In this paper we examine vestibular and visual processing at the center of human vestibular cortex in the Sylvian fissure. We find that two areas, referred to as parieto-insular vestibular cortex (PIVC) and posterior insular cortex (PIC), are located in the Sylvian fissure and exhibit different functional specializations. Our results suggest a more complex organization at the putative core of human vestibular cortex than previously assumed.
Keywords: area PIC, area PIVC, caloric vestibular stimulation, Sylvian fissure, vestibular cortex
Abstract
Unlike other sensory systems, the cortical organization of the human vestibular system is not well established. A central role is assumed for the region of the posterior Sylvian fissure, close to the posterior insula. At this site, activation during vestibular stimulation has been observed in previous imaging studies and labeled as the parieto-insular vestibular cortex area (PIVC). However, vestibular responses are found in other parts of the Sylvian fissure as well, including a region that is referred to as the posterior insular cortex (PIC). The anatomical and functional relationship between PIC and PIVC is still poorly understood, because both areas have never been compared in the same participants. Therefore, to better understand the apparently more complex organization of vestibular cortex in the Sylvian fissure, we employed caloric and visual object motion stimuli during functional magnetic resonance imaging and compared location and function of PIVC and PIC in the same participants. Both regions responded to caloric vestibular stimulation, but only the activation pattern in right PIVC reliably represented the direction of the caloric stimulus. Conversely, activity in PIVC was suppressed during stimulation with visual object motion, whereas PIC showed activation. Area PIC is located at a more posterior site in the Sylvian fissure than PIVC. Our results suggest that PIVC and PIC should be considered separate areas in the vestibular Sylvian network, both in terms of location and function.
NEW & NOTEWORTHY
In this paper we examine vestibular and visual processing at the center of human vestibular cortex in the Sylvian fissure. We find that two areas, referred to as parieto-insular vestibular cortex (PIVC) and posterior insular cortex (PIC), are located in the Sylvian fissure and exhibit different functional specializations. Our results suggest a more complex organization at the putative core of human vestibular cortex than previously assumed.
a central function of the vestibular sensory system is to signal our position and acceleration in space. This information is of critical relevance for guiding locomotion and for processing self-motion signals (for a recent review, see Greenlee et al. 2016). Despite the obvious importance of vestibular signals, the cortical organization of the vestibular system is not yet well established. Unlike other sensory systems, the number of vestibular cortical areas and the information they represent are still under debate (Dieterich and Brandt 2015; Guldin and Grüsser 1998; Kahane et al. 2003; Lopez and Blanke 2011; Lopez et al. 2012).
There is accumulating evidence that the potential hub of the cortical vestibular network is located in a region including the posterior part of the Sylvian fissure (also called lateral sulcus), perisylvian cortex, and the posterior insula. At this site, functional imaging studies in humans found activation during vestibular stimulation (Bense et al. 2001; Dieterich et al. 2003; Eickhoff et al. 2006; Fasold et al. 2002; Lobel et al. 1998) and labeled it the parieto-insular vestibular cortex (PIVC), following terminology in the primate brain (Akbarian et al. 1988; Chen et al. 2010; Grüsser et al. 1990; Shinder and Newlands 2014).
However, the vestibular network in the posterior Sylvian fissure is likely more complex and might include at least one additional area, named posterior insular cortex (PIC), that responds to vestibular stimuli as well as to visual object motion and potentially serves as a site of multisensory integration (Frank et al. 2014).
The anatomical and functional relationship between PIC and PIVC is still unclear, because both areas have not yet been investigated in the same participant. Therefore, in this study we aimed to define and compare the regions known as PIVC and PIC directly in the same participants. With this aim, we employed caloric vestibular and visual object motion stimuli during functional magnetic resonance imaging (fMRI) and examined functional responses of PIC and PIVC, using univariate and multivariate analysis.
METHODS
Participants
Fifteen participants (7 females, 20–45 yr old) were recruited for the study. All participants reported to be right-handed and gave informed written consent. The study was approved by the local ethics committee at the University of Regensburg.
Vestibular Localizer
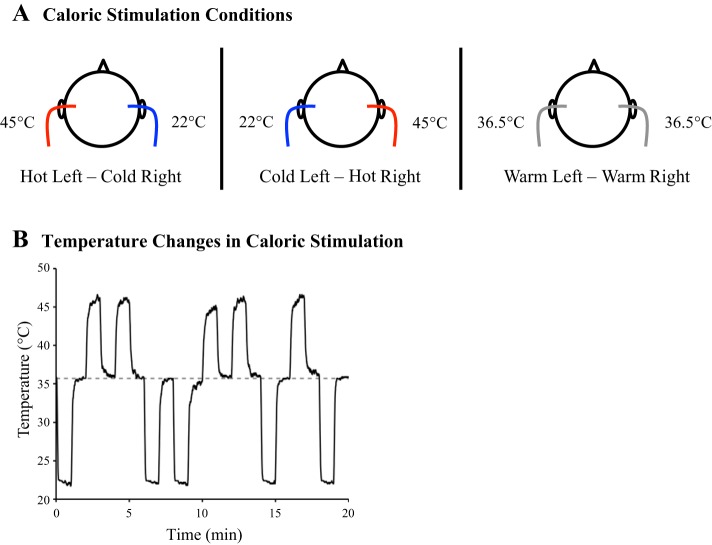
Area PIVC is primarily characterized by strong responsiveness to vestibular cues. Therefore, for the definition of area PIVC, a vestibular localizer involving bithermic caloric stimulation was performed. Caloric stimulation was carried out with a custom-made, MRI-compatible caloric stimulation system. Details of this system have been described elsewhere (see Frank and Greenlee 2014). In brief, two barrels of distilled hot (48°C) and cold water (20°C) in the scanner control room were connected to a switching panel, which controlled the flow of water to the left and right ear canals of the participant in the scanner. The switching panel was operated manually by the experimenter. By opening or closing the flow of water to each ear canal, three different temperature states could be realized: hot in the left ear and simultaneously cold in the right ear, cold in the left ear and simultaneously hot in the right ear, and a mixture of both temperatures in each ear (warm, near body temperature) (Fig. 1A). It is important to note that our system only applied the temperature of the water to the surface of the ear canal (via small glass pods installed in the MRI ear protection), while the water circulated back through return tubes to a collecting barrel in the scanner control room. Temperatures at the stimulation pods in the ear canal were 45°C (hot), 22°C (cold), and 36.5°C (warm) and required a ramp of 10 s to reach steady state levels, as measured in a volunteer participant in the scanner room (Fig. 1B). Temperatures for hot and cold were chosen such that their mixture would yield a baseline matching regular body temperature in the outer ear. As such this condition served as a vestibular-stimulation baseline. Body temperature in the outer ear was measured in five volunteer participants. In each participant temperature was 36.5°C (see Fig. 1B, dashed gray line) and stable over the course of 1 min of recordings. In previous reports we have shown that caloric stimulation with this device may elicit sensations of self motion (Frank et al. 2014) and caloric nystagmus (Frank and Greenlee 2014) in the participant.
