Abstract
Background
Lauha bhasma is one of the herbo-metallic preparations used in Ayurveda, a traditional Indian system of medicine for treating various ailments such as anemia, diarrhea, hyperlipidemia and diabetes.
Objective
To establish standard manufacturing procedure of Teekshna lauha bhasma and analyze its physico-chemical properties.
Materials and methods
The preparation of T. lauha bhasma (calx of iron [Fe] turning) involves samanya shodhana, vishesha shodhana followed by bhanupaka, sthalipaka and putapaka with Triphala kwatha as a medium under temperature of 650 °C in electric muffle furnace (EMF) and maintained for 1 h. T. lauha bhasma were subjected to different physico-chemical characterization using X-ray fluorescence spectrophotometer and scanning electron microscopy.
Results and discussion
The results suggest that these steps are necessary to obtain a good quality of bhasma and also make it acceptable for trituration during Bhasmikarana process. It is found that T. lauha bhasma was prepared properly in 20 puta at a temperature of 650 °C. The particle size of 20 puta T. lauha bhasma is 100–500 nm in range.
Conclusion
Pharmaceutical procedures given in Ayurvedic texts are necessary to prepare pakwa jambu phala varna T. lauha bhasma that complies with all the classical bhasma pariksha and modern analytical parameters in 20 puta at a temperature of 650 °C maintained for 1 h in EMF.
Keywords: Calx of iron turning, Lauha bhasma, Scanning electron microscope, Shodhana, Teekshna lauha, Trividh lauhapaka, X-ray fluorescence
Introduction
Ayurveda, the science of life, is a comprehensive medical system that has been the traditional system of healthcare in India for more than 5000 years. The basic aim of this science is to maintain healthcare by balancing the physical, mental, and spiritual functions of the human body [1], [2]. Rasashastra, an integral part of Ayurveda, deals with the drugs of mineral origin, and details their varieties, characteristics, processing techniques, properties, therapeutic uses, possibilities of developing adverse effects and their management, etc. in a comprehensive way [3]. Ayurvedic experts have estimated that 35–40% of the approximately 600 medicines mentioned in the Ayurvedic formulary may contain at least one metal [4]. Ayurvedic medicines are mostly Rasoushadhies (herbo-mineral) and they play an important role in Ayurvedic therapeutics because of their qualities such as Alpamatropayogitvat (low dose), Arucher-aprasangata (good palatability) and Kshipramarogayadayitvat (fast acting) [5]. Rasayana (immunomodulation and anti-aging quality) and Yogavahi (ability to target drugs to the site) are characteristics of a properly made herbo-mineral preparation, which is also nontoxic, readily absorbable, adaptable, and assimilable in the body [6]. Bhasmas are herbo-metallic ashes in which the metal is calcined along with various herbal ingredients to form organometallic complexes [7]. These complexes should neither contain free metal nor contain free organic constituents, whose presence in bhasma indicates improper calcination [8].
Iron (Fe) is an essential element for almost all living organisms as it participates in a wide variety of metabolic processes, including oxygen transport, deoxyribonucleic acid synthesis, and electron transport [9]. The incinerated Fe preparations of Ayurveda are known as lauha bhasma (Fe calx) [10]. It is a herbo-metallic calx that has several therapeutic applications. Pandit et al. reported that, lauha bhasma for hematinic activity and hemoglobin regeneration efficacy on agar gel diet and phlebotomy induced Fe deficiency anemia in rats and reported significant hematinic and hemoglobin regeneration efficiency in comparison to control and standard ferrous sulfate containing drug [11]. Antibacterial activity of lauha bhasma was reported by Tambekar and Dahikar [12], In the Samhita period Fe (Ayas-Lauha) was used in the form of fine powder. Later, Rasashastra classical texts explained the shodhana (purification) and marana (incineration) methods [13]. According to Rasa Ratna Samuchchhaya, kanta lauha (magnetite Fe ore) is considered as best raw material variety of Fe-for lauha bhasma [14]. However in the absence of kanta lauha, Teekshna lauha (Fe turning) is used for the preparation of lauha bhasma. Now-a-days, in many Ayurvedic pharmacies and industries lauha bhasma is prepared from T. lauha and the preparation protocol for bhasma varies from manufacturer to manufacturer; there are many Ayurvedic texts describing different methods of preparation of lauha bhasma [15], [16] and it plays a major role in deciding the therapeutic efficacy, as well as the toxic effects of bhasmas. The conventional puta (using electric muffle furnace [EMF]) method of heating is very easy and convenient to regulate temperature in closed atmosphere as comparative to traditional puta (using cow dung). Hence, in this study standard manufacturing procedure of T. lauha bhasma (calx of Fe turning) was established by following the guidelines of Ayurvedic formulary of India by adopting various procedures such as samanya shodhana (normal purification), vishesha shodhana (special purification), trividh lauhapaka, that is, bhanupaka (exposure to sunlight), sthalipaka (roasting in an Fe pan), and putapaka (calcination) using EMF. This study also attempts to characterize physico-chemical properties of T. lauha bhasma through conventional studies for studying the quality of bhasma Nischandratvam (lusterless), Apunarbhava (metal irreversibility test), Varitaratvam (floating test), and detailed information on elemental composition and particle size of T. lauha bhasma has been evaluated by Bhargava et al. using modern analytical instruments like scanning electron microscope (SEM) and X-ray fluorescence (XRF) spectrophotometer [17], [18].
Materials and methods
Procurement of raw material
The authenticated raw materials; T. lauha (Fe turnings) were collected from the Department of Metallurgy, IIT (BHU); Tila taila and Triphala were collected from the Ayurvedic pharmacy, BHU; Kulattha collected from local market, Varanasi and Gomutra (cow's urine) were collected from Dairy farm, Institute of Agricultural Sciences, BHU, Varanasi.
