Abstract
Background
Gmelina arborea (GA) is widely used in traditional medicine for treating a number of ailments including gastrointestinal tract disorders.
Objective
To evaluate the gastroprotective effect of GA stem bark against ethanol-induced gastric ulcer in Wistar rats.
Materials and methods
All animals were fasted for 36 h and received GA extract 250 and 500 mg/kg body weight (bw), 1 h before the administration of ethanol. The animals received ranitidine 50 mg/kg bw which served as the standard. The rats were sacrificed after 4 h. Then, the injuries to the gastric mucosa were estimated through gross evaluation of ulcer lesions and histology. The antioxidant parameters such as level of lipid peroxidation, superoxide dismutase (SOD), reduced glutathione (GSH), and glutathione peroxidase (GPx) in gastric tissue were also determined.
Results
GA treatment at a dose of 500 mg/kg bw offered 91.98% inhibition of ulcer formation, which is higher than that of ranitidine. The ethanol treatment extensively increased lipid peroxidation and it was significantly (P < 0.01) reduced in GA-treated group that eventually helped to prevent free radical accumulation. The GA enhanced the gastric mucosal antioxidant system, as indicated by a dose-dependent increase in the level/activities of GSH, GPx, and SOD. GA also attenuated the severity of histological signs of cell damage. Further, GA extract showed in-vitro 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity with IC50 value of 124.39 μg/ml.
Conclusion
The results indicate that the gastroprotective effect of GA is probably related to its antioxidant activities that protect gastric mucosa against oxidative damage and antilipid peroxidative activity that maintain membrane integrity.
Keywords: Antioxidant system, Ethanol, Gastric ulcer, Gmelina arborea, Lipid peroxidation
1. Introduction
Gastric ulcer is one of the most widespread diseases in the world and occurs with stress, nonsteroidal anti-inflammatory drugs, Helicobacter pylori infection, and alcohol ingestion [1]. Ulceration occurs when there is an imbalance between aggressive (acid-pepsin secretions) and protective factors such as mucus secretion, mucosal barrier, cell regeneration, blood flow, and prostaglandins [2]. Most of the drugs used for the treatment of gastric ulcers, show numerous adverse effects [3]. In the search for new drugs, metabolites derived from plants used in traditional medicine provided an alternative source of therapeutic drugs [4]. Plant extracts containing a wide variety of antioxidants such as phenolic and flavonoid compounds, are some of the most attractive sources of new drugs and have been shown to produce promising results in the treatment of gastric ulcers [5].
Gmelina arborea (GA), Gambhari in Sanskrit, a popular commercial timber grows naturally in the warm temperate regions of Mediterranean and South Asia [6]. The plant is widely used in Ayurveda, one of the major traditional forms of medicine in India. The root of the plant is a member of “brihat panchamoola,” which is a major constituent of many ayurvedic preparations [7] used for treating chronic fever, hemorrhages, urinary tract infections, anuria, etc. The plant forms one of the ingredients of Dashamoolarishta—a reputed restorative tonic and Shriparnyadi Kwath—prescribed in bilious fever [8]. The bark is bitter, tonic, and stomachic and is useful in curing fever and dyspepsia [9]. The Ayurvedic Pharmacopoeia of India recommends the use of bark and stem in treating inflammatory diseases and edema [10].
GA has been widely used in Ayurveda and Siddha for curing gastrointestinal tract disorders as well as, the leaf juice is used for ulcer treatment [8]. The plant is reported to contain a plenty of phytochemicals such as alkaloids, flavonoids [11], lignans, and iridoid glycosides [6]. Arboreol, paulownin, gmelinol, and epieudesmin [12] are reported to be in the heartwood of the plant. Tyrosol (2-[4-hydroxyphenyl] ethanol), balanophonin, gmelinol, phenylethanoid glycoside, 2,6-dimethoxy-p-benzoquinone, and 3,4,5-trimethoxyphenol were identified in the bark [13]. Considering its medicinal value and poor availability of scientific data to prove the gastroprotective effect, the present study was conducted to evaluate the antiulcer properties of 70% methanolic extract of GA stem bark against ethanol-induced gastric ulcer in rats and its comparison with that of the standard drug ranitidine.