Fig. 1.
Design of caloric vestibular stimulation. A: caloric stimulation conditions (circle = schematic participant head in bird's eye view). Bithermic caloric stimulation was performed with hot (∼45°C) in the left ear and cold (∼22°C) in the right ear (left) or cold in the left ear and hot in the right ear (middle), in different trials. Each trial with caloric stimulation was followed by a baseline trial with warm (∼36.5°C) in both ears (right). B: temperature changes in the ear canal during caloric stimulation, as measured in one ear of a sample participant outside the MRI-scanner. One run of caloric stimulation was performed (20 min), with 60-s long blocks of caloric stimulation (hot in one ear and cold in the other) alternating with 60-s long baseline blocks (warm in both ears). There were 5 trials with hot left and cold right and 5 trials with temperatures presented vice versa, in random order but always followed by a baseline trial. Please note that temperature changes were only measured in 1 ear. Dashed line = average body temperature in the outer ear of 5 sample participants, measured outside the scanner before the vestibular experiment. Baseline temperatures for caloric stimulation were chosen to match the average body temperature in the ear canals.
With this system a block-design experiment was performed with 60-s long blocks of caloric stimulation. In each run, 10 trials with caloric stimulation (5 trials with hot left and cold right, 5 trials with cold left and hot right) and 10 baseline trials (warm in both ears) were performed. Caloric trials were presented in random order, while each caloric trial was followed by a baseline trial. Each participant completed two runs (40 min in total). Participants had no explicit task and were asked to keep their eyes closed but to stay alert. After scanning, participants filled out a questionnaire inquiring about their sensations during caloric stimulation (see Stephan et al. 2005).
The design of this localizer has limitations: participants had their eyes closed during caloric stimulation but kept eyes open in the visual object motion localizer (see below). Furthermore, requiring participants to report on their sensations during caloric stimulation at the end of the session renders it difficult to estimate the stability of sensations over trials. Finally, since we were unable to perform eye-tracking recordings for the current study, we cannot estimate the amount of elicited caloric nystagmus, including duration and presence of nystagmus during baseline trials.
Despite these limitations, our goal in this study was to use a canonical caloric stimulation design, following previous descriptions, to produce optimal vestibular stimulation conditions for PIVC (e.g., Bense et al. 2001; Dieterich et al. 2003; Fasold et al. 2002; Indovina et al. 2005; Lobel et al. 1998; Stephan et al. 2005). In these studies, participants did not perform any task related to their sensations during caloric stimulation and they kept their eyes closed. Furthermore, activations in PIC in the current study (see below) are comparable to those reported in our previous work (Frank et al. 2014), where participants had their eyes open during caloric stimulation and reported on their sensations after each stimulation trial. Finally, if nystagmus was present and carried over from stimulation into baseline trials, it would be a constant factor over the course of the experiment and hence could not account for activation differences between stimulation and baseline trials. Therefore, we believe that the current design introduces no major limitations with respect to the interpretation of the results.
Visual Object Motion Localizer
Area PIC is primarily characterized by its pronounced responsiveness to visual object motion cues (Beer et al. 2009; Claeys et al. 2003; Orban et al. 2003; Sunaert et al. 1999). Therefore, a standard visual object motion localizer was used to define PIC in individual participants (see Frank et al. 2014). In this localizer 12-s long blocks of dot motion alternated with blocks of static dots for baseline. During visual motion blocks 200 white dots (diameter: 0.2°) moved with a speed of 15°/s in 1 of 12 different translational directions (each presenting 100% coherent motion in 1 of the 12 possible directions) for 1 s each. Successive movement directions alternated between clock- and counterclockwise sequence in different motion blocks, thereby avoiding direction-specific motion adaptation. Dots had a limited random lifetime of 167–333 ms and did not spatially overlap. Each motion block was followed by a baseline block with static dots. Overall, there were 24 blocks with visual motion and 24 baseline static blocks. One run (9.6 min) was performed. Participants were asked to maintain central fixation and to press a button whenever the fixation spot flickered, which occurred at random intervals. Stimuli were generated using Psychtoolbox (Brainard 1997; Pelli 1997), running in MATLAB (The MathWorks, Natick, MA), and back-projected onto a translucent circular screen, located at the back of the scanner bore. Participants viewed the screen with a head-coil mounted mirror (viewing distance: 63 cm). The resulting diameter of the circular visual field with object motion was 30°.
This localizer was designed to activate visual motion processing without any accompanying sensations of self motion induced by the visual stimulus. This approach was chosen to make the definition of PIC more comparable to previous descriptions of this area in studies employing basic object motion cues that do not elicit self-motion sensations (Beer et al. 2009; Claeys et al. 2003; Frank et al. 2014; Orban et al. 2003; Sunaert et al. 1999). The probability of self-motion sensations in the current localizer was minimized by switching coherent motion directions every second. During debriefing after scanning, none of our participants reported sensations of self motion during the localizer scan.
