Abstract
This review summarizes current knowledge of the biology, pathology and clinical understanding of lymphatic invasion and metastasis in pancreatic cancer. We discuss the clinical and biological consequences of lymphatic invasion and metastasis, including paraneoplastic effects on immune responses and consider the possible benefit of therapies to treat tumors that are localized to lymphatics. A review of current techniques and methods to study interactions between tumors and lymphatics is presented.
Keywords: Lymphatics, Lymph node, Pancreatic Cancer
Introduction
As the fourth leading cause of cancer-related deaths (a five-year survival rate of less than 7%), pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal forms of cancers in the United States [1]. Worldwide, PDAC results in over 330,000 deaths annually [2]. Unlike the stable or decreasing trends of incidence and death rates for other cancers, the incidence of PDAC continues to rise, underscoring the critical need for new and effective therapies for this disease [1]. Unfortunately, most PDAC cases are diagnosed when the primary tumor has already spread to regional and distant locations [1] eliminating surgery as a curative treatment option. Cancer metastasis into and through the lymphatic vasculature and lymph nodes occurs frequently in PDAC patients [3–5] and is strongly correlated with poor prognosis [6–9]. Evaluating lymph node status has been proven to be a significant factor when determining therapy selection for cancer patients [10–12].
The lymphatic vasculature offers the most direct route from the primary tumor to the frequentlyinvaded draining lymph nodes during PDAC metastasis. The lymphatic system is responsible for maintenance of tissue fluid homeostasis, absorption of dietary fat, and leukocyte and antigen transport from tissues to lymph nodes for the initiation of immune responses [13–15]. Originating in nearly all vascularized tissues, blind-ended lymphatic capillaries, or initial lymphatics, are specialized for the uptake of interstitial fluids, macromolecules, and leukocytes. They are composed of a single layer of endothelial cells with discontinuous intercellular junctions and lack a basement membrane [16,17]. The endothelial membrane of the initial lymphatics is attached to the extracellular matrix (ECM) via anchoring filaments, which facilitate the opening of the lymphatic lumen during increased interstitial fluid pressure [18,19]. Upon entry into the lymphatic capillaries, lymph and its macromolecular and cellular contents are transported to larger pre-collecting lymphatic vessels and then to collecting vessels, composed of not just the endothelial layer but also smooth muscles to facilitate flow and bi-leaflet valves to prevent backflow [20–22]. The afferent collecting lymphatics enter the lymph nodes where the lymph is filtered, and upon exiting the lymph nodes through the efferent collecting vessels, the lymph passes through the major trunks of the lymphatic system, the thoracic duct and the right lymphatic trunk, and is then returned to the circulatory system [14,23].
The network of lymphatic vasculature and lymph nodes responsible for draining the pancreas is quite complex. In the normal pancreas, the lymphatic vessels are typically located near blood vessels and are often found in the interlobular spaces of the pancreas [24]. Classification of pancreatic nodes has not been uniformly standardized, although pancreatic lymph nodes are generally divided into regions based upon their location around the pancreas and the areas of drainage of the pancreas: head/neck, body/tail, left side, or right side (reviewed in [25,26]). Studies correlating primary tumor location and lymph node involvement following resection have helped to identify the regional patterns and probabilities of lymph node metastasis, but more analysis will need to done for consistent accurate prediction of lymph node involvement [27–30].
Although clinicians and researchers understand the importance of lymphatic invasion and lymph node involvement for pancreatic cancer patient prognosis and therapy selection, the biological processes that govern lymphatic invasion and metastasis remain under-studied. For example, there is currently disagreement within the field as to whether lymphatic vessel expansion at the primary tumor site and draining lymph node is necessary for lymph node metastasis. Also, it has not been conclusively determined whether metastasis to the lymph nodes is a sequential step in distant organ spread or a final destination for tumor cells to promote immunosuppression. The potential role of lymphatics supporting immune suppression has led to questions of how normal and tumor-associated lymphatic endothelia may contribute to immune modulation within the tumor microenvironment and invaded lymph nodes either through trafficking functions or direct interactions with immune cells. These and many more questions have yet to be fully explained: how does the lymphatic endothelium regulate the entry of tumor cells into vessels; how do tumor cells evade immune cell recognition within lymphatic vessels and lymph nodes; what are the therapeutic implications of targeting lymphangiogenesis or other lymphatic-directed functions in patients with PDAC? This review summarizes our current knowledge of the role of the lymphatic system in pancreatic cancer progression and metastasis and examines research techniques and clinical procedures used in this field of study. A better understanding of the processes of lymphatic invasion and lymph node metastasis in PDAC will significantly contribute to our overall understanding of this deadly disease and provide the groundwork for the development of novel efficacious therapies.
Pancreatic Tumor Resection and Lymphadenectomy
Surgical resection of pancreatic adenocarcinoma was first brought to clinical practice by Walther Kausch in Berlin in 1909 [31]. Beginning in the mid-1930s, American surgeon Allen O. Whipple further employed and modified the pancreat(ic)oduodenectomy (PD) procedure that would bear his name; he eventually condensed the surgery into a single operation, the first of which was successfully performed in 1940 [32]. In a traditional PD the head of the pancreas is removed along with the duodenum, gall bladder, and end of the common bile duct [27,33–42]. Several timely surgical advances facilitated increased success of the PD as performed by Whipple and his contemporaries including the first successful duodenectomy in a canine, the discovery that direct immediate restoration of biliary and pancreatic secretions into the gastrointestinal tract was not necessary for survival of patients, and the use of non-dissolvable silk suture rather than the more temporary catgut [32,43]. Additional scientific breakthroughs critical for decreased perioperative morbidity and mortality included the discovery and synthesis of vitamin K, the discovery of insulin, and the description of human blood types and subsequent establishment of blood banks [32,43]. Today, a broad range of similar pancreatic resection procedures are in use in modern surgical practices around the world. Differences in primary tumor placement within the pancreas—head/neck vs. body/tail—and tumor invasion into surrounding tissues and organs often necessitate customization of resection [44–55] beyond the traditional PD to such procedures as distal pancreatectomy with or without splenectomy [41,56], pancreaticogastrostomy [35], pylorus-preserving PD [37,38,40], pylorus-resecting PD [40], subtotal stomach-preserving PD, pancreatojejunostomy, duodenum-preserving head resection, wedge resection of inferior vena cava, and total [39] or regional [57] pancreatectomy [58,59].
As with surgical treatment of other malignancies, one of the most controversial aspects of modern pancreatic ductal adenocarcinoma resection has been the extent to which surrounding connective tissue and lymph nodes should be removed. Evidence suggests that metastasis to lymph nodes is an early event in pancreatic cancer progression, and presence of tumor cells in lymph nodes represents one of the most negative prognostic factors with respect to patient outcomes [8,27,29,37,60–63]. Conservative surgical views support the standard PD with loco-regional lymphadenectomy [27,31,34,36,38,42,56,59,64–74], while others, most notably numerous Japanese groups, advocate that a more radical PD with extensive removal of retroperitoneal soft tissue and extended lymphadenectomy [27,34,39,42,75–84] results in better patient outcomes. Collected studies in Table 1 [8,27,31,34,36,37,39,40,42,59,64–75,77–99] demonstrate the broad range of study designs and conclusions that have fueled this debate. A recent set of randomized, controlled clinical trials from several centers around the world and a mathematical model of outcomes prediction have concluded that extended lymphadenectomy does not improve survival over traditional, more conservative resection and that quality-of-life may be decreased with more radical surgery [67–70,72,74,81,83,87,88,95]. Leading international surgical groups have also applied their expertise to the ongoing conversation in this field. They have recently identified lymph node stations to be included in standard lymphadenectomy for head (5, 6, 8a, 12b1, 12b2, 12c, 13a, 3b, 14a, 14b, 17a, and 17b) and body/tail (10, 11, 18) pancreatic cancer resections [94] and have released recommendations suggesting discontinued use of extended lymphadenectomy for treatment of PDAC [34,73,94]. The occasional case report continues to demonstrate the biological diversity of pancreatic malignancy and challenge these recommendations. In 2013 a Japanese group reported that extended lymphadenectomy in a welldifferentiated, chemotherapy-responsive PDAC with para-aortic lymph node metastases resulted in patient survival of over ten years [75]. Peparini, et al. 2015 [93], addressed para-aortic lymph node involvement (stations 16a2 and 16b1), stating that involvement of these lymph nodes may be due to direct invasion of the primary pancreatic tumor as opposed to dissemination and seeding of migratory cancer cells in the traditional definition of metastasis and that their removal may favorably impact R margin status. Clinical trials and expert consensus recommendations consistently recommend standard lymphadenectomy over more radical resection strategies, but, as each case of pancreatic cancer is truly a unique disease, circumstances in which extended lymph node removal is beneficial may be more clearly defined in the future.
Table 1.
Standard (sLE) vs. Extended (eLE) Lymphadenectomy for Treatment of PDAC: Results and Recommendations of Collected Studies
| Citation | Study Type |
Results/Conclusions | Supported LE Type |
|---|---|---|---|
| Masui 2013 | CR | In selected cases eLE can result in long-term patient survival: here well- differentiated, chemotherapy-responsive pancreatic cancer with PALN metastasis |
Extended |
| Pedrazzoli 1998 | P | Survival trends toward an increase in node positive patients with PD + eLE and retroperitoneal tissue removal over sLE; No increase in operative morbidity/mortality |
Extended |
| Riall 2005 | P | Survival trends toward an increase in pylorus-preserving PD with retroperitoneal LE and distal gastrectomy in PDAC patients, but this may be due to increased positive resection margins in standard resection group |
Extended |
| Benassai 1999 | R | Survival increased with eLE but may be due to patient selection; Additional prospective randomized trials are necessary |
Extended |
| Ishikawa 1988 | R | eLE and connective tissue removal is recommended for regional control of small (< 4 cm) tumors |
Extended |
| Manabe 1989 | R | Survival increased with radical pancreatectomy including soft tissue removal and eLE beyond suspected positive lymph nodes |
Extended |
| Meriggi 2007 | R | Extended peripancreatic and locoregional LE including removal of nerve and connective tissue reduces local recurrence in tumors < 4 cm in diameter |
Extended |
| Nakao 1995 | R | eLE including PALNs should be performed in patients with pancreas head cancer |
Extended |
| Ohta 1993 | R | eLE and removal of retroperitoneal connective tissue is recommended for curative resection in tumors macroscopically confined to pancreas |
Extended |
| Fernández-Cruz 1999 | Rv | Removal of primary tumor as well as eLE and removal of nerve plexus, soft tissue, and portions of nearby blood vessels are necessary to prevent tumor recurrence and spread |
Extended |
| Samra 2008 | Rv | Modified en bloc resection (including removal of lymphatics and neural tissue associated with superior mesenteric artery and retropancreatic tissue) may be best for PDAC of pancreas head |
Modified radical/ Intermediate |
| Tol 2014 | Exp | International Group on Pancreatic Surgery accepted Japanese Pancreas Society lymph node classification system, defined standard and extended LE procedures, and recommended standard LE for PDAC |
Standard |
| Farnell 2005 | P | Quality-of-life is decreased in patients following eLE with no survival benefit; No further trials should be performed comparing these two surgical methods |
Standard |
| Gerdes 2005 | P | Radical LE not recommended in cases of pylorus-preserving PD | Standard |
| Jang 2014 | P | Prospective randomized clinical trial found no improvement in survival with eLE over sLE while eLE increased morbidity |
Standard |
| Nimura 2012 | P | No difference in 5-year or disease-free survival or number of involved lymph nodes; Local recurrence higher and quality-of-life lower with eLE; eLE not indicated based on trials |
Standard |
| Pissas 1984 | P | Describes pancreatic lymphatic drainage patterns and involved lymph nodes with discussion of appropriate surgical procedures to remove specific lymph node clusters; eLE may not be beneficial because of close proximity of pancreatic lymphatics and thoracic duct allowing early circulation of tumor cells |
Standard |
| Yeo 2002 | P | Extending pylorus-preserving PD with retroperitoneal LE and distal gastrectomy does not improve survival and increases morbidity in patients with periampullary carcinomas; eLE may show some benefit in PDAC with long-term follow-up |
Standard |
| Henne-Bruns 1998 | R | Retroperitoneal eLE does not improve survival over regional LE for pancreatic head tumors following R0 partial duodeno-pancreatectomy |
Standard |
| Henne-Bruns 2000 | R | Survival after partial PD does not improve with eLE and retroperitoneal tissue removal over regional LE |
Standard |
| Hirata 1997 | R | eLE does not always improve PDAC outcomes and may be responsible for increased post-operative mortality |
Standard |
| Kanda 2011 | R | A thorough but not radical degree of LE is recommended with differential dissections indicated for head vs. body/tail tumors |
Standard |
| Pawlik 2005 | R | eLE may only benefit 0.3% of patients; Too large of a population size would be necessary to sufficiently power a prospective trial making it infeasible |
Standard |
| Shimada 2006 | R | eLE does not improve outcomes in the presence of positive PALNs and is not recommended |
Standard |
| Dasari 2015 | Rv | Meta-analysis of randomized clinical trials; eLE does not improve survival over sLE but increases morbidity |
Standard |
| Evans 2009 | Rv | Survival is not improved with eLE over sLE; Recommend sLE during PDAC PD |
Standard |
| Farnell 2008 | Rv | Recommends standard PD without eLE based on survival and quality-of- life outcomes of four randomized clinical trials |
Standard |
| Fujii 2013 | Rv | Title somewhat misleading; Advocates LE with sufficient removal of LNs to provide accurate prognosis (to include lymph node ratio metric) but does not recommend extensive eLE |
Standard |
| Iqbal 2009 | Rv | Meta-analysis 1988–2005; eLE is associated with increased patient morbidity including delayed gastric emptying with no increase in survival |
Standard |
| Ke 2014 | Rv | eLE is associated with poor post-operative quality-of-life; sLE is recommended for patients with PDAC of pancreas head |
Standard |
| Michalski 2007 | Rv | Survival not improved and quality-of-life decreased with eLE; Not indicated except perhaps in the setting of additional randomized controlled trials |
Standard |
| Pederzoli 1997 | Rv | Current data does not show benefit of extensive LE; Additional prospective randomized trials are necessary |
Standard |
| Pedrazzoli 2015 | Rv | Lymph node stations 6, 8a, 8p, 12a, 12b, 12c, 13a, 13b, 14a, 14b, 14c, 14d, 16b1, 17a, and 17b should be removed as part of a sLE to accurately stage disease and decrease metastasis risk |
Standard |
| Peparini 2015 | Rv | Lymph node stations 16a2 and 16b1 (PALNs) should be included in sLE to minimize local invasion and improve resection margins |
Standard |
| Schoellhammer 2015 | Rv | eLE should not be implemented for PDAC patients due to lack of improvement in patient survival |
Standard |
| Sergeant 2013 | Rv | Sufficient evidence does not exist to indicate eLE over sLE for treatment of PDAC of pancreas head |
Standard |
| Svoronos 2014 | Rv | Five-year overal survival was not improved with eLE and eLE patients experienced a significant increase in post-operative diarrhea; sLE with PD should be used to treat PDAC of pancreas head |
Standard |
|
The studies below do not directly support one type of LE over another but provide pertinent results that should be considered in a discussion of this topic. | |||
| Hirono 2012 | P | Intraoperative physiological fluorescence imaging identified 7 lymphatic drainage pathways from pancreatic uncinate process; Removal of PALNs and skeletonization of superior mesenteric artery may both be beneficial |
|
| Imai 2010 | P | CT, MRI, and FDG-PET cannot accurately detect presence or absence of lymph node metastases; Intraoperative examination of frozen sections is recommended |
|
| Kocher 2007 | P | Sentinel pancreas lymph nodes were not identified intraoperatively; Prediction of positive lymph nodes to guide selective resection was not possible |
|
| Nguyen 2003 | P | There is no difference in quality-of-life metrics in standard vs. radical resection 2.2 years following surgery |
|
| Roche 2003 | P | Preoperative CT does not accurately predict positive lymph nodes especially in the case of micrometastasis and should not preclude curative resection or direct LE decisions |
|
| Yeo 1999 | P | Extending pylorus-preserving PD with retroperitoneal LE and distal gastrectomy does not increase morbidity and mortality over standard resection; More time and greater numbers of patients are needed to assess survival benefit |
|
| Bittner 1989 | R | Surgery for pancreatic cancer does not increase morbidity or mortality over other abdominal oncologic surgeries; Resection only benefits TNM stage I population |
|
| Doi 2007 | R | PALN metastasis correlated with increased mortality; Upon intraoperative confirmation of PALN metastasis alternative treatment strategies should be considered due to short survival duration even with eLE |
|
CR: Case report, Exp: Expert consensus statement, LE: Lymphadenectomy–(e) Extended, (s) Standard, P: Prospective study, PALN: Paraaortic lymph node, PD: Pancreat(ic)oduodenectomy, PDAC: Pancreatic ductal adenocarcinoma, R: Retrospective study, Rv: Review
Outcomes Prediction: Lymphatic-Specific Metrics
Outcomes prediction for pancreatic cancer patients has traditionally been based on stage classification according to the TNM (tumor, node, metastasis) system at diagnosis [100]. Pancreatic cancer is rarely diagnosed in a pre-metastatic state. Comprehensive examination of lymph node and lymphatic vessel involvement would provide clinicians with important information about the progression characteristics of an individual patient’s tumor such as its pattern and route of spread, likelihood of local/distant recurrence, and potential immunomodulatory effects. Four outcomes predictive metrics specifically address lymph node/lymphatic vessel involvement: lymph node disease (LND), lymph node burden (LNB), lymph node ratio (LNR), and lymphatic vessel invasion (LVI). Each of these measures provides distinct information regarding disease pathology and may be useful in refining prognoses. LND is defined as the confirmed presence of metastatic tumor cells in at least one lymph node. The total number of positive lymph nodes confirmed at resection constitutes LNB. LNR is the ratio of the number of positive nodes to the total number of nodes examined [101]. LNR has been shown to be an effective tool to further stratify the TNM stage N1 patient population for outcomes prediction while decreasing likelihood of understaging and stage migration [102–104]. LVI may refer to lymphatic vessel invasion as determined by immunohistochemical staining of tissue sections or more broadly, to lymphovascular invasion, which may or may not distinguish between invaded hematogenous and lymphogenous vessels [63,105]. The relative prognostic value of each of these lymph node-/lymphatic vasculature-specific metrics is controversial. Prospective and retrospective clinical studies evaluating the utility of these criteria are collected in Table 2 [6,33,38,44,51,62,63,101–104,106–114]. Contradictory conclusions from these studies highlight the remaining need for additional work before use of these metrics is informative in the general clinical setting.
Table 2.
Lymphatic-Specific Outcomes Metrics: Lymph Node Disease (LND), Lymph Node Burden (LNB), Lymph Node Ratio (LNR), Lymphatic Vessel Invasion (LVI)
| Analyzed/Significant Metrics* | |||||
|---|---|---|---|---|---|
| Citation | Conclusions/Recommendations | LND | LNB | LNR | LVI |
| Ausborn 2013 | High LNR is associated with decreased overall survival of PDAC patients only when 53BP1 expression is low |
||||
| Berger 2004 | LNR > 0.15, not number of LNs examined, predicts overall survival and disease-free survival in patients with PDAC |
||||
| Bhatti 2010 | LNR is a better predictor of overall survival than LNB or LND in PDAC |
||||
| Chen 2010 | Lymphovascular invasion (may be blood or lymphatic vessels) predicts 5-year survival in periampullary cancer and PDAC patients |
||||
| House 2007 | Presence of LND predicts survival in PDAC patients; In node positive disease, LNB and LNR are good predictors of survival |
||||
| John 2013 | LNB and LNR can be used to predict cancer-specific survival in patients with PDAC of pancreas head regardless of resection margin status |
||||
| Konstantinidis 2010 | Regional lymph node metastasis and direct lymph node invasion equally negatively impact survival; LNR ≥ 0.2, LNB, and LVI are useful predictors of prognosis of PDAC patients |
||||
| La Torre 2011 | LND and LNR are both predictive of prognosis in PDAC patients; LNR maintains significance on multivariate analysis |
||||
| Murakami 2010 | LND and LNB, not LNR, predict overall survival in PDAC patients; LNR also significant in univariate analysis |
||||
| Nakagohri 2006 | LND and LVI are predictors of 3-year survival; LND is best predictor of all analyzed for PDAC patients |
||||
| Pawlik 2007 | LNR predicts prognosis and disease-specific survival in PDAC patients |
||||
| Riediger 2009 | LNR, not LND or LNB, predicts overall survival in PDAC patients | ||||
| Robinson 2012 | LND and LNR are good predictors of 5-year survival of PDAC patients; LNR better when > 0.15 |
||||
| Schwarz 2006 | Number of lymph nodes examined and number of negative lymph nodes identified predict survival for exocrine pancreatic cancer patients |
||||
| Sergeant 2009 | Extracapsular LNR predicts overall survival as does overall LNR; LNB predicts disease-free survival in PDAC patients |
||||
| Sierzega 2006 | LND predicts prognosis in patients with PDAC of pancreas head; In node positive disease, LNR > 0.2 predicts survival; Specifically, Group 8 and 12 lymph nodes were prognostic |
||||
| Slidell 2008 | Number of positive LNs, presence of LND, and LNR are significantly associated with survival in PDAC patients |
||||
| Smith 2014 | New predictive tool available for LNR and survival that incorporates specific patient metrics and includes population characteristics |
||||
| Tol 2015 | LNR above 0.18 predicts 3-year survival in PDAC patients | ||||
| Yamamoto 2014 | LNB and LNR are predictors of PDAC patient prognosis, not LVI; LNR is best predictor of all metrics examined |
||||
White: metric not analyzed; Light gray: metric analyzed but not statistically significant; Dark gray: statistically significant metric
A major challenge in pancreatic cancer biology and treatment is the presence in lymph nodes of single or small clusters of tumor cells that are not detected by routine histopathological staining techniques [76,115–118]. Similar to insufficient surgical removal of primary lymph nodes [84,102–104,112,113], failure to detect occult micrometastatic deposits in nodes may result in patient misclassification, understaging, and improperly informed outcomes prediction. Descriptions of “skip metastases” in which primary lymph nodes are negative but metastases are established in secondary nodes or distant organ sites [68,119,120] may be attributable, at least in part, to this phenomenon. Emerging immunohistochemical and molecular techniques such as epithelial cell adhesion molecule (EpCAM/BerEP4) [115,116], cytokeratin [76,117], and CA19-9 staining [117], and polymerase chain reaction for mutant K-Ras [118] have demonstrated efficacy in occult tumor cell detection in nodes, but their adaptation from the research laboratory to practical clinical application will require more extensive study.
