This study investigates the relationship of nociceptive afferent density and subjective pain evaluation. Using subjective pain reports alongside corneal confocal microscopy of small-diameter afferents, we show that lower pain estimates are related to lower peripheral nerve density. These findings provide an integrative perspective on pain processing and highlight a multilevel approach to understanding how physiology influences pain experience.
Keywords: nerve growth factor-β gene mutation, corneal confocal microscopy, pain evaluation, Situational Pain Questionnaire, C-fiber
Abstract
The rare nerve growth factor-β (NGFB) mutation R221W causes a selective loss of thinly myelinated fibers and especially unmyelinated C-fibers. Carriers of this mutation show altered pain sensation. A subset presents with arthropathic symptoms, with the homozygous most severely affected. The aim of the present study was to investigate the relationship between peripheral afferent loss and pain evaluation by performing a quantification of small-fiber density in the cornea of the carriers, relating density to pain evaluation measures. In vivo corneal confocal microscopy (CCM) was used to quantify C-fiber loss in the cornea of 19 R221W mutation carriers (3 homozygous) and 19 age-matched healthy control subjects. Pain evaluation data via the Situational Pain Questionnaire (SPQ) and the severity of neuropathy based on the Neuropathy Disability Score (NDS) were assessed. Homozygotes, heterozygotes, and control groups differed significantly in corneal C-nerve fiber density, with the homozygotes showing a significant afferent reduction. Importantly, peripheral C-fiber loss correlated negatively with pain evaluation, as revealed by SPQ scores. This study is the first to investigate the contribution of small-fiber density to the perceptual evaluation of pain. It demonstrates that the lower the peripheral small-fiber density, the lower the degree of reported pain intensity, indicating a functional relationship between small-fiber density and higher level pain experience.
NEW & NOTEWORTHY
This study investigates the relationship of nociceptive afferent density and subjective pain evaluation. Using subjective pain reports alongside corneal confocal microscopy of small-diameter afferents, we show that lower pain estimates are related to lower peripheral nerve density. These findings provide an integrative perspective on pain processing and highlight a multilevel approach to understanding how physiology influences pain experience.
the nerve growth factor -β (NGFB) mutation R221W is rare, with a population of 66 living carriers (of whom 3 are homozygous) in the Norrbotten region in northern Sweden, arising from a founder effect as far back as the 17th century (Minde et al. 2004, 2009). Despite its rarity, the R221W mutation offers vital clinical and preclinical insight for pain syndromes by revealing relationships between small-fiber density and pain perception. The R221W mutation (Capsoni et al. 2011; Einarsdottir et al. 2004) selectively affects the density of thin-diameter sensory afferents (Crowley et al. 1994; Larsson et al. 2009; Minde et al. 2004) with severe loss of unmyelinated C-fibers and comparatively moderate loss of thinly myelinated Aδ-fibers (Crowley et al. 1994; Larsson et al. 2009; Minde et al. 2004, 2006). It does not affect large myelinated Aβ-fibers (Minde et al. 2004), which are typically negative for the primary NGF receptor TrkA in the cell bodies (Patapoutian and Reichardt 2001). Rat cell line models suggest that this missense point mutation affects the cleavage of pro-NGF, limiting the availability of functional mature NGF in the extracellular space (Carvalho et al. 2011; Larsson et al. 2009). The R221W mutation carriers' reduced nociceptive afferent density may thus be a consequence of insufficient trophic support from NGF during development, with possible additional effects of altered regulatory signaling in NGF-mediated nociceptive and inflammatory pathways in adulthood.
The three known homozygous carriers are severely affected, presenting with debilitating and progressive degrees of painless fractures, joint deformation, Charcot arthropathies, bone necrosis, and osteochondritis, resulting in limited mobility (Minde et al. 2004, 2006, 2009). These clinical sequelae in homozygotes follow an autosomal recessive heritability pattern. Based on this clinical presentation of painless fractures without anhidrosis or mental retardation, these patients have been classified as having hereditary somatosensory and autonomic neuropathy type V (HSAN-V; Minde 2006; Minde et al. 2004). Yet, of the 63 currently identified heterozygous carriers, most do not present with pain- or inflammation-related deficits and have been identified only through pedigree and genetic screening. A proportion of these (19) are vulnerable to carpal tunnel syndrome (Hellgren T, Svensson O, Minde J, unpublished data; Minde et al. 2009).
This clinical variability may be underpinned by a uniquely broad range of peripheral unmyelinated afferent density in the R221W carrier population as a whole, thus providing an important window on the role of C-afferent density in pain perception. A previous study (Minde et al. 2004) has quantified small-diameter fiber density in a small sample (3 homozygous and 3 heterozygous carriers) by using electron microscopy of sural nerve biopsies. However, to more completely characterize the relationship between small-fiber reduction and clinical status and pain evaluation, we performed a systematic characterization of fiber peripheral density in a larger sample of carriers.