Scanning Parameters
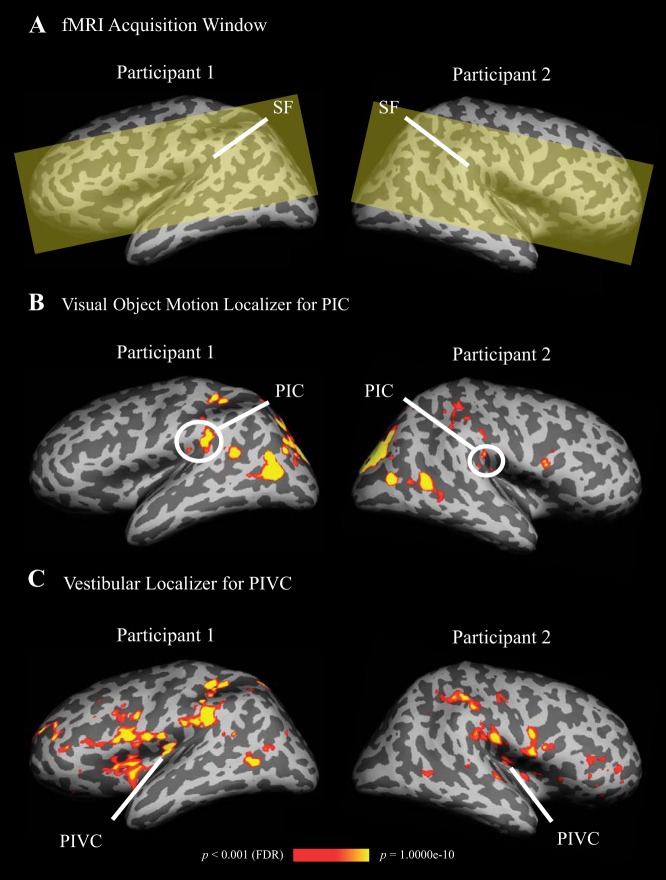
Magnetic-resonance imaging was conducted with a 3-Tesla Allegra head-only scanner (Siemens, Erlangen, Germany), using a single-channel head coil. Functional imaging data were collected with a T2*-weighted echoplanar imaging sequence covering the region surrounding the Sylvian fissure (Fig. 2A) [time-to-repeat (TR) = 1 s, time-to-echo (TE) = 30 ms, flip angle (FA) = 90°, image matrix (IM) = 64 × 64 voxels, voxel-size = 3 × 3 × 3 mm, 16 axial slices, no interslice gap]. A high-resolution anatomical scan of each participant's brain was acquired with a magnetization prepared rapid gradient echo (MP-RAGE) sequence (TR = 2.25 s, TE = 2.6 ms, FA = 9°, IM = 256 × 256 voxels, voxel-size = 1 × 1 × 1 mm, 256 sagittal slices, no interslice gap).
Fig. 2.
Definition of areas posterior insular cortex (PIC) and parieto-insular vestibular cortex (PIVC) by means of activations in visual object motion and caloric stimulation, shown on inflated left and right hemispheres of two sample participants. PIC and PIVC were also activated in the other hemisphere of the shown participants, however, not at the conservative threshold (p < 0.001, false-discovery-rate corrected), used here for displaying purpose. A: the functional MRI acquisition window (yellow shading) was restricted to the region surrounding the Sylvian fissure (SF). B: significantly stronger activation in PIC during visual object motion compared with baseline (=static objects). Color-coding ranges from red to yellow and indicates increasing levels of statistical significance (scale bar shown at bottom). In both sample participants PIC was split in anterior and posterior clusters. C: significantly stronger activation in PIVC during caloric stimulation compared with baseline (=warm in both ears). Color coding as in B.
Imaging Data Analysis
General.
Anatomical and functional MRI data were analyzed with FreeSurfer and the FSFAST toolbox (Martinos Center for Biomedical Imaging, Charlestown, MA). High-resolution anatomical images of each participant's brain were reconstructed and inflated (Dale et al. 1999; Fischl et al. 1999). Functional images were motion corrected to the first functional volume, coregistered to the reconstructed individual brain, smoothed with a three-dimensional Gaussian kernel (full-width at half maximum = 5 mm), and intensity normalized.
Univariate analysis.
Functional localizer scans were analyzed using a general linear model (GLM) approach with a block design. Each GLM model contained a linear scanner drift predictor and motion-correction parameters as regressors-of-no-interest.
In the object motion localizer there were two regressors-of-interest, one for blocks with moving dots, one for blocks with static dots (=baseline). To define area PIC activity during visual motion was contrasted with baseline.
In the vestibular localizer there were three regressors-of-interest, two for caloric stimulation trials (hot in the left ear and cold in the right ear, cold in the left ear and hot in the right ear) and one for baseline (warm in both ears) (see Fig. 1A). In each case only the 50-s long periods of steady-state temperatures were modeled in the regressor. For the definition of area PIVC, activity during caloric stimulation (hot left and cold right + cold left and hot right) was contrasted with baseline.
Since both localizer scans involved standard AB-block designs with partially predictable stimulus presentations (because stimulation A was always followed by baseline B), one might wonder about any effects related to stimulus order (for instance, as revealed by a comparison of early vs. late presentations). However, when parameter contrasts (stimulation minus baseline) were computed separately for the first and second half of each run and compared, there were no significant differences. It is therefore unlikely that stimulus order had an influence on activations reported below.
Random-effects group analyses were performed for the visual object motion and vestibular localizers to estimate average locations of PIC and PIVC. For the visual localizer a larger pool of participants, who completed this localizer scan between the years 2011 and 2015 for various studies in our laboratory, was included (n = 86, including participants from the current study, 44 females, 18–54 yr old). This was necessary because PIC is small and varies substantially in location between participants, which renders detection in a group analysis difficult (Frank et al. 2014; Orban et al. 2003; Sunaert et al. 1999).
To avoid circularity, region-specific blood oxygenation-dependent (BOLD) percent-signal change activations for the comparison of stimulation with baseline were only computed in the localizer that was not used for definition of PIC and PIVC. That is, BOLD percent-signal changes in stimulation and baseline were only computed for PIC in the vestibular localizer and for PIVC in the object motion localizer. Therefore, for each voxel in PIC and PIVC β-estimates for regressors of interest in the GLM were converted to BOLD percent-signal change (referenced to implicit baseline) and pooled across left and right hemisphere voxels or across voxels in the hemisphere where the region could be defined (see below). Activity, based on these average scores, was then contrasted between conditions-of-interest using paired-sample t-tests. For the analysis of activity differences due to the side where hot and cold caloric stimulations occurred (hot left-cold right and vice versa), PIC and PIVC were analyzed separately for left and right hemispheres. This was necessary to preserve information about the side of stimulation in each region (either hot ipsilateral and cold contralateral or vice versa).
