Abstract
Leukoplakia is the most common precursor lesion of oral squamous cell carcinoma (OSCC). Currently, the risk of progression to OSCC is assessed based on histopathologic examination alone. However, this method fails to identify the subset of microscopically innocuous leukoplakia that ultimately transforms to OSCC. The aim of this study was to determine if microRNAs (miRNAs) can be utilized to identify non- and low-grade dysplastic oral lesions at risk for cancer progression. A retrospective study of genome-wide miRNA expression level analyses was performed in the training cohort (n=20) using deep sequencing formalin-fixed paraffin embedded incisional biopsy tissues from patients with oral leukoplakic lesions diagnosed with non- or low-grade dysplasia and known clinical outcome. The promising miRNA candidates were then evaluated in the validation cohort (n=80) using quantitative real-time PCR (qRT-PCR). Four promising miRNAs-208b-3p, 204-5p, 129-2-3p and 3065-5p were identified. Combining these four miRNAs as a panel with age and histologic diagnosis (p<0.004), our final model had a predictive value for the area under the receiver operating characteristic (ROC) curve (AUC) of 0.792, sensitivity of 76.9% and specificity of 73.7% to accurately identify non- and low-grade dysplastic lesions at risk of cancer progression, which is a significant improvement over histopathologic examination alone (AUC of 0.645). While further investigation is needed, discovery of predictive markers that can accurately identify histologically innocuous oral lesions at high risk for progression to OSSC will significantly improve clinical outcome by means of early intervention.
Keywords: microRNA, deep sequencing, qRT-PCR, predictive markers, oral leukoplakia, oral epithelial dysplasia, oral squamous cell carcinoma
Introduction
Oral squamous cell carcinoma (OSCC) is the 6th leading cause of cancer related deaths worldwide [1,2]. Over 30,000 people in the United States are diagnosed with OSCC each year [1-4]. Half of those newly diagnosed with oral cancer will die of the disease [1-4]. Since the majority of OSCC develop from a precursor lesion [4,5], accurate identification, followed by complete surgical removal of the precursor lesion will effectively halt its malignant progression, offering the best hope at reducing OSCC associated morbidity and mortality.
Most precursor lesions of OSCC clinically present as a white patch known as leukoplakia [4-16]. Upon microscopic examination, most leukoplakia (80%) appear to be non-dysplastic or low-grade dysplastic lesions, whereupon the patients are diagnosed with epithelial hyperplasia and/or hyperkeratosis and placed under ‘close observation’ without further treatment [14]. Moderate to high-grade epithelial dysplasia is seen in ~17% of leukoplakia [15], in which case the lesion is completely removed by surgery [16]. The remaining 3% of cases are OSCC [14]. While moderate to high-grade epithelial dysplasia will most likely progress to OSCC within 4-5 years, some of the non-dysplastic and low-grade dysplastic lesions also undergo malignant transformation [8-16]. Hence histopathologic examination alone is insufficient for identification of leukoplakic lesions that will progress of OSCC, especially if the leukoplakia lacks atypical cellular features at the time of initial histopathologic assessment.
It is these histologically innocuous lesions that are at greatest need for intervention since without the presence of significant histologic dysplasia, these lesions could be erroneously interpreted as being harmless and not managed properly. Currently there are no established guidelines for managing non- and low-grade dysplastic lesions. Treatment options vary from no treatment to complete surgical excision to laser ablation [17]. Moreover, there is no established guideline for patient follow-up duration or frequency [17]. Accurate identification of non-dysplastic and low-grade dysplastic lesions at risk for malignant transformation will ensure proper clinical management of these premalignant lesions.