In the present study, we used corneal confocal microscopy (CCM), a clinical ophthalmic test for the assessment of small-fiber structure. This noninvasive method has been applied to quantify C-fiber loss in neuropathy secondary to diabetes and other etiologies (Einarsdottir et al. 2004; Minde et al. 2004, 2006, 2009; Quattrini et al. 2007; Tavakoli et al. 2009, 2010, 2012). To further investigate the contribution of C-fiber density to clinical pain assessment scores and subjective pain evaluation, we also administered the Neuropathy Disability Score (NDS; Young et al. 1993) and the Situational Pain Questionnaire (SPQ; Clark and Yang 1983).
METHODS
Participants.
Nineteen individuals (11 males; 3 homozygous carriers; mean age 50 ± 4.9 yr, mean ± SE) with a mutation of the NGFB gene were investigated in this study. Nineteen healthy age-matched controls for the CCM investigation and 12 healthy age-matched controls for the SPQ questionnaire were included in the analysis. Ethical approval was obtained by the ethics board of Gothenburg University. Participants gave informed consent in accordance with the Declaration of Helsinki and were compensated at 200 Swedish crowns per hour. The control subjects for the CCM investigation were recruited and assessed for a clinical study at the University of Manchester and are part of a normative data set for corneal nerve images (Tavakoli et al. 2015). The CCM data were collected in 2 days at the Department of Orthopedics, Gällivare Hospital, Sweden. The homozygous individuals (2 males born in 1990 and 1968, 1 female born in 1983) do not share parents and are only distantly related. Each individual presents with painless fractures, osteochondritis, bone necrosis, and neuropathic joint destruction. Compared with the homozygous carriers, the symptomatic heterozygote carriers in this sample have later onset of symptoms (20–70 yr) and less severe clinical signs. Although not suffering from painless fractures, they can manifest Charcot arthropathies at single or multiple joints, particularly in the lower extremities. Cognitive functions and basic reflexes are normal in carriers (Einarsdottir et al. 2004; Minde et al. 2004, 2006, 2009). A list of the most relevant clinical features of R221W carriers is provided in Table 1.
Table 1.
Clinical features of the NGFB carriers
| Mutation | Gender | DoB | Age of Onset, yr | Charcot Joint | Fractures | NDS | MNSI | CTS |
|---|---|---|---|---|---|---|---|---|
| Ho | F | 1983 | 7 | Knee, ankle, hip | Yes | 7 | 4.5 | No |
| Ho | M | 1990 | 4 | Knee, ankle, spine | Yes | 3 | 4.5 | No |
| Ho | M | 1948 | 7 | Knee, ankle, spine, elbow | Yes | 5 | 3 | No |
| He | M | 1996 | No | No | 0 | No | ||
| He | M | 1992 | No | No | 0 | 0 | No | |
| He | M | 1989 | No | No | 0 | 1 | ||
| He | M | 1985 | No | No | 0 | 0 | No | |
| He | M | 1981 | No | No | 0 | 0 | No | |
| He | F | 1967 | No | No | 0 | 2 | Yes | |
| He | F | 1962 | 15 | Hip | No | 1 | ||
| He | F | 1960 | No | No | 2 | 2 | Yes | |
| He | M | 1958 | No | No | 1 | 1 | Yes | |
| He | F | 1952 | 20 | Knee | No | 2 | 0 | No |
| He | M | 1951 | 20 | Knee | No | 0 | 1 | Yes |
| He | F | 1946 | Gonarthrosis | No | 1 | 2 | No | |
| He | F | 1942 | No | No | 5 | 3 | Yes | |
| He | F | 1936 | Gonarthrosis | no | 2 | 0 | No | |
| He | M | 1934 | No | No | 5 | 1.5 | Yes | |
| He | M | 1933 | 50 | Knee | No | 3 | Yes |
NGFB, nerve growth factor-β; DoB, date of birth; NDS, Neuropathy Disability Score; MNSI, Michigan Neuropathy Screening Instrument; CTS, carpal tunnel syndrome; Ho, homozgyous; He, heterozygous. MNSI scores were collected in a previous investigation (Larsson et al. 2009). CTS scores were collected in a separate study (Hellgren T, Svensson O, Minde J, unpublished data).
Corneal confocal microscopy.
