Abstract
We have recently reported the expression of functional cannabinoid CB2 receptors (CB2Rs) in midbrain dopamine (DA) neurons in mice. However, little is known whether CB2Rs are similarly expressed in rat brain since significant species differences in CB2 receptor structures and expression are found. In situ hybridization and immunohistochemical assays detected CB2 gene and receptors in DA neurons of the ventral tegmental area (VTA), which was up-regulated in cocaine self-administration rats. Electrophysiological studies demonstrated that activation of CB2Rs by JWH133 inhibited VTA DA neuronal firing in single dissociated neurons. Systemic administration of JWH133 failed to alter, while local administration of JWH133 into the nucleus accumbens inhibited cocaine-enhanced extracellular DA and intravenous cocaine self-administration. This effect was blocked by AM630, a selective CB2 receptor antagonist. These data suggest that CB2Rs are expressed in VTA DA neurons and functionally modulate DA neuronal activities and cocaine self-administration behavior in rats.
Keywords: Cocaine, dopamine, JWH133, cannabinoid, CB2 receptor, self-administration
Introduction
Marijuana has been used by humans for centuries for medical and recreational purposes. However, the neurobiological mechanisms underlying marijuana’s actions are still not fully understood (Maldonado et al., 2011). Δ9-tetrahydrocannabinol (Δ9-THC) is the major psychoactive ingredient of marijuana (Mechoulam and Parker, 2012). The pharmacological action of Δ9-THC is mediated predominantly by activation of cannabinoid CB1 and CB2 receptors (CB1Rs and CB2Rs) (Maldonado et al., 2011; Mechoulam and Parker, 2012). Since CB1Rs are highly expressed in the brain (Glass et al., 1997; Glass and Felder, 1997; Mackie, 2005, 2008) , while CB2Rs are found mainly in peripheral tissues (Griffin et al., 2000; Munro et al., 1993), it has heretofore been generally believed that the central psychoactive effects of Δ9-THC or cannabis are mediated by activation of brain CB1Rs (Howlett et al., 2002; Maldonado et al., 2011). However, this long-held view has been challenged by recent findings that CB2Rs are also expressed in mouse brain and functionally involved in several dopamine (DA)-related CNS disorders such as Parkinson’s disease (Garcia et al., 2015; Ortega-Alvaro et al., 2011), depression (Garcia-Gutierrez et al., 2010), anxiety (Garcia-Gutierrez et al., 2011, 2012; Garcia-Gutierrez and Manzanares, 2011), and schizophrenia (Navarrete et al., 2012; Ortega-Alvaro et al., 2011).
In addition, brain CB2Rs are recently reported to be involved in drug abuse and addiction. Systemic or intracranial local administration of CB2R agonists inhibits cocaine or alcohol self-administration, cocaine- or alcohol-induced conditioned place preference (CPP), and alcohol-induced locomotor sensitization in mice (Al Mansouri et al., 2014; Ignatowska-Jankowska et al., 2013; Ortega-Alvaro et al., 2015; Xi et al., 2011; Zhang et al., 2015; Zhang et al., 2014). Congruently, overexpression of CB2Rs in mouse brain decreases cocaine self-administration and cocaine-enhanced locomotion (Aracil-Fernandez et al., 2012). Further, genetic deletion of CB2Rs (CB2-KO mice) caused increased response to ethanol (Ortega-Alvaro et al., 2015), but decreased response to nicotine in the CPP and self-administration paradigms (Ignatowska-Jankowska et al., 2013; Navarrete et al., 2013). More recent research suggests that CB2Rs are expressed in midbrain DA neurons in mice and activation of CB2Rs in the VTA inhibits DA neuronal firing and cocaine self-administration (Aracil-Fernandez et al., 2012; Xi et al., 2011; Zhang et al., 2015; Zhang et al., 2014). In addition, CB2Rs were also expressed in pyramidal neurons and modulate excitatory synaptic transmission in the hippocampus of mice (Kim and Li, 2015; Li and Kim, 2015).
In contrast to the above findings in mice, systemic administration of CB2R agonists or antagonists fail to alter cocaine or nicotine self-administration under fixed-ratio reinforcement schedules in rats (Adamczyk et al., 2012; Gamaleddin et al., 2012). Paradoxically, CB2R antagonists were reported to attenuate cocaine-induced reinstatement of drug-seeking behavior (Adamczyk et al., 2012) and cocaine-induced conditioned locomotion in rats (Blanco-Calvo et al., 2014). The reasons underlying such conflicting findings in rats and mice are unclear. We have recently reported species differences in the structure and expression of CB2 genes and receptors in rats and mice (Liu et al., 2009; Zhang et al., 2015), suggesting that the findings observed in one species (mice) cannot be simply generalized to another species (rats). One hypothesis is that brain CB2Rs may have different cellular distributions in the brains of rats and mice, and therefore, produce different behavioral effects in the above experiments. To the best of our knowledge, there is no evidence demonstrating whether CB2Rs are expressed in VTA DA neurons in rats.
In the present study, we first used duplex in situ hybridization, quantitative RT-PCR, and double-label immunohistochemistry (IHC) assays to study CB2R gene and receptor expression in the brain and in VTA DA neurons in rats. We then examined whether cocaine self-administration alters brain CB2R expression and whether the selective CB2R agonist JWH133 alters VTA DA neuronal firing in single dissociated neurons. Lastly, we examined whether systemic or local administration of JWH133 into the nucleus accumbens (NAc) alters cocaine-enhanced extracellular DA and intravenous cocaine self-administration in rats.
Materials and Methods
Animals
Male Long-Evans rats (Charles River, Raleigh, NC) were used in the present study. Animals were housed in a fully-accredited animal facility and were maintained with food and water available in the home cage. The experimental procedures followed the Guide for the Care and Use of Laboratory Animals of the U.S. National Research Council (1996) and were approved by the Animal Care and Use Committee of the National Institute on Drug Abuse or by the Institutional Animal Care and Use Committee of the Barrow Neurological Institute (for the electrophysiological experiments). Wistar rats were used for the electrophysiological experiments based on the availability of this strain in Dr. Wu’s laboratory. This should not be a concern since there is little evidence demonstrating that CB2Rs display strain differences in receptor structure and function (Zhang et al., 2015).
Experiment 1: RNAscope In situ Hybridization (ISH) Assay
Drug naïve rats and cocaine self-administration rats (see procedures for cocaine self-administration below under “Experiment 6” were deeply anaesthetized 24 hours after the last cocaine self-administration with 100 mg/kg pentobarbital and transcardially perfused with saline to remove all blood from the brain. Whole brain tissues were removed and snap frozen on dry ice. The fresh frozen tissue sections (12 µm thick) were mounted on positively charged microscopic glass slides (Thermo Fisher Scientific, Waltham, MA). Both the CB2R RNA probe (1935–2843 bp of the Rattus norvegicus CB2R, located on 3′ UTR, NM001164142.2) and dopamine transporter (DAT) probe [827–1913 bp of Rattus norvegicus solute carrier family 6, NM_012694.2] were designed and provided by Advanced Cell Diagnostics, Inc. (Hayward, CA). The experimental procedures followed the manufacturer’s instructions. Stained slides were cover-slipped with fluorescent mounting medium (ProLong Gold Antifade Reagent, P36930, Thermo Fisher Scientific, Waltham, MA) and scanned into digital images using an Olympus FluoView FV1000 confocal microscope (Olympus Corporation of the Americas, Center Valley, PA). For each sample, three adjacent sections were stained using the CB2 and DAT RNAscope probes. Ubiquitin C (UBC) was used as an endogenous positive control to assess RNA probe integrity. The bacterial gene dapB served as a negative control to assess background staining (Wang et al., 2012).
RNAscope fluorescent images were captured at 40× magnification using manufacturer-provided software on an Olympus FluoView FV1000 confocal microscope. Images were saved as 16-bit TIFF files and enhanced by using Adobe Photoshop CS5 software for analysis. ImageJ software was used to analyze the fluorescent particles. The same parameters were used to quantify the fluorescent particles or fluorescent “dots”. Individual DA neurons were identified based on DAT staining. Pixels in each DA neuron were counted. Each pixel is assumed to represent a single molecule of CB2 mRNA (Wang et al., 2012).
Experiment 2: Quantitative RT-PCR
The general procedures for quantitative real-time polymerase chain reaction (qRT-PCR) assay of brain CB2 mRNA were as described previously (Liu et al., 2009). Three groups of rats (i.e., drug naïve, oral sucrose self-administration, and intravenous cocaine self-administration) were used to study the effects of cocaine or sucrose self-administration on brain CB2 mRNA expression. After 4 weeks of intravenous cocaine (0.5 mg/kg/infusion, 3 hrs per session) or oral sucrose (0.1 ml 5% sucrose solution per delivery) self-administration, rat brains were perfused with saline at 24 hrs after the last cocaine or sucrose self-administration. Brains were then removed, and the prefrontal cortex, striatum, and midbrain were dissected. A rat CB2 TaqMan probe (TGGGCCCAGTCCT) that recognizes the conjunction region of encoding exons 2 and 3 was used to detect CB2 mRNA expression in each brain region using the forward primer (GCCACCCAGCAAACATCTAT) and reverse primer (GACAGGCTTTGGCTGCTTCTAC). Rat β-actin-mRNA was used as an endogenous control.
