Abstract
Background
Samasharkara Churna, a polyherbal Ayurvedic formulation, is prescribed for treating various conditions such as asthma and cough. Literature review suggested that characterization parameters of Samasharkara Churna are not reported.
Objective
To report characteristic parameters of Samasharkara Churna to conform its identity, quality and purity.
Materials and Methods
Samasharkara Churna was evaluated for pharmacognostic, physicochemical, microbiological, and chromatographic parameters.
Results
The chromatographic analysis was able to showed presence of all ingredients in Samasharkara Churna.
Conclusion
The characterization parameters presented in this paper may serve as standard reference for the quality control analysis of Samasharkara Churna.
Keywords: Samasharkara churna, Pharmacognostic, Toxicological, Chromatography, Thermogravimetry
1. Introduction
According to World Health Organization (WHO); traditional, complementary, alternative, or non-conventional medicines are used by 70–95% of global population particularly in developing countries for their healthcare [1]. Moreover, the use of herbal medicines has increased remarkably in line with the global trend of people returning to natural therapies [2]. The growing use of botanicals (drug and other products derived from plants) by the public is forcing moves to assess the health claims of these agents and to develop standards of quality and manufacture.
Standardization of herbal medicines is the process of prescribing a set of standards or inherent characteristics, constant parameters, definitive qualitative and quantitative values that carry an assurance of quality, efficacy, safety and reproducibility. A herbal product cannot be considered scientifically valid if the drug tested has not been authenticated and characterized in order to ensure reproducibility in the manufacturing of the product. Moreover, many dangerous and lethal side effects have recently been reported, including direct toxic effects, allergic reactions, effects from contaminants, and interactions with herbal drugs [2]. On this background, standardization is an important step for the establishment of a consistent biological activity, a consistent chemical profile, or simply a quality assurance program for production and manufacturing of a herbal drug [3].
The Indian system of medicine, mainly comprising of Ayurveda, Siddha and Unani, is one of the oldest holistic management system with thoroughly documented remedies. Ayurveda, a part of cultural heritage of India, is widely respected for its uniqueness and global acceptance as it offers natural ways to treat diseases and promote healthcare [4]. Unfortunately, standardization and quality control have remained grey areas in the preparation of Ayurvedic medicines. Till date, most of the ayurvedic formulations are lacking in their defined quality control parameters and method of its evaluation [5].
Asthma is one of the most common chronic diseases affecting an estimated 300 million people worldwide and ranks third responsible for hospitalization [6], [7]. The prevalence of asthma is increasing in most countries, especially among children. Asthma is a significant burden, not only in terms of health care costs but also of lost productivity and reduced participation in family life [8]. Asthma is not just a public health problem for high income countries, it occurs in all countries regardless of level of development. Over 80% of asthma deaths occur in low and lower-middle income countries [9]. Plant-based medicines are the 3rd most popular choice of both adults (11%) and children (6%) suffering from asthma.
Samasharkara Churna, an ayurvedic polyherbal churna (fine powder) formulation, is prescribed by Ayurvedic physicians for treating conditions such as Asthma (Shwasa Roga in Ayurveda) and cough [10]. It contains six ingredients, viz., Lavanga (Syzygium aromaticum), Jatiphala (Myristica fragrans), Pippali (Piper longum), Maricha (Piper nigrum), Mahaushadha (Zingiber officinale) and Sita (Sugar) mixed in equal proportion by weight. There is lack of information regarding scientific analysis of Samasharkara Churna, hence characterisation of Samasharkara Churna was planned to conform its identity, quality and purity.
2. Materials & methods
2.1. Plant materials
All the ingredients of Samasharkara Churna were procured from the local market of Bhubaneswar, Odisha, India, and were authenticated by botanist Miss. Rashmibala Sahoo, Scientific officer of the Department of Botany, State Drug Testing & Research Laboratory (ISM), Bhubaneswar, Odisha, India. Voucher specimens of these ingredients have been deposited in the Department of Pharmacognosy, State drug Testing & Research Laboratory (ISM), Bhubaneswar, Odisha, India, for future reference.
2.2. Methods
2.2.1. Preparation of Samasharkara Churna
The Samasharkara Churna was prepared as per the standard method described in Ayurvedic Formulary of India. As per the literature, all the ingredients were shade dried and powdered separately, passed through #80 sieve, and then mixed together in required proportions to get uniformly blended churna [11].
2.3. Pharmacognostical study
2.3.1. Determination of foreign matter
Total 100 g of the sample was spread out in a thin layer. The foreign matter was detected by inspection with the unaided eye or by the use of a lens (6×), separated, weighed and the percentage foreign matter was calculated [12].
2.3.2. Organoleptic parameters
The organoleptic characters like colour, odour, taste, appearance and texture of the ingredients and formulation samples were evaluated based on the reported method [13].
2.3.3. Fluorescence analysis
Fluorescence characters of powdered materials in different standard reagent solutions towards ordinary visible light and Ultra Violet light (both long 365 nm and short 254 nm wave lengths) were observed [14].
2.3.4. Microscopic study of Samasharkara Churna
Five mg of the sieved (#80) powder samples (churna and ingredients) were taken and washed with plain water. Then the samples were treated separately with iodine, chloral hydrate, pholorglucinol or potassium iodide; a drop of glycerine was added and mounted. The powder sample characters were then observed by Carl Zeiss binocular microscope attached with camera according to standard method [15], [16].
2.4. Physicochemical investigation
Different physicochemical investigations of churna and its raw materials were carried out using standard pharmacopoeial methods, including determination of alcohol soluble extractives, water soluble extractives, total ash, acid insoluble ash, loss on drying and pH determinations [17], [18].
2.5. Determination of physical characteristics of powder
Physical characteristics like bulk density, tap density, angle of repose, Hausner's ratio and Carr's index were determined for the churna formulations [19].
2.6. Qualitative phytochemical investigation
Comparative qualitative chemical tests were carried out for Samasharkara Churna and its ingredients on their different extracts of various polarities. These phytochemical screening included tests for alkaloids, tannins, steroid, glycoside, flavonoids, saponins, carbohydrates, terpenoids and proteins [20].
2.7. Determination of toxic contaminants
2.7.1. Heavy metal determination
Heavy metal analysis was performed using PERKIN ELMER AAS-200 instrument. As per protocol, sample digestion was carried out by multi-acid digestion system for Lead (Pb), Cadmium (Cd), Copper (Cu), Zinc (Zn), Nickel (Ni) and Chromium (Cr) [21]. After completion of digestion process, the filtered samples were analysed by Atomic Absorption Spectrometer (AAS). However being volatile, Mercury (Hg) and Arsenic (As) were digested using Nitric acid-Hydrochloric Acid-Potassium Permanganate system before analysis [22]. The Mercury Vapour Atomization (MVA) and Hybrid Vapour Generation (HVG) attachments were utilised for AAS analysis of Hg and As respectively. The standards of Lead (Pb), Cadmium (Cd), Arsenic (As), Mercury (Hg), Cupper (Cu), Zinc (Zn), Nickel (Ni) and Chromium (Cr) were purchased from Merck, Germany and utilised for development of the respective calibration curves for these metals.