Materials
EMF – inner hearth (length: 15 cm, breadth: 19 cm, depth: 30 cm, and maximum temperature capacity: 1000 °C), khalva yantra (mortar – length: 36 cm, breadth: 21 cm, thickness: 3 cm, depth: 11 cm; pestle – length: 13 cm, and diameters: 7 cm).
Methods
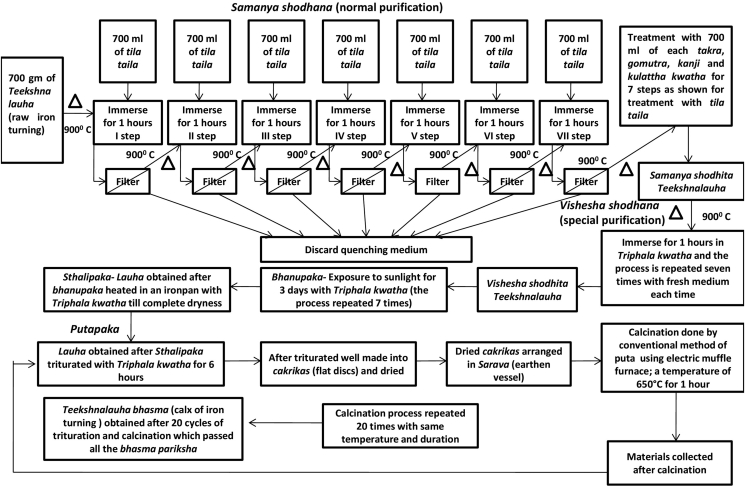
The preparation of T. lauha bhasma (calx of Fe turning) was carried out in Laboratory of Department of Rasa Shastra, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India by following the procedure described in the Ayurvedic formulary of India [19]. It involves the following major steps; samanya shodhana (normal purification), vishesha shodhana (special purification), trividh lauhapaka, that is, bhanupaka (exposure to sunlight), sthalipaka (roasting in an Fe pan), and putapaka (calcination) (Fig. 1). T. lauha bhasma was subjected to various organoleptic and physico-chemical analysis such as color, taste, texture, loss on drying [20], ash value [21], acid insoluble ash [21], and water soluble ash [21]. Modern analytical instruments such as XRF and SEM were employed to determine the elemental composition and particle size respectively.
Fig. 1.
Flow diagram for the preparation of Teekshna lauha bhasma.
Samanya shodhana
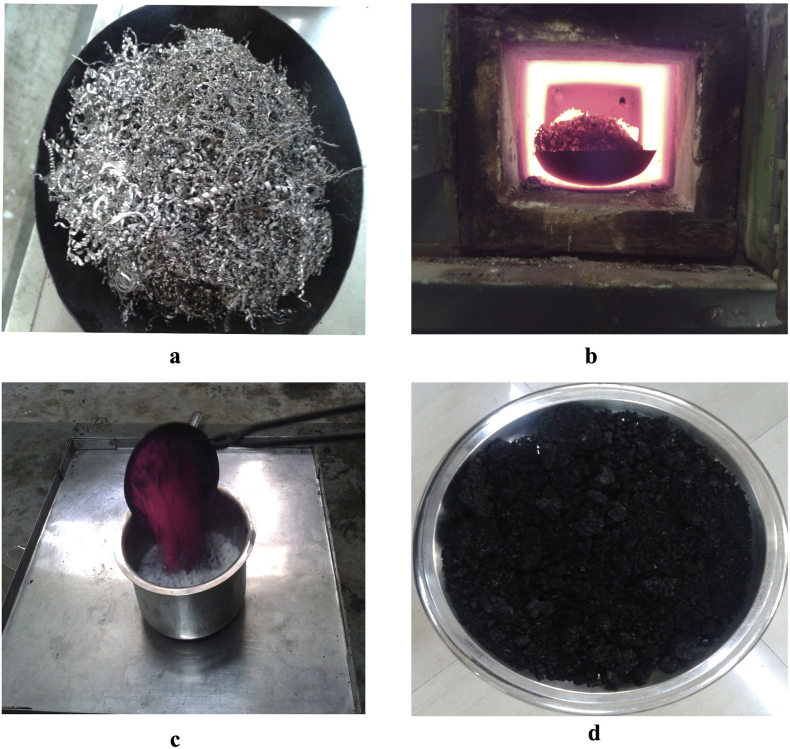
In samanya shodhana process, 700 g of raw material (Fe turning) was heated in EMF till red hot condition (∼875–900 °C) and immersed in 700 ml of each medium viz. tila taila (sesame oil), takra [22] (buttermilk), Gomutra (cow's urine), kanji [23] (rice gruel), and kulattha kwatha [24] (decoction of horse gram) and kept for self-cooling (approximately 1 h) at room temperature (Fig. 2). This quenching process was repeated for seven times consecutively in tila taila followed by seven times consecutively in takra, gomutra, kanji, and kulattha kwatha by using fresh media every time. After completion of the process, material was filtered by Fe mesh and dried under sunlight. The material obtained at this stage is called samanya shodhita lauha.
Fig. 2.
Shodhana process of Teekshna lauha (iron turning). (a) Raw Teekshna lauha (iron turning), (b) red hot iron turning, (c) quenching process, (d) Teekshna lauha after shodhana.
Vishesha shodhana
In this purification step, quenching was done in Triphala kwatha. It was prepared by taking coarse powders of three myrobalans, taken without seed: Haritaki (Terminalia chebula Retz.), Bibhitaki (Terminalia bellirica [Gaertn.] Roxb.), and Amalaki (Phyllanthus emblica L.) in equal quantity (each 1 kg) and boiled in 24 L of water till reduction to 1/4th of the original volume of water to obtain Triphala kwatha. Using this, repeated quenching process of samanya shodhita lauha was done. This purification step was repeated seven times using freshly prepared Triphala kwatha. The lauha churna (coarse powder of Fe turning) obtained at this stage is called vishesha shodhita lauha.