Lymphangiogenesis
Signaling and Regulation
While clearly akin to angiogenesis in many regards, progress to define the process of lymphangiogenesis has revealed distinct molecular mechanisms that direct its inception, regulation, and roles in inflammatory disease and malignancy. Like angiogenesis, new lymphatic vessel growth can be directed by many growth factors and regulated by intra- and extracellular signaling mechanisms. Primary growth factors associated with lymphangiogenesis include vascular endothelial growth factor-A (VEGF-A), -C, and -D signaling via vascular endothelial growth factor receptor-2 (VEGFR-2) and -3 and neuropilin-2 (Nrp-2) [121–126], and angiopoietin-1 (Ang-1) and -2 signaling through receptor Tie-2 [127,128].
Recent evidence shows that in addition to the VEGFs and angiopoietins, several other chemical messengers are also capable of directly or indirectly inducing lymphangiogenesis in vitro and/or in vivo in experimental model systems. Such mediators include growth factors fibroblast growth factor-2 (FGF-2) [129], platelet-derived growth factor-BB (PDGF-BB) [130,131], nerve growth factor (NGF) [132], insulin-like growth factor-1 (IGF-1) and -2 [133], and hepatocyte growth factor (HGF) [134]; inflammatory cytokines interleukin-1 β (IL-1β) [135,136] and tumor necrosis factor- α (TNF-α) [135–137]; and other non-traditional signaling molecules lipid sphingosine-1-phosphate [138], cyclooxygenase 2 (Cox2), and EP3/4 [139]. A role for integrins in lymphangiogenesis is also emerging with evidence of binding of VEGF-A, -C, and -D to lymphatic endothelial-specific integrin-α9β1 [140,141]. While each of these factors does induce new lymphatic vessel growth, not all lymphangiogenesis is created equal; a study of corneal lymphangiogenesis in response to VEGF-A, VEGF-C, or FGF-2-loaded micropellets has revealed differences in both structure and function of lymphatic vessels and the proportion of blood to lymphatic vessels induced by these growth factors [142]. In the case of indirect stimulation of lymphangiogenesis, paracrine signals such as IL-1β [135,136] and TNF-α [135–137] can drive increased expression of the VEGFs, most notably VEGF-C. This likely occurs through activation of the NFκB promoter to induce VEGF-C expression [137]. Other indirect inducers of lymphangiogenesis include Cox2 and EP3/4, which may increase expression of VEGF-C and -D to modulate cell growth during inflammation [139], and NGF, which increased expression of VEGF-C, but not VEGF-A, in a mouse corneal model of lymphangiogenesis [132]. Some studies have attributed the secondary production of VEGFs, specifically VEGF-C, to an infiltrating macrophage population during periods of inflammation and malignancy [135,138,143,144]. Huang, et al., have also identified B cells and dendritic cells (DCs) as candidate immune cell populations that may secrete VEGF-A, -C, and -D to influence lymphatic vessel organization and growth [138]. B cells have also been shown to modulate lymphangiogenesis within lymph nodes in the context of tissue inflammation following experimental immunization [145]. In addition to their role in secretion of lymphangiogenic growth factors, Hall, et al., have shown that tissue macrophages may directly contribute to new lymphatic vessel growth by transdifferentiation into a lymphatic endothelial progenitor-like phenotype and incorporation into growing vessels [146]. The roles of tumor-associated macrophages (TAMs) in lymphangiogenesis in the tumor microenvironment are discussed in more detail below.
Lymphangiogenesis is further controlled by regulation of growth factor and cytokine receptors on the lymphatic endothelial surface. Gene expression of VEGFR-1, -2, and -3 and Nrp-1, and -2 is regulated by transcription factors GATA-binding protein 2 (GATA2) and LIM domain only 2 (Lmo2) to influence both angiogenesis and lymphangiogenesis [147]. Another transcription factor, COUP transcription factor 2 (COUP-TFII), increases expression of Nrp-2 to augment VEGF-C signaling [148]. VEGFR-2 and -3 signaling is further modulated by Bone marrow kinase in X chromosome (BMX) following its upregulation upon VEGF-A stimulation of lymphatic endothelial cells (LECs) [149]. H-, N-, and K-Ras can also regulate VEGFR-3 signaling by inducing the up- or downregulation of that receptor [150]. Another mechanism of VEGFR-3 pathway signaling regulation in LECs is the IL-1β-dependent induction of miRNA-1236 [151] and the molecular scaffolding protein ASK-1 interacting protein-1 (AIP-1) [152]. The Slit2-Robo4 signaling axis has been shown to regulate surrogate lymphangiogenesis behaviors in lung LECs in culture by modulating VEGF-C/VEGFR-3 pathway signaling [153]. NFκB pathway signaling has been shown to further modulate inflammatory lymphangiogenesis by upregulating prospero-related homeobox-1 (Prox-1) and VEGFR-3 in a mouse model of peritonitis [138].
Tumor-associated Lymphangiogenesis
The discussion above has primarily focused on the regulation of inflammatory lymphangiogenesis typical of an injury or infection. A related, but in many ways physiologically distinct process, is that of tumor-associated lymphangiogenesis (TALA). Factors elaborated by tumor cells and other supporting cell types of the tumor microenvironment, such as cancer-associated fibroblasts (CAFs), TAMs, and DCs, interact with cognate receptors on the lymphatic endothelium both locally and in lymph nodes to influence lymphangiogenesis, lymph node metastasis, and tumor progression. Studies of human pancreatic cancer tissues have identified a role for TALA in lymph node metastasis and patient outcomes. Kurahara, et al. [154], found that high lymphatic vessel density (LVD) in PDAC head tumors predicted increased lymph node metastasis and decreased survival. They also showed increased LVD within metastatic lymph nodes [154]. Wang, et al., found that increased peritumoral LVD in human pancreatic carcinoma tissues correlated with unfavorable tumor differentiation status, increased LVI, and more lymph node metastasis, while this was not the case for intratumoral LVD [53]. These data highlight the importance of peripancreatic lymphatics in the progression and metastasis of pancreatic cancer and their potential utility as both a predictor of patient outcomes and a possible therapeutic target.
As in inflammatory lymphangiogenesis, the VEGF-C/-D signaling pathways appear to play an important role in TALA, although the exact mechanisms of their activity remain somewhat less clear. Kurahara, et al., found increased VEGF-C and -D expression in patient PDAC tumor margins compared to the tumor interior, and they reported that high VEGF-C and -D expression in tumor margins correlated with increased LVI (VEGF-C) and lymph node metastasis (VEGF-C and -D) and decreased five-year survival; expression levels of these proteins did not correlate with either hematogenous invasion or distant metastasis [48]. A similar study of patient samples also showed increased VEGF-C and -D immunostaining at pancreatic adenocarcinoma tumor margins that correlated with increased LVD, lymphatic and blood vessel invasion, lymph node metastasis, and overall survival [155]. Von Marschall, et al., corroborated these findings with their evidence of increased VEGF-D and VEGFR-3 expression in human PDAC tissue and of increased LVI, presence of intra- and peritumoral lymphatics, and lymph node metastasis [156]. Deletion of VEGF-D in mice resulted in impaired peritumoral lymphangiogenesis and decreased lymph node metastasis while having no effect on lymphatic development or inflammatory lymphangiogenesis suggesting a tumor microenvironment-specific role for VEGF-D signaling [157]. In a Rip1Tag2 model of pancreatic β-cell carcinogenesis, Kopfstein, et al., showed that VEGF-D expression in these tumors induced peritumoral lymphangiogenesis and lymph node and lung metastases [158]; a very similar study examining the role of VEGF-C in this context found increased lymphangiogenesis and lymph node metastasis but not distant metastases [159]. In an orthotopic PDAC model, treatment with anti-VEGF-C shRNA decreased tumoral LVD and inhibited tumor growth [160]. A role for microRNAs may also exist in the regulation of pancreatic TALA. Keklikoglou, et al., recently described a mechanism of regulation of VEGF-C production in PDAC cells by miR-206. They showed that, in addition to regulating K-Ras and annexin-A2 gene expression, restoration of miR-206 expression blocked tumor-associated angiogenesis and lymphangiogenesis, and its overexpression in pancreatic cancer cell lines disrupted the cell cycle restricting proliferation, impaired migration and invasion in vitro,and delayed tumor xenograft growth in vivo [161]. While the role of hypoxia in lymphangiogenesis remains unclear, HIF-1α expression has been shown to correlate with VEGF-C expression in PDAC of the pancreatic head and may be responsible for increased lymphangiogenesis and LN metastasis [57]. Contrary to these studies, Sipos, et al., examined the expression levels of lymphangiogenic factors, LVD, and effects on lymph node metastasis in human PDAC and orthotopic PDAC mouse models and found that VEGF-C and -D were not overexpressed in tumor tissues and that LVD within tumors was decreased while peritumoral LVD was increased. They found no correlation between LVD or expression of VEGF-C or -D and rate of lymph node metastasis or patient outcomes and concluded that PDAC metastasis is independent of lymphangiogenesis [162].
In vitro experiments have examined the effects of tumor-secreted VEGF-C on LEC surrogate lymphangiogenesis behaviors. Supernatant from a high VEGF-C-secreting cell line, MiaPaCa-2, increased LEC migration, and MiaPaCa-2 co-culture with LECs increased LEC tubulogenesis [163]. These effects may be dependent on KAI-1 regulation as overexpression of that gene in MiaPaCa-2 resulted in decreased VEGF-C secretion, lymphangiogenesis, and lymph node metastasis [164]. Re-expression of tumor suppressor p16 in a MiaPaCa-2 orthotopic model had no effect on levels of VEGF-C or -D, but nevertheless resulted in decreased lymphangiogenesis, LVD, and lymph node metastasis suggesting an alternate mechanism of regulation [165].
Overall, many studies examining the relationships among VEGF-C/-D expression and lymphatic-related phenotypes have found that high VEGF-C/-D levels correlate with increased lymphangiogenesis, lymphatic vessel invasion, and lymph node metastasis (or their surrogate in vitro counterpart behaviors). Whether a direct pathway can be drawn from tumor-associated lymphangiogenesis, to tumor cell invasion into lymphatic vessels, to tumor cell trafficking to lymph nodes, to establishment of lymph node metastases, to tumor cell exit of the lymph node by blood or lymphatic vessels and seeding of metastases at distant sites, to direct effects on patient outcomes is still unclear. Some of the studies we have discussed have supported portions of this pathway from lymphangiogenesis to distant metastases, but other data suggest that disease progression does not necessarily follow this linear sequence—i.e. the concept that lymphangiogenesis may not be required for lymphatic vessel invasion due to entry into pre-existing lymphatics, or the possibility of trafficking of tumor cells to lymph nodes through blood vessels, or the results from Sipos, et al. [162], showing that lymph node metastasis and patient prognosis are not linked to VEGF-C/-D levels. Also complicating this discussion is the fact that tumor cells may themselves respond to VEGF-C/-D signals in an autocrine manner further influencing their metastatic behaviors. Additional studies to systematically dissect each of the biological components of this proposed metastatic pathway are needed to concretely define their connections and contributions to disease progression.
Traditional neural signaling molecules also act to influence lymphatic vessel biology in the tumor microenvironment. Suppression of neural cell adhesion molecule (NCAM) induced VEGF-C and -D expression resulting in increased lymphangiogenesis and lymph node metastasis in the Rip1Tag2 mouse model [166], while the presence of NCAM expression in pancreatic cancer tissues from patients correlated with better prognosis [167]. In another example derived from the Rip1Tag2 model, Slit2 induced Robo1 in LECs to increase lymphangiogenesis and lymph node metastasis [168]. As previously mentioned, signaling of Slit2 through another receptor, Robo4, may also influence lymphangiogenesis behaviors such as growth, migration, and tubulogenesis, by modulating VEGF-C/VEGFR-3 signaling [153]. Nrp-2, a classical semaphorin receptor and VEGF pathway co-receptor, has also been shown to be a key regulator of TALA. It is expressed on intra- and peritumoral lymphatic vessels and lymph nodes; blocking its function in vivo decreased TALA, impaired tumor-associated LV function, and reduced lymph node and distant metastases [169]. These effects may be the result of impaired lymphatic sprouting [170]. Nrp-1 and -2 are also expressed on pancreatic tumor cells themselves [171,172]. In a model of colorectal cancer, TALA was stimulated by upregulation of Nrp-2 in LECs, and LVD correlated with the level of Nrp-2 expression; this Nrp-2 induction was mediated by integrin-α9β1 signaling in a VEGF-C/VEGFR-3 pathway-independent manner [173].
Other signaling pathways have also been implicated in regulation of TALA. In a mouse model of pancreatic β-cell carcinoma, both Ang-1 and -2 induced peritumoral lymphangiogenesis, but this new lymphatic vessel growth did not result in increased metastasis to either local lymph nodes or distant sites [174]. Ang-2 expression in orthotopic PDAC xenografts resulted in increased LVD and lymphatic metastasis, and high levels of Ang-2 in patient serum samples correlated with lymph node metastasis and decreased survival. In MiaPaCa-2 cells, Ang-2 altered message levels of cytoskeletal and motility pathway molecules as well as decreasing expression of tumor suppressor genes [175]. The transforming growth factor- β (TGF-β) pathway may also be involved in TALA as expression of endoglin on intra- and peritumoral blood and lymphatic vessels in PDAC correlated with poor patient prognosis [176].
PDAC Invasion of Lymphatic Vessels and Metastasis to the Lymph Nodes
Background
Lymphatic vessel invasion and subsequent metastasis to the lymph nodes are early and significant events frequently observed during pancreatic cancer progression [4,5]. Although lymphatic invasion and metastasis to the lymph nodes does not directly contribute to PDAC morbidity in patients, these pathologies are important indicators of the metastatic potential of this disease. In the clinical setting, lymph node status is used to assess disease progression, to select appropriate therapies, and to predict survival [11,12]. Nearly all studies concur that lymph node status correlates with poor prognosis for pancreatic cancer patients [6,8–10]. Studies also agree that invasion of lymph nodes by PDAC occurs most frequently through the lymphatic vasculature rather than through direct/contiguous extension of the primary tumor to the lymph node [109,177,178]. However, the prognostic value of mode of lymph node invasion is arguable: some studies report poorer overall survival in patients with lymphatic vessel-directed metastasis as compared to direct invasion [178], while other reports show no survival difference between the two modes of lymph node invasion [109,177]. Although lymph node invasion by PDAC occurs most frequently through the lymphatic vasculature, the LVD at the tumor site has not been conclusively correlated with either lymph node metastasis or prognosis due to conflicting study results [53,156,162,179]. This is also true for studies examining the expression of pro-lymphangiogenic factors such as VEGF-C and -D [162,180,181] (and in pancreatic endocrine tumors [182]). The lack of standardized protocols for quantifying LVD in patients makes comparative analysis among collected data sets difficult. Some studies enumerate only intratumoral lymphatics in whole tumor sections, while others examine tumor margins for peritumoral lymphatics, and still others examine the sum of lymphatic vessels in both regions. In the continued absence of a standardized method, LVD has limited value as a metric for assessing pancreatic cancer progression.
PDAC tumors are often hypovascular with only sporadic blood and lymphatic vessels found among the tumor cells [183]. These intratumoral lymphatic vessels are typically collapsed and nonfunctional due to direct compression by the tumor cells and the high internal pressure of the PDAC tumor microenvironment [180,184,185]. However, even in the absence of functioning intratumoral lymphatic vessels, tumor cells are still capable of disseminating to lymph nodes, although identification of reliable sentinel lymph nodes remains challenging [27]. The lymphatic vessels located at the tumor margins are frequently described as enlarged with open lumens capable of being filled with tumor cells [180,184], and drainage studies show that these peritumoral lymphatic vessels are, in fact, functional [185]. Sipos and colleagues demonstrated that even in the absence of elevated LVD values and active lymphangiogenesis, PDAC patients still frequently presented with lymph node metastases [162]. This suggests that PDAC cells are capable of invading the pre-existing lymphatic vasculature, especially enlarged vessels at tumor margins.
Mechanisms/Players
Mechanisms regulating lymphatic invasion are not completely understood, but are gaining increasing research interest. Most of our knowledge of vascular invasion has come from studies of the blood vasculature that are now being extended to studies of lymphatic vessel properties and function. Initially, invasion of lymphatic vessels by tumor cells was considered to be a passive process with increased interstitial fluid pressure driving tumor cells into draining lymphatic vessels [186]. Although increased interstitial pressure may contribute to tumor cell invasion, the concept of lymphatic-mediated tumor metastasis as a process that utilizes a “path of least resistance” is greatly oversimplified, and proteomics studies have identified distinctions between primary pancreatic tumors and their corresponding lymph node lesions [187]. Comparisons of pancreas tumors with and without lymph node metastases revealed differences in protein expression intrinsic to these two pathological tumor presentations [188]. In an effort to better understand the potential drivers of lymphatic metastasis, results of studies of leukocyte intravasation into lymphatic vessels are now being examined for commonalities to tumor cell intravasation. Three key molecular players of invasion have emerged as likely candidates in the regulation of tumor-lymphatic interactions and metastasis: chemokine signaling, paired binding of adhesion protein partners, and alterations in lymphatic vessel barrier integrity.
a) Chemokines
Chemokines secreted by lymphatic endothelial cells contribute to inflammation and initiation of immune responses in part by regulating the chemotaxis of antigen presenting cells to the lymph nodes. These same molecules are also being studied for similar roles in tumor metastasis to lymph nodes. Two widely researched candidate chemokines are CCL21 and CXCL12 and their respective G-protein coupled receptors (GPCRs), CCR7 and CXCR4.
During normal immune responses, lymphatic endothelial cells secrete CCL21 to increase migration of CCR7+ DCs toward the vessel and then to guide DCs to the lymph nodes [189,190]. Tumor cells, including those of pancreatic cancer, overexpress CCR7 and are capable of responding to CCL21 cues to facilitate their dissemination to the lymph nodes [191–195]. Guo, et al., noted a correlation between CCR7 expression in tumor cells and frequency of lymph node metastasis in pancreatic cancer patients [196]. Sperveslage, et al., confirmed these results and also demonstrated that lymphatic vessels of PDAC patients had significantly higher expression of CCL21 compared to lymphatic vessels of the normal pancreas. Expression of CCL21 in lymphatic vessels correlated with increased lymphatic invasion and lymph node metastasis in these patients, as did overexpression of CCR7 in pancreatic tumor cells in vivo [197].
The expression of CCL21 in lymphatic endothelial cells is regulated by numerous inflammatory cytokines including TNF-α and IL-1β and is also influenced by increases in transmural flow [198], both of which are often present in tumor microenvironments. In vitro co-culture work has demonstrated that CCR7-expressing tumor cells have increased chemotaxis toward CCL21-expressing lymphatic endothelial cells [199–201]. This chemotactic axis is used by tumor cells specifically for invasion into lymphatic vessels; tumor cell chemoattraction to blood endothelial cells does not use this mechanism [200,202]. Blocking CCR7 or CCL21 expression and/or function inhibits lymphatic vessel invasion and metastasis to the lymph nodes in vitro and in vivo [201,203,204]. This chemokine signaling axis appears to be regulated by and to work in concert with VEGF-C to synergistically promote lymphatic invasion of CCR7+ and VEGFR-3+ tumor cells [200].
Another chemokine axis that influences lymphatic metastasis is the CXCL12-CXCR4 axis. It has been widely documented that CXCR4-expressing tumor cells, including PDAC cells, home to organs with high CXCL12 expression, such as the lungs, bone marrow, and lymph nodes [54,195,205,206]. In PDAC patient tissues, high expression of CXCR4 was found in tumors, while lymph nodes expressed high levels of CXCL12 [54,207]. This expression pattern positively correlated with increased LVD values in the pancreas, lymph node metastasis frequency, and poor disease prognosis. Tumor-associated, but not normal uninflamed, LECs secrete ample amounts of CXCL12 in the tumor microenvironment and attract CXCR4+ tumor cells to lymphatic vessels and lymph nodes [208,209]. Blocking the CXCR4-CXCL12 signaling axis has resulted in impaired lymph node metastasis in numerous tumor models [210–212]. An in vitro breast cancer model demonstrated that CXCL12-treated LECs permitted greater transendothelial migration by breast cancer cells, and this permissiveness could be reversed by blocking CXCR4 in the LECs [213]. An in vivo model of melanoma demonstrated that stem-like, dual positive CD133+/CXCR4+ tumor cells were strongly associated with CXCL12-producing LECs and that these cells were resistant to chemotherapy [208]. Combinational treatment with a CXCR4 antagonist relieved this resistance and increased the efficacy of chemotherapy thereby reducing tumor growth and metastasis. This study suggested that CXCL12 secretion from lymphatic vessels supported a pro-metastatic and pro-survival niche for tumor cells. Further studies are required to elucidate whether or not these types of mechanisms are employed in PDAC and/or its tumor microenvironment.
b) Adhesion Proteins
Physical interactions between tumor cells and lymphatic endothelial cells may be another crucial regulator of tumor cell intravasation. Adhesion molecules such as E-selectin, intercellular adhesion molecule 1 (ICAM-1), and vascular adhesion molecule 1 (VCAM-1) are typically used by DCs to gain entry into inflamed lymphatic vessels during migration toward lymph nodes [198,214]. Mounting evidence indicates that these same leukocyte adhesion molecules may also be important for controlling tumor cell entry into lymphatic vessels [215–217]. In a non-inflamed state, the lymphatic endothelium does not express or only very weakly expresses these adhesion molecules [214,218]. Inflammatory conditions—such as those found during infection or tumor development—or a wound healing response quickly increase the expression of these molecules on the lymphatic endothelium [198,214]. Increased transmural flow, also characteristic of an inflamed microenvironment, upregulates ICAM-1 and Eselectin expression on an in vitro lymphatic endothelium resulting in increased DC binding [198]. A recent report shows that binding and transendothelial migration of breast cancer cells is also influenced by in vitro fluid flow, although the mechanisms governing these behaviors have not been elucidated [219]. When placed in co-culture with tumor cells, LECs display marked upregulation of adhesion molecules. Kawai, et al. (2008 and 2009), have demonstrated that invasive breast cancer cells, which express the αLβ2 ligand for ICAM-1, are capable of inducing the expression of E-selectin and ICAM-1 on lymphatic endothelial cells. They also demonstrated that blocking ICAM-1 impaired the ability of these tumor cells to bind to a lymphatic endothelium [217,220]. Studies of the ability of adhesion proteins on lymphatic vessels to regulate tumor cell entry should be expanded to pancreatic cancer cell lines to determine if PDAC tumor cells can use similar mechanisms to bind and gain access to the lymphatic vasculature.
c) Lymphatic Vessel Barrier Integrity
The intrinsic cellular and molecular organizational characteristics of lymphatic vessels facilitate entry of immune cells and fluids from a collecting tissue bed—properties that may also allow these vessels to support tumor cell metastasis. The initial lymphatic capillaries within tissues are composed of only a single layer of endothelial cells with loose junctions between neighboring cells [15,221]. Unlike the tightly-formed, continuously-arranged junctions between neighboring endothelial cells of the blood vasculature [222], the junctional proteins—vascular endothelial cadherin (VE-cadherin), platelet/endothelial cell adhesion molecule-1 (PECAM-1), claudins, occludins, etc. —of initial lymphatic vessels are discontinuously arranged, creating gaps between overlapping lymphatic endothelial cells [16]. These discontinuous junctions along with preformed openings in the basement membrane [17] enable uptake of macromolecules, fluids, and cells by the initial lymphatic capillaries. As lymph and cells are transported up the lymphatic vasculature to the collecting lymphatic vessels, the discontinuous intercellular junctions become more constant and successive to prevent leakage prior to arrival at the lymph nodes [16].