Experiment 3: Immunohistochemistry (IHC) Assay
We used the Alomone CB2R antibody (ACR-002, Alomone Labs, Jerusalem, Israel) to detect CB2R-immunostaining in VTA DA neurons. The epitope of this antibody is located at the intracellular 3rd loop (residues 228–242). Compared to many other antibodies, this antibody displayed a high degree of CB2R specificity (Zhang et al., 2014).
After brain perfusion, the brains were removed and placed in 20% sucrose phosphate buffer at 4°C overnight. Coronal sections were cut at 10 µm on a cryostat (CM3050S, Leica Microsystems, Nussloch, Germany). Midbrain sections containing the VTA were blocked and floated in 5% BSA and 0.5% Triton X-100 phosphate buffer for 2 hr at room temperature before being incubated with primary antibodies.
IHC assays were performed using Alomone CB2 antibody (1:250) and TH-antibody (1:500) to detect CB2R-immunostaining in VTA DA neurons, astrocytes or microglia, respectively. After washes, sections were further incubated with a mixture of the secondary antibodies – Alexa Fluor 488 goat anti-rabbit for CB2Rs and Alexa Fluor 568 goat anti-mouse for TH – in 5% BSA and 0.5% Triton X-100 phosphate buffer at room temperature for 2 hrs. Sections were then washed, mounted, and cover slipped. Fluorescent images were taken with a fluorescence microscope (Nikon Eclipse 80i, Melville, NY, USA) or an Olympus FluoView FV1000 confocal microscope (Olympus Corporation of the Americas, Center Valley, PA).
Experiment 4: Electrophysiology Study
To determine whether functional CB2Rs are expressed in VTA DA neurons in rats, we employed perforated (amphotericin B) patch-clamp recording in single dissociated VTA DA neurons, which were obtained through an enzymatic/mechanical dissociation process as described previously (Wu et al., 2004; Yang et al., 2009). Phenotypic identification of DA neurons was based on the following three criteria: i) electrophysiology: DA neurons exhibit low spontaneous firing rates (1–3 Hz) with long action potential (AP) duration and a distinctive H-current; ii) pharmacology: DA neuron firing is inhibited by DA or D2 receptor agonists; and iii) IHC staining: recorded neurons were TH-positive. Coronal midbrain slices (400 µm thickness) were prepared from 14- to 21-day old rats as described previously (Wu et al., 2004; Yang et al., 2009). Briefly, under isoflurane anesthesia, brain tissue was quickly removed and bathed in ice-cold (4°C) artificial cerebrospinal fluid (aCSF) that was composed of (mM): 124 NaCl, 3 KCl, 26 NaHCO3, 2.0 MgSO4, 1.2 NaH2PO4, 2.4 CaCl2, and 10 glucose; bubbled with 95% O2-5% CO2 (carbogen). Using a vibratome 1000 (Vibratome 1000 plus; Jed Pella Inc., Redding, CA), several 400 µm coronal sections containing the VTA were cut, transferred to a pre-incubation chamber and incubated at room temperature (22 ± 1°C) in aCSF for at least 60 min. Then, brain slices were incubated in aCSF containing 1 mg/6 mL pronase (Calchem, La Jolla, CA, USA) at 31°C for 25–35 min. The VTA area from each slice was then punched out using a well-polished needle punch. Each punched tissue fragment was transferred to a 35-mm culture dish filled with well-oxygenated standard extracellular solution composed of (mM): 150 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose and 10 HEPES; pH adjusted to 7.4 with Tris-base. Each tissue fragment was then mechanically dissociated using a fire-polished micro-Pasteur pipette. Isolated single cells usually adhered to the bottom of the dish within 30 min and were then used for patch-clamp recording at room temperature within 4 hours. Perforated-patch whole-cell recording techniques (Wu et al., 2004; Yang et al., 2009) were employed to determine the effects of JWH133 and/or AM630 on VTA DA neuronal firing under current-clamp recording mode. Pipettes (3–5 MΩ) used for perforated-patch recordings were filled with intracellular recording solution, which contained (mM): 140 K-gluconate, 10 KCl, 5 MgCl2, and 10 HEPES; pH 7.2 (with Tris-OH) freshly supplemented before use with amphotericin B to 200 µg/mL from a 40 mg/mL DMSO stock. After tight seal (>2 G) formation, it usually took about 5–20 min to convert to perforated-patch mode, and an access resistance of 20–60 MΩ was accepted to initiate experiments. Series resistance was not compensated in this study. Data were filtered at 2 kHz, acquired at 10 kHz and digitized on-line (Digidata 1322 series A/D board, Axon Instruments, Foster City, CA). After stable recording for several minutes (baseline), JWH133 or AM630 was bath-applied to the recorded neuron for 1–2 min and then washed out. Rapid application of drugs was performed using a computer-controlled "U-tube" system that allowed for complete exchange of solution surrounding the recorded cell within 30 ms. Data were acquired by Clamplex9.2 (Axon Instruments) via a Digidata 1322 series A/D board set to a sampling frequency of 10 kHz, filtered in Clampfit 9.2 using an 8-pole Bessel filter and a 1-kHz low-pass filter, and stored on hard media for subsequent off-line analysis.
Experiment 5: In vivo Microdialysis
Surgery
Naive rats were prepared for experimentation by biolateral surgical implantation of intracranial guide cannulae (20 gauge, 14 mm; Plastics One, Roanoke, VA, USA) into NAc (AP +1.6, ML ± 2.0, DV −4.0 mm, 6° from vertical) under sodium pentobarbital anaesthesia (65 mg/kg, i.p.) using standard aseptic surgical techniques. The intracranial guide cannulae were fixed to the skull with 4 stainless steel jeweler’s screws (Small Parts Inc., Miami Lakes, FL, USA) and dental acrylic.
Microdialysis procedure
The night before the experiment, concentric microdialysis probes (each with 2 mm of dialysis membrane at the tip) were inserted 2 mm beyond the tips of the guide cannulae into the NAc. At 12 hrs after probe implantation, dialysis buffer (5 mM KCl, 140 mM NaCl, 1.4 mM CaCl2 1.2 mM MgCl2, 5.0 mM glucose, plus 0.2 mM phosphate-buffered saline to give a pH of 7.4) was perfused through the probe at a rate of 2 µL/min via a syringe pump (Bioanalytical Systems, W. Lafayette, IN). At 2 hrs after start of perfusion, microdialysis samples were collected every 20 min into 10 µl 0.1 M perchloric acid to prevent DA degradation. After collection, samples were frozen at −80°C. Dialysate DA was measured using high pressure liquid chromatography (HPLC) with electrochemical detection, as we have reported previously (Li et al., 2009).
Quantification of DA
Dialysate DA was measured using the ESA electrochemical detection system (ESA Inc., Chelmsford, MA). The DA mobile phase contained 4.76 mM citric acid, 150 mM Na2HPO4, 3 mM sodium dodecyl sulfate, 50 mM EDTA, 10% methanol, and 15% acetylnitrile, pH5.6. DA was separated using an ESA MD-150 × 3.2 mm reverse phase column and oxidized/reduced using an ESA Coulochem III detector. Three electrodes were used: a pre-injection port guard cell (+0.25 V) to oxidize the mobile phase, a reduction analytical electrode (E1, −0.1V), and an oxidation analytical electrode (E2, 0.2 V). The area under curve (AUC) of the DA peak was measured using an “EZChrom Elite” ESA chromatography data system. DA values were quantified with an external standard curve (generated by three standard concentrations: 10, 100, 1000 pM). The limit of detection for DA was ~1 pM.
Experiment 6: Intravenous Cocaine Self-Administration
Surgery
Animals were prepared for experimentation by surgical catheterization of the right external jugular vein. The jugular catheters were constructed of microrenathane (Braintree Scientific Inc., Braintree, MA, USA), and catheterization was performed under sodium pentobarbital anaesthesia (65 mg/kg, i.p.) using standard aseptic surgical techniques. To determine loci of action in rat brain, 2 additional groups of rats were also surgically implanted with intracranial guide cannulae (20 gauge, 14 mm; Plastics One, Roanoke, VA) into the NAc (shell) (AP +1.7 mm, ML ±2.0 mm, DV −5.0 mm, 6° angle from vertical), with intracranial target coordinates based on the atlas of Paxinos and Watson. Both the self-administration cannulae and intracranial guide cannulae were fixed to the skull with 4 stainless steel jeweler’s screws (Small Parts Inc., Miami Lakes, FL) and dental acrylic. During experimental sessions, each self-administration catheter was connected to an injection pump via tubing encased in a protective metal spring from the head-mounted connector to the top of the experimental chamber. To help prevent clogging, the catheters were flushed daily with a gentamicin-heparin-saline solution (0.1 mg/ml gentamicin, 30 IU/ml heparin; ICN Biochemicals, Cleveland, OH).