Bhanupaka
Triphala kwatha was prepared by heating equal quantity of Triphala to vishesha shodhita lauha churna with two parts of water and reduced to 1/4th of original volume. This Triphala kwatha was added to lauha obtained after vishesha shodhana and allowed to dry under sunlight. It took a maximum of 3 days for complete drying of Triphala kwatha. This process was repeated seven times to yield intermediate after bhanupaka (Fig. 3a).
Fig. 3.
Trividh lauhapaka. (a) Bhanupaka, (b) Sthalipaka, (c) after Sthalipaka, (d) after 1 puta, (e) after 5 puta, (f) after 10 puta, (g) after 15 puta, (h) after 20 puta.
Sthalipaka
In this step, Triphala kwatha was prepared by taking Triphala 3 times of lauha obtained after bhanupaka and 16 times of water was added to it. The whole material was boiled in a stainless steel container to reduce the volume to 1/8th of the original volume of water. Lauha obtained after bhanupaka was washed with hot water and placed in a sthali (Fe pan), to which above freshly prepared Triphala kwatha was added and intense heating was given for complete evaporation of water contents of Triphala kwatha. On complete drying of the material, again Triphala kwatha was added and subjected to heat. This process required 4 h 30 min for complete drying of Triphala kwatha. The whole process was repeated seven times to yield intermediate after Sthalipaka (Fig. 3b and c).
Putapaka
In traditional literature, the process of puta (calcination) refers to controlled heating of herbo-mineral preparations and allowing the preparation to cool to room temperature. [25] According to Rasa tarangini, lauha bhasma should be prepared by triturating with specific media according to disease and subjecting to puta, but in case of unavailability of the specific media the bhasma should be prepared by Triphala kwatha. [24], [26] In this process, freshly prepared Triphala kwatha was mixed with lauha obtained after sthalipaka in mechanized khalva yantra and trituration was done with a frequency of 60 times/min. The paste formed during this trituration was made into cakrikas (pellets) and dried under sunlight. After complete drying of cakrikas, it was taken in an earthen vessel (sarava) and covered with another inverted earthen vessel. The space between the two earthen vessels was covered with clay smeared cloth; this specific process is known as Sarava samputikarana (sealed earthen pot). After this, it was subjected to puta in horizontal EMF and temperature was allowed to gradually rise to 650 °C in 2 h and maintained for 1 h [27]. The furnace was then switched off and allowed for self-cooling. The next day, pellets were collected from sarava and again triturated with Triphala kwatha. Same process of puta was repeated for 20 times to obtain T. lauha bhasma of desired quality.
Observations and results
Samanya shodhana of T. lauha (Fe turning) was carried out as per Ayurvedic formulary of India where the sequential quenching process was done in different media by following conventional order. Metallic luster was lost after quenching in tila taila and prominent cracks and flakes were seen after quenching in takra and gomutra. The maximum part was in powder form after quenching in kulattha kwatha. At the end of samanya shodhana, it was observed that, the hard shining Fe metal was converted into black colored lusterless powder of brittle material. After the process of vishesha shodhana, brittleness and weight of T. lauha increases and the dark black color powder was formed. It was observed that pH of media increases after every quenching of shodhana process (Table 1, Table 2).
Table 1.
Observations made during samanya and vishesha shodhana of Teekshna lauha (iron turning).
| Media | Quantity (ml) | Initial weight (g) | Final weight (g) | Gain/loss (g) | Actual percentage change |
|---|---|---|---|---|---|
| Tila taila | 700 | 700 | 740 | 40 ↑ | 5.7 ↑ |
| Takra | 700 | 740 | 780 | 40 ↑ | 5.7 ↑ |
| Gomutra | 700 | 780 | 746 | 34 ↓ | 4.4 ↓ |
| Kanji | 700 | 746 | 740 | 6 ↓ | 0.9 ↓ |
| Kulattha kwatha | 700 | 740 | 740 | No change | No change |
| Triphala kwatha | 700 | 740 | 850 | 110 ↑ | 15% ↑ |
Table 2.
Changes in pH of media before and after quenching during samanya and vishesha shodhana of Teekshna lauha (iron turning).
| Media | Before | After |
|---|---|---|
| Takra | 3.5 | 4 |
| Gomutra | 7.5 | 8.3 |
| Kanji | 3 | 4 |
| Kulattha kwatha | 6.4 | 7.4 |
| Triphala kwatha | 2.8 | 3.3 |
During the process of bhanupaka while adding Triphala kwatha in shodhita lauha churna, color changes from greenish brown to black (Fig. 3a). After complete sun drying, lauha churna gets converted into a big cluster. The product obtained after bhanupaka was more brittle in nature and big particles of lauha churna were converted into fine particles. In sthalipaka, during heating lauha churna was adhering to the surface of container and fumes increased with the duration of heating (Fig. 3b). At the end of sthalipaka the color changes from dark black to brick brown in color and lauha churna was converted into powder form completely (Fig. 3c). Various observations during bhanupaka and sthalipaka are tabulated in Table 3.
Table 3.
Observations made during bhanupaka and sthalipaka of vishesha shodhita Teekshna lauha.
| Pharmaceutical procedure | Media (Triphala kwatha in ml) | Initial weight (g) | Final weight (g) | Gain/loss (g) | Actual percentage change |
|---|---|---|---|---|---|
| Bhanupaka | 425 | 850 | 1250 | 400↑ | 47↑ |
| Sthalipaka | 7500 | 1250 | 885 | 365↓ | 29↓ |
During Putapaka (calcination), lauha churna obtained after sthalipaka was triturated with triphala kwatha. In first puta, it was taking 8 h to get converted into paste like structure and also difficult to make pellets. After putapaka the color of pellets changed from brick brown to dark brown (Fig. 3d–h). After every puta, it was easy to make pellets due to reduction in particles size and also color changes. Physical characteristics of the material recorded down after every puta which was shown in Table 4, Table 5. T. lauha bhasma was passed by various classical bhasma pariksha (Table 6). The results of various physiochemical parameters color, taste, texture, loss on drying, ash value, acid insoluble ash, water soluble ash, particle size (Fig. 4), and elemental composition are tabulated in Table 7, Table 8.