2.7.2. Microbial limit test
Microbial analysis was carried out as per standard procedure mentioned in Ayurvedic Pharmacopoeia of India. It included total bacterial count, total fungal count, presence of pathogens like Escherichia coli, Salmonella ebony, Pseudomonas aeruginosa, and Staphylococcus aureus [23].
2.7.3. Pesticide residues study
Pesticide residues were analysed using an Agilent 7000 Triple Quad GC/MS system equipped with a multimode inlet (MMI) using a DB-5 (30 m × 0.25 mm × 0.25 μm) capillary column. The samples for analysis were prepared by QuEChERS method [24]. As per the standard study protocol, the GC oven temperature was programmed for linear increase from 50 °C (1 min hold) to 130 °C at 10 °C/min, 130–250 °C at 5°/min and then 250–300 °C, at 10 °C/min. The injector temperature was 270 °C, with a split 40: 1 and Helium carrier gas at 1.1 mL/min flow. The mass spectrometric detector was operated in the GC MSMS mode with 70 eV ionization energy, ion source temperature at 230 °C, and the quadrupolar mass detector at 150 °C. Internal standards of pp'DDT and Chlorpyrifos were procured from sigma (Aldrich) were utilised [25]. The tested pesticides included the following:
Organochlorine pesticides: Butachlor; α-HCH; β-HCH; γ-HCH (Lindane); δ-HCH; O, P′-DDT; P, P′-DDT; O, P′-DDE; P, P′-DDE; O, P′-DDD; P, P′-DDD; α- Endosulfan; β- Endosulfan; Endosulfan sulphate; Aldrin; Dieldrin; Endrin; Endrin aldehyde; Endrin ketone; Cis- Chlordane; Trans- Chlordane; Heptachlor; Heptachlor epoxide; Methoxychlor; Dicofol; Alachlor; Chlorthalonil; Chlorobenzilate; Dichlofluanid and Vinclozolin.
Organophosphorous pesticides: Acephate; Dichlorvos; Ethion; Fenitrothion; Methamidophos; Phosalone; Profenofos; Quinalphos; Triazophos; Chlorfenvinphos; Chlorpyriphos; Chlorpyriphos-methyl; Coumaphos; Diazinon; Dimethoate; Disulfoton; Ethoprophos; Fenchlorphos; Iprobenphos; Monocrotophos; Malathion; Malaoxon; Mevinphos; Omethoate; Methyl paraoxon; Parathion-ethyl; Parathion-methyl; Prothiofos; Phorate; Phoratesulfone; Phoratesulfoxide; Phosphamidon and Triadimefon.
Pyrethroids pesticides: Cypermethrin; Deltamethrin; Fenpropathrin; Permethrin; Cyfluthrin; Cyhalothrin-λ; Fenvalerate; Esfenvalerate and Fluvalinate.
2.7.4. Aflatoxin determination
Extraction and Clean-Up: The sample preparation was carried out by Immuno affinity Column Liquid Chromatography as per the reported literature [26].
HPLC Analysis: Aflatoxins were determined by a Waters Alliance 2695 HPLC instrument using a Luna C18 column (Phenomenex) of dimensions 4.6 × 150 mm × 5 μ coupled with a Waters 2475 Fluorescence detector containing Cobra cell. In this method, 40 μl of the samples were injected into the HPLC column heated at 40 °C. The mobile phase was taken as water: methanol solution (60:40, v/v) with 119 mg of potassium bromide and 350 μl of 4 M nitric acid were added to the 1 L of mobile phase for post column electrochemical derivatization of fluorescence detector. The flow rate was kept 1 ml/min with a total runtime of 20 min where the retention times were found to be 7.5 min, 9.38 min, 11.44 min and 14.5 min for Aflatoxins G2, G1, B2 and B1 respectively. The excitation wavelength and the emission wavelength for fluorescent detection were set at 362 nm and 455 nm respectively. The calibration standards were procured from Sigma Aldrich [27].
2.8. Chromatographically analysis
2.8.1. Thin layer chromatographic (TLC) study
Sample Preparation: Accurately weighed 1 g samples of churna and its ingredients were separately dissolved in 20 ml methanol and refluxed on water bath at 90–100 °C for 15 min. They were filtered and evaporated up to 5 ml in porcelain dish and taken for TLC profiling.
Solvent System: The solvent system- Toluene: Ethyl Acetate: Glacial acetic Acid (9:1:0.5 v/v) showing best separation, selected through trial and error, was used for developing the TLC plates.
Development: Methanolic extracts were applied on 0.2 mm precoated Silica Gel 60 F254 plates (Merck KGaA) and developed in the above mentioned solvent system.
Visualization: The developed TLC plates were examined under ultraviolet light at 254 nm and 366 nm. Then the plates were derivatized with anisaldehyde-sulphuric acid reagent followed by heating at 110 °C till the development of coloured spots and visualised in daylight [28]. The colour and Rf values of the resolved spots were noted.
2.8.2. HPTLC analysis
Stationary phase: Silica Gel 60F254 pre-coated aluminium plates (MerckKGaA) of 10 × 10 cm and 0.2 mm thickness were pre-washed by methanol and activated at 60 °C for 5 min prior to chromatography.
Sample Preparation: Methanolic extracts were diluted in methanol up to concentration of 1 mg/ml and passed through 0.45 Millipore filters.
Application of sample: 2 μl of each ingredient samples of 8 mm width was applied by auto sampler system-CAMAG Linomat 5. However Samasharkara Churna sample was applied at amounts of 2 μl, 4 μl and 8 μl.
Development: HPTLC plates were developed in CAMAG glass twin-through chamber (20 × 10 cm) previously saturated with the solvent for 60 min maintained at 60 °C and 40% relative humidity (RH). The development distance was kept to be 9 cm.
Mobile Phase: Toluene: Ethyl acetate: Glacial acetic acid (9:1:0.5 v/v).
Visualization: At wavelengths of 254 nm, 366 nm and 540 nm, before and after spraying with anisaldehyde-sulphuric acid reagent.
2.9. Thermogravimetric analysis
The thermogravimetric analysis (TGA) is used to determine total weight change in the churna formulations during thermal treatments. An accurately weighed quantity of sample was heated in alumina crucibles with a heating rate of 10 °C/min from room temperature to 1200 °C in a N2 atmosphere using a thermo gravimetric analyzer TGA/STDTA851e (METTLER, Switzerland). The biochar yield was calculated by mass balance. The data was interpreted using STARe SW 12.10 software (METTLER) [18], [29].
3. Results
3.1. Foreign matter
The tested foreign matter content in the ingredients of Samasharkara Churna was less than 0.5% (w/w). Sugar did not have any foreign matter. Limits for foreign matter of these ingredients were 0.5–2% as per Ayurvedic Pharmacopoeia of India (API).
