Abstract
Take-all disease of Poaceae is caused by Gaeumannomyces graminis (Magnaporthaceae). Four varieties are recognised in G. graminis based on ascospore size, hyphopodial morphology and host preference. The aim of the present study was to clarify boundaries among species and varieties in Gaeumannomyces by combining morphology and multi-locus phylogenetic analyses based on partial gene sequences of ITS, LSU, tef1 and rpb1. Two new genera, Falciphoriella and Gaeumannomycella were subsequently introduced in Magnaporthaceae. The resulting phylogeny revealed several cryptic species previously overlooked within Gaeumannomyces. Isolates of Gaeumannomyces were distributed in four main clades, from which 19 species could be delimited, 12 of which were new to science. Our results show that the former varieties Gaeumannomyces graminis var. avenae and Gaeumannomyces graminis var. tritici represent species phylogenetically distinct from G. graminis, for which the new combinations G. avenae and G. tritici are introduced. Based on molecular data, morphology and host preferences, Gaeumannomyces graminis var. maydis is proposed as a synonym of G. radicicola. Furthermore, an epitype for Gaeumannomyces graminis var. avenae was designated to help stabilise the application of that name.
Key words: Cryptic species, Gaeumannomyces graminis, Magnaporthaceae, Phylogeny, Triticum
Taxonomic novelties: New genera: Falciphoriella M. Hern.-Restr. & Crous, Gaeumannomycella M. Hern.-Restr. & Crous
New species: Falciphoriellasolaniterrestris M. Hern.-Restr. & Crous; Gaeumannomycellacaricis M. Hern.-Restr. & Crous; Gaeumannomycesarxii M. Hern.-Restr. & Crous; G. australiensis M. Hern.-Restr. & Crous; G. californicus M. Hern.-Restr. & Crous; G. ellisiorum M. Hern.-Restr. & Crous; G. floridanus M. Hern.-Restr. & Crous; G. fusiformis M. Hern.-Restr. & Crous; G. glycinicola M. Hern.-Restr., G. Canning & Crous; G. graminicola M. Hern.-Restr. & Crous; G. hyphopodioides M. Hern.-Restr. & Crous; G. oryzicola M. Hern.-Restr. & Crous; G. setariicola M. Hern.-Restr. & Crous; G. walkeri M. Hern.-Restr. & Crous
New combinations: Gaeumannomyces tritici (J. Walker) M. Hern.-Restr. & Crous, Gaeumannomyces avenae (E. M. Turner) M. Hern.-Restr. & Crous
Typification: Epitypification: Gaeumannomyces graminis var. avenae (E. M. Turner) Dennis
Introduction
Take-all is one of the most important root diseases in cereal crops and grasses, caused by Gaeumannomyces graminis. Taxonomic placement of Gaeumannomyces graminis at the variety level has been a research topic for many decades. Based on morphology, pathogenicity and host preference, four varieties of this species can be recognised (Turner, 1940, Walker, 1972, Yao et al., 1992). The type variety Gaeumannomyces graminis var. graminis (Ggg) causes crown (black) sheath rot of rice, dieback in Bermuda grass, take-all root rot of St. Augustine grass or root decline of other warm-season turf grasses (Walker, 1972, Walker, 1981, Elliott, 1991, Ward and Bateman, 1999). It is the least aggressive and is also often found as a weak pathogen or saprobe on cereals, grasses and soybeans (Walker, 1980, Roy et al., 1982, Ward and Bateman, 1999). Gaeumannomyces graminis var. avenae (Turner, 1940, Dennis, 1960) (Gga) causes take-all of oats and take-all patch of turfgrasses, although it can also infect wheat, rye and barley. Gaeumannomyces graminis var. tritici (Walker 1972) (Ggt) is the most aggressive variety and is known as the wheat take-all fungus. It infects mainly wheat but can also infect triticale, barley and rye as well as other cereals and grasses (Walker, 1980, Ward and Bateman, 1999, Freeman and Ward, 2004). Take-all of wheat is the most important root disease of wheat worldwide. Gaeumannomyces graminis var. maydis (Yao et al. 1992) (Ggm) is the most recently described variety and causes take-all of maize but also can slightly infect Sorghum and other cereals.
The sexual morph in Gaeumannomyces is characterised by the production of globose or pyriform, immersed ascomata with a conical to cylindrical neck, and fusiform, multiseptate and hyaline ascospores. Asexual morphs are characterised by phialidic conidiogenous cells with refractive collarettes and lunate or phialophora-like conidia. For a long time the asexual morphs in Gaeumannomyces were referred to Phialophora, but based on morphology, Gams (2000) proposed the genus Harpophora to accommodate the phialidic asexual morphs in Magnaporthaceae. However, Harpophora became the later synonym of Gaeumannomyces, following the Melbourne code (Luo et al. 2015c).
Hyphopodia are commonly found in this genus and in other members of Magnaporthaceae. This feature has been used as a taxonomic character to differentiate some of the varieties in G. graminis. The asexual morph of Ggg has been reported to have lobed hyphopodia (Walker, 1980, Ward and Bateman, 1999, Freeman and Ward, 2004). On the other hand Ggt, Gga and Ggm are characterised by the production of simple hyphopodia in the substrate (Walker, 1972, Yao et al., 1992).
However, differentiation among isolates of Gaeumannomyces based on disease symptoms, host range, cultural and/or morphological characteristics is difficult, time consuming and is in many cases inconclusive (Ulrich et al., 2000, Freeman and Ward, 2004). Different molecular techniques have been used to identify species and varieties in Gaeumannomyces, for example RAPD (Wetzel et al., 1996, Augustin et al., 1999, Ulrich et al., 2000), RFLP (Bateman et al., 1992, Tan et al., 1994, Ward and Akrofi, 1994), amplification of specific gene sequences within the ITS nrDNA (Bryan et al., 1995, Ward and Bateman, 1999, Ulrich et al., 2000), or avenacinase-like genes (Rachdawong et al. 2002). Those studies revealed that Ggt and Gga form a monophyletic clade, whereas Ggg appears to be polyphyletic, with high variability among isolates (Elliott et al., 1993, Ward and Akrofi, 1994, Fouly et al., 1996, Tan, 1997, Ward and Bateman, 1999, Fouly and Wilkinson, 2000, Saleh and Leslie, 2004, Sadeghi et al., 2012). In addition, Ggm is related to another maize root pathogen named G. radicicola (Luo et al. 2015c), formerly recognised as Harpophora radicicola and H. zeicola (Ward and Bateman, 1999, Gams, 2000). Phylogenetic studies also revealed new lineages in Gaeumannomyces referred to as “Phialophora sp. GP57” (Ward & Bateman 1999) and “group E” (Ulrich et al. 2000). Nevertheless, no formal names or combinations have been proposed.
The genus Gaeumannomyces (Magnaporthaceae, Magnaporthales), was established by von Arx & Olivier (1952) to accommodate Ophiobolus graminis, formerly described as Rhaphidophora graminis. Besides G. graminis and G. radicicola, this genus includes other root-infecting pathogens such as G. wongoonoo; the cause of a patch disease of Stenotaphrum secundatum (buffalo grass) (Wong 2002) and G. caricis occurring on Carex spp. (Cyperaceae) (Walker 1980). Endophytic and saprobic fungi have been found in this genus as well, for example G. amomi, described as endophytic in Amomum and Alpinia (Zingiberaceae) (Bussaban et al. 2001), and the saprobic G. licualae, an unusual Gaeumannomyces species collected from palm (Licuala sp.), known only from the type locality; Brunei Darussalam (Fröhlich & Hyde 2000).
The number of taxa in Magnaporthaceae with phialophora-, and harpophora-like asexual morphs has been increasing in the past 20 years, together with the introduction of new genera, e.g. Falciphora (Yuan et al., 2010, Luo et al., 2015c), Magnaporthiopsis (Luo & Zhang 2013), and Pseudophialophora (Luo et al., 2014, Luo et al., 2015b), with a high number of cryptic species among those genera.
Other studies relocated some species previously accommodated in Gaeumannomyces for example; G. incrustans was transferred to Magnaporthiopsis (Luo & Zhang 2013). Slopeiomyces and Kohlmeyeriopsis were proposed as new genera to accommodate G. cylindrosporus and G. medullaris respectively (Klaubauf et al. 2014).
The aims of the present study were: (1) to explore the diversity of Gaeumannomyces isolates, collected from diverse geographic origins and from different hosts; (2) to determine the phylogenetic relationships of the isolates using a multi-locus sequence alignment consisting of partial gene sequences of LSU (28S nrDNA), ITS (internal transcribed spacers and intervening 5.8S nrRNA gene), tef1 (translation elongation factor 1-alpha) and rpb1 (RNA polymerase II large subunit); (3) to resolve the taxonomy of Gaeumannomyces by adopting a polyphasic approach; and (4) to designate epitypes and reference sequences for species of Gaeumannomyces.
Materials and methods
Isolates and morphological analysis
A total of 83 strains identified as Gaeumannomyces or Harpophora (Phialophora) from different localities and hosts were examined (Table 1). Specimens were obtained from the culture collection of the CBS-KNAW Fungal Biodiversity Centre (CBS), Utrecht, The Netherlands, the Monica Elliott personal collection, University of Florida, USA, the working collection of P.W. Crous (CPC) housed at CBS, and the Rothamsted plant pathology culture collection, Department of Plant Biology and Crop Science, Rothamsted Research, Harpenden, Herts, UK.
Table 1.
Isolates used in this study and their GenBank accession numbers. Newly generated sequences are indicated in bold.
| Species | Old name/Received as | Strain number1 | Status2 | Country | Host, substrate | GenBank accession numbers3 |
|||
|---|---|---|---|---|---|---|---|---|---|
| LSU | ITS | RPB1 | TEF1 | ||||||
| Buergenerula spartinae | Buergenerula spartinae | ATCC 22848 | USA | Spartina alterniflora, leaves | DQ341492 | JX134666 | JX134720 | – | |
| Bussabanomyces longisporus | Bussabanomyces longisporus | CBS 125232 | T | Thailand | Amomum siamense, leaves | KM484951 | KM484832 | KM485046 | – |
| Falciphora oryzae | Harpophora oryzae | CBS 125863, R5-6-1 | T | China | Oryza sativa, root, endophytic | KJ026705 | EU636699 | KJ026706 | JN857963 |
| Falciphoriella solaniterrestris | Gaeumannomyces sp. | CBS 117.83 | T | Netherlands | Soil in potato field | KM484959 | KM484842 | KM485058 | – |
| Gaeumannomycella caricis | Gaeumannomyces sp. | CBS 388.81 | T | UK | Carex rostrata | KM484960 | KM484843 | KX306674 | – |
| Gaeumannomyces graminis var. graminis | CPC 26262, CBS 141374 | UK | Carex rostrata | KX306548 | KX306478 | KX306671 | KX306675 | ||
| Gaeumannomyces amomi | Gaeumannomyces amomi | CBS 109354, CMUZE0002, BCC 4066 | Thailand | Amomun sp., endophytic in leaves | DQ341493 | AY265318 | – | KX306679 | |
| G. arxii | Gaeumannomyces graminis var. graminis | CBS 902.73, DAR 17502 | Australia | Stenotaphrum secundatum (buffalo grass) | KM484953 | KM484836 | KM485052 | KX306680 | |
| Gaeumannomyces graminis var. graminis | CBS 903.73, DAR 23471 | T | Australia | Pennisetum clandestinum, (kikuyu grass), stolon | KM484854 | KM484837 | KM485053 | KX306681 | |
| Gaeumannomyces graminis var. avenae | CPC 26054, CBS 141375 | USA | Stenotaphrum secundatum | KX306549 | KX306479 | KX306618 | KX306682 | ||
| G. australiensis | Gaeumannomyces graminis var. graminis | CPC 26058, DAR 32100, CBS 141387 | T | Australia | Triticum aestivum | KX306550 | KX306480 | KX306619 | KX306683 |
| G. avenae | Gaeumannomyces graminis var. avenae | CBS 187.65 | Netherlands | Avena sativa, root | JX134680 | JX134668 | JX134722 | JX134694 | |
| Gaeumannomyces graminis var. avenae | CBS 870.73, DAR 20999 | Australia | Avena sativa | DQ341495 | KM484833 | KM485048 | KX306684 | ||
| Gaeumannomyces graminis var. avenae | CPC 26253 | Australia | Agrostis (bent grass) | KX306551 | KX306481 | – | KX306685 | ||
| Gaeumannomyces graminis var. avenae | CPC 26254 | Australia | Agrostis (bent grass) | KX306552 | KX306482 | – | – | ||
| Gaeumannomyces graminis var. avenae | CPC 26255 | Australia | Agrostis (bent grass) | KX306553 | KX306483 | KX306620 | KX306686 | ||
| Gaeumannomyces graminis var. avenae | CPC 26256 | UK | Avena sativa | KX306554 | KX306484 | – | – | ||
| Gaeumannomyces graminis var. avenae | CPC 26257, CBS 141376 | Ireland | Avena sativa (winter Oats) | KX306555 | KX306485 | KX306621 | KX306687 | ||
| Gaeumannomyces graminis var. avenae | CPC 26258 | ET | Ireland | Avena sativa (winter Oats) | KX306556 | KX306486 | KX306622 | KX306688 | |
| Gaeumannomyces graminis var. avenae | CPC 26259 | Ireland | Triticum aestivum (winter wheat) | KX306557 | KX306487 | – | – | ||
| Gaeumannomyces graminis var. avenae | CPC 26260 | Ireland | Turf | KX306558 | KX306488 | KX306623 | KX306689 | ||
| Gaeumannomyces graminis var. avenae | CPC 26261 | UK | Turf | KX306559 | KX306489 | KX306624 | KX306690 | ||
| G. californicus | Gaeumannomyces graminis var. graminis | CPC 26044, CBS 141377 | T | USA | Stenotaphrum secundatum | KX306560 | KX306490 | KX306625 | KX306691 |
| G. ellisiorum | Gaeumannomyces graminis var. graminis | CBS 387.81 | T | UK | Deschampsia caespitosa, dead culm and sheath | KM484952 | KM484835 | KM485051 | KX306692 |
| G. floridanus | Gaeumannomyces graminis var. graminis | CPC 26037, CBS 141378 | T | USA | Stenotaphrum secundatum | KX306561 | KX306491 | KX306626 | KX306693 |
| G. fusiformis | Gaeumannomyces graminis var. graminis | CPC 26068, CBS 141379 | T | USA | Oryza sativa | KX306562 | KX306492 | KX306627 | KX306694 |
| Gaeumannomyces glycinicola | Gaeumannomyces graminis var. graminis | CPC 26057, DAR 28746 | T | USA | Glycine max | KX306563 | KX306493 | KX306628 | KX306695 |
| Gaeumannomyces graminis var. graminis | CPC 26266, CBS 141380 | USA | Glycine max | KX306564 | KX306494 | KX306629 | KX306696 | ||
| G. graminicola | Gaeumannomyces graminis var. graminis | CBS 352.93 | T | Netherlands | Ctenanthe sp., stem base | DQ341496 | KM484834 | KM485050 | KX306697 |
| Gaeumannomyces graminis var. graminis | CPC 26025, CBS 141381 | USA | Stenotaphrum secundatum | KX306565 | KX306495 | KX306630 | KX306698 | ||
| Gaeumannomyces graminis var. graminis | CPC 26036, CBS 141382 | USA | Stenotaphrum secundatum | KX306566 | KX306496 | KX306631 | KX306699 | ||
| Gaeumannomyces graminis var. graminis | CPC 26056, CBS 141383 | USA | Eremochloa ophiuroides | KX306567 | KX306497 | KX306632 | KX306700 | ||
| G. graminis | Gaeumannomyces graminis var. graminis | CPC 26020, CBS 141384 | USA | Cynodon dactylon × C. transvaalensis | KX306568 | KX306498 | KX306633 | KX306701 | |
| Gaeumannomyces graminis var. graminis | CPC 26027 | USA | Cynodon dactylon × C. transvaalensis | KX306569 | KX306499 | KX306634 | KX306702 | ||
| Gaeumannomyces graminis var. graminis | CPC 26029 | USA | Cynodon dactylon × C. transvaalensis | KX306570 | KX306500 | KX306635 | KX306703 | ||
| Gaeumannomyces graminis var. graminis | CPC 26033, CBS 141385 | USA | Cynodon dactylon × C. transvaalensis | KX306571 | KX306501 | KX306636 | KX306704 | ||
| Gaeumannomyces graminis var. graminis | CPC 26035, CBS 141386 | USA | Cynodon dactylon × C. transvaalensis | KX306572 | KX306502 | KX306637 | KX306705 | ||
| Gaeumannomyces graminis var. graminis | CPC 26039 | USA | Cynodon dactylon × C. transvaalensis | KX306573 | KX306503 | KX306638 | KX306706 | ||
| Gaeumannomyces graminis var. graminis | CPC 26042 | USA | Cynodon dactylon × C. transvaalensis | KX306574 | KX306504 | KX306639 | KX306707 | ||
| Gaeumannomyces graminis var. graminis | CPC 26045 | USA | Cynodon dactylon × C. transvaalensis | KX306575 | KX306505 | KX306640 | KX306708 | ||
| G. hyphopodioides | Phialophora radicicola | CBS 350.77, G6, ATCC 28234, IMI 187786 | T | UK | Zea mays, root | KX306576 | KX306506 | KM009192 | KM009204 |