Table 4.
Changes in weight of Teekshna lauha bhasma before and after puta during putapaka.
| Puta | Media (Triphala kwatha in ml) | Initial weight (g) | Final weight (g) | Loss (g) | Actual percentage change |
|---|---|---|---|---|---|
| 1st | 325 | 885 | 839 | 46 ↓ | 5 ↓ |
| 2nd | 325 | 839 | 836 | 3 ↓ | 0.35 ↓ |
| 3rd | 325 | 836 | 832 | 4 ↓ | 0.47 ↓ |
| 4th | 325 | 832 | 830 | 2 ↓ | 0.25 ↓ |
| 5th | 325 | 830 | 828 | 2 ↓ | 0.25 ↓ |
| 6th | 350 | 828 | 815 | 13 ↓ | 1.5 ↓ |
| 7th | 350 | 815 | 813 | 2 ↓ | 0.25 ↓ |
| 8th | 350 | 813 | 813 | No change | No change |
| 9th | 350 | 813 | 810 | 3 ↓ | 0.35 ↓ |
| 10th | 400 | 810 | 810 | No change | No change |
| 11th | 400 | 810 | 790 | 20 ↓ | 2.4 ↓ |
| 12th | 400 | 790 | 786 | 4 ↓ | 0.47 ↓ |
| 13th | 400 | 786 | 783 | 3 ↓ | 0.35 ↓ |
| 14th | 400 | 783 | 783 | No change | No change |
| 15th | 425 | 783 | 780 | 3 ↓ | 0.35 ↓ |
| 16th | 425 | 780 | 770 | 10 ↓ | 1.2 ↓ |
| 17th | 425 | 770 | 769 | 1 ↓ | 0.1 ↓ |
| 18th | 450 | 769 | 766 | 3 ↓ | 0.35 ↓ |
| 19th | 450 | 766 | 765 | 1 ↓ | 0.1 ↓ |
| 20th | 450 | 765 | 765 | No change | No change |
Table 5.
Observations recorded before and after puta of Teekshna lauha bhasma during putapaka.
| Puta cycle | Before puta | After puta |
|---|---|---|
| 1st | Brick brown color, on trituration color changes to black | Dark brown color |
| 2nd | Pellets were made easily and rough in consistency | Pellets were easily breakable by hand and color was bluish black but surface of pellets was brown |
| 3rd | After trituration color turns to grayish black | Pellets become soft and color changes to more bluish black |
| 4th | Particle size increases and soft in consistency | Pellets were fragile and color was same as previous puta |
| 5th | Duration of trituration decreases and pellets were made easily | Color of pellets were nearer to blackish red, that is, pakwa jambu phala varna and soft in consistency |
| 6th | Lauha bhasma was very soft and quantity of media increases for trituration | Color was blackish red and pellets were easily breakable |
| 7th | Again on trituration, color was black and very soft in consistency | Color was very much similar as previous puta and 15% rekhapurnatva test positive |
| 8th | Color was bluish black before trituration | Pellets were easily broken by mild pressure of finger and color was blackish red. 20% rekhapurnatva passed |
| 9th | Lauha bhasma was very soft in consistency | 25% rekhapurnatva and other findings are same as previous puta |
| 10th | More quantity of liquid media required for trituration and it was easily to make pellets | Hardness of pellets was increased. Color of pellets was turned to blackish red and black spots were found over few pellets. 35% rekhapurnatva passed |
| 11th | Bhasma was brownish red color and became more rekhapurnatva | Pellets were still very hard and color was blackish red and 45% rekhapurnatva test positive |
| 12th | Color was same and after trituration material becomes stickier | Pellets were hard and bhasma achieved 50% rekhapurnatva and 30% varitar |
| 13th | Bhasma was blackish red colored and metallic taste present | Color maintained and pellets were mild hard |
| 14th | Same as previous | Color was blackish red and bhasma achieved 60% rekhapurnatva and 40% varitar |
| 15th | Quantity of liquid media increases for trituration | Pellets were little hard and blackish spots were found on the surface. Color of bhasma was blackish red and 50% varitara test positive |
| 16th | After trituration material were stickier in nature and difficult to make pellets | Color of bhasma was completely blackish red, that is, pakvajambuphalavarna. 65% rekhapurnata and 60% varitara test positive |
| 17th | On trituration gritty appearance of bhasma was observed. Color maintained | Color of bhasma maintained and metallic taste present. 70% rekhapurnata. Pellets were now soft |
| 18th | Color of bhasma maintained and soft in consistency. On trituration quantity of media increases | 75% rekhapurnata and 70% varitara test positive. Metallic taste present |
| 19th | Color and softness of pellets maintained | 90% rekhapurnata and 75% varitara test positive. Metallic taste absent |
| 20th | Same as previous | Bhasma passed all bhasma pariksha with 95% rekhapurnata and 80% varitara test positive |
Table 6.
Analysis of Teekshna lauha bhasma by ancient methods.
| Test | Teekshna lauha bhasma |
|---|---|
| Rekhapurnatvam | The Lauha bhasma was rubbed in between index finger and thumb. It enters into the ridges of the finger – positive |
| Varitaratvam | A small amount of Lauha bhasma was carefully sprinkled on the water. It was found that 80% bhasma was floating on the water surface – positive |
| Nirdhumatvam | The Lauha bhasma was sprinkled on red hot coal. It did not emit smoke – positive |
| Nischandratvam | It was not having any lustre found positive |
| Apunarbhava | The Lauha bhasma was triturated with Gunja (Abrus precatorius L.), Goghrita (cow ghee), Madhu (honey), Tankana (Borax), Guggulu (Commiphora wightii [Arn.] Bhandari), and made pellets. Then it was get subjected to puta on 650 °C in EMF maintained for 1 h. Next day after self-cooling pellets were triturated and not any agglomeration was found |
EMF = Electric muffle furnace.