Carriers and healthy subjects underwent corneal assessment with the Heidelberg retina tomograph (HRT III, Rostock cornea module) in vivo corneal confocal microscopy, based on an established protocol (Tavakoli and Malik 2011). The subjects' eyes were anesthetized using a drop of oxybuprocaine hydrochloride (0.4%; Bausch & Lomb), and Viscotears (carbomer polyacrylic acid, 2 mg/g; Alcon) was applied on the front of the eye for lubrication. For each subject, a sterile, disposable Perspex cap (TomoCap, Heidelberg Engineering) was placed over the objective lens, and a drop of Viscotears gel was placed on the tip of the lens. The subject was instructed to fixate on a target with the eye not being examined. Several scans of the entire depth of the cornea were recorded by turning the fine focus of the objective lens backward and forward for 2 min using the section mode, which enables manual acquisition and storage of single images of all corneal layers. This provides en face two-dimensional images with a lateral resolution of 2 mm/pixel and a final image size of 400 × 400 pixels of the subbasal nerve plexus of the cornea from each subject. Six images per subject from the center of the cornea were selected and examined in a masked and randomized fashion using purpose-written, proprietary software (CCMetrics; M. A. Dabbah, Imaging Science, University of Manchester). C-fibers are mainly located in the subbasal plexus between the Bowman's layer and the corneal basal epithelium, whereas Aδ-fibers are located in the stromal layer (Guthoff et al. 2005; Muller et al. 1997). Each main fiber is constituted of a bundle of unmyelinated axons (Al-Aqaba et al. 2010). Three corneal nerve parameters were quantified from the subbasal plexus: 1) corneal nerve fiber density (CNFD), the total number of main fibers per square millimeter of corneal tissue (Al-Aqaba et al. 2010); 2) corneal nerve branch density (CNBD), the number of branches emanating from all main fibers trunks per square millimeter of corneal tissue; and 3) corneal nerve fiber length (CNFL), the total length of all main fibers and branches (mm/mm2) within the area of corneal tissue. The presence and density of Langerhans cells (LCs) in the same images from Bowman's layer were also assessed (Tavakoli et al. 2011). In addition, the presence of Aδ-fibers was also assessed via the examination of images from the stromal layer, although in this study an exact quantification was not performed due to current methodological limitations (Patel and McGhee 2009). Stromal rather than subbasal nerves appear more robust in surviving postmortem change; therefore, most of our knowledge from corneal A δ-fibers is from in vitro studies (Al-Aqaba et al. 2010).
Corneal sensitivity.
Corneal sensitivity was quantified using a non-contact corneal aesthesiometer (NCCA; Glasgow Caledonian University), which uses a puff of air through a 0.5-mm-diameter bore, lasting 0.9 s, and exerts a force expressed in the millibar range. The stimulus jet is positioned 1 cm from the eye, and the air jet is aligned to the center of the cornea. The subject feels and acknowledges a sensation on the cornea, which is most commonly described as being “cold” or as a “'breeze.” With the use of a staircase method, each subject is presented with a supramaximal stimulus followed by stimuli of reduced strength until the subject does not feel the air jet anymore. The whole process is repeated three times to derive a threshold. The coefficient of variation for NCCA was 5.6%. Both eyes were tested, and the results are the average of both eye assessments.
Neuropathy Disability Score.
The NDS is a widely used tool that assesses neuropathy severity and is based on clinical neurological examination findings. It includes evaluation of vibration, pin prick, and temperature perception as well as the presence or absence of ankle reflexes to establish the severity of neuropathy: NDS 0–2, no neuropathy; NDS 3–5, mild neuropathy; NDS 6–8, moderate neuropathy; and NDS 9–10, severe neuropathy.
Situational Pain Questionnaire.
A Swedish translation of the original 30-item SPQ was administered to the subjects (Clark and Yang 1983). The SPQ consists of a series of situations describing low-pain (“I have been bitten by a mosquito”) or high-pain events (“The dentist drills in one of my teeth without anesthesia”). Subjects estimate the amount of pain they might feel in those situations on a Likert scale from 1 (“non-noticeable”) to 10 (“worst possible pain”). The SPQ addresses the ability of the subjects to discriminate hypothetical painful from nonpainful situations as well as the amount of pain they think they would experience in them. The interpretation of the SPQ is based on a model of signal detection theory that allows the quantification of the ability to discriminate between the two categories of events and the amount of pain perceived via two scores: P(A) and B (Danziger et al. 2006; Green and Swets 1966; Wickens 2001). P(A) captures the degree of discrimination between high-pain and low-pain situations, whereas the B score reflects the degree to which situations are considered as painful. A high B score reflects an underestimate of pain, whereas a low score reflects a good estimate of pain (for more details, see Danziger et al. 2006).
Data analysis.
Statistical comparisons between homozygous carriers, heterozygous carriers, and controls were performed with the Statistical Package for the Social Sciences (SPSS, Chicago, IL; version 20) using the nonparametric independent-samples Kruskal-Wallis and Mann-Whitney tests. Of all the CCM parameters investigated, particular focus was put on the nerve fiber density (CNFD). Correlation between CNFD, NDS, and SPQ results was investigated with a Spearman's correlation test.
RESULTS
NGFB carriers show a reduced density of C-fibers.
