Abstract
Purpose
Aujeszky's disease (AD) is an economically important disease affecting both wild and domestic pigs of the species Sus scrofa. A previous study yielded serological evidence of AD in Korean wild boars, which could spread AD to other animals. A new Aujeszky's disease virus (ADV) bait vaccine is required to prevent AD outbreaks in swine. In the present study, we investigated the safety and immunogenicity of a gE-deleted marker vaccine, strain YS-400, in young domestic pigs.
Materials and Methods
The YS-400 strain was propagated in Vero cells, and the trial ADV bait vaccine (a vaccine blister in a matrix including an attractant) was prepared. Pigs were orally immunized with the vaccine (2 mL, 107.5 TCID50/mL) delivered using a syringe or in the bait vaccine. The animals were observed for 9 weeks after vaccination, and immunogenicity was assessed using a virus neutralization (VN) test and enzyme linked immunosorbent assay.
Results
The YS-400 strain was non-pathogenic to pigs when given orally and induced high VN titers (1:32-1:128) 6 weeks post-administration. Of the pigs given the ADV bait vaccine twice or three times, 40% were seropositive by 2 weeks, and 100% were seropositive by 7 weeks after the first dose. Pigs that consumed the AD bait vaccine three times developed VN titers that were slightly higher than those of pigs given the vaccine twice.
Conclusion
Domestic pigs given the trial ADV bait vaccine exhibited no adverse effects and developed high VN titers against ADV, indicating that the YS-400 strain is safe and can prevent ADV infection in domestic pigs.
Keywords: Aujeszky's disease virus, Immunity, Oral vaccine
Introduction
Aujeszky's disease (AD), also known as pseudorabies, is caused by Aujeszky's disease virus (ADV), an alpha-herpesvirus of the family Herpesviridae with a positive double-stranded DNA genome about 145 kb in length [1]. ADV can infect several types of animal, including cattle, sheep, goats, raccoons, opossums, rats, and mice, causing fatal disease. ADV causes economically significant illness in domestic pigs and can become latent in the trigeminal ganglia of naturally infected pigs, both domestic and wild. The symptoms of AD depend on the pig's age; piglets under 2 weeks of age die from central nervous system problems, including lack of coordination, tremors, paddling, and convulsions. Fattening pigs infected with ADV principally develop respiratory illnesses, and naturally infected sows in their second or third trimester manifest reproductive failures, including abortion, stillbirth, and weak piglets [2].
If a country is recognized as AD-free, the swine industry will continuously thrive, and pork producers can sell their products on international markets. Thus, many countries (including Korea) have instigated national AD eradication programs that involve the use of gE-deleted vaccines, the culling of pigs positive for the anti-ADV-gE antibody, and the restriction of the movement of pigs from infected farms, rendering domestic pigs AD-free in many parts of the world [3,4,5]. AD has caused economic losses to the Korean swine industry since ADV infection in pigs was first reported in 1987. In 2000, the Korean government decided to launch an AD eradication program using a non-virulent vaccine. This intensive program has dramatically decreased the incidence of disease in domestic pigs [6]. However, ADV infections have been reported in wild boars (Sus scrofa) worldwide, including Korea [4,6,7,8].
To maintain its AD-free status, a country must comply with several requirements, including periodic serological surveys, a ban on further AD vaccination, and the instigation of measures preventing the transmission of ADV from wild boars to domestic pigs. All Korean domestic pigs on all farms undergo annual ADV sero-surveillance, and the inactivated vaccine has not been given to domestic pigs since 2010. ADV infections in wild boar populations have been serologically documented in the United States and many European countries [9]. Between 2% and 5% of Korean wild boars are infected [6]. Therefore, it is necessary to take measures to prevent the transmission of ADV from wild to domestic pigs. Oral vaccination is a valuable strategy for controlling infectious diseases in wildlife. Oral rabies vaccination has helped prevent the spread of disease in wild animals in European countries and the United States [10]. Oral vaccination of wild boars with the classical swine fever virus has been used successfully in European countries to target populations for which parenteral vaccines are not practicable [11].
Oral ADV vaccination of domestic pigs with the YS-400 strain has not yet been reported. In the present study, the safety and immunogenicity of a gE-deleted ADV vaccine, strain YS-400, were evaluated in young domestic pigs.