We assessed microRNA (miRNA)-based markers that can increase the predictive value when combined with histopathologic examination to accurately identify non- or low-grade dysplastic lesion that will ultimately progress to OSCC. miRNAs are small non-coding RNA molecules that function as post-transcriptional regulators capable of causing both gene silencing and activation [18]. They are involved in multiple critical biological processes, including cellular proliferation, apoptosis, differentiation, and carcinogenesis [19-22]. A limited number of studies have examined differential miRNA expression levels in precancerous lesions in the esophagus, cervix, lung and stomach [23-28]. While there are reports of miRNA expression profiles in oral leukoplakia that progressed to OSCC [29-36], only a couple of these studies focused on low-grade dysplastic lesions that progressed to cancer [34-36]. Moreover, while the majority of other studies of oral leukoplakia utilized TaqMan low density arrays, we employed genome wide deep sequencing to determine a miRNA expression profile unique to histologically innocuous (non- and low-grade dysplastic lesions) leukoplakias that ultimately progress to cancer. The advantage of this next-generating sequencing technology is that it is capable of detecting all miRNAs present in the samples, thereby increasing testing coverage [37]. Development of a clinically applicable method to identify histologically innocuous precancerous lesions will be a valuable modality for clinicians in guiding patient management.
Materials and methods
Subjects
Following institutional review board approval at the Columbia University Medical Center, we conducted a retrospective search of our pathology database to identify 100 adult patients ≥21 years old, with a clinical leukoplakia diagnosed as ‘epithelial hyperplasia’, ‘epithelial hyperplasia with hyperkeratosis’, ‘epithelial atypia limited to the basal cell region’ or ‘mild epithelial dysplasia’ prior to 2008 and which also had a minimum of 5-year follow-up information available. Only the patients who had an incisional biopsy of the leukoplakia were selected. As an excisional biopsy could account for the reason why the leukoplakia did not progress to cancer, the patients who had excisional biopsy were excluded from the study. Identified patients were stratified into the following two groups; Group 1 ‘Progressive Group’ (patients with leukoplakia that progressed to OSCC within 5 years) and Group 2 ‘Non-Progressive Group’ (patients with leukoplakia that did not progress to OSCC within 5 years). The age and gender of the patient, histologic diagnosis, and the location of lesion were recorded. Archived formalin-fixed paraffin-embedded (FFPE) tissue blocks were retrieved for all subjects.
Total RNA extraction
Ten 10-μm sections were obtained from archived FFPE precursor tissue samples for all subjects. For each sample, a representative section was stained with H&E and reviewed by a pathologist to confirm the histologic diagnosis. Total RNA was isolated from tissues using RNeasy FFPE kit (Qiagen Inc., Valencia, CA) following the manufacturer’s protocol and yield was quantitated by Nanodrop, and samples were stored at -80°C.
microRNA quality control, library preparation and sequencing
Deep sequencing analysis was performed as previously described [38] in the training set of 20 patients; 10 from Group 1 (progressive group) and 10 from Group 2 (non-progressive group). For quantification and quality control, total RNAs were tested using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) and the Qubit 2.0 Fluorometer (Life Technologies, Grand Island, NY). Total RNA (minimum of 2 μg) ranging from 18 to 30 nt were gel-purified and ligated to 3’ and 5’adaptors. Ligation products were reverse transcribed, then amplified for 16 cycles using the adaptor primers, and the fragments around 150 bp were isolated from PAGE-gels using a TruSeq Small RNA Sample Prep Kit (Illumina, San Diego, CA). Libraries were sequenced on an Illumina HiSeq 2500 platform-50 SR that allows for 1x50 base-pair single-end reads in the New York Genome Center. The data was deposited in the publicly available Gene Expression Omnibus (GEO) database (GSE62809).
microRNA mapping and differential expression analysis
Adaptors were removed and low quality tags were filtered with FASTX-Toolkit (http://hannonlab.cshl.edu/fastx_toolkit/). Reads were processed with the pipeline miraligner, which mapped them to the miRBase v.20 sequences. The number of reads per miRNA was first assessed and analyzed using the bioconductor package DESeq to compare miRNA expression between the two groups. In addition to normalizing between the samples, DESeq performed a statistical test of differential expression under the hypothesis of a negative binomial distribution of the reads. To select the top miRNAs with prognostic value, those with significant (unadjusted p<0.05) and >1.25 log2-fold change of the different mean normalized expression levels between the two groups were identified. The association between selected miRNAs and their role in carcinogenesis was also investigated through a literature search.