Self-administration apparatus
Intravenous (i.v.) self-administration experiments were conducted in operant response test chambers (32 × 25 × 33 cm) from Med Associates Inc. (Georgia, VT). Each test chamber had 2 levers: 1 active and 1 inactive, located 6.5 cm above the floor. Pressing the active lever activated the infusion pump; Pressing the inactive lever was counted but had no consequence. A cue light and a speaker were located 12 cm above the active lever. The house light was turned on at the start of each 3 hr test session. Scheduling of experimental events and data collection was accomplished using Med Associates software.
Self-administration procedure
After recovery from surgery, each rat was placed into a test chamber (day time - dark phase) and allowed to lever-press for i.v. cocaine (1 mg/kg/infusion) delivered in 0.08 ml over 4.6 sec, on a fixed ratio 1 (FR1) reinforcement schedule. Each cocaine infusion was associated with presentation of a stimulus light and tone. During the 4.6 sec infusion time, additional responses on the active lever were recorded but did not lead to additional infusions. Each session lasted 3 hr. FR1 reinforcement was used for 3–5 days until stable cocaine self-administration was established: operationally defined as a minimum of 20 presses on the active lever per test session, less than 10% variability in inter-response interval, less than 10% variability in number of infusions taken, and less than 10% variability in number of presses on the active lever for at least 3 consecutive days. Subjects were then allowed to continue self-administration cocaine (0.5 mg/kg/infusion) under FR2 reinforcement. This dose of cocaine was chosen based on previous studies showing that 0.5 mg/kg/infusion of cocaine lies within the middle range of the descending limb of the cocaine dose-response self-administration curve, where reliable dose-dependent effects are observed (Xi et al., 2005). To avoid cocaine overdose, each animal was limited to a maximum of 50 cocaine injections per 3hr session.
Evaluating the effects of JWH133 on cocaine self-administration
We have recently reported that systemic administration of JWH133 or AM630 failed to alter cocaine self-administration (Zhag et al., 2015). In this experiment, we further observed the effects of local administration of JWH133 into the NAc on cocaine self-administration under FR2 (within-subjects design, n=10) or progressive-ratio (within-subjects design, n=7) reinforcement, respectively. On the test day, each animal randomly received bilateral intracranial microinjections of vehicle or one of two doses of JWH133 (1 or 3 µg/µl/side, 30 min prior to test). A total of 0.5 µl was injected into each NAc over 1 min, and the injectors were then kept in place for an additional 1 min. The doses of JWH133 were based on pilot preliminary observations and a previous study (Xi et al., 2011). Cannula placements were verified after the completion of experiments by standard histological and anatomic localization techniques.
Sucrose self-administration
To determine whether brain CB2R up-regulation is cocaine-specific and whether drug self-administration procedure alters CB2R expression, we further observed the effects of operant sucrose self-administration on brain CB2R mRNA expression in rats. The sucrose self-administration procedures were identical to the procedures for cocaine self-administration except that no i.v. surgery was performed on the animals in the sucrose experiment and that active lever presses led to delivery of 0.1 ml of 5% sucrose solution into a liquid food tray on the operant chamber wall.
Drugs
Cocaine HCl (Sigma-Aldrich) was dissolved in physiological saline. JWH133 and AM630 were obtained from Tocris Bioscience division of Biotechnology (Minneapolis, MN) and dissolved in Tocrisolve-100 (vehicle).
Statistical Analysis
Data are presented as means ± S.E.M.. One-way analysis of variance (ANOVA) was used to determine the significance of changes in each measurement described above. Post-hoc individual group comparisons were carried out using the Student-Newman Keuls method.
Results
CB2 mRNA is expressed in rat VTA DA neurons
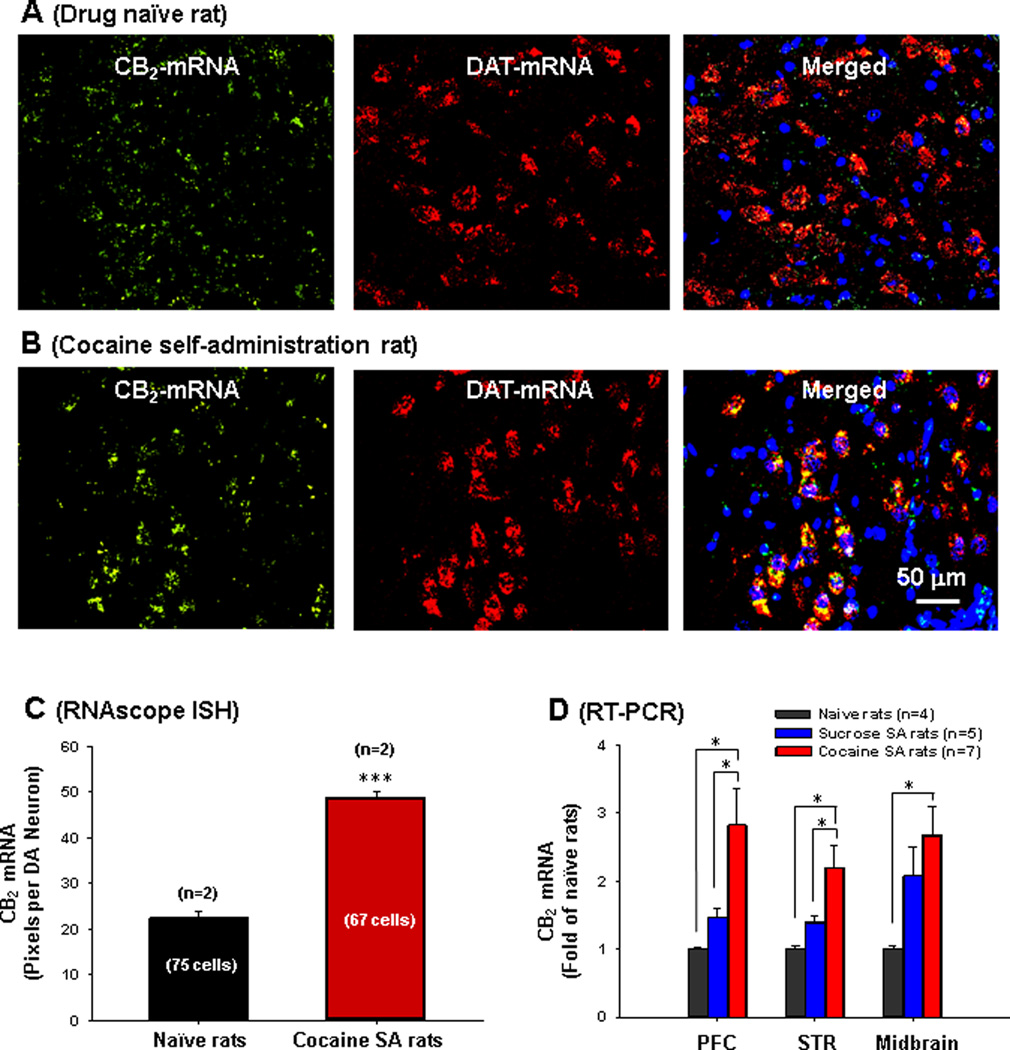
To study whether rat CB2 (rCB2) gene is present in VTA DA neurons, we used a novel, highly sensitive RNA in situ hybridization (ISH) method (RNAscope) (Wang et al., 2012). We initially designed 3 RNAscope probes that target different gene sequences. We found only one probe that targets the 3′-untranslated region (UTR) to work well. This probe is long (908 bp) and displays high rCB2 specificity since it does not hybridize with the CB1 or other genes as determined by cross-checking the host genomic background (Advanced Cell Diagnositics, Inc., Hayward, CA). Figure 1 shows CB2 mRNA-staining (green dots) in VTA DA (DAT-mRNA-positive) neurons (yellow dots in merged images) and also in VTA non-DA cells (DAT-mRNA-negative, green dots around blue nuclei in merged images) in drug naïve (Fig. 1A) and in cocaine self-administration (Fig. 1B) rats. CB2 mRNA and DAT-mRNA are co-localized in VTA DA neurons. Quantitative dot/pixel counting indicated that cocaine self-administration significantly up-regulated CB2 mRNA expression in VTA DA neurons (Fig. 1C, F1, 140=126.78, p<0.001). To further confirm this finding, we used quantitative RT-PCR to compare CB2 mRNA expression in various brain regions. We found that cocaine, but not sucrose, self-administration significantly up-regulated CB2 mRNA expression in prefrontal cortex (PFC, F2, 13=6.2, p<0.05), striatum (including the NAc, F2, 13=6.55, p<0.05) and VTA-containing midbrain tissues (Fig. 1D, F2, 13=5.23, p<0.05).