Materials and Methods
Cells and viruses
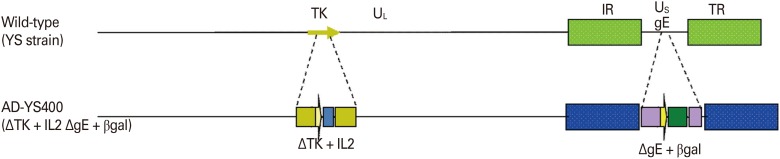
Vero cells (an African green monkey kidney cell line, ATCC CCL81) were maintained in α-minimum essential medium (MEM; Gibco BRL, Grand Island, NY, USA) containing 5% (v/v) fetal bovine serum (Gibco BRL), penicillin (100 IU/mL), streptomycin (100 µg/mL), and amphotericin B (0.25 µg/mL) at 37℃ under 5% (v/v) CO2. A gE-deleted ADV (YS-400 strain) in which parts of the gE and TK genes were deleted and the interleukin-2 (IL2) and β-galactosidase genes inserted was constructed in 2005 via homologous recombination (Fig. 1). The ADV used in the virus neutralization (VN) test was the Yangsan strain isolated from a pig in July 1987. The virus was propagated in Vero cells cultivated in α-MEM. Uninfected cultures served as negative controls.
Fig. 1. Schematic diagram of construction of the recombinant Aujeszky's disease virus (ADV) vaccine strain. The YS-400 strain lacks parts of the wild-type TK and gE genes and carries recombinant interleukin 2 (IL2) and beta galactosidase (βgal) genes. The vaccine is based on the wild-type Yangsan ADV strain.
Preparation of the AD bait vaccine
To propagate the gE-deleted ADV (the YS-400 strain), Vero cells grown in α-MEM were washed three times with phosphate buffered saline and inoculated with virus. After viral adsorption, α-MEM was added and the cells incubated until the cytopathic effect (CPE) attained 90%. The cells were harvested, frozen, and thawed three times and centrifuged (3,000 ×g, 30 minutes) to remove cellular debris. The vaccine was titrated in 96-well microplates (10-fold dilutions). The viral titer determined by the CPE was calculated using the method of Reed and Muench. The ADV bait vaccine consists of a blister containing the vaccine strain and a matrix that includes an attractant.
Experimental design
Experiment 1
Young pigs (11 weeks of age) seronegative for ADV were housed in a compartment. Six underwent oral vaccination with 2 mL (107.5 TCID50/mL) of the YS-400 strain using a syringe without a needle. Vaccination was repeated 2 weeks later. Blood was taken at 0, 2, 4, and 6 weeks post-inoculation (WPI). Two pigs served as controls (no treatment). All pigs underwent physical examination for 6 WPI.
Experiment 2
Young pigs (11 weeks of age) seronegative for ADV were divided into three groups, and two groups were given the AD bait vaccine mentioned above. The ADV titer within a blister of the AD bait vaccine was 107.5 TCID50/mL. Groups 1 and 2 each included five pigs given the vaccine twice or three times, respectively. The control group contained four untreated pigs. After consuming the baits, all pigs were behaviorally monitored. Any adverse effect, including anorexia, prostration, anxiety, agitation, aggression, or paralysis, was noted daily. Blood was collected from all pigs (including the controls) 0, 1, 2, 4, 5, 7, and 9 weeks after vaccination.
Serological assays (VN and enzyme linked immunosorbent assay tests)
The VN test was performed in 96-well microplates using Vero cells. Each serum sample (including the negative controls) was evaluated in duplicate, and serial twofold dilutions. ADV (Yangsan strain; ca. 100 TCID50/50 µL) was added to each well. After 60 minutes of incubation at 37℃, 0.1 mL of a Vero cell suspension (4×105 cells/mL) was added to each well. The microplates were incubated for 72 hours in a humidified incubator under 5% (v/v) CO2 at 37℃, and virus-induced CPE was microscopically evaluated. Each titer was the reciprocal of the highest serum dilution that completely inhibited the CPE. Each serum was diluted from 1:1 to 1:128. A VN titer ≥1:1 was considered positive.
Commercially available enzyme linked immunosorbent assay (ELISA) kits (IDEXX Lab, Westbrook, ME, USA) were used to detect ADV-specific anti-gE and -gB antibodies following the manufacturer's instructions. The two blocking ELISA kits for the detection of antibodies against gE or gB of ADV were developed using monoclonal antibodies directed to gE or gB. So, the absorbance of the ELISA kit is inversely proportional to the amount of bound antibody. Detection of ADV-specific anti-gE-antibody in wild pigs is indicative of ADV infection; the anti-gB antibody is associated with viral neutralization. The positive of ADV gB and the negative of gE antibodies in domestic pigs indicates a response to a gE-deleted vaccine.