qRT-PCR for microRNAs
Selected top-ranked miRNAs were quantified using TaqMan MicroRNA Assays Kits (Applied Biosystems, Foster City, CA) as described previously [38], in the validation set with 80 patients, consisting of 40 patients in Group 1 (progressive cases) and 40 patients in Group 2 (non-progressive cases). Input RNA was reverse transcribed using the TaqMan miR Reverse Transcription Kit and miR-specific stem-loop primers for the selected four miRs in a small-scale RT reaction. For quantification, diluted RT product was combined with PCR assay reagents and real-time PCR carried out on an ABI7900HT thermocycler. The endogenous control (RNU48) showed even expression levels between the two groups. Test samples were assayed in duplicate with the laboratory blinded to survival status and with 5% triplication after relabeling. The coefficient of variation was calculated and values <5% was considered acceptable. Data was analyzed with SDS Relative Quantification Software version 2.2.2 (Applied BioSystems) to determine the threshold cycle (Ct). The fold change was determined by the 2-ΔΔCt method [39]. A two-sample t test was used to compare the normalized expression levels between the two groups.
microRNA target prediction
MicroRNA target analysis was performed by the TargetScanHuman release 7.0 (www.targetscan.org) to compare potential target genes affected by the top four miRNAs with the NCBI reference (human genome build 36). The biologic processes, molecular functions, cellular components, and protein classes of these genes were examined utilizing the Panther web site (Supplemental Table 1).
Statistical analysis
Univariate logistic regression models were first used to test if the selected miRNAs were independent factors of 5-year progression status. A multiple logistic regression model was then used to build a prediction model. Histologic findings were incorporated into the model by assigning a numeric value of ‘1’ to diagnoses of hyperkeratosis, ‘2’ to epithelial hyperplasia with hyperkeratosis, ‘3’ to epithelial atypia and ‘4’ to mild epithelial dysplasia. Using the multiple logistic regression model, we constructed receiver-operating characteristic (ROC) curves and calculated the area under the curve (AUC) and the sensitivity and specificity with 0.5 predicted probability of progression to OSCC as the cutoff point. P<0.05 was considered statistically significant. Statistical analyses were conducted using SAS 9.3 (SAS Institute).
Results
Deep sequencing, mapping of miRNAs and selection of predictive miRNAs
Age, gender, histopathologic diagnosis and location of lesion are listed in Table 1 for the 20 subjects in the training set and 80 subjects in the validation. For deep sequencing, the total number of reads obtained ranged from 3,229,855 to 39,216,964 for the 20 tissue samples. After removing adaptors and filtering out low quality tags, 973,424 - 31,446,445 clean reads were obtained (~30.14-80.19%). From these reads, the mapping rate to miRNA ranged from 0.89 to 12.71, and the rate to miRalligner, which distinguish -3p and -5p sequences, ranged from 1.48 to 14.75. The length distribution analysis revealed a peak at 22 nt, which is the size of most known microRNAs. For each sample, ~30 thousand to 11 million sequence reads mapped to the human genome were obtained, which included miRNA, rRNA, Mt_rRNA, snoRNA, snRNA, tRNA. A total of 1755 miRNAs were detected in each sample using the miRaligner and the number of reads of each miRNA ranged from 0 to 138,335.
Table 1.