2. Materials and methods
2.1. Plant material and extraction
The stem bark of GA was collected from the institute's ayurvedic garden. The plant was authenticated by Dr. P. Sujanapal, Scientist—B, Silviculture Department, KFRI, Peechi, Thrissur—680 653, Kerala (Voucher specimen No: KFRI/SILVA/GEN/07/11). The stem bark was dried at 45–50 °C for 7 days, powdered, and extracted with 70% methanol using Soxhlet apparatus. The extract was filtered, evaporated to dryness, and the dried extract was re-dissolved in distilled water for further studies.
2.2. 2,2-Diphenyl-1-picrylhydrazyl radical scavenging activity
The antioxidant activity of the GA extract was measured by the scavenging activity of the stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical [14]. The radical form of DPPH has an absorption peak at 515 nm, which reduces upon reduction by an antioxidant compound. Different concentration of the extract was incubated with freshly prepared DPPH solution in a total volume of 2 ml (0.25 g/l in methanol). Absorbance at 515 nm was measured 20 min after starting the reaction. Vitamin C was used as a standard. Concentration of extract necessary to decrease the initial concentration of DPPH by 50% (IC50) was calculated.
2.3. Experimental animals
Wistar rats were purchased from the Small Animal Breeding Station, Kerala Veterinary and Animal Sciences University, Mannuthy, Kerala, India. The animals were maintained under standardized environmental conditions (temperature: 22–30 °C, relative humidity: 60–70%, and 12 h of dark/light cycle) with free access to standard rat feed (Lipton, India) and water ad libitum. They were kept in a group of three in polypropylene cages with husk paddy as the bedding with stainless steel top grill having facilities for providing food and water. All animal experiments were conducted during the present study got prior permission from Institutional Animal Ethical Committee (IAEC) (Register Number: ACRC/IAEC/15/02-[2]) and followed the internationally accepted laboratory animal use and care guidelines and rules of IAEC.
2.4. Acute oral toxicity
For acute oral toxicity analysis, female Wistar rats (170–180 g) were divided into five groups of five animals each. Before the initiation of experiment, the animals were fasted overnight and then a single dose of GA extract at concentrations 50, 500, 1000, 2000, and 5000 mg/kg body weight (bw) was administered orally. The animals were observed for behavioral changes and mortality, periodically for the first 24 h and then daily for 14 days according to the Organisation for Economic Cooperation and Development-423 Guideline. Changes in body weight, food, and water intake of the animals were also recorded during the period.
2.5. Experimental design
Thirty male Wistar rats, weighing 180–200 g were randomly divided into five groups of six animals each.
-
•
Group I: Normal – 1 ml distilled water
-
•
Group II: Control – 1 ml distilled water
-
•
Group III: Standard – Ranitidine (50 mg/kg bw.)
-
•
Group IV: G. arborea extract low concentration (GALC) (250 mg/kg bw.)
-
•
Group V: G. arborea extract high concentration (GAHC) (500 mg/kg bw.).
Before the start of the experiment, the rats were deprived of food for 36 h and water for 12 h. After 1 h of oral treatment as per the above schedule, all groups except Group I (normal) were administered with 1 ml of 80% ethanol orally to induce gastric ulcer. The animals were sacrificed after 4 h of ethanol administration, with an overdose of ether. Stomach of each experimental animal was carefully dissected and opened along the greater curvature. The stomachs were washed with ice-cold normal saline (0.9%).
2.6. Gross lesion evaluation
The ulcer index (U.I.) was calculated by severity of gastric mucosal lesions graded as erosions, 1 mm or less Grade 1; 1–2 mm Grade 2, and more than 2 mm Grade 3. Calculation of U.I was done according to Main and Whittle [15]. The percentage inhibition (I%) of ulcer formation was calculated by the formula, I% = ([UI of control − UI of test]/UI of control) × 100.
2.7. Biochemical analysis
Mucosa of glandular stomach homogenate (10% in Tris buffer, pH 7.0) was prepared and used for the biochemical analysis of lipid peroxidation by measuring the color produced by the reaction of thiobarbituric acid with malondialdehyde (MDA) [16]. The supernatant obtained after centrifugation of homogenate at 10,000 rpm for 1 h was used for further estimations. Reduced glutathione (GSH) was measured by its reaction with 5,5’-dithio-bis-(2-nitrobenzoic acid) to give a yellow colored complex [17]. Glutathione peroxidase (GPx) was estimated by measuring the amount of unconsumed GSH, as the enzyme degrades hydrogen peroxide in the presence of GSH [18]. Superoxide dismutase (SOD) was assayed based on the ability of the enzyme to inhibit superoxide radical mediated reduction of nitroblue tetrazolium salt [19]. Total protein was also measured [20].