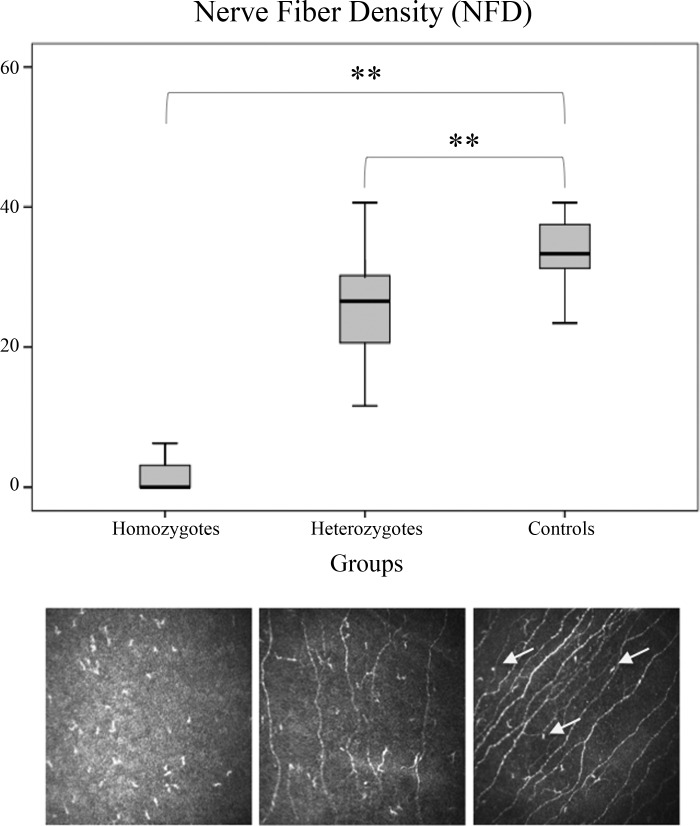
The R221W mutation carriers showed a significant decrease in C-fiber density (CNFD) compared with healthy individuals [χ2(2) = 16.2, P = 0.0003, with a mean rank score of 2 for the homozygous carriers, 15.16 for the heterozygous carriers, and 25.92 for the controls; Fig. 1]. For normative values, see also Tavakoli et al. (2015). The CNFD scores varied greatly between the carriers with the lowest value of 0 in two of the homozygous and the maximum value of 40 in one of the male heterozygous, although the nonparametric Levene's test showed no significant difference in the homogeneity of variance (P = 0.1). In addition, nerve branch density and nerve fiber length were also significantly reduced in the carriers (see Table 2). In seven of the carriers, no Aδ-fibers were observed, although an exact quantification in this case was not methodologically possible. Langerhans cell presence and density of cells was significantly higher in the carriers compared with the control subjects (P < 0.001). No difference in corneal nerve parameters between males and females (P > 0.1) was observed. CNFD correlated positively with CNBD (rs = 0.62, P < 0.01) and CNFL (rs = 0.86, P < 0.01). Corneal sensitivity correlated significantly with CNFD (rs = −0.66, P < 0.01) and CNFL (rs = −0.52, P < 0.059).
Fig. 1.
Box plot showing CNFD values (top) and CCM images (bottom) for homozygous (left), heterozygous (center), and control (right) subjects. NGFB carriers show a significant reduction of C-afferent density in the cornea. **P < 0.01. Arrows indicate Langerhans cells.
Table 2.
Corneal sensitivity and corneal nerve morphological parameters in NGFB carriers and controls
|
P Value |
||||||
|---|---|---|---|---|---|---|
| Parameter | Homozygous Carriers | Heterozygous Carriers | Controls | Homozygous vs. Heterozygous | Control vs. Homozygous | Control vs. Heterozygous |
| NCCA-R, mbar | 5.55 (1.54–12.50) | 1.30 (0.68–4.85) | 0.56 (0.12–0.88) | 0.073 | 0.006 | <0.001 |
| CNFD, fibers/mm2 | 0 (0–6.25) | 26.56 (11.61–40.62) | 33.33 (23.44–40.62) | 0.007 | 0.006 | 0.002 |
| CNBD, nerve branches/mm2 | 0 (0–0) | 10.68 (2.08–23.96) | 58.33 (15–183.33) | 0.007 | 0.006 | <0.001 |
| CNFL, mm/mm2 | 0 (0–1.42) | 12.22 (3.87–16.05) | 23.73 (13.94–32.58) | 0.007 | 0.006 | <0.001 |
| LCs, %presence | 100 | 93.7 | 20 | |||
| LC density, cells/mm2 | 158.13 ± 83.46 | 93.09 ± 24.83 | 14.1 ± 4.13 | 0.342 | 0.0001 | 0.0001 |
| Stromal Aδ-fibers, %presence | 0 | 56 | 100 | |||
Data are medians with interquartile range (IQR) in parentheses, percentages, or means ± SE for parameters in homozygous carriers (n = 3), heterozygous carriers (n = 16), and healthy controls (n = 19). NCCA-R, non-contact corneal aesthesiometer-right side; CNFD, nerve fiber density; CNBD, nerve branch density; CNFL, nerve fiber length; LC, Langerhans cells.