Demographic and clinicopathologic characteristics of training and validation sets
| Training Set (n=20) | Validation Set (n=77) | ||||
|---|---|---|---|---|---|
|
|
|||||
| Non-progressive Group (n=10) | Progressive Group (n=10) | Non-progressive Group (n=39) | Progressive Group (n=38) | P-value* | |
| Age mean (SD) | 58.9 (11.1) | 63.2 (23.2) | 59.62 (12.6) | 66.5 (17.5) | 0.050 |
| Gender % male | 40% | 50% | 33% | 35% | 0.813 |
| Histologic Diagnosis | 0.058 | ||||
| Hyperkeratosis | 1 (10%) | 2 (20%) | 17 (43%) | 11 (28%) | |
| Epithelial hyperplasia and hyperkeratosis | 3 (30%) | 3 (30%) | 9 (23%) | 6 (15%) | |
| Epithelial atypia of the basal cell layer | 4 (40%) | 2 (20%) | 10 (25%) | 9 (23%) | |
| Mild epithelial dysplasia | 2 (20%) | 3 (30%) | 4 (10%) | 14 (35%) | |
| Location | 0.256 | ||||
| High risk site (tongue, floor of mouth) | 7 (70%) | 7 (70%) | 21 (53%) | 26 (65%) | |
| Low risk site (buccal mucosa, vestibule, gingiva, palate, lip mucosa) | 3 (30%) | 3 (30%) | 19 (47.5%) | 14 (35%) | |
Two-sample t-test was used to compare means between non-progressive and progressive groups and Pearson’s Chi-square test was used to compare proportions between non-progressive and progressive groups within the validation set (n=80).
A total of 4 miRNAs (miRs-129-2-3p, 204-5p, 208b-3p and 3065-5p) were identified to be differentially expressed in the progressive group (Group 1) compared to non-progressive group (Group 2) with at least 1.25 log2 fold change with unadjusted p<0.05 and to be expressed in close to (at least 80%) if not all samples. The mean expression levels and the fold changes of the 4 selected miRNAs are listed in Table 2. There were no miRNAs with false discovery rate adjusted p<0.05. One of the miRNAs (miR-208b-3p) was overexpressed in the progressive group and 3 were underexpressed (miRs-129-2-3p, 204-5p and 3065-5p) in the progressive group as shown in Figure 1.
Table 2.
Mean expression levels of the 4 selected miRNAs and the fold change between the two groups by deep sequencing in the testing set with 20 patients and qRT-PCR in the validation set with 77 patients
| miRNAs | Methods | Non-progressive Group mean (SD) | Progressive Group mean (SD) | Fold-change | P-value** |
|---|---|---|---|---|---|
| miR-129-2-3p | Deep Seq (n=20) | 13.28 (9.67) | 2.75 (2.78) | 0.21 | 0.0001 |
| qRT-PCR (n=77) | 6.81 (1.98) | 6.21 (1.42) | 1.36 | 0.361 | |
| miR-204-5p | Deep Seq (n=20) | 848.03 (1117) | 239.16 (537.9) | 0.28 | 0.007 |
| qRT-PCR (n=77) | 3.55 (1.83) | 2.88 (1.70) | 1.60 | 0.250 | |
| miR-208b-3p | Deep Seq (n=20) | 51.86 (58.46) | 132.73 (169.1) | 2.56 | 0.003 |
| qRT-PCR (n=77) | 6.24 (1.89) | 5.43 (1.99) | 1.73 | 0.049 | |
| miR-3065-5p | Deep Seq (n=20) | 14.03 (9.36) | 5.01 (3.47) | 0.36 | 0.005 |
| qRT-PCR (n=77) | 6.23 (1.78) | 6.61 (1.79) | 0.86 | 0.564 |
*Mean count for deep sequencing and mean normalized Ct value for qRT-PCR.
p-value is unadjusted for multiple comparisons and is based on two-sample t-test comparing the means of the normalized miRNA expression levels.
Figure 1.