Fig. 4.
Scanning electron micrographs of 20 puta Teekshna lauha bhasma.
Table 7.
Physico-chemical analysis of Teekshna lauha bhasma.
| Test | Result |
|---|---|
| Color | Pakva jambu phala varna |
| Taste | Tasteless |
| Texture | Amorphous |
| Loss on drying (%) | 0.31 |
| Ash value (%) | 98.15 |
| Acid insoluble ash (%) | 27.50 |
| Water soluble ash (%) | 30.26 |
| Particle size | 100–500 nm |
Table 8.
Results of XRF analysis showing elemental composition.
| Element | Raw iron turning (%) | After sthalipaka (%) | After 10 puta (%) | After 20 puta (%) |
|---|---|---|---|---|
| Fe | 98.10 | 58.18 | 80.92 | 70.26 |
| Si | 0.40 | 3.74 | 1.44 | 0.96 |
| Al | 0.13 | 1.26 | 0.49 | 0.31 |
| Ca | 0.074 | 0.30 | 1.32 | 1.50 |
| Mn | 0.75 | 1.36 | 1.77 | 1.63 |
| Others elementsa | 0.54 | 35.16 | 14.06 | 74.66 |
Other elements are P, Cl, Ni, Ar, S, K, Tb, Sm, W, Dy, Cu, Zn, Gd, Co, Rb, Sr, Ti, Er, Ga, Y, and Na. XRF = X-ray fluorescence.
Discussion
Bhasma preparations involve the conversion of the metal into its mixed oxides, during which, the zero valent metal state is converted to a higher oxidation state. The significance of this “Bhasmikarana” is that the toxic nature of the resulting metal oxide is completely destroyed while introducing the medicinal properties into it [28]. A bhasma means a fine ash obtained though incineration [29]. Selection of raw material is the most important step in the bhasma preparation. Authentic raw material with high quality assures producing safe and efficacious finished product. In traditional literature, kanta lauha (magnetite Fe ore) and T. lauha (Fe turnings) is considered as best raw material for lauha bhasma preparation [30]. The availability of kanta lauha (magnetite Fe ore) is rare, that is why, in many Ayurvedic pharmacies and industries, lauha bhasma is prepared from T. lauha. Hence in this study, a step is made to find out the best quality of T. lauha bhasma using EMF. In this regard, raw material T. lauha was tested by classical method, as well as by modern analytical techniques [18], [31]. In classical method, Kalka of Kasisa (ferrous sulphate, FeSO4.7H2O) and Amalaki (P. emblica L.) fruit pulp was applied over the surface of T. lauha and after some time conical protrusion (girisringa) are seen over the surface of lauha, it shows the sample of T. lauha is genuine. The elemental analysis through XRF study also revealed that raw Fe turning contains 98.10% of Fe (Table 8) [18]. All the other necessary media for pharmaceutical procedure such as kanji, takra, Triphala kwatha, etc., that were used at various stages were prepared under observation.
Shodhana (purification/detoxification) is the foremost step in the preparation of lauha bhasma because impure lauha bhasma on administration causes Hritpida (chest-pain), Agnimandya (indigestion), Apasmar (epilepsy), Shotha (oedema), Napunsakata (impotency), Prameha (diabetes), Ashmari (renal calculus), and even death also [32], [33]. For the process of samanya and vishesha shodhana, the process of nirvapa (quenching) was adapted. It is one of the foremost step in the purification process of T. lauha in which the metal is heated up to red hot condition and immediately immersed in various plants and animal media (sesame oil, butter milk, cow urine, cow milk, etc.) and kept for self-cooling [34]. It removes the inorganic impurities and incorporates beneficial organic moieties into the metal which render them suitable for further process of preparation of bhasma (grinding with plant drug and repeated calcination) [35]. In this study, T. lauha (Fe turning) was heated till they were red hot and poured in different media, that is, tila taila, takra, gomutra, kanji, kulattha kwatha, and Triphala kwatha (7 times each). At each time, sufficient quantity of media was taken which was approximately 700 ml. The average temperature of the heating device and surface of container was 1000 °C and 950 °C, respectively. The average temperature of red hot Fe turning recorded in EMF was 900 °C. The use of a particular media and particular sequence is notable. The probable concept behind using such variation may be removal of impurities from the drug in a particular acidic or alkaline media and also reduction in particle size of drug [36]. After heating, immediate cooling in liquid media leads to decrease in tension and increase in compression force. Repetition in heating and cooling causes disruption in compression tension equilibrium and leads to increased brittleness, reduction in hardness, and finally reduction in the particle size [37]. After removing the hydrophobic impurities using sesame oil (tila taila) treatment, oxide scales are formed due to atmospheric oxidation of the raw material, are removed by treatment with aqueous media viz., butter milk (takra), cow urine, rice gruel (kanji), and horse gram decoction, which are traditionally known for this property [25]. The formation of oxides scales are also responsible for increase in pH after every purification steps. Oxides (Fe oxides) are mainly basic in nature, so it can raise the pH after every quenching. In each of the steps in samanya shodhana (normal purification), progressive increase in surface area and reduction in particle size, probably due to micro cracks formed during heat treatment is observed. At the end of shodhana process weight gain was observed, it may be due to addition of contents of quenching media (Table 1, Table 2).
During the process of bhanupaka, Triphala kwatha was added to vishesha shodhita lauha and allowed to dry under sunlight. The role of sunlight during bhanupaka has very specific reason. It has been widely established that the metallic Fe is toxic [38]. Hence, Fe supplements should contain Fe in the form of complex. The ultraviolet radiation present in the sunlight reduces the oxidation state of Fe in the presence of Vitamin C present in the Triphala decoction thereby improving the bioavailability [39]. In this process, more time is available for the reaction between Triphala and lauha churna, it causes an increase in the weight of lauha churna, which may be due to the addition of solid contents of Triphala kwatha. In sthalipaka, lauha obtained after bhanupaka was mixed with Triphala kwatha in an Fe pan and intense heating was given up to complete dryness. After sthalipaka weight of lauha churna was decreased due to burning of solid contents of Triphala kwatha (Table 3).