| Gaeumannomyces graminis var. tritici | CBS 541.86 | Germany | Triticum aestivum, seedling | KX306577 | KX306507 | KX306641 | KX306709 | ||
| “Phialophora sp. lobed hyphopodia” | CPC 26247, CBS 141388 | UK | Triticum aestivum | KX306578 | KX306508 | KX306642 | KX306710 | ||
| “Phialophora sp. lobed hyphopodia” | CPC 26248 | UK | Triticum aestivum | KX306579 | KX306509 | – | – | ||
| “Phialophora sp. lobed hyphopodia” | CPC 26249 | UK | Triticum aestivum | KX306580 | KX306510 | – | KX306711 | ||
| “Phialophora sp. lobed hyphopodia” | CPC 26250 | UK | Avena sativa | KX306581 | KX306511 | – | KX306712 | ||
| “Phialophora sp. lobed hyphopodia” | CPC 26252 | Poland | Triticum aestivum | KX306582 | KX306512 | KX306643 | KX306713 | ||
| Gaeumannomyces graminis var. graminis | CPC 26264, CBS 141389 | UK | Triticum aestivum (winter wheat) | KX306583 | KX306513 | KX306644 | KX306714 | ||
| Gaeumannomyces graminis var. graminis | CPC 26265 | UK | Triticum aestivum | KX306584 | KX306514 | – | KX306715 | ||
| Gaeumannomyces graminis var. graminis | CPC 26267 | Australia | Pennisetum clandestinum | KX306585 | KX306515 | KX306645 | KX306716 | ||
| G. oryzicola | Gaeumannomyces graminis var. graminis | CPC 26063, CBS 141390 | T | USA | Oryza sativa | KX306586 | KX306516 | KX306646 | KX306717 |
| Gaeumannomyces oryzinus | Gaeumannomyces graminis var. graminis | CBS 235.32 | USA | Oryza sativa | JX134681 | JX134669 | KM485049 | JX134695 | |
| Gaeumannomyces graminis var. graminis | CPC 26030, CBS 141391 | The Bahamas | Cynodon dactylon × C. transvaalensis | KX306587 | KX306517 | KX306647 | KX306718 | ||
| Gaeumannomyces graminis var. graminis | CPC 26031 | USA | Oryza sativa | KX306588 | KX306518 | KX306648 | KX306719 | ||
| Gaeumannomyces graminis var. graminis | CPC 26032 | USA | Oryza sativa | KX306589 | KX306519 | KX306649 | KX306720 | ||
| Gaeumannomyces graminis var. graminis | CPC 26043, CBS 141392 | USA | Oryza sativa | KX306590 | KX306520 | KX306650 | KX306721 | ||
| Gaeumannomyces graminis var. graminis | CPC 26065 | USA | Oryza sativa | KX306591 | KX306521 | KX306651 | KX306722 | ||
| Gaeumannomyces graminis var. graminis | CPC 26066 | USA | Oryza sativa | KX306592 | KX306522 | KX306652 | KX306723 | ||
| Gaeumannomyces graminis var. graminis | CPC 26067, CBS 141393 | USA | Oryza sativa | KX306593 | KX306523 | KX306653 | KX306724 | ||
| G. radicicola | Phialophora zeicola | CBS 149.85, PREM 45754 | South Africa | Zea mays | KM484961 | KM484844 | KM485060 | KM009205 | |
| Phialophora radicicola | CBS 296.53, MUCL 28970 | T | Canada | Zea mays, root | KM484962 | KM484845 | KM485061 | KM009206 | |
| Gaeumannomyces graminis var. maydis | W4066B | China | Zea mays | – | AJ010035 | – | – | ||
| Gaeumannomyces graminis var. maydis | Ggm02 | – | – | – | AY120939 | – | – | ||
| G. setariicola | Gaeumannomyces graminis var. graminis | CPC 26059, PRRI 4754, CBS 141394 | T | South Africa | Setaria italica | KX306594 | KX306524 | KX306654 | KX306725 |
| Gaeumannomyces graminis var. tritici | CBS 186.65 | Netherlands | Hordeum vulgare | KM484955 | KM484838 | KM485054 | KX306726 | ||
| G. tritici | Gaeumannomyces graminis var. tritici | CBS 247.29 | Netherlands | Triticum sp. | KM484956 | KM484839 | KM485055 | KX306727 | |
| Gaeumannomyces graminis var. tritici | CBS 249.29, IMI 083849 | – | Triticum aestivum | KM484957 | KM484840 | KM485056 | KX306728 | ||
| Gaeumannomyces graminis var. tritici | CBS 273.36 | Argentina | Triticum aestivum | KX306595 | KX306525 | KX306655 | KX306730 | ||
| Gaeumannomyces graminis var. tritici | CBS 905.73, DAR 23140 | Australia | Triticum aestivum | KM484958 | KM484841 | KM485057 | KX306731 | ||
| Gaeumannomyces graminis var. tritici | CBS 131293 | USA | Triticum sp. | KX306596 | KX306526 | KX306656 | KX306729 | ||
| Gaeumannomyces graminis var. avenae | CPC 26069, CBS 141395 | USA | – | KX306597 | KX306527 | KX306657 | KX306732 | ||
| Gaeumannomyces graminis var. tritici | CPC 26268, CBS 141396 | Australia | Triticum aestivum | KX306598 | KX306528 | KX306658 | KX306733 | ||
| Gaeumannomyces graminis var. tritici | CPC 26269, CBS 141397 | Brazil | Triticum aestivum | KX306599 | KX306529 | – | – | ||
| Gaeumannomyces graminis var. tritici | CPC 26270 | UK | Hordeum vulgare | KX306600 | KX306530 | KX306659 | KX306734 | ||
| Gaeumannomyces graminis var. tritici | CPC 26271 | UK | Triticum aestivum | KX306601 | KX306531 | – | KX306735 | ||
| Gaeumannomyces graminis var. tritici | CPC 26272 | UK | Hordeum vulgare (winter barley) | KX306602 | KX306532 | KX306660 | KX306736 | ||
| Gaeumannomyces graminis var. tritici | CPC 26273, CBS 141398 | UK | Elymus repens (couch grass) | KX306603 | KX306533 | KX306661 | KX306737 | ||
| Gaeumannomyces graminis var. tritici | CPC 26274 | Australia | – | KX306604 | KX306534 | KX306662 | KX306738 | ||
| Gaeumannomyces graminis var. tritici | CPC 26275 | UK | Bromus sp. (Brome grass) | KX306605 | KX306535 | KX306663 | – | ||
| Gaeumannomyces graminis var. tritici | CPC 26276 | Brazil | – | KX306606 | KX306536 | KX306664 | KX306739 | ||
| Gaeumannomyces graminis var. tritici | CPC 26277 | UK | Elymus repens (couch grass) | KX306607 | KX306537 | KX306665 | KX306740 | ||
| Gaeumannomyces tritici | Gaeumannomyces graminis var. tritici | CPC 26278 | UK | Agropyron sp. | KX306608 | KX306538 | KX306666 | KX306741 | |
| Gaeumannomyces graminis var. tritici | CPC 26280 | UK | – | KX306609 | KX306539 | KX306667 | KX306742 | ||
| Gaeumannomyces graminis var. tritici | CPC 26281 | UK | – | KX306610 | KX306540 | KX306668 | KX306743 | ||
| Gaeumannomyces graminis var. tritici | CPC 26282, CBS 141399 | UK | Triticum aestivum (winter wheat) | KX306611 | KX306541 | – | KX306744 | ||
| Gaeumannomyces graminis var. tritici | CPC 26283 | UK | Triticum aestivum (winter wheat) | KX306612 | KX306542 | KX306669 | KX306745 | ||
| Gaeumannomyces graminis var. tritici | R3-111a-1 | USA | Triticum aestivum | – | – | Genome | Genome | ||
| Gaeumannomyces walkeri | Gaeumannomyces incrustans | CPC 26028, CBS 141400 | T | USA | Stenotaphrum secundatum | KX306613 | KX306543 | KX306670 | KX306746 |
| G. wongoonoo | Gaeumannomyces wongoonoo | BRIP 60376 | Australia | Buffalo grass | KP162146 | KP162137 | – | – | |
| Kohlmeyeriopsis medullaris | Gaeumannomyces medullaris | CBS 117849, JK5528S | T | USA | Juncus roemerianus | KM484968 | KM484852 | KM485068 | – |
| Magnaporthiopsis incrustans | Gaeumannomyces incrustans | M35 | – | – | JF414892 | JF414843 | JF710437 | – | |
| M. maydis | Magnaporthiopsis maydis | CBS 662.82A | T | Egypt | Zea mays | KM484971 | KM484856 | KM485072 | – |
| Harpophora sp. | CBS 133165, ATCC MYA-3356 | Israel | Zea mays | KX306614 | KX306544 | – | – | ||
| M. poae | Magnaporthe poae | M48 | USA | Poa pratensis | – | JF414837 | JF710434 | – | |
| M. rhizophila | Magnaporthe poae | M23 | – | Poa pratensis | JF414846 | JF414834 | JF710432 | – | |
| Magnaporthiopsis sp. | Gaeumannomyces graminis var. graminis | CPC 26038 | USA | Cynodon dactylon × C. transvaalensis | KX306615 | KX306545 | KX306672 | KX306676 | |
| Nakataea oryzae | Nakataea oryzae | CBS 252.34 | Burma | Oryza sativa | KM484976 | KM484862 | KM485078 | – | |
| Neogaeumannomyces bambusicola | Neogaeumannomyces bambusicola | MFLUCC 110390 | T | Thailand | Dead culm of bamboo (Bambusae) | KP744492 | KP744449 | – | – |
| Omnidemptus affinis | Omnidemptus affinis | ATCC 200212 | T | Australia | Panicum effusum var. effusum, grass leaves | KX134686 | JX134674 | JX134728 | – |
| Pseudophialophora eragrostis | Pseudophialophora eragrostis | CM12m9 | T | USA | Eragrostis sp. | KF689638 | KF689648 | KF689618 | KF689628 |
| Pyricularia grisea | Pyricularia grisea | BR0029 | Brazil | Digitaria sanguinalis | KM484995 | KM484880 | KM485100 | – | |
| Pyricularia grisea | CR0024 | South Korea | Lolium perenne | KM484997 | KM484882 | KM485102 | – | ||
| Slopeiomyces cylindrosporus | Gaeumannomyces cylindrosporus | CBS 609.75 | T | UK | Grass root, associated with Phialophora graminicola | KM485040 | KM484944 | KM485158 | – |
| Magnaporthaceae, incertae sedis | Phialophora sp. | CPC 26284, GP57, CBS 141401 | UK | Triticum aestivum | KX306616 | KX306546 | – | KX306677 | |
| Gaeumannomyces caricis | CPC 26245, CBS 141402 | UK | Carex acutiformis | KX306617 | KX306547 | KX306673 | KX306678 | ||
ATCC: American Type Culture Collection, Virginia, USA; BCC: BIOTEC Culture Collection, National Center for Genetic Engineering and Biotechnology (BIOTEC), Bangkok, Thailand; BRIP: Queensland Plant Pathology Herbarium, Brisbane, Australia; CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CPC: Culture collection of Pedro Crous, housed at CBS; DAR: Plant Pathology Herbarium, Orange Agricultural Institute, Forest Road, Orange. NSW 2800, Australia; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, United Kingdom; MFLUCC: Mae Fah Luang University Culture Collection, Chiang Rai, Thailand; MUCL: Université Catholique de Louvain, Louvain-la-Neuve, Belgium; PREM: South African National Collection of Fungi (NCF), Mycology Unit, Biosystematics Division, Plant Protection Institute, Agricultural Research Council, Roodeplaat, Pretoria, South Africa.
T: ex-type strain; ET: ex-epitype strain.
ITS: internal transcribed spacer regions 1 & 2 including 5.8S nrRNA gene; LSU: 28S large subunit of the nrRNA gene; rpb1: partial RNA polymerase II largest subunit; tef1: partial translation elongation factor 1-α.
Isolates were cultured on 2 % potato dextrose agar (PDA), 2 % malt extract agar (MEA; Oxoid) and oatmeal agar (OA; Crous et al. 2009), and incubated at 25 °C under daylight conditions for 1–3 wk; UV light conditions were used for some isolates to induce sporulation. After 7 d of incubation the colony diameters were measured and the colony morphologies described. Colony colours on the surface and reverse of inoculated media were assessed according to the colour charts of Rayner (1970). Micromorphological descriptions and 30 measurements of relevant features were carried out from mature cultures mounted in clear lactic acid. For ascomata, measurements were taken from 5 to 10 structures depending on availability. Observations and photomicrographs were made with a Nikon SMZ1500 stereo-microscope, and with a Nikon Eclipse Ni microscope, using a DS-Ri2 digital camera (Nikon, Tokyo, Japan) and NIS-Elements imaging software v. 4.20. Reference strains were deposited in the CBS culture collection. Taxonomic information and nomenclature for new species were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004).
DNA isolation, amplification and sequences alignment
Genomic DNA was extracted from fungal colonies growing on MEA using the Wizard® Genomic DNA purification kit (Promega, Madison, USA), according to the manufacturer's protocols. Procedures for amplifying and sequencing the internal transcribed spacer nrDNA including the intervening 5.8S nrDNA (ITS) and partial large subunit nrDNA (28S nrDNA; LSU), were performed as described in Hernández-Restrepo et al. (2016). Part of the largest subunit of the RNA polymerase II gene (rpb1) was amplified and sequenced as described in Klaubauf et al. (2014). Translation elongation factor 1-α gene (tef1), corresponding to the section 983–1567 bp, was amplified and sequenced as described in Rehner & Buckley (2005). Sequences were edited and consensus sequences constructed using SeqMan Pro (DNASTAR, Madison, WI, USA) and deposited in GenBank (Table 1).
To further study the phylogenetic relationships, additional homologous sequences of members of Magnaporthales were retrieved from GenBank and combined with those generated during the present study (Table 1). Sequence alignments were performed with MAFFT v. 7 (Katoh & Standley 2013) using the defaults settings and adjusted by hand in MEGA v. 6.06 (Tamura et al. 2013).
Phylogenetic analysis
A draft phylogeny based on the ITS sequences was first generated to infer a preliminary phylogenetic placement of the studied isolates (data not shown). Phylogenetic relationships of Gaeumannomyces spp. and related genera in Magnaporthaceae were resolved by combined analyses of ITS, LSU, tef1, and rpb1 sequences. The first dataset combining LSU and rpb1 sequences was used to infer the generic relationship among all the isolates within genera belonging to Magnaporthaceae. A second combined dataset based on LSU, ITS, tef1 and rpb1 sequences was used to resolve the taxonomy of Gaeumannomyces sensu stricto (s. s.) at species level.
Phylogenetic analyses of both individual and combined aligned data consisted of Bayesian inference (BI), Maximum Parsimony (MP), Maximum-Likelihood (ML), and neighbour-joining (NJ) analyses. Substitution models for each sequence dataset were inferred with MrModeltest2 v. 2.3 (Nylander 2004). The BI was addressed using MrBayes v. 3.2.1 (Ronquist et al. 2012). The Markov Chain Monte Carlo sampling (MCMC) analysis of four chains started in parallel from a random tree topology. The number of generations was set at 10 million and the run was stopped automatically when the average standard deviation of split frequencies fell below 0.01. Trees were saved each 1 000 generations. Burn-in was set at 25 % after which the likelihood values were stationary and the remaining trees were used to calculate posterior probabilities (BPP).
The ML analyses, including 1 000 bootstrap replicates, were conducted using RAxML on the CIPRES portal (www.phylo.org) using RAxML-HPC BlackBox v. 8.2.6. A general time reversible model (GTR) was applied with a gamma-distributed rate variation. The MP and NJ analyses with the Kimura 2-parameter and the HKY85 substitution model using PAUP v. 4.0b10 (Swofford 2003) were performed as described by Crous et al. (2006).
Results
Phylogeny
The first dataset consisted of 64 aligned LSU and rpb1 sequences of members of Magnaporthaceae, including the outgroup Pyricularia grisea represented by two strains (BR0029 and CR0024). Based on the results of MrModeltest, the GTR+I+G model with inverse gamma-distributed was selected as best fit model for BI. This dataset included 1 368 characters, from which 424 constitute unique site patterns. A total of 2 130 trees were sampled after the burn-in with a stop value of 0.01. In the MP analyses, 948 characters were constant, 66 were variable and parsimony uninformative while 354 were parsimony informative. A total of 48 equally most parsimonious trees were retained from this analysis (Tree length = 1 253, CI = 0.516, RI = 0.787, and RC = 0.407). The topology of the MP tree confirmed those of BI and ML trees for the distinction of 14 well-supported monophyletic clades, and therefore only the Bayesian tree with MP and RAxML bootstrap support values (MPBS and MLBS, respectively) and Bayesian posterior probabilities (BPP) are shown in Fig. 1. This analysis delimited 14 generic clades in Magnaporthaceae. The majority of the isolates cluster in Gaeumannomyces s. s. However one strain, CPC 26038, clustered in Magnaporthiopsis while CPC 26284 [=GP57 Phialophora sp. in Ward & Bateman (1999)], CPC 26245 (identified as G. caricis), CBS 117.83, and CBS 388.81 together with CPC 26262, were placed in separate clades distinct from other genera in Magnaporthaceae. Two new genera are introduced here (see Taxonomy section); Falciphoriella to accommodate CBS 117.83, and Gaeumannomycella to accommodate the isolates CBS 388.81 and CPC 26262. Cultures CPC 26284 and CPC 26245, identified as Phialophora sp. and G. caricis respectively, represent distinct lineages in Magnaporthaceae, but unfortunately these cultures proved to be sterile and thus await future taxonomic treatment until sporulating material is collected.