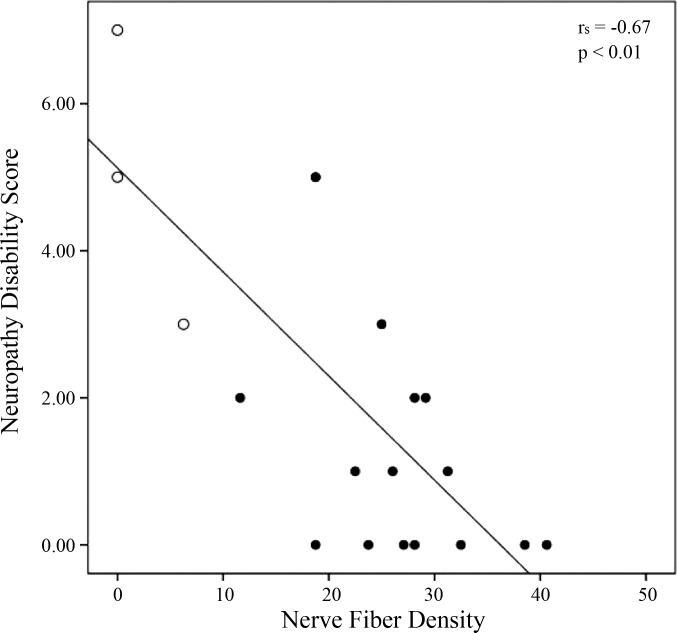
Nerve fiber density correlates with NDS.
The maximum disability score is 10, which would indicate complete loss of sensation to all sensory modalities and absent reflexes. The carriers show a significant negative correlation between peripheral C-fibers density to NDS (rs = −0.67, P < 0.01; Fig. 2). Although the NDS test is not specific for fiber type, these findings suggest a relationship between peripheral density and severity of the neuropathy: the lower the density of small fibers, the higher the severity of the neuropathy.
Fig. 2.
Correlation between CNFD and NDS scores. The carriers show a significant negative correlation between C-fiber density and the severity of neuropathic symptoms (rs = −0.67, P < 0.01). Open circles represent homozygous carriers' scores.
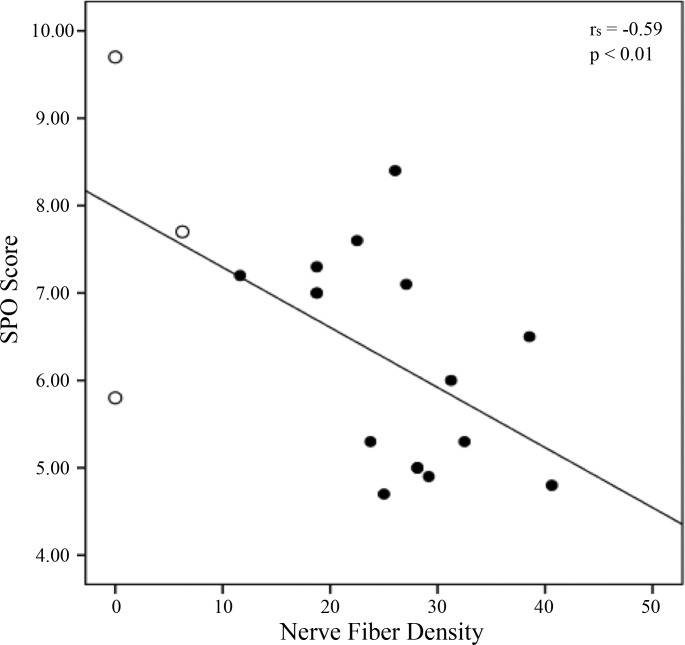
Nerve fiber density correlates with estimation of pain.
The SPQ looks at the ability to discriminate painful vs. innocuous situations via the P(A) score and at the intensity of the imagined pain via the B score. Median scores for P(A) = 0.91 [interquartile range (IQR) = 0.7–0.9] and B = 6.50 (IQR = 5.1–7.2) were observed in the carriers. More specifically, the homozygous carriers (n = 3) scored P(A) = 0.74 (IQR = 0.7–0.8) and B = 7.67 (IQR = 6.7–8.7), whereas the heterozygous carriers (n = 16) scored P(A) = 0.91 (IQR = 0.8–0.9) and B = 6.25 (IQR = 5–7.1). Scores from control subjects (n = 12) were also considered for comparisons: P(A) = 0.92 (IQR = 0.91–0.97) and B = 5 (IQR = 4.8–6.1). The homozygous carriers scored a significantly higher B value than controls (U = 4, P = 0.04), indicating a lower degree of pain imagined in highly painful situations, whereas a trend for significance was seen between the heterozygous and the controls (U = 66, P = 0.053). Regarding the P(A) score, no significant difference was found between the three groups (P = 0.05) and no correlation was observed between the CNFD score and the P(A) value (P > 0.05), indicating that the ability to discriminate between imaginary painful and innocuous situations was intact and did not correlate to the density of C-fibers at the periphery. However, a significant negative correlation between nerve fiber density and B score (rs = −0.59, P < 0.01; Fig. 3) was observed in the carriers, indicating that, on average, the lower the degree of pain imagined, the greater the loss of small fibers measured in the cornea.