Figure 1.
RNA in situ hybridization results, illustrating cannabinoid CB2 mRNA expression (green) in VTA DA neurons of naïve rats (A) and cocaine self-administration rats (B) measured by RNAscope ISH. A DAT probe was used to label VTA DA neurons (red) and DAPI was used to label cell nuclei (blue). C: Quantitative assay results in RNAscope ISH assays, illustrating that cocaine self-administration significantly increased CB2 mRNA expression in VTA DA neurons. D: Quantitative RT-PCR results, illustrating that chronic cocaine self-administration (4 weeks) significantly up-regulated CB2 mRNA expression in prefrontal cortex (PFC), striatum (STR) and midbrain. *p<0.05, ***p<0.001, compared with naïve rats or sucrose SA rats.
CB2R proteins are expressed in VTA DA neurons
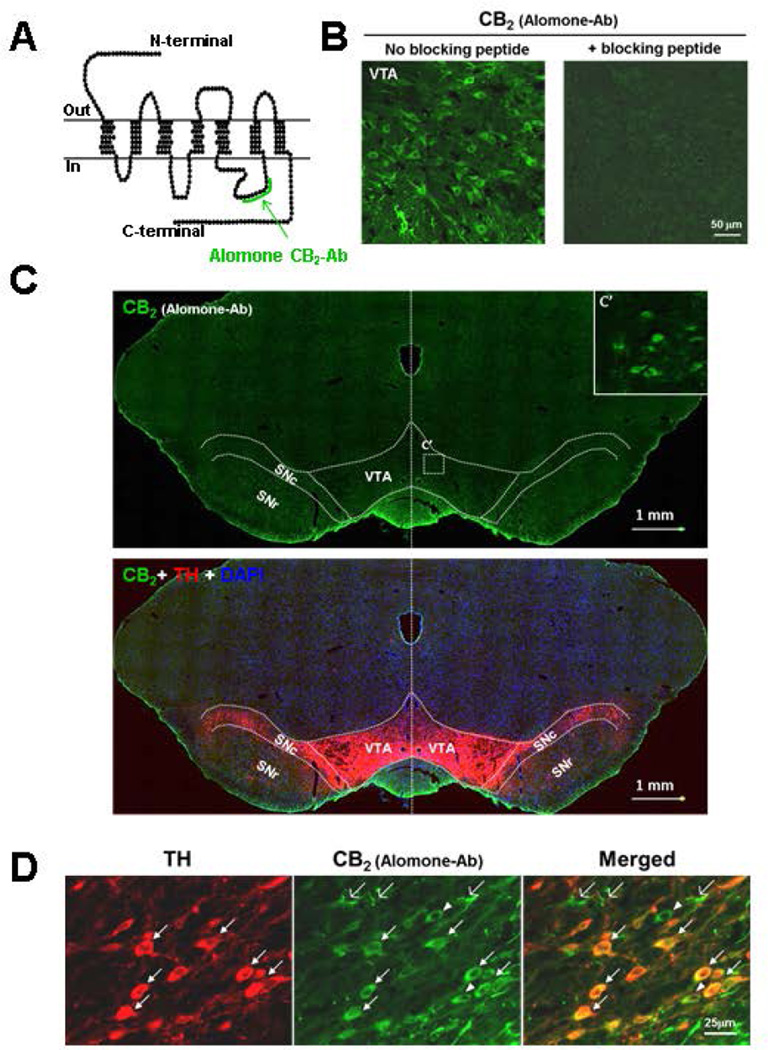
We then examined CB2R protein expression in VTA DA neurons using IHC assays. Figure 2A shows the binding site (epitope) of the Alomone rCB2 antibody used to detect CB2-immunostaining in this study. Figure 2B shows rCB2-immunostaining with this antibody in the absence or presence of immune peptide, illustrating that rCB2R-staining was detected in VTA neurons and was blocked by an immune peptide that preabsorbed the rCB2 antibody. Figure 2C shows rCB2 and tyrosine hydroxylase (TH) immunostaining in the whole midbrain, illustrating that rCB2-immunostaining is very weak or undetectable under low magnification (10×) in the VTA and substantia nigra pars compacta (SNc) of rats. However, under high magnification (40×), rCB2-immunostaining was clearly seen in neuronal cells in the VTA (see the inserted image in Fig. 2C – upper panel). Figure 2–D/E shows double-label immunostaining, illustrating that CB2-immunostaining is co-localized with TH in VTA DA neurons (TH-positive, white arrows). In addition, CB2R-immunostaining is also seen in TH-negative non-DA neurons (white arrow-heads).
Figure 2.
CB2R-immunostaining in VTA of rats, illustrating the binding sites (epitopes) of Alomone rCB2 antibody on CB2Rs (A). Preabsorption of Alomone rCB2-antibody by immune peptide blocked rCB2-staining in VTA DA neurons (B). CB2R- and tyrosine hydroxylase (TH)-staining in midbrain VTA and SNc under low magnification (10×) (C). D: The Alomone rCB2R-antibody detected rCB2R-immunostaining under high magnification (40×) in VTA DA neurons (TH-positive, marked by white filled arrows), non-DA neurons (TH-negative, green cells indicated by white triangles) and glial cells (indicated by white open arrows). Ab=antibody.
Activation of CB2Rs inhibits VTA DA neuronal firing
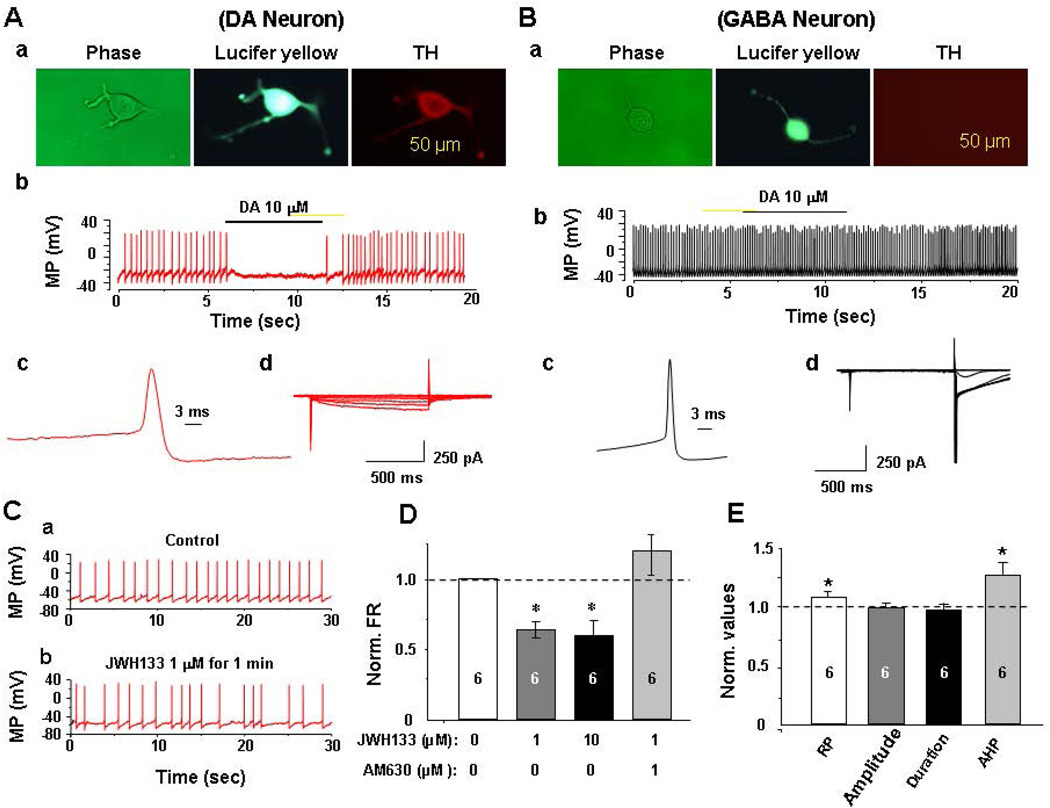
Next, we examined whether activation of CB2Rs in the VTA functionally alters neuronal firing in single dissociated VTA DA neurons using patch-clamp recording. Identification of DA neurons was based on the following three criteria (Wu et al., 2004; Yang et al., 2009): 1) electrophysiology: DA neurons exhibited low spontaneous firing rates (1–3 Hz, Fig. 3Ab) with long action potential (AP) duration (Fig. 3A–c), and a distinctive H-current (Fig. 3A–d), while GABA neurons exhibited relatively high firing rates (Fig. 3Bb) with short AP duration (Fig. 3B–c), and no H-current (Fig. 3B–d); 2) pharmacology: DA (or D2 receptor agonist, not shown here) administration inhibited DA (Fig. 3A–b), but not GABA (Fig. 3B–b), neuronal firing; and 3) IHC staining: the recorded neuron was labeled with Lucifer yellow and showed positive TH-immunostaining (Fig. 3A–a); the GABA neurons showed TH-negative (Fig. 3B–a). Figure 3C shows representative recording of low firing rate of a DA neuron (~1 Hz) (Fig. 3C–a); bath-applied JWH133 (1 µM) reduced the neuronal firing rate (Fig. 3C–b). Figure 3D shows that JWH133 dose-dependently inhibited VTA DA neuronal firing, an effect that was blocked by co-administration of AM630 (a selective CB2R antagonist, 1 µM). Figure 3E shows that JWH133 (1 µM) hyperpolarized the resting membrane potential (RP) and augmented the after-hyperpolarization potential (AHP), but had no effect on action potential amplitude or duration.