3.2. Organoleptic parameters
In organoleptic evaluation, the prepared in-house Samasharkara Churna was found to be yellowish brown in colour with characteristic odour and tasted sweet. Organoleptic inferences of the churna along with its ingredients are given in the Supplementary Table 1.
3.3. Fluorescence analysis
In our study, the fluorescence behaviour of the powdered samples as such as well as after treatment with different reagent solutions towards ordinary light and ultraviolet light (both long 365 nm and short 254 nm wave lengths) were observed and exhibited characteristic colours as reported in Table 1.
Table 1.
Fluorescence analysis of Samasharkara Churna
| Powdered drug | Visible/day light | Ultra violet light |
|
|---|---|---|---|
| 254 nm | 366 nm | ||
| Powder as such | Crimson to dark brown | Light yellow | Light yellow |
| Powder + conc. HCl | Yellow | Green | Green |
| Powder + 10% K 2Cr 2O7 | Yellow | Fluorescent green | Brown |
| Powder + 1 M NaOH | Red brown | Deep green | Fluorescent green |
| Powder + AgNO3 | Light yellow | Yellow | Yellow |
| Powder + conc. HNO3 | Orange yellow | Black | Fluorescent green |
| Powder + conc. H2SO4 | Dark brown | Greenish black | Black |
| Powder + Br2 water | Light brown | Fluorescent green | Fluorescent green |
| Powder + Methanol | Light brown | Fluorescent green | Fluorescent green |
| Powder + CH3COOH | Light brown | Yellow | Yellow |
| Powder + NH3 | Yellow | Fluorescent green | Yellow |
| Powder + I2 | Light purple | No colour | No colour |
3.4. Microscopic study of Samasharkara Churna
In the powder microscopic analysis of Samasharkara Churna (Fig. 1), the diagnostic characters such as presence of pollen grains, tracheids with spiral thickenings, simple fibre and oil glands indicated the presence of Lavanga (S. aromaticum). Simple and compound starch grains, cut fragments of scalariform tracheids and fibre, oil globule and sclereids indicated the presence of Jatiphala (M. fragrans). Starch grains, tannin content, fragment of pitted vessels and oil globule were suggestive of Pippali (P. longum). Beaker shaped stone cells, black debris along with parenchyma cells, starch grains, simple fibre, oil globules and vessels of vascular strands suggested presence of Maricha (P. nigrum). Fragmented vessel elements, oleoresin content, parenchyma cells with starch grain, fibres and oil globules observed in the sample were suggestive of Shunthi (Z. officinale).
Fig. 1.
Photographs of powder microscopic of Samasharkara Churna: (a) Pollen grains of Lavanga, (b) Tracheids with spiral thickenings of Lavanga, (c) Fibre of Lavanga, (d) Oil glands of Lavanga, (e) Oil globules of Jatiphala, (f) Cut fragments of scalariform tracheids and fibre oil globules of Jatiphala (g) Simple and compound starch grains oil globules of Jatiphala (h) Sclereids oil globules of Jatiphala, (i) Oil globules of Pippali, (j) Tannin content of Pippali, (k) Starch grains of Pippali, (l) Fragments of pitted vessels of Pippali, (m) Beaker-shaped stone cell of Maricha, (n) Simple fibre of Maricha, (o) Black debris along with parenchyma cells of Maricha, (p) Vessels of vascular strands of Maricha, (q) Starch grains of Shunthi, (r) Fragmented vessel elements of Shunthi, (s) Oil globule of Shunthi, (t) Fibre of Shunthi.
3.5. Physicochemical investigation
Physicochemical analysis of Samasharkara Churna showed water soluble extractive 16.314% w/w, ethanol soluble extractive 20.248% w/w, total ash content 2.343% w/w, acid insoluble ash 0.441% w/w, pH of 6.697 and loss on drying of 7.97% w/w at 105 °C.
3.6. Qualitative phytochemical investigation
The comparative phytochemical evaluation of Samasharkara Churna and its individual ingredients in solvents of different polarity are done and presented in Table 2. The preliminary phytochemical screening of different extracts of Samasharkara Churna revealed the presence of phytoconstituents like alkaloids, tannins, steroids, terpenoids glycoside, flavonoids, saponins, carbohydrates, and proteins.
Table 2.
Phytochemical investigation of raw materials present in Samasharkara Churna
| Material | Extracts | Phytoconstituents present |
|---|---|---|
| Lavanga | Aqueous Extract | A, T, G, F, Sa, P |
| Methanolic Extract | A, T, G, F, Sa, P | |
| Ethyl acetate Extract | T, F, Sa, C, P | |
| Chloroform Extract | T, Sa, C | |
| Pet. Ether Extract | St | |
| Jatiphala | Aqueous Extract | T, Sa, P |
| Methanolic Extract | A, T, G, F, Sa, C | |
| Ethyl acetate Extract | Sa, C, P | |
| Chloroform Extract | A, St, G, F, C | |
| Pet. Ether Extract | St | |
| Pippali | Aqueous Extract | G, Sa, C, P |
| Methanolic Extract | A, Sa | |
| Ethyl acetate Extract | G, Sa, C | |
| Chloroform Extract | A, F, Sa, C | |
| Pet. Ether Extract | St | |
| Maricha | Aqueous Extract | A, T, G, Sa, P |
| Methanolic Extract | A, G, Sa, P | |
| Ethyl acetate Extract | Te, P | |
| Chloroform Extract | St, F, Sa, C, P | |
| Pet. Ether Extract | St | |
| Shunthi | Aqueous Extract | A, T, G, Sa, P |
| Methanolic Extract | G, Sa, P | |
| Ethyl acetate Extract | G, Sa, P | |
| Chloroform Extract | C | |
| Pet. Ether Extract | – | |
| Samasharkara Churna | Aqueous Extract | A, T, G, F, Sa, C, P |
| Methanolic Extract | A, T, G, F, Sa, P | |
| Ethyl acetate Extract | St, G, F, C, P | |
| Chloroform Extract | St, G, F, Sa, C, P | |
| Pet. Ether Extract | St |
A: Alkaloids, T: Tannins, St: Steroid, G: Glycoside, F: Flavonoids, Sa: Saponins, C: Carbohydrates, P: Proteins, Te: Terpenoids.
3.7. Determination of physical characteristics of powder
The flow ability of the in-house churna formulation was found to be poor with tap density of 0.462 ± 0.017 and bulk density (0.382 ± 0.013). This was further confirmed by high values of Hausner's ratio (1.578 ± 0.003) and Carr's index (34.66 ± 1.73).
