Introduction
Mohs surgery and repair of lower eyelid surgical defects present unique reconstruction challenges because of risk for ectropion. There are several options to avoid this adverse outcome, but when a patient declines surgical repair, there are no alternatives aside from second-intention healing with or without adjunctive therapy. One promising method is use of dehydrated human amnion/chorion membrane (dHACM) allograft (EpiFix®, MiMedx Group Inc, Marietta, GA). To address the issue of a paucity of nonsurgical repair options, the author presents 3 cases of relatively superficial lower eyelid defects after Mohs micrographic surgery that were successfully repaired using dHACM.
Case reports
Case 1
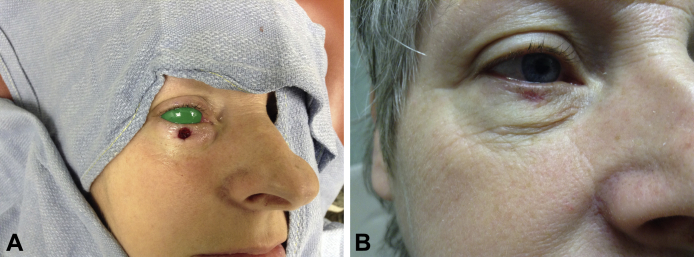
An 82-year-old white woman with multiple medical problems (including a previous Mohs procedure and surgical repair for a basal cell carcinoma of the forehead) and chronic tobacco use presented to the Mohs surgical clinic with a nodular and infiltrative basal cell carcinoma on the right lower eyelid. After 3 stages of Mohs surgery, there was a 2.5- × 1.5-cm surgical defect involving almost the entire lower eyelid, coming within 0.8 cm of the eyelid margin and minimal extension into the underlying orbicularis oculi. The patient refused surgical reconstruction because of significant pain and difficulty healing subsequent to a previous large Mohs defect repair. Second-intention healing was discouraged because of ectropion risk; thus, the decision to use dHACM was made, and a single dHACM allograft was applied. The allograft was trimmed to slightly larger than the surgical defect (2 mm larger than the defect circumferentially), and a silicone dressing was placed on the wound (Fig 1, A). The surgical site was cleaned weekly with sterile saline, and another silicone dressing was placed until the wound was healed. The patient reported negligible pain or bleeding compared with the previous Mohs procedure. There were no complications, significant scarring, or ectropion formation. The lesion took 45 days to heal (Fig 1, B).
Fig 1.
Case 1. A, Final surgical defect after Mohs surgery. B, Postoperative day 45 with the use of a dHACM allograft.
Case 2
A 52-year-old white woman female with medical history significant for type II diabetes, was referred by the ophthalmology department to the Mohs clinic to remove a nodular basal cell carcinoma on the right lower eyelid. After 1 stage of Mohs surgery, the patient had a 0.6- × 0.6-cm surgical defect directly under the tarsal plate and had minimal extension into the underlying orbicularis oculi. A wedge repair or a full-thickness skin graft was initially planned by the ophthalmology department, but the patient declined surgical repair. The patient agreed to try a dHACM allograft over second intention because of ectropion risk. A dHACM allograft was trimmed to slightly larger than the surgical defect (2 mm larger than the defect circumferentially) and was placed on the wound (Fig 2, A). The lesion healed completely in 15 days with no complications, minimal scarring, and no ectropion formation (Fig 2, B). The patient denied any significant pain or bleeding. Postoperatively, a silicone dressing was placed on the wound, but it fell off after 2 days. Going forward, daily application of petroleum jelly and a standard adhesive bandage were used until the wound healed.
Fig 2.
Case 2. A, Final surgical defect after Mohs surgery. B, Postoperative day 15 with the use of a dHACM allograft.