2.8. Histopathological analysis
Histological evaluation was performed on the glandular stomach of rats. The tissue samples were preserved in 10% buffered formalin and processed for routine paraffin block preparation. Sections about 5 μm in thickness were cut and stained with hematoxylin and eosin [21].
2.9. Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical comparisons were made using one-way analysis of variance followed by Student's t-test or Dunnett's multiple comparison test. A value of P < 0.05 was considered to indicate statistical significance.
3. Results
3.1. Acute oral toxicity
The GA extract at all tested doses did not produce any signs of toxicity and mortality. Therefore, the LD50, if any, should be more than 5000 mg/kg bw.
3.2. 2,2-Diphenyl-1-picrylhydrazyl radical scavenging activity
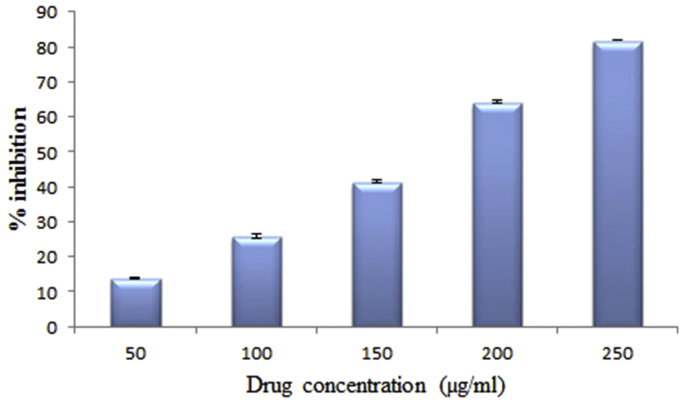
The effect of antioxidants on DPPH radical scavenging was thought to be due to their hydrogen donating ability. The scavenging effect of GA extract on DPPH radical increased with an increasing concentration of the extract. The IC50 value of the extract was found to be 124.39 μg/ml (Fig. 1) and that of Vitamin C was 2.5 μg/ml.
Fig. 1.
Effect of Gmelina arborea on 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity. Values are expressed as mean ± standard deviation for six animals.
3.3. Gross evaluation of gastric lesions
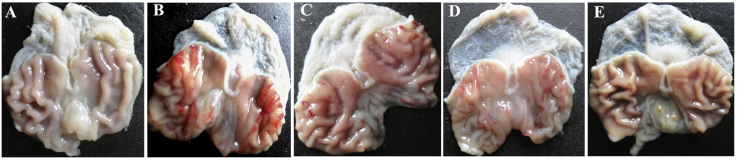
The ulcer lesions were elongated (1–10 mm long), parallel to the long axis of the stomach and were hemorrhagic. The ethanol alone treated control group shows extensive and visible hemorrhagic lesions of gastric mucosa. The GA extract significantly suppressed the formation of the ulcers, especially at its highest dosage. The extract significantly reduced the ulcer size and severity in a dose-dependent manner. The protection properties of GAHC-treated group appeared slightly superior to that of the standard group (Fig. 2).
Fig. 2.
Effect of Gmelina arborea on the prevention of gastric ulcer. Gastric mucosa of (A) normal animals showing absence of ulcers; (B) ethanol alone administered control animals showing severe ulceration as reddish mucosal lesions; (C) standard drug ranitidine (50 mg/kg bw.) treated animals with less intense ulceration; (D) G. arborea extract low concentration (GALC – 250 mg/kg bw.) showing mild ulceration with short mucosal lesions; (E) G. arborea extract high concentration (GAHC – 500 mg/kg bw.) displaying minor ulceration.
3.4. Ulcer index (U.I.) and percentage inhibition (I %)
The ethanol alone treated control group shows extensive and visible hemorrhagic lesions of gastric mucosa. Ethanol administration produced gastric damage with an U.I. of 1.66 ± 0.05, while all the treated groups showed a significant (P < 0.01) reduction in ulcer index. I% of ulcer formation was highest for the GAHC group (91.98%), whereas the ranitidine group and GALC-treated group exhibited only 86.9 and 86.4% inhibition, respectively (Table 1).