Fig. 3.
Correlation between CNFD and SPQB scores. The degree of pain imagined is negatively correlated to the average loss of C-fibers in the carriers (rs = −0.59, P < 0.01). Open circles represent homozygous carriers' scores.
DISCUSSION
These findings indicate that C-afferent nerve fiber density affects acute pain evaluation in carriers of the R221W mutation. Individual variability of carriers' CNFD is related to variability on clinical and subjective measures of acute pain (NDS and SPQ). This is the first study to show a direct relationship between peripheral C-fiber afferent density, assessed using CCM, and the evaluation of acute pain. The cornea is the most highly innervated tissue in the human body. It contains myelinated Aδ-fibers, which are large-diameter (6 μm), straight nerves that respond primarily to mechanical stimuli, and unmyelinated C-fibers, which are small-diameter (2–4 μm), beaded nerves that respond to thermal and chemical stimuli (Muller et al. 1997). The nerves penetrate the cornea radially, and ∼70–80 nerve trunks, which contain 900-1,200 myelinated and unmyelinated axons, enter the midstroma (Muller et al. 1997). The myelinated nerve bundles lose their myelin sheaths and perineurium early after entering the stroma, and all the nerve bundles lose their Schwann cell sheath before penetrating Bowman's layer to enter the epithelium, allowing the cornea to remain transparent (Tavakoli and Malik 2011).
The homozygous carriers showed a marked reduction of small fibers compared with both heterozygous carriers and healthy controls, with almost a complete lack of C-afferents in the cornea. The heterozygous group was more variable, with individual values ranging from low CNFD approaching that of the homozygote group to high CNFD approaching that of healthy controls. This suggests that in homozygotes the consequences of the R221W mutation on thin-diameter sensory afferents are severe and definite. In contrast, the consequences are milder on average, yet more variable, in the heterozygote group.
The density of corneal nerve fibers (CNFD) was negatively correlated with NDS scores of neuropathy severity in the carrier group, indicating that the lower the CNFD, the higher the severity of symptoms. The SPQ questionnaire provided a self-reported measure of pain intensity estimates in the context of potential painful situations. The groups did not significantly differ on the ability to distinguish written descriptions of painful from nonpainful situations [SPQ P(A)]. However, differences in pain intensity estimates for painful situations (SPQ B) were observed, especially in the homozygotes whose ratings were significantly lower than the controls. This might indicate a general pain underestimation bias among carriers as a group, which is especially pronounced among the homozygotes. In the whole carrier group, this underestimation bias also correlated with small-fiber CNFD: the lower the amount of fibers in the periphery, the lower the pain intensity estimates.
The R221W carriers' reduced small-fibers density was previously assessed through sural biopsies in six carriers (Minde et al. 2009). Although crucial in characterizing the peripheral physiology of the R221W mutation, this assessment was limited in scope, partly due to the invasive nature of nerve biopsy. Furthermore, the biopsy data were skewed to a more severely affected sample, consisting of three homozygotes and three heterozygotes. CCM allowed us to noninvasively assess more detailed morphological properties of small-fiber epithelial C-afferents in a broader sample and to take variability into account, especially among heterozygote carriers.
The haplotype of the R221W mutation is on chromosome 1p11.2-p13.2, restricted to the 8.3-Mb region, flanked by the single nucleotide polymorphism (SNP) markers rs2490334 and rs2275607. The mutation involves a basic arginine (CGG) to a nonpolar tryptophan (TGG) substitution at position 221 in the NGFB amino acid sequence. Rat cell line models suggest that this missense point mutation affects the cleavage of pro-NGF and that the resulting intracellular accumulation of pro-NGF limits the availability of mature NGF in the extracellular space (Carvalho et al. 2011; Larsson et al. 2009).
NGF plays a role not only in trophic support and cell differentiation in development but also in pain and inflammation in adulthood (Capsoni et al. 2011; Lewin and Moshourab 2004). The R221W mutation carriers' phenotype may thus be a consequence of lack of trophic support during development, resulting in reduced nociceptive afferent density. It may also involve altered regulatory signaling in nociceptive pathways during pain and injury. NGF binding to the TrkA receptor was unchanged in rat cell lines and transgenic mouse models of the mutation (Capsoni et al. 2011; Larsson et al. 2009). However, in the transgenic mouse model (R100W), the mutated form of NGF exhibited a reduced ability to activate downstream TrkA-dependent signaling pathways, in particular PLC-γ, suggesting that the carriers' phenotype might be explained by altered nociceptive regulatory actions (Capsoni et al. 2011; Larsson et al. 2009).