Data suggest that tumor cells are capable of modulating the barrier integrity of the lymphatic endothelium to further facilitate lymphatic vessel invasion [223]. Lipoxygenase secretion by breast cancer cells has been shown to disrupt VE-cadherin junctions and induce endothelial cell repulsion, resulting in breaches in the lymphatic endothelium. Tumor-secreted VEGF-C also facilitates invasion by creating leaky lymphatic vasculature. VEGF-C induces the internalization of VE-cadherin, which, in turn, promotes tumor cell transendothelial migration [224,225]. In a pancreatic tumor model, inhibiting Ang-2 signaling with a soluble Tie-2 receptor decreased lymphatic-directed metastasis to the lymph nodes [175]. This result may be explained by studies demonstrating that Ang-2 disrupts the barrier integrity of the lymphatic endothelium and increases lymphatic permeability through phosphorylation of VE-cadherin resulting in button-junction formation in the initial lymphatic capillaries [226].
Lymphatic Vasculature and the PDAC Microenvironment
The PDAC microenvironment is arguably one of the most complex of any tumor microenvironment, replete with CAFs, immunosuppressive leukocytes, tumor-associated blood/lymphatic endothelial networks, nerves, and a considerably dense ECM compartment (Figure 1). Each of these components facilitates PDAC progression and dissemination and has the capacity to influence the normal lymphatic vasculature within the pancreas.
Figure 1. Pancreatic tumor microenvironment and lymph node metastasis.
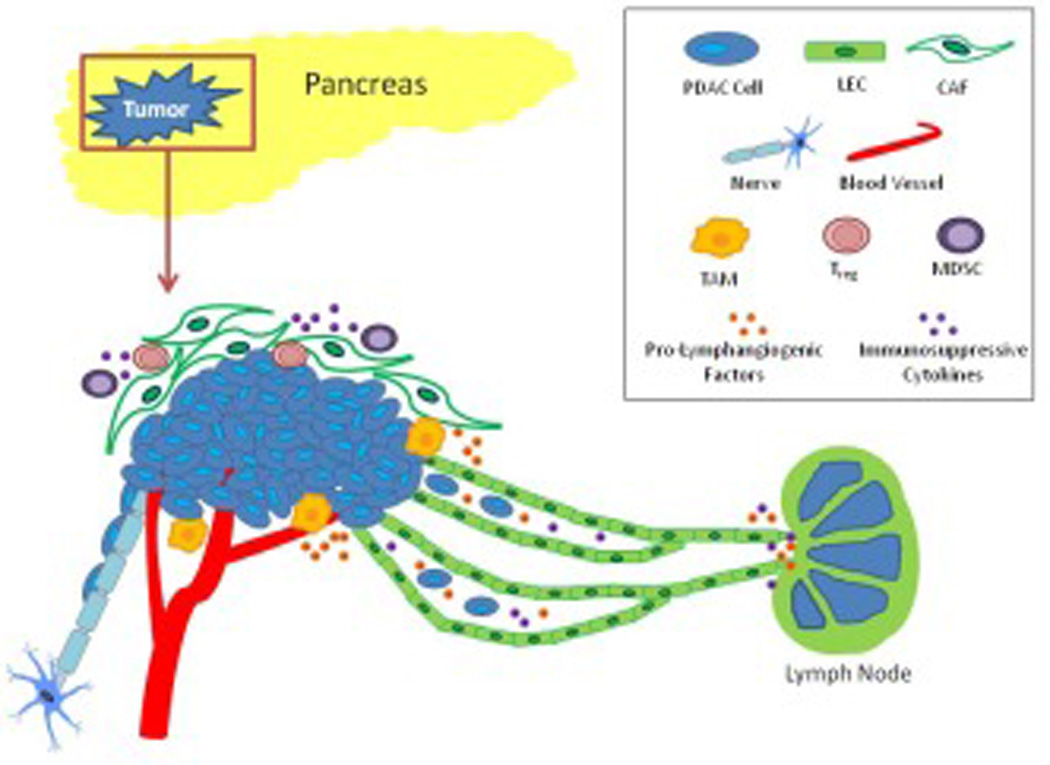
Cells of the tumor microenvironment are essential contributors to tumor growth, lymphatic invasion, and lymph node metastasis. CAFs and TAMs secrete pro-lymphangiogenic factors and proteases needed for lymphangiogenesis and metastasis. Lymphatic vessels act as conduits not only for tumor cell metastasis, but also for immunosuppressive cell and cytokine transport to lymph nodes. Nerves are also another route for pancreatic tumor metastasis and can communicate with lymphatic vessels to facilitate tumor metastasis from one network to the other.
Cancer-Associated Fibroblasts
One of the most striking features of PDAC is the robust desmoplastic reaction seen within the primary tumor. Due to their abundance in the PDAC microenvironment, CAFs exert a strong influence over other microenvironmental cell types including the lymphatic endothelium [227]. One of the main protein regulators of desmoplasia in PDAC is sonic hedgehog (Shh) [228,229]. Bailey, et al. (2009), noted that Shh signaling in the CAFs of PDAC tumors led to the creation of a pro-angiogenic and prolymphangiogenic stromal compartment. When Shh signaling was inhibited in the CAFs, LVD decreased and lymph node metastasis was reduced. Data such as these suggest that CAFs primarily influence the lymphatic endothelium via secretion of various effector proteins. It has been demonstrated that CAFs of various tumor types, including PDAC, secrete a wide range of pro-lymphangiogenic factors such as VEGF-C, VEGF-D [230,231], VEGF-A [232], epidermal growth factor (EGF) [233], PDGF, and FGF [183]. CAFs also secrete chemokines, including CXCL12, which has been shown to correlate with increased tumor aggressiveness, LVD values, and lymph node metastases in PDAC patient tissues [54,234]. In addition to their direct action on lymphatic endothelia, many of these same secreted factors as well as proinflammatory cytokines allow CAFs to indirectly support lymphangiogenesis and lymphatic vessel invasion through the recruitment of pro-lymphangiogenic immune cells such as TAMs and DCs [235,236]. Lastly, CAFs secrete matrix metalloproteinases (MMPs) and other proteases that remodel the ECM of tumors [237]. This remodeling promotes tumor invasion of stroma and tumor vasculature and releases sequestered growth factors and cytokines from the ECM for tumor growth, angiogenesis, and lymphangiogenesis. A recent study by Shi, et al. highlights an additional protease-related mechanism by which CAFs may influence pancreatic cancer progression and lymphatic metastasis. Specific pancreatic stromal compartment deletion of protease-activated receptor-2 (PAR-2), a GPCR highly expressed in PDAC, resulted in decreased primary tumor size (due to anti-angiogenesis effects) but increased LVD and lymph node metastases [238].
Immune Cells and Immune Regulation
One of the main functions of lymphatic vessels is to transport leukocytes to lymph nodes for immune response initiation, uniquely positioning LECs to modulate immune responses in ways that may support tumor progression. As immune cell trafficking conduits, LECs are responsible for the transport of both antigens and antigen presenting cells (APCs), such as DCs, to the lymph nodes for immune response optimization [190]. By regulating the expression and secretion of various chemokines in response to inflammation, injury, or tumor development, LECs can alter the recruitment of immune cells to the lymph nodes, and, as a result, influence the ensuing immune response (reviewed in [239,240]). Partially due to lymphatic-directed recruitment, tumor-draining lymph nodes demonstrate a more immunosuppressive environment as compared to normal lymph nodes with an increased presence of regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), immature and tolerogenic DCs, and immunosuppressive cytokines [241–244]. These immunosuppressive cells and cytokines accumulate in the lymph as a result of increased lymphatic drainage from the tumor site [245]. Within the lymph nodes TGF-β, a major driver of immune suppression, supports the differentiation and activation of Tregs as well as promoting tolerogenic and immature phenotypes of DCs [246]. As Tregs differentiate and accumulate, they secrete more TGF-β to further drive immune suppression. IL-10 is another factor that supports the accumulation of immunosuppressive cells in the lymph nodes by promoting Treg activity [247] and tolerogenic DC function [247,248]. Indoleamine 2,3-dioxygenase increases the generation of Tregs in the lymph nodes [249,250], while concurrently inhibiting effector T cell activity [251]. Other factors implicated in the accumulation of immunosuppressive cells in lymph nodes include IL-4, VEGF-A, and prostaglandin E2 [252].
In addition to cellular and cytokine transport, LECs also transport tissue antigens (and in the case of cancer, tumor antigens) from peripheral tissues to lymph nodes. Studies have demonstrated that LECs, particularly those in the lymph nodes, are capable of scavenging these tissue and tumor antigens and cross-presenting them on major histocompatibility complex-I (MHC-I) [253,254]. This can lead to immune tolerance through deletion of naive CD8+ T cells as LECs lack co-stimulatory molecules needed to activate the T cells and instead express programmed death-ligand 1 (PD-L1), an inhibitory signal for T cells [255]. LECs can also present scavenged exogenous tissue/tumor antigens on MHC-II molecules and likely induce immune tolerance through interactions with the inhibitory lymphocyte activation gene-3 (LAG-3) protein on CD8+ T cells [256]. These studies shed light on the phenomenon that when tumor cells are denied lymphatic vessel experience, such as through direct implantation into lymph nodes, tumor immunity is impaired through a robust CD8+ T cell response [257]. LECs also modulate immune responses by inhibiting DC maturation [242]. Binding of DCs to the lymphatic endothelium via macrophage-1 antigen (Mac-1) and ICAM-1-mediated interactions during transendothelial migration can reduce the expression of co-stimulatory molecules on DCs needed for T cell activation. Studies such as these inspire new ideas regarding increased lymphangiogenesis at the tumor periphery and draining lymph nodes, suggesting that it may influence tumor progression in two ways: 1) increasing metastatic routes for dissemination and 2) immune suppression through increased antigen scavenging and decreased DC maturation leading to T cell inhibition and immune tolerance [258]. Further investigation is needed to substantiate the immunosuppressive properties of the lymphatic endothelium and its specific contribution to disease progression as a component of the tumor microenvironment.
A reciprocal concept in relation to the capacity of LECs to affect immunity is that of immune cells inducing effects on LECs. One such tumor infiltrating immune cell type, TAMs, can be found in many tumor microenvironments, including PDAC [259–262], and their presence often correlates with poor patient prognosis [263–266]. TAMs promote tumor lymphangiogenesis through two mechanisms: paracrine secretion of pro-lymphangiogenic factors and transdifferentiation into LEC-like progenitor cells. TAMs secrete high levels of VEGF-C and -D, which, in turn, increases LVD in and around tumors [259,260,267,268]. Indeed, TAM density has been shown to significantly correlate with increased LVD, lymphatic vessel invasion, and lymph node metastasis in many cancers [259,268–271]. Inhibition or depletion of TAMs from tumor microenvironments significantly reduced LVD values and decreased the incidence of lymph node metastases compared to tumors with TAMs present [272–274]. However, depletion of TAMs did not completely inhibit lymph node metastasis as tumor cells were still able to invade pre-existing lymphatic vessels. These macrophages also secrete proteases such as MMP-2, MMP-9, and plasmin/urokinase plasminogen activator (uPA) that remodel the extracellular microenvironment and release sequestered growth factors for lymphangiogenesis [275,276]. The plasmin/uPA system is also important for the proteolytic maturation of VEGF-C and -D increasing their affinity for VEGFR-3 [277]. It has yet to be determined if TAMs secrete any of the other factors known to promote lymphangiogenesis. The second way TAMs contribute to lymphangiogenesis is by transdifferentiating into LEC-like progenitors both in inflammatory and tumor settings [146,273,278,279]. Transdifferentiated macrophages undergo genetic reprogramming [146] with increased expression of lymphatic markers Lyve-1, Prox-1, podoplanin, and VEGFR-3 [146,273,280,281]. Expression of LEC markers enables TAMs to physically incorporate into the newly developing lymphatic vasculature. The percentage of transdifferentiated TAMs within these newly formed lymphatic vessels is often less than 10% [273,280] suggesting the main mechanism by which TAMs promote tumorassociated lymphangiogenesis is through secretion of pro-lymphangiogenic factors.
Lymphatic Vessel-Nerve Interactions
In addition to lymph node metastasis, one of the most devastating pathologies of PDAC is perineural invasion. The peripancreatic region is densely innervated, housing large nerve plexuses with sensory, sympathetic, and parasympathetic nerves extending into the pancreas [82,282,283]. Incidence of perineural invasion in PDAC approaches 100% [44,82] and has been implicated in local and distant recurrence [75,82,284,285] after resection and neuropathic pain [286,287]. In an effort to decrease these effects, it has been suggested that nerve tissue be removed as part of radical pancreatic resection procedures [71,75,82,84,285,288]. The interactions between nerves and the lymphatic vasculature within and around the pancreas are poorly understood. Studies have shown that lymphatic vessels are, in fact, innervated [289,290] and have suggested that these connections may represent an additional route of metastatic dissemination of tumor cells from either network to the other [77,291]. It is well documented that vasculature and nerves can respond to the same molecular cues—termed neurovascular guidance molecules—for development and remodeling [132,168–170,292,293], and many of these molecules, such as Nrp-1 and -2, NGF, brain-derived neurotrophic factor (BDNF), FGF, IGF-2, Netrin-1, Semaphorin-3A, Ephrin receptor B4, Slit2, and Robo1 may be differentially expressed or have altered signaling functions in the pancreatic tumor microenvironment, implicating them in cancer progression [171,172,294–300]. As well, somatic alterations in axon guidance pathway genes are observed in a subset of pancreatic cancer genomes [301]. Remarkably, Chen, et al., showed that in the absence of both perineural and lymphovascular invasion, the five-year survival rate for pancreatic adenocarcinoma patients was 71% [63]. These studies underscore the importance of both lymphatics and nerves in the pancreatic tumor microenvironment and highlight the need for further mechanistic work interrogating the specific contributions of these tissue networks to disease progression and metastasis.
Comparative Tools and Models to Study Lymphatic Biology and Tumor-Lymphatic Interactions
The discoveries of lymphatic endothelium markers such as lymphatic vessel hyaluronan receptor-1 (Lyve-1) [302], Prox-1 [303], VEGFR-3 [304], and podoplanin (PDPN) [305–307] have facilitated the development of new research methodologies and models with which to study lymphatic vessel biology under homeostasis and various disease pathologies as well as interactions between lymphatic endothelial cells, immune cells, and tumor cells in vivo. The mouse cornea and skin have emerged as two popular mammalian platforms for this type of work. Historically used for studies of angiogenesis, the murine corneal model system has proven equally informative for studies of lymphatic biology because of its unique characteristics. The normal healthy cornea harbors a single limbal lymphatic vessel ring at its periphery and is otherwise devoid of lymphatic vessels. Upon insult or injury, inflammatory lymphangiogenesis occurs resulting in extension of newly-synthesized lymphatic vessels from the limbal arcade toward the site of the stimulus. Corneal injury can be recapitulated experimentally by placement of sutures, mechanical debridement, or chemical burn. A refinement of this inflammatory model enabling more mechanistic dissection of lymphatic vessel behavior is the corneal micropocket assay in which a micropellet can be loaded with a protein or drug of interest and implanted into the cornea [132,308,309]. Further modifications of traditional acute inflammatory protocols can induce wound recovery [132,310] and recurrent inflammation [132,311]—two additional distinct physiological microenvironments with implications for wound healing, chronic inflammatory disease, and tumor microenvironment research. Anatomical sites commonly used in skin imaging studies include murine dorsal surface, foot pad, and pinna. Unlike the cornea, the skin is vascularized with a dense network of lymphatic capillaries under steady state conditions. This presents an ideal system for studies of lymphatic vessel homeostasis and remodeling, local inflammatory lymphangiogenesis, and endothelium-immune/tumor interactions.
Both cornea and skin have also been employed in real time live-imaging and intravital microscopy studies [17,312–314]. Early experiments of this type relied on injection and uptake of large fluorescent conjugate molecules such as FITC-dextran or explant immunostaining (Reviewed in [315]) to label vasculature and other tissue antigens, but recently several genetically engineered mouse models [316–321] have enabled more sophisticated lymphatic vessel-specific experimental designs. In these immunocompetent models, fluorescent protein expression is driven by lymphatic endothelium-specific promoters such as Prox-1, Lyve-1, or VEGFR-3 in either a constitutive or inducible manner. Inducible systems offer the advantages of titration and temporal control of fluorescence expression within the lymphatic endothelial compartment. Fluorescently labeled tumor or immune cells may be delivered to and tracked in either cornea or skin providing insight into intravasation/extravasation behavior, cell trafficking and fate, and spread to draining lymph nodes. Translation of these techniques to studies of the pancreatic lymphatic vasculature specifically would provide insight into organ-specific lymphatic vessel biology and pancreatic tumor microenvironment contributions to lymphatic remodeling and lymphatic-mediated metastasis. We suggest combination of several existing technologies to examine these questions. First, crossing a spontaneous pancreatic ductal adenocarcinoma model containing a fluorescent reporter gene, such as the PKCY [322] or KPCT mouse [323], with one of the available lymphatic-specific reporter mice would facilitate visualization of cells of pancreatic origin and lymphatic vessels in two colors. Implantation of a pancreas window [324] in these animals could enable long term intravital microscopy studies of lymphatic vessel biology and tumor metastasis throughout the course of disease progression, from PanIN formation through advanced metastatic disease. Finally, use of the CLARITY technique as previously described for brain [325] in concert with multiphoton microscopy and immunofluorescent staining would allow deep tissue visualization and reconstruction of full lymphatic vascular networks as well as detection of other important microenvironmental structures (such as nerve and blood vascular networks) and signaling molecules both peri- and intratumorally.
Other research and pre-clinical imaging models have further studied lymphatic vessel- and lymph node-related pathologies in cancer. High resolution MRI has proven an effective non-invasive strategy for mapping involved mouse lymph nodes in pancreatic ductal adenocarcinoma [326]. Multiphoton laser scanning microscopy work in a model of melanoma showed that functional lymphatic vessels are not present within the tumor proper and that functional peri-tumoral lymphatic vessels are sufficient to mediate metastasis [185]. Other methods of lymph node and metastasis imaging (reviewed in [315]) have included injection of dyes or radiotracers such as Lymphoseek for lymphoscintigraphy, injection and uptake of cancer-specific radio-labeled antibodies and their accumulation in affected lymph nodes, injection of fluorescent antibody conjugates against the lymphatic endothelium in combination with fluorescent reporter-expressing pancreatic cancer cells, and use of combinatorial bioluminescence and fluorescence resonance energy transfer (BRET-FRET) nanoparticles for mapping lymphovascular and node networks [327]. Fluorescence lifetime imaging microscopy (FLIM)-FRET [328], optical coherence tomography [329], optical frequency domain imaging [330], photoacoustic tomography [331], higherorder harmonics generation [332], and Raman spectroscopy [333] imaging technologies offer other options for reconstructive deep tissue imaging and analysis of single cell signaling within an intact tumor microenvironment. Jeong and Jones, et al., have also established a chronic lymph node window to facilitate long-term live imaging microscopy studies of lymph node biology, angiogenesis and lymphangiogenesis, and nodal deposition of metastatic tumor cells [334]. Application of CRISPR-Cas9 gene editing technology [335] may also prove useful in generating new pre-clinical models suitable for lymphatic vessel imaging in disease.
Clinical Imaging Techniques to Detect Pancreatic Cancer Lymph Node Metastasis
Despite research advances in comparative lymphatic vessel imaging, clinical imaging of pancreatic cancer patient lymphatic networks and lymph node status has remained challenging. Several groups have examined the utility of traditional clinical imaging platforms for detection of lymph node metastasis with limited success. Roche, et al., have shown that examination of peripancreatic lymph nodes by CT cannot accurately predict presence of metastatic deposits [85]. Similarly, Imai, et al., showed that CT, MRI, and FDG-PET were not consistently accurate in predicting pre-operative para-aortic lymph node involvement in pancreatic cancer patients [86]. Conversely, another group had some success using endoscopic ultrasound (EUS) to differentiate benign and cancerous lymph nodes and to identify diseased pancreas; this technique has not been fully developed for widespread clinical use [336]. Cesmebasi, et al., have recently reviewed other advances in clinical imaging techniques including EUS and lymphotropic nanoparticle-enhanced MRI, reporting that further refinement of these techniques may make them promising options to identify patterns of pancreatic cancer spread [25]. Other groups have focused efforts on imaging routes of pancreatic drainage in attempts to identify sentinel lymph nodes for pancreatic tumors arising in various locations within the pancreas. Injection of indocyanine green fluorescent dye into the pancreatic surface during PD surgery allowed visualization of pancreatic lymphatic vessels intraoperatively and resulted in identification of seven routes of lymphatic drainage highlighting the complexity of the pancreatic lymphatic vascular network [40]. In a similar study methylene blue dye was injected peri- and intratumorally during pancreatic cancer resection, but the authors concluded that detection of patterns of pancreatic lymphatic drainage and sentinel lymph node identification were not feasible with this protocol [39]. Another group injected activated carbon particles or regular insulin colloid at resection and examined their patterns of spread to surgicallyremoved lymph nodes by histology. They documented uptake in several groups of lymph nodes and recommended new radical resection guidelines based on their findings [30]. Development and testing of additional lymphatic imaging technologies and their adaptation to pancreatic adenocarcinoma patients may make pre-operative identification of lymph node metastases a reality in the future [337–339].