Table 1.
Effect of GA on stabilization of gastric mucosa in ethanol-induced gastric ulcer.
| Groups | Ulcer index | Percentage of inhibition |
|---|---|---|
| Control | 1.66 ± 0.05 | 0 |
| Standard | 0.217 ± 0.02a | 86.9 |
| GALC | 0.225 ± 0.03a | 86.4 |
| GAHC | 0.133 ± 0.05a | 91.98 |
Values are expressed as mean ± SD for 6 animals in each group.
SD: Standard deviation, GA: Gmelina arborea, GALC: Gmelina arborea extract low concentration, GAHC: Gmelina arborea extract high concentration.
P < 0.01, when compared to control.
3.5. Gastric mucosal lipid peroxidation and antioxidant status
The concentration of MDA, a major product of lipid peroxidation, was increased in the ethanol alone treated group, whereas it was significantly reduced in GA-treated groups in a dose dependent manner. Animals treated with the standard drug ranitidine also displayed MDA level closer to normal. The antioxidant defense systems such as reduced GSH, SOD, and GPx level were drastically reduced in the control group, whereas the treated groups restored the enzyme activity to the normal level in a dose-dependent manner (Table 2).
Table 2.
Effect of GA on mucosal lipid peroxidation and antioxidant status.
| Groups | MDA (nmol/mg of protein) | GSH (nmol/mg of protein) | GPx (U/mg of protein) | SOD (U/mg of protein) |
|---|---|---|---|---|
| Normal | 0.522 ± 0.016 | 34.522 ± 1.10 | 28.563 ± 0.481 | 1.018 ± 0.016 |
| Control | 1.432 ± 0.072a | 20 ± 0.874a | 18.317 ± 0.303a | 0.548 ± 0.011a |
| Standard | 0.579 ± 0.05b | 31.251 ± 0.935b | 23.981 ± 0.711b | 0.897 ± 0.062b |
| GALC | 0.822 ± 0.048b | 30.421 ± 0.644b | 23.867 ± 0.341b | 0.706 ± 0.074b |
| GAHC | 0.376 ± 0.047b | 35 ± 0.668b | 28.305 ± 1.02b | 1.034 ± 0.088b |
Values are expressed as mean ± SD for 6 animals in each group.
SD: Standard deviation, GA: Gmelina arborea, GALC: Gmelina arborea extract low concentration, GAHC: Gmelina arborea extract high concentration, MDA: Malondialdehyde, GPx: Glutathione peroxidase, SOD: Superoxide dismutase.
P < 0.01, when compared to normal.
P < 0.01, when compared to control.
3.6. Histopathology
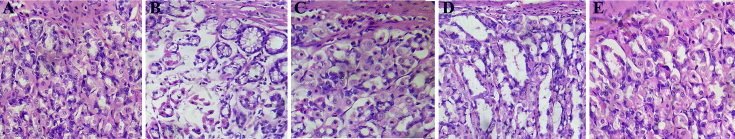
When compared to the normal group, the ulcer-induced ethanol alone treated group showed atrophy of the lining cells of the mucosal glands, vacuolation in several areas, leukocyte infiltration, and severe necrosis. The GALC-treated group also showed necrosis and inflammatory changes. There was a significant reduction of such pathological features in the histology of the GAHC- and ranitidine-treated group (Fig. 3).
Fig. 3.
The protective effect of Gmelina arborea on histological evaluation of rats' stomach in ethanol-induced ulcer model. Microscopic appearance of H & E stained (×400) gastric mucosa of (A) normal animals showing normal appearance (B) ethanol alone administered control animals displaying severe erosions associated with vacuole formation, inflammatory changes, and severe necrosis. (C) standard drug ranitidine (50 mg/kg bw.) treated animals showing mild vacuolation and inflammatory changes, but no necrosis (D) G. arborea extract low concentration (GALC – 250 mg/kg bw.) treated animals showing mild necrosis and vacuolation (E) G. arborea extract high concentration (GAHC – 500 mg/kg bw.) treated animals showing almost normal appearance of gastric mucosa.