Case 3
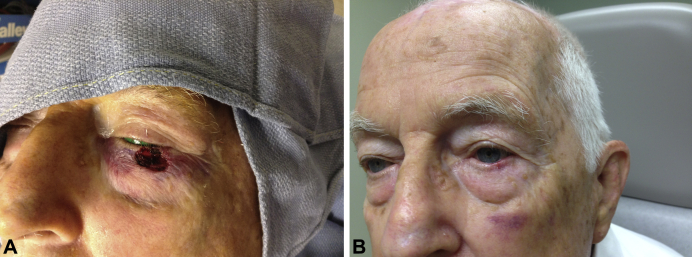
An 86-year-old white man with multiple medical problems presented to the Mohs surgical clinic with a nodular basal cell carcinoma on the left lower eyelid. After 3 stages of Mohs surgery, the patient had a 1.2- × 1.1-cm surgical defect that involved the tarsal plate but spared the eyelid margin and had minimal extension into the underlying orbicularis oculi. Because of the patient's use of clopidogrel bisulfate and aspirin, significant bleeding occurred throughout the Mohs surgical process. Because of this bleeding, it was decided to use a dHACM allograft over a wedge repair or a full-thickness skin graft. Second-intention healing was not used because of possible ectropion formation. A dHACM allograft was trimmed to slightly larger than the surgical defect (2 mm larger than the defect circumferentially) and was placed on the wound (Fig 3, A). The lesion healed completely in 18 days with no complications, minimal scarring, and no ectropion formation (Fig 3, B). The patient denied any significant pain or bleeding. A silicone dressing was placed on the wound and was to be replaced 5 days postoperatively. However, the dressing fell off in 3 days. Going forward, daily application of petroleum jelly and a standard adhesive bandage were used until the wound healed.
Fig 3.
Case 3. A, Final surgical defect after Mohs surgery. B, Postoperative day 18 with the use of a dHACM allograft.
Discussion
Mohs micrographic surgery is a precise surgical excision technique used to remove cutaneous malignancies. The objective of the Mohs procedure is to remove small layers of tissue until cancer-free margins are achieved to preserve the surrounding healthy tissue as much as possible. After Mohs surgery, depending on the size, depth, and location of the surgical wound, reconstruction options include secondary intention healing, primary closure with sutures, or local skin flaps or grafts.1, 2 Surgical defects near the eye present unique challenges for the surgeon owing to risk for ectropion formation. Considering this risk, repair options are limited and secondary intention healing is discouraged.3, 4 In this case series, we describe results of 3 patients with basal cell carcinoma and conditions associated with poor healing who had Mohs surgery and successful repairs with dHACM allografts.
Human amniotic membrane has been used clinically in a variety of applications for more than 100 years, but there are limited data showing the utility in the repair of cutaneous eyelid surgical defects.5 Amniotic membrane has been identified as a potent facilitator of wound healing in a variety of circumstances, including lower extremity ulcers, conjunctival reconstruction, burns, gynecologic surgery, and orthopedic surgery.6, 7, 8, 9, 10, 11, 12 Key features of amniotic membrane include an immunologically privileged state, a reservoir of multiple growth factors involved with tissue regeneration, and modulation of inflammatory processes. Although application of human amniotic membrane has been used successfully in a variety of wounds,13 difficulty in obtaining, preparing, and storing the material and a concern for potential for infectious disease transmission, has precluded its widespread use.14 Using dHACM minimizes the issues that arise with use of fresh amniotic membrane. The tissue is processed according to the American Association of Tissue Banks standards and regulated under Section 361 of the Public Health Service Act.15 Placental materials are obtained from screened and tested donors to ensure safety.13 The tissue is then minimally manipulated using a proprietary process to create a tissue allograft comprised of processed, dried, and sterilized human amnion/chorion membranes.16 Growth factors, cytokines, and chemokines remain present in the material after the proprietary processing.17 The process also allows for storage at ambient temperature for up to 5 years and the ability to make material available in multiple sizes and configurations for use in a variety of wound types.
Although the exact protocol of graft application and wound care differed in the cases above, the recommended protocol is as follows. Standard informed consent is obtained from the patient and then grafts are cut to overlay the wound edge by 2 mm circumferentially. The graft is then placed in the wound and is rehydrated by applying 1 drop of sterile saline per 1 × 1 cm of surface area of graft or by applying half a pea-size amount of hydrogel per 1 × 1 cm of surface area of graft. The graft is then secured by placing a standard pressure dressing on the wound. The graft does not need to be sutured into place. In our cases, we used silicone dressings, but a standard nonadherent contact layer covered by a pressure dressing is adequate. The dressing is then changed in the clinic every 5 to days until healed. The 2 small cases that were approximately 1 × 1 cm took 15 to 18 days to heal, whereas the larger 2.5- × 1.5-cm case took 45 days to heal. It is anticipated that for every 1.0- × 1.0- × 0.5-cm section (length × width × depth), it should take 2 weeks to heal with 1 graft application. However, more precise data on wound care and healing times with varying graft application frequency need to be determined through a larger cohort of patients.