3.8. Microbial limit test
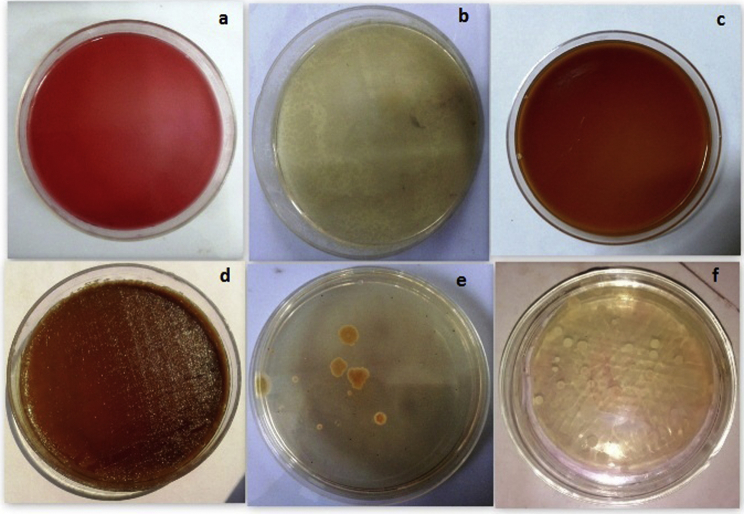
The microbial profile of the Samasharkara Churna was found to be satisfactory with total microbial plate count, Yeast and moulds counts being 40 CFU/mL (below API limit of NMT 105 CFU/mL), total yeast and mould were 11 CFU/mL (below API limit of NMT 103 CFU/mL). Moreover, the pathogenic bacteria, i.e. Salmonella, Pseudomonas, Staphylococcus and E. coli were found to be absent (Fig. 2).
Fig. 2.
Photographs of microbiological limit test in Samasharkara Churna. (a) Escherichia coli, (b) Pseudomonas aeruginosa, (c) Salmonella ebony, (d) Staphylococcus aureus, (e) Total fungal count, (f) Total bacterial count.
3.9. Heavy metal determination
In the present evaluation, the presence of heavy metals namely cadmium, arsenic, mercury, lead, copper, zinc, nickel and chromium in Samasharkara Churna was detected within the limits specified, whenever available (Table 3).
Table 3.
Heavy metal analysis of Samasharkara Churna.
| Sr. No. | Heavy metal | Standard limit∗ (ppm) | Observed Value (ppm) |
|---|---|---|---|
| 1 | Arsenic | 3 ppm | 1.35 ppm |
| 2 | Lead | 10 ppm | 3.46 ppm |
| 3 | Mercury | 1 ppm | 0.05 ppm |
| 4 | Cadmium | 0.3 ppm | 0.11 ppm |
| 5 | Nickel | NA | 4.23 ppm |
| 6 | Zinc | NA | 11.45 ppm |
| 7 | Copper | NA | 9.51 ppm |
| 8 | Chromium | NA | 8.20 ppm |
As per limits mentioned in Ayurvedic Pharmacopoeia of India (API). NA -Not available in Ayurvedic Pharmacopoeia of India.
3.10. Aflatoxins determination
The different objectionable Aflatoxins viz., B1 (MDL 0.5 ppm), B2 (MDL 0.1 ppm), G1 (MDL 0.5 ppm) and G2 (MDL 0.1 ppm) were found to be below limit of detection (MDL) mentioned in Ayurvedic Pharmacopoeia of India (API).
3.11. Pesticides residues study
In our evaluation of pesticide residues in Samasharkara Churna, various pesticide classes like- Organochlorine pesticides, Organophosphorus pesticides and Pyrethroids were not detected (with detection limit of 0.01 mg/kg).
3.12. TLC and HPTLC study
The preliminary TLC was carried out to compare the TLC profiles of the methanolic extracts of Samasharkara Churna with that of its ingredients using Toluene: Ethyl acetate: Glacial acetic acid (9:1:0.5 v/v) solvent system. The developed TLC spots were visualised in normal daylight by derivatization with anisaldehyde sulphuric acid reagent. The comparative TLC profile showed good separation of its components with five unique spots at Rf: 0.4, 0.44, 0.48, 0.58 and 0.71, indicating presence of Lavanga, Jatiphala, Pippali, Maricha and Shunthi respectively. The results of the TLC study are shown in Table 4.
Table 4.
TLC screening of raw materials vs. Samasharkara Churna
| Rf Values | |||||
|---|---|---|---|---|---|
| Track A (Lavanga) | Track B (Jatiphala) | Track C (Pippali) | Track D (Maricha) | Track E (Shunthi) | Track S (Samasharkara Churna) |
| 0.28 | 0.44 | 0.36 | 0.4 | 0.41 | 0.4 |
| 0.31 | 0.46 | 0.44 | 0.43 | 0.45 | 0.44 |
| 0.38 | 0.75 | 0.48 | 0.5 | 0.48 | 0.48 |
| 0.4 | – | – | 0.58 | 0.7 | 0.58 |
| 0.46 | – | – | 0.62 | 0.76 | 0.71 |
| 0.6 | – | – | 0.67 | – | 0.75 |
| 0.71 | – | – | – | – | – |
| 0.8 | – | – | – | – | – |
| 0.82 | – | – | – | – | – |
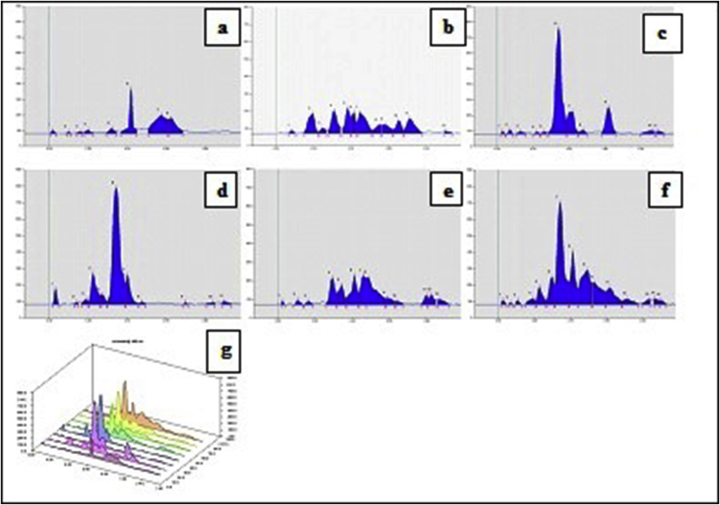
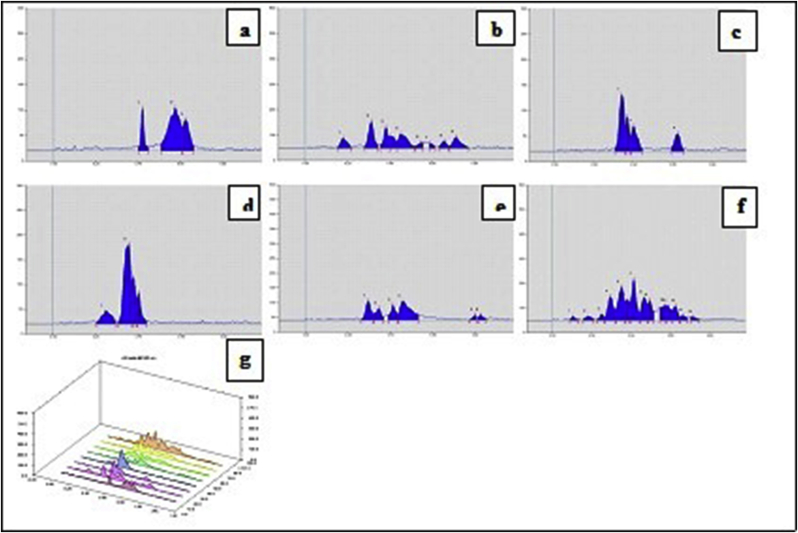
HPTLC chromatogram of the methanolic extract of Samasharkara Churna with Toluene: Ethyl acetate: Glacial acetic acid (9:1:0.5 v/v) showed unique spots of each and every ingredient in formulation on the basis of comparative Rf values. Plate images, 3D chromatograms at scanned wavelengths (i.e. 254 nm 366 nm and 540 nm) before and after derivatization with anisaldehyde sulphuric acid reagent are shown in Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8. Comparative Rf values of ingredients and Samasharkara Churna are also provided in Supplementary Tables 2–Supplementary Table 6.