4. Discussion
The effect of ethanol-induced ulcer is proved by its rapid penetration into gastric mucosa, thereby increasing mucosal permeability and discharge of vasoactive mediators such as endothelin-1, leukotrienes C4, and histamine. These vasoactive mediators induce blood flow stasis in mucus membrane, which increase the lesions in mucosa [22], [23]. In addition, ethanol causes reduction in mucus production and increase in the production of reactive oxygen species that result in rupture of blood vessels, thus contributes to hemorrhage, tissue necrosis, and finally disrupting the protective mucosal barrier [24].
The present study clearly indicates the effectiveness of GA extract in protecting the gastric mucosa from ethanol-induced ulcer. This can be evident from the gross evaluation of the rats’ stomach where the treated group showed significant suppression of ulcer formation and reduction in ulcer size in a dose-dependent manner.
Oxidative stress plays an important role in the pathogenesis of various diseases including gastric ulcer. Antioxidants have been reported to play a significant role in the protection of gastric mucosa against various necrotic agents. Ethanol-induced gastric injury in rats can be inhibited by the administration of antioxidants [25]. GA stem bark was found to have good free radical scavenging activity as evidenced by DPPH assay. Thus, the gastroprotective effect of GA could be attributed to its antioxidant properties which may be due to the presence of phytochemicals such as lignans.
It has been suggested that reactive oxygen species, primarily hydroxyl radicals, superoxide anions, and lipid peroxides are the harmful species identified to cause gastric ulcer development [26]. Free radical buildup can ultimately cause depletion in the tissue antioxidant status leading to lipid peroxidation. In the current study, the ethanol treatment extensively increased lipid peroxidation and it was significantly reduced by GA extract in a dose-dependent way. The rise in the generation of lipid peroxides indicates the involvement of free radicals in the process of ulceration. Henceforth, the antilipid peroxidative activity of the extract could have resulted in the inhibition of ulceration.
Antioxidants scavenge free radical formation and thus play a major role in the protection of cellular damage. The treatment with plant extract enhanced the antioxidant levels/activities of reduced GSH, SOD, and GPx that will eventually help to prevent the free radical generation, which may occur during ulcer development.
5. Conclusion
The results of the study can be suggestive of the gastroprotective activity of G. arborea stem bark extract against ethanol-induced injury and this can be credited to its ability to enhance antioxidant defense system and also to antilipid peroxidative activity. A further detailed study on various other parameters is required to throw more light on the exact mechanism involved in gastroprotection.
Conflicts of interest
None declared.
Acknowledgment
The authors are grateful to UGC, Government of India, for the financial support as research grant (No.F.2-19/2006 (SA-I)).
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore
References
- 1.Ogawa K., Oyagi A., Tanaka J., Kobayashi S., Hara H. The protective effect and action mechanism of Vaccinium myrtillus L. on gastric ulcer in mice. Phytother Res. 2011;25:1160–1165. doi: 10.1002/ptr.3413. [DOI] [PubMed] [Google Scholar]
- 2.Lima Z.P., Severi J.A., Pellizzon C.H., Brito A.R., Solis P.N., Cáceres A. Can the aqueous decoction of mango flowers be used as an antiulcer agent? J Ethnopharmacol. 2006;106:29–37. doi: 10.1016/j.jep.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe M.M., Sachs G. Acid suppression: optimizing therapy for gastroduodenal ulcer healing, gastroesophageal reflux disease, and stress-related erosive syndrome. Gastroenterology. 2000;118(2 Suppl. 1):S9–S31. doi: 10.1016/s0016-5085(00)70004-7. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez-Mendoza M.E., Reyes-Ramírez A., Cruz Antonio L., Martínez Jiménez L., Rodríguez-Silverio J., Arrieta J. Bioassay-guided isolation of an anti-ulcer compound, tagitinin C, from Tithonia diversifolia: role of nitric oxide, prostaglandins and sulfhydryls. Molecules. 2011;16:665–674. doi: 10.3390/molecules16010665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiruma-Lima C.A., Gracioso J.S., Toma W., Almeida A.B., Paula A.C., Brasil D.S. Gastroprotective effect of aparisthman, a diterpene isolated from Aparisthmium cordatum, on experimental gastric ulcer models in rats and mice. Phytomedicine. 2001;8:94–100. doi: 10.1078/0944-7113-00017. [DOI] [PubMed] [Google Scholar]