Results
Safety and immunogenicity of the ADV YS-400 strain given orally to pigs
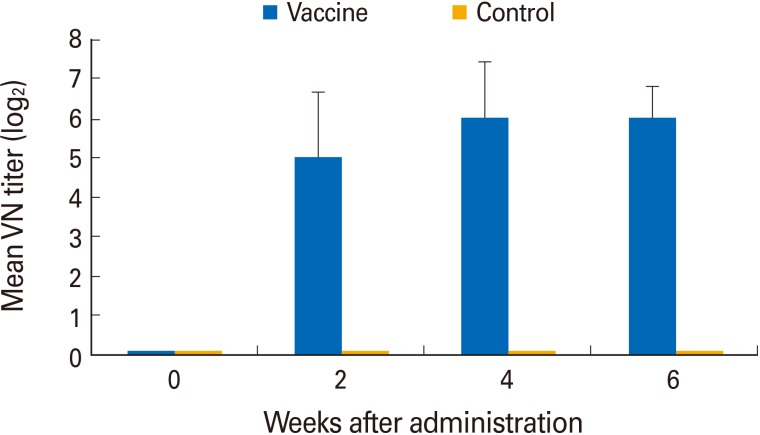
All pigs were seronegative for ADV prior to vaccine administration, but they seroconverted 14 days after vaccination via the oral route (Fig. 2). No vaccinated or unvaccinated pig exhibited clinical signs of AD. All pigs in experiment 1 that received the YS-400 strain orally developed high VN titers ranging from 1:8 to 1:128 (geometric mean, 1:32) by 2 weeks after vaccination and a geometric mean VN titer of 1:64 (range, 1:32 to 1:128) by 6 weeks. The two control pigs remained seronegative throughout the experiment.
Fig. 2. Immunogenicity of the YS-400 strain given via the oral route using a syringe. Six young pigs were vaccinated twice with 2 mL of the YS-400 strain and developed virus neutralization (VN) titers of 1:8-1:128 against Aujeszky's disease virus by 2 weeks after vaccination. All control pigs were negative in terms of VN titer throughout the experiment.
Immunogenicity of the ADV YS-400 strain in pigs given the AD bait vaccine
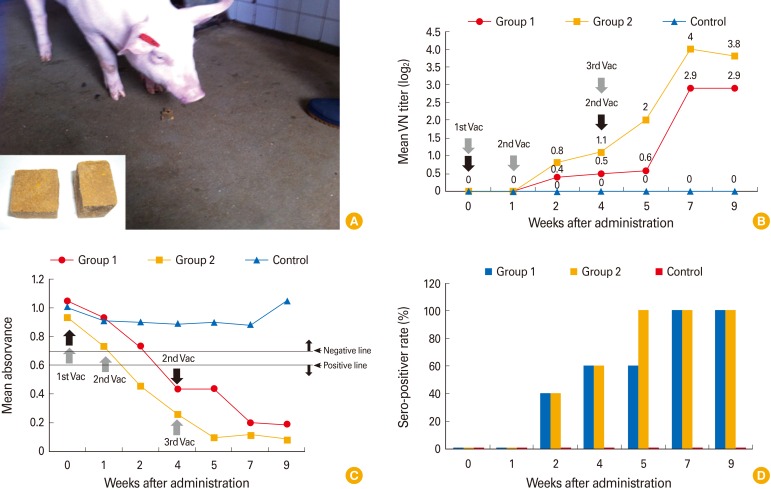
All pigs in group 1 in experiment 2 given the AD bait vaccine twice attained ADV geometric mean VN titers of 1:0.5 ranging from negative to 1:2 by 4 weeks after the first dose and of 1:2.9 ranging from 1:1 to 1:32 by 7 weeks after the first dose (Fig. 3A, B). All pigs in group 1 seroconverted by 7 weeks after the first vaccination. Pigs in group 2 given the AD vaccine three times attained ADV geometric mean VN titers of 1:1.1 ranging from negative to 1:4 by 3 weeks after the first dose and of 1:4 ranging from 1:8 to 1:64 by 7 weeks after the first dose. All serum samples were subjected to an ELISA detecting ADV-specific anti-gB antibodies. As shown in Fig. 3C, pigs in group 1 developed positive reactions to ADV by 3 weeks after the first dose, but pigs in group 2 were positive at 2 weeks. Pigs vaccinated three times had earlier and higher positive titers than did pigs vaccinated twice. In addition, all serum samples were evaluated by an ELISA detecting ADV anti-gE antibodies; no animal was positive.