In the human carriers, however, the wide phenotypic variance and the sharp differences in clinical status between heterozygote and homozygote carriers suggest a gene dosage or codominance effect. Heterozygote carriers do not manifest severe pain indifference, inflammation, or arthropathy, although as a group they show subtle effects that are related to their degree of epithelial C-afferent innervation, and some individuals develop arthropathies with increasing age (Minde et al. 2009). In this study, corneal fiber loss was regarded as an index of the general reduction in nerve fibers that was observed in the sural nerve biopsy samples (Minde et al. 2004). It is reasonable to expect that such a general, congenital reduction of nociceptive fibers would result in atypical functional physiology of the nerve pathways, including downstream signaling in the relevant subcortical nuclei, as well as the relevant cortical projections. We thus predicted that the R221W mutation would not only affect nociceptive processing at the level of sensory perception but also bias pain processing at the level of experience, memory, and behavior. The results from the SPQ show that carriers are biased to underestimate the painfulness of hypothetical situations, despite being able to accurately differentiate painful from nonpainful situations. This indicates that the reduced CNFD associated with the R221W mutation might bias pain processing at the central, and probably cortical, level. This central-level bias may also influence carriers' behavioral responses to pain; for example, underestimation of acute pain could imply underreaction to pain-relevant stimuli.
The extreme inflammation and injury in homozygote carriers' load-bearing joints may reflect the cumulative effects of even subtle deficits in the ability to produce appropriate and timely behavioral responses to nociceptive signals. Radin et al. (1990) termed this “microklutziness.” In this scenario, arthropathic symptoms are the result of wear and tear on the joints as a consequence of poor behavioral adjustments to pain and discomfort, creating negative feedback loops of inflammation and further injury. We observed a high incidence of Langerhans cells in the carriers, a type of epithelial dendritic cell (Sere et al. 2012). These antigen-presenting dendritic cells are released as part of the immune response to injury and lodge throughout the body. This may bear some relationship to the carriers' history of pain events. However, Langerhans density did not correlate with surgical history or any of the measures collected in this study.
CCM allows the collection of several parameters, of which we focused only on the CNFD since evidence from previous studies had shown reduced small fibers' density in the carriers (Minde et al. 2004). Future studies should provide further insights on whether other CCM parameters might correlate to components of pain perception.
This study demonstrates that corneal confocal microscopy can be used as a noninvasive technique to assess relationships between afferent density and morphology and clinical or preclinical presentation in pain syndromes or in neuropathic conditions with consequences for patient pain. It reveals a relationship between CNFD and clinical, subjective, and behavioral pain outcomes. These findings have wider implications for understanding the contribution of peripheral afferent populations to pain-related pathways across levels of the nervous system and the nociceptive mechanisms involved in acute, superficial pain evaluation.
GRANTS
The study was funded by Swedish Research Council Distinguished Young Investigator Award FF-2013-687 (to I. Morrison). M. Tavakoli acknowledges support from the National Institute for Health Research Wellcome Trust Clinical Research Facility. M. Tavakoli acknowledges and is grateful for the support of Heidelberg Engineering UK and Sweden.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.P., A.M., and I.M. conception and design of research; I.P. and M.T. analyzed data; I.P., M.T., A.M., J.M., and I.M. interpreted results of experiments; I.P. prepared figures; I.P. and I.M. drafted manuscript; I.P., M.T., A.M., J.M., and I.M. edited and revised manuscript; I.P., M.T., A.M., J.M., and I.M. approved final version of manuscript; M.T. and A.M. performed experiments.
ACKNOWLEDGMENTS
We thank the study participants for their time and for supporting the study.
REFERENCES
- Al-Aqaba MA, Alomar T, Miri A, Fares U, Otri AM, Dua HS. Ex vivo confocal microscopy of human corneal nerves. Br J Ophthalmol 94: 1251–1257, 2010. [DOI] [PubMed] [Google Scholar]
- Capsoni S, Covaceuszach S, Marinelli S, Ceci M, Bernardo A, Minghetti L, Ugolini G, Pavone F, Cattaneo A. Taking pain out of NGF: a “painless” NGF mutant, linked to hereditary sensory autonomic neuropathy type V, with full neurotrophic activity. PLoS One 6: e17321, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho OP, Thornton GK, Hertecant J, Houlden H, Nicholas AK, Cox JJ, Rielly M, Al-Gazali L, Woods CG. A novel NGF mutation clarifies the molecular mechanism and extends the phenotypic spectrum of the HSAN5 neuropathy. J Med Genet 48: 131–135, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WC, Yang JC. Applications of sensory detection theory to problems in laboratory and clinical pain. In: Pain Measurement and Assessment, edited by Melzack R. New York: Raven, 1983, p. 15–25. [Google Scholar]
