The biochemical and radiological responses to radionuclide therapy with 177Lu-PSMA-617 targeting prostate-specific membrane antigen (PSMA) make it a promising approach to the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC) [1]. However, PSMA-617 has been reported to have slower tumour accumulation and clearance kinetics than PSMA-11, and the latter is still therefore the preferred diagnostic agent when labelled with generator-produced 68Ga which has a short half-life (68 min) [2]. A PSMA-targeting 18F-labelled PET tracer could be produced with higher activity in a cyclotron and the half-life (110 min) would allow both late imaging beyond 1 h after injection and shipping to satellite institutions. However, the structure of the currently most-used 18F-labelled PSMA tracer, 18F-DCFPyl, is different from that of PSMA-617, and like PSMA-11 it might be a suboptimal surrogate for stratifying patients according to their suitability for therapy with 177Lu-PSMA-617 [3].
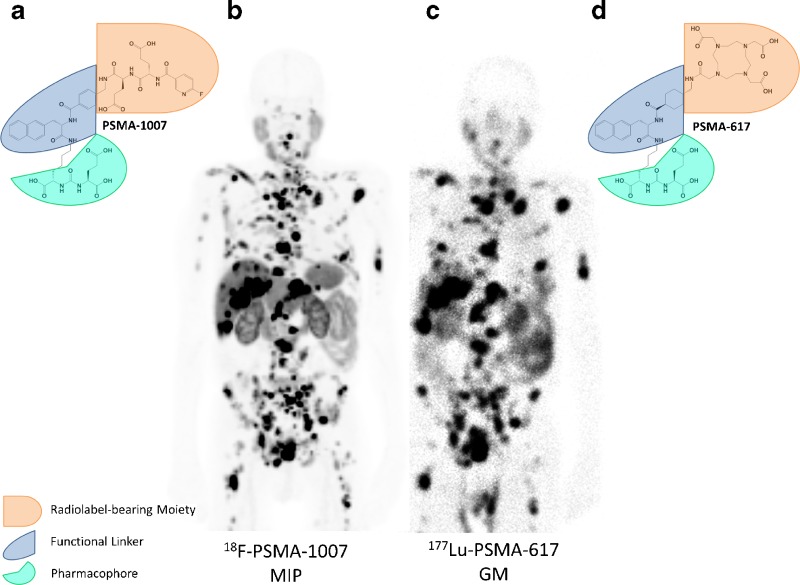
Based on the scaffold of PSMA-617, the novel compound 18F-PSMA-1007 was developed. As shown in the image (a, d), PSMA-1007 shares the Glu-urea-Lys motif targeting the catalytic domain of PSMA and the naphthalene-based linker region considered to cotarget the hydrophobic accessory pocket [4], while in the radiolabel-bearing moiety glutamic acids were added to mimic the carboxylic acid groups of the DOTA chelator to retain the polar charge influencing clearance kinetics.
The image also shows a patient with mCRPC who was staged using 18F-PSMA-1007 (b PET 1 h after injection, maximum intensity projection) and treatment with 177Lu-PSMA-617 (c planar scan 24 h after injection, geometric mean). In analogy to the chemical structure, the uptake in tumour and normal organs is very similar with the two compounds.
Thus, 18F-PSMA-1007 and 177Lu-PSMA-617 seem to be a perfect theragnostic tandem. Due to the preferred physical characteristics of 18F for PET imaging and the possibility for large-scale production in a cyclotron, 18F-PSMA-1007 is also a promising alternative to 68Ga-PSMA-11 for diagnostic purposes. However, non-inferior diagnostic accuracy has still to be proven in a larger cohort.
Compliance with ethical standards
Ethical approval
As this is a retrospective case report of a patient in regular clinical care but not a clinical trial, ethical approval was not needed.
Informed consent
Written informed consent for imaging with an experimental tracer and publication of the individual patient history was obtained.
Footnotes
18F-PSMA-1007 is the subject of a patent application (EP 15 002 800.9, DKFZ)
References
- 1.Kratochwil C, Giesel FL, Stefanova M, Benešová M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with Lu-177 labeled PSMA-617. J Nucl Med. 2016 doi: 10.2967/jnumed.115.171397. [DOI] [PubMed] [Google Scholar]
- 2.Afshar-Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, Neels OC, et al. The theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med. 2015;56(11):1697–1705. doi: 10.2967/jnumed.115.161299. [DOI] [PubMed] [Google Scholar]
- 3.Szabo Z, Mena E, Rowe SP, Plyku D, Nidal R, Eisenberger MA, et al. Initial evaluation of [(18)F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17(4):565–574. doi: 10.1007/s11307-015-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barinka C, Byun Y, Dusich CL, Banerjee SR, Chen Y, Castanares M, et al. Interactions between human glutamate carboxypeptidase II and urea-based inhibitors: structural characterization. J Med Chem. 2008;51:7737–7743. doi: 10.1021/jm800765e. [DOI] [PMC free article] [PubMed] [Google Scholar]