Fig. 3.
HPTLC Finger prints of Samasharkara Churna and its ingredients at 254 nm before derivatization. (a) Lavanga, (b) Jatiphala, (c) Pippali, (d) Maricha, (e) Shunthi, (f) Samasharkara Churna, (g) 3D Chromatogram.
Fig. 4.
HPTLC Finger prints of Samasharkara Churna and its ingredients at 254 nm after derivatization. (a) Lavanga, (b) Jatiphala, (c) Pippali, (d) Maricha, (e) Shunthi, (f) Samasharkara Churna, (g) 3D Chromatogram.
Fig. 5.
HPTLC Finger prints of Samasharkara Churna and its ingredients at 366 nm before derivatization. (a) Lavanga, (b) Jatiphala, (c) Pippali, (d) Maricha, (e) Shunthi, (f) Samasharkara Churna, (g) 3D Chromatogram.
Fig. 6.
HPTLC Finger prints of Samasharkara Churna and its ingredients at 366 nm after derivatization. (a) Lavanga, (b) Jatiphala, (c) Pippali, (d) Maricha, (e) Shunthi, (f) Samasharkara Churna, (g) 3D Chromatogram.
Fig. 7.
HPTLC Finger prints of Samasharkara Churna and its ingredients at 540 nm after derivatization. (a) Lavanga, (b) Jatiphala, (c) Pippali, (d) Maricha, (e) Shunthi, (f) Samasharkara Churna, (g) 3D Chromatogram.
Fig. 8.
(a). Photographs of HPTLC plates of Samasharkara Churna and its ingredients at 254 nm before derivatization. (A) Lavanga, (B) Jatiphala, (C) Pippali, (D) Maricha, (E) Shunthi, (S1) Samasharkara Churna (2 μl), (S2) Samasharkara Churna (4 μl), (S3) Samasharkara Churna (8 μl). (b). Photographs of HPTLC plates of Samasharkara Churna and its ingredients at 366 nm before derivatization. (A) Lavanga, (B) Jatiphala, (C) Pippali, (D) Maricha, (E) Shunthi, (S1) Samasharkara Churna (2 μl), (S2) Samasharkara Churna (4 μl), (S3) Samasharkara Churna (8 μl).
3.13. Thermogravimetric analysis
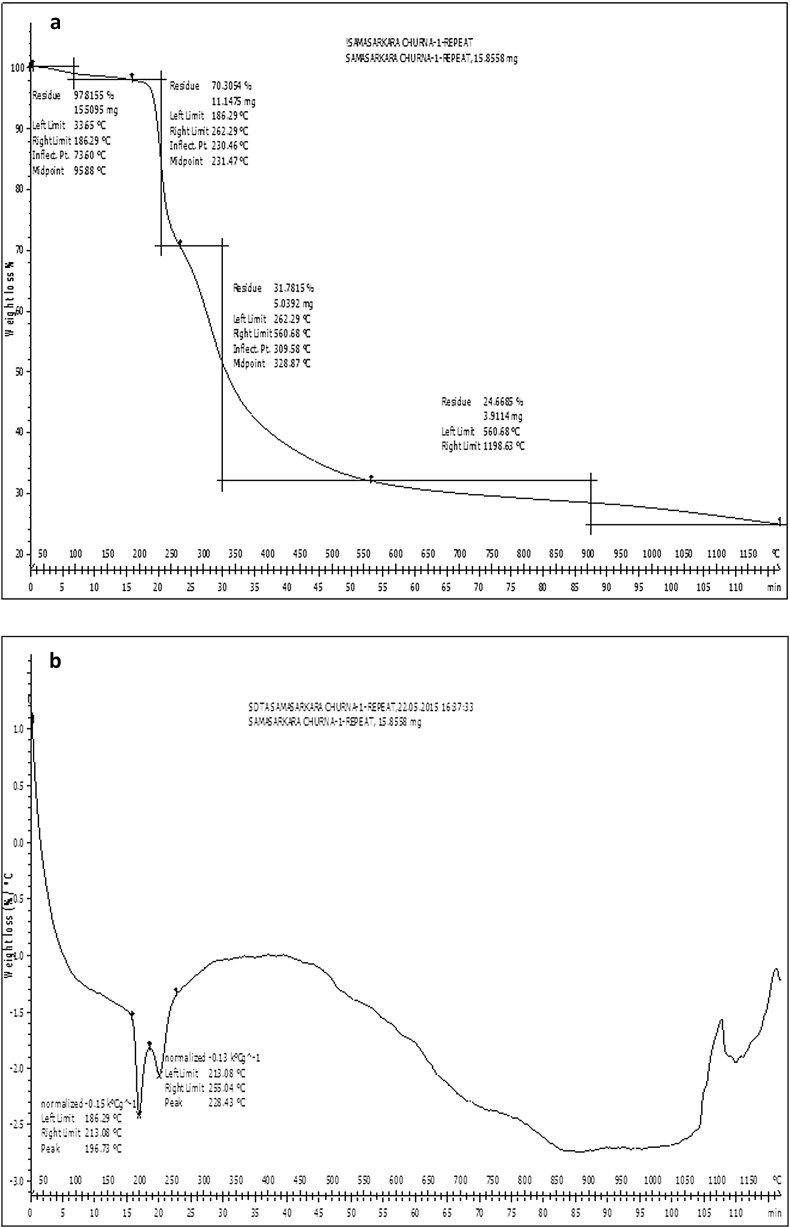
The pyrolytic characteristics of the Samasharkara Churna were examined by the TGA technique and its thermogravimetry (TG) and derivative thermogravimetric (DTG) curves are shown in Fig. 9a and Fig. 9b. TG curve showed a plot of Temperature Vs Weight of the sample and is displayed as Z shaped curve from left to right. Again, the DTG curve (Fig. 9b) shown by the rate of mass loss Vs temperature confirmed presence of two endothermic peaks at 196 °C and 228°. However, no further peaks were detected within the entire DTG curve.