Regions-of-interest.
Regions-of-interest (ROIs) were defined on each participant's inflated cortical surfaces at a false-discovery-rate corrected threshold of p < 0.05. The definition of area PIC was guided by previous descriptions (Beer et al. 2009; Claeys et al. 2003; Frank et al. 2014; Orban et al. 2003; Sunaert et al. 1999). In two participants PIC could only be defined in one hemisphere.
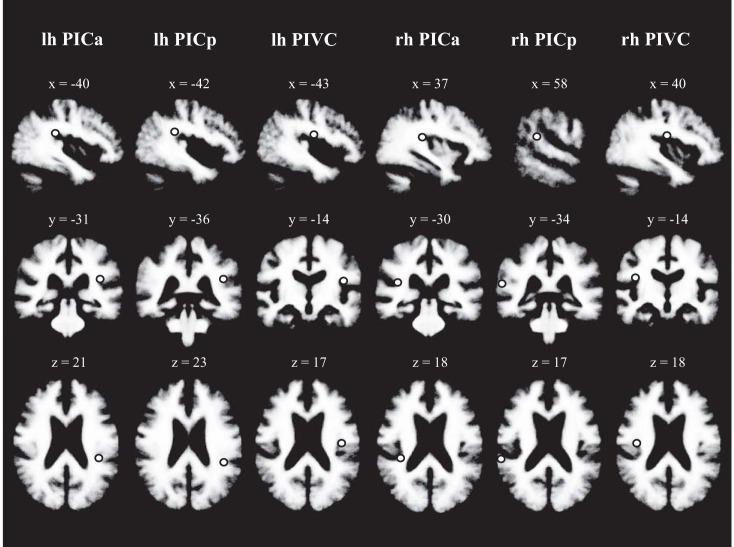
In the majority of participants activations in the object motion localizer were distributed across two or more separate patches in the posterior Sylvian fissure. These patches could be grouped into an anterior and a posterior cluster (see Fig. 2B for two example participants, and Fig. 3B for a similar pattern in the group analysis). Anterior and posterior PIC clusters were evident in 10 out of 15 participants for the left hemisphere and in 12 out of fifteen participants for the right hemisphere. We did not observe any functional differences between anterior and posterior PIC in the univariate and multivariate analyses and therefore report results on PIC combined across clusters. Center coordinates (based on the random-effects group analysis, see Fig. 3B) and anatomical locations in volumetric space (see Fig. 4) are reported separately for anterior and posterior PIC, to provide a reference for localizing activations in future studies. The center of anterior PIC had the following Talairach-coordinates (in mm): X = −40, Y = −31, Z = 21 (left); X = 37, Y = −30, Z = 18 (right). Anterior PIC was ∼20 mm (left) and ∼22 mm (right) anterior to the posterior ending of the Sylvian fissure (Euclidean distance between Talairach-coordinates). The center of posterior PIC had the following Talairach-coordinates: X = −42, Y = −36, Z = 23 (left); X = 58, Y = −34, Z = 17 (right). Posterior PIC in the left hemisphere was ∼15 mm anterior to the posterior ending of the Sylvian fissure. In the right hemisphere posterior PIC was located right at the posterior end of the Sylvian fissure. Anterior and posterior PICs were ∼6 mm (left) and ∼21 mm (right) apart.
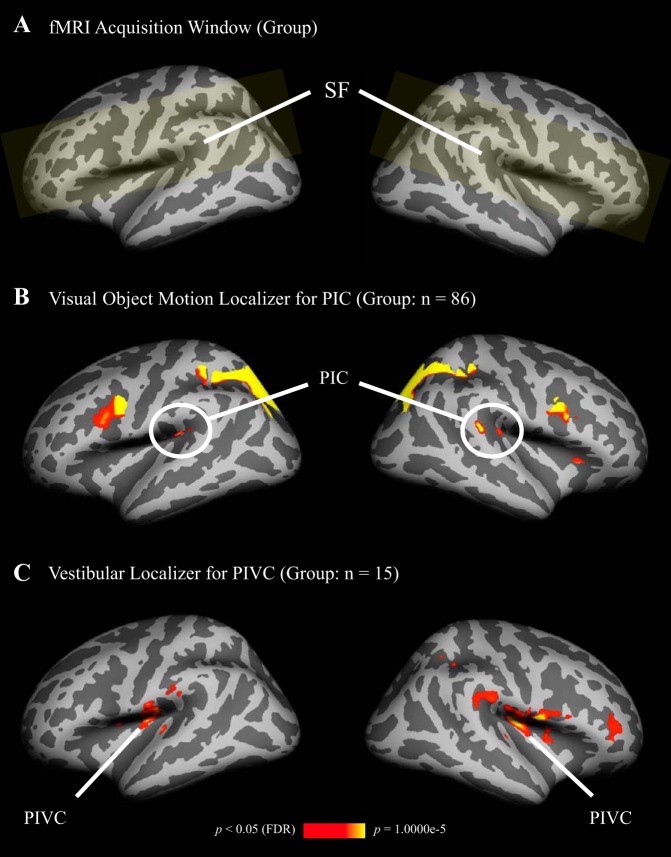
Fig. 3.
Average locations of areas PIC and PIVC, revealed by random-effects group analyses, and shown on inflated left and right hemispheres of the Freesurfer template brain (color coding as in Fig. 2). A: common acquisition window (yellow shading) was restricted to the region surrounding the SF, following the data acquisition window for the current study (see Fig. 2A). B: significantly stronger activation in PIC during visual object motion compared with static objects. Since area PIC is small and varies substantially in location between participants, 86 subjects, who completed the object motion localizer between the years 2011 and 2015 for various studies in our laboratory, were included to reveal activation in PIC on the group level. One anterior and one posterior cluster for PIC emerged (see also Fig. 2B). C: significantly stronger activation in PIVC during caloric stimulation compared with baseline (n = 15).