Triphala mainly consists of tannins and ascorbic acid. The absorption of food Fe can be greatly influenced by other constituents in the diet, such as ascorbic acid (Vitamin C) and phenolics. Ascorbic acid increases the bioavailability of Fe by converting Fe3+ to Fe2+, while phenolics can reduce the bioavailability of Fe by binding to its phenolics (e.g., tannins). Excess of ascorbic acid and/or a lack of dietary tannins have both been suggested as contributing to clinical/pathological Fe storage disease [36]. Five hundred milligram of Fe causes severe toxicity, leads to shock, metabolic acidosis, and liver damage [38]. In other words, this may also be taken as the various constituents of Triphala have antagonizing activity. Thus, too much Fe absorption is prevented. Triphala is a mild laxative and thereby counteracts the constipating property of Fe and thus be beneficial due to which ancient scholars of Ayurveda might have mentioned Triphala in maximum lauha bhasma preparations [13]. For the putapaka process instead of traditional method, to provide controlled and regulated heat and with a view of standardizing a modified EMF was used. Lauha bhasma were prepared on same temperature, that is, 650 °C and the duration of peak temperature was 1 h for all puta. The amount of liquid media (Triphala kwatha) for trituration increases after every successive puta, this may be because of decrease in particle size causes increases in surface area which are responsible for more absorption of Triphala kwatha during trituration (Table 4). [40] During first few puta, metallic luster was observed on the surface of pellets, later on it disappeared. Luster is the physical character of metal, when the metal transforms to compound form then its luster is lost. Appearance of luster after first few puta indicates lauha was still persistent in metallic form, later on it completely transformed to lusterless compounds. After trituration cakrikas (pellets) were formed and sarava samputikarana was done by earthen vessels that are responsible for homogenous distribution of heating and also does not react with the material because of inert property of earthen vessel. After every puta, weight of lauha bhasma decreases due to burning of solid contents of Triphala kwatha (Table 4). After 5th puta, color was nearer to blackish red and pellets were fragile and soft. In the 7th puta 1st time rekhapurnatva (the bhasma enter the ridges of finger) test presence which shows the reduction in particle size of bhasma. In 10th puta, pellets were hard and after 11th puta color was almost blackish red, that is, pakwa jambu phala varna which suggests the formation of an entirely new compound. On 15th puta, it was observed that hardness of pellets decreases and bhasma passed 50% varitara (the bhasma floats on the still water surface) test. After 20th puta bhasma passed all bhasma pariksha with 95% Rekhapurnata and 80% varitara test and color of bhasma was blackish red, that is, pakwa jambu phala varna (Table 5, Table 6). The color of lauha bhasma is purple (Pakwa jambu phala varna), it may be considered as a mixture of ferrous oxide, ferrous sulphide, ferric oxide and other trace elements. Ferrous oxide and ferrous sulphide are black in color, and ferric oxide is red in color. Combination of all these compounds makes the lauha bhasma purple in color. Several physical and chemical parameters have been described for ascertaining the purity of lauha bhasma [19]. Physical parameters include luster, color, fineness, floatability, etc., while chemical parameters include test of lauha bhasma for irreversibility to metallic state, floatability taste, etc. [19] In this study, after quality check done by classical parameters (Table 6), T. lauha bhasma were subjected to different physico-chemical characterization studies using modern analytical tools. Our results showed negligible moisture content (0.31% loss on drying), total ash value (98.15%), water soluble ash (30.26%), and lower solubility in acid (27.50%) (Table 7). The results are comparable to the reported values [41]. The total ash value is useful in determining the purity of bhasma and indicates the absence of free organic moieties. During the preparation of lauha bhasma, large quantity of Triphala were added resulting in the formation of complexes between the constituents of Triphala and the metal. The formation of coordination compounds will be precluded however, if the bhasmas are not prepared properly, resulting in lower total ash content [41]. Lower acid-insoluble ash indicates higher bioavailability of the drug [42] and lower value of loss on drying indicates the absence of moisture in the drug. The results of XRF study revealed that, Fe present in the elemental form along with its impurities in the raw Fe turnings is converted into different forms of Fe oxide in final bhasma were estimated by X-ray diffraction [18], [43]. Other impurities such as Si, Al, etc., are also get oxidized after repeated trituration and calcination. Triphala which is used extensively in preparation of the bhasma for puta and also for trituration between puta consist of a number of minor elements such as Na, K, Mg, Ca, Cl, and P; and 23 trace AI, Ba, Br, Cd, Co, Cr, Cs, Cu, Fe, Eu, Hf, Hg, La, Mn, Ni, P, Pb, Rb, Sb, Se, Th, V, and Zn elements [18]. These get oxidized during puta and remain as integral part of the final bhasma. The SEM of 20 puta T. lauha bhasma showed irregular aggregates of various sizes and shapes with nano structure on the surfaces (100–500 nm) (Fig. 4) [17]. The role of nano structured materials as therapeutic agents has been reasonably established [44], [45] and we also presumed that the efficacy of lauha bhamsa may be attributed to the presence of nanostructures.
Limitations of the study: Particle size after each puta was not measured. It can be analyzed at regular intervals to provide leads for further studies.
Conclusion
Lauha bhasma preparation includes major steps such as samanya shodhana (normal purification), vishesha shodhana (special purification), and trividh lauhapaka, that is, bhanupaka (exposure to sunlight), sthalipaka (frying in an Fe pan), and putapaka (calcination). It is essential to follow all these procedures as per Ayurvedic guidelines to get good quality of bhasma. It is also very important to understand each and every step to set up the standards for bhasma preparation. This work has resulted in establishing standard manufacturing procedure of Teekshna lauha bhasma (calx of Fe turning) by adapting conventional method of puta using EMF. This study revealed that a temperature of 650 °C with peak duration of temperature 1 h in 20 puta is sufficient to obtain purple colored, that is, pakwa jambu phala varna Teekshna lauha bhasma which passed the bhasma parikshas. Our results, also evaluated the physico-chemical properties of Teekshna lauha bhasma, based on AYUSH guidelines, as well as modern analytical tools.
