Abstract
It has been estimated that one-third of schizophrenia patients are treatment resistant (TRS). Recent studies have shown that functional connectivity (FC) can be used for measuring connections between brain regions in diseased states. White, Wigton, Joyce, Collier, Fornito, and Shergill (Neuropsychopharmacology. First published September 9, 2015; doi:10.1038/npp.2015.277) used FC to identify differences between schizophrenia patients responding to antipsychotic treatment and TRS patients. Their results support the idea that the groups differ not only in treatment response but also neurophysiologically through differences in FC.
Keywords: functional connectivity, nigrostriatal, schizophrenia, treatment resistant
schizophrenia encompasses a wide range of symptoms and can be divided into three categories: positive, negative, and cognitive (Fornito et al. 2013; Kapur 2003; van den Heuvel and Fornito 2014). Positive symptoms are auditory and visual hallucinations, perceptions, delusions, and atypical beliefs (van den Heuvel and Fornito 2014), whereas negative symptoms involve social withdrawal, neglect, and loss of motivation (Kapur 2003). Current treatments involve a variety of antipsychotic medications that function primarily as dopamine antagonists but also possess additional antagonist properties against the cholinergic, serotonergic, and adrenergic systems (Heinz and Schlagenhauf 2010). Currently, the main drug to treat these patients is clozapine, a second-generation atypical antipsychotic medication that is a broad-acting antagonist targeting NMDA receptors, serotonergic receptors, α-adrenergic receptors, and acetylcholine receptors (Elkis 2007). However, ∼20–30% of patients are unresponsive to these drugs and considered to have treatment-resistant schizophrenia (TRS). TRS patients experience auditory and visual hallucinations uncontrolled by pharmacological treatments (Nakajima et al. 2015). Attempts have been made to characterize the underlying neurophysiology of schizophrenia to better understand this illness and possibly find a biomarker associated with TRS. In their study, White et al. (2015) use functional imaging to identify functional connectivity (FC) differences within the striatal pathway and reveal different neurophysiology between the treatment-responsive and -resistant groups.
Many techniques have been used to probe and identify morphology, connectivity, and broad global communication in schizophrenia, including diffuse weighted imaging, graph theory, and FC (van den Heuvel and Fornito 2014). Recently, efforts have been aimed specifically at characterizing differences in neurophysiological pathways between individuals who respond to treatment and TRS patients. Such efforts may reveal differentially affected neural substrates between treatment-responsive schizophrenia and TRS. Results of these studies may explain why current treatments are inefficacious in this subset of patients (Sarpal et al. 2016). Through FC analysis, it may become apparent that TRS patients possess differentially affected neurophysiology revealing novel targets and providing further insight into TRS.
The symptoms of schizophrenia, particularly the positive symptoms, may be related to aberrant activity in striatum, and striatal dopamine levels have been shown to be elevated in schizophrenia (Fornito et al. 2013). In striatal system, dopamine controls reward, goal-mediated behavior, anticipation of reward, and possibly salience acquisition (Haber and Knutson 2010; Kapur 2003). In addition, dopamine is implicated in the default mode network (DMN), which is essentially the restful state of the brain (Garrity et al. 2007). Disruption of the DMN and dopaminergic pathways, which include the mesolimbic and corticostriatal systems, are believed to lead to delusions and hallucinations (Garrity et al. 2007). Increased dopamine levels and oscillatory activity in regions of the DMN such as the hippocampus, parahippocampus, and cingulate gyrus (Garrity et al. 2007) may cause saliency attachment to inappropriate stimuli, leading to psychotic symptoms (Kapur 2003). Dysfunction within the DMN in schizophrenia is supported through MRI and FC studies, where there is decreased anatomical connectivity between the parietal regions and the prefrontal cortex (PFC) and increased connectivity between the ventral caudate and insular regions (Fornito et al. 2013; Skudlarski et al. 2010). The dorsal caudate modulates activity of the ventral caudate (Fornito et al. 2013), controlling signaling preferentially through GABAergic neurons (Haber and Knutson 2010). A recent study demonstrated decreased connectivity between the dorsal caudate and PFC and increased connectivity between the ventral caudate and PFC (Fornito et al. 2013). Decreased connectivity of the dorsal corticostriatal pathway may alter modulation of the ventral corticostriatal pathway and lead to greater striatal dopamine, which has been observed in patients (Fornito et al. 2013). As previously mentioned, the increase in dopamine is related to psychosis and thus makes a preferable target for treatment.