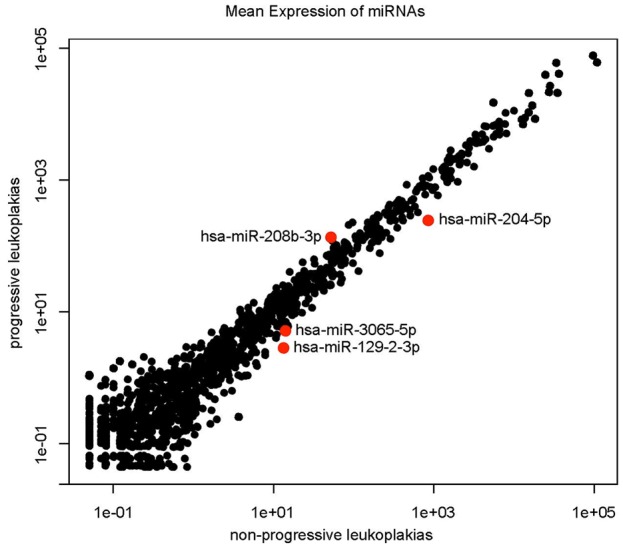
Scatterplot demonstrating overexpression of miRNA-208-3p and underexpression of miRNAs-204-5p, 3065-5p and 129-2-3p in the Progressive Group (Group 1) compared to the Non-Progressive Group (Group 2) in the training set (n=20) as measured by deep sequencing.
Putative target genes for the selected microRNAs
The biologic characteristics of 8, 74, 59, and 96 conserved genes potentially targeted by miRNAs-129-2-3p, 204-5p, 208b-3p and 3065-5p, respectively, were evaluated using TargetScanHuman release 7.0 (www.targetscan.org) and NCBI database (human genome build 36). Enriched genes were associated with multiple biologic pathways including pathways involving regulation of transcription, VEGF activity, Activin, IL-6, Notch signaling pathways, p53 regulation, Wnt/beta-catenin signaling cascade, cell proliferation, cell differentiation, cellular adhesion, apoptosis, cell migration, tumor suppression, and oncogenic signaling (Supplemental Table 1).
Evaluation of the top four miRNA expression profiles by RT-qPCR in the validation set
To confirm overexpression of miR-208b-3p and underexpression of miRs-204-5p, 129-2-3p and 3065-5p, these four miRNAs were quantified using qRT-PCR in the validation set with an additional 40 patients that progressed to OSCC (Group 1) and 40 patients that did not progress to OSCC (Group 2).
Only two of the four selected microRNAs showed changes in expression level consistent with that of the deep sequencing; miR-208b-3p was overexpressed and miR-3065-5p was underexpressed in the progressive group (Table 2). This finding is consistent with the putative role of these miRNAs; miR-208b-3p with oncogenic function, and miR-3065-5p with tumor suppressor role (Table 3). Although statistically insignificant, the other two miRNAs, miR-129-2-3p and miR-204-5p, showed overexpression in the progressive group (significant underexpression of these two microRNAs were demonstrated in the deep sequencing analysis), despite their presumptive role as tumor suppressors.
Table 3.
Univariate analysis of microRNAs associated with cancer progression in the validation set (n=77)
| miRNAs | OR (95% CI) | P-valuea | Chromosomal Location | Putative function | Expression in Progressive Group |
|---|---|---|---|---|---|
| miR-129-2-3p | 1.785 (1.686-1.694) | 0.361 | 11p11.2 | Tumor suppressor | increased |
| miR-204-5p | 2.184 (1.78-2.243) | 0.250 | 9q21.12 | Tumor suppressor | increased |
| miR-208b-3p | 1.472 (1.236-2.061) | 0.049 | 14q11.2 | Oncogenic | increased |
| miR-3065-5p | 1.605 (1.569-1.469) | 0.564 | 17q25.3 | Tumor suppressor | decreased |
p<0.05 is considered to be significant.