Fig. 1.
Phylogenetic tree inferred from a Bayesian analysis based on a concatenated alignment of LSU and rpb1 sequences of 64 strains representing Magnaporthaceae family. The Maximum Parsimony and RAxML bootstrap support values (MPBS, MLBS) and Bayesian posterior probabilities (BPP) are given at the nodes (MPBS/MLBS/BPP). Some branches were shortened to fit them to the page – these are indicated by two diagonal lines with the number of times a branch was shortened indicated next to the lines. Ex-type or ex-epitype strains are indicated as (T) and (ET) respectively. The tree was rooted with Pyricularia grisea (BR0029 and CR0024).
Gaeumannomyces s. s. was analysed in detail to calculate the phylogenetic differences among the varieties of Gaeumannomyces and other species included in the genus, i.e. G. amomi, G. radicicola and G. wongoonoo. This dataset consisted of 74 aligned sequences including two outgroups Falciphora oryzae (CBS 125863) and Pseudophialophora eragrostis (CM12m9). This dataset consisted in total of 2 634 characters (882 bp from the LSU, 719 bp from ITS, 1 041 bp from tef1 and 1 044 bp from rpb1) of which 961 constitute unique site patterns. Based on the results of MrModeltest, the GTR+I+G model with inverse gamma-distributed was selected as best fit model for BI. For the multi-locus analyses, a total of 4 068 trees were sampled after the burn-in with a stop value of 0.01. In the MP analyses, 2 046 characters were constant, 322 were variable and parsimony uninformative while 266 were parsimony informative. A maximum of 1 000 equally most parsimonious trees were retained from this analysis (Tree length = 1 010, CI = 0.754, RI = 0.915, and RC = 0.690). The topology of the BI tree was congruent to that of ML and MP trees and therefore only the Bayesian tree with BPP and MPBS values are indicated in Fig. 2. Gaeumannomyces isolates are distributed in four main clades designated here as Graminis, Oryzinus, Radicicola, and Tritici. Naming was based on the oldest species described in the clade, except for the tritici clade which was chosen based on the most phytopathogenic important species G. tritici (the wheat take-all fungus). Clade tritici consists of G. tritici, G. avenae (both elevated here to species status, formerly recognised as varieties of G. graminis), G. amomi and four new species described here as G. arxii, G. ellisiorum, G. glycinicola and G. walkeri. Clade graminis consists of G. graminis and three new species described here as G. californicus, G. australiensis and G. oryzicola. Clade oryzinus consists of G. oryzinus and three new species described here as G. floridanus, G. fusiformis and G. graminicola. Clade radicicola consists of G. radicicola, G. wongoonoo and two new species described here as G. hyphopodioides and G. setariicola.
Fig. 2.
Phylogenetic tree inferred from a Bayesian analysis based on a concatenated alignment of LSU, ITS, tef1 and rpb1 sequences of 74 strains of Gaeumannomyces. The Bayesian posterior probabilities (BPP) and Maximum Parsimony bootstrap support values (MPBS) are given at the nodes (BPP/MPBS). Ex-type or ex-epitype strains are indicated as (T) and (ET) respectively. The tree was rooted with Falciphora oryzae (CBS 125863) and Pseudophialophora eragrostis (CM19M9).
Taxonomy
Based on DNA sequence data and variation in morphology among the isolates studied, two new genera in Magnaporthaceae are introduced with a harpophora-like asexual morph, namely Falciphoriella and Gaeumannomycella. The Gaeumannomyces s. s. analysis resolved a total of 19 species, 12 of which are introduced as new species; and two new combinations are proposed. All the novelties, as well as epitypifications, are described and illustrated below. The main morphological characters of accepted species in Gaeumannomyces are provided in Table 2. The identity of some isolates could not be resolved in the present study, mostly because they remained sterile in culture; their identities will be resolved in future studies.
Table 2.
Overview of the main characters of Gaeumannomyces species.
| Clade | Species | Sexual |
Asexual |
Hyphopodia |
Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ascomata (μm) | Asci (μm) | Ascospores (μm) | # of Septa | Conidiogenous cells (μm) | Conidial size (μm) | Conidial shape1 | Size (μm) | Shape,2 colour | |||
| Graminis | G. australiensis | Not observed | 6.5–27.5 × 1.5–3 | 5–11 × 1–2 | L, C | 18.5–25 × 21.5–23 | L, brown | This study | |||
| G. californicus | Not observed | 4.5–24 × 1.5–4 | 4–11 × 1–1.5 | L, F | 25–32.5 × 24–30 | L, brown | This study | ||||
| G. graminis | 200–300 × 150–200 | 80–110 × 10–13 | 70–110 × 2.5–4 | 3 | – | – | – | 17–27 × 20–30 | S–L | Walker (1980) | |
| Not observed | 7–30 × 1.5–4 | 4–10 × 1–2 | L | Not observed | This study | ||||||
| G. oryzicola | 110–413 × 112–525 | 118–148 × 14–16 | 92.5–120 × 4–6 | 0–5 | 7.5–20.5 × 2–2.5 | 5–9 × 1.5–2.5 | F, L | Not observed | This study | ||
| Oryzinus | G. floridanus | Not observed | 7–14.5 × 2–3.5 | 5–11 × 1–1.5 | L | 18–27 × 14.5–26.5 | L, hyaline, brown | This study | |||
| G. fusiformis | Not observed | 5–9.5 × 1.5–2 | F | Not observed | This study | ||||||
| G. graminicola | Not observed | 5–20 × 2–4.5 | 5–11.5 × 1–2 | L, C | 16.5–24 × 15.5–23.5 | L, brown | This study | ||||
| G. oryzinus | 187–415 | (72)87–130 × 7–16 | 70–112 × 2–4.6 | 3–5 | – | – | – | – | Walker (1972) (as Ggg) | ||
| – | 113–173.5 × 14.5–24 | 96–116 × 3.5–5.5 | 0–3 | 5–21 × 2–5 | 5–11 × 1–2.5 | L, F, C | 19–45 × 15.5–36 | L, brown | This study | ||
| Radicicola | G. hyphopodioides | Not observed | 7–21 × 2–4 | 5.5–10.5 × 1–2 | L, F | 17–28 × 18–25 | S–L, hyaline, brown | This study | |||
| G. radicicola | 200–450 diam | 60–100 × 9–12 | 55–85 × 2.5–4 | 10–23 × 3–4 | 5–9 × 0.7–1.5 | L | S–slightly L | Yao et al. (1992) (as Ggm), Cain (1952) (as Phialophora) | |||
| G. setariicola | Not observed | 6.5–28.5 × 2–4 | 4–12 × 1–2 | L | Not observed | This study | |||||
| G. wongoonoo | 300–650 × 90–160 | 80–140 × 10–14 | 36–75 × 3–5 | 5–8 (12) | – | 5–12.5 × 3–5 | – | 20 diam | S–L | Wong (2002) | |
| Tritici | G. amomi | 500–650 × 300–400 | 100–130 × 12.5–15 | 70–100 × 4–5 | 3–6 | – | – | – | 24–34 × 30–38 | L | Bussaban et al. (2001) |
| G. arxii | Not observed | 6–23 × 2–5 | 4–10 × 1–2 | L, F | Not observed | This study | |||||
| G. avenae | 300–500 × 250–400 | (90)110–160 × 12–16 | (85)100–130 (140) × 3–5 | (3)5–13 | – | – | – | 7–15 × 4–8 | S | Walker, 1972, Walker, 1981 | |
| G. ellisiorum | Not observed | 5–18 × 3–4 | 4–9 × 1–2 | L | 19.5–35.5 × 16.5–30 | S–L, hyaline | This study | ||||
| G. glycinicola | – | – | 71.6 ± 6.8 × 2.6 ± 0.5 | – | – | – | – | – | – | Roy et al. (1982) (as Ggg) | |
| Not observed | Not observed | 22.5–43 × 15–34 | L, hyaline, brown | This study | |||||||
| G. tritici | 150–500 | (65)90–136 × 10–15 | 60–118 × 3–4 | (2–3)5–9 (12) | – | – | – | – | S | Walker (1972) | |
| G. walkeri | Not observed | 6–23 × 2–3.5 | 5–14 × 1–1.5 (at 8 days fusiform 7.5–11 × 2–3) | F, L | 20–31 × 18.5–24.5 | L, brown | This study | ||||
L = lobed hyphopodia, S = simple hyphopodia.
L = lunate conidia, F = fusiform conidia, and C = cylindrical conidia.
Sordariomycetes, Magnaporthales, Magnaporthaceae
Falciphoriella M. Hern.-Restr. & Crous, gen. nov. MycoBank MB816902.
Etymology: Morphologically similar to the genus Falciphora.
Mycelium consisting of septate, branched, smooth, hyaline to subhyaline. Conidiophores differentiated, indeterminate, branched, hyaline to pale brown. Conidiogenous cells phialidic, hyaline to pale brown, solitary or grouped, terminal or intercalary, cylindrical, lageniform, to conical, straight or curved with a cylindrical to funnel-shaped collarette. Conidia mainly fusiform sometimes obovoid, slightly curved at the ends, usually pointed base, hyaline. Hyphopodia not observed.
Type species: Falciphoriella solaniterrestris M. Hern.-Restr. & Crous
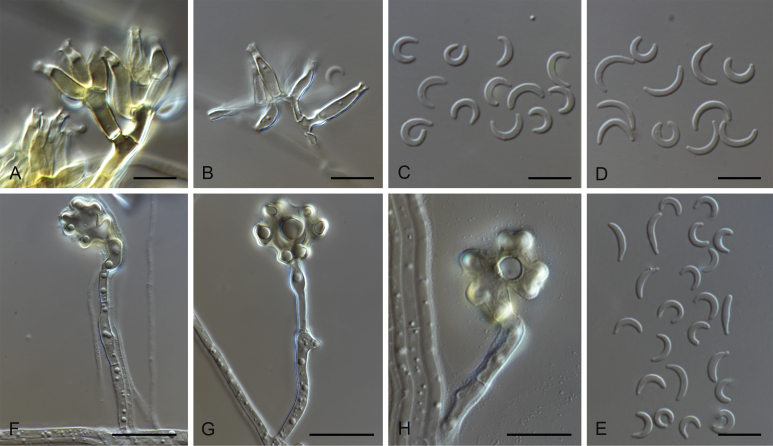
Falciphoriella solaniterrestris M. Hern.-Restr. & Crous, sp. nov. MycoBank MB816903. Fig. 3.
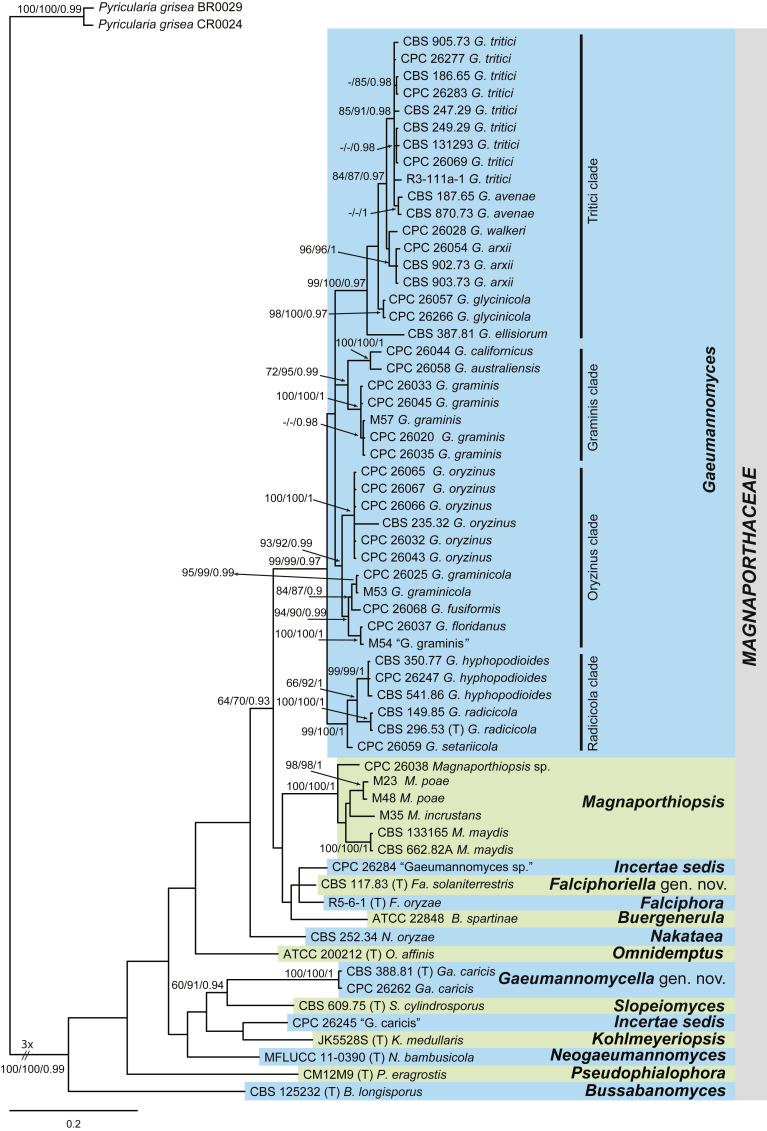
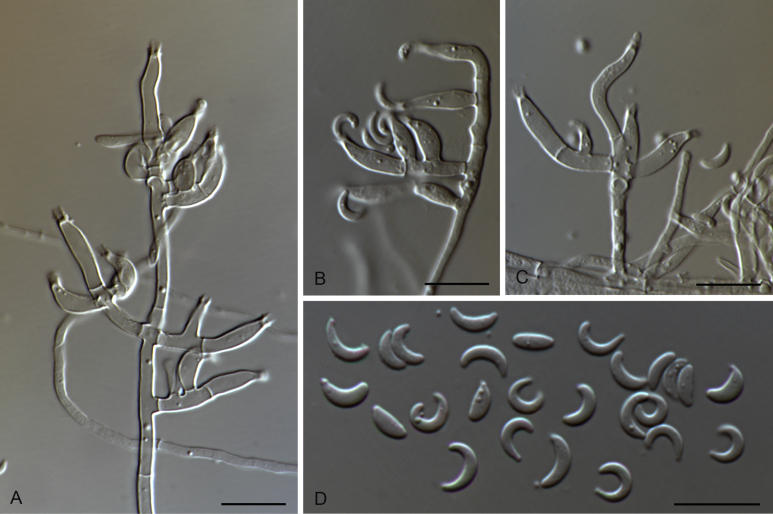
Fig. 3.
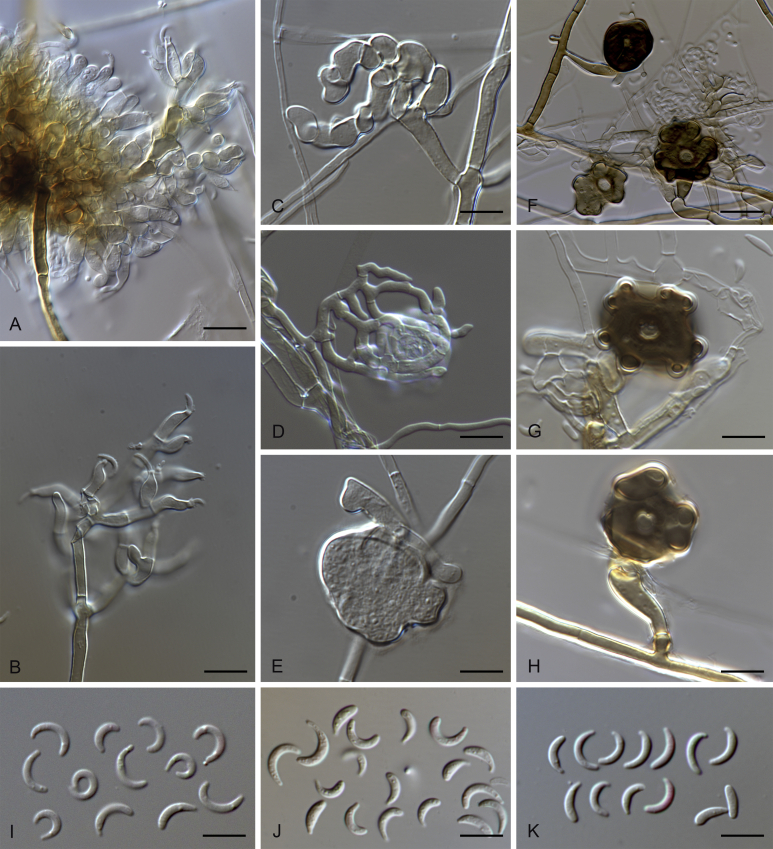
Falciphoriella solaniterrestris (CBS 117.83). A–C. Conidiophores and conidiogenous cells. D. Conidia. Scale bars: A, C, D = 10 μm; B = 5 μm.
Etymology: Referring to the substrate solani – Solanum the Latin generic name of potato, and terrestris – from soil, since this species was isolated from soil in a potato field.