Figure 3.
Electrophysiological assays in rats, illustrating that activation of CB2Rs by JWH133 inhibits neuronal firing in single dissociated VTA DA neurons. A: Identification of VTA DA neurons, illustrating a representative dissociated DA neuron (phase contrast, Lucifer yellow labeled and TH stained images, Aa), the effect of DA on neuronal firing (Ab), and on action potential (AP) profiles. VTA DA neurons exhibited low firing rates (1–3 Hz) with relatively long AP duration (Ac) and a characteristic H-current (Ad). B: Identification of VTA GABA neurons, illustrating a representative dissociated GABA neuron (Ba), the effect of DA on neuronal firing (Bb), and AP profiles. VTA GABA neurons exhibited high firing rates (>7 Hz, Bb) with short AP durations (Bc) and did not show H-currents (Bd). C: Representative trace of spontaneous neuronal firing before (Ca) and after (Cb) JWH133 (1 µM). D: group mean data, illustrating that JWH133 dose-dependently inhibited VTA DA neuronal firing, an effect that was blocked by AM630. E: Bath-applied JWH133 (1 µM) hyperpolarized resting membrane potential (RP), potentiated the after-hyperpolarization potential (AHP), but had no effect on AP amplitude and duration. The number in the each column indicates neuronal numbers tested for each treatment. *p<0.05, compared to baseline.
Activation of CB2Rs inhibits DA release in the nucleus accumbens (NAc)
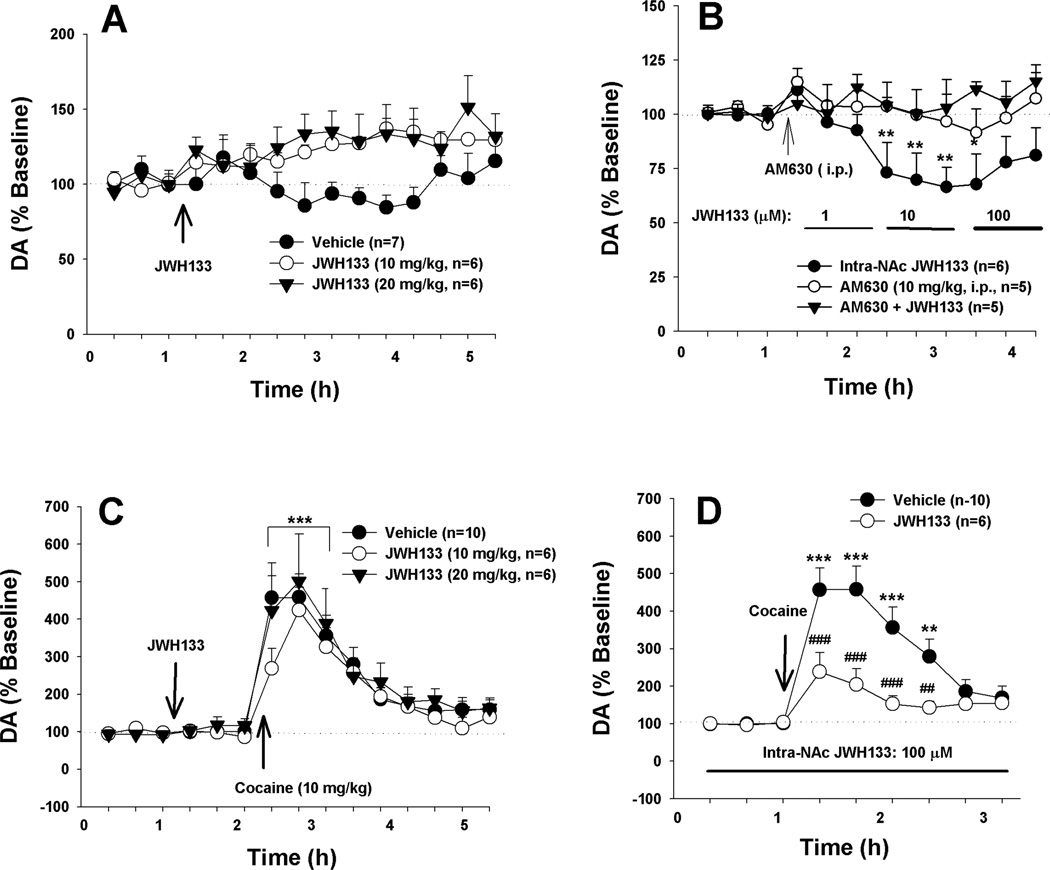
Figure 4A shows that systemic administration of JWH133 (10, 20 mg/kg) failed to alter basal extracellular DA level compared to pre-JWH133 baselines. However, local perfusion of JWH133 into the NAc significantly lowered extracellular DA level (Fig. 4B, F2,13=4.10, p<0.01). Post-hoc individual group comparisons revealed a significant reduction in extracellular DA after 10 µM or 100 µM JWH133 compared to baseline level before JWH133 perfusion. This effect was blocked by pretreatment with AM630 before intra-NAc local JWH133 perfusion. AM630 alone (10 mg/kg, i.p.) had no effect on extracellular NAc DA (Fig. 4B).
Figure 4.
Effects of JWH133 on basal and cocaine-enhanced extracellular DA in the NAc of rats. A: Systemic administration of JWH133 did not significantly alter basal extracellular DA as compared to pre-JWH133 baseline. B: Intra-NAc local perfusion of JWH133 significantly reduced extracellular DA. C: Systemic administration of JWH133 failed to alter cocaine-enhanced extracellular DA. D: Local perfusion of JWH133 (100 µM) into the NAc attenuated cocaine-induced increases in extracellular DA. * p<0.05, ** p<0.01, ***p<0.001, compared to pre-cocaine or pre-JWH133 baseline; ##p<0.01, ###p<0.001, compared to vehicle control group.
Consistent with the above findings, systemic administration of JWH133 (10, 20 mg/kg, i.p., 40 min prior to cocaine) failed to alter cocaine-induced increases in extracellular DA (Fig. 4C, F2,19=0.62, p>0.05). However, intra-NAc local administration of JWH133 (100 µM) significantly attenuated cocaine-induced increases in extracellular DA (Fig. 4D). Two-way ANOVA for repeated measures over time revealed a significant JWH133 treatment main effect (F1,14=11.25, p<0.01), time main effect (F8,112=26.29, p<0.001); and treatment × time interaction (F8,112=8.96, p<0.001). Post-hoc individual group comparisons revealed a significant reduction in cocaine-enhanced DA in the presence of JWH133 in the NAc.