With FC, data analysis can be used to identify disease-specific changes (Fornito et al. 2013) and measures of treatment outcome (Sarpal et al. 2016) and may be combined with anatomical data to yield more useful information about changes to brain areas in schizophrenia (Skudlarski et al. 2010). Importantly, similar patterns of connectivity are seen in first relatives of individuals with schizophrenia, implying a genetic aspect to schizophrenia that is associated with altered brain states. Furthermore, using FC analysis, Fornito et al. (2013) detected disease-specific differences between relatives and individuals; specifically, in schizophrenia patients there was increased FC between the ventral caudate and the dorsolateral PFC. In addition, FC may reflect changes in disease state in response to treatment, whereby increases between regions that were hypoconnected and decreases between regions that were hyperconnected in patients with a first episode of schizophrenia showed concomitant improvement with treatment (Sarpal et al. 2015). Taken together, FC analysis can be used to identify disease-specific differences between patients with schizophrenia and patients without schizophrenia and reveal genetic components of schizophrenia using related family members, and it may be used to identify efficacy of treatment, as well.
Unfortunately, little research has been done looking differences between treatment-responsive patients and TRS patients using FC (Nakajima et al. 2015). Auditory verbal hallucinations (AVH) are persistent hallucinations, and up to one-quarter of patients with AVH may have TRS (Alonso-Solis et al. 2015). One study utilized patients with AVH and examined FC differences in the DMN between TRS and treatment-responsive patients (Alonso-Solis et al. 2015). The results revealed that the DMN was more active in patients with AVH. Indeed, increased activity in the DMN is associated with greater psychosis (Alonso-Solis et al. 2015). Of importance, patients with AVH had increased connectivity between regions of the dorsomedial PFC and insular cortex, as well as decreased connectivity between the ventromedial PFC and cingulate cortex (Alonso-Solis et al. 2015). These two regions are involved not only in the DMN but also in the saliency network (Menon and Uddin 2010), suggesting that dysfunction of these networks may result in persistent psychotic symptoms.
Despite evidence suggesting network connectivity differences between treatment-responsive and TRS patients, there has yet to be a study directly comparing striatal connectivity between the two subsets of schizophrenia patients using FC analysis. The efforts put forth by White et al. (2015) were threefold. First, they investigated the differences in corticostriatal FC between treatment-responsive and TRS patients. They targeted the corticostriatal pathway, because the striatum is involved in various processes, including learning, and has densely innervated dopaminergic pathways (Haber and Knutson 2010). Second, they compared differences in connectivity between treatment-responsive and TRS patients with those of controls to investigate differences in frontospatial disruption in schizophrenia. There have been multitudes of imaging studies comparing schizophrenia patients with controls (for review see Nakajima et al. 2015), yet White et al. (2015) compared the groups with controls to further identify differences between treatment-responsive and TRS patients. Finally, they investigated the relationship between positive symptoms and connectivity between groups to see if positive symptoms predicted FC differences.