Fig. 3. The trial Aujeszky's disease bait vaccine and the immunogenicity thereof in domestic pigs. The pig is consuming a bait vaccine containing the YS-400 strain (A). The mean virus neutralization (VN) titers (B) and enzyme linked immunosorbent assay titers (C) in groups vaccinated twice (group 1) or three times (group 2) and the seropositive rates attained by each group (D).
Of the pigs in groups 1 and 2, 40% were seropositive by 2 weeks after the first dose and 100% were seropositive by 7 weeks after the first dose (Fig. 3D). Pigs in group 2 seroconverted earlier, at 5 weeks after the first dose, than did pigs in group 1. All four control pigs remained ADV-seronegative throughout the experiment, confirming that no contact transmission had occurred between vaccinated and control animals.
Discussion
Many countries have instigated specific AD control programs to attain AD-free status, and ADV has been eliminated from the domestic pig populations of several countries worldwide [12,13]. Thus, national AD eradication programs have been successful. A total of 110,000 pigs on all pig farms in Korea have been surveyed annually for ADV since 2008, and no seropositive response has been detected [6]. Although vaccination against AD is not formally banned, no domestic pig has been inoculated with the inactivated gE-deleted AD vaccine since 2010. However, in countries with wild boar populations, several measures must be implemented to prevent transmission of ADV from boars to domestic pigs [14]. Although a vaccine marker does not contribute to vaccine efficacy, such a marker renders it possible to differentiate infected from vaccinated animals. Oral vaccination of wild animals has successfully controlled rabies in carnivores and classical swine fever virus in boars [10,11]. Wild boars immunized with the live attenuated ADV Bartha strain are protected against highly virulent ADV. Boars immunized using a syringe or by consuming a blister developed comparable VN titers [15,16].
In the present study, the YS-400 strain, in which the gE and TK genes are partly deleted and the IL2 and beta-galactosidase genes inserted, served as both the orally administered and bait vaccine. The first experiment evaluated vaccine safety and immunogenicity in pigs given the (oral) vaccine using a syringe. No pig given 107.5 TCID50/mL orally (twice) developed any adverse respiratory symptom, and all orally vaccinated pigs developed high mean VN titers (1:64) 4 weeks after vaccination. Interestingly, the mean VN titer (thus, 1:64) induced was similar to that of wild boars given the Bartha strain orally [15], indicating that oral vaccination of domestic pigs using the YS-400 strain induced strong immunity.
The second experiment was designed to determine whether the ADV bait vaccine containing the YS-400 strain induced a high-level antibody response. At 9 weeks after vaccination, the VN titers of pigs given the vaccine three times were higher than those of pigs given the vaccine twice. The serological data (the ELISA results) showed similar trends. In addition, pigs vaccinated three times attained 100% seropositivity earlier (by 7 weeks after vaccination) than did pigs vaccinated twice. It was earlier reported that large quantities of ADV were necessary to adequately infect animals and that the oral route required more vaccine than did the intranasal route [17]. It may be that a large quantity of ADV is required to induce an adequate immune response in domestic pigs. Therefore, we will soon prepare an AD bait vaccine containing over 108.0 TCID50/mL for testing on Korean wild boars. Although both humoral and cellular immunity play roles in protection, it is likely that a high VN titer contributes to the protection of pigs against challenge with virulent ADV [18,19]. However, we did not conduct efficacy testing in immunized pigs. Therefore, a further study on the efficacy of the ADV bait vaccine is required. Importantly, no contact animal seroconverted, indicating that the YS-400 strain may not be directly transmitted to contact pigs.
In conclusion, the YS-400 strain was safe and immunogenic in domestic pigs. After oral inoculation, a high-level immune response was evident, and two or three doses of the AD bait vaccine induced high VN titers in domestic pigs. This indicates that, after efficacy testing in the near future, the YS-400 strain can be used as an AD bait vaccine in domestic pigs and is thus a candidate for use in wild boars.
Footnotes
No potential conflict of interest relevant to this article was reported.
This work was financially supported by a grant (No. F-1543083-2013-13-01) from the Animal and Plant Quarantine Agency, Ministry of Agriculture, Food and Rural Affairs (MAFRA), Republic of Korea.