Therapy
Due to advanced stage at diagnosis and its complex microenvironmental organization, pancreatic ductal adenocarcinoma has proven to be very difficult to treat. Surgical removal of the tumor is the most effective option, but only approximately 15% of cases are considered resectable [340,341]. Of those cases in which resection is an option, incomplete removal of microscopic disease (R1 residual margin status) only slightly improves patient survival over those cases presenting with unresectable metastatic disease [342,343]. Non-surgical options for pancreatic cancer include radiation, chemotherapy, or a combination of both. Some approved chemotherapies for the treatment of pancreatic cancer are the use of FOLFIRINOX, gemcitabine, albumin-bound paclitaxel, cisplatin, and oxaliplatin (as well as others) [344,345]. However, these drugs have had limited success in prolonging patient survival. Development of targeted therapies that specialize in blocking crucial molecular pathways of the pancreatic tumor and its microenvironment is becoming an increasingly attractive therapeutic option.
Anti-angiogenic therapies were originally developed to starve tumors of important nutrients and oxygen and to reduce the number of potential routes for dissemination. However, clinical trials demonstrated that, when used alone, anti-angiogenic therapy was not sufficient to improve patient survival. Unexpectedly though, the results indicated that anti-angiogenic therapy significantly improved survival of patients with solid tumors when used in combination with conventional chemotherapies [346–348]. These findings led to the evolution of the current vascular normalization theory: the use of anti-angiogenic therapy to block aberrant tumor angiogenesis and alleviate vessel dysfunction [349]. By restoring the balance between pro- and anti-angiogenic factors, anti-angiogenic therapies improved vessel organization, stabilized cell-to-cell junctions, increased pericyte coverage, and, consequently, reduced fluid leakage. All these factors, in turn, relieved blood flow irregularities resulting in improved delivery of chemotherapy to all parts of the tumor [350]. Unfortunately, in the setting of pancreatic cancer, anti-angiogenic therapies have had either no effect or only transient effects on improving patient survival even when used in combination with standard chemotherapies [351–355]. PDAC tumors are unusually hypovascular and desmoplastic negating the ability of even normalized vessels to deliver therapy [356]. The failure of anti-angiogenic therapy in PDAC may also be the result of tumor cells circumventing the VEGF-A/VEGFR-1 blockade through autocrine or paracrine secretion of alternative angiogenic factors, such as the prototypical lymphangiogenic factors which have overlapping angiogenic functions [355,357–359].
Targeting the tumor lymphatic vasculature as a treatment for cancer is beginning to gain interest among both basic and clinical research groups with the primary focus on anti-lymphangiogenic therapies. Lymphangiogenic growth factors are not critical for the maintenance of adult lymphatic vessels in homeostasis. This allows for extended treatment with anti-lymphangiogenic therapies in tumor settings without disruption of pre-existing vessels and with minimal drug-induced toxicities [360,361]. Numerous pre-clinical in vivo studies have demonstrated that blocking pro-lymphangiogenic factors VEGF-C and VEGF-D and their receptor VEGFR-3 significantly reduces tumor lymphangiogenesis and lymph node metastases in many tumor types including pancreatic [157], breast [362–365], melanoma [361], renal [366], lung [225,367], gastric [368,369], prostate [370], hepatocellular [371], and bladder [372]. Other protein targets of lymphangiogenesis that have shown promise in inhibiting lymphatic metastasis in vivo include the VEGFR-3 co-receptor Nrp-2 [169,173] and the angiopoietins Ang-1 and -2 [373,374]. Currently, two humanized neutralizing antibodies are in clinical trials for patients with solid tumors: VGX-100, which inhibits VEGF-C (NCT01514123) and IMC-3C5, which inhibits VEGFR-3 (NCT01288989).
The blockade of a single VEGF/VEGFR pathway will likely be insufficient to inhibit tumor lymphangiogenesis and lymph node metastasis due to the multiple compensatory and overlapping roles of the VEGF ligands and receptors [23,126,358,375]. Other growth mechanisms outside of VEGF/VEGFR signaling may also regulate lymphangiogenesis in the tumor setting, such as PDGF-BB/PDGFR [130] and FGF/FGFR [376]. Receptor tyrosine kinase inhibitors (RTKIs) often target multiple receptors allowing them to inhibit several signaling pathways simultaneously—including the VEGFR pathways. Both preclinical comparative studies and clinical trials have determined the safety and efficacy of numerous anti-angiogenic/-lymphangiogenic RTKIs for the treatment of cancer including foretinib [377], cediranib [378,379], and axitinib [380–382]. Some of these RTK inhibitors have also been approved for clinical use. Sorafenib, which inhibits VEGFR-1 and -3, PDGFR-β, FGFR-1, and Raf proteins, has been approved for renal cell (RCC) and hepatocellular carcinomas [383–385]; sunitinib, which inhibits VEGFR-1 and -3, and PDGFR-α and -β, has been approved to treat pancreatic neuroendocrine tumors, RCC, and gastrointestinal stromal tumors [386–389]; and pazopanib, which inhibits VEGFR-1 and -3, PDGFR-α and -β, and FGFR, has been approved to treat RCC and soft tissue sarcoma [390–392] (RTKIs further reviewed in [13]). Vatalanib, which inhibits VEGFR-1, -2, and -3, and PDGFR-β, is currently in clinical trials for the treatment of various solid tumors including pancreatic, ovarian, and breast cancers. This RTKI has been shown to directly inhibit angiogenesis, lymphangiogenesis, and tumor growth in preclinical models of pancreatic cancer as well as other cancer models [393–397]. In a recent clinical trial, vatalanib resulted in a partial or stable response for some metastatic pancreatic cancer patients who had initially failed gemcitabine treatment [398]. Many of these lymphangiogenic receptor-targeting RTKIs hold promise for the treatment of early-diagnosed and resectable cancers [23]. Unfortunately, these are not typical characteristics of pancreatic cancer, and, consequently, many of these drugs have failed to significantly improve pancreatic cancer patient survival [381,399–401].
Lymphangiogenesis is not the only manner by which the lymphatic vasculature can promote tumor progression. As discussed previously, pre-existing lymphatic vessels can directly facilitate metastasis by transporting tumor cells to distant sites. Lymphatic endothelia may also contribute to immune suppression by altering DC and T cell responses. However, these functions are poorly understood and much more work needs to be done to determine if these functions can be specifically targeted in lymphatic vessels for the treatment of cancer.
Using Lymphatic Vessels to Deliver Therapies to Lymph Nodes
In pancreatic cancer, metastasis to lymph nodes and distant sites has often already occurred by the time of diagnosis. Anti-lymphangiogenic therapies may inhibit further tumor cell dissemination but will do little to reduce the growth of metastatic tumors that have already seeded at distant sites [225,379,402]. Successful treatment of tumor-invaded lymph nodes has been particularly difficult to achieve. Resection of invaded lymph nodes would intuitively seem to be a promising strategy; however, as discussed above, current clinical imaging technologies cannot reliably detect single cell or microscopic lymph node metastases [337,338], and excision of an excessive number of lymph nodes is controversial due to conflicting evidence regarding its survival benefits and concerns about post-operative quality-of-life [70,403]. Also, conventional intravenously-administered therapies display poor access to lymphatic vessels and lymph nodes resulting in sub-optimal drug concentrations within lymph nodes [404]. This enables tumor cells present within lymph nodes to evade treatment and potentiate future recurrence. Using the lymphatic vasculature as a delivery system for cancer therapies to the lymph nodes has gained increasing interest. For therapies to be effectively taken up by lymphatic vessels and not blood capillaries requires specific characteristics of drug formulations such as being of a particular size and molecular weight, lipophilicity and surface charge of the drug carrier, and concentrations of the drug and carrier (reviewed in [405,406]). A few anti-cancer drugs have been formulated to target the lymphatic system and have shown promise in vivo: a methyl poly(ethylene glycol)distearoylphosphatidylethanolamine micelle containing doxorubicin reduced the size of lymph node metastases in a melanoma model [407]; a PEGylation of interferon-α2 demonstrated anti-tumor efficacy in the lymph nodes of rats with breast cancer [408]; cisplatin conjugated to a copolymer block of poly(ethylene oxide)-block-poly(lysine) successfully treated lymph node metastases in a model of squamous cell carcinoma [409]; and gemcitabine loaded onto magnetic multiwalled carbon nanotubes (mMWNTs) resulted in better uptake of gemcitabine in the lymph nodes and regression of lymph node metastases in a subcutaneous model of pancreatic cancer [372]. The field of lymphatic-based drug delivery is still in its infancy and more studies are required to demonstrate efficacy and feasibility in patients.
Conclusions and Perspectives
Advances in surgical, radiation, and chemotherapeutic treatment regimens for pancreatic cancer have not greatly impacted overall survival rates for patients afflicted with this devastating disease. Early spread of tumor cells to lymph nodes and distant sites often precludes curative resection and facilitates cancer chemoresistance and immune evasion. While not the sole route of spread, the lymphatic system represents one of the major understudied players in the tumor microenvironment and in the process oftumor metastasis. The intrinsic functional and structural characteristics of the lymphatic system suggest roles in immune regulation (especially immune suppression), cell trafficking, and interactions with other tissue networks and cell types. The distortion of the physiological process of lymphangiogenesis in the tumor microenvironment and its consequent effects on the biology of the lymphatic system are complex and complicate targeting strategies. Clinical efforts to detect and use lymph node status to inform treatment decisions, guide surgical lymphadenectomy, and stratify patients into prognostic cohorts have improved but remain inconsistent across groups and are not yet standardized or broadly applied. Identification, refinement, and directed studies of lymphatic-specific metrics have highlighted the importance of these criteria as additional prognostic factors in this disease. Development of pre-clinical models of tumor-associated lymphangiogenesis and lymph node metastasis and new lymphatic-directed clinical therapeutics represent two significant areas of current research. Live imaging studies in genetically engineered models that recapitulate both molecular and behavioral signatures of specific cancer types will improve our understanding of the earliest events in tumor-associated lymphangiogenesis, tumor-lymphatic interactions, and lymph node invasion. Translation of these and other preclinical findings to clinically-relevant diagnostic criteria or therapeutic interventions remains an underexplored but promising strategy to ultimately improve PDAC patient quality-of-life and outcomes.
Highlights.
We review the clinical relationship between pancreatic cancer metastasis to lymph nodes, clinical staging, and clinical outcome
We review the clinical, pathological, molecular and biological features of lymphangiogenesis, lymphatic invasion and metastasis by pancreatic tumors
We review the biological consequences of lymphatic invasion and metastasis including immunosuppression and tumor sequestration
We review strategies to image and treat metastases in lymph nodes
Acknowledgments
Financial Support: This work was supported by grants from the National Institutes of Health. The National Cancer Institute: R01CA57362, SPORE (1P50CA127297), Early Detection Research Network (5U01CA111294), Tumor Microenvironment Network (U54 CA163120), Cancer Center Support Grant 5P30 CA036727, Training Grant T32CA009476.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer. J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer. J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Katz MH, Hwang R, Fleming JB, Evans DB. Tumor-node-metastasis staging of pancreatic adenocarcinoma. CA Cancer. J. Clin. 2008;58:111–125. doi: 10.3322/CA.2007.0012. [DOI] [PubMed] [Google Scholar]
- 4.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 5.DiMagno EP, Reber HA, Tempero MA. AGA technical review on the epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. american gastroenterological association. Gastroenterology. 1999;117:1464–1484. doi: 10.1016/s0016-5085(99)70298-2. [DOI] [PubMed] [Google Scholar]
- 6.Robinson SM, Rahman A, Haugk B, French JJ, Manas DM, Jaques BC, Charnley RM, White SA. Metastatic lymph node ratio as an important prognostic factor in pancreatic ductal adenocarcinoma. Eur. J. Surg. Oncol. 2012;38:333–339. doi: 10.1016/j.ejso.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Kedra B, Popiela T, Sierzega M, Precht A. Prognostic factors of long-term survival after resective procedures for pancreatic cancer. Hepatogastroenterology. 2001;48:1762–1766. [PubMed] [Google Scholar]
- 8.Benassai G, Mastrorilli M, Mosella F, Mosella G. Significance of lymph node metastases in the surgical management of pancreatic head carcinoma. J. Exp. Clin. Cancer Res. 1999;18:23–28. [PubMed] [Google Scholar]
- 9.Delcore R, Rodriguez FJ, Forster J, Hermreck AS, Thomas JH. Significance of lymph node metastases in patients with pancreatic cancer undergoing curative resection. Am. J. Surg. 1996;172:463–468. doi: 10.1016/S0002-9610(96)00237-1. discussion 468-9. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Luo G, Guo M, et al. Lymph node status predicts the benefit of adjuvant chemoradiotherapy for patients with resected pancreatic cancer. Pancreatology. 2015;15:253–258. doi: 10.1016/j.pan.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Nathanson SD, Shah R, Rosso K. Sentinel lymph node metastases in cancer: Causes, detection and their role in disease progression. Semin. Cell Dev. Biol. 2015;38:106–116. doi: 10.1016/j.semcdb.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Kawada K, Taketo MM. Significance and mechanism of lymph node metastasis in cancer progression. Cancer Res. 2011;71:1214–1218. doi: 10.1158/0008-5472.CAN-10-3277. [DOI] [PubMed] [Google Scholar]
- 13.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer. 2014;14:159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 14.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 15.Maby-El Hajjami H, Petrova TV. Developmental and pathological lymphangiogenesis: From models to human disease. Histochem. Cell Biol. 2008;130:1063–1078. doi: 10.1007/s00418-008-0525-5. [DOI] [PubMed] [Google Scholar]
- 16.Baluk P, Fuxe J, Hashizume H, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J. Exp. Med. 2009;206:2925–2935. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerli R, Solito R, Weber E, Agliano M. Specific adhesion molecules bind anchoring filaments and endothelial cells in human skin initial lymphatics. Lymphology. 2000;33:148–157. [PubMed] [Google Scholar]
- 19.Solito R, Alessandrini C, Fruschelli M, Pucci AM, Gerli R. An immunological correlation between the anchoring filaments of initial lymph vessels and the neighboring elastic fibers: A unified morphofunctional concept. Lymphology. 1997;30:194–202. [PubMed] [Google Scholar]
- 20.Bazigou E, Wilson JT, Moore JE., Jr Primary and secondary lymphatic valve development: Molecular, functional and mechanical insights. Microvasc. Res. 2014;96:38–45. doi: 10.1016/j.mvr.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von der Weid PY, Zawieja DC. Lymphatic smooth muscle: The motor unit of lymph drainage. Int. J. Biochem. Cell Biol. 2004;36:1147–1153. doi: 10.1016/j.biocel.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Leak LV, Burke JF. Fine structure of the lymphatic capillary and the adjoining connective tissue area. Am. J. Anat. 1966;118:785–809. doi: 10.1002/aja.1001180308. [DOI] [PubMed] [Google Scholar]
- 23.Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene. 2012;31:4499–4508. doi: 10.1038/onc.2011.602. [DOI] [PubMed] [Google Scholar]
- 24.O'Morchoe CC. Lymphatic system of the pancreas. Microsc. Res. Tech. 1997;37:456–477. doi: 10.1002/(SICI)1097-0029(19970601)37:5/6<456::AID-JEMT9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Cesmebasi A, Malefant J, Patel SD, Du Plessis M, Renna S, Tubbs RS, Loukas M. The surgical anatomy of the lymphatic system of the pancreas. Clin. Anat. 2015;28:527–537. doi: 10.1002/ca.22461. [DOI] [PubMed] [Google Scholar]
- 26.Isaji S, Kawarada Y, Uemoto S. Classification of pancreatic cancer: Comparison of japanese and UICC classifications. Pancreas. 2004;28:231–234. doi: 10.1097/00006676-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Kanda M, Fujii T, Nagai S, et al. Pattern of lymph node metastasis spread in pancreatic cancer. Pancreas. 2011;40:951–955. doi: 10.1097/MPA.0b013e3182148342. [DOI] [PubMed] [Google Scholar]
- 28.Sun W, Leong CN, Zhang Z, Lu JJ. Proposing the lymphatic target volume for elective radiation therapy for pancreatic cancer: A pooled analysis of clinical evidence. Radiat. Oncol. 2010;5 doi: 10.1186/1748-717X-5-28. 28-717X-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujita T, Nakagohri T, Gotohda N, Takahashi S, Konishi M, Kojima M, Kinoshita T. Evaluation of the prognostic factors and significance of lymph node status in invasive ductal carcinoma of the body or tail of the pancreas. Pancreas. 2010;39:e48–e54. doi: 10.1097/MPA.0b013e3181bd5cfa. [DOI] [PubMed] [Google Scholar]
- 30.Nagakawa T, Kobayashi H, Ueno K, Ohta T, Kayahara M, Miyazaki I. Clinical study of lymphatic flow to the paraaortic lymph nodes in carcinoma of the head of the pancreas. Cancer. 1994;73:1155–1162. doi: 10.1002/1097-0142(19940215)73:4<1155::aid-cncr2820730406>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 31.Gerdes B, Ramaswamy A, Bartsch DK, Rothmund M. Peripyloric lymph node metastasis is a rare condition in carcinoma of the pancreatic head. Pancreas. 2005;31:88–92. doi: 10.1097/01.mpa.0000168221.97967.98. [DOI] [PubMed] [Google Scholar]
- 32.Whipple AO. The rationale of radical surgery for cancer of the pancreas and ampullary region. Ann. Surg. 1941;114:612–615. doi: 10.1097/00000658-194111440-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhatti I, Peacock O, Awan AK, Semeraro D, Larvin M, Hall RI. Lymph node ratio versus number of affected lymph nodes as predictors of survival for resected pancreatic adenocarcinoma. World J. Surg. 2010;34:768–775. doi: 10.1007/s00268-009-0336-4. [DOI] [PubMed] [Google Scholar]
- 34.Sergeant G, Melloul E, Lesurtel M, Deoliveira ML, Clavien PA. Extended lymphadenectomy in patients with pancreatic cancer is debatable. World J. Surg. 2013;37:1782–1788. doi: 10.1007/s00268-013-2064-z. [DOI] [PubMed] [Google Scholar]
- 35.Zacharias T, Jaeck D, Oussoultzoglou E, Neuville A, Bachellier P. Impact of lymph node involvement on long-term survival after R0 pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas. J. Gastrointest. Surg. 2007;11:350–356. doi: 10.1007/s11605-007-0113-3. [DOI] [PubMed] [Google Scholar]
- 36.Henne-Bruns D, Vogel I, Luttges J, Kloppel G, Kremer B. Surgery for ductal adenocarcinoma of the pancreatic head: Staging, complications, and survival after regional versus extended lymphadenectomy. World J. Surg. 2000;24:595–601. doi: 10.1007/s002689910089. discussion 601-2. [DOI] [PubMed] [Google Scholar]
- 37.Doi R, Kami K, Ito D, Fujimoto K, Kawaguchi Y, Wada M, Kogire M, Hosotani R, Imamura M, Uemoto S. Prognostic implication of para-aortic lymph node metastasis in resectable pancreatic cancer. World J. Surg. 2007;31:147–154. doi: 10.1007/s00268-005-0730-5. [DOI] [PubMed] [Google Scholar]
- 38.Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakashima A, Yuasa Y, Kondo N, Ohge H, Sueda T. Number of metastatic lymph nodes, but not lymph node ratio, is an independent prognostic factor after resection of pancreatic carcinoma. J. Am. Coll. Surg. 2010;211:196–204. doi: 10.1016/j.jamcollsurg.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 39.Kocher HM, Sohail M, Benjamin IS, Patel AG. Technical limitations of lymph node mapping in pancreatic cancer. Eur. J. Surg. Oncol. 2007;33:887–891. doi: 10.1016/j.ejso.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 40.Hirono S, Tani M, Kawai M, Okada K, Miyazawa M, Shimizu A, Uchiyama K, Yamaue H. Identification of the lymphatic drainage pathways from the pancreatic head guided by indocyanine green fluorescence imaging during pancreaticoduodenectomy. Dig. Surg. 2012;29:132–139. doi: 10.1159/000337306. [DOI] [PubMed] [Google Scholar]
- 41.Duanmin H, Chao X, Qi Z. eEF1A2 protein expression correlates with lymph node metastasis and decreased survival in pancreatic ductal adenocarcinoma. Hepatogastroenterology. 2013;60:870–875. doi: 10.5754/hge12869. [DOI] [PubMed] [Google Scholar]