Description on MEA. Mycelium consisting of septate, branched, smooth, hyaline to subhyaline, 1.5–4.5 μm diam hyphae. Conidiophores differentiated, indeterminate, branched, hyaline to pale brown. Conidiogenous cells phialidic, hyaline to pale brown, solitary or grouped, terminal or intercalary, cylindrical, lageniform, to conical, straight or curved, 5–29 × 1.5–3.5 μm, cylindrical to funnel-shaped collarette up to 2.5 μm, 1–2 μm diam. Conidia mainly fusiform sometimes obovoid, slightly curved at the ends, usually pointed base, hyaline, 5–13 × 1–2 μm. Hyphopodia not observed.
Culture characteristics: After 7 d at 25 °C: On PDA reaching 35 mm diam, aerial mycelium moderate, cottony, vinaceous buff, submerged mycelium dark, margin effuse, rhizoid; reverse no change. On MEA reaching 50 mm diam, aerial mycelium abundant, dense in the centre, cottony, submerged mycelium dark, margin effuse; reverse sepia in the centre, colourless to the periphery. On OA reaching 50 mm diam, flat, aerial mycelium moderate, cottony, white, submerged mycelium pale luteous in the centre, colourless to the periphery, margin effuse; reverse colourless to yellow.
Specimen examined: Netherlands, Prov. Groningen, Groningen, isolated from soil in potato field, Jul. 1982, isol. by H. Nielander (holotype, CBS H-22572, culture ex-type CBS 117.83).
Notes: Falciphoriella solaniterrestris is introduced for a fungus isolated from soil in a potato field in the Netherlands. The isolate CBS 117.83, formerly identified as Gaeumannomyces sp. (Klaubauf et al. 2014), formed a separated branch distant from Gaeumannomyces in our phylogenetic tree (Fig. 1) and represents a new genus in Magnaporthaceae.
Gaeumannomycella M. Hern.-Restr. & Crous, gen. nov. MycoBank MB816904.
Etymology: Morphologically similar to the genus Gaeumannomyces.
Mycelium consisting of septate, branched, smooth, hyaline to brown, hyphae. Conidiophores slightly differentiated and hyaline. Conidiogenous cells phialidic, scarce, formed close to the hyphopodia, hyaline to pale brown, mostly grouped, terminal sometimes intercalary, ampulliform, lageniform or conical, straight or curved, with inconspicuous collarette. Conidia lunate or cylindrical, hyaline. Hyphopodia hyaline to brown when mature, lobed.
Type species: Gaeumannomycella caricis M. Hern.-Restr. & Crous
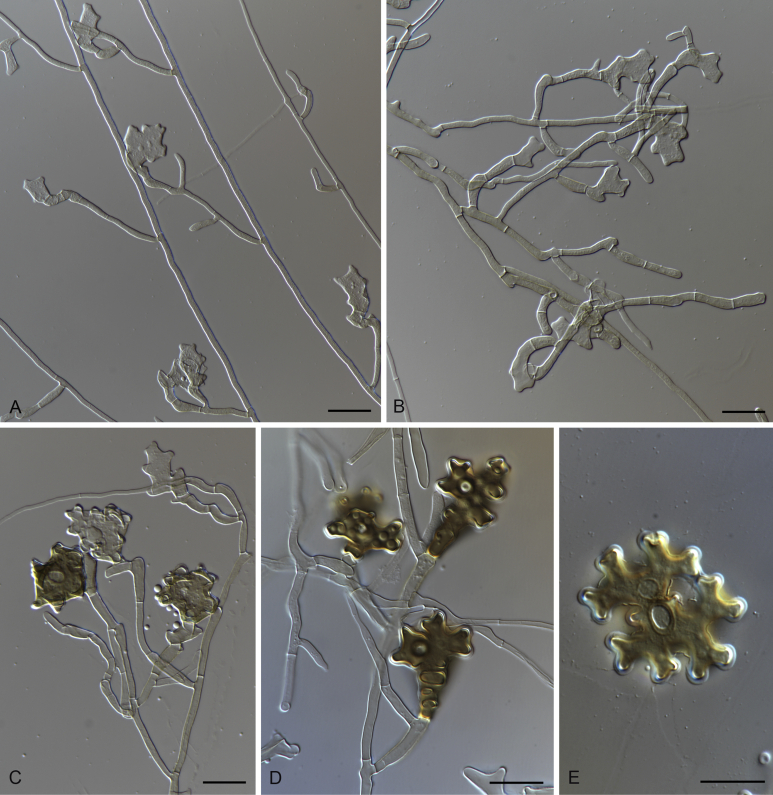
Gaeumannomycella caricis M. Hern.-Restr. & Crous, sp. nov. MycoBank MB816905. Fig. 4.
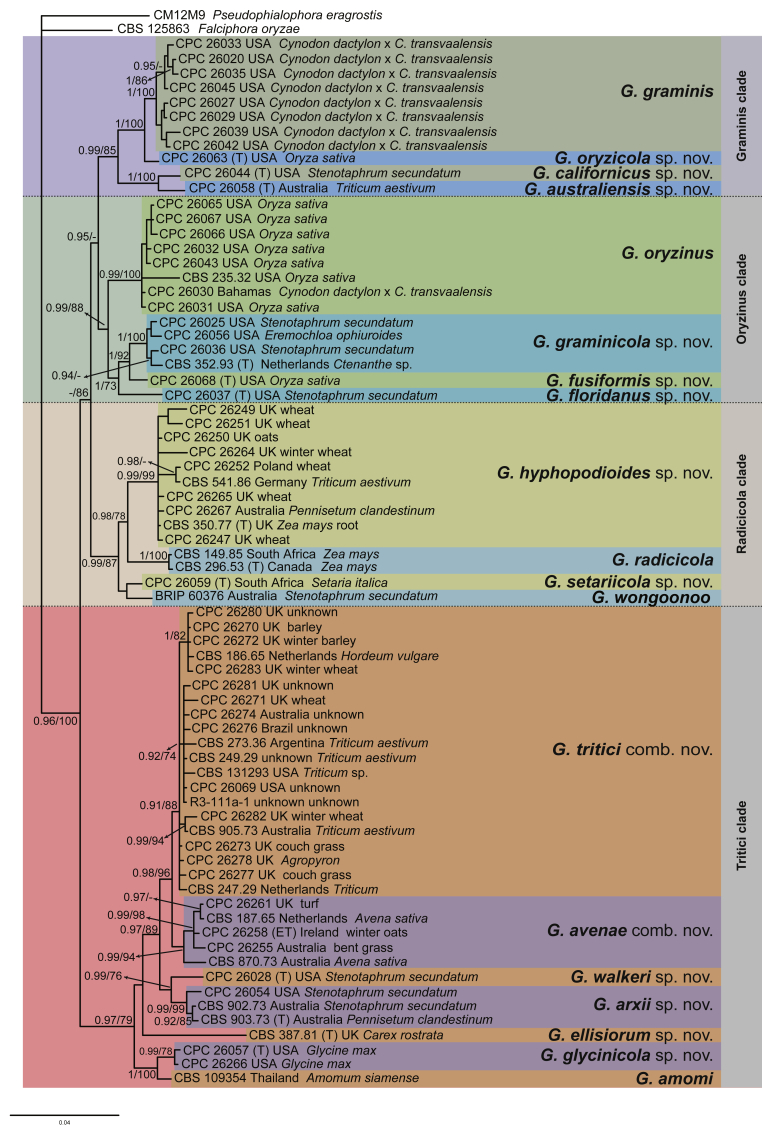
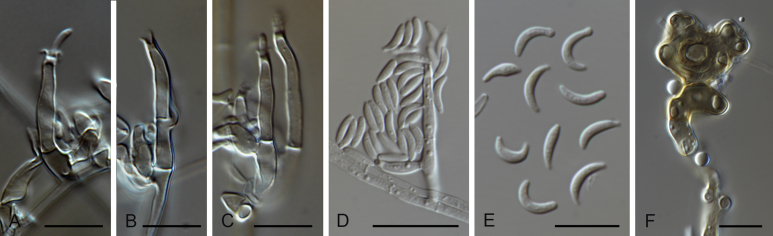
Fig. 4.
Gaeumannomycella caricis (CBS 388.81). A, B. Conidiogenous cells. C. Conidia. D–H. Hyphopodia. Scale bars: A–H = 10 μm.
Etymology: Referring to the substrate Carex rostrata from which the species was isolated for the first time.
Description on PDA. Mycelium consisting of septate, branched, smooth, hyaline to brown, 1.5–6.5 μm diam hyphae. Conidiophores slightly differentiated, hyaline. Conidiogenous cells phialidic, scarce, formed close to the hyphopodia, hyaline to pale brown, mostly grouped, terminal sometimes intercalary, ampulliform, lageniform or conical, straight or curved, 6.5–12 × 3–4 μm, inconspicuous collarette up to 1 μm long, 1 μm diam. Conidia lunate or cylindrical, hyaline, 6.5–9.5 × 1–2 μm. Hyphopodia hyaline to brown, lobed at maturity, 15–31 × 10–23 μm.
Culture characteristics: After 7 d at 25 °C: On PDA reaching 35 mm diam, flat, aerial mycelium scarce to moderate, cottony, white, pale grey, submerged mycelium dark or white, margin effuse, rhizoid; reverse dark. On MEA reaching 36 mm diam, elevated, aerial mycelium moderate to abundant dense, cottony, white, submerged mycelium dark, margin effuse, rhizoid; reverse dark in the centre colourless to the periphery. On OA reaching 40 mm diam, elevate, aerial mycelium moderate to abundant, cottony to funiculose, submerged mycelium dark, margin effuse, rhizoid; reverse dark.
Specimens examined: UK, Wales, Powys, Llyn Ebyr, isolated from Carex rostrata, 28 May 1979, M.B. Ellis (holotype, CBS H-22575, culture ex-type CBS 388.81); Powys, Llyn Ebyr, isolated from Carex rostrata, 3 Jan. 1980, unknown collector, CPC 26262 = CBS 141374.
Notes: Gaeumannomycella caricis is only known occurring on Carex rostrata. This new species is represented by two strains isolated from the UK. It is morphologically similar to Gaeumannomyces since it produces a harpophora-like asexual state and lobed hyphopodia, but was phylogenetically considerably different. In the phylogenetic tree (Fig. 1), Slopeiomyces is shown to be the sister clade of Gaeumannomycella.
Gaeumannomyces Arx & D.L. Olivier, Trans. Br. mycol. Soc. 35: 32. 1952.
= Rhaphidophora Ces. & De Not., Sfer. Ital.: 79. 1863.
= Rhaphidospora Fr., Summa veg. Scand., Section Post. (Stockholm): 401. 1849.
Mycelium mainly immersed, consisting of branched, septate, hyaline to brown hyphae. Sexual morph. Ascomata perithecial, superficial and submerged, globose, subglobose to elliptical, with a cylindrical neck, dark brown to black. Peridium textura epidermoidea. Paraphyses hyaline, septate, often constricted at the septa, widest at the base and gradually narrow at the apex, dissolving at maturity. Asci numerous, unitunicate, cylindrical to elongated clavate, shortly stalked, with apical refringent ring, 8 ascospores. Ascospores faintly tinted yellowish in mass, hyaline to pale brown, vacuolated, slightly curved to sinuate, ends rounded, widest in the middle, tapering toward the base, septate, septa often indistinct. Asexual morph harpophora-, phialophora-like. Conidiophores branched, verticillate, indeterminate often reduced to conidiogenous cells, hyaline to brown. Conidiogenous cells phialidic, borne directly from the mycelium or on pale brown conidiophores, solitary or in dense clusters, individual phialides lageniform, cylindrical, straight or slightly curved tapering to a short cylindrical to funnel-shaped or hardly visible collarette. Conidia dimorphic (A) hyaline, ovoid to cylindrical, straight to curved, tapering to an often acute base, solitary, grouped in slimy heads and/or (B) hyaline, falcate to lunate or usually strongly curved in a semicircle with varying degrees of curvature, solitary, arranged in heads at the apex. Hyphopodia when present hyaline or becoming brown when mature, simple or lobed. Sclerotia present or absent.
Type species: Gaeumannomyces graminis (Sacc.) Arx & D.L. Olivier
Gaeumannomyces amomi Bussaban et al., Nova Hedwigia 73: 488. 2001.
Specimen examined: Thailand, Chiang Mai, Doi Suthep Pui national Park, isolated from Alpinia malaccensis, endophytic in leaves, Aug. 1999, B. Bussaban (CBS 109354).
Notes: This species was described as an endophyte from leaves and pseudo-stem of Amomum siamense and Alpinia malaccensis in Thailand (Bussaban et al. 2001). It differs from G. graminis in having wider ascospores, more septa and being the only Gaeumannomyces species reported from Zingiberaceae.
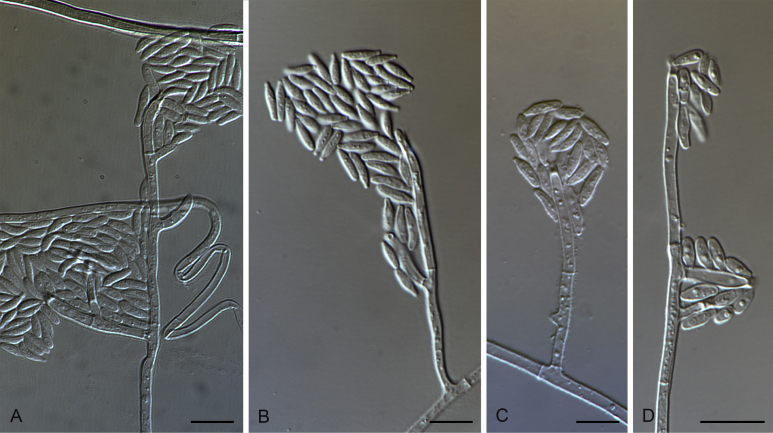
Gaeumannomyces arxii M. Hern.-Restr. & Crous, sp. nov. MycoBank MB816890. Fig. 5.
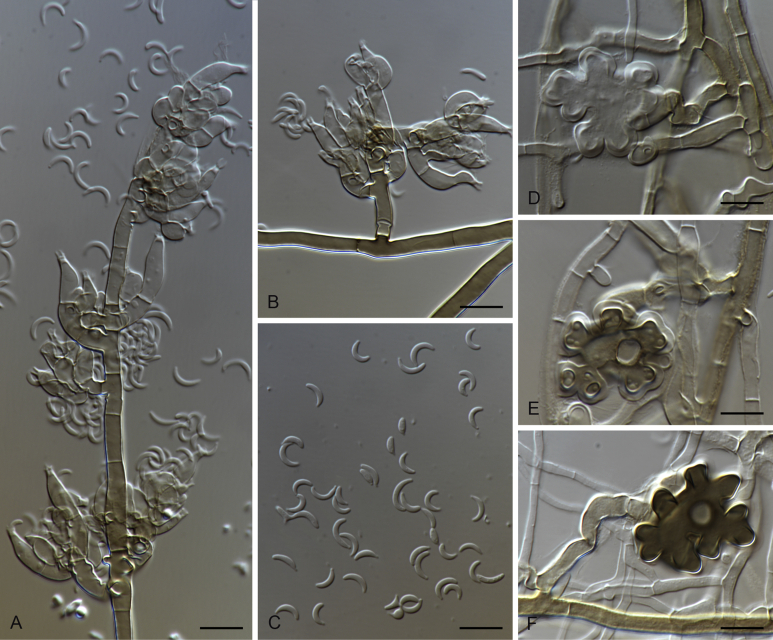
Fig. 5.
Gaeumannomyces arxii (CBS 903.73). A–C. Conidiophores and conidiogenous cells. D. Conidia. Scale bars: A–D = 10 μm.
Etymology: Name after Josef Adolph von Arx, a distinguished mycologist who together with D.L. Olivier introduced the genus Gaeumannomyces.
Description on MEA. Mycelium consisting of septate, branched, smooth, hyaline to pale brown, 1–5 μm diam hyphae. Conidiophores erect, simple or branched sometimes reduced to a conidiogenous cells. Conidiogenous cells phialidic, terminal or intercalary, hyaline, cylindrical to lageniform, straight to curved, 6–23 × 2–5 μm, with a cylindrical to funnel-shaped, refractive collarette up to 3 μm long, 1.5–3.5 μm wide. Conidia lunate, fusiform, tapering to pointed base, hyaline, 4–10 × 1–2 μm. Hyphopodia not observed.
Culture characteristics: After 7 d at 25 °C: On PDA reaching 72 mm, flat, mycelium mostly submerged, grey olivaceous or greyish sepia in the centre, aerial mycelium scarce and white, margin effuse to irregular, rhizoid; reverse light olivaceous to white greyish in the centre, periphery no change. On MEA reaching 64 mm, elevated, cottony to funiculose, aerial mycelium white, submerged mycelium black, and margin effuse to rhizoid; reverse centre dark, white to the periphery; or flat, velvety, mycelium aerial white, mycelium mostly submerged, margin effuse to rhizoid; reverse white. On OA reaching 70 mm, glabrous, white to colourless, submerged mycelium dark, margin effuse with rhizoid zones; reverse no change.
Specimens examined: Australia, New South Wales, Turramurra, isolated from Pennisetum clandestinum (kikuyu grass), stolon, 11 Aug. 1972, J. Walker & P. Wong (holotype, CBS H-22573, culture ex-type CBS 903.73); Wagga Wagga, isolated from Stenotaphrum secundatum (buffalo grass), 23 Jul. 1969, J. Kuiper, CBS 902.73. USA, California, isolated from Stenotaphrum secundatum, 1991, H. Wilkinson, CPC 26054 = CBS 141375.