Multiple logistic regression analysis was performed to construct a predictive model of cancer progression using the top 4 miRNAs together with age, gender, histologic diagnosis, and location of the lesion. After model selection procedures, the final predictive model included the four miRNA marker panel (miRs-208b-3p, 3065-5p, 129-2-3p and 204-5p), age and histologic diagnosis. In the final model the endogenous control was undetectable in three samples. Since normalization could not be performed for miRNA expression level in those samples, they were eliminated (n=77). The AUC of the ROC curve of the final model (p<0.004 for the 6 predictors combined) was 0.792, with a sensitivity of 76.9% and specificity of 73.7% (Figure 2). Interestingly, this predictive value was stronger than that histology alone (AUC of the ROC curve = 0.646 with sensitivity of 57.5% and specificity of 65.0%), which is utilized in current clinical practice as a sole source of oral cancer progression predictor. As an exploratory study, we performed Maximum Likelihood Estimates analysis for the 6 predictors included in the final model in an attempt to generate a meaningful risk score formula that can be utilized in a clinical setting. The following formula was constructed from the analysis:
Figure 2.
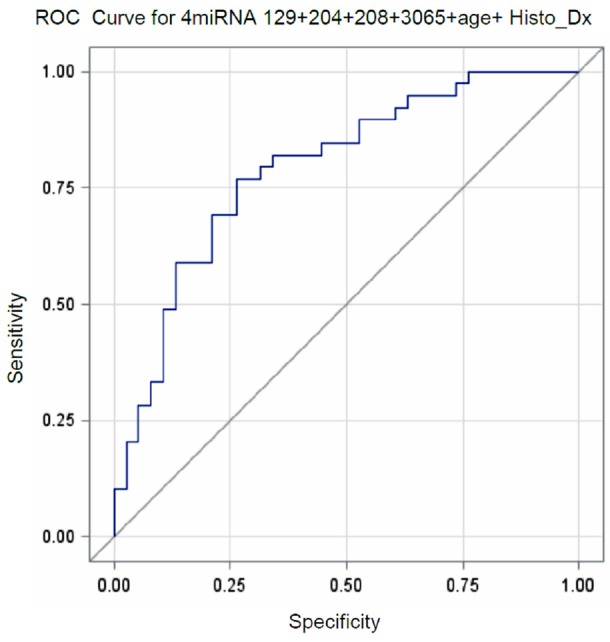
ROC curve for miRNA levels combined with age and histologic diagnosis that can identify progressive oral precursor lesions in the validation set (n=77).
Score of cancer progression = -1.837 - (0.147 x miR-129b-3p) - (0.320 x miR-204-5p) - (0.312 x miR-208b-3p) + (0.348 x miR-3065-5p) + (0.048 x age) + (0.160 x histologic diagnosis level2) + (0.259 x histologic diagnosis level3) + (1.803 x histologic diagnosis level4)
Risk of cancer progression = exp(Score)/{1+exp(Score)}
From this analysis, -1.837 is the intercept, and the higher risk of cancer progression was associated with over expression of miR-3065-5p and underexpression of miRs-129-2-3p, 204-5p and 208b-3p, as well as older age. For the histologic diagnosis, increase in assigned number [1=epithelial hyperplasia, 2=epithelial hyperplasia with hyperkeratosis, 3=cellular atypia, 4=epithelial dysplasia] was associated with increased risk of cancer progression. When the risk score of cancer progression was calculated for all subjects in the validation set (n=77), the mean risk score in the Progressive group was 0.773 (63% likelihood) versus those in the Non-Progressive group -0.680 (38% likelihood). Using the mean (-0.282) as a cutoff point, 31 of the 39 progressive cases (80%) were correctly identified as high risk for cancer progression. In the non-progressive group, 24 of the 38 cases (63%) were properly identified as minimal risk of cancer progression.