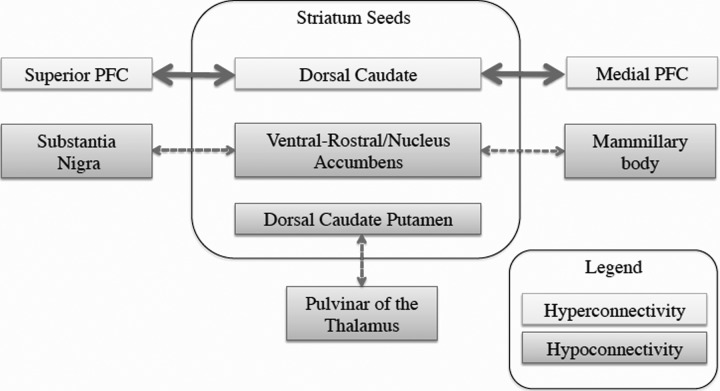
White et al. (2015) found that the TRS patients exhibited increased connectivity in the dorsal caudate with the medial and superior PFC. In addition, White et al. (2015) observed decreased connectivity between the ventral striatum and substantia nigra and between the dorsal caudate and the pulvinar of the thalamus in TRS patients compared with treatment-responsive patients (Fig. 1). Increased positive symptoms were associated with decreased connectivity between the ventral striatum and both the precuneus and cingulate. In addition, greater positive symptom severity was associated with increased connectivity between the dorsal striatum and precuneus, posterior cingulate, and medial PFC. Finally, antipsychotic dose was found to inversely predict FC, mainly within the ventral stratum; however, direct comparisons between groups were not performed because of methodological limitations.
Fig. 1.
Regional functional connectivity differences of treatment-resistant schizophrenia compared with treatment-responsive schizophrenia. PFC, prefrontal cortex.
In light of these findings, White et al. (2015) conclude that the ventral striatum and substantia nigra hypoconnectivity may contribute to a potential mechanism for TRS. The ventral striatum is a key region that projects both the substantia nigra compacta and the reticulata (Haber and Knutson 2010). The ventral striatum forms a loop by projecting to the substantia nigra compacta and reticulata, which in turn projects back to the dorsal striatum (Haber and Knutson 2010). This essentially forms feedback loops, starting with the ventral striatum and progressing to the dorsal striatum, that are organized topographically across the substantia nigra, forming striato-nigro-striatal connections. Given the wide range of projections of the ventral striatum and dorsal striatum, their role in learning, and the implications in saliency (Haber and Knutson 2010), any decoupling between these regions is likely to have larger implications on global functioning. Furthermore, White et al. (2015) reported hyperconnectivity between the dorsal striatum, precuneus, and inferior parietal lobe associated with increased positive symptom severity. This is consistent with previous research showing that decoupling of this network is associated with increased positive symptoms (Alonso-Solis et al. 2015; Fornito et al. 2013; Garrity et al. 2007). Taken together, the findings of White et al. (2015) indicate that there are differences in connectivity that distinguish between TRS and treatment-responsive patients and that decoupling of connectivity within the DMN is associated with increased positive symptoms that may be related to TRS.
Despite the characterization of TRS, to date no longitudinal studies have investigated changes in TRS using FC. To understand this disorder in full, understanding changes that occur over time is critical, since biomarkers may be discovered that allow for preferential treatment to TRS (Fornito et al. 2013; Sarpal et al. 2015). Additionally, some studies have investigated antipsychotic treatment and changes to FC with treatment-responsive schizophrenia patients (Sarpal et al. 2015); however, this has yet to be done with TRS, limiting the potential power of FC as an investigational tool. Also, FC offers limited insight into the underlying mechanisms of differences in connectivity. Therefore, using anatomical methods such as DTI and FC in treatment-responsive and TRS patients (Skudlarksi et al. 2010) may offer increased resolution of affected pathways. Admittedly, it should be noted that neuroimaging studies have limitations, which may explain some discrepant findings. For instance, corticostriatal connectivity between the ventral striatum and the dorsomedial PFC (dmPFC) has been reported to be both increased (Fornito et al. 2013) and decreased (White et al. 2015). White and colleagues (2015) offer insight into nigrostriatal decoupling between TRS and treatment-responsive schizophrenia, and their findings may be beneficial as biomarkers for predicting individuals who are more unlikely to respond to medication. Clearly, there are differences in nigrostriatal and corticostriatal systems between treatment-responsive and TRS patients, and future studies are needed to find other regions that may be affected in TRS and determine the reasons for the connectivity dysregulation.