Fig. 4.
Average locations of areas PIC and PIVC in volumetric space, shown on the Freesurfer template brain. Locations indicated by the dot correspond to center-coordinates of PIC and PIVC activation clusters revealed in the random-effects group analysis (see Fig. 3). Participant's left side is depicted on the right. PICa and PICp, anterior and posterior PIC clusters (Fig. 3B). Talairach-coordinates (in mm) are inserted.
For the definition of area PIVC, in addition to previous descriptions (e.g., Deutschländer et al. 2002; Dieterich et al. 2003; Eickhoff et al. 2006; Lopez and Blanke 2011; Lopez et al. 2012; zu Eulenburg et al. 2012), results of a random-effects group analysis of the vestibular localizer were used (Fig. 3C). The group analysis revealed activation likely corresponding to the average location of PIVC across participants and this location guided the definition of PIVC in each participant's individual activations. PIVC could be defined in each participant's left and right hemispheres. The center of PIVC (based on activations in the random-effects group analysis in Fig. 3C) had the following Talairach-coordinates: X = −43, Y = −14, Z = 17 (left); X = 40, Y = −14, Z = 18 (right). PIVC was ∼32 mm (left) and ∼27 mm (right) anterior to the posterior ending of the Sylvian fissure. The distance between the center of PIVC and anterior PIC was ∼18 mm (left) and ∼16 mm (right). PIVC and posterior PIC were ∼23 mm (left) and ∼27 mm (right) apart.
Multivariate analysis.
Multivariate fMRI pattern analysis of the vestibular localizer was performed with the PyMVPA toolbox running in Python (Hanke et al. 2009). Before the analysis motion corrected and unsmoothed fMRI data were analyzed following the GLM approach described above, however, each caloric stimulation trial was modeled with a separate regressor. The resulting β-estimates (each representing activation in a different caloric stimulation trial) were then combined into a time series and z-scored separately for trials in run 1 and run 2 to remove any leftover run-related variance. After this preprocessing, data were analyzed with a standard linear support vector machine. We performed a two-way classification for fMRI patterns representing on which side hot and cold caloric stimulation occurred (hot left and cold right vs. cold left and hot right, see Fig. 1A, left and middle). Chance level for classification accuracy was 50%. The classifier was first trained on all except one trial and then a prediction about the condition associated with the fMRI-pattern of the left-out trial was made. The prediction could either be correct or incorrect. This procedure was reiterated until each trial had served as left-out trial and the sum of correct predictions, across trials, was divided by the total number of trials to compute average classification accuracy (in percentage correct). This was done for each participant and ROI.
For statistical assessment, we employed permutation tests (following Stelzer et al. 2013). Therefore, for each participant and ROI, condition labels were randomly permuted across trials and classified as described above. This procedure was repeated 1,000 times, resulting in a chance distribution of classification accuracy for each participant. A chance distribution on the group level was computed by randomly drawing (with replacement) a classification result from each participant's 1,000 iterations that were then averaged across participants. This was repeated 105 times, resulting in a chance distribution of classification accuracy across participants for each ROI. Classification performance using correct condition labels with a probability that exceeded 95% of the chance distribution was considered significant (P < 0.05).
Multivariate analyses can be influenced by the size of an ROI. Since PIVC tended to be larger, on average (size in functional voxels ± SD; left: 67 ± 48, right: 64 ± 48), than PIC (left: 45 ± 29, right: 45 ± 21), we ran a control analysis where PIVC was reduced in size until it matched the smaller size of PIC. Therefore, for each participant and hemisphere, voxels were randomly sampled from PIVC until the number of voxels matched with PIC. We report the range of classification results across five iterations of this random sampling procedure.
RESULTS
Sensations During Caloric Stimulation
During postscan questioning, all participants (n = 15) indicated that they noticed the stimulation. No participant fell asleep during the scan or reported feelings of vertigo and/or dizziness. In most participants (12/15) caloric stimulation was accompanied by sensations of self motion. Self motion was experienced as rotation (10/15), tilt (1/15), or a combination of both (1/15), and affected the head (10/15) or the whole body (2/15). Participants described self-motion sensations along yaw (6/15), roll (3/15), and pitch axes (1/15), or along combinations of yaw and roll (1/15) and yaw and pitch (1/15). When asked whether the self-motion sensation was towards or away from the side of cold stimulation, six participants indicated self motion away from cold, two experienced self motion towards cold, and four participants reported alternating directions.
Functional Imaging Results
Figure 2 shows activations in two sample participants during localizer scans for areas PIC (contrast: visual object motion > static) and PIVC (contrast: caloric stimulation > baseline). Note that the significant activation in PIC was split across at least two patches in both participants.
Figure 3 shows activations for the same localizers in a random-effects group analysis. Group results for the PIC localizer are based on a larger sample of participants (n = 86, including participants from the current study), who performed the same localizer scan for earlier studies from our laboratory. When the group analysis was limited to participants in the current study (n = 15), no activation at the PIC location was observed. Similarly to Fig. 2, the group analysis revealed two PIC patches (anterior and posterior) in each hemisphere. Local statistical maxima in these patches remained separate even when the statistical threshold was relaxed. Partial volume effects, caused by inflating gyri and sulci, cannot account for the separation because clusters remained separate when the surface of the brain was not inflated. Anterior and posterior PIC patches in the left hemisphere were each located at the posterior end of the Sylvian fissure. In the right hemisphere, posterior PIC was located at a more posterior site than in the left hemisphere, at the junction of the Sylvian fissure with the supramarginal gyrus. Figure 4 shows the center locations of anterior and posterior PIC and PIVC from the group analyses in volumetric space.
For participants in the current study who completed the vestibular localizer scan, area PIC was significantly more activated during caloric stimulation than during baseline [t(14) = 2.78, P = 0.01; Fig. 5A], replicating our previous results (Frank et al. 2014). Conversely, activation of area PIVC was significantly lower during periods of visual object motion compared with static [t(14) = −4.40, P < 0.001; Fig. 5B], which agrees with earlier reports (Brandt et al. 1998; Deutschländer et al. 2002; Kleinschmidt et al. 2002).