- 42.Henne-Bruns D, Vogel I, Luttges J, Kloppel G, Kremer B. Ductal adenocarcinoma of the pancreas head: Survival after regional versus extended lymphadenectomy. Hepatogastroenterology. 1998;45:855–866. [PubMed] [Google Scholar]
- 43.Howard JM. Development and progress in resective surgery for pancreatic cancer. World J. Surg. 1999;23:901–906. doi: 10.1007/s002689900597. [DOI] [PubMed] [Google Scholar]
- 44.Sergeant G, Ectors N, Fieuws S, Aerts R, Topal B. Prognostic relevance of extracapsular lymph node involvement in pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2009;16:3070–3079. doi: 10.1245/s10434-009-0627-x. [DOI] [PubMed] [Google Scholar]
- 45.Dansranjavin T, Mobius C, Tannapfel A, Bartels M, Wittekind C, Hauss J, Witzigmann H. E-cadherin and DAP kinase in pancreatic adenocarcinoma and corresponding lymph node metastases. Oncol. Rep. 2006;15:1125–1131. [PubMed] [Google Scholar]
- 46.Kamisawa T, Isawa T, Koike M, Tsuruta K, Okamoto A. Hematogenous metastases of pancreatic ductal carcinoma. Pancreas. 1995;11:345–349. doi: 10.1097/00006676-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Formentini A, Prokopchuk O, Strater J, Kleeff J, Grochola LF, Leder G, Henne-Bruns D, Korc M, Kornmann M. Interleukin-13 exerts autocrine growth-promoting effects on human pancreatic cancer, and its expression correlates with a propensity for lymph node metastases. Int. J. Colorectal Dis. 2009;24:57–67. doi: 10.1007/s00384-008-0550-9. [DOI] [PubMed] [Google Scholar]
- 48.Kurahara H, Takao S, Maemura K, Shinchi H, Natsugoe S, Aikou T. Impact of vascular endothelial growth factor-C and -D expression in human pancreatic cancer: Its relationship to lymph node metastasis. Clin. Cancer Res. 2004;10:8413–8420. doi: 10.1158/1078-0432.CCR-04-0379. [DOI] [PubMed] [Google Scholar]
- 49.Ohta T, Terada T, Nagakawa T, Tajima H, Itoh H, Fonseca L, Miyazaki I. Pancreatic trypsinogen and cathepsin B in human pancreatic carcinomas and associated metastatic lesions. Br. J. Cancer. 1994;69:152–156. doi: 10.1038/bjc.1994.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu QH, Shi ML, Bai J, Zheng JN. Identification of ANXA1 as a lymphatic metastasis and poor prognostic factor in pancreatic ductal adenocarcinoma. Asian Pac. J. Cancer. Prev. 2015;16:2719–2724. doi: 10.7314/apjcp.2015.16.7.2719. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto Y, Ikoma H, Morimura R, et al. The clinical impact of the lymph node ratio as a prognostic factor after resection of pancreatic cancer. Anticancer Res. 2014;34:2389–2394. [PubMed] [Google Scholar]
- 52.Pignatelli M, Ansari TW, Gunter P, Liu D, Hirano S, Takeichi M, Kloppel G, Lemoine NR. Loss of membranous E-cadherin expression in pancreatic cancer: Correlation with lymph node metastasis, high grade, and advanced stage. J. Pathol. 1994;174:243–248. doi: 10.1002/path.1711740403. [DOI] [PubMed] [Google Scholar]
- 53.Wang Z, Wu J, Li G, Zhang X, Tong M, Wu Z, Liu Z. Lymphangiogenesis and biological behavior in pancreatic carcinoma and other pancreatic tumors. Mol. Med. Rep. 2012;5:959–963. doi: 10.3892/mmr.2012.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cui K, Zhao W, Wang C, Wang A, Zhang B, Zhou W, Yu J, Sun Z, Li S. The CXCR4-CXCL12 pathway facilitates the progression of pancreatic cancer via induction of angiogenesis and lymphangiogenesis. J. Surg. Res. 2011;171:143–150. doi: 10.1016/j.jss.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Tempia-Caliera AA, Horvath LZ, Zimmermann A, Tihanyi TT, Korc M, Friess H, Buchler MW. Adhesion molecules in human pancreatic cancer. J. Surg. Oncol. 2002;79:93–100. doi: 10.1002/jso.10053. [DOI] [PubMed] [Google Scholar]
- 56.Nagai K, Doi R, Koizumi M, Masui T, Kawaguchi Y, Yoshizawa A, Uemoto S. Noninvasive intraductal papillary mucinous neoplasm with para-aortic lymph node metastasis: Report of a case. Surg. Today. 2011;41:147–152. doi: 10.1007/s00595-009-4210-7. [DOI] [PubMed] [Google Scholar]
- 57.Tao J, Li T, Li K, Xiong J, Yang Z, Wu H, Wang C. Effect of HIF-1alpha on VEGF-C induced lymphangiogenesis and lymph nodes metastases of pancreatic cancer. J. Huazhong Univ. Sci. Technolog Med. Sci. 2006;26:562–564. doi: 10.1007/s11596-006-0520-9. [DOI] [PubMed] [Google Scholar]
- 58.Kobayashi K, Sadakari Y, Ohtsuka T, Takahata S, Nakamura M, Mizumoto K, Tanaka M. Factors in intraductal papillary mucinous neoplasms of the pancreas predictive of lymph node metastasis. Pancreatology. 2010;10:720–725. doi: 10.1159/000320709. [DOI] [PubMed] [Google Scholar]
- 59.Shimada K, Sakamoto Y, Sano T, Kosuge T. The role of paraaortic lymph node involvement on early recurrence and survival after macroscopic curative resection with extended lymphadenectomy for pancreatic carcinoma. J. Am. Coll. Surg. 2006;203:345–352. doi: 10.1016/j.jamcollsurg.2006.05.289. [DOI] [PubMed] [Google Scholar]
- 60.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J. Gastrointest. Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210-1. [DOI] [PubMed] [Google Scholar]
- 61.Yamamoto S, Tomita Y, Hoshida Y, et al. Increased expression of valosin-containing protein (p97) is associated with lymph node metastasis and prognosis of pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2004;11:165–172. doi: 10.1245/aso.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 62.Nakagohri T, Kinoshita T, Konishi M, Takahashi S, Gotohda N. Nodal involvement is strongest predictor of poor survival in patients with invasive adenocarcinoma of the head of the pancreas. Hepatogastroenterology. 2006;53:447–451. [PubMed] [Google Scholar]
- 63.Chen JW, Bhandari M, Astill DS, Wilson TG, Kow L, Brooke-Smith M, Toouli J, Padbury RT. Predicting patient survival after pancreaticoduodenectomy for malignancy: Histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB (Oxford) 2010;12:101–108. doi: 10.1111/j.1477-2574.2009.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pederzoli P, Bassi C, Falconi M, Pedrazzoli S. Does the extent of lymphatic resection affect the outcome in pancreatic cancer? Digestion. 1997;58:536–541. doi: 10.1159/000201498. [DOI] [PubMed] [Google Scholar]
- 65.Pissas A. Anatomoclinical and anatomosurgical essay on the lymphatic circulation of the pancreas. Anat. Clin. 1984;6:255–280. doi: 10.1007/BF01654459. [DOI] [PubMed] [Google Scholar]
- 66.Hirata K, Sato T, Mukaiya M, Yamashiro K, Kimura M, Sasaki K, Denno R. Results of 1001 pancreatic resections for invasive ductal adenocarcinoma of the pancreas. Arch. Surg. 1997;132:771–776. doi: 10.1001/archsurg.1997.01430310085018. discussion 777. [DOI] [PubMed] [Google Scholar]
- 67.Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, Coleman J, Abrams RA, Hruban RH. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: Randomized controlled trial evaluating survival, morbidity, and mortality. Ann. Surg. 2002;236:355–366. doi: 10.1097/00000658-200209000-00012. discussion 366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farnell MB, Pearson RK, Sarr MG, DiMagno EP, Burgart LJ, Dahl TR, Foster N, Sargent DJ Pancreas Cancer Working Group. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138:618–628. doi: 10.1016/j.surg.2005.06.044. discussion 628-30. [DOI] [PubMed] [Google Scholar]
- 69.Pawlik TM, Abdalla EK, Barnett CC, Ahmad SA, Cleary KR, Vauthey JN, Lee JE, Evans DB, Pisters PW. Feasibility of a randomized trial of extended lymphadenectomy for pancreatic cancer. Arch. Surg. 2005;140:584–589. doi: 10.1001/archsurg.140.6.584. discussion 589-91. [DOI] [PubMed] [Google Scholar]
- 70.Michalski CW, Kleeff J, Wente MN, Diener MK, Buchler MW, Friess H. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br. J. Surg. 2007;94:265–273. doi: 10.1002/bjs.5716. [DOI] [PubMed] [Google Scholar]
- 71.Samra JS, Gananadha S, Hugh TJ. Surgical management of carcinoma of the head of pancreas: Extended lymphadenectomy or modified en bloc resection? ANZ J. Surg. 2008;78:228–236. doi: 10.1111/j.1445-2197.2008.04426.x. [DOI] [PubMed] [Google Scholar]
- 72.Farnell MB, Aranha GV, Nimura Y, Michelassi F. The role of extended lymphadenectomy for adenocarcinoma of the head of the pancreas: Strength of the evidence. J. Gastrointest. Surg. 2008;12:651–656. doi: 10.1007/s11605-007-0451-1. [DOI] [PubMed] [Google Scholar]
- 73.Evans DB, Farnell MB, Lillemoe KD, Vollmer C, Jr, Strasberg SM, Schulick RD. Surgical treatment of resectable and borderline resectable pancreas cancer: Expert consensus statement. Ann. Surg. Oncol. 2009;16:1736–1744. doi: 10.1245/s10434-009-0416-6. [DOI] [PubMed] [Google Scholar]
- 74.Nimura Y, Nagino M, Takao S, et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: Long-term results of a japanese multicenter randomized controlled trial. J. Hepatobiliary. Pancreat. Sci. 2012;19:230–241. doi: 10.1007/s00534-011-0466-6. [DOI] [PubMed] [Google Scholar]
- 75.Masui T, Kubota T, Aoki K, et al. Long-term survival after resection of pancreatic ductal adenocarcinoma with para-aortic lymph node metastasis: Case report. World J. Surg. Oncol. 2013;11 doi: 10.1186/1477-7819-11-195. 195-7819-11-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katuchova J, Bober J, Katuch V, Radonak J. Significance of lymph node micrometastasis in pancreatic cancer patients. Eur. Surg. Res. 2012;48:10–15. doi: 10.1159/000334171. [DOI] [PubMed] [Google Scholar]
- 77.Ishikawa O, Ohhigashi H, Sasaki Y, Kabuto T, Fukuda I, Furukawa H, Imaoka S, Iwanaga T. Practical usefulness of lymphatic and connective tissue clearance for the carcinoma of the pancreas head. Ann. Surg. 1988;208:215–220. doi: 10.1097/00000658-198808000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manabe T, Ohshio G, Baba N, Miyashita T, Asano N, Tamura K, Yamaki K, Nonaka A, Tobe T. Radical pancreatectomy for ductal cell carcinoma of the head of the pancreas. Cancer. 1989;64:1132–1137. doi: 10.1002/1097-0142(19890901)64:5<1132::aid-cncr2820640528>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 79.Ohta T, Nagakawa T, Ueno K, Kayahara M, Mori K, Kobayashi H, Takeda T, Miyazaki I. The mode of lymphatic and local spread of pancreatic carcinomas less than 4.0 cm in size. Int. Surg. 1993;78:208–212. [PubMed] [Google Scholar]
- 80.Nakao A, Harada A, Nonami T, Kaneko T, Murakami H, Inoue S, Takeuchi Y, Takagi H. Lymph node metastases in carcinoma of the head of the pancreas region. Br. J. Surg. 1995;82:399–402. doi: 10.1002/bjs.1800820340. [DOI] [PubMed] [Google Scholar]
- 81.Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, Kloppel G, Dhaene K, Michelassi F. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: A multicenter, prospective, randomized study. lymphadenectomy study group. Ann. Surg. 1998;228:508–517. doi: 10.1097/00000658-199810000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fernandez-Cruz L, Johnson C, Dervenis C. Locoregional dissemination and extended lymphadenectomy in pancreatic cancer. Dig. Surg. 1999;16:313–319. doi: 10.1159/000018741. [DOI] [PubMed] [Google Scholar]
- 83.Riall TS, Cameron JL, Lillemoe KD, Campbell KA, Sauter PK, Coleman J, Abrams RA, Laheru D, Hruban RH, Yeo CJ. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma–part 3: Update on 5-year survival. J. Gastrointest. Surg. 2005;9:1191–1204. doi: 10.1016/j.gassur.2005.08.034. discussion 1204-6. [DOI] [PubMed] [Google Scholar]
- 84.Meriggi F, Gramigna P, Forni E. Extended lymphadenectomy in cephalic pancreatoduodenectomy. personal observations. Hepatogastroenterology. 2007;54:549–555. [PubMed] [Google Scholar]
- 85.Roche CJ, Hughes ML, Garvey CJ, Campbell F, White DA, Jones L, Neoptolemos JP. CT and pathologic assessment of prospective nodal staging in patients with ductal adenocarcinoma of the head of the pancreas. AJR Am. J. Roentgenol. 2003;180:475–480. doi: 10.2214/ajr.180.2.1800475. [DOI] [PubMed] [Google Scholar]
- 86.Imai H, Doi R, Kanazawa H, et al. Preoperative assessment of para-aortic lymph node metastasis in patients with pancreatic cancer. Int. J. Clin. Oncol. 2010;15:294–300. doi: 10.1007/s10147-010-0066-5. [DOI] [PubMed] [Google Scholar]
- 87.Yeo CJ, Cameron JL, Sohn TA, Coleman J, Sauter PK, Hruban RH, Pitt HA, Lillemoe KD. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: Comparison of morbidity and mortality and short-term outcome. Ann. Surg. 1999;229:613–622. doi: 10.1097/00000658-199905000-00003. discussion 622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nguyen TC, Sohn TA, Cameron JL, Lillemoe KD, Campbell KA, Coleman J, Sauter PK, Abrams RA, Hruban RH, Yeo CJ. Standard vs. radical pancreaticoduodenectomy for periampullary adenocarcinoma: A prospective, randomized trial evaluating quality of life in pancreaticoduodenectomy survivors. J. Gastrointest. Surg. 2003;7:1–9. doi: 10.1016/s1091-255x(02)00187-7. discussion 9-11. [DOI] [PubMed] [Google Scholar]
- 89.Bittner R, Roscher R, Safi F, Dopfer HP, Scholzel E, Beger HG. Effect of tumor size and lymph node status on the prognosis of pancreatic cancer. Chirurg. 1989;60:240–245. [PubMed] [Google Scholar]
- 90.Dasari BV, Pasquali S, Vohra RS, Smith AM, Taylor MA, Sutcliffe RP, Muiesan P, Roberts KJ, Isaac J, Mirza DF. Extended versus standard lymphadenectomy for pancreatic head cancer: Meta-analysis of randomized controlled trials. J. Gastrointest. Surg. 2015;19:1725–1732. doi: 10.1007/s11605-015-2859-3. [DOI] [PubMed] [Google Scholar]
- 91.Pedrazzoli S. Extent of lymphadenectomy to associate with pancreaticoduodenectomy in patients with pancreatic head cancer for better tumor staging. Cancer Treat. Rev. 2015;41:577–587. doi: 10.1016/j.ctrv.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 92.Schoellhammer HF, Goldner BS, Kim J, Singh G. Beyond the whipple operation: Radical resections for cancers of the head of the pancreas. Indian. J. Surg. Oncol. 2015;6:41–46. doi: 10.1007/s13193-013-0258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peparini N. Mesopancreas: A boundless structure, namely the rationale for dissection of the paraaortic area in pancreaticoduodenectomy for pancreatic head carcinoma. World J. Gastroenterol. 2015;21:2865–2870. doi: 10.3748/wjg.v21.i10.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tol JA, Gouma DJ, Bassi C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: A consensus statement by the international study group on pancreatic surgery (ISGPS) Surgery. 2014;156:591–600. doi: 10.1007/978-1-4939-1726-6_59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jang JY, Kang MJ, Heo JS, et al. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann. Surg. 2014;259:656–664. doi: 10.1097/SLA.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 96.Svoronos C, Tsoulfas G, Katsourakis A, Noussios G, Chatzitheoklitos E, Marakis NG. Role of extended lymphadenectomy in the treatment of pancreatic head adenocarcinoma: Review and meta-analysis. ANZ J. Surg. 2014;84:706–711. doi: 10.1111/ans.12423. [DOI] [PubMed] [Google Scholar]
- 97.Ke K, Chen W, Chen Y. Standard and extended lymphadenectomy for adenocarcinoma of the pancreatic head: A meta-analysis and systematic review. J. Gastroenterol. Hepatol. 2014;29:453–462. doi: 10.1111/jgh.12393. [DOI] [PubMed] [Google Scholar]
- 98.Fujii T. Extended lymphadenectomy in pancreatic cancer is crucial. World J. Surg. 2013;37:1778–1781. doi: 10.1007/s00268-013-2039-0. [DOI] [PubMed] [Google Scholar]
- 99.Iqbal N, Lovegrove RE, Tilney HS, Abraham AT, Bhattacharya S, Tekkis PP, Kocher HM. A comparison of pancreaticoduodenectomy with extended pancreaticoduodenectomy: A meta-analysis of 1909 patients. Eur. J. Surg. Oncol. 2009;35:79–86. doi: 10.1016/j.ejso.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 100.Santi I, Brandt A, Hemminki K. What is the major prognostic factor in tumor-node-metastasis staging of pancreatic adenocarcinoma? Ann. Surg. Oncol. 2011;18:300–301. doi: 10.1245/s10434-010-1189-7. [DOI] [PubMed] [Google Scholar]
- 101.John BJ, Naik P, Ironside A, Davidson BR, Fusai G, Gillmore R, Watkins J, Rahman SH. Redefining the R1 resection for pancreatic ductal adenocarcinoma: Tumour lymph nodal burden and lymph node ratio are the only prognostic factors associated with survival. HPB (Oxford) 2013;15:674–680. doi: 10.1111/hpb.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Berger AC, Watson JC, Ross EA, Hoffman JP. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am. Surg. 2004;70:235–240. discussion 240. [PubMed] [Google Scholar]
- 103.Slidell MB, Chang DC, Cameron JL, Wolfgang C, Herman JM, Schulick RD, Choti MA, Pawlik TM. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: A large, population-based analysis. Ann. Surg. Oncol. 2008;15:165–174. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 104.Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 105.Safuan S, Storr SJ, Patel PM, Martin SG. A comparative study of adhesion of melanoma and breast cancer cells to blood and lymphatic endothelium. Lymphat Res. Biol. 2012;10:173–181. doi: 10.1089/lrb.2012.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tol JA, Brosens LA, van Dieren S, van Gulik TM, Busch OR, Besselink MG, Gouma DJ. Impact of lymph node ratio on survival in patients with pancreatic and periampullary cancer. Br. J. Surg. 2015;102:237–245. doi: 10.1002/bjs.9709. [DOI] [PubMed] [Google Scholar]
- 107.Ausborn NL, Wang T, Wentz SC, Washington MK, Merchant NB, Zhao Z, Shyr Y, Chakravarthy AB, Xia F. 53BP1 expression is a modifier of the prognostic value of lymph node ratio and CA 19-9 in pancreatic adenocarcinoma. BMC Cancer. 2013;13 doi: 10.1186/1471-2407-13-155. 155-2407-13-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith BJ, Mezhir JJ. An interactive bayesian model for prediction of lymph node ratio and survival in pancreatic cancer patients. J. Am. Med. Inform. Assoc. 2014;21:e203–e211. doi: 10.1136/amiajnl-2013-002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Konstantinidis IT, Deshpande V, Zheng H, Wargo JA, Fernandez-del Castillo C, Thayer SP, Androutsopoulos V, Lauwers GY, Warshaw AL, Ferrone CR. Does the mechanism of lymph node invasion affect survival in patients with pancreatic ductal adenocarcinoma? J. Gastrointest. Surg. 2010;14:261–267. doi: 10.1007/s11605-009-1096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Riediger H, Keck T, Wellner U, zur Hausen A, Adam U, Hopt UT, Makowiec F. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J. Gastrointest. Surg. 2009;13:1337–1344. doi: 10.1007/s11605-009-0919-2. [DOI] [PubMed] [Google Scholar]
- 111.La Torre M, Cavallini M, Ramacciato G, Cosenza G, Rossi Del Monte S, Nigri G, Ferri M, Mercantini P, Ziparo V. Role of the lymph node ratio in pancreatic ductal adenocarcinoma. impact on patient stratification and prognosis. J. Surg. Oncol. 2011;104:629–633. doi: 10.1002/jso.22013. [DOI] [PubMed] [Google Scholar]
- 112.House MG, Gonen M, Jarnagin WR, D'Angelica M, DeMatteo RP, Fong Y, Brennan MF, Allen PJ. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J. Gastrointest. Surg. 2007;11:1549–1555. doi: 10.1007/s11605-007-0243-7. [DOI] [PubMed] [Google Scholar]
- 113.Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: Information from a large US population database. Ann. Surg. Oncol. 2006;13:1189–1200. doi: 10.1245/s10434-006-9016-x. [DOI] [PubMed] [Google Scholar]
- 114.Sierzega M, Popiela T, Kulig J, Nowak K. The ratio of metastatic/resected lymph nodes is an independent prognostic factor in patients with node-positive pancreatic head cancer. Pancreas. 2006;33:240–245. doi: 10.1097/01.mpa.0000235306.96486.2a. [DOI] [PubMed] [Google Scholar]
- 115.Bogoevski D, Yekebas EF, Schurr P, Kaifi JT, Kutup A, Erbersdobler A, Pantel K, Izbicki JR. Mode of spread in the early phase of lymphatic metastasis in pancreatic ductal adenocarcinoma: Prognostic significance of nodal microinvolvement. Ann. Surg. 2004;240:993–1000. doi: 10.1097/01.sla.0000145922.25106.e3. discussion 1000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Milsmann C, Fuzesi L, Werner C, Becker H, Horstmann O. Significance of occult lymphatic tumor spread in pancreatic cancer. Chirurg. 2005;76:1064–1072. doi: 10.1007/s00104-005-1041-y. [DOI] [PubMed] [Google Scholar]
- 117.Ridwelski K, Meyer F, Fahlke J, Kasper U, Roessner A, Lippert H. Value of cytokeratin and ca 19-9 antigen in immunohistological detection of disseminated tumor cells in lymph nodes in pancreas carcinoma. Chirurg. 2001;72:920–926. doi: 10.1007/s001040170089. [DOI] [PubMed] [Google Scholar]
- 118.Demeure MJ, Doffek KM, Komorowski RA, Wilson SD. Adenocarcinoma of the pancreas: Detection of occult metastases in regional lymph nodes by a polymerase chain reaction-based assay. Cancer. 1998;83:1328–1334. [PubMed] [Google Scholar]
- 119.Mao C, Domenico DR, Kim K, Hanson DJ, Howard JM. Observations on the developmental patterns and the consequences of pancreatic exocrine adenocarcinoma. findings of 154 autopsies. Arch. Surg. 1995;130:125–134. doi: 10.1001/archsurg.1995.01430020015001. [DOI] [PubMed] [Google Scholar]
- 120.Golse N, Lebeau R, Lombard-Bohas C, Hervieu V, Ponchon T, Adham M. Lymph node involvement beyond peripancreatic region in pancreatic head cancers: When results belie expectations. Pancreas. 2013;42:239–248. doi: 10.1097/MPA.0b013e31825f80a9. [DOI] [PubMed] [Google Scholar]
- 121.Veikkola T, Jussila L, Makinen T, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20:1223–1231. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 123.Oh SJ, Jeltsch MM, Birkenhager R, McCarthy JE, Weich HA, Christ B, Alitalo K, Wilting J. VEGF and VEGF-C: Specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev. Biol. 1997;188:96–109. doi: 10.1006/dbio.1997.8639. [DOI] [PubMed] [Google Scholar]