Notes: Gaeumannomyces arxii is represented by two strains from Stenotaphrum secundatum and another one from Pennisetum clandestinum from USA and Australia. This species was placed in the Tritici clade with G. walkeri as sister species. Both species were isolated from Stenotaphrum secundatum. Nevertheless, G. walkeri had brown and lobed hyphopodia, while in G. arxii hyphopodia were not observed. Some minor differences in the conidial morphology were noted between these two species. Gaeumannomyces walkeri had cylindrical to fusiform conidia after 8 d, and at 14 d conidia were mostly lunate and longer than G. arxii, where conidia are mostly lunate at 8 and 14 d.
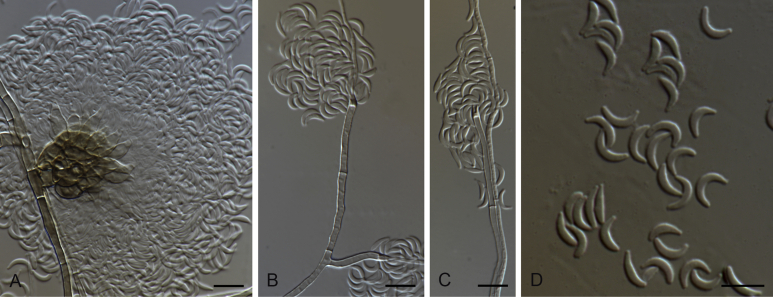
Gaeumannomyces australiensis M. Hern.-Restr. & Crous, sp. nov. MycoBank MB816906. Fig. 6.
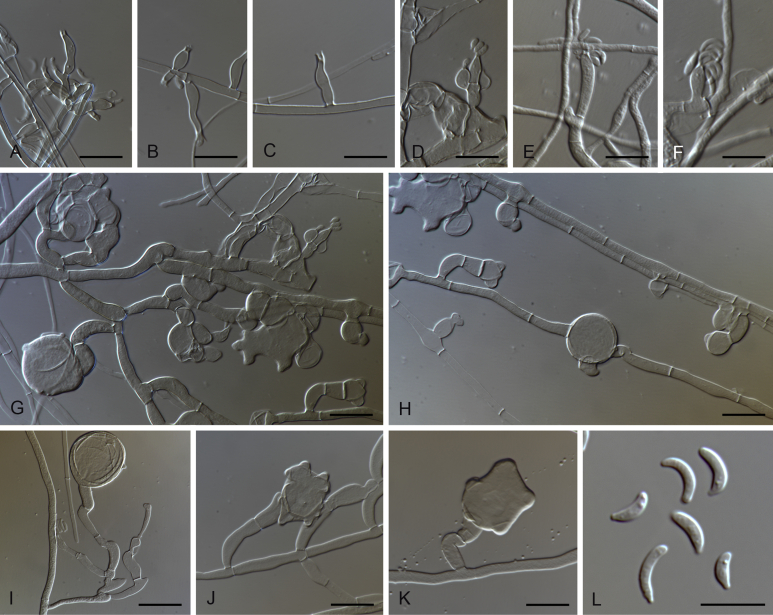
Fig. 6.
Gaeumannomyces australiensis (CPC 26058). A–C. Conidiogenous cells. D. Conidiogenous cells and conidia. E. Conidia. F. Hyphopodium. Scale bars: A–C = 5 μm; D–F = 10 μm.
Etymology: Named after Australia, the country where this fungus was collected.
Description on MEA. Mycelium consisting of septate, branched, smooth, hyaline to subhyaline, 1–4 μm diam hyphae. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, scarce, hyaline to pale brown, solitary or grouped, terminal or intercalary, cylindrical, sometimes lageniform, straight or curved, 6.5–27.5 × 1.5–3 μm, cylindrical to funnel-shaped collarette up to 2.5 μm long, 1–2 μm diam. Conidia lunate, allantoid, hyaline, 5–11 × 1–1.5 μm. Hyphopodia hyaline becoming brown when mature, lobed, 18.5–25 × 21.5–23 μm.
Culture characteristics: After 7 d at 25 °C: On PDA reaching 65 mm diam, flat, aerial mycelium scarce and white, submerged mycelium dark (isabelline), margin effuse, rhizoid; reverse no change. On MEA reaching 60 mm diam, aerial mycelium abundant, cottony, pale greenish grey, margin effuse, rhizoid; reverse centre fuscous periphery amber white to white. On OA reaching 55 mm diam, aerial mycelium white, submerged mycelium dark, smoke grey, margin effuse; reverse pale olivaceous grey.
Specimen examined: Australia, New South Wales, isolated from Triticum aestivum, unknown date, J. Walker (holotype, CBS H-22581, culture ex-type CBS 141387 = CPC 26058).
Notes: This is a single-isolate species collected on Triticum from Australia. This strain was placed in the Graminis clade with G. californicus as sister species (Fig. 2).
Gaeumannomyces avenae (E.M. Turner) Hern.-Restr. & Crous, comb. et stat. nov. MycoBank MB816891.
≡ Ophiobolus graminis var. avenae E.M. Turner, Trans. Br. mycol. Soc. 24: 279. 1941 [1940].
= Gaeumannomyces graminis var. avenae (E.M. Turner) Dennis, British Cup Fungi & their Allies: 202. 1960.
Type details: Original collection lost. Neotype in Kew. UK, Scotland, Applecross, West ross, on Avenae sativa, 29 Sep. 1946, RWG Dennis, K(M) (slides as DAR 32104). Ireland, Killinick, Wexford, isolated from winter oats, 11 Sept. 1990, unknown collector (epitype designated here, CBS H-22587, MBT 371909, culture ex-epitype CPC 26258).
Additional specimens examined: Australia, New South Wales, isolated from Agrostis (bentgrass), 11 Nov. 1980, unknown collector, CPC 26253; CPC 26254; CPC 26255; Western Australia, 25 km W of Mt. Barker, isolated from Avena sativa, Dec. 1963, deposited by J. Walker, CBS 870.73. Ireland, Killinick, Wexford, isolated from winter oats, 11 Sept. 1990, unknown collector, CPC 26257; CPC 26259; Killarney, Kerry, isolated from turf, 11 Sep. 1990, unknown collector, CPC 26260. Netherlands, Oostelijk Flevoland, isolated from Avena sativa, root, unknown date, isol. M. Gerlagh, CBS 187.65. UK, England, Gleadthorpe, Notts, isolated from Avena sativa, 10 Jul. 1990, unknown collector CPC 26256 = CBS 141376; Macclesfield, Cheshire, isolated from turf, 11 Sep. 1990, unknown collector, CPC 26261.
Notes: In our phylogenetic tree (Fig. 2), G. avenae is represented by five isolates, formerly identified as Gga, and is placed in the Tritici clade with G. tritici as sister species. Isolates were collected growing on Avenae sativa and grasses; from Australia, Ireland, the Netherlands and the UK.
Dennis (1960) proposed Gga (=Ophiobolus graminis var. avenae E.M. Turner 1940) for those strains of G. graminis with larger ascospores and occurring on oats. This fungus causes take-all of oats and take-all patch of turfgrasses. Walker, 1972, Walker, 1980 distinguished Gga from Ggg by the former producing simple hyphopodia, and distinguished Gga from Ggt, the fungus that causes wheat take-all, on the basis of longer mean ascospores length, and pathogenicity to oats. Nevertheless, Gga can also infect grasses and which seems to be much more important hosts than oats.
Previous studies demonstrated that oats and wheat take-all fungi are closely related but separated from G. graminis (Walker, 1972, Walker, 1981, Bryan et al., 1995, Fouly and Wilkinson, 2000, Saleh and Leslie, 2004). Gaeumannomyces tritici and G. avenae are more virulent species and have simple hyphopodia, but ascospores are larger in G. avenae (Walker 1972). In addition, Rachdawong et al. (2002) differentiated G. avenae (as Gga) and G. tritici (as Ggt) based on sequences of avenacinase-like genes. A recent phylogenomic study by Luo et al. (2015a) included isolates from all three varieties, which revealed considerable differences among them. Our multi-locus analysis combining LSU, ITS, rpb1 and tef1 also showed differences in these two clades, and therefore we propose G. avenae comb. et stat. nov. to accommodate this species.
Gaeumannomyces californicus M. Hern.-Restr. & Crous, sp. nov. MycoBank MB816892. Fig. 7.
Fig. 7.
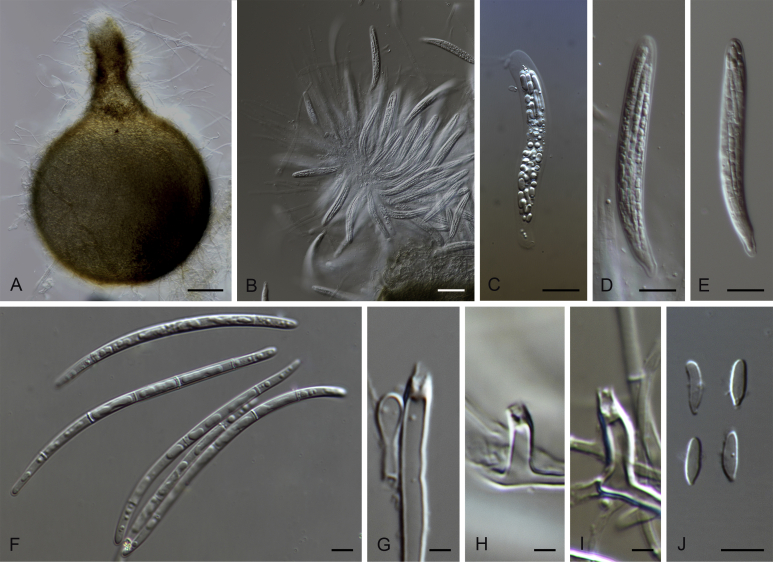
Gaeumannomyces californicus (CPC 26044). A, B. Conidiophores and conidiogenous cells. C. Conidia. D, E. Hyphopodia. Scale bars: A–F = 10 μm.
Etymology: Named after California, the state in the USA where the sample was collected.
Description on MEA. Mycelium consisting of septate, branched, smooth, hyaline to brown, 1.5–4.5 μm diam hyphae. Conidiophores more or less differentiated, verticillate. Conidiogenous cells phialidic, hyaline to pale brown, solitary or grouped, terminal or intercalary, lageniform, cylindrical, straight or curved, 4.5–24 × 1.5–4 μm, cylindrical to funnel-shaped collarette up to 2.5 μm, 1–2 μm wide. Conidia lunate, allantoid or fusiform, hyaline, 4–11 × 1–1.5 μm. Hyphopodia hyaline, becoming brown when mature, lobed, 25–32.5 × 24–30 μm.
Culture characteristics: After 7 d at 25 °C: On PDA reaching 85 mm diam, flat, aerial mycelium scarce, cottony, white, submerged mycelium grey olivaceous, margin effuse, rhizoid; reverse smoke grey. On MEA reaching 85 mm diam, aerial mycelium abundant, cottony to funiculose, white, smoke grey, submerged mycelium dark, margin effuse, rhizoid; reverse olivaceous. On OA reaching 85 mm diam, flat, aerial mycelium moderate to abundant, cottony to funiculose, white, submerged mycelium dark, olivaceous black, margin effuse, rhizoid; reverse centre no change, periphery olivaceous.
Specimen examined: USA, California, isolated from Stenotaphrum secundatum, 1992, M. Elliott (holotype, CBS H-22574, culture ex-type CBS 141377 = CPC 26044).
Notes: This species is represented by one strain isolated from Stenotaphrum secundatum, placed in the Graminis clade with G. australiensis as sister species (Fig. 2). In culture G. californicus produces long and branched conidiophores, and lunate to fusiform conidia; being different from G. australiensis, in which the conidiophores are mostly reduced to conidiogenous cells and conidia are lunate to cylindrical.
Gaeumannomyces ellisiorum M. Hern.-Restr. & Crous, sp. nov. MycoBank MB816893. Fig. 8.
Fig. 8.
Gaeumannomyces ellisiorum (CBS 387.81). A–F. Conidiogenous cells. G–K. Hyphopodia. L. Conidia. Scale bars: A–L = 10 μm.
Etymology: Named after M.B. & J.P Ellis, who collected this fungus in the UK.
Description on PDA. Mycelium consisting of septate, branched, smooth, hyaline to pale brown, 1.5–3.5 μm diam hyphae. Conidiophores reduced to conidiogenous cells. Conidiogenous cells phialidic, scarce, terminal or intercalary, hyaline, clustered often solitary, cylindrical to lageniform, 5–18 × 3–4 μm, with a cylindrical, refractive collarette, up to 2.5 μm long, 1–2 μm diam. Conidia lunate, allantoid strong to slightly curved, to fusiform with one side straighter than the other, hyaline, 4–9 × 1–2 μm. Hyphopodia at the beginning formed as chlamydospores-like structures, globose, 1–3 cells, intercalary often terminal, hyaline, becoming lobed and pale brown hyphopodia 19.5–35.5 × 16.5–30 μm.
Culture characteristics: After 7 d at 25 °C: On PDA reaching 80 mm diam, cottony, aerial mycelium white, submerged mycelium buff, margin effuse; reverse colourless (dark under inoculum). On MEA reaching 70 mm diam, cottony, aerial mycelium abundant, dense, and white, margin effuse; reverse apricot. On OA reaching 90 mm diam, cottony-funiculose, moderate, colourless.
Specimen examined: UK, Suffolk, Wolves Wood Reserve, isolated from Deschampsia caespitosa, dead culm and sheath, 9 Sep. 1979, M.B. & J.P. Ellis (holotype, CBS H-22576, culture ex-type CBS 387.81).
Notes: This species was previously identified as Ggg, and is only known from the type locality, growing on dead culms and sheaths of Deschampsia caespitose. In the multigene phylogeny, isolate CBS 387.81 was considerably genetically distant from other Gaeumannomyces species, and formed a separate branch in the Tritici clade (Fig. 2).
Gaeumannomyces floridanus M. Hern.-Restr. & Crous, sp. nov. MycoBank MB816894. Fig. 9.
Fig. 9.
Gaeumannomyces floridanus (CPC 26037). A, B. Conidiogenous cells and conidia. C. Conidiogenous cells. D. Hyphopodia. E. Conidia. Scale bars: A–D. = 10 μm.
Etymology: Named after Florida, the state in the USA where the sample was collected.
Description on MEA. Mycelium consisting of septate, branched, smooth, hyaline to brown, 1.7–5 μm diam hyphae. Conidiophores more or less differentiated, simple or verticillate, hyaline to light brown. Conidiogenous cells phialidic, scarce, hyaline to pale brown, solitary or in groups, cylindrical, lageniform or clavate, straight or curved, 7–14.5 × 2–3.5 μm, inconspicuous collarette. Conidia lunate, slightly to strongly curved, hyaline, 5–11 × 1–1.5 μm. Hyphopodia lobed, hyaline becoming brown when mature, 18–27 × 14.5–26.5 μm.
Culture characteristics: After 7 d at 25 °C: On PDA reaching 85 mm diam, aerial mycelium scarce, white, submerged mycelium dark (greyish sepia), margin effuse, rhizoid; reverse greyish sepia. On MEA reaching 70 mm diam, aerial mycelium abundant, cottony, submerged mycelium mouse grey, margin entire, rhizoid; reverse fuscous. On OA reaching 85 mm diam, aerial mycelium moderate, mouse grey, submerged mycelium dark, margin effuse, rhizoid; reverse mouse grey, olivaceous grey, colourless to the periphery.
Specimen examined: USA, Florida, isolated from Stenotaphrum secundatum, 1992, M. Elliott (holotype, CBS H-22577, culture ex-type CBS 141378 = CPC 26037).
Notes: This species is known only from the type locality, Florida (USA). It is located on a separate branch in the Oryzinus clade (Fig. 2), and is introduced here as new species. The strain CPC 26037 formed a sub-clade together with G. graminicola and G. fusiformis. Gaeumannomyces floridanus is distinguished from G. fusiformis by its lunate conidia, and from G. graminicola in their hyphopodial pigmentation, being hyaline and brown in G. floridanus and brown in G. graminicola.
Gaeumannomyces fusiformis M. Hern.-Restr. & Crous, sp. nov. MycoBank MB816895. Fig. 10.
Fig. 10.
Gaeumannomyces fusiformis (CPC 26068). A–D. Conidiophores, conidiogenous cells and conidia. Scale bars: A–D = 10 μm.
Etymology: The name refers to the presence of fusiform conidia.
Description on MEA. Mycelium consisting of septate, branched, smooth, hyaline to brown, 1.5–5 μm diam hyphae. Conidiophores erect, simple or branched sometimes reduced to conidiogenous cells. Conidiogenous cells phialidic, terminal or intercalary, hyaline, cylindrical, straight to curved, 5–28 × 1.5–5 μm, with a cylindrical, refractive collarette, up to 2.5 μm, 1–2 μm diam. Conidia fusiform, tapering at the base, hyaline, 5–9.5 × 1–2.5 μm. Hyphopodia not observed.
Culture characteristics: After 7 d at 25 °C: On PDA reaching 90 mm diam, aerial mycelium cottony, white, submerged mycelium rhizoid, hazel, margin rhizoid; reverse pale isabelline. On MEA reaching 60 mm diam, cottony, aerial mycelium moderate, white to grey, margin effuse; reverse umber in the centre, paler to the periphery. On OA reaching 90 mm diam, aerial mycelium scarce to moderate, cottony to funiculose, white, submerged mycelium olivaceous; reverse isabelline.