Fig. 5.
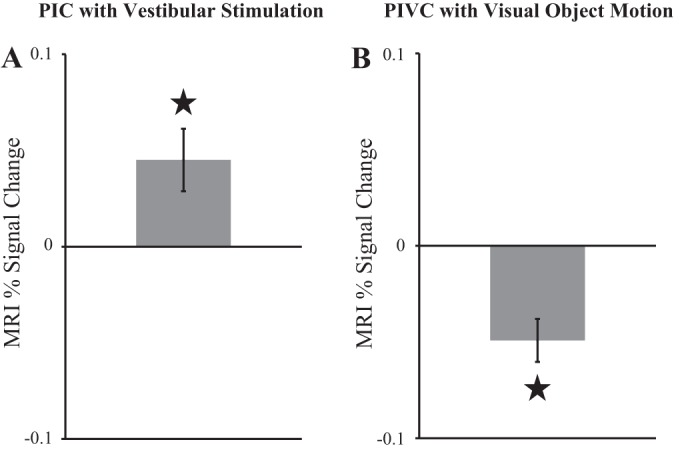
Results of univariate fMRI analyses of the vestibular and visual localizers. To avoid circularity, activations in areas PIC and PIVC were only analyzed in the localizer scan that was not used for region of interest definition. Activations were computed separately for stimulation and baseline conditions (in units MRI percent-signal change from implicit baseline). For displaying purposes activation during baseline was subtracted from activation during stimulation. Therefore, positive values reflect more activation during stimulation vs. baseline and negative values reflect stronger activation in baseline vs. stimulation. Shown are mean values with standard error of the mean (SE). ⋆Significant effects. A: vestibular activation in PIC during caloric stimulation vs. baseline. B: deactivation of PIVC in visual object motion vs. static baseline.
Univariate parameter estimates in PIVC and PIC were not significantly different between the two caloric stimulation conditions [left PIVC: t(14) = −0.09, P = 0.93, right PIVC: t(14) = −0.22, P = 0.83, left PIC: t(13) = −1.71, P = 0.11], except in right PIC where activation was significantly more pronounced for cold left and hot right vs. hot left and cold right [t(13) = 2.95, P = 0.01]. A more sensitive multivariate classification analysis showed that the activation pattern in right PIVC represented on which side hot and cold stimulation occurred (Fig. 6): there was significant above-chance classification of activation patterns corresponding to hot left and cold right vs. cold left and hot right in right PIVC (average classification accuracy: M = 63.0%, permutation test: P = 0.003). There was a trend for similar results in right PIC (M = 57.8%); however, permutation tests did not indicate that classification accuracy was significantly different from chance level (P = 0.11). Classifications were also not significantly different from chance in left PIVC (M = 54.1%, P = 0.45) or in left PIC (M = 53.9%, P = 0.43). Similar results were obtained in a control analysis where PIVC was matched in size with PIC. Average classification accuracies, across participants, in five iterations, each with a new random selection of voxels from PIVC, ranged from 62.5% (P = 0.005) to 62.8% (P = 0.003) in right PIVC and from 54.4% (P = 0.43) to 55.1% (P = 0.33) in left PIVC.
Fig. 6.
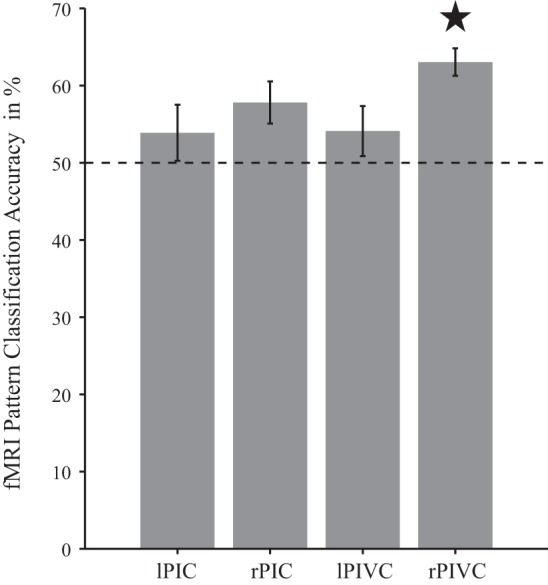
Representation of caloric stimulation conditions in areas PIVC and PIC. Multivariate fMRI-pattern analysis revealed above-chance classification of caloric stimulation conditions in right PIVC but not in left PIVC or in PIC (however, there was a nonsignificant trend in right PIC). ★Significant above-chance (=50%, dashed line) classification between activation patterns corresponding to hot left and cold right vs. cold left and hot right. Displayed are mean classification accuracies with SE.
DISCUSSION
In this study, two areas in the region of the posterior Sylvian fissure, referred to as PIC and PIVC, were examined in the same participants using vestibular and visual stimuli. The results point to similarities between PIC and PIVC, as both areas respond to caloric stimulation. However, there were also differences: the activation pattern of PIVC in the right hemisphere represented on which side hot and cold stimulation occurred, whereas the activation pattern of PIC did not exhibit this differential information. During visual object motion, activity in PIVC was suppressed and in PIC increased. PIC, even if referred to as “posterior insular cortex,” was located in the posterior Sylvian fissure and at a more posterior site than PIVC. In the majority of cases, PIC was split across separate anterior and posterior clusters. Overall, the results suggest a complex organization of the vestibular and visual networks in the Sylvian fissure. The vestibular network appears to consist of at least two anatomically and functionally separate areas, referred to as PIVC and PIC. The visual network overlaps with the vestibular network in area PIC.
The activity pattern in right PIVC represented on which side hot and cold stimulation occurred. It could be argued that this representation is related to the thermal sensations of hot and cold evoked by caloric stimuli (Craig et al. 2000). However, we think it is more likely that the pattern corresponds to the vestibular effects of caloric stimulation, in particular because the hot-cold representation was only found in right PIVC. This finding fits well with the majority of studies reporting a dominant role of PIVC in the right hemisphere of right-handed participants in vestibular processing (Dieterich et al. 2003; Eickhoff et al. 2006; Lopez and Blanke 2011; zu Eulenburg et al. 2012). Therefore, we suggest that the activation pattern in right PIVC reflects vestibular information, potentially related to the direction of self-motion sensations during caloric stimulation.