- 124.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc. Natl. Acad. Sci. U. S. A. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Favier B, Alam A, Barron P, et al. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108:1243–1250. doi: 10.1182/blood-2005-11-4447. [DOI] [PubMed] [Google Scholar]
- 126.Bjorndahl MA, Cao R, Burton JB, Brakenhielm E, Religa P, Galter D, Wu L, Cao Y. Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res. 2005;65:9261–9268. doi: 10.1158/0008-5472.CAN-04-2345. [DOI] [PubMed] [Google Scholar]
- 127.Morisada T, Oike Y, Yamada Y, et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- 128.Yan ZX, Jiang ZH, Liu NF. Angiopoietin-2 promotes inflammatory lymphangiogenesis and its effect can be blocked by the specific inhibitor L1–10. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H215–H223. doi: 10.1152/ajpheart.00895.2011. [DOI] [PubMed] [Google Scholar]
- 129.Kubo H, Cao R, Brakenhielm E, Makinen T, Cao Y, Alitalo K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cao R, Bjorndahl MA, Religa P, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer. Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 131.Miyazaki H, Yoshimatsu Y, Akatsu Y, Mishima K, Fukayama M, Watabe T, Miyazono K. Expression of platelet-derived growth factor receptor beta is maintained by Prox1 in lymphatic endothelial cells and is required for tumor lymphangiogenesis. Cancer. Sci. 2014;105:1116–1123. doi: 10.1111/cas.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fink DM, Connor AL, Kelley PM, Steele MM, Hollingsworth MA, Tempero RM. Nerve growth factor regulates neurolymphatic remodeling during corneal inflammation and resolution. PLoS One. 2014;9:e112737. doi: 10.1371/journal.pone.0112737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bjorndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ, Cao Y. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc. Natl. Acad. Sci. U. S. A. 2005;102:15593–15598. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cao R, Bjorndahl MA, Gallego MI, Chen S, Religa P, Hansen AJ, Cao Y. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood. 2006;107:3531–3536. doi: 10.1182/blood-2005-06-2538. [DOI] [PubMed] [Google Scholar]
- 135.Peppicelli S, Bianchini F, Calorini L. Inflammatory cytokines induce vascular endothelial growth factor-C expression in melanoma-associated macrophages and stimulate melanoma lymph node metastasis. Oncol. Lett. 2014;8:1133–1138. doi: 10.3892/ol.2014.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ristimaki A, Narko K, Enholm B, Joukov V, Alitalo K. Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J. Biol. Chem. 1998;273:8413–8418. doi: 10.1074/jbc.273.14.8413. [DOI] [PubMed] [Google Scholar]
- 137.Du Q, Jiang L, Wang X, Wang M, She F, Chen Y. Tumor necrosis factor-alpha promotes the lymphangiogenesis of gallbladder carcinoma through nuclear factor-kappaB-mediated upregulation of vascular endothelial growth factor-C. Cancer. Sci. 2014;105:1261–1271. doi: 10.1111/cas.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang WC, Nagahashi M, Terracina KP, Takabe K. Emerging role of sphingosine-1-phosphate in inflammation, cancer, and lymphangiogenesis. Biomolecules. 2013;3 doi: 10.3390/biom3030408. 10.3390/biom3030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hosono K, Suzuki T, Tamaki H, Sakagami H, Hayashi I, Narumiya S, Alitalo K, Majima M. Roles of prostaglandin E2–EP3/EP4 receptor signaling in the enhancement of lymphangiogenesis during fibroblast growth factor-2-induced granulation formation. Arterioscler. Thromb. Vasc. Biol. 2011;31:1049–1058. doi: 10.1161/ATVBAHA.110.222356. [DOI] [PubMed] [Google Scholar]
- 140.Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and -D are ligands for the integrin alpha9beta1. J. Biol. Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Oommen S, Gupta SK, Vlahakis NE. Vascular endothelial growth factor A (VEGF-A) induces endothelial and cancer cell migration through direct binding to integrin {alpha}9{beta}1: Identification of a specific {alpha}9{beta}1 binding site. J. Biol. Chem. 2011;286:1083–1092. doi: 10.1074/jbc.M110.175158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ. Res. 2004;94:664–670. doi: 10.1161/01.RES.0000118600.91698.BB. [DOI] [PubMed] [Google Scholar]
- 143.Murakami M, Zheng Y, Hirashima M, Suda T, Morita Y, Ooehara J, Ema H, Fong GH, Shibuya M. VEGFR1 tyrosine kinase signaling promotes lymphangiogenesis as well as angiogenesis indirectly via macrophage recruitment. Arterioscler. Thromb. Vasc. Biol. 2008;28:658–664. doi: 10.1161/ATVBAHA.107.150433. [DOI] [PubMed] [Google Scholar]
- 144.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, Jessberger R, Merad M, Randolph GJ. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 146.Hall KL, Volk-Draper LD, Flister MJ, Ran S. New model of macrophage acquisition of the lymphatic endothelial phenotype. PLoS One. 2012;7:e31794. doi: 10.1371/journal.pone.0031794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Coma S, Allard-Ratick M, Akino T, van Meeteren LA, Mammoto A, Klagsbrun M. GATA2 and Lmo2 control angiogenesis and lymphangiogenesis via direct transcriptional regulation of neuropilin-2. Angiogenesis. 2013;16:939–952. doi: 10.1007/s10456-013-9370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lin FJ, Chen X, Qin J, Hong YK, Tsai MJ, Tsai SY. Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J. Clin. Invest. 2010;120:1694–1707. doi: 10.1172/JCI40101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jones D, Xu Z, Zhang H, He Y, Kluger MS, Chen H, Min W. Functional analyses of the bone marrow kinase in the X chromosome in vascular endothelial growth factor-induced lymphangiogenesis. Arterioscler. Thromb. Vasc. Biol. 2010;30:2553–2561. doi: 10.1161/ATVBAHA.110.214999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ichise T, Yoshida N, Ichise H. H-, N- and kras cooperatively regulate lymphatic vessel growth by modulating VEGFR3 expression in lymphatic endothelial cells in mice. Development. 2010;137:1003–1013. doi: 10.1242/dev.043489. [DOI] [PubMed] [Google Scholar]
- 151.Jones D, Li Y, He Y, Xu Z, Chen H, Min W. Mirtron microRNA-1236 inhibits VEGFR-3 signaling during inflammatory lymphangiogenesis. Arterioscler. Thromb. Vasc. Biol. 2012;32:633–642. doi: 10.1161/ATVBAHA.111.243576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou HJ, Chen X, Huang Q, et al. AIP1 mediates vascular endothelial cell growth factor receptor-3-dependent angiogenic and lymphangiogenic responses. Arterioscler. Thromb. Vasc. Biol. 2014;34:603–615. doi: 10.1161/ATVBAHA.113.303053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yu J, Zhang X, Kuzontkoski PM, Jiang S, Zhu W, Li DY, Groopman JE. Slit2N and Robo4 regulate lymphangiogenesis through the VEGF-C/VEGFR-3 pathway. Cell. Commun. Signal. 2014;12 doi: 10.1186/1478-811X-12-25. 25-811X-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kurahara H, Takao S, Shinchi H, et al. Significance of lymphangiogenesis in primary tumor and draining lymph nodes during lymphatic metastasis of pancreatic head cancer. J. Surg. Oncol. 2010;102:809–815. doi: 10.1002/jso.21744. [DOI] [PubMed] [Google Scholar]
- 155.Zhang B, Zhao WH, Zhou WY, Yu WS, Yu JM, Li S. Expression of vascular endothelial growth factors-C and -D correlate with evidence of lymphangiogenesis and angiogenesis in pancreatic adenocarcinoma. Cancer Detect. Prev. 2007;31:436–442. doi: 10.1016/j.cdp.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 156.Von Marschall Z, Scholz A, Stacker SA, et al. Vascular endothelial growth factor-D induces lymphangiogenesis and lymphatic metastasis in models of ductal pancreatic cancer. Int. J. Oncol. 2005;27:669–679. [PubMed] [Google Scholar]
- 157.Koch M, Dettori D, Van Nuffelen A, et al. VEGF-D deficiency in mice does not affect embryonic or postnatal lymphangiogenesis but reduces lymphatic metastasis. J. Pathol. 2009;219:356–364. doi: 10.1002/path.2605. [DOI] [PubMed] [Google Scholar]
- 158.Kopfstein L, Veikkola T, Djonov VG, Baeriswyl V, Schomber T, Strittmatter K, Stacker SA, Achen MG, Alitalo K, Christofori G. Distinct roles of vascular endothelial growth factor-D in lymphangiogenesis and metastasis. Am. J. Pathol. 2007;170:1348–1361. doi: 10.2353/ajpath.2007.060835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mandriota SJ, Jussila L, Jeltsch M, et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shi Y, Tong M, Wu Y, et al. VEGF-C ShRNA inhibits pancreatic cancer growth and lymphangiogenesis in an orthotopic fluorescent nude mouse model. Anticancer Res. 2013;33:409–417. [PubMed] [Google Scholar]
- 161.Keklikoglou I, Hosaka K, Bender C, et al. MicroRNA-206 functions as a pleiotropic modulator of cell proliferation, invasion and lymphangiogenesis in pancreatic adenocarcinoma by targeting ANXA2 and KRAS genes. Oncogene. 2015;34:4867–4878. doi: 10.1038/onc.2014.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sipos B, Kojima M, Tiemann K, et al. Lymphatic spread of ductal pancreatic adenocarcinoma is independent of lymphangiogenesis. J. Pathol. 2005;207:301–312. doi: 10.1002/path.1840. [DOI] [PubMed] [Google Scholar]
- 163.Ochi N, Matsuo Y, Sawai H, Yasuda A, Takahashi H, Sato M, Funahashi H, Okada Y, Manabe T. Vascular endothelial growth factor-C secreted by pancreatic cancer cell line promotes lymphatic endothelial cell migration in an in vitro model of tumor lymphangiogenesis. Pancreas. 2007;34:444–451. doi: 10.1097/mpa.0b13e31803dd307. [DOI] [PubMed] [Google Scholar]
- 164.Liu X, Guo XZ, Li HY, Chen J, Ren LN, Wu CY. KAI1 inhibits lymphangiogenesis and lymphatic metastasis of pancreatic cancer in vivo. Hepatobiliary. Pancreat. Dis. Int. 2014;13:87–92. doi: 10.1016/s1499-3872(14)60012-6. [DOI] [PubMed] [Google Scholar]
- 165.Schulz P, Scholz A, Rexin A, Hauff P, Schirner M, Wiedenmann B, Detjen K. Inducible re-expression of p16 in an orthotopic mouse model of pancreatic cancer inhibits lymphangiogenesis and lymphatic metastasis. Br. J. Cancer. 2008;99:110–117. doi: 10.1038/sj.bjc.6604457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Crnic I, Strittmatter K, Cavallaro U, Kopfstein L, Jussila L, Alitalo K, Christofori G. Loss of neural cell adhesion molecule induces tumor metastasis by up-regulating lymphangiogenesis. Cancer Res. 2004;64:8630–8638. doi: 10.1158/0008-5472.CAN-04-2523. [DOI] [PubMed] [Google Scholar]
- 167.Tezel E, Kawase Y, Takeda S, Oshima K, Nakao A. Expression of neural cell adhesion molecule in pancreatic cancer. Pancreas. 2001;22:122–125. doi: 10.1097/00006676-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 168.Yang XM, Han HX, Sui F, Dai YM, Chen M, Geng JG. Slit-robo signaling mediates lymphangiogenesis and promotes tumor lymphatic metastasis. Biochem. Biophys. Res. Commun. 2010;396:571–577. doi: 10.1016/j.bbrc.2010.04.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Caunt M, Mak J, Liang WC, et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer. Cell. 2008;13:331–342. doi: 10.1016/j.ccr.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 170.Xu Y, Yuan L, Mak J, et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J. Cell Biol. 2010;188:115–130. doi: 10.1083/jcb.200903137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fukahi K, Fukasawa M, Neufeld G, Itakura J, Korc M. Aberrant expression of neuropilin-1 and-2 in human pancreatic cancer cells. Clin. Cancer Res. 2004;10:581–590. doi: 10.1158/1078-0432.ccr-0930-03. [DOI] [PubMed] [Google Scholar]
- 172.Dallas NA, Gray MJ, Xia L, et al. Neuropilin-2-mediated tumor growth and angiogenesis in pancreatic adenocarcinoma. Clin. Cancer Res. 2008;14:8052–8060. doi: 10.1158/1078-0432.CCR-08-1520. [DOI] [PubMed] [Google Scholar]
- 173.Ou JJ, Wei X, Peng Y, Zha L, Zhou RB, Shi H, Zhou Q, Liang HJ. Neuropilin-2 mediates lymphangiogenesis of colorectal carcinoma via a VEGFC/VEGFR3 independent signaling. Cancer Lett. 2015;358:200–209. doi: 10.1016/j.canlet.2014.12.046. [DOI] [PubMed] [Google Scholar]
- 174.Fagiani E, Lorentz P, Kopfstein L, Christofori G. Angiopoietin-1 and-2 exert antagonistic functions in tumor angiogenesis, yet both induce lymphangiogenesis. Cancer Res. 2011;71:5717–5727. doi: 10.1158/0008-5472.CAN-10-4635. [DOI] [PubMed] [Google Scholar]
- 175.Schulz P, Fischer C, Detjen KM, et al. Angiopoietin-2 drives lymphatic metastasis of pancreatic cancer. FASEB J. 2011;25:3325–3335. doi: 10.1096/fj.11-182287. [DOI] [PubMed] [Google Scholar]
- 176.Yoshitomi H, Kobayashi S, Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Miyazaki M. Specific expression of endoglin (CD105) in endothelial cells of intratumoral blood and lymphatic vessels in pancreatic cancer. Pancreas. 2008;37:275–281. doi: 10.1097/mpa.0b013e3181690b97. [DOI] [PubMed] [Google Scholar]
- 177.Buc E, Couvelard A, Kwiatkowski F, Dokmak S, Ruszniewski P, Hammel P, Belghiti J, Sauvanet A. Adenocarcinoma of the pancreas: Does prognosis depend on mode of lymph node invasion? Eur. J. Surg. Oncol. 2014;40:1578–1585. doi: 10.1016/j.ejso.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 178.Pai RK, Beck AH, Mitchem J, Linehan DC, Chang DT, Norton JA, Pai RK. Pattern of lymph node involvement and prognosis in pancreatic adenocarcinoma: Direct lymph node invasion has similar survival to node-negative disease. Am. J. Surg. Pathol. 2011;35:228–234. doi: 10.1097/PAS.0b013e318206c37a. [DOI] [PubMed] [Google Scholar]
- 179.Zorgetto VA, Silveira GG, Oliveira-Costa JP, Soave DF, Soares FA, Ribeiro-Silva A. The relationship between lymphatic vascular density and vascular endothelial growth factor A (VEGF-A) expression with clinical-pathological features and survival in pancreatic adenocarcinomas. Diagn. Pathol. 2013;8 doi: 10.1186/1746-1596-8-170. 170-1596-8-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schneider M, Buchler P, Giese N, Giese T, Wilting J, Buchler MW, Friess H. Role of lymphangiogenesis and lymphangiogenic factors during pancreatic cancer progression and lymphatic spread. Int. J. Oncol. 2006;28:883–890. [PubMed] [Google Scholar]
- 181.Tang RF, Itakura J, Aikawa T, Matsuda K, Fujii H, Korc M, Matsumoto Y. Overexpression of lymphangiogenic growth factor VEGF-C in human pancreatic cancer. Pancreas. 2001;22:285–292. doi: 10.1097/00006676-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 182.Rubbia-Brandt L, Terris B, Giostra E, Dousset B, Morel P, Pepper MS. Lymphatic vessel density and vascular endothelial growth factor-C expression correlate with malignant behavior in human pancreatic endocrine tumors. Clin. Cancer Res. 2004;10:6919–6928. doi: 10.1158/1078-0432.CCR-04-0397. [DOI] [PubMed] [Google Scholar]
- 183.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin. Cancer Res. 2012;18:4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Olszewski WL, Stanczyk M, Gewartowska M, Domaszewska-Szostek A, Durlik M. Lack of functioning intratumoral lymphatics in colon and pancreas cancer tissue. Lymphat Res. Biol. 2012;10:112–117. doi: 10.1089/lrb.2012.0008. [DOI] [PubMed] [Google Scholar]
- 185.Padera TP, Kadambi A, di Tomaso E, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 186.Hartveit E. Attenuated cells in breast stroma: The missing lymphatic system of the breast. Histopathology. 1990;16:533–543. doi: 10.1111/j.1365-2559.1990.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 187.Naidoo K, Jones R, Dmitrovic B, Wijesuriya N, Kocher H, Hart IR, Crnogorac-Jurcevic T. Proteome of formalin-fixed paraffin-embedded pancreatic ductal adenocarcinoma and lymph node metastases. J. Pathol. 2012;226:756–763. doi: 10.1002/path.3959. [DOI] [PubMed] [Google Scholar]
- 188.Cui Y, Wu J, Zong M, Song G, Jia Q, Jiang J, Han J. Proteomic profiling in pancreatic cancer with and without lymph node metastasis. Int. J. Cancer. 2009;124:1614–1621. doi: 10.1002/ijc.24163. [DOI] [PubMed] [Google Scholar]
- 189.Vigl B, Aebischer D, Nitschke M, Iolyeva M, Rothlin T, Antsiferova O, Halin C. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood. 2011;118:205–215. doi: 10.1182/blood-2010-12-326447. [DOI] [PubMed] [Google Scholar]
- 190.Tal O, Lim HY, Gurevich I, Milo I, Shipony Z, Ng LG, Angeli V, Shakhar G. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J. Exp. Med. 2011;208:2141–2153. doi: 10.1084/jem.20102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Irino T, Takeuchi H, Matsuda S, et al. CC-chemokine receptor CCR7: A key molecule for lymph node metastasis in esophageal squamous cell carcinoma. BMC Cancer. 2014;14 doi: 10.1186/1471-2407-14-291. 291-2407-14-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hwang TL, Lee LY, Wang CC, Liang Y, Huang SF, Wu CM. CCL7 and CCL21 overexpression in gastric cancer is associated with lymph node metastasis and poor prognosis. World J. Gastroenterol. 2012;18:1249–1256. doi: 10.3748/wjg.v18.i11.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhao B, Cui K, Wang CL, Wang AL, Zhang B, Zhou WY, Zhao WH, Li S. The chemotactic interaction between CCL21 and its receptor, CCR7, facilitates the progression of pancreatic cancer via induction of angiogenesis and lymphangiogenesis. J. Hepatobiliary. Pancreat. Sci. 2011;18:821–828. doi: 10.1007/s00534-011-0395-4. [DOI] [PubMed] [Google Scholar]
- 194.Gunther K, Leier J, Henning G, Dimmler A, Weissbach R, Hohenberger W, Forster R. Prediction of lymph node metastasis in colorectal carcinoma by expression of chemokine receptor CCR7. Int. J. Cancer. 2005;116:726–733. doi: 10.1002/ijc.21123. [DOI] [PubMed] [Google Scholar]
- 195.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 196.Guo J, Lou W, Ji Y, Zhang S. Effect of CCR7, CXCR4 and VEGF-C on the lymph node metastasis of human pancreatic ductal adenocarcinoma. Oncol. Lett. 2013;5:1572–1578. doi: 10.3892/ol.2013.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sperveslage J, Frank S, Heneweer C, et al. Lack of CCR7 expression is rate limiting for lymphatic spread of pancreatic ductal adenocarcinoma. Int. J. Cancer. 2012;131:E371–E381. doi: 10.1002/ijc.26502. [DOI] [PubMed] [Google Scholar]
- 198.Miteva DO, Rutkowski JM, Dixon JB, Kilarski W, Shields JD, Swartz MA. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ. Res. 2010;106:920–931. doi: 10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Emmett MS, Lanati S, Dunn DB, Stone OA, Bates DO. CCR7 mediates directed growth of melanomas towards lymphatics. Microcirculation. 2011;18:172–182. doi: 10.1111/j.1549-8719.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Issa A, Le TX, Shoushtari AN, Shields JD, Swartz MA. Vascular endothelial growth factor-C and C-C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res. 2009;69:349–357. doi: 10.1158/0008-5472.CAN-08-1875. [DOI] [PubMed] [Google Scholar]
- 201.Shields JD, Emmett MS, Dunn DB, Joory KD, Sage LM, Rigby H, Mortimer PS, Orlando A, Levick JR, Bates DO. Chemokine-mediated migration of melanoma cells towards lymphatics–a mechanism contributing to metastasis. Oncogene. 2007;26:2997–3005. doi: 10.1038/sj.onc.1210114. [DOI] [PubMed] [Google Scholar]
- 202.Pang MF, Georgoudaki AM, Lambut L, et al. TGF-beta1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene. 2015 May 11; doi: 10.1038/onc.2015.133. [Epub ahead of print] [ http://www.nature.com/onc/journal/vaop/ncurrent/full/onc2015133a.html] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yu S, Duan J, Zhou Z, Pang Q, Wuyang J, Liu T, He X, Xinfa L, Chen Y. A critical role of CCR7 in invasiveness and metastasis of SW620 colon cancer cell in vitro and in vivo. Cancer. Biol. Ther. 2008;7:1037–1043. doi: 10.4161/cbt.7.7.6065. [DOI] [PubMed] [Google Scholar]
- 204.Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J. Natl. Cancer Inst. 2001;93:1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- 205.Kaifi JT, Yekebas EF, Schurr P, Obonyo D, Wachowiak R, Busch P, Heinecke A, Pantel K, Izbicki JR. Tumor-cell homing to lymph nodes and bone marrow and CXCR4 expression in esophageal cancer. J. Natl. Cancer Inst. 2005;97:1840–1847. doi: 10.1093/jnci/dji431. [DOI] [PubMed] [Google Scholar]
- 206.Cardones AR, Murakami T, Hwang ST. CXCR4 enhances adhesion of B16 tumor cells to endothelial cells in vitro and in vivo via beta(1) integrin. Cancer Res. 2003;63:6751–6757. [PubMed] [Google Scholar]
- 207.Wehler T, Wolfert F, Schimanski CC, et al. Strong expression of chemokine receptor CXCR4 by pancreatic cancer correlates with advanced disease. Oncol. Rep. 2006;16:1159–1164. [PubMed] [Google Scholar]
- 208.Kim M, Koh YJ, Kim KE, Koh BI, Nam DH, Alitalo K, Kim I, Koh GY. CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche. Cancer Res. 2010;70:10411–10421. doi: 10.1158/0008-5472.CAN-10-2591. [DOI] [PubMed] [Google Scholar]
- 209.Hirakawa S, Detmar M, Kerjaschki D, et al. Nodal lymphangiogenesis and metastasis: Role of tumor-induced lymphatic vessel activation in extramammary paget's disease. Am. J. Pathol. 2009;175:2235–2248. doi: 10.2353/ajpath.2009.090420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liu X, Xiao Q, Bai X, et al. Activation of STAT3 is involved in malignancy mediated by CXCL12-CXCR4 signaling in human breast cancer. Oncol. Rep. 2014;32:2760–2768. doi: 10.3892/or.2014.3536. [DOI] [PubMed] [Google Scholar]