Fig. 9.
(a). Thermogravimetric (TG) curve of the Samasharkara Churna. (b). Differential thermogravimetric (DTG) curve of the Samasharkara Churna.
4. Discussion
The work dealt with detailed evaluation of Samasharkara Churna following the guidelines prescribed by Ayurvedic Pharmacopoeia of India (API). Standardization guidelines provided by World Health Organization (WHO), European Agency for Evaluation of Medicinal Products (EMEA) and United States Pharmacopoeias (USP) have also been considered. As part of the procedure, the churna was tested for relevant physical, chemical and analytical parameters for its safety and consistent efficacy through quality control measures [30], [31], [32].
Herbal drugs should be free from foreign matters such as other parts of the same plant or other plants, moulds or insects, including excreta and visible contaminant such as sand and stones, poisonous and harmful foreign matter and chemical residues. In our study, the tested foreign matter content in the ingredients of Samasharkara Churna was found to be below the limits for foreign matter of these ingredients as per API.
Organoleptic properties are the aspects of food or other substances as experienced by the senses, including taste, sight, smell, and touch. Deviation in these properties gives a primary indication about quality variation. Again, fluorescence is the phenomenon exhibited by various chemicals, which do visibly fluoresce in light. Some plant constituents show fluorescence in the visible range of daylight. Moreover, the ultraviolet light produces fluorescence in many more natural products or they may often be converted into fluorescent derivatives by applying different reagents. Many crude drugs are assessed qualitatively in this way and it is an important parameter of pharmacognostical evaluation. Hence the characteristic organoleptic properties and fluorescence behaviour of Samasharkara Churna are reported in our work.
Powder microscopy is used to study the specific microscopic characters of medicinal plants using different staining reagent. These studies provide suitable diagnostic tool for the standardization as well as identification of adulterants. This method is also very useful in conforming presence of ingredients of a polyherbal powder. In our powder microscopic analysis, the microscopic features present in Samasharkara Churna confirmed the presence of all of its herbal ingredients (Fig. 1).
Established preliminary and physicochemical standards give important information for further investigations and facilitate the identification of formulations in routine industrial production. The test for percentage of moisture content (loss on drying) determines both water and volatile matter. Total ash measures the amount of materials remaining after ignition. Acid insoluble ash measures the amount of silica present especially, sand and siliceous matter. Extractive values are useful for evaluation consistency of nature and amount of chemical constituents present in drug.
Considering the importance of these physicochemical parameters, Samasharkara Churna was characterised by evaluating water soluble extractive, ethanol soluble extractive, total ash content, acid insoluble ash, pH and loss on drying at 105 °C.
Phytochemicals are chemical compounds that occur naturally in plants. Factors such as geographical location, harvest time, part used and method of isolation affect chemical composition of the crude material separated from the plant. In this relation, the comparative phytochemical evaluation of Samasharkara Churna and its individual ingredients in solvents of different polarity are carried out. Phytoconstituents like alkaloids, flavonoids, terpenoids etc. have been previously found to be useful in management of asthma [33], [34]. Presence of the said constituents in Samasharkara Churna, as reported by its phytochemical evaluation, may be responsible for its usefulness in the treatment of Asthma (Shwasa Roga).
Powder flow is a key requirement for pharmaceutical manufacturing process. Understanding of powder flow is crucial during mixing, packaging, and transportation. There are various compendia methods available to measure the powder flow such as: measurement of angle of repose, bulk density, tapped density, Carr's compressibility index and Hausner's ratio. The bulk density of a powder is the ratio of the mass of an untapped powder sample and its volume including the contribution of the inter-particulate void volume. Hence, the bulk density depends on both the density of powder particles and the spatial arrangement of particles in the powder bed. The tapped density is an increased bulk density attained after mechanically tapping a container containing the powder sample. Particle size influences flowability. Fine particles with smaller bulk/tapped density are less free-flowing, whereas larger denser particles tend to be free flowing. Again, Hausner ratio of <1.25 indicates a powder that is free flowing whereas >1.25 indicates poor flow ability. Again, smaller the Carr's Index the better the flow properties. For example 5–15 indicates excellent, 12–16 good, 18–21 fair and >23 poor flow. In our work, the low values of tap density and bulk density as well as high Hausner's ratio and Carr's index indicated poor flow ability of Samasharkara Churna.
Because of their origin, herbal drugs are subjected to contamination by microorganisms from soil, air and water which may present potentially pathogenic microorganisms to man. The presence of microbial contaminants in herbal products can reduce or even inactivate the therapeutic activity of the products and has the potential to adversely affect patients taking these medicines. Thus, manufacturers should ensure the lowest possible level of microorganisms in the raw material, finished dosage forms and the packaging components to maintain appropriate quality, safety and efficacy of the natural products [23], [35]. The microbial load for Samasharkara Churna was found to be within the limit of API limits with specified pathogens i.e. Salmonella, Pseudomonas, Staphylococcus and E. coli were found to be absent. This indicated its microbiological safety in human use.
The contaminations of heavy toxic metals in plants could develop serious health problems because there is a narrow concentration range between the deficiency and toxicity levels of the heavy metals in humans [36]. The WHO has emphasized on various standard techniques for the analysis of toxic heavy metals in plant products to ascertain their safety [37]. Aflatoxins, the secondary metabolites produced by the Aspergillus species (namely A. flavus, A. parasiticus) contaminate a variety of agricultural and food commodities. Aflatoxins are classified into a number of subtypes. However, the most important ones are B1, B2, G1 and G2. These mycotoxins are recognized to be hepatotoxins and carcinogens for humans. WHO also urges the levels of Aflatoxins to be reduced to as low as reasonably achievable [38]. Pesticides are often used in order to improve productivity and profit margins in the production of medicinal plants. Contamination of medicinal plants with these pesticide residues poses a significant health risk. WHO (2009) estimated that globally, every year, 3 million people suffer health effects from exposure to pesticides and a minimum of 300,000 people die, of which, 99% belonging to low and middle-income countries [39]. Hence it is important to develop effective method for the detection of these compounds. As per the AOAC (Association of Official Analytical Chemists) guidelines, the presence of residues of pesticides of major three pesticide classes i.e. organochlorines, organophosphates and pyrethroids were tested in Samasharkara Churna by GC/MS technique [40].
The results of evaluation of residues of these objectionable toxicants in i.e., heavy metals, aflatoxins and pesticide residues in Samasharkara Churna revealed that all these toxic contaminants are within the API acceptable range.