Specimen examined: USA, Arkansas, isolated from Oryza sativa, 1992, C. Rothrock G-8 (holotype, CBS H-22578, culture ex-type CBS 141379 = CPC 26068).
Notes: This is a single-isolate species isolated from Oryza sativa and phylogenetically placed in the Oryzinus clade with G. graminicola as sister group (Fig. 2). Morphologically it is distinct from G. graminicola and other species in the genus since it produces fusiform instead of lunate conidia.
Gaeumannomyces glycinicola M. Hern.-Restr., G. Canning & Crous, sp. nov. MycoBank MB816907. Fig. 11.
Fig. 11.
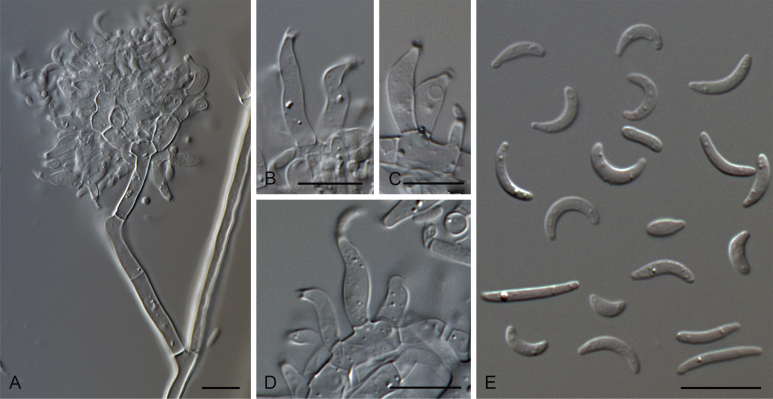
Gaeumannomyces glycinicola (CPC 26266). A–E. Hyphopodia. Scale bars: A–E = 10 μm.
Etymology: The name refers to the host genus Glycine, from which this species was isolated.
Description on MEA. Mycelium consisting of septate, branched, smooth, straight or flexuous, hyaline to brown, 1.5–4 μm diam hyphae. Hyphopodia hyaline getting dark brown when mature, lobed, 22.5–43 × 15–34 μm diam. Conidiophores and conidia not observed.
Culture characteristics: After 7 d at 25 °C: On PDA reaching 90 mm diam, aerial mycelium scarce, white, submerged mycelium rhizoid, pale cinnamon, margin rhizoid; reverse pale cinnamon. On MEA reaching 70 mm diam, cottony, aerial mycelium abundant, dense, white, submerged umber, margin effuse; reverse interweave, umber. On OA reaching 90 mm diam, cottony, moderate and colourless.
Specimens examined: USA, Indiana, isolated from Glycine max, 1974, D. Huber (holotype, CBS H-22579, culture ex-type CPC 26057 = DAR 28746); isolated from Glycine max (pods of soybean), 1974, unknown collector, CPC 26266 = CBS 141380.
Notes: Isolates CPC 26057 and CPC 26266, formerly classified as Ggg, grouped in the Tritici clade with G. amomi as sister group (Fig. 2). Gaeumannomyces glycinicola shows different ecological preferences compared to G. amomi. Gaeumannomyces glycinicola is the only Gaeumannomyces species reported from a dicotyledonous plant whereas G. amomi has been reported as an endophyte in Amomum siamense (Bussaban et al. 2001). In our study both isolates remained sterile on all media and conditions tested. Nevertheless, Roy et al. (1982) studied soybean isolates from Midwest USA (identified as Ggg) and described perithecia as globose to ellipsoidal with cylindrical necks, pale to dark brown. Ascospores filiform, attenuated toward one end, measuring 71.6 ± 6.8 × 2.6 ± 0.5 μm, hyaline and multiseptate. Hyphopodia with one or more lobes, and brown. Although G. glycinicola is similar to G. graminis in hyphopodial morphology, and overlaps in ascospore dimensions, in our analyses G. glycinicola was phylogenetically distant from G. graminis (Fig. 2). Pathogenicity tests demonstrated that isolates from soybean produce the typical take-all symptoms on wheat, causing mild to severe infections, but disease symptoms were not observed on soybean leaves, stems or roots (Roy et al. 1982). On the other hand, G. graminis is not able to infect wheat. The presence of brown, lobed hyphopodia distinguishes G. glycinicola from G. tritici which produces simple hyphopodia as well as different aminopeptidase profiles (Roy et al. 1982).
Gaeumannomyces graminicola M. Hern.-Restr. & Crous, sp. nov. MycoBank MB816896. Fig. 12.
Fig. 12.
Gaeumannomyces graminicola (CBS 352.93, CPC 26056, CPC 26025, CPC 26036). A, B. Conidiogenous cells. C–E. Conidia. F–H. Hyphopodia. Scale bars: A–H = 10 μm.
Etymology: Named after the grass hosts from which it was isolated.
Description on MEA. Mycelium consisting of septate, branched, smooth, hyaline to brown, 1–4 μm diam hyphae. Conidiophores more or less differentiated, verticillate. Conidiogenous cells phialidic, hyaline to pale brown, solitary or grouped, terminal, sometimes intercalary, cylindrical, lageniform, 5–20 × 2–4.5 μm, collarette up to 3 μm long, 1–2.5 μm diam. Conidia lunate, slightly or strongly curved, hyaline, 5–11.5 × 1–2 μm. Hyphopodia lobed, brown, 16.5–24 × 15.5–23.5 μm.
Culture characteristics: After 7 d at 25 °C: On PDA reaching 74 mm diam, flat, aerial mycelium scarce, cottony, white, submerged mycelium dark, in the centre hazel, grey, isabelline, olivaceous grey, buff to the periphery, margin effuse, rhizoid; reverse fuscous black, mouse grey or isabelline in the centre, no change to the periphery. On MEA reaching 76 mm diam, aerial mycelium moderate, cottony to funiculose, white, mouse grey to pale mouse grey, submerged mycelium dark (mouse grey), margin effuse, rhizoid; reverse centre fuscous, periphery amber white to white. On OA reaching 77 mm diam, flat, aerial mycelium scarce to moderate or abundant, cottony to funiculose, white, submerged mycelium dark, olivaceous grey, olivaceous black, dark mouse grey, margin effuse, rhizoid; reverse olivaceous, mouse grey, leaden grey, no change to the periphery.
Specimens examined: Netherlands, near Barendrecht, isolated from Ctenanthe, stem base, isol. J.W. Veenbaas-Rijks (holotype, CBS H-22580, culture ex-type CBS 352.93). USA, Florida, isolated from Stenotaphrum secundatum, 1988, M. Elliott, CPC 26022; 1990 M. Elliott, CPC 26025 = CBS 141381; 1991, M. Elliott, CPC 26036 = CBS 141382; Georgia, isolated from Eremochloa ophiuroides, 1994, H. Wilkinson, CPC 26056 = CBS 141383.
Notes: This species is represented by four isolates placed in the Oryzinus clade (Fig. 2). The strains were isolated from different grasses; i.e. Ctenanthe, Stenotaphrum, and Eremochloa from The Netherlands and USA. Formerly they were identified as Ggg; however the phylogenetic analyses place this species distant from G. graminis.
Gaeumannomyces graminis (Sacc.) Arx & Oliver, Trans. Br. mycol. Soc. 35: 32. 1952. Fig. 13.
Fig. 13.
Gaeumannomyces graminis (CPC 26035). A–C. Conidiophores, conidiogenous cells and conidia. D. Conidia. Scale bars: A–D = 10 μm.
Basionym: Rhaphidophora graminis Sacc., Fungi venet. nov. vel. Crit., Sér. 2: 307. 1875.
≡ Ophiobolus graminis (Sacc.) Sacc., Reliq. Libert 2: no. 134. 1875.
≡ Ophiochaeta graminis (Sacc.) Hara, Journal of Plant Protection, Tokyo 3: 342. 1916.
≡ Gaeumannomyces graminis (Sacc.) Arx & D.L. Olivier, Trans. Br. Mycol. Soc. 35: 32. 1952. var. graminis
≡ Sphaeria cariceti Berk. & Broome, Ann. Mag. nat. Hist., Ser. 3 7: 455. 1861.
≡ Ophiobolus cariceti (Berk. & Broome) Sacc., Syll. fung. (Abellini) 2: 349. 1883.
≡ Linocarpon cariceti (Berk. & Broome) Petr., Sydowia 6: 387. 1952.
≡ Gaeumannomyces cariceti (Berk. & Broome) Lar.N. Vassiljeva, Nizshie Rasteniya, Griby i Mokhoobraznye Dalnego Vostoka Rossii, Griby. Tom 4. Pirenomitsety i Lokuloaskomitsety (Sankt-Peterburg) 4: 146. 1998.
Type details: Saccardo, P.A. 1875. Fungi veneti novi vel critici. Series II. Nuovo Giornale Botanico Italiano. 7:299–329 [307–308] in PAD. Slides as DAR 21032. On Cynodon or Agropyron, Selva, Treviso, Italy, Oct. ? 1874.
Description on MEA. Mycelium consisting of septate, branched, smooth, hyaline to pale brown, 1–4 μm diam hyphae. Conidiophores differentiated, branched often verticillate, hyaline, pale brown to brown. Conidiogenous cells phialidic, solitary or grouped, terminal, hyaline to pale brown, cylindrical to lageniform, straight or curved, 7–30 × 1.5–4 μm, with a cylindrical to conical, refractive, collarette up to 3.5 μm long, 1–1.7 μm wide. Conidia lunate, allantoid, hyaline, 4–10 × 1–2 μm. Hyphopodia not observed.
Culture characteristics: After 7 d at 25 °C: On PDA reaching 60 mm diam, aerial mycelium scarce to moderate, cottony, olivaceous grey, buff or isabelline, submerged mycelium darker, margin diffuse to rhizoid; reverse centre olivaceous grey, colourless to the periphery. On MEA reaching 62 mm diam, aerial mycelium abundant to moderate, cottony, pale olivaceous grey, darker to the periphery, submerged mycelium dark, margin effuse, rhizoid; reverse fuscous dark, rhizoid to the periphery. On OA reaching 62 mm diam, flat to cottony, greenish grey to grey olivaceous in the centre, white to colourless to the periphery, aerial mycelium moderate to abundant, white, submerged mycelium dark in the centre, margin effuse; reverse pale mouse grey.
Additional specimens examined: USA, Florida, isolated from Cynodon dactylon × C. transvaalensis, 1987, M. Elliott, CPC 26020 = CBS 141384; 1991, M. Elliott, CPC 26027; CPC 26029; CPC 26033 = CBS 141385; CPC 26035 = CBS 141386; 1992, M. Elliott, CPC 26039; CPC 26042; CPC 26045.
Notes: Isolates formerly identified as Ggg segregated into different species in the phylogenetic tree (Fig. 2). Gaeumannomyces graminis, the type species of the genus was originally described from Italy, on Cynodon or Agropyron. Unfortunately an epitype cannot be proposed at present since the isolates studied here are from a different geographic origin (USA). Based on host affinities we consider G. graminis s. s. as those strains isolated from Cynodon represented here by eight strains. The sister species was G. oryzicola which shows perithecia and an asexual morph in culture, characterised by conidiogenous cells scarce and cylindrical, with conidia fusiform, straight to slightly curved, while in G. graminis the perithecia were not observed in any of the studied isolates, and the asexual morph sometimes presents brown conidiophores with lunate conidia.
Gaeumannomyces graminis is a widespread species with a wide host range, variable pathogenicity, and high morphological and genetic diversity (Walker, 1972, Walker, 1980, Bryan et al., 1995 Fouly et al. 1996, Ward and Bateman, 1999, Saleh and Leslie, 2004, Zhang et al., 2011, Sadeghi et al., 2012). Gaeumannomyces graminis, formerly recognised as the variety graminis, is characterised by perithecia immersed in culm and leaf sheath tissue, associated with a superficial mycelium producing both pale and brown hyphopodia. The asci are unitunicate, with an apical refractive ring and ascospores filiform, septate, hyaline, measuring (70–)80–105(–110) × 2–3(–4) μm (Walker 1980). “Phialophora sp. (with lobed hyphopodia)” has been tentatively referred to as the asexual morph of G. graminis based on morphological observations of the asexual morph (Walker 1980). With the available data at that moment, Walker (1980) did not introduce a new species for “Phialophora sp. lobed hyphopodia”. Nevertheless, in our study, strains identified as “Phialophora sp. lobed hyphopodia” from the UK, Poland, Australia and Germany were placed in the clade Radicicola (Fig. 2), and are here introduced as a new species to accommodate those isolates (see G. hyphopodioides).
Gaeumannomyces hyphopodioides M. Hern.-Restr. & Crous, sp. nov. MycoBank MB816897. Fig. 14.
Fig. 14.
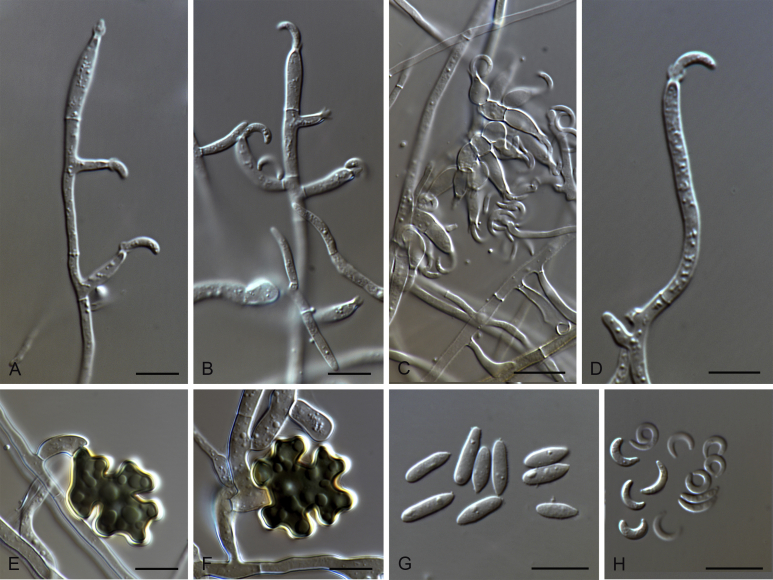
Gaeumannomyces hyphopodioides (CBS 541.86, CBS 350.77, CPC 26248, CPC 26267) A, B. Conidiophores. C–D. Mycelium. E. Young hyphopodium. F–H. Hyphopodia. I–K. Conidia. Scale bars: A–H = 10 μm.
Etymology: hyphopodium – referring to the first approximation to this species “Phialophora sp. lobed hyphopodia” (Walker 1981).
= Phialophora radicicola var. radicicola sensu Deacon (1974) and subsequent British workers; NOT P. radicicola Cain var. radicicola (Cain 1952).
Description on PDA. Mycelium consisting of septate, branched, smooth, hyaline to red brown, 1–4 μm diam hyphae. Conidiophores differentiated, branched often verticillate, brown, sometimes reduced to conidiogenous cells. Conidiogenous cells phialidic, terminal or intercalary, hyaline to pale brown, cylindrical to lageniform, straight or curved, 7–21 × 2–4 μm, with a cylindrical to funnel-shaped collarette, up to 2.5 μm long, 1–2.5 μm diam. Conidia lunate, slightly to strongly curved, fusiform, allantoid, hyaline, 5.5–10.5 × 1–2 μm. Hyphopodia lobed, dark brown, 17–28 × 18–25 μm.
Culture characteristics: After 7 d at 25 °C: On PDA reaching 85 mm diam, aerial mycelium abundant, cottony, white to grey, submerged mycelium hazel, olivaceous, dull green, margin effuse, rhizoid; reverse centre cinnamon, hazel, dark green, grey olivaceous, umber, dark olivaceous, colourless to the periphery. On MEA reaching 35–65 mm diam, aerial mycelium moderate, cottony, white to pale mouse grey, submerged mycelium grey to olivaceous grey, margin effuse; reverse dark (fuscous, olivaceous grey, dark brown). On OA reaching 20–55 mm diam, aerial mycelium scarce, white to grey, submerged mycelium grey, olivaceous black, margin effuse, rhizoid; reverse dark (olivaceous grey) or pale olivaceous, mouse grey, colourless to the periphery.
Specimens examined: Australia, New South Wales, isolated from Pennisetum clandestinum, 24 Oct. 1977, unknown collector, CPC 26267. Germany, Monheim, isolated from Triticum aestivum, seedling, unknown date, isol. A. Walz, CBS 541.86. Poland, Pulawy, isolated from wheat, 18 Oct. 1979, unknown collector, CPC 26252. UK, Butt Furlong, Woburn, Beds, isolated from oats, 27 Apr. 1983, unknown collector, CPC 26250; Essex, isolated from Zea mays, root, May 1972, J.W. Deacon G6 (holotype, CBS H-22582, culture ex-type CBS 350.77 = ATCC 28234 = IMI 187786); Hertfordshire, Fosters West, RRes, isolated from wheat, 11 Oct. 1985, unknown collector, CPC 26247 = CBS 141388; 29 Sep. 1989, unknown collector, CPC 26248; CPC 26249; West Barnfield, RRes, isolated from winter wheat, 9 Feb. 1990, unknown collector, CPC 26264 = CBS 141389; CPC 26265.
Notes: This species forms a distinct subclade in the Radicicola clade (Fig. 2) together with G. radicicola (ex-type culture CBS 296.53 and CBS 149.85), G. wongoonoo (BRIP 60376) and G. setariicola (CPC 26059). It is represented by strains isolated from Zea mays, Triticum, Avena, and Pennisetum, mainly from the UK, and others from Australia, Germany, and Poland.