- 6.Tiwari N., Yadav A.K., Srivastava P., Shanker K., Verma R.K., Gupta M.M. Iridoid glycosides from Gmelina arborea. Phytochemistry. 2008;69:2387–2390. doi: 10.1016/j.phytochem.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Sharma P.C., Yelne M.B., Dennis T.J. Central Council for Research in Ayurveda and Siddha, Government of India; New Delhi: 2001. Database on medicinal plants used in ayurveda. [Google Scholar]
- 8.Khare C.P. Springer Science & Business Media; New York: 2004. Indian herbal remedies: rational western therapy, ayurvedic, and other traditional usage, botany; p. 523. [Google Scholar]
- 9.Nambiar V.P. Orient Blackswan; Ernakulam: 1995. Indian medicinal plants: a compendium of 500 species; p. 592. [Google Scholar]
- 10.Khare C.P. Springer Science & Business Media; New Delhi: 2008. Indian medicinal plants: an illustrated dictionary; p. 900. [Google Scholar]
- 11.Kulkarni Y.A., Panjabi R., Patel V., Tawade A., Gokhale A. Effect of Gmelina arborea Roxb in experimentally induced inflammation and nociception. J Ayurveda Integr Med. 2013;4:152–157. doi: 10.4103/0975-9476.118697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawamura F., Ohara S., Nishida A. Antifungal activity of constituents from the heartwood of Gmelina arborea: part 1. Sensitive antifungal assay against Basidiomycetes. Holzforschung. 2004;58:189–192. [Google Scholar]
- 13.Falah S., Katayama T., Suzuki T. Chemical constituents from Gmelina arborea bark and their antioxidant activity. J Wood Sci. 2008;54:483–489. doi: 10.3923/pjbs.2008.2007.2012. [DOI] [PubMed] [Google Scholar]
- 14.Aquino R., Morelli S., Lauro M.R., Abdo S., Saija A., Tomaino A. Phenolic constituents and antioxidant activity of an extract of Anthurium versicolor leaves. J Nat Prod. 2001;64:1019–1023. doi: 10.1021/np0101245. [DOI] [PubMed] [Google Scholar]
- 15.Main I.H., Whittle B.J. Investigation of the vasodilator and antisecretory role of prostaglandins in the rat gastric mucosa by use of non-steroidal anti-inflammatory drugs. Br J Pharmacol. 1975;53:217–224. doi: 10.1111/j.1476-5381.1975.tb07351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 17.Moron M.S., Depierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 18.Hafeman D.G., Sunde R.A., Hoekstra W.G. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J Nutr. 1974;104:580–587. doi: 10.1093/jn/104.5.580. [DOI] [PubMed] [Google Scholar]
- 19.McCord J.M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 20.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 21.Bancroft J., Stevens A. Churchill Livingstone; New York: 1982. Theory and practice of histological techniques; p. 662. [Google Scholar]
- 22.Al-wabel N., Hassan A., Abbas H., Muosa H. Antiulcerogenic effect of camel milk against ethanol induced gastric ulcers in rats. Webmedcentral Vet Med. 2012;3:1–12. [Google Scholar]
- 23.Wallace J.L. Nonsteroidal anti-inflammatory drugs and the gastrointestinal tract. Mechanisms of protection and healing: current knowledge and future research. Am J Med. 2001;110:19S–23S. doi: 10.1016/s0002-9343(00)00631-8. [DOI] [PubMed] [Google Scholar]
- 24.Lewis D.A., Hanson P.J. Anti-ulcer drugs of plant origin. Prog Med Chem. 1991;28:201–231. doi: 10.1016/s0079-6468(08)70365-5. [DOI] [PubMed] [Google Scholar]
- 25.Abdulla M.A., Ahmed K.A., Al-Bayaty F.H., Masood Y. Gastroprotective effect of Phyllanthus niruri leaf extract against ethanol-induced gastric mucosal injury in rats. Afr J Pharm Pharmacol. 2010;4:5. [Google Scholar]
- 26.Smith G.S., Mercer D.W., Cross J.M., Barreto J.C., Miller T.A. Gastric injury induced by ethanol and ischemia-reperfusion in the rat. Differing roles for lipid peroxidation and oxygen radicals. Dig Dis Sci. 1996;41:1157–1164. doi: 10.1007/BF02088232. [DOI] [PubMed] [Google Scholar]