However, we want to point out that although the majority of participants reported sensations of self motion during caloric stimulation, the perceived direction of self motion varied between participants. Moreover, participants reported retrospectively on their overall sensations after the end of scanning, not after each stimulation trial. Therefore, the intertrial variability in self-motion sensations cannot be estimated. Since we were unable to perform eye tracking in the scanner, we also cannot exclude the possibility that the activation pattern in PIVC is associated with the direction of caloric nystagmus. Differing from our earlier work (Frank et al. 2014), we were aiming to perform vestibular stimulation without any explicit task in the current study, to avoid task-related cognitive processing in addition to the processing of the sensory stimulus. Overall, our goal was to follow more closely the approach of previous studies, which defined PIVC by means of vestibular stimulation while the participant was not performing any particular task (e.g., Dieterich et al. 2003; Stephan et al. 2005). Nevertheless, caloric stimulation appears to generate a distinguishable activation pattern related to the side of hot and cold stimulation in right PIVC, across trials and participants. It remains a goal for future studies to relate these differential activation patterns to neural computations occurring in PIVC during caloric stimulation and to participants' sensations.
In contrast to PIVC, the activation pattern in PIC did not contain information about the side of hot and cold stimulation, although there was a trend for such information in right PIC, where stronger overall activations for cold left and hot right vs. hot left and cold right were observed. Therefore, there are indications for a more detailed representation in right PIC as well; however, the results are less clear than in right PIVC.
A functional dissociation between PIC and PIVC was evident in the visual object motion localizer, where area PIVC was, in strong contrast to PIC, deactivated during visual object motion compared with static. Single-cell recordings in primates (Chen et al. 2010) and functional imaging studies in humans (Brandt et al. 1998; Deutschländer et al. 2002; Kleinschmidt et al. 2002) repeatedly reported little activation or even deactivation in PIVC during stimulation with visual object motion cues [but note that PIVC neurons appear to become activated when a moving object is actively tracked (see Shinder and Newlands 2014) or during full field visual motion (Akbarian et al. 1988)]. Some imaging studies in humans found activation in the posterior Sylvian fissure during self-motion sensations induced by visual motion cues (see Cardin and Smith 2010; Huang et al. 2015; Uesaki and Ashida 2015). The reported location of this activation coincides well with the location of PIC (Figs. 2B and 3B), and these activations might therefore be located in or at least overlap with PIC rather than PIVC, which also fits to the proposed role of PIC as a site of integration between visual and vestibular information (Billington and Smith 2015; Frank et al. 2014). It is important to note that PIC, as defined in this study, is at the location erroneously designated as PIVC in previous studies (Cardin and Smith 2010; Huang et al. 2015; Uesaki and Ashida 2015).
Similarly, activations observed in retroinsular cortex related to processing head and gaze movements (Petit and Beauchamp 2003), and visual gravitational motion (Indovina et al. 2005), at a more posterior site in the Sylvian fissure, might be better associated with PIC rather than PIVC.
Finally, the putative separation of PIC into an anterior and a posterior cluster is a new observation. Previous studies investigating PIC, including our own work, noticed that PIC was sometimes scattered across two or more clusters in individual participants and tended to vary in location between participants, rendering the identification of PIC in a random-effects group analysis difficult. Orban et al. (2003) included visual object motion localizer scans from 30 participants and found PIC activation (labeled as “PIVC” in their study) only in the posterior end of the Sylvian fissure of the left hemisphere. In our study, using object motion localizer data from 86 participants, we were able to identify PIC in a group analysis in each hemisphere. Moreover, PIC appeared to be split across an anterior and a posterior cluster (Fig. 3B). This separation might be caused by intersubject variability in the exact location of a single PIC cluster; however, we also observed a similar anterior-posterior separation in the majority of individual participants (see Fig. 2B, for 2 examples). There were no obvious functional differences in vestibular processing between the anterior and posterior PIC clusters. Future studies, employing richer stimulus sets, might further examine whether the putative separation and functional differences between anterior and posterior PIC exist, in particular with respect to the processing of visual object motion cues with and without accompanying self-motion sensations.
In conclusion, we find evidence that PIVC and PIC are both part of the vestibular network in the Sylvian fissure but exhibit different functional specialization. Together with different anatomical locations, PIVC and PIC should therefore be considered separate areas. Overall, our results suggest the existence of a more complex organization at the putative core of human vestibular cortex than previously assumed.
GRANTS
M. W. Greenlee was supported by Deutsche Forschungsgemeinschaft (GR988/20-2).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.M.F., A.M.W., and M.W.G. conception and design of research; S.M.F. and A.M.W. performed experiments; S.M.F. analyzed data; S.M.F. and M.W.G. interpreted results of experiments; S.M.F. prepared figures; S.M.F. drafted manuscript; S.M.F., A.M.W., and M.W.G. edited and revised manuscript; S.M.F., A.M.W., and M.W.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Christian Renner and Peter Fuchs from the workshop of the University of Regensburg for constructing the caloric stimulation device and for assistance with temperature measurements. We also thank four anonymous reviewers for helpful comments on a previous version of the manuscript.