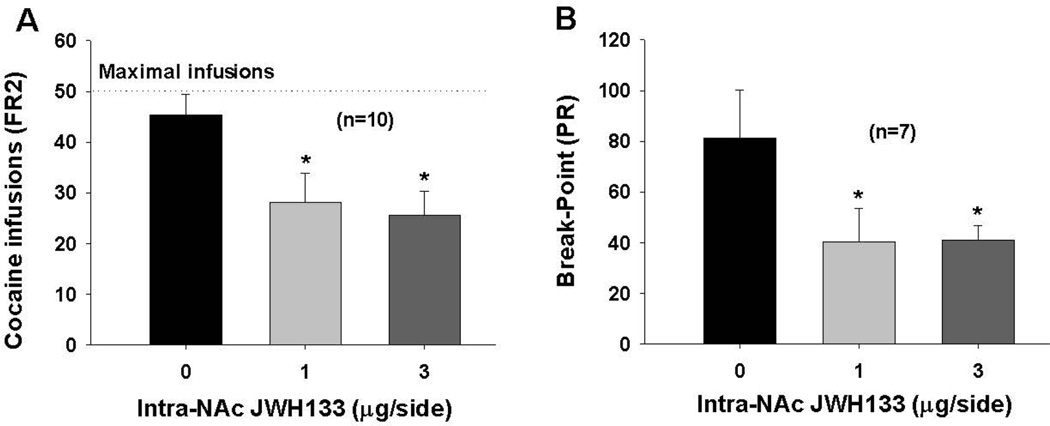
CB2R activation inhibits cocaine self-administration
We have recently reported that systemic administration of JWH133 has no effect on intravenous cocaine self-administration (Zhang et al., 2015). Here, we further examined whether local administration of JWH133 into the NAc inhibits cocaine self-administration. Figure 5 shows that bilateral microinjections of JWH133 (1, 3 µg/µl/side) into the NAc significantly inhibited cocaine self-administration under FR2 reinforcement schedule (F2,27 = 4.10 , p<0.05, one-way ANOVA). Figure 5B shows that microinjections of JWH133 into the NAc also significantly lowered the break-point levels for cocaine self-administration under progressive-ratio (PR) reinforcement (F2,18=5.21, p<0.01).
Figure 5.
Effects of local intra-NAc administration of JWH133 on cocaine self-administration in rats. A: Microinjections of JWH133 into the NAc inhibited cocaine self-administration under FR2 reinforcement; B: intra-NAc microinjections of JWH133 lowered break-point levels for cocaine self-administration under PR reinforcement conditions. *p<0.05, compared to vehicle control group.
Discussion
The major findings of the present study include: 1) CB2R mRNA and proteins are expressed in VTA DA neurons (and also non-DA neurons) in rats; 2) cocaine self-administration up-regulates CB2 mRNA expression in the PFC and striatum and in VTA DA neurons; and 3) activation of CB2Rs by JWH133 inhibits VTA DA neuronal firing, NAc DA release, and intravenous cocaine self-administration. These findings suggest that CB2Rs are expressed in VTA DA neurons and functionally modulate mesolimbic DA release and cocaine self-administration behavior in rats.
To determine whether CB2Rs are expressed in rat VTA DA neurons, we used multiple approaches, including highly sensitive RNAscope ISH, qRT-PCR, IHC, electrophysiology, neurochemistry, behavioral pharmacology, use of the highly-selective CB2 receptor agonist (JWH133) and antagonist (AM630), and multiple methods of drug delivery (systemic, intra-NAc, bath-applied). All the present findings converge to support the same conclusion – that functional CB2Rs are expressed in the mesolimbic DA system. We note that antibody specificity has been an issue in this field for many years (Ashton, 2012; Atwood and Mackie, 2010). We have tested up to 10 different CB2 antibodies in the IHC assays by the use of WT, CB1-KO and CB2-KO mice as controls. We found none of them to be absolutely CB2-specific. However, the presently-used Alomone CB2 antibody displays a high degree of CB2 specificity, as assessed by a ~70% reduction in the density of CB2-immunostaining in VTA DA neurons in CB2-KO mice compared to that in WT mice (Zhang et al., 2014). Due to this IHC limitation, we were forced to use additional approaches to study CB2R expression in VTA DA neurons.
Importantly, the CB2 mRNA signal detected by RNAscope ISH technology is highly rCB2-specific. This technique uses unique 20 double Z probe design across 1 kb region of CB2R 3'-UTR that allows simultaneous target-signal amplification and background suppression. Thus, it tremendously lowers nonspecific binding and significantly increases target-signaling specificity. It is therefore highly sensitive to very low RNA signals, even from a single copy of a gene (Wang et al., 2012). A computer-screened probe assay also supports the specificity of the presently-utilized CB2 RNAscope probe since it exhibits no homology or cross hybridization to the cannabinoid CB1 or other genes. Using both ISH and IHC assays, we detected clear CB2 gene and receptor expression in VTA DA neurons in rats. This is consistent with other recent reports that CB2 mRNA is expressed in VTA (DA) neurons in mice (Aracil-Fernandez et al., 2012; Zhang et al., 2015; Zhang et al., 2014), in hippocampal neurons in mice (Kim and Li, 2015; Li and Kim, 2015), and in striatal GABAergic neurons in non-human primates (Lanciego et al., 2011; Sierra et al., 2015).
The above findings using ISH and IHC assays are further supported by our electrophysiological results demonstrating that bath-applied JWH133 significantly inhibits VTA DA neuronal firing and produced VTA DA neuronal hyperpolarization in single dissociated VTA DA neurons – providing direct functional evidence supporting CB2R expression in VTA DA neurons in rats. This is further supported by our finding that intra-NAc local administration of JWH133 significantly inhibited basal DA release, cocaine-induced increase in NAc DA, and cocaine self-administration. These effects were blocked by co-administration of AM630, a selective CB2R antagonist, suggesting CB2R-mediated effects. All these findings support a common conclusion – CB2Rs are expressed and are functional in VTA DA neurons in rats. We have recently reported that JWH133 is a highly-selective CB2R agonist since deletion of CB2Rs, not CB1Rs, abolished the action produced by JWH133 (Xi et al., 2011; Zhang et al., 2014). Due to lack of CB2-KO rats as controls in the present study, in theory, we cannot completely exclude possible involvement of non-CB1 and non-CB2 receptor mechanisms in JWH133 action in rats. In addition, CB2 mRNA and immunostaining are also seen in some non-DA neurons in the VTA, suggesting that non-DA mechanisms may also contribute to the behavioral effects of JWH133 in the above cocaine self-administration experiments.
We reiterate that systemic administration of JWH133 had no effect on either DA release in the NAc (present study) or intravenous cocaine self-administration in rats (Zhang et al., 2015). Similarly, systemic administration of AM1241, another putative CB2R agonist, failed to alter nicotine self-administration in rats (Gamaleddin et al., 2012). The reason(s) for the ineffectiveness are unclear. One possibility is that JWH133 may have relatively poor bioavailability in rats. Therefore, higher systemic doses of JWH133 may be required to produce significant neurochemical and behavioral effects. Unfortunately, we cannot test this hypothesis since a higher dose (50 mg/kg) of JWH133 produced significant sedation and locomotor impairment in our pilot studies. The second possibility is that CB2Rs have broad distributions in rat brain. Thus, JWH133-induced reduction in NAc DA release and cocaine self-administration may be compromised by its actions in other brain regions after systemic administration. A third possibility is species differences in CB2R structures in rats and mice (Liu et al., 2009; Zhang et al., 2015). There are four CB2 mRNA isoforms identified in rats, but only two isoforms identified in mice (Liu et al., 2009; Zhang et al., 2015). CB2Rs display 90%, 81% and 79% homology in amino acid sequences between rat and mouse, human and rat, and human and mouse, respectively. More importantly, rat CB2R is 13 amino acids longer in its intracellular C-terminal than mouse CB2Rs (Zhang et al., 2015). These data suggest that CB2Rs may have different affinity or sensitivity to JWH133 or other ligands in rats and mice. In consistent with this view, JWH133 was reported to be more selective for hCB2Rs (200-fold) over rCB2Rs, while AM630 is more potent at rCB2Rs than at hCB2Rs (Huffman, 2005; Marriott et al., 2006). AM1241 has higher affinity for hCB2Rs over rCB2Rs and over mCB2Rs (Bingham et al., 2007; Yao et al., 2006). Finally, the present studies demonstrate that CB2Rs display similar cellular distributions and functioning in VTA DA neurons in rats and mice, suggesting that the ineffectiveness of JWH133 after systemic administration in rats is more likely due to other mechanisms, rather than different CB2R distributions in VTA DA neurons in rats and mice. Taken together, all these findings suggest that ligand binding and efficacy data cannot be automatically extrapolated from one species to another; thus, species-specific CB2R agonists or antagonists may be required in medication development for treatment of CNS disorders that are therapeutically amenable to CB2R-mediated manipulations.
We note that the present finding that intra-NAc microinjections of JWH133 significantly inhibit cocaine self-administration appears different from our previous reports that systemic administration of JWH133 produced biphasic effects – lower doses increased, while higher doses decreased break-point for cocaine self-administration under progressive-ratio (PR) reinforcement (Zhang et al., 2015). This may be related to the fact that PR cocaine self-administration is very sensitive to small changes in drug’s rewarding efficacy (Richardson and Roberts, 1996). Thus, it is likely that the enhanced PR break-point could be a compensatory response in motivation for drug-taking to reduced cocaine reward after low doses of JWH133, while the reduction in PR break-point could be simply interpreted as cessation of cocaine-taking due to reinforcement blockade after high doses of JWH133 (Zhang et al., 2015). Thus, all these findings support a common conclusion that activation of brain CB2Rs inhibits cocaine reward, which may leads to different alterations in cocaine self-administration under different experimental conditions.