TLC and HPTLC are important tools by which the quality control and fingerprint of herbs can be maintained. TLC/HPTLC has excellent resolution and, therefore, permits simultaneous identification of a wide range of substances in a single run. They also help to identify the individual herbs in herbal formulations. The main objective of the TLC/HPTLC study of Samasharkara Churna was to develop unique TLC spots in the formulation as identifier of its every ingredient. By trial with various solvent combinations, it was possible to establish a common TLC/HPTLC solvent system of Toluene: Ethyl acetate: Glacial acetic acid (9:1:0.5 v/v) which established the presence of every ingredient of Samasharkara Churna in its methanolic extract.
TGA is a rapid, reliable and reproducible technique, which is used for material characterization in pharmaceutical applications. Loss in weight over specific temperature provides information of composition of the sample and thermal stability. Thermo characterizations of medicinal plant materials provide information about the contents of volatile and heat liable compounds present in them. This could give major support to the analysis of plant materials during standardization. TG curve of Samasharkara Churna (Fig. 9a) obtained can be categorized into four stages. The first decomposition stage is present up to approximately 186 °C with negligible mass loss of 2.2% indicating low hygroscopic nature of the product. The weight loss in this stage might be attributed to loss of volatile oils mainly the monoterpenes. However, the principal weight loss mainly occurred in the temperature range of 196°C–560 °C, covering second and third decomposition stages, which is attributed to the decomposition of plant materials as well as the evolution of turbostratic crystallites during pyrolysis [41]. The second stage represented the degradation of micro and macro compound degradation in the range of 186–262 °C corresponding to mass losses of 27.5%. The third mass loss stage between 262 °C and 560 °C correlated the non-degradable residues mainly mineral residues with mass losses of 38.5%. In the final (fourth) degradation stage of 560–1200 °C, there was weight stabilization of the biochar with mass losses of only 7.1% due to decomposition of more heat-resistant components like lignin. The total mass losses during four stages were about 75.3%. The evaluation of the thermal instability of Samasharkara Churna by its DTG curve (Fig. 9b) has confirmed two endothermic peaks at 196 °C and 228 °C within the second decomposition stage of 186–262 °C of TG curve. These two peaks indicate the rate of mass loss due to decomposition in Samasharkara Churna is maximum at these two temperatures. However, no further peaks were detected within the entire DTG curve.
5. Conclusion
Samasharkara Churna was characterized on the basis of the pharmacognostic, physicochemical, pharmaceutical, microbiological, toxicological and chromatographic parameters. The analytical specifications were established for the product with respect to quality based raw materials. The chromatographic data showed presence of all ingredients in the churna formulation Samasharkara with their unique Rf value. This study may serve as standard reference and the standard operating procedures to be adopted for quality control analysis of various churna formulations.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jaim.2015.11.004.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Organoleptic properties of raw materials and Samasharkara Churna.
HPTLC screening of raw materials vs. Samasharkara Churna at 254 nm before derivatization.
HPTLC screening of raw materials vs. Samasharkara Churna at 254 nm after derivatization.
HPTLC screening of raw materials vs. Samasharkara Churna at 366 nm before derivatization.
HPTLC screening of raw materials vs. Samasharkara Churna at 366 nm after derivatization.
HPTLC screening of raw materials vs. Samasharkara Churna at 540 nm after derivatization.
References
- 1.World Health Organization (WHO) World Health Organization Publications; Geneva: Switzerland: 2011. The World medicines situation, traditional medicines: global situation, issues and challenges. [Google Scholar]
- 2.Vaidya A.D.B., Devasagayam T.P.A. Current status of herbal drugs in India: an overview. J Clin Biochem. 2007;41(1):1–11. doi: 10.3164/jcbn.2007001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhope S.G., Nagore D.H., Kuber V.V., Gupta P.K., Patil M.J. Design and development of a stable polyherbal formulation based on the results of compatibility studies. Pharmacogn Res. 2011;3(2):122–129. doi: 10.4103/0974-8490.81960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukherjee P.K., Houghton P.J. Pharmaceutical Press, Royal Pharmaceutical Society of Great Britain; UK: 2009. Evaluation of herbal medicinal products - perspectives of quality, safety and efficacy; pp. 3–12. [Google Scholar]
- 5.Sahoo N., Manchikanti P., Dey S. Herbal drugs: standards and regulation. Fitoterapia. 2010;6(81):462–471. doi: 10.1016/j.fitote.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Acharya V.T. Chaukhamba Surbharti Prakashan; Varanasi: 2011. Commentary: Ayurveda Deepika of Chakrapani on CharakSamhita of Charaka, Sharirasthana; p. 535. [Google Scholar]
- 7.Arora P., Ansari S.H. Quality standard parameters of an anti-asthmatic ayurvedic formulation “Kanakasava”. Int J Pharmacogn Phytochem Res. 2014-15;6(4):983–987. [Google Scholar]
- 8.American Lung Association . American Lung Association, Epidemiology & Statistics Unit. Research and Program Services; USA: 2007. Trends in Asthma morbidity and mortality. [Google Scholar]
- 9.Defrances C.J., Cullen K.A., Kozak L.J. National Hospital Discharge Survey: 2005. Annual summary with detailed diagnosis and procedure data. Vital Health Stat. 2007;12:165. National Centre for Health Statistics. [PubMed] [Google Scholar]
- 10.Anonymous . 1st ed. Ministry of Health and Family Welfare. Department of AYUSH, Government of India; New Delhi: 2011. Ayurvedic formulary of India; p. 169. Part 3. [Google Scholar]
- 11.Anonymous . 1st ed. vol. 2. Ministry of Health and Family Welfare. Department of AYUSH, Government of India; New Delhi: 2008. (Ayurvedic Pharmacopoeia of India). Part 2. [Google Scholar]
- 12.Zhang J., Wider B., Shang H., Li X., Ernst E. Quality of herbal medicines: challenges and solutions. Complement Ther Med. 2012;20(1–2):100–106. doi: 10.1016/j.ctim.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Patra K.C., Kumar K.J., Suresh P. Standardization of a polyherbal Siddha formulation, Amukkara Choornam. Indian J Tradit Knowl. 2009;8(3):449–452. [Google Scholar]