Source of support
None.
Conflicts of interest
None declared.
Acknowledgment
Authors are thankful to Dr. Subhash Chandra Bhargava (Ayurvedic Medical Officer, Kshipra, Dewas, Madhya Pradesh), Dr. KRC Reddy (Professor, Department of Rasa Shastra, Banaras Hindu University, Varanasi) and his team for partial support in analytical data during preparation of the manuscript.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Bhanu P. Use of metals in ayurvedic medicine. Indian J Hist Sci. 1997;32:1–28. [Google Scholar]
- 2.Sushruta . 11th ed. Chaukhamba Sanskrit Sansthan; Varanasi: 2010. Sushruta Samhitaa Sutra Sthana (Doshadhatumalakshayavridhi Vigyaniya) Verse- 15/ 48 Ayurved Tatva Sandipikahindi commentary by Kaviraja Ambika Dutta Shastri; p. 84. [Google Scholar]
- 3.Galib, Barve M., Mashru M., Jagtap C., Patgiri B.J., Prajapati P.K. Therapeutic potentials of metals in ancient India: a review through Charaka Samhita. J Ayurveda Integr Med. 2011;2:55–63. doi: 10.4103/0975-9476.82523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gogtay N.J., Bhatt H.A., Dalvi S.S., Kshirsagar N.A. The use and safety of non-allopathic Indian medicines. Drug Saf. 2002;25:1005–1019. doi: 10.2165/00002018-200225140-00003. [DOI] [PubMed] [Google Scholar]
- 5.Rasa Vagbhatta, Rasa Ratna Samuccaya, commentary by Siddhinandan Mishra, Verse 28/1. Varanasi, India: Choukhambha Orientalia; Reprint 2011. p. 633.
- 6.Sarkar P.K., Chaudhary A.K. Ayurvedic bhasmas: the most ancient application of nano medicine. J Sci Ind Res. 2010;69:901–905. [Google Scholar]
- 7.Rajendran N., Pemiah B., Rajan K.S., Krishnan U.M., Sethuraman S., Krishnaswamy S. Role of gallic acid in the preparation of an iron–based Indian traditional medicine – Lauhabhasma. Int J Pharm Pharm Sci. 2012;4:45–48. [Google Scholar]
- 8.Nagarajan S., Pemiah B., Krishnan U.M., Rajan K.S., Krishnaswamy S., Sethuraman S. Physico- chemical characterization of lead based Indian traditional medicine – Naga bhasma. Int J Pharm Pharm Sci. 2012;4:69–74. [PMC free article] [PubMed] [Google Scholar]
- 9.Abbaspour N., Hurrell R., Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19:164–174. [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar P.K., Prajapati P.K., Choudhary A.K., Shukla V.J., Ravishankar B. Haematinic evaluation of lauha bhasma and mandur bhasma on HgCl2 – induced anaemia in rats. Indian J Pharm Sci. 2007;69:791–795. [Google Scholar]
- 11.Pandit S., Biswas T.K., Debnath P.K., Saha A.V., Chowdhury U., Shaw B.P. Chemical and pharmacological evaluation of different ayurvedic preparations of iron. J Ethnopharmacol. 1999;65:149–156. doi: 10.1016/s0378-8741(99)00003-3. [DOI] [PubMed] [Google Scholar]
- 12.Tambekar D.H., Dahikar S.B. Screening antibacterial activity of some bhasma against enteric pathogens. Recent Res Sci Technol. 2010;2:59–62. doi: 10.4103/2231-4040.79801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta K.L., Pallavi G., Patgiri B.J., Galib, Prajapati P.K. Critical review on the pharmaceutical vistas of Lauha Kalpas (iron formulations) J Ayurveda Integr Med. 2012;3:21–28. doi: 10.4103/0975-9476.93944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vagbhatta Rasa. Choukhambha Orientalia; Varanasi, India: 2011. Rasa Ratna Samuccaya, commentary by Siddhinandan Mishra, Verse 5/96; p. 163. [Google Scholar]
- 15.Rasa Vagbhatta, Rasa Ratna Samuccaya, commentary by Siddhinandan Mishra, Verse 5/98-132. Varanasi, India: Choukhambha Orientalia; Reprint 2011. p. 164–168.
- 16.Sharma S. Rasa Tarangini, 20/15-44. 11th ed. New Delhi: Motilal Banarasidas; Reprint 2012. p. 494–500.
- 17.Bhargava S.C., Prakash R., Sastry G.V.S., Reddy K.R.C. Banaras Hindu University, Department of Rasa Shastra Institute of Medical Sciences; Varanasi: 2010. Pharmaceutical standardization and characterization of Lauha bhasma; pp. 143–145. MD Ayurveda thesis. [Google Scholar]
- 18.Bhargava S.C., Reddy K.R., Sastry G.V.S. Identification studies of Lauha bhasma by X ray diffraction and X ray fluorescence. AYU. 2012;33:143–145. doi: 10.4103/0974-8520.100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anonymous . Department of Ayush, Ministry of H and FW; 2003. Ayurvedic formulary of India; pp. 624–628. Part 1. 2nd ed. India: Government of India. [Google Scholar]
- 20.Anonymous . Department of Ayush, Ministry of H and FW; 2007. Ayurvedic pharmacopoeia of India; p. 214. Part 2. 2nd ed., Vol. 1. India: Government of India. [Google Scholar]
- 21.Anonymous . Department of Ayush, Ministry of H and FW; 2007. Ayurvedic pharmacopoeia of India; p. 213. Part 2. 2nd ed., Vol. 1. India: Government of India. [Google Scholar]
- 22.Sushruta . 11th ed. Chaukhamba Sanskrit Sansthan; Varanasi: 1997. Sushruta Samhitaa Sutra Sthana (Dravdravya Vidhi) Verse- 45/ 85 Ayurved Tattva Sandipika Hindi commentary by Kaviraja Ambika Dutta Shastri; p. 227. [Google Scholar]
- 23.Anonymous . Department of Ayush, Ministry of H and FW; 2007. Ayurvedic pharmacopoeia of India; p. 249. Part 2. 2nd ed., Vol. 1. India: Government of India. [Google Scholar]
- 24.Sharangdhar, Sharangdhar Samhita, Madhyama Khanda, Verse 2/1 Jiwanprada Hindi commentary by Shailaja Srivastava. Varanasi: Choukhambha Orientalia; Reprint 2009. p. 135.