REFERENCES
- Akbarian S, Berndl K, Grüsser OJ, Guldin W, Pause M, Schreiter U. Responses of single neurons in the parietoinsular vestibular cortex of primates. Ann NY Acad Sci 545: 187–202, 1988. [DOI] [PubMed] [Google Scholar]
- Beer AL, Watanabe T, Ni R, Sasaki Y, Andersen GJ. 3D surface perception from motion involves a temporal-parietal network. Eur J Neurosci 30: 703–713, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI). J Neurophysiol 85: 886–899, 2001. [DOI] [PubMed] [Google Scholar]
- Billington J, Smith AT. Neural mechanisms for discounting head-roll-induced retinal motion. J Neurosci 35: 4851–4856, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Brandt T, Bartenstein P, Janek A, Dieterich M. Reciprocal inhibitory visual-vestibular interaction. Brain 121: 1749–1758, 1998. [DOI] [PubMed] [Google Scholar]
- Cardin V, Smith AT. Sensitivity of human visual and vestibular cortical regions to egomotion-compatible visual stimulation. Cereb Cortex 20: 1964–1973, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, DeAngelis GC, Angelaki DE. Macaque parieto-insular vestibular cortex: responses to self-motion and optic flow. J Neurosci 30: 3022–3042, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeys KG, Lindsey DT, de Schutter E, Orban GA. A higher order motion region in human inferior parietal lobule: evidence from fMRI. Neuron 40: 631–642, 2003. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci 3: 184–190, 2000. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9: 179–194, 1999. [DOI] [PubMed] [Google Scholar]
- Deutschländer A, Bense S, Stephan T, Schwaiger M, Brandt T, Dieterich M. Sensory system interactions during simultaneous vestibular and visual stimulation in PET. Hum Brain Mapp 16: 92–103, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Bartenstein P, Brandt T. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb Cortex 13: 994–1007, 2003. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Brandt T. The bilateral central vestibular system: its pathways, functions, and disorders. Ann NY Acad Sci 1343: 10–26, 2015. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Weiss PH, Amunts K, Fink GR, Zilles K. Identifying human parieto-insular vestibular cortex using fMRI and cytoarchitectonic mapping. Hum Brain Mapp 27: 611–621, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasold O, von Brevern M, Kuhberg M, Ploner CJ, Villringer A, Lempert T, Wenzel R. Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. Neuroimage 17: 1384–1393, 2002. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage 9: 195–207, 1999. [DOI] [PubMed] [Google Scholar]
- Frank SM, Baumann O, Mattingley JB, Greenlee MW. Vestibular and visual responses in human posterior insular cortex. J Neurophysiol 112: 2481–2491, 2014. [DOI] [PubMed] [Google Scholar]
- Frank SM, Greenlee MW. An MRI-compatible caloric stimulation device for the investigation of human vestibular cortex. J Neurosci Meth 235: 208–218, 2014. [DOI] [PubMed] [Google Scholar]
- Greenlee MW, Frank SM, Kaliuzhna M, Blanke O, Bremmer F, Churan J, Cuturi LF, MacNeilage PR, Smith AT. Multisensory integration in self motion perception. Multisens Res 29: 525–556, 2016. [Google Scholar]
- Grüsser OJ, Pause M, Schreiter U. Localization and responses of neurones in the parieto-insular vestibular cortex of awake monkeys (Macaca fascicularis). J Physiol 430: 537–557, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldin WO, Grüsser OJ. Is there a vestibular cortex? Trends Neurosci 21: 254–259, 1998. [DOI] [PubMed] [Google Scholar]
- Hanke M, Halchenko YO, Sederberg PB, Hanson SJ, Haxby JV, Pollmann S. PyMVPA: A python toolbox for multivariate pattern analysis of fMRI data. Neuroinformatics 7: 37–53, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RS, Chen CF, Sereno MI. Neural substrates underlying the passive observation and active control of translational egomotion. J Neurosci 35: 4258–4267, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indovina I, Maffei V, Bosco G, Zago M, Macaluso E, Lacquaniti F. Representation of visual gravitational motion in the human vestibular cortex. Science 308: 416–419, 2005. [DOI] [PubMed] [Google Scholar]
- Kahane P, Hoffmann D, Minotti L, Berthoz A. Reappraisal of the human vestibular cortex by cortical electrical stimulation study. Ann Neurol 54: 615–624, 2003. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Thilo KV, Büchel C, Gresty MA, Bronstein AM, Frackowiak RS. Neural correlates of visual-motion perception as object- or self-motion. Neuroimage 16: 873–882, 2002. [DOI] [PubMed] [Google Scholar]
- Lobel E, Kleine JF, Le Bihan D, Leroy-Willig A, Berthoz A. Functional MRI of galvanic vestibular stimulation. J Neurophysiol 80: 2699–2709, 1998. [DOI] [PubMed] [Google Scholar]
- Lopez C, Blanke O. The thalamocortical vestibular system in animals and humans. Brain Res Rev 67: 119–146, 2011. [DOI] [PubMed] [Google Scholar]
- Lopez C, Blanke O, Mast FW. The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neuroscience 212: 159–179, 2012. [DOI] [PubMed] [Google Scholar]
- Orban GA, Fize D, Peuskens H, Denys K, Nelissen K, Sunaert S, Todd J, Vanduffel W. Similarities and differences in motion processing between the human and macaque brain: evidence from fMRI. Neuropsychologia 41: 1757–1768, 2003. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997. [PubMed] [Google Scholar]
- Petit L, Beauchamp MS. Neural basis of visually guided head movements studied with fMRI. J Neurophysiol 89: 2516–2527, 2003. [DOI] [PubMed] [Google Scholar]
- Shinder ME, Newlands SD. Sensory convergence in the parieto-insular vestibular cortex. J Neurophysiol 111: 2445–2464, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer J, Chen Y, Turner R. Statistical inference and multiple testing correction in classification-based multi-voxel pattern analysis (MVPA): random permutations and cluster size control. Neuroimage 65: 69–82, 2013. [DOI] [PubMed] [Google Scholar]
- Stephan T, Deutschländer A, Nolte A, Schneider E, Wiesmann M, Brandt T, Dieterich M. Functional MRI of galvanic vestibular stimulation with alternating currents at different frequencies. Neuroimage 26: 721–732, 2005. [DOI] [PubMed] [Google Scholar]
- Sunaert S, Van Hecke P, Marchal G, Orban GA. Motion-responsive regions of the human brain. Exp Brain Res 127: 355–370, 1999. [DOI] [PubMed] [Google Scholar]
- Uesaki M, Ashida H. Optic-flow selective cortical sensory regions associated with self-reported states of vection. Front Psychol 6: 775, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zu Eulenburg P, Caspers S, Roski C, Eickhoff SB. Meta-analytical definition and functional connectivity of the human vestibular cortex. Neuroimage 60: 162–169, 2012. [DOI] [PubMed] [Google Scholar]