Walker (1980) referred to this species as “Phialophora sp. (with lobed hyphopodia)”. He found this species morphologically similar to the superficial mycelia present in Ggg. Nevertheless, he noticed that the isolates of “Phialophora sp. (with lobed hyphopodia)” from France, England and Australia from different substrates never developed perithecia. Our results show that G. hyphopodioides is different from G. graminis and is phylogenetically closer to G. radicicola than G. graminis. Gaeumannomyces hyphopodioides is different from G. radicicola in having lobed hyphopodia; McKeen (1952) described G. radicicola as having simple, brown hyphopodia (as chlamydospores with a pore). In addition some differences in pathogenicity are reported. Gaeumannomyces radicicola has been associated with root rot in corn (Cain, 1952, McKeen, 1952). The strain CBS 350.77 of G. hyphopodioides isolated from corn exhibits low virulence (Deacon, 1973, Walker, 1980).
Two of the isolates studied by Walker (1980) are represented in our tree as CBS 350.77 and CPC 26267. Walker (1980) found that the British (CBS 350.77), and the Australian (CPC 26267) isolates had identical serological tests. In our study those strains are placed in G. hyphopodioides together with other isolates from the UK, Poland and Germany.
Gaeumannomyces oryzicola M. Hern.-Restr. & Crous, sp. nov. MycoBank MB816898. Fig. 15.
Fig. 15.
Gaeumannomyces oryzicola (CPC 26063). A. Perithecium. B–E. Asci. F. Ascospores. G–I. Conidiogenous cells. J. Conidia. Scale bars: A, B = 50 μm; C–E = 20 μm, F–J = 10 μm.
Etymology: Named after the host from which it was isolated, Oryza.
Description on MEA. Mycelium consisting of septate, branched, smooth, hyaline to brown, 2–6 μm diam hyphae. Ascomata perithecial, superficial and submerged, globose, subglobose to elliptical, 110–413 × 112–525 μm with a cylindrical neck, dark brown, 22–30 × 38–47 μm. Peridium textura epidermoidea. Paraphyses hyaline, septate, dissolving at maturity. Asci numerous, unitunicate, cylindrical to elongated clavate, shortly stalked, with apical refringent ring, 8 ascospores, 118–148 × 14–16 μm. Ascospores faintly tinted yellowish in mass, hyaline to pale brown, vacuolated, slightly curved to sinuate, ends rounded, 92.5–120 × 4–6, 0–5-septate, septa often indistinct. Conidiophores if present slightly differentiated. Conidiogenous cells phialidic, terminal or intercalary, hyaline, cylindrical, 7.5–20.5 × 2–2.5 μm, with a cylindrical collarette, up to 3 μm long, 1.5–2 μm diam. Conidia lunate, allantoid to fusiform, hyaline, 5–9 × 1.5–2.5 μm. Hyphopodia not observed.
Specimen examined: USA, Texas, isolated from Oryza sativa, prior to 1992, J. Krausz (holotype, CBS H-26063, culture ex-type CBS 141390 = CPC 26063).
Notes: Gaeumannomyces oryzicola is represented by a single isolate in the Graminis clade. In the phylogenetic tree (Fig. 2), it clustered as the sister species of G. graminis.
Gaeumannomyces oryzinus (Sacc.) Schrantz., Bull. trimest. Soc. mycol. Fr. 76: 337. 1961. Fig. 16.
Fig. 16.
Gaeumannomyces oryzinus (CBS 235.32, CPC 26032, CPC 26065, CPC 26067) A. Perithecium. B–G. Asci. H–I. Ascospores. J–M, O, Q–S. Conidiogenous cells. N, P, T. Conidia. U, V. Hyphopodia. Scale bars: A–C = 50 μm; D–I = 20 μm, J–V = 10 μm.
Basionym: Ophiobolus oryzinus Sacc., Nuovo Giornale Botanico Italiano 23: 203. 1916.
≡ Linocarpon oryzinum (Sacc.) Petr., Sydowia 6: 387. 1952.
≡ Gaeumannomyces oryzinus (Sacc.) Schrantz as “oryzinum”, Bull. trimest. Soc. mycol. Fr. 76: 337. 1961.
= Linospora pulchella Speg. Anal. Mus. Nac. Hist. Nat. Buenos Aires 23: 71. 1912.
Description on MEA. Mycelium consisting of septate, branched, smooth, hyaline to brown, 1.5–6 μm diam hyphae. Ascomata perithecial, superficial and submerged, globose, subglobose to elliptical, with a cylindrical neck, dark brown to black. Peridium textura epidermoidea. Paraphyses hyaline, septate, often constricted at the septa, widest at the base and gradually narrow at the apex, dissolving at maturity. Asci numerous, unitunicate, cylindrical to elongated clavate, shortly stalked, with apical refringent ring, 8 ascospores, 113–173.5 × 14.5–24. Ascospores faintly tinted yellowish in mass, hyaline to pale brown, vacuolated, slightly curved to sinuate, ends rounded, widest in the middle, tapering toward the base, 96–116 × 3.5–5.5, 0–3-septate, septa often indistinct. Conidiophores if present slightly differentiated. Conidiogenous cells phialidic, terminal or intercalary, pale brown sometimes hyaline, cylindrical to lageniform, straight or curved, 5–21 × 2–5 μm, with a cylindrical to funnel-shaped collarette, up to 2.8 μm long, 1–2 μm diam. Conidia lunate, allantoid to fusiform, hyaline, 5–11 × 1–2.5 μm. Hyphopodia if present lobed, brown, 19–45 × 15.5–36 μm diam.
Culture characteristics: After 7 d at 25 °C: On PDA reaching 79 mm diam, aerial mycelium scarce to moderate, white to pale grey, submerged mycelium dark (dark to grey olivaceous, isabelline, olivaceous, smoke grey). On MEA reaching 80 mm diam, aerial mycelium moderate to abundant, cottony to funiculose, mouse grey, pale mouse grey, isabelline, pale olivaceous grey, greenish olivaceous, smoke grey, to the periphery white, submerged mycelium dark (fuscous, isabelline, mouse grey), margin effuse, rhizoid; reverse fuscous in the centre, white to the periphery or colourless. On OA reaching 85 mm diam, aerial mycelium moderate, mouse grey, submerged mycelium dark, margin effuse, rhizoid; reverse mouse grey, olivaceous grey, colourless to the periphery.
Specimens examined: Bahamas, New Providence, isolated from Cynodon dactylon × C. transvaalensis, 1991, M. Elliott, CPC 26030 = CBS 141391. USA, Arkansas, Stuttgart, isolated from Oryza sativa, Nov. 1931, E.C. Tullis, CBS 235.32; Florida, isolated from Oryza sativa, 1991, M. Elliott, CPC 26031; CPC 26032; 1992, L. Datnoff, CPC 26043 = CBS 141392; Arkansas, isolated from Oryza sativa, 1992, C. Rothrock, CPC 26065; CPC 26066; CPC 26067 = CBS 141393.
Notes: In our phylogenetic tree G. oryzinus is represented by seven isolates on Oryza sativa from the USA and one isolate on Cynodon from The Bahamas. Among the USA strains, CBS 235.32 was also studied by Walker (1972) as BRIP 3517.
Gaeumannomyces oryzinus was introduced as Ophiobolus oryzinus by Saccardo in 1916, growing on rotting Oryza sativa culms in the Philippines. Later it was treated as a synonym of Ggg by Walker (1972), who studied the holotypes of both species and concluded that they were the same species. Nevertheless, our phylogenetic studies demonstrate that G. graminis and G. oryzinus are distinct species.
Other species isolated from Oryza sativa are different from G. oryzinus; for instance, G. fusiformis has fusiform conidia and in G. oryzicola the ascospores are larger and have more septa (92.5–120 × 4–6 μm; 0–5 septa), and phylogenetically distant, being placed in the Graminis clade (Fig. 2).
Gaeumannomyces radicicola (Cain) J. Luo & N. Zhang, Mycologia 107: 644. 2015. Fig. 17.
Fig. 17.
Gaeumannomyces radicicola (CBS 296.53). A. Conidiophores. B–D. Conidiogenous cells. E. Conidia. Scale bars: A–E = 10 μm.
Basionym: Phialophora radicicola Cain, Canad. J. Bot. 30: 340. 1952.
≡ Phialophora radicicola var. radicicola Cain, Canad. J. Bot. 30: 340. 1952. [NOT Phialophora radicicola var. graminicola, Deacon 1974].
= Harpophora radicicola (Cain) W. Gams, Stud. Mycol. 45: 192. 2000.
= Phialophora zeicola Deacon & D.B. Scott, Trans. Br. Mycol. Soc. 81: 256. 1983.
≡ Harpophora zeicola (Deacon & D.B. Scott) W. Gams, Stud. Mycol. 45: 192. 2000.
= Gaeumannomyces graminis var. maydis J.M. Yao, Yong C. Wang & Y.G. Zhu, Acta Mycol. Sin. 11: 99. 1992. [Type details. China, Province Liaoning, Tiling, Xu Heng-wu. On basal internodes of Zea mays. Shenyang Agricultural University, MHSAU 3805].
Specimens examined: Canada, Ontario, Chatham, isolated from Zea mays, root, 1950, R.F. Cain (isotype of Phialophora radicicola CBS H-7592, CBS H-7593, culture ex-isotype of Phialophora radicicola, CBS 296.53). South Africa, unknown locality, isolated from Zea mays, Feb. 1984 (isotype of Phialophora zeicola CBS H-7597, culture ex-isotype of Phialophora zeicola, CBS 149.85).
Notes: Gaeumannomyces radicicola was described as a corn root-rot pathogen in Canada (Cain, 1952, McKeen, 1952). Later Yao et al. (1992) introduce Ggm for the take-all fungus of maize as a new variety of G. graminis. Morphologically it is characterised by perithecia, asci and ascospores typical for Gaeumannomyces, with a phialophora-like asexual morph and simple to slightly lobed hyphopodia.
Based on ITS sequence analyses Ward & Bateman (1999) concluded that Ggm and G. radicicola (represented by isolates of P. radicicola and P. zeicola) were conspecific, but the authors did not formally propose the synonymy. Comparing those GenBank sequences with our dataset, we introduce Ggm as synonym of G. radicicola.
Gaeumannomyces setariicola M. Hern.-Restr. & Crous, sp. nov. MycoBank MB816899. Fig. 18.
Fig. 18.
Gaeumannomyces setariicola (CPC 26059). A–D. Conidiophores and conidia. E. Conidia. Scale bars: A–E = 10 μm.
Etymology: The name refers to the host genus Setaria, from which this species was isolated.
Description on MEA. Mycelium consisting of septate, branched, smooth, hyaline to brown, 1.2–4 μm diam hyphae. Conidiophores simple or verticillate, often reduced to conidiogenous cells. Conidiogenous cells mono- or poly-phialidic, terminal or intercalary, hyaline, cylindrical to lageniform, straight to curved, 6.5–28.5 × 2–4 μm, with a cylindrical to funnel-shaped, refractive collarette, up to 3 μm long, 1.5–2.5 μm diam. Conidia lunate, allantoid to fusiform strong to slightly curved, tapered at the base, hyaline, 4–12 × 1–2 μm. Hyphopodia not observed.
Culture characteristics: After 7 d at 25 °C: On PDA reaching 85 mm diam, flat, aerial mycelium scarce, light isabelline in the centre, smoke grey to the periphery, submerged mycelium darker, margin rhizoid; reverse isabelline. On MEA reaching 75 mm diam, cottony, aerial mycelium abundant, pale greenish grey, margin rhizoid; reverse fuscous in the centre, white-amber to the periphery. On OA reaching 65 mm diam, flat, aerial mycelium scarce, colourless, submerged mycelium with grey olivaceous “zones”; reverse similar.
Specimen examined: South Africa, Limpopo province, Warmbaths (current name is Bela-Bela), isolated from Setaria italica, 1981, D.B. Scott (holotype, CBS H-22584, culture ex-type CBS 141394 = PRRI 4754 = CPC 26059).
Notes: This species is represented by one strain isolated from Setaria italica in the Radicicola clade (Fig. 2). Gaeumannomyces setariicola showed the typical characteristics of harpophora-like fungi; however, hyphopodia were not observed.
Gaeumannomyces tritici (J. Walker) Hern.-Restr. & Crous, comb. et stat. nov. MycoBank MB816900.
Basionym: Gaeumannomyces graminis var. tritici J. Walker, Trans. Br. Mycol. Soc. 58: 439. 1972.
Type details: Australia, New South Wales, Dubbo, on wheat, 20 Oct. 1969, GM Murray, DAR 17916.
Additional specimens examined: Argentina, La Pampa, isolated from Triticum aestivum, 9 Feb. 1935, isol. L. Grodsinsky, CBS 273.36. Australia, South Australia, Mortlock, isolated from Triticum aestivum, 16 Dec. 1980, unknown collector, CPC 26268 = CBS 141396; Western Australia, Carnamah, isolated from Triticum aestivum, 28 Oct. 1970, A. Parker, DAR 23140 = CBS 905.73; unknown locality, unknown substrate, 29 Nov. 1983, unknown collector, CPC 26274. Brazil, Espumoso, isolated from wheat, 9 Feb. 1982, unknown collector, CPC 26269 = CBS 141397; unknown locality, unknown substrate, 9 Feb. 1982, D. Hornby Girua, CPC 26276. Netherlands, Oostelijk Flevoland, isolated from Hordeum vulgare, isol. M. Gerlagh No. My53g, CBS 186.65; unknown locality, isolated from Triticum, unknown date, dep. H.A. Diddens, CBS 247.29. UK, Far Field II Woburn, Beds, isolated from Elymus repens (couch grass), 9 Jun. 1988, unknown collector, CPC 26273 = CBS 141398; Peterborough, unknown substrate, 1999, unknown collector, CPC 26280; Hertfordshire, RRes, isolated from Bromus sp. (brome grass), 26 Feb. 1982, unknown collector, CPC 26275; isolated from Agropyron, 26 Feb. 1982, unknown collector, CPC 26278; unknown substrate, 1992, unknown collector, CPC 26281; Great Harpenden, isolated from couch grass, 21 Aug. 1980, unknown collector, CPC 26277; New Zealand fields, RRes, isolated from Triticum aestivum (winter wheat), 1 Sept. 2012, G. Canning, CPC 26282 = CBS 141399; CPC 26283; Pastures, isolated from wheat, 24 Jun. 1988, unknown collector, CPC 26271; Summerdells, isolated from Hordeum vulgare (winter barley), 4 Mar. 1987, unknown collector, CPC 26272. USA, Montana, isolated from Triticum sp., unknown date, Juhnke, CBS 131293; unknown locality, unknown substrate, prior to 1987, R. Smiley, CPC 26069 = CBS 141395. Unknown country, unknown locality, isolated from Triticum aestivum, Dec. 1929, isol. C.A. Jörgensen, CBS 249.29.
Notes: Ggt was introduced as a variety of G. graminis (for a misapplied Ophiobolus graminis) for the wheat take-all fungus (Walker 1972). Walker (1972) distinguished Ggt from Ggg and Gga in their hyphopodial morphology, ascospore size and pathogenicity. In Ggt hyphopodia are not lobed as in Ggg, ascospores are shorter than in Gga, and Ggt is pathogenic to wheat. In our study, isolates received as Ggt grouped in a clade (Fig. 2), representing different species from G. graminis and G. avenae, and here we propose G. tritici comb. et stat. nov. for those isolates.
Gaeumannomyces tritici is the most aggressive species in the genus, is widespread, and found mainly on Triticum, but was also reported growing on other hosts as well. In our phylogenetic tree this species was represented by isolates from Triticum, Hordeum, Elymus repens and Agropyron.
Gaeumannomyces walkeri M. Hern.-Restr. & Crous, sp. nov. MycoBank MB816901. Fig. 19.
Fig. 19.
Gaeumannomyces walkeri (CPC 26028). A–D. Conidiophores and conidiogenous cells. E, F. Hyphopodia. G. Conidia at 7 days. H. Conidia at 14 days. Scale bars: A–H = 10 μm.
Etymology: Named after John Walker, for his contributions to understanding the taxonomy and pathology of Gaeumannomyces.
Description on MEA. Mycelium consisting of septate, branched, smooth, hyaline to brown, 1–4.5 μm diam hyphae. Conidiophores semi- to macronematous branched often verticillate. Conidiogenous cells phialidic, terminal or intercalary, hyaline, cylindrical to lageniform, straight or curved, 6–23 × 2–3.5 μm, with a funnel-shaped collarette, up to 2.5 μm long, 1–2.5 μm diam. Conidia initially (8 d) fusiform, 7.5–11 × 2–3 μm, becoming lunate, slightly to strongly curved, allantoid to fusiform, sinuous, hyaline, 5–14 × 1–1.5 μm. Hyphopodia lobed, brown, 20–31 × 18.5–24.5 μm.
Culture characteristics: After 7 d at 25 °C: On PDA reaching 65 mm diam, flat, aerial mycelium scarce, pale olivaceous in the centre, colourless to the periphery, margin effuse; reverse pale olivaceous. On MEA reaching 70 mm diam, cottony, funiculose aerial mycelium abundant, white, margin rhizoid; reverse umber, darker in the centre. On OA reaching 60 mm diam, cottony, aerial mycelium moderate, white, submerged mycelium grey olivaceous; reverse isabelline.