References
- 1.Mettenleiter TC. Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis: state of the art, June 1999. Vet Res. 2000;31:99–115. doi: 10.1051/vetres:2000110. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari M, Gualandi GL, Corradi A, et al. Experimental infection of pigs with a thymidine kinase negative strain of pseudorabies virus. Comp Immunol Microbiol Infect Dis. 1998;21:291–303. doi: 10.1016/s0147-9571(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 3.Muller T, Batza HJ, Schluter H, Conraths FJ, Mettenleiter TC. Eradication of Aujeszky's disease in Germany. J Vet Med B Infect Dis Vet Public Health. 2003;50:207–213. doi: 10.1046/j.1439-0450.2003.00666.x. [DOI] [PubMed] [Google Scholar]
- 4.Muller T, Hahn EC, Tottewitz F, et al. Pseudorabies virus in wild swine: a global perspective. Arch Virol. 2011;156:1691–1705. doi: 10.1007/s00705-011-1080-2. [DOI] [PubMed] [Google Scholar]
- 5.Wang CH, Yuan J, Qin HY, et al. A novel gE-deleted pseudorabies virus (PRV) provides rapid and complete protection from lethal challenge with the PRV variant emerging in Bartha-K61-vaccinated swine population in China. Vaccine. 2014;32:3379–3385. doi: 10.1016/j.vaccine.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Yang DK, Nah JJ, Kim HH, et al. Seroepidemiological aurvey of Aujeszky's sisease cirus in wild boar (Sus scrofa) and raccoon dogs (Nyctereutes procyonoides koreensis) in Korea. J Bacteriol Virol. 2014;44:336–341. [Google Scholar]
- 7.Pannwitz G, Freuling C, Denzin N, et al. A long-term serological survey on Aujeszky's disease virus infections in wild boar in East Germany. Epidemiol Infect. 2012;140:348–358. doi: 10.1017/S0950268811000033. [DOI] [PubMed] [Google Scholar]
- 8.Boadella M, Gortazar C, Vicente J, Ruiz-Fons F. Wild boar: an increasing concern for Aujeszky's disease control in pigs? BMC Vet Res. 2012;8:7. doi: 10.1186/1746-6148-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sedlak K, Bartova E, Machova J. Antibodies to selected viral disease agents in wild boars from the Czech Republic. J Wildl Dis. 2008;44:777–780. doi: 10.7589/0090-3558-44.3.777. [DOI] [PubMed] [Google Scholar]
- 10.Mainguy J, Fehlner-Gardiner C, Slate D, Rudd RJ. Oral rabies vaccination in raccoons: comparison of ONRAB(R) and RABORAL V-RG(R) vaccine-bait field performance in Quebec, Canada and Vermont, USA. J Wildl Dis. 2013;49:190–193. doi: 10.7589/2011-11-342. [DOI] [PubMed] [Google Scholar]
- 11.Kaden V, Lange E, Fischer U, Strebelow G. Oral immunisation of wild boar against classical swine fever: evaluation of the first field study in Germany. Vet Microbiol. 2000;73:239–252. doi: 10.1016/s0378-1135(00)00148-6. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Lopez B, Carpenter TE, Sanchez-Vizcaino JM. Risk assessment and cost-effectiveness analysis of Aujeszky's disease virus introduction through breeding and fattening pig movements into Spain. Prev Vet Med. 2009;90:10–16. doi: 10.1016/j.prevetmed.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Miller GY, Tsai JS, Forster DL. Benefit-cost analysis of the national pseudorabies virus eradication program. J Am Vet Med Assoc. 1996;208:208–213. [PubMed] [Google Scholar]
- 14.World Organisation for Animal Health. Manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees) 7th ed. Paris: World Organisation for Animal Health; 2012. pp. 97–111. [Google Scholar]
- 15.Maresch C, Lange E, Teifke JP, et al. Oral immunization of wild boar and domestic pigs with attenuated live vaccine protects against Pseudorabies virus infection. Vet Microbiol. 2012;161:20–25. doi: 10.1016/j.vetmic.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 16.An TQ, Peng JM, Tian ZJ, et al. Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg Infect Dis. 2013;19:1749–1755. doi: 10.3201/eid1911.130177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakubik J. Comparative susceptibility of rabbits, rats, mice and pigs to infection with Aujeszky diseases virus (ADV) in the development of an efficacy test for ADV vaccines. Zentralbl Veterinarmed B. 1977;24:764–766. doi: 10.1111/j.1439-0450.1977.tb01051.x. [DOI] [PubMed] [Google Scholar]
- 18.Alva-Valdes R, Glock RD, Kluge JP, Hill HT. The effects of challenge on the humoral and cellular immune responses in pseudorabies vaccinated swine. Can J Comp Med. 1983;47:451–455. [PMC free article] [PubMed] [Google Scholar]
- 19.Pol JM, Gielkens AL, van Oirschot JT. Comparative pathogenesis of three strains of pseudorabies virus in pigs. Microb Pathog. 1989;7:361–371. doi: 10.1016/0882-4010(89)90039-9. [DOI] [PubMed] [Google Scholar]