One potential future brain area that may offer insight into TRS is the insula. The insula is a critical structure coupled with the cingulate cortex that modulates both bottom-up and top-down processing of stimuli, playing a key role in attributing saliency to appropriate stimuli (Menon and Uddin 2010). Specifically, the anterior insula and the anterior cingulate cortex (ACC) compose the saliency network (SN). The SN integrates bottom-up sensory processes, controls top-down output, and more importantly as shown by Granger causality analysis, controls the switch from a restful state, or DMN, to attentive state, or the central executive network (CEN) (Menon and Uddin 2010; Sridharan et al. 2008). Dysfunction of the saliency network may underlie psychosis and has been found in studies of schizophrenia. For example, ACC connectivity is affected in schizophrenia (Garrity et al. 2007; Sarpal et al. 2015; Skudlarski et al. 2010), implicating dysfunction of the SN. Furthermore, regions of the SN are associated with TRS markers including AVH (Alonso-Solis et al. 2015) and positive symptoms (White et al. 2015). In patients with AVH, there is increased FC with the insular cortex and dmPFC, a critical region of the CEN (Alonso-Solis et al. 2015; Menon and Uddin 2010), suggesting changes in these regions may partially underlie the etiology of TRS. Taken together, the evidence presented indicates differentially affected neurophysiology between TRS and treatment-responsive patients and indicates that future studies are needed to characterize other potential regions and systems in TRS, such as the insula cortex and the saliency network.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.P. and N.S. conception and design of research; S.P. and N.S. drafted manuscript; N.S. prepared figures; N.S. edited and revised manuscript; N.S. approved final version of manuscript.
REFERENCES
- Alonso-Solis A, Vives-Gilabert Y, Grasa E, Portella MJ, Rabella M, Sauras RB, Roldán A, Núñez-Marín F, Gómez-Ansón B, Pérez V, Alvarez E, Corripio I. Resting-state functional connectivity alterations in the default network of schizophrenia patients with persistent auditory verbal hallucinations. Schizophr Res 161: 261–268, 2015. [DOI] [PubMed] [Google Scholar]
- Elkis H. Treatment-resistant schizophrenia. Psychiatr Clin North Am 30: 511–533, 2007. [DOI] [PubMed] [Google Scholar]
- Fornito A, Harrison BJ, Goodby E, Dean A, Ooi C, Nathan PJ, Lennox BR, Jones PB, Suckling J, Bullmore ET. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry 70: 1143–1151, 2013. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry 164: 450–457, 2007. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35: 4–26, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull 36: 472–485, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 160: 13–23, 2003. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214: 655–667, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Takeuchi H, Plitman E, Fervaha G, Gerretsen P, Caravaggio F, Chung JK, Iwata Y, Remington G, Graff-Guerrero A. Neuroimaging findings in treatment-resistant schizophrenia: a systematic review: Lack of neuroimaging correlates of treatment-resistant schizophrenia. Schizophr Res 164: 164–175, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpal DK, Argyelan M, Robinson DG, Szeszko PR, Karlsgodt KH, John M, John M, Weissman N, Gallego JA, Kane JM, Lencz T, Malhotra AK. Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am J Psychiatry 173: 69–77, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpal DK, Robinson DG, Lencz T, Argyelan M, Ikuta T, Karlsgodt K, Gallego JA, Kane JM, Szeszko PR, Malhotra AK. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry 72: 5–13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, Pearlson G. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry 68: 61–69, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA 105: 12569–12574, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Fornito A. Brain networks in schizophrenia. Neuropsychol Rev 24: 32–48, 2014. [DOI] [PubMed] [Google Scholar]
- White TP, Wigton R, Joyce DW, Collier T, Fornito A, Shergill SS. Dysfunctional striatal systems in treatment-resistant schizophrenia. Neuropsychopharmacology. First published September 9, 2015; doi: 10.1038/npp.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]