- 14.Mulla S.K., Swamy P. Preliminary pharmacognostical and phytochemical evaluation of Portulaca quadrifida Linn. Scopus. 2010;2(3):1699–1702. [Google Scholar]
- 15.Evans W.C. 15th ed. Baillere Tindall; London: 1983. Trees and Evans Pharmacognosy; pp. 538–547. [Google Scholar]
- 16.Singh S., Machawal L., Chauhan M.G. Pharmacognostic study of male leaves of Trichosanthes dioica Roxb. With special emphasis on microscopic technique. J Pharmacogn Phytother. 2010;2(5):71–75. [Google Scholar]
- 17.Lohar D.R. Pharmacopoeial Laboratory for Indian Medicine, AYUSH. Ministry of Health and Family Welfare. Government of India; Ghaziabad: 2011. Protocol for testing of Ayurvedic, Siddha and Unani medicines; p. 20. [Google Scholar]
- 18.Satheesh N.V.M., Upadhyaya K., Bisht A. Phytochemical screening and standardization of polyherbal formulation for dyslipidemia. Int J Pharm Pharm Sci. 2011;3(3):235–238. [Google Scholar]
- 19.Sharma R., Amin H., Galib, Prajapati P.K. Validation of standard manufacturing procedure of Guduchi satva (aqueous extract of Tinospora cordifolia (Willd.) Miers) and its tablets. Anc Sci Life. 2013;33(1):27–34. doi: 10.4103/0257-7941.134564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallis T.E. J & A Churchill Ltd; Gloucester Place. London: 1953. Practical pharmacognosy; pp. 57–78. [Google Scholar]
- 21.Rout K.K., Parida S., Mishra S.K. Standardization of the ayurvedic formulation Haridra Khanda using high-performance thin-layer chromatography-densitometry. J AOAC Int. 2008;5(7):1162–1168. [PubMed] [Google Scholar]
- 22.Maranhao T.A., Silva J.S.A., Andrade R.M., Bascunan V.L.A.F., Oliveira F.J.S., Curtius A.J. Determination of As and Hg in acetic acid extract by vapor generation coupled to atomic spectrometry for solid waste classification. Microchem J. 2013;106:139–146. [Google Scholar]
- 23.Rajput S., Tripathi M.K., Tiwari A.K., Dwivedi N., Tripathi S.P. Scientific evaluation of Panchkola Churna – an Ayurvedic polyherbal drug formulation. Indian J Tradit Knowl. 2012;11(4):697–703. [Google Scholar]
- 24.Rai V., Kakkar P., Misra C., Ojha S.K., Srivastava N., Mehrotra S. Metals and organochlorine pesticide residues in some herbal ayurvedic formulations. Bull Environ Contam Toxicol. 2007;79(3):269–272. doi: 10.1007/s00128-007-9063-4. [DOI] [PubMed] [Google Scholar]
- 25.Baragi C.U., Baragi C.P., Vyas K.M., Shukla V.J. Standardization and quality control parameters of Dashanga Kwatha Ghana tablet: an ayurvedic formulation. Int J Ayurveda Res. 2011;2(1):42–47. doi: 10.4103/0974-7788.83190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stroka J., Anklam E., Jorissen U., Gilbert J. Immuno-affinity column clean-up with liquid chromatography post column bromination for determination of Aflatoxins in peanut butter, pistachio paste, fig paste and paprika powder: collaborative study. J Assoc Anal Commun Interact. 2000;83(2):320–340. [PubMed] [Google Scholar]
- 27.Siddique N.A., Mujeeba M., Ahmada S., Pandab B.P., Makhmoorb M. Determination of aflatoxins in medicinal plants by high-performance liquid chromatography–tandem mass spectrometry. J Pharm Sci. 2013;16(2):321–330. doi: 10.18433/j3mk6p. [DOI] [PubMed] [Google Scholar]
- 28.Rasheed N., Gupta V.C. Standardization of a compound Unani herbal formulation “Qurs-e-Luk” with modern techniques. Phcog Res. 2010;2:237–241. doi: 10.4103/0974-8490.69115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wesolowski M., Konieczyriski P. Relation between thermal decomposition of medicinal herbs and their non-metals concentration. J Therm Anal Calorim. 1998;54:219–226. [Google Scholar]
- 30.Anonymous . 1999. European community; European agency for the evaluation of medicinal products; pp. 56–60. EMEA/HMPWG25/99. [Google Scholar]
- 31.World Health Organization (WHO) World Health Organization Publications; Geneva: Switzerland: 1998. Quality control methods for medicinal plant materials. 8-9; 22-25: 61–63. [Google Scholar]
- 32.Mukherjee P.K. Quality control of herbal drugs. Business Horizons Pharmaceutical Publishers; New Delhi: 2002. United States Pharmacopoeia (USP) pp. 192–193. [Google Scholar]
- 33.Park H.S., Kim S.R., Kim J.O., Lee Y.C. The roles of phytochemicals in bronchial asthma. Molecules. 2010;15:6810–6834. doi: 10.3390/molecules15106810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W., Wang Y., He D.D., Li S.P., Zhu Y.D., Jiang B. Antitussive, expectorant, and bronchodilating effects of quinazoline alkaloids (±)-vasicine, deoxyvasicine, and (±)-vasicinone from aerial parts of Peganum harmala L. Phytomedicine. 2015;22(12):1088–1095. doi: 10.1016/j.phymed.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Kalaiselvan V., Shah A.K.K., Patel F.B., Shah C.N.B., Kalaivani M., Rajasekaran A. Quality assessment of different marketed brands of Dasamoolaristam, an Ayurvedic formulation. Int J Ayurveda Res. 2010;1(1):10–13. doi: 10.4103/0974-7788.59937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.En Z., Vasidov A., Tsipin V. Study of element uptake in plants from the soil to assess environmental contamination by toxic elements. Nucl Instr Meth Phys Res Sec A. 2003;505(1–2):462–465. [Google Scholar]
- 37.Ajasa O.M., Bello M.O., Ibrahim A.O. Heavy trace metals and macronutrients status in herbal plants of Nigeria. Food Chem. 2004;85:67–71. [Google Scholar]
- 38.Siruguri V., Kumar P.U., Raghu P. Aflatoxin contamination in stored rice variety PAU 201 collected from Punjab, India. Indian J Med Res. 2012;136(1):89–97. [PMC free article] [PubMed] [Google Scholar]
- 39.Eyer P. The role of oximes in the management of organophosphorous pesticide poisoning. Toxicol Rev. 2003;22:165–190. doi: 10.2165/00139709-200322030-00004. [DOI] [PubMed] [Google Scholar]
- 40.Ahmad A., Husain A., Mujeeb M., Khan S.A., Alhadrami H.A.A., Bhandari A. Quantification of total phenol, flavonoid content and pharmacognostical evaluation including HPTLC fingerprinting for the standardization of Piper nigrum Linn fruits. Asian Pac J Trop Biomed. 2015;5(2):101–107. [Google Scholar]
- 41.Keiluweit M., Nico P.S., Johnson M.G., Kleber M. Dynamic molecular structure of plant biomass-derived black carbon (biochar) Environ Sci Tech. 2010;44(4):1247–1253. doi: 10.1021/es9031419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Organoleptic properties of raw materials and Samasharkara Churna.
HPTLC screening of raw materials vs. Samasharkara Churna at 254 nm before derivatization.
HPTLC screening of raw materials vs. Samasharkara Churna at 254 nm after derivatization.
HPTLC screening of raw materials vs. Samasharkara Churna at 366 nm before derivatization.
HPTLC screening of raw materials vs. Samasharkara Churna at 366 nm after derivatization.
HPTLC screening of raw materials vs. Samasharkara Churna at 540 nm after derivatization.