This small sample of patients with superficial lower eyelid defects benefited from dHACM use by having a shorter procedure and avoiding large flap or graft procedures, while experiencing minimal pain, no complications, and negligible scarring. Further evaluation of the use of dHACM after Mohs surgery with regard to the benefits of rapid wound closure, patient satisfaction, and pain and scar reduction is warranted. In addition, a cost-effectiveness analysis with comparison to other allograft products should be conducted with a larger study population. A dHACM allograft appears to be an effective closure option for Mohs surgical defects in patients with superficial lower eyelid defects.
Footnotes
Funding sources: None.
Conflicts of interest: Oliver J. Wisco is a consultant to MiMedx Group, Inc.
References
- 1.Rullan P.P., Vallbona C., Rullan J.M., Mansbridge J.N., Morhenn V.B. Use of gelatin sponges in Mohs micrographic surgery defects and staged melanoma excisions: a novel approach to secondary wound healing. J Drugs Dermatol. 2011;10:68–73. [PubMed] [Google Scholar]
- 2.Zhang A.Y., Meine J.G. Flaps and grafts reconstruction. Dermatol Clin. 2011;29:217–230. doi: 10.1016/j.det.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Mathijssen I.M., van der Meulen J.C. Guidelines for reconstruction of the eyelids and canthal regions. J Plast Reconstr Aesthet Surg. 2010;63:1420–1433. doi: 10.1016/j.bjps.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 4.Kimyai-Asadi A., Goldberg L.H., Peterson S.R., Silapint S., Jih M.H. The incidence of major complications from Mohs micrographic surgery performed in office-based and hospital-based settings. J Am Acad Dermatol. 2005;53:628–634. doi: 10.1016/j.jaad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharjee K., Singh M., Bhattacharjee H. Amniotic membrane graft to reconstruct divided nevi of eyelids. BMJ Case Rep. 2015 doi: 10.1136/bcr-2014-209020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parolini O., Solomon A., Evangelista M., Soncini M. Human term placenta as a therapeutic agent: from the first clinical applications to future perspectives. In: Berven E., editor. Human Placenta: Structure and Development. Nova Science Publishers; Hauppauge, NY: 2010. pp. 1–48. [Google Scholar]
- 7.John T. Human amniotic membrane transplantation: past, present, and future. Ophthalmol Clin North Am. 2003;16:43–65. doi: 10.1016/s0896-1549(02)00110-4. [DOI] [PubMed] [Google Scholar]
- 8.Niknejad H., Peirovi H., Jorjani M., Ahmadiani A., Ghanavi J., Seifalian A.M. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99. doi: 10.22203/ecm.v015a07. [DOI] [PubMed] [Google Scholar]
- 9.Bennett J.P., Matthews R., Faulk W.P. Treatment of chronic ulceration of the legs with human amnion. Lancet. 1980;1:1153–1156. doi: 10.1016/s0140-6736(80)91616-5. [DOI] [PubMed] [Google Scholar]
- 10.Baradaran-Rafii A., Aghayan H., Arjmand B., Javadi M. Amniotic membrane transplantation. Iran J Ophthalmic Res. 2007;2:58–75. [Google Scholar]
- 11.Adly O.A., Moghazy A.M., Abbas A.H. Assessment of amniotic and polyurethane membrane dressings in the treatment of burns. Burns. 2010;36:703–710. doi: 10.1016/j.burns.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Tao H., Fan H. Implantation of amniotic membrane to reduce postlaminectomy epidural adhesions. Eur Spine J. 2009;18:1202–1212. doi: 10.1007/s00586-009-1013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruss J.S., Jirsch D.W. Human amniotic membrane: a versatile wound dressing. Can Med Assoc J. 1978;118:1237–1246. [PMC free article] [PubMed] [Google Scholar]
- 14.Shores J.T., Gabriel A., Gupta S. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20:493–508. doi: 10.1097/01.ASW.0000288217.83128.f3. [DOI] [PubMed] [Google Scholar]
- 15.American Association of Tissue Banks Standards. Available from: www.fda.gov/BiologicsBloodVaccines/Tissue. Accessed June 25, 2014
- 16.Zelen C.M., Snyder R.J., Serena T.E., Li W.W. The use of human amnion/chorion membrane in the clinical setting for lower extremity repair: a review. Clin Podiatr Med Surg. 2015;32:135–146. doi: 10.1016/j.cpm.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Koob T.J., Rennert R., Zabek N. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J. 2013;10:493–500. doi: 10.1111/iwj.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]