Finally, we should point out that brain CB2 mRNA levels are very low compared to levels in spleen (Zhang et al., 2014). However, low densities of CB2 mRNA in brain do not necessarily mean low levels of CB2R expression. Actually, a moderate density of CB2R-immunostaining is detected in DA neurons under high magnification (40×) in the VTA (present study) (Aracil-Fernandez et al., 2012; Zhang et al., 2014) and in neurons in many other brain regions (Ashton et al., 2006; Baek et al., 2013; Gong et al., 2006). Although antibody specificity is of concern due to a lack of full CB2-KO mice as controls, it would be imprudent to conclude that the immunostaining detected in the above experiments are absolutely not CB2-specific and that low levels of mRNA certainly imply low levels of CB2R expression. Brain opioid receptor mRNA levels are also very low and there is no highly-selective opioid receptor antibody available (Mansour et al., 1995; Mansour et al., 1994). Yet, the presence of functional opioid receptors in brain is not disputed, as they are detected by many other functional and pharmacological methods such as autoradiography. Although autoradiography assays have not been used to study brain CB2R expression, multiple radiolabel CB2R agonists have been developed and have been successfully used to study brain CB2R expression by microPET imaging techniques (Hortala et al., 2014; Horti et al., 2010; Savonenko et al., 2015; Yrjola et al., 2015). Second, many recent studies suggest that brain CB2Rs are inducible or up-regulated in response to various types of insults (Mechoulam and Parker, 2013; Pacher and Mechoulam, 2011; Shohami et al., 2011), such as chronic pain (Beltramo et al., 2006; Luongo et al., 2010), ischemia-induced hypoxia (Ashton et al., 2007), neuroinflammation (Atwood and Mackie, 2010; Benito et al., 2008), and several chronic CNS disorders such as Alzheimer’s disease, HIV-induced encephalitis and multiple sclerosis (Benito et al., 2008). In the present study, we found that chronic cocaine self-administration also significantly up-regulated CB2 mRNA expression in brain and in VTA DA neurons. Such CB2R up-regulation observed in cocaine self-administration rats may in part explain the ameliorative effects of CB2R ligands on cocaine or alcohol self-administration (Al Mansouri et al., 2014; Xi et al., 2011; Zhang et al., 2015; Zhang et al., 2014), cocaine- or nicotine-induced CPP (Ignatowska-Jankowska et al., 2013; Navarrete et al., 2013), cocaine-induced reinstatement of drug-seeking behavior (Adamczyk et al., 2012), and cocaine-induced conditioned locomotion (Blanco-Calvo et al., 2014).
In summary, the present findings demonstrate that cannabinoid CB2 gene and receptors are expressed and up-regulated by cocaine in midbrain VTA DA neurons in rats. Activation of CB2Rs inhibits VTA DA neuronal firing, NAc DA release and DA-regulated cocaine self-administration behavior. Given the important role of DA and cannabinoid CB2Rs in substance abuse and addiction, food-taking and other DA-related neuropsychiatric disorders, the present findings suggest that brain CB2Rs may constitute new targets in medication development for treatment of such CNS disorders.
Acknowledgments
This research was supported by the Intramural Research Program (IRP) of the National Institute on Drug Abuse (NIDA), National Institutes of Health (NIH). The electrophysiological studies were supported by the Barrow Neuroscience Foundation (M.G.) and the Barrow Neurological Institute-Bristol-Myers Squibb Seed Fund (J.W.).
References
- Adamczyk P, Miszkiel J, McCreary AC, Filip M, Papp M, Przegalinski E. The effects of cannabinoid CB1, CB2 and vanilloid TRPV1 receptor antagonists on cocaine addictive behavior in rats. Brain Res. 2012;1444:45–54. doi: 10.1016/j.brainres.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Al Mansouri S, Ojha S, Al Maamari E, Al Ameri M, Nurulain SM, Bahi A. The cannabinoid receptor 2 agonist, beta-caryophyllene, reduced voluntary alcohol intake and attenuated ethanol-induced place preference and sensitivity in mice. Pharmacology, biochemistry, and behavior. 2014;124:260–268. doi: 10.1016/j.pbb.2014.06.025. [DOI] [PubMed] [Google Scholar]
- Aracil-Fernandez A, Trigo JM, Garcia-Gutierrez MS, Ortega-Alvaro A, Ternianov A, Navarro D, Robledo P, Berbel P, Maldonado R, Manzanares J. Decreased cocaine motor sensitization and self-administration in mice overexpressing cannabinoid CB(2) receptors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:1749–1763. doi: 10.1038/npp.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton JC. The use of knockout mice to test the specificity of antibodies for cannabinoid receptors. Hippocampus. 2012;22:643–644. doi: 10.1002/hipo.20946. [DOI] [PubMed] [Google Scholar]
- Ashton JC, Friberg D, Darlington CL, Smith PF. Expression of the cannabinoid CB2 receptor in the rat cerebellum: an immunohistochemical study. Neuroscience letters. 2006;396:113–116. doi: 10.1016/j.neulet.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Ashton JC, Rahman RM, Nair SM, Sutherland BA, Glass M, Appleton I. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neuroscience letters. 2007;412:114–117. doi: 10.1016/j.neulet.2006.10.053. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. British journal of pharmacology. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek JH, Darlington CL, Smith PF, Ashton JC. Antibody testing for brain immunohistochemistry: Brain immunolabeling for the cannabinoid CB2 receptor. J Neurosci Methods. 2013;216:87–95. doi: 10.1016/j.jneumeth.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, Reggiani A. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- Benito C, Tolon RM, Pazos MR, Nunez E, Castillo AI, Romero J. Cannabinoid CB2 receptors in human brain inflammation. British journal of pharmacology. 2008;153:277–285. doi: 10.1038/sj.bjp.0707505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham B, Jones PG, Uveges AJ, Kotnis S, Lu P, Smith VA, Sun SC, Resnick L, Chlenov M, He Y, Strassle BW, Cummons TA, Piesla MJ, Harrison JE, Whiteside GT, Kennedy JD. Species-specific in vitro pharmacological effects of the cannabinoid receptor 2 (CB2) selective ligand AM1241 and its resolved enantiomers. British journal of pharmacology. 2007;151:1061–1070. doi: 10.1038/sj.bjp.0707303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Calvo E, Rivera P, Arrabal S, Vargas A, Pavon FJ, Serrano A, Castilla-Ortega E, Galeano P, Rubio L, Suarez J, Rodriguez de Fonseca F. Pharmacological blockade of either cannabinoid CB1 or CB2 receptors prevents both cocaine-induced conditioned locomotion and cocaine-induced reduction of cell proliferation in the hippocampus of adult male rat. Frontiers in integrative neuroscience. 2014;7:106. doi: 10.3389/fnint.2013.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamaleddin I, Zvonok A, Makriyannis A, Goldberg SR, Le Foll B. Effects of a selective cannabinoid CB2 agonist and antagonist on intravenous nicotine self administration and reinstatement of nicotine seeking. PloS one. 2012;7:e29900. doi: 10.1371/journal.pone.0029900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Garcia-Bueno B, Zoppi S, Leza JC, Manzanares J. Chronic blockade of cannabinoid CB(2) receptors induces anxiolytic-like actions associated to alterations in GABA(A) receptors. Br J Pharmacol. 2011;165:951–964. doi: 10.1111/j.1476-5381.2011.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Garcia-Bueno B, Zoppi S, Leza JC, Manzanares J. Chronic blockade of cannabinoid CB2 receptors induces anxiolytic-like actions associated with alterations in GABA(A) receptors. British journal of pharmacology. 2012;165:951–964. doi: 10.1111/j.1476-5381.2011.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Manzanares J. Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. Journal of psychopharmacology. 2011;25:111–120. doi: 10.1177/0269881110379507. [DOI] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Perez-Ortiz JM, Gutierrez-Adan A, Manzanares J. Depression-resistant endophenotype in mice overexpressing cannabinoid CB(2) receptors. British journal of pharmacology. 2010;160:1773–1784. doi: 10.1111/j.1476-5381.2010.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MC, Cinquina V, Palomo-Garo C, Rabano A, Fernandez-Ruiz J. Identification of CB2 receptors in human nigral neurons that degenerate in Parkinson's disease. Neurosci Lett. 2015;587:1–4. doi: 10.1016/j.neulet.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Griffin G, Tao Q, Abood ME. Cloning and pharmacological characterization of the rat CB(2) cannabinoid receptor. J Pharmacol Exp Ther. 2000;292:886–894. [PubMed] [Google Scholar]