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD, Phillips HS. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 76: 1001–1011, 1994. [DOI] [PubMed] [Google Scholar]
- Danziger N, Prkachin KM, Willer JC. Is pain the price of empathy? The perception of others' pain in patients with congenital insensitivity to pain. Brain 129: 2494–2507, 2006. [DOI] [PubMed] [Google Scholar]
- Einarsdottir E, Carlsson A, Minde J, Toolanen G, Svensson O, Solders G, Holmgren G, Holmberg D, Holmberg M. A mutation in the nerve growth factor beta gene (NGFB) causes loss of pain perception. Hum Mol Genet 13: 799–805, 2004. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: John Wiley and Sons, 1966. [Google Scholar]
- Guthoff RF, Wienss H, Hahnel C, Wree A. Epithelial innervation of human cornea: a three-dimensional study using confocal laser scanning fluorescence microscopy. Cornea 24: 608–613, 2005. [DOI] [PubMed] [Google Scholar]
- Larsson E, Kuma R, Norberg A, Minde J, Holmberg M. Nerve growth factor R221W responsible for insensitivity to pain is defectively processed and accumulates as proNGF. Neurobiol Dis 33: 221–228, 2009. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Moshourab R. Mechanosensation and pain. J Neurobiol 61: 30–44, 2004. [DOI] [PubMed] [Google Scholar]
- Minde J, Andersson T, Fulford M, Aguirre M, Nennesmo I, Remahl IN, Svensson O, Holmberg M, Toolanen G, Solders G. A novel NGFB point mutation: a phenotype study of heterozygous patients. J Neurol Neurosurg Psychiatry 80: 188–195, 2009. [DOI] [PubMed] [Google Scholar]
- Minde J, Svensson O, Holmberg M, Solders G, Toolanen G. Orthopedic aspects of familial insensitivity to pain due to a novel nerve growth factor beta mutation. Acta Orthop 77: 198–202, 2006. [DOI] [PubMed] [Google Scholar]
- Minde J, Toolanen G, Andersson T, Nennesmo I, Remahl IN, Svensson O, Solders G. Familial insensitivity to pain (HSAN V) and a mutation in the NGFB gene. A neurophysiological and pathological study. Muscle Nerve 30: 752–760, 2004. [DOI] [PubMed] [Google Scholar]
- Minde JK. Norrbottnian congenital insensitivity to pain. Acta Orthop 77: 2–32, 2006. [PubMed] [Google Scholar]
- Muller LJ, Vrensen GF, Pels L, Cardozo BN, Willekens B. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci 38: 985–994, 1997. [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol 11: 272–280, 2001. [DOI] [PubMed] [Google Scholar]
- Patel DV, McGhee CJ. In vivo confocal microscopy of corneal stromal nerves in patients with peripheral neuropathy. Arch Neurol 66: 1179–1180, 2009. [DOI] [PubMed] [Google Scholar]
- Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, Marshall A, Boulton AJ, Efron N, Malik RA. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes 56: 2148–2154, 2007. [DOI] [PubMed] [Google Scholar]
- Radin EL, Yang KH, Riegger C, Kish VL, O'Connor JJ. Relationship between lower limb dynamics and knee joint pain. J Orthop Res 9: 398–405, 1991. [DOI] [PubMed] [Google Scholar]
- Sere K, Baek JH, Ober-Blobaum J, Muller-Newen G, Tacke F, Yokota Y, Zenke M, Hieronymus T. Two distinct types of Langerhans cells populate the skin during steady state and inflammation. Immunity 37: 905–916, 2012. [DOI] [PubMed] [Google Scholar]
- Tavakoli M, Boulton AJ, Efron N, Malik RA. Increased Langerhan cell density and corneal nerve damage in diabetic patients: role of immune mechanisms in human diabetic neuropathy. Cont Lens Anterior Eye 34: 7–11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli M, Ferdousi M, Petropoulos IN, Morris J, Pritchard N, Zhivov A, Ziegler D, Pacaud D, Romanchuk K, Perkins BA, Lovblom LE, Bril V, Singleton JR, Smith G, Boulton AJ, Efron N, Malik RA. Normative values for corneal nerve morphology assessed using corneal confocal microscopy: a multinational normative data set. Diabetes Care 38: 838–843, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli M, Marshall A, Banka S, Petropoulos IN, Fadavi H, Kingston H, Malik RA. Corneal confocal microscopy detects small-fiber neuropathy in Charcot-Marie-Tooth disease type 1A patients. Muscle Nerve 46: 698–704, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli M, Marshall A, Thompson L, Kenny M, Waldek S, Efron N, Malik RA. Corneal confocal microscopy: a novel noninvasive means to diagnose neuropathy in patients with Fabry disease. Muscle Nerve 40: 976–984, 2009. [DOI] [PubMed] [Google Scholar]
- Tavakoli M, Quattrini C, Abbott C, Kallinikos P, Marshall A, Finnigan J, Morgan P, Efron N, Boulton AJ, Malik RA. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care 33: 1792–1797, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli M, Malik RA. Corneal confocal microscopy: a novel non-invasive technique to quantify small fibre pathology in peripheral neuropathies. J Vis Exp: 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens TD. Elementary Signal Detection Theory. New York: Oxford University Press, 2001. [Google Scholar]
- Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 36: 150–154, 1993. [DOI] [PubMed] [Google Scholar]