Discussion
The deep sequencing analyses in the training set of 20 subjects revealed the overexpression of miR-208b-3p and underexpression of miR-204-5p, 129-2-3p and 3065-5p in the non- and low-grade dysplastic lesions that ultimately progressed to cancer. Indeed, the biologic characteristics of conserved genes potentially targeted by these miRNAs are associated with multiple critical biological processes related to carcinogenesis and tumor suppression. miR-208-3p has a presumptive oncogenic role and its overexpression has been linked to increased cellular proliferation, cell cycle progression, and tumorigenicity in hepatic tissue [40] and esophageal squamous cells [41]. SOX6 tumor suppressor gene is directly targeted by miR-208, and hence, overexpression of miR-208 leads to downregulation of SOX6 protein, which results in downregulation of p21, upregulation of cyclin D1, and deregulation Rb via phosphorylation [41].
miR-3065-5p is a relatively novel microRNA with a limited number of reports. Expression of miR-3065-5p was much lower in metastatic prostate cancer cells compared to those that did not metastasize. Hence, miR-3065-5p is thought to have a tumor-suppressive effect, contributing to reduction of cell migration and tissue invasion [42]. miR-129-2-3p is a negative regulator of SOX4, an oncogene [4]. Hypermethylation of its promoter region and subsequent gene silencing of miR-129-2-3p leads to overexpression of SOX4 and Cdk6, an event reported in tumorigenesis of endometrium [43], stomach [44], and liver [45]. Epigenetic alteration, such as hypermethylation of the promoter region, has been reported to be an early event in oral carcinogenesis [46]. Similarly, miR-204-5p is thought to have a tumor suppressor function. Its downregulation via hypermethylation is associated with increased metastasis and decreased overall survival in breast cancer [47], endometrial carcinoma [48], and colorectal cancer [49]. Researchers have shown that miR-204 is located at the genomic imbalanced 9q21.1-22.3 locus associated with genetic predisposition for head and neck cancer [50].
In the validation cohort (n=77), miR-208-5p expression levels showed a significant increase in the progressive group compared to that of the non-progressive group, concordant with the deep sequencing data. There was underexpression of miR-3065-5p in the progressive group, as observed in the deep sequencing analysis, although it was not statically significant. In contrast to the deep sequencing data, overexpression of miR-129-2-3p and miR-204-5p were detected in the progressive group in the validation cohort. Similar discrepancies between the deep sequencing and qRT-PCR data have been reported by Schee et al [50]. In theory, lack of specificity of the qRT-PCR assay for miR-129-2-3p and miR-204-5p might explain such discrepancies [50]. Alternatively, the small sample size in our training set may have led to false positives.
Despite the discrepancies in expression profile of microRNAs between the training and validation cohorts, when the four miRNAs were combined as a panel together with age and histologic diagnosis, the AUC of the ROC curve was 0.792, with a sensitivity of 76.9% and specificity of 73.7% (p<0.004) to identify non- and low-grade dysplastic lesions that progress to cancer. As previously mentioned, histopathologic examination alone has limitations in identifying leukoplakic lesion that will progress of OSCC, especially if the leukoplakia lacks significant atypical cellular features at the time of initial histopathologic assessment. Limitations of histopathology were also observed in our study; the predictive value of the histopathology alone was 0.645 (AUC of the ROC curve). If the four miRNA marker panel is added to the histopathologic examination, which can be obtained via qRT-PCR with relative ease, together with patient’s age, the predictive power to identify non- and low-grade dysplastic lesions at higher risk for malignant transformation will increase from 0.645 to 0.792.
For clinical practicality, we conducted an exploratory study to assess the feasibility of developing a parsimonious risk score formula from our final model. The main purpose of risk score formula is to translate miRNA expression levels assessed by qRT-PCR at the time of initial biopsy into a score that reflects the patient’s risk of cancer progression. Using the risk score formula, 31 of the 39 progressive cases (80%) were accurately identified as high risk for cancer progression. Conversely, 24 of the 38 non-progressive cases (63%) were properly identified as minimal risk of cancer progression. Those cases identified as high risk would have been treated aggressively initially which would potentially have prevented carcinoma development. The 63% of the low cancer progression risk group could have potentially been spared from unnecessary surgical excision. Further investigation is being planned to determine if our model can be utilized as a predictive modality for progression in early oral leukoplakia. This study is limited by small sample size. Also there are limitations in terms of generalizability as this is a single institutional study. However, our study is unique in that it assesses genome-wide miRNA expression profiles to identify a panel of miRNAs that may be utilized as a cancer progression predictive modality in non- and low-grade dysplastic lesions. We have plans to perform an internal validation and test the repeatability of the final risk score model in a larger set of samples. We will then test the model in a multicenter setting prior to conducting a large-scale prospective study. If validated, we will have obtained a useful predictive modality that can be applied in the clinic, which will guide appropriate management of patients at risk of developing oral cancer.