- Hortala L, Arnaud J, Roux P, Oustric D, Boulu L, Oury-Donat F, Avenet P, Rooney T, Alagille D, Barret O, Tamagnan G, Barth F. Synthesis and preliminary evaluation of a new fluorine-18 labelled triazine derivative for PET imaging of cannabinoid CB2 receptor. Bioorganic & medicinal chemistry letters. 2014;24:283–287. doi: 10.1016/j.bmcl.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Horti AG, Gao Y, Ravert HT, Finley P, Valentine H, Wong DF, Endres CJ, Savonenko AV, Dannals RF. Synthesis and biodistribution of [11C]A-836339, a new potential radioligand for PET imaging of cannabinoid type 2 receptors (CB2) Bioorganic & medicinal chemistry. 2010;18:5202–5207. doi: 10.1016/j.bmc.2010.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacological reviews. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Huffman JW. CB2 receptor ligands. Mini Rev Med Chem. 2005;5:641–649. doi: 10.2174/1389557054368844. [DOI] [PubMed] [Google Scholar]
- Ignatowska-Jankowska BM, Muldoon PP, Lichtman AH, Damaj MI. The cannabinoid CB receptor is necessary for nicotine-conditioned place preference, but not other behavioral effects of nicotine in mice. Psychopharmacology (Berl) 2013;229:591–601. doi: 10.1007/s00213-013-3117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Li Y. Chronic activation of CB2 cannabinoid receptors in the hippocampus increases excitatory synaptic transmission. The Journal of physiology. 2015;593:871–886. doi: 10.1113/jphysiol.2014.286633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, Barroso-Chinea P, Rico AJ, Conte-Perales L, Callen L, Roda E, Gomez-Bautista V, Lopez IP, Lluis C, Labandeira-Garcia JL, Franco R. Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. J Psychopharmacol. 2011;25:97–104. doi: 10.1177/0269881110367732. [DOI] [PubMed] [Google Scholar]
- Li X, Hoffman AF, Peng XQ, Lupica CR, Gardner EL, Xi ZX. Attenuation of basal and cocaine-enhanced locomotion and nucleus accumbens dopamine in cannabinoid CB1-receptor-knockout mice. Psychopharmacology. 2009;204:1–11. doi: 10.1007/s00213-008-1432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kim J. Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience. 2015;311:253–267. doi: 10.1016/j.neuroscience.2015.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Pan CH, Hishimoto A, Li CY, Xi ZX, Llorente-Berzal A, Viveros MP, Ishiguro H, Arinami T, Onaivi ES, Uhl GR. Species differences in cannabinoid receptor 2 (CNR2 gene): identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes, brain, and behavior. 2009;8:519–530. doi: 10.1111/j.1601-183X.2009.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luongo L, Palazzo E, Tambaro S, Giordano C, Gatta L, Scafuro MA, Rossi FS, Lazzari P, Pani L, de Novellis V, Malcangio M, Maione S. 1-(2',4'-dichlorophenyl)-6-methyl-N-cyclohexylamine-1,4-dihydroindeno[1,2-c]pyraz ole-3-carboxamide, a novel CB2 agonist, alleviates neuropathic pain through functional microglial changes in mice. Neurobiology of disease. 2010;37:177–185. doi: 10.1016/j.nbd.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handbook of experimental pharmacology. 2005:299–325. doi: 10.1007/3-540-26573-2_10. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors: where they are and what they do. Journal of neuroendocrinology. 2008;(20 Suppl 1):10–14. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Berrendero F, Ozaita A, Robledo P. Neurochemical basis of cannabis addiction. Neuroscience. 2011;181:1–17. doi: 10.1016/j.neuroscience.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. Journal of chemical neuroanatomy. 1995;8:283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. The Journal of comparative neurology. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Marriott KS, Huffman JW, Wiley JL, Martin BR. Synthesis and pharmacology of 11-nor-1-methoxy-9-hydroxyhexahydrocannabinols and 11-nor-1-deoxy-9-hydroxyhexahydrocannabinols: new selective ligands for the cannabinoid CB2 receptor. Bioorganic & medicinal chemistry. 2006;14:2386–2397. doi: 10.1016/j.bmc.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA. The Endocannabinoid System and the Brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annual review of psychology. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Navarrete F, Perez-Ortiz JM, Manzanares J. Cannabinoid CB(2) receptor-mediated regulation of impulsive-like behaviour in DBA/2 mice. British journal of pharmacology. 2012;165:260–273. doi: 10.1111/j.1476-5381.2011.01542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete F, Rodriguez-Arias M, Martin-Garcia E, Navarro D, Garcia-Gutierrez MS, Aguilar MA, Aracil-Fernandez A, Berbel P, Minarro J, Maldonado R, Manzanares J. Role of CB2 cannabinoid receptors in the rewarding, reinforcing, and physical effects of nicotine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:2515–2524. doi: 10.1038/npp.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Alvaro A, Aracil-Fernandez A, Garcia-Gutierrez MS, Navarrete F, Manzanares J. Deletion of CB2 cannabinoid receptor induces schizophrenia-related behaviors in mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:1489–1504. doi: 10.1038/npp.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Alvaro A, Ternianov A, Aracil-Fernandez A, Navarrete F, Garcia-Gutierrez MS, Manzanares J. Role of cannabinoid CB2 receptor in the reinforcing actions of ethanol. Addiction biology. 2015;20:43–55. doi: 10.1111/adb.12076. [DOI] [PubMed] [Google Scholar]
- Pacher P, Mechoulam R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Progress in lipid research. 2011;50:193–211. doi: 10.1016/j.plipres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of neuroscience methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Melnikova T, Wang Y, Ravert H, Gao Y, Koppel J, Lee D, Pletnikova O, Cho E, Sayyida N, Hiatt A, Troncoso J, Davies P, Dannals RF, Pomper MG, Horti AG. Cannabinoid CB2 Receptors in a Mouse Model of Abeta Amyloidosis: Immunohistochemical Analysis and Suitability as a PET Biomarker of Neuroinflammation. PloS one. 2015;10:e0129618. doi: 10.1371/journal.pone.0129618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohami E, Cohen-Yeshurun A, Magid L, Algali M, Mechoulam R. Endocannabinoids and traumatic brain injury. British journal of pharmacology. 2011;163:1402–1410. doi: 10.1111/j.1476-5381.2011.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra S, Luquin N, Rico AJ, Gomez-Bautista V, Roda E, Dopeso-Reyes IG, Vazquez A, Martinez-Pinilla E, Labandeira-Garcia JL, Franco R, Lanciego JL. Detection of cannabinoid receptors CB1 and CB2 within basal ganglia output neurons in macaques: changes following experimental parkinsonism. Brain structure & function. 2015;220:2721–2738. doi: 10.1007/s00429-014-0823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. The Journal of molecular diagnostics : JMD. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, George AA, Schroeder KM, Xu L, Marxer-Miller S, Lucero L, Lukas RJ. Electrophysiological, pharmacological, and molecular evidence for alpha7-nicotinic acetylcholine receptors in rat midbrain dopamine neurons. The Journal of pharmacology and experimental therapeutics. 2004;311:80–91. doi: 10.1124/jpet.104.070417. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. The European journal of neuroscience. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL. Brain cannabinoid CB2 receptors modulate cocaine's actions in mice. Nat Neurosci. 2011;14:1160–U1216. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Hu J, Lucero L, Liu Q, Zheng C, Zhen X, Jin G, Lukas RJ, Wu J. Distinctive nicotinic acetylcholine receptor functional phenotypes of rat ventral tegmental area dopaminergic neurons. The Journal of physiology. 2009;587:345–361. doi: 10.1113/jphysiol.2008.162743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao BB, Mukherjee S, Fan Y, Garrison TR, Daza AV, Grayson GK, Hooker BA, Dart MJ, Sullivan JP, Meyer MD. In vitro pharmacological characterization of AM1241: a protean agonist at the cannabinoid CB2 receptor? British journal of pharmacology. 2006;149:145–154. doi: 10.1038/sj.bjp.0706838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjola S, Sarparanta M, Airaksinen AJ, Hytti M, Kauppinen A, Pasonen-Seppanen S, Adinolfi B, Nieri P, Manera C, Keinanen O, Poso A, Nevalainen TJ, Parkkari T. Synthesis, in vitro and in vivo evaluation of 1,3,5-triazines as cannabinoid CB2 receptor agonists. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2015;67:85–96. doi: 10.1016/j.ejps.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Bi GH, Li X, Li J, Qu H, Zhang SJ, Li CY, Onaivi ES, Gardner EL, Xi ZX, Liu QR. Species Differences in Cannabinoid Receptor 2 and Receptor Responses to Cocaine Self-Administration in Mice and Rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40:1037–1051. doi: 10.1038/npp.2014.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ, Gardner EL, Wu J, Xi ZX. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E5007–E5015. doi: 10.1073/pnas.1413210111. [DOI] [PMC free article] [PubMed] [Google Scholar]