- 211.Uchida D, Onoue T, Kuribayashi N, Tomizuka Y, Tamatani T, Nagai H, Miyamoto Y. Blockade of CXCR4 in oral squamous cell carcinoma inhibits lymph node metastases. Eur. J. Cancer. 2011;47:452–459. doi: 10.1016/j.ejca.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 212.Chu H, Zhou H, Liu Y, Liu X, Hu Y, Zhang J. Functional expression of CXC chemokine recepter-4 mediates the secretion of matrix metalloproteinases from mouse hepatocarcinoma cell lines with different lymphatic metastasis ability. Int. J. Biochem. Cell Biol. 2007;39:197–205. doi: 10.1016/j.biocel.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 213.Yagi H, Tan W, Dillenburg-Pilla P, Armando S, Amornphimoltham P, Simaan M, Weigert R, Molinolo AA, Bouvier M, Gutkind JS. A synthetic biology approach reveals a CXCR4-G13-rho signaling axis driving transendothelial migration of metastatic breast cancer cells. Sci. Signal. 2011;4:ra60. doi: 10.1126/scisignal.2002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J. Exp. Med. 2006;203:2763–2777. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yan J, Jiang Y, Ye M, Liu W, Feng L. The clinical value of lymphatic vessel density, intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 expression in patients with oral tongue squamous cell carcinoma. J. Cancer. Res. Ther. 2014;10(Suppl):C125–C130. doi: 10.4103/0973-1482.145827. [DOI] [PubMed] [Google Scholar]
- 216.Viola K, Kopf S, Huttary N, et al. Bay11-7082 inhibits the disintegration of the lymphendothelial barrier triggered by MCF-7 breast cancer spheroids; the role of ICAM-1 and adhesion. Br. J. Cancer. 2013;108:564–569. doi: 10.1038/bjc.2012.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kawai Y, Kaidoh M, Ohhashi T. MDA-MB-231 produces ATP-mediated ICAM-1-dependent facilitation of the attachment of carcinoma cells to human lymphatic endothelial cells. Am. J. Physiol. Cell. Physiol. 2008;295:C1123–C1132. doi: 10.1152/ajpcell.00247.2008. [DOI] [PubMed] [Google Scholar]
- 218.Sawa Y, Sugimoto Y, Ueki T, Ishikawa H, Sato A, Nagato T, Yoshida S. Effects of TNF-alpha on leukocyte adhesion molecule expressions in cultured human lymphatic endothelium. J. Histochem. Cytochem. 2007;55:721–733. doi: 10.1369/jhc.6A7171.2007. [DOI] [PubMed] [Google Scholar]
- 219.Pisano M, Triacca V, Barbee KA, Swartz MA. An in vitro model of the tumor-lymphatic microenvironment with simultaneous transendothelial and luminal flows reveals mechanisms of flow enhanced invasion. Integr. Biol. (Camb) 2015;7:525–533. doi: 10.1039/c5ib00085h. [DOI] [PubMed] [Google Scholar]
- 220.Kawai Y, Kaidoh M, Yokoyama Y, Sano K, Ohhashi T. Chemokine CCL2 facilitates ICAM-1-mediated interactions of cancer cells and lymphatic endothelial cells in sentinel lymph nodes. Cancer. Sci. 2009;100:419–428. doi: 10.1111/j.1349-7006.2008.01064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Leak LV. The structure of lymphatic capillaries in lymph formation. Fed. Proc. 1976;35:1863–1871. [PubMed] [Google Scholar]
- 222.Dejana E, Orsenigo F, Molendini C, Baluk P, McDonald DM. Organization and signaling of endothelial cell-to-cell junctions in various regions of the blood and lymphatic vascular trees. Cell Tissue Res. 2009;335:17–25. doi: 10.1007/s00441-008-0694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kerjaschki D, Bago-Horvath Z, Rudas M, et al. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J. Clin. Invest. 2011;121:2000–2012. doi: 10.1172/JCI44751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tacconi C, Correale C, Gandelli A, Spinelli A, Dejana E, D'Alessio S, Danese S. Vascular endothelial growth factor C disrupts the endothelial lymphatic barrier to promote colorectal cancer invasion. Gastroenterology. 2015;148:1438.e8–1451.e8. doi: 10.1053/j.gastro.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 225.He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T, Alitalo K. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65:4739–4746. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- 226.Zheng W, Nurmi H, Appak S, et al. Angiopoietin 2 regulates the transformation and integrity of lymphatic endothelial cell junctions. Genes Dev. 2014;28:1592–1603. doi: 10.1101/gad.237677.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hanahan D, Coussens LM. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer. Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 228.Tian H, Callahan CA, DuPree KJ, Darbonne WC, Ahn CP, Scales SJ, de Sauvage FJ. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4254–4259. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, Ouellette MM, Hollingsworth MA. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin. Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Duong T, Koopman P, Francois M. Tumor lymphangiogenesis as a potential therapeutic target. J. Oncol. 2012;2012:204946. doi: 10.1155/2012/204946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Koyama H, Kobayashi N, Harada M, et al. Significance of tumor-associated stroma in promotion of intratumoral lymphangiogenesis: Pivotal role of a hyaluronan-rich tumor microenvironment. Am. J. Pathol. 2008;172:179–193. doi: 10.2353/ajpath.2008.070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mace TA, Ameen Z, Collins A, et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013;73:3007–3018. doi: 10.1158/0008-5472.CAN-12-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Dadras SS. An unexpected role for EGF in lymphangiogenesis-mediated melanoma metastasis to sentinel lymph nodes. J. Invest. Dermatol. 2013;133:14–16. doi: 10.1038/jid.2012.436. [DOI] [PubMed] [Google Scholar]
- 234.Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. U. S. A. 2013;110:20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Rasanen K, Vaheri A. Activation of fibroblasts in cancer stroma. Exp. Cell Res. 2010;316:2713–2722. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 236.Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One. 2009;4:e7965. doi: 10.1371/journal.pone.0007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shi K, Queiroz KC, Roelofs JJ, van Noesel CJ, Richel DJ, Spek CA. Protease-activated receptor 2 suppresses lymphangiogenesis and subsequent lymph node metastasis in a murine pancreatic cancer model. J. Pathol. 2014;234:398–409. doi: 10.1002/path.4411. [DOI] [PubMed] [Google Scholar]
- 239.Aebischer D, Iolyeva M, Halin C. The inflammatory response of lymphatic endothelium. Angiogenesis. 2014;17:383–393. doi: 10.1007/s10456-013-9404-3. [DOI] [PubMed] [Google Scholar]
- 240.Liao S, von der Weid PY. Lymphatic system: An active pathway for immune protection. Semin. Cell Dev. Biol. 2015;38:83–89. doi: 10.1016/j.semcdb.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science. 2010;328:749–752. doi: 10.1126/science.1185837. [DOI] [PubMed] [Google Scholar]
- 242.Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, Randolph GJ, Skobe M. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via mac-1/ICAM-1-dependent mechanism. J. Immunol. 2009;183:1767–1779. doi: 10.4049/jimmunol.0802167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Munn DH, Mellor AL. The tumor-draining lymph node as an immune-privileged site. Immunol. Rev. 2006;213:146–158. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 244.Inman KS, Francis AA, Murray NR. Complex role for the immune system in initiation and progression of pancreatic cancer. World J. Gastroenterol. 2014;20:11160–11181. doi: 10.3748/wjg.v20.i32.11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am. J. Pathol. 2007;170:774–786. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Seo N, Hayakawa S, Takigawa M, Tokura Y. Interleukin-10 expressed at early tumour sites induces subsequent generation of CD4(+) T-regulatory cells and systemic collapse of antitumour immunity. Immunology. 2001;103:449–457. doi: 10.1046/j.1365-2567.2001.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur. J. Immunol. 1997;27:1229–1235. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 249.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J. Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 251.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 252.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 253.Hirosue S, Vokali E, Raghavan VR, Rincon-Restrepo M, Lund AW, Corthesy-Henrioud P, Capotosti F, Halin Winter C, Hugues S, Swartz MA. Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: A new role for lymphatic endothelial cells. J. Immunol. 2014;192:5002–5011. doi: 10.4049/jimmunol.1302492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, Issa A, Hugues S, Swartz MA. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell. Rep. 2012;1:191–199. doi: 10.1016/j.celrep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 255.Tewalt EF, Cohen JN, Rouhani SJ, et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood. 2012;120:4772–4782. doi: 10.1182/blood-2012-04-427013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Rouhani SJ, Eccles JD, Riccardi P, Peske JD, Tewalt EF, Cohen JN, Liblau R, Makinen T, Engelhard VH. Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nat. Commun. 2015;6:6771. doi: 10.1038/ncomms7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Preynat-Seauve O, Contassot E, Schuler P, Piguet V, French LE, Huard B. Extralymphatic tumors prepare draining lymph nodes to invasion via a T-cell cross-tolerance process. Cancer Res. 2007;67:5009–5016. doi: 10.1158/0008-5472.CAN-06-4494. [DOI] [PubMed] [Google Scholar]
- 258.Swartz MA. Immunomodulatory roles of lymphatic vessels in cancer progression. Cancer. Immunol. Res. 2014;2:701–707. doi: 10.1158/2326-6066.CIR-14-0115. [DOI] [PubMed] [Google Scholar]
- 259.Kurahara H, Takao S, Maemura K, et al. M2-polarized tumor-associated macrophage infiltration of regional lymph nodes is associated with nodal lymphangiogenesis and occult nodal involvement in pN0 pancreatic cancer. Pancreas. 2013;42:155–159. doi: 10.1097/MPA.0b013e318254f2d1. [DOI] [PubMed] [Google Scholar]
- 260.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J. Mammary Gland Biol. Neoplasia. 2002;7:177–189. doi: 10.1023/a:1020304003704. [DOI] [PubMed] [Google Scholar]
- 262.Condeelis J, Pollard JW. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 263.Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S, Takao S. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J. Surg. Res. 2011;167:e211–e219. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 264.Sugimura K, Miyata H, Tanaka K, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y. High infiltration of tumor-associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J. Surg. Oncol. 2015;111:752–759. doi: 10.1002/jso.23881. [DOI] [PubMed] [Google Scholar]
- 265.Jung KY, Cho SW, Kim YA, Kim D, Oh BC, Park do J, Park YJ. Cancers with higher density of tumor-associated macrophages were associated with poor survival rates. J. Pathol. Transl. Med. 2015;49:318–324. doi: 10.4132/jptm.2015.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yuan ZY, Luo RZ, Peng RJ, Wang SS, Xue C. High infiltration of tumor-associated macrophages in triple-negative breast cancer is associated with a higher risk of distant metastasis. Onco Targets Ther. 2014;7:1475–1480. doi: 10.2147/OTT.S61838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wu H, Xu JB, He YL, Peng JJ, Zhang XH, Chen CQ, Li W, Cai SR. Tumor-associated macrophages promote angiogenesis and lymphangiogenesis of gastric cancer. J. Surg. Oncol. 2012;106:462–468. doi: 10.1002/jso.23110. [DOI] [PubMed] [Google Scholar]
- 268.Schoppmann SF, Fenzl A, Nagy K, Unger S, Bayer G, Geleff S, Gnant M, Horvat R, Jakesz R, Birner P. VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: Impact on lymphangiogenesis and survival. Surgery. 2006;139:839–846. doi: 10.1016/j.surg.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 269.Ding M, Fu X, Tan H, Wang R, Chen Z, Ding S. The effect of vascular endothelial growth factor C expression in tumor-associated macrophages on lymphangiogenesis and lymphatic metastasis in breast cancer. Mol. Med. Rep. 2012;6:1023–1029. doi: 10.3892/mmr.2012.1043. [DOI] [PubMed] [Google Scholar]
- 270.Storr SJ, Safuan S, Mitra A, et al. Objective assessment of blood and lymphatic vessel invasion and association with macrophage infiltration in cutaneous melanoma. Mod. Pathol. 2012;25:493–504. doi: 10.1038/modpathol.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zhang BC, Gao J, Wang J, Rao ZG, Wang BC, Gao JF. Tumor-associated macrophages infiltration is associated with peritumoral lymphangiogenesis and poor prognosis in lung adenocarcinoma. Med. Oncol. 2011;28:1447–1452. doi: 10.1007/s12032-010-9638-5. [DOI] [PubMed] [Google Scholar]
- 272.Yang H, Kim C, Kim MJ, Schwendener RA, Alitalo K, Heston W, Kim I, Kim WJ, Koh GY. Soluble vascular endothelial growth factor receptor-3 suppresses lymphangiogenesis and lymphatic metastasis in bladder cancer. Mol. Cancer. 2011;10 doi: 10.1186/1476-4598-10-36. 36-4598-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Ruegg C, Christofori G. Myeloid cells contribute to tumor lymphangiogenesis. PLoS One. 2009;4:e7067. doi: 10.1371/journal.pone.0007067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 275.Marconi C, Bianchini F, Mannini A, Mugnai G, Ruggieri S, Calorini L. Tumoral and macrophage uPAR and MMP-9 contribute to the invasiveness of B16 murine melanoma cells. Clin. Exp. Metastasis. 2008;25:225–231. doi: 10.1007/s10585-007-9136-0. [DOI] [PubMed] [Google Scholar]
- 276.Ran S, Montgomery KE. Macrophage-mediated lymphangiogenesis: The emerging role of macrophages as lymphatic endothelial progenitors. Cancers (Basel) 2012;4:618–657. doi: 10.3390/cancers4030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.McColl BK, Baldwin ME, Roufail S, Freeman C, Moritz RL, Simpson RJ, Alitalo K, Stacker SA, Achen MG. Plasmin activates the lymphangiogenic growth factors VEGF-C and VEGF-D. J. Exp. Med. 2003;198:863–868. doi: 10.1084/jem.20030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hunter KE, Palermo C, Kester JC, Simpson K, Li JP, Tang LH, Klimstra DS, Vlodavsky I, Joyce JA. Heparanase promotes lymphangiogenesis and tumor invasion in pancreatic neuroendocrine tumors. Oncogene. 2014;33:1799–1808. doi: 10.1038/onc.2013.142. [DOI] [PubMed] [Google Scholar]
- 279.Schledzewski K, Falkowski M, Moldenhauer G, et al. Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: Implications for the assessment of lymphangiogenesis. J. Pathol. 2006;209:67–77. doi: 10.1002/path.1942. [DOI] [PubMed] [Google Scholar]
- 280.Lee JY, Park C, Cho YP, Lee E, Kim H, Kim P, Yun SH, Yoon YS. Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation. 2010;122:1413–1425. doi: 10.1161/CIRCULATIONAHA.110.941468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Maruyama K, Ii M, Cursiefen C, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Christians K, Evans DB. Pancreaticoduodenectomy and vascular resection: Persistent controversy and current recommendations. Ann. Surg. Oncol. 2009;16:789–791. doi: 10.1245/s10434-009-0322-y. [DOI] [PubMed] [Google Scholar]
- 283.Bockman DE. Nerves in the pancreas: What are they for? Am. J. Surg. 2007;194(Suppl to October 2007):S61–S64. [Google Scholar]
- 284.Shimada K, Nara S, Esaki M, Sakamoto Y, Kosuge T, Hiraoka N. Intrapancreatic nerve invasion as a predictor for recurrence after pancreaticoduodenectomy in patients with invasive ductal carcinoma of the pancreas. Pancreas. 2011;40:464–468. doi: 10.1097/MPA.0b013e31820b5d37. [DOI] [PubMed] [Google Scholar]
- 285.Kayahara M, Nagakawa T, Ueno K, Ohta T, Takeda T, Miyazaki I. An evaluation of radical resection for pancreatic cancer based on the mode of recurrence as determined by autopsy and diagnostic imaging. Cancer. 1993;72:2118–2123. doi: 10.1002/1097-0142(19931001)72:7<2118::aid-cncr2820720710>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 286.Ceyhan GO, Bergmann F, Kadihasanoglu M, et al. Pancreatic neuropathy and neuropathic pain–a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136:177.e1–186.e1. doi: 10.1053/j.gastro.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 287.Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat. Rev. Cancer. 2011;11:695–707. doi: 10.1038/nrc3131. [DOI] [PubMed] [Google Scholar]
- 288.Kayahara M, Nagakawa T, Konishi I, Ueno K, Ohta T, Miyazaki I. Clinicopathological study of pancreatic carcinoma with particular reference to the invasion of the extrapancreatic neural plexus. Int. J. Pancreatol. 1991;10:105–111. doi: 10.1007/BF02924113. [DOI] [PubMed] [Google Scholar]
- 289.Carlson SL, Albers KM, Beiting DJ, Parish M, Conner JM, Davis BM. NGF modulates sympathetic innervation of lymphoid tissues. J. Neurosci. 1995;15:5892–5899. doi: 10.1523/JNEUROSCI.15-09-05892.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Mignini F, Sabbatini M, Coppola L, Cavallotti C. Analysis of nerve supply pattern in human lymphatic vessels of young and old men. Lymphat Res. Biol. 2012;10:189–197. doi: 10.1089/lrb.2012.0013. [DOI] [PubMed] [Google Scholar]
- 291.Cheng P, Jin G, Hu X, Shi M, Zhang Y, Liu R, Zhou Y, Shao C, Zheng J, Zhu M. Analysis of tumor-induced lymphangiogenesis and lymphatic vessel invasion of pancreatic carcinoma in the peripheral nerve plexus. Cancer. Sci. 2012;103:1756–1763. doi: 10.1111/j.1349-7006.2012.02364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Bouvree K, Brunet I, Del Toro R, et al. Semaphorin3A, neuropilin-1, and PlexinA1 are required for lymphatic valve formation. Circ. Res. 2012;111:437–445. doi: 10.1161/CIRCRESAHA.112.269316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Zhang G, Brady J, Liang WC, Wu Y, Henkemeyer M, Yan M. EphB4 forward signalling regulates lymphatic valve development. Nat. Commun. 2015;6:6625. doi: 10.1038/ncomms7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zhu Z, Friess H, diMola FF, Zimmermann A, Graber HU, Korc M, Buchler MW. Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J. Clin. Oncol. 1999;17:2419–2428. doi: 10.1200/JCO.1999.17.8.2419. [DOI] [PubMed] [Google Scholar]
- 295.Muller MW, Giese NA, Swiercz JM, et al. Association of axon guidance factor semaphorin 3A with poor outcome in pancreatic cancer. Int. J. Cancer. 2007;121:2421–2433. doi: 10.1002/ijc.22949. [DOI] [PubMed] [Google Scholar]
- 296.Li M, Zhao J, Qiao J, Song C, Zhao Z. EphB4 regulates the growth and migration of pancreatic cancer cells. Tumour Biol. 2014;35:6855–6859. doi: 10.1007/s13277-014-1937-6. [DOI] [PubMed] [Google Scholar]
- 297.Li M, Zhao Z. Clinical implications of EphB4 receptor expression in pancreatic cancer. Mol. Biol. Rep. 2013;40:1735–1741. doi: 10.1007/s11033-012-2224-5. [DOI] [PubMed] [Google Scholar]
- 298.He H, Di Y, Liang M, Yang F, Yao L, Hao S, Li J, Jiang Y, Jin C, Fu D. The microRNA-218 and ROBO-1 signaling axis correlates with the lymphatic metastasis of pancreatic cancer. Oncol. Rep. 2013;30:651–658. doi: 10.3892/or.2013.2516. [DOI] [PubMed] [Google Scholar]
- 299.Mancino M, Ametller E, Gascon P, Almendro V. The neuronal influence on tumor progression. Biochim. Biophys. Acta. 2011;1816:105–118. doi: 10.1016/j.bbcan.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 300.Gohrig A, Detjen KM, Hilfenhaus G, et al. Axon guidance factor SLIT2 inhibits neural invasion and metastasis in pancreatic cancer. Cancer Res. 2014;74:1529–1540. doi: 10.1158/0008-5472.CAN-13-1012. [DOI] [PubMed] [Google Scholar]
- 301.Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 304.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. U. S. A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Breiteneder-Geleff S, Matsui K, Soleiman A, Meraner P, Poczewski H, Kalt R, Schaffner G, Kerjaschki D. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am. J. Pathol. 1997;151:1141–1152. [PMC free article] [PubMed] [Google Scholar]
- 306.Wetterwald A, Hoffstetter W, Cecchini MG, Lanske B, Wagner C, Fleisch H, Atkinson M. Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone. 1996;18:125–132. doi: 10.1016/8756-3282(95)00457-2. [DOI] [PubMed] [Google Scholar]
- 307.Schacht V, Ramirez MI, Hong YK, et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D'Amato RJ. A model of angiogenesis in the mouse cornea. Invest. Ophthalmol. Vis. Sci. 1996;37:1625–1632. [PubMed] [Google Scholar]
- 309.Cao R, Lim S, Ji H, Zhang Y, Yang Y, Honek J, Hedlund EM, Cao Y. Mouse corneal lymphangiogenesis model. Nat. Protoc. 2011;6:817–826. doi: 10.1038/nprot.2011.359. [DOI] [PubMed] [Google Scholar]
- 310.Kelley PM, Steele MM, Tempero RM. Regressed lymphatic vessels develop during corneal repair. Lab. Invest. 2011;91:1643–1651. doi: 10.1038/labinvest.2011.121. [DOI] [PubMed] [Google Scholar]
- 311.Kelley PM, Connor AL, Tempero RM. Lymphatic vessel memory stimulated by recurrent inflammation. Am. J. Pathol. 2013;182:2418–2428. doi: 10.1016/j.ajpath.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Li JL, Goh CC, Keeble JL, Qin JS, Roediger B, Jain R, Wang Y, Chew WK, Weninger W, Ng LG. Intravital multiphoton imaging of immune responses in the mouse ear skin. Nat. Protoc. 2012;7:221–234. doi: 10.1038/nprot.2011.438. [DOI] [PubMed] [Google Scholar]