Specimen examined: USA, Alabama, isolated from Stenotaphrum secundatum, 1991, M. Elliott (holotype, CBS H-22586, culture ex-type CBS 141400 = CPC 26028 = FL156).
Note: This species is represented by one strain that is placed in the Tritici clade with G. arxii as sister group (Fig. 2).
Gaeumannomyces wongoonoo P. Wong, Mycol. Res. 106: 861. 2002.
Notes: This species is only known from the type locality, Australia (Wong 2002), and was placed in the Radicicola clade (Fig. 2). Compared with the other species in the clade, G. wongoonoo has shorter (36–75 × 3–5 μm) ascospores than G. radicicola (55–85 × 2.5–4 μm), and wider conidia than other species in this clade (5–12.5 × 3–5 μm, Wong 2002).
Pathogenicity tests demonstrated that this species is pathogenic on Stenotaphrum secundatum (buffalo grass) causing “wongoonoo patch” and it was not pathogenic to wheat or maize (Wong 2002).
Discussion
This is the first study that presents a robust phylogeny using a broad distribution of Gaeumannomyces isolates from different hosts and geographic origins. Based on our phylogenetic analyses two new genera with harpophora-like asexual morphs are introduced in Magnaporthaceae: Falciphoriella and Gaeumannomycella. By combining multi-locus data from ITS, LSU, rpb1 and tef1 sequences with morphological analyses, we were able to delimit 19 species in Gaeumannomyces, 12 of which are formally proposed as new species and two as new combinations. The taxonomic status of two unique phylogenetic lineages (CPC 26245 and CPC 26284) remains unresolved as they were only represented in our tree by single sterile isolates.
Traditionally, isolates of G. graminis had been classified in four varieties; Ggg, Gga, Ggt and Ggm (Turner, 1940, Dennis, 1960, Walker, 1972, Yao et al., 1992). However, this classification was inconsistent with our results. Previous molecular studies had shown Ggg as the genetically most diverse variety (Ward and Bateman, 1999, Ulrich et al., 2000, Freeman and Ward, 2004). Ward & Bateman (1999), based on ITS sequences, recognised three groups in Ggg: Ggg I, Ggg II and Ggg III. Nevertheless, no taxonomic changes or new species were proposed by the authors. These results agree with our phylogenetic analyses; isolates formerly identified as Ggg presented a high genetic diversity and we find 14 cryptic species; named G. arxii, G. australiensis, G. californicus, G. ellisiorum, G. floridanus, G. fusiformis, G. glycinicola, G. graminicola, G. graminis, G. hyphopodioides, G. oryzicola, G. oryzinus, G. setariicola and G. walkeri.
Much confusion has prevailed in the naming of Gaeumannomyces, especially in the varieties of G. graminis. Walker, 1972, Walker, 1980, Walker, 1981 studied type specimens and several collections of Gaeumannomyces in detail. He found that Ophiobolous oryzinus (= Gaeumannomyces oryzinus), described by Saccardo on rotting rice culms from the Philippines, was conspecific with Ggg. Nevertheless, in our phylogenetic analyses strains that were isolated from Oryza sativa, including the CBS 235.35 material studied by Walker (1972), formed a separate clade from G. graminis s. s. representing a different species; resulting in the resurrection of G. oryzinus. On the other hand, the presumed anamorph of Ggg was referred to as “Phialophora sp. with lobed hyphopodia” (Walker, 1980, Walker, 1981). However, our phylogenetic analyses show that isolates identified as “Phialophora sp. with lobed hyphopodia”, form a separate clade and we therefore introduce here as a new species G. hyphopodioides to accommodate those isolates.
An interesting result generated in the present study was that a well-supported clade comprising mainly of wheat and oat isolates, formerly identified as Ggt and Gga, clustered outside the G. graminis clade, and represent different species, G. avenae and G. tritici. This is consistent with previous studies, which indicated that G. avenae and G. tritici are more virulent pathogens than G. graminis. Both present simple hyphopodia and are phylogenetically related (Walker, 1972, Walker, 1980, Ward and Bateman, 1999, Freeman and Ward, 2004, Saleh and Leslie, 2004).
Ggm was introduced for a fungus with simple hyphopodia growing on maize (Yao et al. 1992). Based on ITS sequences (Ward & Bateman 1999) of Ggm, it was shown to be conspecific with G. radicicola, but the authors did not formally propose the synonymy. After comparing those GenBank sequences with our results, here we introduce Ggm as synonym of G. radicicola. Unfortunately no strains of Ggm were available to us to sequence additional loci for the combined analyses.
In the past, ascospore size, hyphopodial morphology and host preference used to be regarded as the most important criteria to discriminate species and varieties of Gaeumannomyces (Turner 1940, Walker, 1972, Walker, 1981, Yao et al., 1992, Deacon, 1973, Deacon, 1974). Ascospores and hyphopodia produced in the natural substrate have proven to be useful in the differentiation of the varieties in G. graminis, but do not always develop in culture. The variability in host range within Gaeumannomyces is so great that grouping isolates based on host origin alone is problematic for predicting pathogenicity and genetic relatedness. Wheat isolates belong mainly to G. tritici, but isolates from this substrate can also be identified as G. hyphopodioides or G. australiensis. Oat isolates grouped mainly in G. avenae, even though one isolate was placed in G. hyphopodioides. Oryza sativa is a common substrate for G. oryzinus, G. oryzicola and G. graminicola. Although strains used in the present study were collected globally, the USA and UK are over-represented whereas Asia, Africa and Central and South America are less well-represented.
Gaeumannomyces spp. are morphologically difficult to distinguish because of their simple morphology, the overlapping of many features and considerable intraspecific variation. Molecular identification is mandatory to classify species in Gaeumannomyces. The four gene loci used in this study were chosen based on their previous use in molecular studies in Magnaporthales (Zhang et al., 2011, Klaubauf et al., 2014). The ITS and rpb1 loci are more or less equal in their ability to distinguish species in this genus (17 / 19 and 15 / 17, respectively), whereas LSU and tef1 are not very successful in distinguishing species in this genus (9 / 19 and 11 / 18, respectively). By combining ITS and rpb1 it is possible to resolve the phylogenetic position of G. oryzicola as an individual species, different from G. oryzinus and G. graminis. Based on ITS sequences G. oryzicola is placed in the G. oryzinus species clade, whereas based on rpb1 sequences it is placed in G. graminis.
In addition to providing a phylogenetic overview of an important phytopathogenic genus, Gaeumannomyces, this study offers reliable sequences and cultures for future studies. The lack of type or reference strains in this genus makes the correct identification of a species difficult and confusing; this was partly addressed in the present study by designating ex-epitype culture for G. avenae. Unfortunately it was not possible to propose epi- or neotypes for all known species, since the geographical origins of included isolates were not the same as described in the protologues (e.g. G. graminis and G. oryzinus).
Acknowledgements
The authors thank the technical staff, Arien van Iperen (cultures and deposit of herbarium samples), and Trix Merkx and Gerard Verkleij (deposit of isolates) for their invaluable assistance. VM was supported by the Biotechnology and Biological Sciences Research Council of the UK (BBSRC) through the Institute Strategic Programme Grant 20:20 Wheat® (BB/J/00426X/1). GC was supported by BBSRC through the research grant “Enhancing diversity in UK wheat through a public sector pre-breeding programme” (BB/I002278/1). We also thank the editors and reviewers for critically reviewing the manuscript.
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
Contributor Information
M. Hernández-Restrepo, Email: m.hernandez@cbs.knaw.nl.
P.W. Crous, Email: p.crous@cbs.knaw.nl.
References
- Augustin C., Ulrich K., Ward E. RAPD-based inter- and intravarietal classification of fungi of the Gaeumannomyces–Phialophora complex. Journal of Phytopathology. 1999;147:109–117. [Google Scholar]
- Bateman G.L., Ward E., Antoniw J.F. Identification of Gaeumannomyces graminis var. tritici and G. graminis var. avenae using a DNA probe and non-molecular methods. Mycological Research. 1992;96:737–742. [Google Scholar]
- Bryan G.T., Daniels M.J., Osbourn A.E. Comparison of fungi within the Gaeumannomyces–Phialophora complex by analysis of ribosomal DNA sequences. Applied and Environmental Microbiology. 1995;61:681–689. doi: 10.1128/aem.61.2.681-689.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussaban B., Lumyong S., Lumyong P. Two new species of endophytes (Ascomycetes) from Zingiberaceae sporulating in culture. Nova Hedwigia. 2001;73:487–493. [Google Scholar]
- Cain R.F. Studies of fungi imperfecti I. Phialophora. Canadian Journal of Botany. 1952;30:338–343. [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50:19–22. [Google Scholar]
- Crous P.W., Verkley G.J.M., Groenewald J.Z. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2009. Fungal biodiversity. (CBS laboratory manuals series 1). [Google Scholar]
- Crous P.W., Wingfield M.J., Mansilla J.P. Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology. 2006;55:99–131. doi: 10.3114/sim.55.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon J.W. Phialophora radicicola and Gaeumannomyces graminis on roots of grasses and cereals. Transactions of the British Mycological Society. 1973;61:471–485. [Google Scholar]
- Deacon J.W. Further studies on Phialophora radicicola and Gaeumannomyces graminis on roots and stem bases of grasses and cereals. Transactions of the British Mycological Society. 1974;63:307–327. [Google Scholar]
- Dennis R.W.G. Ray Society; London: 1960. British cup fungi and their allies: an introduction to the Ascomycetes. [Google Scholar]
- Elliott M.L. Determination of an etiological agent of Bermuda grass decline. Phytopathology. 1991;81:1380–1384. [Google Scholar]
- Elliott M.L., Hagan A.K., Mullen J.M. Association of Gaeumannomyces graminis var. graminis with a St. Augustine grass root rot disease. Plant Disease. 1993;77:206–209. [Google Scholar]
- Fouly H.M., Wilkinson H.T. A group I intron in the nuclear small subunit ribosomal DNA of Gaeumannomyces graminis. Current Microbiology. 2000;40:291–296. doi: 10.1007/s002849910058. [DOI] [PubMed] [Google Scholar]
- Fouly H.M., Wilkinson H.T., Domier L.L. Use of random amplified polymorphic DNA (RAPD) for identification of Gaeumannomyces species. Soil Biology and Biochemistry. 1996;28:703–710. [Google Scholar]
- Freeman J., Ward E. Gaeumannomyces graminis, the take-all fungus and its relatives. Molecular Plant Pathology. 2004;5:235–252. doi: 10.1111/j.1364-3703.2004.00226.x. [DOI] [PubMed] [Google Scholar]
- Fröhlich J., Hyde K.D. Fungal Diversity Press; Thailand: 2000. Palm microfungi. [Google Scholar]
- Gams W. Phialophora and some similar morphologically little-differentiated anamorphs of divergent Ascomycetes. Studies in Mycology. 2000;45:187–199. [Google Scholar]
- Hernández-Restrepo M., Groenewald J.Z., Crous P.W. Taxonomic and phylogenetic re-evaluation of Microdochium, Monographella and Idriella. Persoonia. 2016;36:57–82. doi: 10.3767/003158516X688676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software v. 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaubauf S., Tharreau D., Fournier E. Resolving the polyphyletic nature of Pyricularia (Pyriculariaceae) Studies in Mycology. 2014;79:85–120. doi: 10.1016/j.simyco.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Qiu H., Cai G. Phylogenomic analysis uncovers the evolutionary history of nutrition and infection mode in rice blast fungus and other Magnaporthales. Scientific Reports. 2015;5:9448. doi: 10.1038/srep09448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Walsh E., Blystone D. Five new Pseudophialophora species from grass roots in the oligotrophic pine barrens ecosystem. Fungal Biology. 2015;119:1205–1215. doi: 10.1016/j.funbio.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Luo J., Walsh E., Zhang N. Four new species in Magnaporthaceae from grass roots in New Jersey Pine Barrens. Mycologia. 2014;106:580–588. doi: 10.3852/13-306. [DOI] [PubMed] [Google Scholar]
- Luo J., Walsh E., Zhang N. Toward monophyletic generic concepts in Magnaporthales: species with Harpophora asexual states. Mycologia. 2015;107:641–646. doi: 10.3852/14-302. [DOI] [PubMed] [Google Scholar]
- Luo J., Zhang N. Magnaporthiopsis, a new genus in Magnaporthaceae. Mycologia. 2013;105:1019–1029. doi: 10.3852/12-359. [DOI] [PubMed] [Google Scholar]
- McKeen W.E. Phialophora radicicola Cain, a corn root rot pathogen. Canadian Journal of Botany. 1952;30:338–343. [Google Scholar]
- Nylander J.A.A. Evolutionary Biology Centre, Uppsala University; Sweden: 2004. MrModeltest v2.2. Uppsala: distributed by the author. [Google Scholar]
- Rachdawong S., Cramer C.L., Grabau E.A. Gaeumannomyces graminis vars. avenae, graminis, and tritici identified using PCR amplification of avenacinase-like genes. Plant Disease. 2002;86:652–660. doi: 10.1094/PDIS.2002.86.6.652. [DOI] [PubMed] [Google Scholar]
- Rayner R.W. CMI and British Mycological Society; Kew, Surrey, UK: 1970. A mycological colour chart. [Google Scholar]
- Rehner S.A., Buckley E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 2005;97:84–89. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- Ronquist F., Teslenko M., Mark P. van der. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy K.W., Abney T.S., Huber D.M. Isolation of Gaeumannomyces graminis var. graminis from soybeans in the Midwest. Plant Disease. 1982;66:822–825. [Google Scholar]
- Sadeghi L., Alizadeh A., Safaie N. Genetic diversity of Gaeumannomyces graminis var. tritici populations using RAPD and ERIC markers. Journal of Plant Pathology and Microbiology. 2012;3:143. [Google Scholar]
- Saleh A.A., Leslie J.F. Cephalosporium maydis is a distinct species in the Gaeumannomyces–Harpophora species complex. Mycologia. 2004;96:1294–1305. [PubMed] [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, Massachusetts: 2003. PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods) Version 4. [Google Scholar]
- Tamura K., Stecher G., Peterson D. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M.K. Origin and inheritance of group I introns in 26S rRNA genes of Gaeumannomyces graminis. Journal of Molecular Evolution. 1997;44:637–645. doi: 10.1007/pl00006187. [DOI] [PubMed] [Google Scholar]
- Tan M.K., Wong P.T.W., Holley M.P. Characterization of nuclear ribosomal DNA (rDNA) in Gaeumannomyces graminis and correlation of rDNA variation with G. graminis varieties. Mycological Research. 1994;98:553–561. [Google Scholar]
- Turner E.M. Ophiobolus graminis Sacc. var. avenae var. n. as the cause of take-all or white-heads in Wales. Transactions of the British Mycological Society. 1940;24:269–281. [Google Scholar]
- Ulrich K., Augustin C., Werner A. Identification and characterization of a new group of root-colonizing fungi within the Gaeumannomyces–Phialophora complex. New Phytologist. 2000;145:127–135. [Google Scholar]
- von Arx J.A., Olivier D. The taxonomy of Ophiobolus graminis Sacc. Transactions of the British Mycological Society. 1952;35:29–33. [Google Scholar]
- Walker J. Type studies on Gaeumannomyces graminis and related fungi. Transactions of the British Mycological Society. 1972;58:427–457. [Google Scholar]
- Walker J. Gaeumannomyces, Linocarpon, Ophiobolus and several other genera of scolecospored Ascomycetes and Phialophora conidial states, with a note on hyphopodia. Mycotaxon. 1980;11:1–129. [Google Scholar]
- Walker J. Taxonomy of take-all fungi and related genera and species. In: Asher M.J.C., Shipton P.J., editors. Biology and control of take-all. Academic Press; London, UK: 1981. [Google Scholar]
- Ward E., Akrofi A.Y. Identification of fungi in the Gaeumannomyces–Phialophora complex by RFLPs of PCR amplified ribosomal DNAs. Mycological Research. 1994;98:219–224. [Google Scholar]
- Ward E., Bateman G.L. Comparison of Gaeumannomyces- and Phialophora-like fungal pathogens from maize and other plants using DNA methods. New Phytologist. 1999;141:323–331. doi: 10.1046/j.1469-8137.1999.00346.x. [DOI] [PubMed] [Google Scholar]
- Wetzel H.C., III, Dernoeden P.H., Millner P.D. Identification of darkly pigmented fungi associated with turfgrass roots by mycelial characteristics and RAPD-PCR. Plant Disease. 1996;80:359–364. [Google Scholar]
- Wong P.T.W. Gaeumannomyces wongoonoo sp. nov., the cause of a patch disease of buffalo grass (St Augustine grass) Mycological Research. 2002;106:857–862. [Google Scholar]
- Yao J.M., Wang Y.C., Zhu Y.G. A new variety of the pathogen of maize take-all. Acta Mycologica Sinica. 1992;11:99–104. [Google Scholar]
- Yuan Z.L., Lin F.C., Zhang C.L. A new species of Harpophora (Magnaporthaceae) recovered from healthy wild rice (Oryza granulata) roots, representing a novel member of a beneficial dark septate endophyte. FEMS Microbiology Letters. 2010;307:94–101. doi: 10.1111/j.1574-6968.2010.01963.x. [DOI] [PubMed] [Google Scholar]
- Zhang N., Zhao S., Shen S. A six-gene phylogeny reveals the evolution of mode of infection in the rice blast fungus and allied species. Mycologia. 2011;103:1267–1276. doi: 10.3852/11-022. [DOI] [PubMed] [Google Scholar]