceutical
Key of Definitions for Abbreviations
- ANS
autonomic nervous system
- IC/BPS
interstitial cystitis/bladder pain syndrome
- CPP
chronic pelvic pain
- TTT
tilt table test
- AN
autonomic neuropathy
- HC
healthy controls
- MPP
myofascial pelvic pain
- POTS
postural tachycardia syndrome
- HR
heart rate
- VM
Valsalva maneuver
- DB
deep breathing
- QSART
quantitative sudomotor axon reflex test
- CASS
Composite Autonomic Severity Score
- OH
orthostatic hypotension
- BP
blood pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reynolds GK, Lewis DP, Richardson AM, Lidbury BA. Comorbidity of postural orthostatic tachycardia syndrome and chronic fatigue syndrome in an Australian cohort. J Intern Med. 2014;275:409–417. doi: 10.1111/joim.12161. [DOI] [PubMed] [Google Scholar]
- 2.Chelimsky G, Boyle JT, Tusing L, Chelimsky TC. Autonomic abnormalities in children with functional abdominal pain: coincidence or etiology? J Pediatr Gastroenterol Nutr. 2001;33:47–53. doi: 10.1097/00005176-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Khurana RK, Eisenberg L. Orthostatic and non-orthostatic headache in postural tachycardia syndrome. CEPHALALGIA. 2011;31:409–415. doi: 10.1177/0333102410382792. [DOI] [PubMed] [Google Scholar]
- 4.Hanno PM. Diagnosis of interstitial cystitis. Urol Clin North Am. 1994;21:63–66. [PubMed] [Google Scholar]
- 5.Chelimsky G, et al. Autonomic testing of women with interstitial cystitis/bladder pain syndrome. Clin Auton Res. 2014;24:161–166. doi: 10.1007/s10286-014-0243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giannoccaro MP, Donadio V, Incensi A, Avoni P, Liguori R. Small nerve fiber involvement in patients referred for fibromyalgia. Muscle Nerve. 2014;49:757–759. doi: 10.1002/mus.24156. [DOI] [PubMed] [Google Scholar]
- 7.Chelimsky T, et al. Interstitial Cystitis - Elucidation of Psychophysiologic and Autonomic Characteristics (the ICEPAC Study): design and methods. J Pain Res. 2014;7:243–253. doi: 10.2147/JPR.S58853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.2006 NIDDK International Symposium: Frontiers in Painful Bladder Syndrome and Interstitial Cystitis. 2006 < http://archives.niddk.nih.gov/niddkfrontiers/displaypage.aspx?pagename=niddkfrontiers/index.htm>.
- 9.Adams K, Gregory WT, Osmundsen B, Clark A. Levator myalgia: why bother? International urogynecology journal. 2013;24:1687–1693. doi: 10.1007/s00192-013-2089-8. [DOI] [PubMed] [Google Scholar]
- 10.Zolnoun D, et al. Reliability and reproducibility of novel methodology for assessment of pressure pain sensitivity in pelvis. J Pain. 2012;13:910–920. doi: 10.1016/j.jpain.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfe F, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 12.Low PA, Sletten DM. In: Clinical Autonomic Disorders. Low PA, Benarroch EE, editors. Ch. 11. Lippicott Williams & Wilkins; 2008. pp. 130–163. [Google Scholar]
- 13.Grubb B, Karas B. The potential role of serotonin in the pathogenesis of neurocardiogenic syncope and related autonomic. Journal Interventional Cardiac Electrophysiology. 1998;2:325–332. doi: 10.1023/a:1009792000490. [DOI] [PubMed] [Google Scholar]
- 14.Freeman R, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 15.Kovacic K, et al. Joint hypermobility: a common association with complex functional gastrointestinal disorders. J Pediatr. 2014;165:973–978. doi: 10.1016/j.jpeds.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Staud R. Heart rate variability as a biomarker of fibromyalgia syndrome. Fut Rheumatol. 2008;3:475–483. doi: 10.2217/17460816.3.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thieben MJ, et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clinic proceedings. 2007;82:308–313. doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- 18.Stewart JM, et al. Postural neurocognitive and neuronal activated cerebral blood flow deficits in young chronic fatigue syndrome patients with postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H1185–H1194. doi: 10.1152/ajpheart.00994.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chelimsky G, et al. Co-morbidities of interstitial cystitis. Frontiers in neuroscience. 2012;6:114. doi: 10.3389/fnins.2012.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nickel JC, Tripp DA International Interstitial Cystitis Study, G. Clinical and psychological parameters associated with pain pattern phenotypes in women with interstitial cystitis/bladder pain syndrome. J Urol. 2015;193:138–144. doi: 10.1016/j.juro.2014.07.108. [DOI] [PubMed] [Google Scholar]
- 21.Williams DP, et al. Effects of Chronic Pelvic Pain on Heart Rate Variability in Women. J Urol. 2015;194:1289–1294. doi: 10.1016/j.juro.2015.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chelimsky G, et al. Comorbid Conditions Do Not Differ in Children and Young Adults with Functional Disorders with or without Postural Tachycardia Syndrome. The Journal of pediatrics. 2015;167:120–124. doi: 10.1016/j.jpeds.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 23.Campbell CM, et al. Sleep, Pain Catastrophizing, and Central Sensitization in Knee Osteoarthritis Patients With and Without Insomnia. Arthritis Care Res (Hoboken) 2015;67:1387–1396. doi: 10.1002/acr.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollins M, et al. Perceived intensity and unpleasantness of cutaneous and auditory stimuli: an evaluation of the generalized hypervigilance hypothesis. PAIN. 2009;141:215–221. doi: 10.1016/j.pain.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sowder EGR, Shapiro W, Ebert C. Restoration of vagal tone: A possible mechanism for functional abdominal pain. Appl Psychophysiol Biofeedback. 2010;35:199–206. doi: 10.1007/s10484-010-9128-8. [DOI] [PubMed] [Google Scholar]
- 26.Drummond PD, Finch PM, Smythe GA. Reflex sympathetic dystrophy: The significance of differing plasma catecholamine concentrations in affected and unaffected limbs. Brain. 1991;114:2025–2036. doi: 10.1093/brain/114.5.2025. [DOI] [PubMed] [Google Scholar]
- 27.Pavlov VA, Tracey KJ. Neural circuitry and immunity. Immunol Res. 2015;63:38–57. doi: 10.1007/s12026-015-8718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Napadow V, et al. Evoked pain analgesia in chronic pelvic pain patients using respiratory-gated auricular vagal afferent nerve stimulation. Pain Med. 2012;13:777–789. doi: 10.1111/j.1526-4637.2012.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanudo B, Carrasco L, de Hoyo M, Figueroa A, Saxton JM. Vagal modulation and symptomatology following a 6-month aerobic exercise program for women with fibromyalgia. Clin Exp Rheumatol. 2015;33:S41–S45. [PubMed] [Google Scholar]
- 30.Diveky T, et al. Comparison of heart rate variability in patients with panic disorder during cognitive behavioral therapy program. Psychiatr Danub. 2013;25:62–67. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.