- 313.Kilarski WW, Guc E, Teo JC, Oliver SR, Lund AW, Swartz MA. Intravital immunofluorescence for visualizing the microcirculatory and immune microenvironments in the mouse ear dermis. PLoS One. 2013;8:e57135. doi: 10.1371/journal.pone.0057135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Steven P, Bock F, Huttmann G, Cursiefen C. Intravital two-photon microscopy of immune cell dynamics in corneal lymphatic vessels. PLoS One. 2011;6:e26253. doi: 10.1371/journal.pone.0026253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Tran Cao HS, McElroy M, Kaushal S, Hoffman RM, Bouvet M. Imaging of the interaction of cancer cells and the lymphatic system. Adv. Drug Deliv. Rev. 2011;63:886–889. doi: 10.1016/j.addr.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Choi I, Chung HK, Ramu S, et al. Visualization of lymphatic vessels by Prox1-promoter directed GFP reporter in a bacterial artificial chromosome-based transgenic mouse. Blood. 2011;117:362–365. doi: 10.1182/blood-2010-07-298562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Hagerling R, Pollmann C, Kremer L, Andresen V, Kiefer F. Intravital two-photon microscopy of lymphatic vessel development and function using a transgenic Prox1 promoter-directed mOrange2 reporter mouse. Biochem. Soc. Trans. 2011;39:1674–1681. doi: 10.1042/BST20110722. [DOI] [PubMed] [Google Scholar]
- 318.Martinez-Corral I, Olmeda D, Dieguez-Hurtado R, Tammela T, Alitalo K, Ortega S. In vivo imaging of lymphatic vessels in development, wound healing, inflammation, and tumor metastasis. Proc. Natl. Acad. Sci. U. S. A. 2012;109:6223–6228. doi: 10.1073/pnas.1115542109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Truman LA, Bentley KL, Smith EC, et al. ProxTom lymphatic vessel reporter mice reveal Prox1 expression in the adrenal medulla, megakaryocytes, and platelets. Am. J. Pathol. 2012;180:1715–1725. doi: 10.1016/j.ajpath.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Bianchi R, Teijeira A, Proulx ST, Christiansen AJ, Seidel CD, Rulicke T, Makinen T, Hagerling R, Halin C, Detmar M. A transgenic Prox1-cre-tdTomato reporter mouse for lymphatic vessel research. PLoS One. 2015;10:e0122976. doi: 10.1371/journal.pone.0122976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Fink DM, Connor AL, Kelley PM, Tempero RM, Hollingsworth MA. Inflamed and wound recovered tumor microenvironment contributions to lymphatic-mediated metastasis. American Association for Cancer Research Annual Meeting Proceedings. 2015:1311. [Google Scholar]
- 322.Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Stopczynski RE, Normolle DP, Hartman DJ, Ying H, DeBerry JJ, Bielefeldt K, Rhim AD, DePinho RA, Albers KM, Davis BM. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74:1718–1727. doi: 10.1158/0008-5472.CAN-13-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Ritsma L, Steller EJ, Ellenbroek SI, Kranenburg O, Borel Rinkes IH, van Rheenen J. Surgical implantation of an abdominal imaging window for intravital microscopy. Nat. Protoc. 2013;8:583–594. doi: 10.1038/nprot.2013.026. [DOI] [PubMed] [Google Scholar]
- 325.Chung K, Wallace J, Kim SY, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332–337. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Zhang Z, Procissi D, Li W, Kim DH, Li K, Han G, Huan Y, Larson AC. High resolution MRI for non-invasive mouse lymph node mapping. J. Immunol. Methods. 2013;400–401:23–29. doi: 10.1016/j.jim.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Xiong L, Shuhendler AJ, Rao J. Self-luminescing BRET-FRET near-infrared dots for in vivo lymph-node mapping and tumour imaging. Nat. Commun. 2012;3:1193. doi: 10.1038/ncomms2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Nobis M, McGhee EJ, Morton JP, et al. Intravital FLIM-FRET imaging reveals dasatinib-induced spatial control of src in pancreatic cancer. Cancer Res. 2013;73:4674–4686. doi: 10.1158/0008-5472.CAN-12-4545. [DOI] [PubMed] [Google Scholar]
- 329.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Vakoc BJ, Lanning RM, Tyrrell JA, et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat. Med. 2009;15:1219–1223. doi: 10.1038/nm.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Wang X, Pang Y, Ku G, Xie X, Stoica G, Wang LV. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 2003;21:803–806. doi: 10.1038/nbt839. [DOI] [PubMed] [Google Scholar]
- 332.Wu PC, Hsieh TY, Tsai ZU, Liu TM. In vivo quantification of the structural changes of collagens in a melanoma microenvironment with second and third harmonic generation microscopy. Sci. Rep. 2015;5:8879. doi: 10.1038/srep08879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Krafft C, Popp J. The many facets of raman spectroscopy for biomedical analysis. Anal. Bioanal Chem. 2015;407:699–717. doi: 10.1007/s00216-014-8311-9. [DOI] [PubMed] [Google Scholar]
- 334.Jeong HS, Jones D, Liao S, Wattson DA, Cui CH, Duda DG, Willett CG, Jain RK, Padera TP. Investigation of the lack of angiogenesis in the formation of lymph node metastases. J. Natl. Cancer Inst. 2015;107 doi: 10.1093/jnci/djv155. 10.1093/jnci/djv155. Print 2015 Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Kumon RE, Pollack MJ, Faulx AL, et al. In vivo characterization of pancreatic and lymph node tissue by using EUS spectrum analysis: A validation study. Gastrointest. Endosc. 2010;71:53–63. doi: 10.1016/j.gie.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Nune SK, Gunda P, Majeti BK, Thallapally PK, Forrest ML. Advances in lymphatic imaging and drug delivery. Adv. Drug Deliv. Rev. 2011;63:876–885. doi: 10.1016/j.addr.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Sevick-Muraca EM, Kwon S, Rasmussen JC. Emerging lymphatic imaging technologies for mouse and man. J. Clin. Invest. 2014;124:905–914. doi: 10.1172/JCI71612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Karaman S, Detmar M. Mechanisms of lymphatic metastasis. J. Clin. Invest. 2014;124:922–928. doi: 10.1172/JCI71606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Yeo CJ, Cameron JL. Prognostic factors in ductal pancreatic cancer. Langenbecks Arch. Surg. 1998;383:129–133. doi: 10.1007/s004230050104. [DOI] [PubMed] [Google Scholar]
- 341.Zuckerman DS, Ryan DP. Adjuvant therapy for pancreatic cancer: A review. Cancer. 2008;112:243–249. doi: 10.1002/cncr.23174. [DOI] [PubMed] [Google Scholar]
- 342.Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: Is there a survival difference for R1 resections versus locally advanced unresectable tumors? what is a "true" R0 resection? Ann. Surg. 2013;257:731–736. doi: 10.1097/SLA.0b013e318263da2f. [DOI] [PubMed] [Google Scholar]
- 343.Chang DK, Johns AL, Merrett ND, et al. Margin clearance and outcome in resected pancreatic cancer. J. Clin. Oncol. 2009;27:2855–2862. doi: 10.1200/JCO.2008.20.5104. [DOI] [PubMed] [Google Scholar]
- 344.Tempero MA, Malafa MP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2014: Featured updates to the NCCN guidelines. J. Natl. Compr. Canc Netw. 2014;12:1083–1093. doi: 10.6004/jnccn.2014.0106. [DOI] [PubMed] [Google Scholar]
- 345.Gresham GK, Wells GA, Gill S, Cameron C, Jonker DJ. Chemotherapy regimens for advanced pancreatic cancer: A systematic review and network meta-analysis. BMC Cancer. 2014;14 doi: 10.1186/1471-2407-14-471. 471-2407-14-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 347.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J. Clin. Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 348.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 349.Goel S, Wong AH, Jain RK. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb Perspect. Med. 2012;2:a006486. doi: 10.1101/cshperspect.a006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Sahora K, Schindl M, Kuehrer I, et al. A phase II trial of two durations of bevacizumab added to neoadjuvant gemcitabine for borderline and locally advanced pancreatic cancer. Anticancer Res. 2014;34:2377–2384. [PubMed] [Google Scholar]
- 352.Sohal DP, Metz JM, Sun W, et al. Toxicity study of gemcitabine, oxaliplatin, and bevacizumab, followed by 5-fluorouracil, oxaliplatin, bevacizumab, and radiotherapy, in patients with locally advanced pancreatic cancer. Cancer Chemother. Pharmacol. 2013;71:1485–1491. doi: 10.1007/s00280-013-2147-4. [DOI] [PubMed] [Google Scholar]
- 353.Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: Phase III trial of the cancer and leukemia group B (CALGB 80303) J. Clin. Oncol. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J. Clin. Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 355.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Tamburrino A, Piro G, Carbone C, Tortora G, Melisi D. Mechanisms of resistance to chemotherapeutic and anti-angiogenic drugs as novel targets for pancreatic cancer therapy. Front. Pharmacol. 2013;4:56. doi: 10.3389/fphar.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Chien MH, Ku CC, Johansson G, Chen MW, Hsiao M, Su JL, Inoue H, Hua KT, Wei LH, Kuo ML. Vascular endothelial growth factor-C (VEGF-C) promotes angiogenesis by induction of COX-2 in leukemic cells via the VEGF-R3/JNK/AP-1 pathway. Carcinogenesis. 2009;30:2005–2013. doi: 10.1093/carcin/bgp244. [DOI] [PubMed] [Google Scholar]
- 358.Scavelli C, Vacca A, Di Pietro G, Dammacco F, Ribatti D. Crosstalk between angiogenesis and lymphangiogenesis in tumor progression. Leukemia. 2004;18:1054–1058. doi: 10.1038/sj.leu.2403355. [DOI] [PubMed] [Google Scholar]
- 359.Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi JH, Claesson-Welsh L, Alitalo K. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14389–14394. doi: 10.1073/pnas.95.24.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Karpanen T, Wirzenius M, Makinen T, Veikkola T, Haisma HJ, Achen MG, Stacker SA, Pytowski B, Yla-Herttuala S, Alitalo K. Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation. Am. J. Pathol. 2006;169:708–718. doi: 10.2353/ajpath.2006.051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Lin J, Lalani AS, Harding TC, et al. Inhibition of lymphogenous metastasis using adenoassociated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005;65:6901–6909. doi: 10.1158/0008-5472.CAN-05-0408. [DOI] [PubMed] [Google Scholar]
- 362.He XW, Liu T, Chen YX, Cheng DJ, Li XR, Xiao Y, Feng YL. Calcium carbonate nanoparticle delivering vascular endothelial growth factor-C siRNA effectively inhibits lymphangiogenesis and growth of gastric cancer in vivo. Cancer Gene Ther. 2008;15:193–202. doi: 10.1038/sj.cgt.7701122. [DOI] [PubMed] [Google Scholar]
- 363.Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y, Pytowski B, Skobe M. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 2006;66:2650–2657. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- 364.Krishnan J, Kirkin V, Steffen A, Hegen M, Weih D, Tomarev S, Wilting J, Sleeman JP. Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res. 2003;63:713–722. [PubMed] [Google Scholar]
- 365.Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Yla-Herttuala S, Jaattela M, Alitalo K. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–1790. [PubMed] [Google Scholar]
- 366.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat. Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 367.He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J. Natl. Cancer Inst. 2002;94:819–825. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- 368.Chen Z, Varney ML, Backora MW, Cowan K, Solheim JC, Talmadge JE, Singh RK. Down-regulation of vascular endothelial cell growth factor-C expression using small interfering RNA vectors in mammary tumors inhibits tumor lymphangiogenesis and spontaneous metastasis and enhances survival. Cancer Res. 2005;65:9004–9011. doi: 10.1158/0008-5472.CAN-05-0885. [DOI] [PubMed] [Google Scholar]
- 369.Shimizu K, Kubo H, Yamaguchi K, et al. Suppression of VEGFR-3 signaling inhibits lymph node metastasis in gastric cancer. Cancer. Sci. 2004;95:328–333. doi: 10.1111/j.1349-7006.2004.tb03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Burton JB, Priceman SJ, Sung JL, Brakenhielm E, An DS, Pytowski B, Alitalo K, Wu L. Suppression of prostate cancer nodal and systemic metastasis by blockade of the lymphangiogenic axis. Cancer Res. 2008;68:7828–7837. doi: 10.1158/0008-5472.CAN-08-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Thelen A, Scholz A, Benckert C, von Marschall Z, Schroder M, Wiedenmann B, Neuhaus P, Rosewicz S, Jonas S. VEGF-D promotes tumor growth and lymphatic spread in a mouse model of hepatocellular carcinoma. Int. J. Cancer. 2008;122:2471–2481. doi: 10.1002/ijc.23439. [DOI] [PubMed] [Google Scholar]
- 372.Yang F, Jin C, Yang D, Jiang Y, Li J, Di Y, Hu J, Wang C, Ni Q, Fu D. Magnetic functionalised carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment. Eur. J. Cancer. 2011;47:1873–1882. doi: 10.1016/j.ejca.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 373.Neal J, Wakelee H. AMG-386, a selective angiopoietin-1/-2-neutralizing peptibody for the potential treatment of cancer. Curr. Opin. Mol. Ther. 2010;12:487–495. [PubMed] [Google Scholar]
- 374.Holopainen T, Saharinen P, D'Amico G, et al. Effects of angiopoietin-2-blocking antibody on endothelial cell-cell junctions and lung metastasis. J. Natl. Cancer Inst. 2012;104:461–475. doi: 10.1093/jnci/djs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Da MX, Wu Z, Tian HW. Tumor lymphangiogenesis and lymphangiogenic growth factors. Arch. Med. Res. 2008;39:365–372. doi: 10.1016/j.arcmed.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 376.Cao R, Ji H, Feng N, et al. Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc. Natl. Acad. Sci. U. S. A. 2012;109:15894–15899. doi: 10.1073/pnas.1208324109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Chen HM, Tsai CH, Hung WC. Foretinib inhibits angiogenesis, lymphangiogenesis and tumor growth of pancreatic cancer in vivo by decreasing VEGFR-2/3 and TIE-2 signaling. Oncotarget. 2015;6:14940–14952. doi: 10.18632/oncotarget.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Heckman CA, Holopainen T, Wirzenius M, Keskitalo S, Jeltsch M, Yla-Herttuala S, Wedge SR, Jurgensmeier JM, Alitalo K. The tyrosine kinase inhibitor cediranib blocks ligand-induced vascular endothelial growth factor receptor-3 activity and lymphangiogenesis. Cancer Res. 2008;68:4754–4762. doi: 10.1158/0008-5472.CAN-07-5809. [DOI] [PubMed] [Google Scholar]
- 379.Padera TP, Kuo AH, Hoshida T, Liao S, Lobo J, Kozak KR, Fukumura D, Jain RK. Differential response of primary tumor versus lymphatic metastasis to VEGFR-2 and VEGFR-3 kinase inhibitors cediranib and vandetanib. Mol. Cancer. Ther. 2008;7:2272–2279. doi: 10.1158/1535-7163.MCT-08-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Grunwald V, Merseburger AS. Axitinib for the treatment of patients with advanced metastatic renal cell carcinoma (mRCC) after failure of prior systemic treatment. Onco Targets Ther. 2012;5:111–117. doi: 10.2147/OTT.S23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Spano JP, Chodkiewicz C, Maurel J, et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: An open-label randomised phase II study. Lancet. 2008;371:2101–2108. doi: 10.1016/S0140-6736(08)60661-3. [DOI] [PubMed] [Google Scholar]
- 382.Rixe O, Bukowski RM, Michaelson MD, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: A phase II study. Lancet Oncol. 2007;8:975–984. doi: 10.1016/S1470-2045(07)70285-1. [DOI] [PubMed] [Google Scholar]
- 383.Reataza M, Imagawa DK. Advances in managing hepatocellular carcinoma. Front. Med. 2014;8:175–189. doi: 10.1007/s11684-014-0332-4. [DOI] [PubMed] [Google Scholar]
- 384.Procopio G, Verzoni E, Testa I, Nicolai N, Salvioni R, Debraud F. Experience with sorafenib in the treatment of advanced renal cell carcinoma. Ther. Adv. Urol. 2012;4:303–313. doi: 10.1177/1756287212457216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 386.Khagi S, Saif MW. Pancreatic neuroendocrine tumors: Targeting the molecular basis of disease. Curr. Opin. Oncol. 2015;27:38–43. doi: 10.1097/CCO.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 387.Detry B, Blacher S, Erpicum C, et al. Sunitinib inhibits inflammatory corneal lymphangiogenesis. Invest. Ophthalmol. Vis. Sci. 2013;54:3082–3093. doi: 10.1167/iovs.12-10856. [DOI] [PubMed] [Google Scholar]
- 388.Mankal P, O'Reilly E. Sunitinib malate for the treatment of pancreas malignancies–where does it fit? Expert Opin. Pharmacother. 2013;14:783–792. doi: 10.1517/14656566.2013.776540. [DOI] [PubMed] [Google Scholar]
- 389.Kodera Y, Katanasaka Y, Kitamura Y, Tsuda H, Nishio K, Tamura T, Koizumi F. Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3. Breast Cancer Res. 2011;13:R66. doi: 10.1186/bcr2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Ahn HK, Choi JY, Kim KM, et al. Phase II study of pazopanib monotherapy in metastatic gastroenteropancreatic neuroendocrine tumours. Br. J. Cancer. 2013;109:1414–1419. doi: 10.1038/bjc.2013.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Verweij J, Sleijfer S. Pazopanib, a new therapy for metastatic soft tissue sarcoma. Expert Opin. Pharmacother. 2013;14:929–935. doi: 10.1517/14656566.2013.780030. [DOI] [PubMed] [Google Scholar]
- 392.Schutz FA, Choueiri TK, Sternberg CN. Pazopanib: Clinical development of a potent antiangiogenic drug. Crit. Rev. Oncol. Hematol. 2011;77:163–171. doi: 10.1016/j.critrevonc.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 393.Baker CH, Solorzano CC, Fidler IJ. Blockade of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling for therapy of metastatic human pancreatic cancer. Cancer Res. 2002;62:1996–2003. [PubMed] [Google Scholar]
- 394.Solorzano CC, Baker CH, Bruns CJ, Killion JJ, Ellis LM, Wood J, Fidler IJ. Inhibition of growth and metastasis of human pancreatic cancer growing in nude mice by PTK 787/ZK222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Cancer Biother. Radiopharm. 2001;16:359–370. doi: 10.1089/108497801753354267. [DOI] [PubMed] [Google Scholar]
- 395.Sini P, Samarzija I, Baffert F, et al. Inhibition of multiple vascular endothelial growth factor receptors (VEGFR) blocks lymph node metastases but inhibition of VEGFR-2 is sufficient to sensitize tumor cells to platinum-based chemotherapeutics. Cancer Res. 2008;68:1581–1592. doi: 10.1158/0008-5472.CAN-06-4685. [DOI] [PubMed] [Google Scholar]
- 396.Lin B, Podar K, Gupta D, et al. The vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584 inhibits growth and migration of multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2002;62:5019–5026. [PubMed] [Google Scholar]
- 397.Drevs J, Hofmann I, Hugenschmidt H, Wittig C, Madjar H, Muller M, Wood J, Martiny-Baron G, Unger C, Marme D. Effects of PTK787/ZK 222584, a specific inhibitor of vascular endothelial growth factor receptor tyrosine kinases, on primary tumor, metastasis, vessel density, and blood flow in a murine renal cell carcinoma model. Cancer Res. 2000;60:4819–4824. [PubMed] [Google Scholar]
- 398.Dragovich T, Laheru D, Dayyani F, et al. Phase II trial of vatalanib in patients with advanced or metastatic pancreatic adenocarcinoma after first-line gemcitabine therapy (PCRT O4-001) Cancer Chemother. Pharmacol. 2014;74:379–387. doi: 10.1007/s00280-014-2499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: A double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–262. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 400.Cardin DB, Goff L, Li CI, et al. Phase II trial of sorafenib and erlotinib in advanced pancreatic cancer. Cancer. Med. 2014;3:572–579. doi: 10.1002/cam4.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Goncalves A, Gilabert M, Francois E, et al. BAYPAN study: A double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann. Oncol. 2012;23:2799–2805. doi: 10.1093/annonc/mds135. [DOI] [PubMed] [Google Scholar]
- 402.Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, Pytowski B, Fukumura D, Padera TP, Jain RK. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: Therapeutic implications. Cancer Res. 2006;66:8065–8075. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- 403.Witte MH, Dellinger MT, McDonald DM, Nathanson SD, Boccardo FM, Campisi CC, Sleeman JP, Gershenwald JE. Lymphangiogenesis and hemangiogenesis: Potential targets for therapy. J. Surg. Oncol. 2011;103:489–500. doi: 10.1002/jso.21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.O'Hagan D, Christy N, Davis S. Particulates and lymphatic drug delivery. In: Charman W, Stella V, editors. Lymphatic Transport of Drugs. Boca Raton, FL, USA: CRC Press Inc; 1992. pp. 279–280.pp. 315 [Google Scholar]
- 405.Singh I, Swami R, Khan W, Sistla R. Lymphatic system: A prospective area for advanced targeting of particulate drug carriers. Expert Opin. Drug Deliv. 2014;11:211–229. doi: 10.1517/17425247.2014.866088. [DOI] [PubMed] [Google Scholar]
- 406.Ali Khan A, Mudassir J, Mohtar N, Darwis Y. Advanced drug delivery to the lymphatic system: Lipid-based nanoformulations. Int. J. Nanomedicine. 2013;8:2733–2744. doi: 10.2147/IJN.S41521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Li X, Dong Q, Yan Z, Lu W, Feng L, Xie C, Xie Z, Su B, Liu M. MPEG-DSPE polymeric micelle for translymphatic chemotherapy of lymph node metastasis. Int. J. Pharm. 2015;487:8–16. doi: 10.1016/j.ijpharm.2015.03.074. [DOI] [PubMed] [Google Scholar]
- 408.Kaminskas LM, Ascher DB, McLeod VM, Herold MJ, Le CP, Sloan EK, Porter CJ. PEGylation of interferon alpha2 improves lymphatic exposure after subcutaneous and intravenous administration and improves antitumour efficacy against lymphatic breast cancer metastases. J. Control. Release. 2013;168:200–208. doi: 10.1016/j.jconrel.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Dunne AA, Boerner HG, Kukula H, Schlaad H, Wiegand S, Werner JA, Antonietti M. Block copolymer carrier systems for translymphatic chemotherapy of lymph node metastases. Anticancer Res. 2007;27:3935–3940. [PubMed] [Google Scholar]