- 25.Krishnamachary B., Rajendran N., Pemiah B., Krishnaswamy S., Krishnan U.M., Sethuraman S. Scientific validation of the different purification steps involved in the preparation of an Indian ayurvedic medicine, Lauha bhasma. J Ethnopharmacol. 2012;142:98–104. doi: 10.1016/j.jep.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Sharma S. Rasa Tarangini, 20/39. 11th ed. New Delhi: Motilal Banarasidas; Reprint 2012. p. 500.
- 27.Singh T.R., Gupta L.N., Kumar V., Kumar N. Characterization of an ayurvedic drug (Shilajatwadi Lauha): an approach to standardization. Int J Res Ayurveda Pharm. 2014;5:424–427. [Google Scholar]
- 28.Wadekar M.P., Rode C.V., Bendale Y.N., Patil K.R., Prabhune A.A. Preparation and characterization of a copper based Indian traditional drug: Tamra Bhasma. J Pharm Biomed. 2005;39:951–955. doi: 10.1016/j.jpba.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Subbarayappa B.V. Siddha medicine: an overview. Lancet. 1997;350:1841–1844. doi: 10.1016/s0140-6736(97)04223-2. [DOI] [PubMed] [Google Scholar]
- 30.Sharma S. Rasa Tarangini, 20/7. 11th ed. New Delhi: Motilal Banarasidas; Reprint 2004. p. 489.
- 31.Sharma S. Rasa Tarangini, 20/8. 11th ed. New Delhi: Motilal Banarasidas; Reprint 2004. p. 491.
- 32.Vagbhatta Rasa. Choukhambha Orientalia; Varanasi, India: 2011. Rasa Ratna Samuccaya, commentary by Siddhinandan Mishra, Verse 5/97; p. 163. [Google Scholar]
- 33.Mishra Siddhinandan. Choukhambha Orientalia; Varanasi, India: 2013. Ayurvediya Rasashastra; p. 453. [Google Scholar]
- 34.Sharma S. Rasa Tarangini, 2/40. 11th ed. New Delhi: Motilal Banarasidas; Reprint 2012. p. 19.
- 35.Devanathan R. Concept of Bhasmikaran. Int J Res Ayuveda Pharm. 2011;2:18–23. [Google Scholar]
- 36.Singh N., Reddy K.R. Pharmaceutical study of Lauha Bhasma. AYU. 2010;31:387–390. doi: 10.4103/0974-8520.77157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarkar P., Choudhary A.K., Shukla V.J., Ravishankar B., Prajapti P.K. Department of RS and BK Including Drug Research; Jamnagar, Gujarat: 2005. A comparative pharmaceutico-pharmaco-clinical study of Lauha Bhasma and Mandoora Bhasma w.s.r. to its Panduhara effect; p. 234. MD dissertation. IPGT&RA, G Ay U. [Google Scholar]
- 38.Goyer R.A., Klaassen C.D., Amdur M.O., Doull J., editors. Toxic Effects of Metals. Toxicology. 3rd ed. McGraw Hill; New York: 1986. pp. 842–843. [Google Scholar]
- 39.Krishnamachary B., Purushothaman A.K., Pemiah B., Krishnaswamy S., Krishnan U.M., Sethuraman S. Bhanupaka: a green process in the preparation of an Indian ayurvedic medicine, Lauha Bhasma. J Chem. 2013;2013:1–8. [Google Scholar]
- 40.Singh T.R., Gupta L.N., Kumar V., Singh R.S., Kumar N. Banaras Hindu University, Department of Rasa Shastra Institute of Medical Sciences; Varanasi: 2014. Comparative pharmaceutical and pharmacological evaluation of Shilajatvadi Lauha and modified Shilajatvadi Lauha: a preclinical study; pp. 162–184. MD Ayurveda thesis. [Google Scholar]
- 41.Krishnamachary B., Pemiah B., Krishnaswamy S., Krishnan U.M., Sethuraman S., Sekar R.K. Elucidation of a core shell model for Lauha bhasma through physio-chemical characterization. Int J Pharm Pharm Sci. 2012;4:644–649. [Google Scholar]
- 42.Chaudhary A., Prakash B. Scientific validated approach for application of Mandura bhasma a review. Electron J Pharmacol Ther. 2010;3:35–40. [Google Scholar]
- 43.Singh N., Reddy K.R., Prasad N.K., Singh M. Chemical characterization of Lauha bhasma by X ray diffraction and vibrating sample magnetometry. Int J Ayurvedic Med. 2010;1:143–149. [Google Scholar]
- 44.Rathod K.B., Patel M.B., Parmar P.K., Kharadi S.R., Patel P.V., Patel K.S. Glimpses of current advances of nanotechnology in therapeutics. Int J Pharm Pharm Sci. 2011;3:8–12. [Google Scholar]
- 45.Lakshmi N.R., Swaminathan S., Udaykumar R., Uma M.K. Development of a liposomal nanodelivery system for nevirapine. J Biomed Sci. 2010;17:57. doi: 10.1186/1423-0127-17-57. [DOI] [PMC free article] [PubMed] [Google Scholar]