Acknowledgements
We thank Irina Gurvich for the technical assistance. This work was supported by the NIH/NIDCR R03-DE-023820 (to E. Philipone) and P30ES009089.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64. 31. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Kademani D. Oral cancer. Mayo Clin Proc. 2007;82:878–887. doi: 10.4065/82.7.878. [DOI] [PubMed] [Google Scholar]
- 3.Rodu B, Cole P. Oral cavity and pharynx-throat cancer in the United States, 1973-2003. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:653–658. doi: 10.1016/j.tripleo.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Sciubba JJ. Oral cancer. The importance of early diagnosis and treatment. Am J Clin Dermatol. 2001;2:239–251. doi: 10.2165/00128071-200102040-00005. [DOI] [PubMed] [Google Scholar]
- 5.Lee JJ, Hong WK, Hittelman WN, Mao L, Lotan R, Shin DM, Benner SE, Xu XC, Lee JS, Papadimitrakopoulou VM, Geyer C, Perez C, Martin JW, El-Naggar AK, Lippman SM. Predicting cancer development in oral leukoplakia: ten years of translational research. Clin Cancer Res. 2000;6:1702–1710. [PubMed] [Google Scholar]
- 6.Bouquot JE, Gorlin RJ. Leukoplakia, lichen planus, and other oral keratoses in 23,616 white Americans over the age of 35 years. Oral Surg Oral Med Oral Pathol. 1986;61:373–381. doi: 10.1016/0030-4220(86)90422-6. [DOI] [PubMed] [Google Scholar]
- 7.Bouquot JE. Oral leukoplakia and erythroplakia: a review and update. Pract Periodontics Aesthet Dent. 1994;6:9–17. quiz 19. [PubMed] [Google Scholar]
- 8.Schepman KP, van der Meij EH, Smeele LE, van der Waal I. Malignant transformation of oral leukoplakia: a follow-up study of a hospital-based population of 166 patients with oral leukoplakia from The Netherlands. Oral Oncol. 1998;34:270–275. [PubMed] [Google Scholar]
- 9.Silverman S Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation. A follow-up study of 257 patients. Cancer. 1984;53:563–568. doi: 10.1002/1097-0142(19840201)53:3<563::aid-cncr2820530332>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Cowan CG, Gregg TA, Napier SS, McKenna SM, Kee F. Potentially malignant oral lesions in northern Ireland: a 20-year population-based perspective of malignant transformation. Oral Dis. 2001;7:18–24. [PubMed] [Google Scholar]
- 11.Mehanna HM, Rattay T, Smith J, McConkey CC. Treatment and follow-up of oral dysplasia - a systematic review and meta-analysis. Head Neck. 2009;31:1600–1609. doi: 10.1002/hed.21131. [DOI] [PubMed] [Google Scholar]
- 12.Speight PM. Update on oral epithelial dysplasia and progression to cancer. Head Neck Pathol. 2007;1:61–66. doi: 10.1007/s12105-007-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouquot JE, Speight PM, Farthing PM. Epithelial dysplasia of the oral mucosa-diagnostic problems and prognostic features. Curr Diagn Pathol. 2006;12:11–21. [Google Scholar]
- 14.Waldron CA, Shafer WG. Leukoplakia revisited. A clinicopathologic study 3256 oral leukoplakias. Cancer. 1975;36:1386–1392. doi: 10.1002/1097-0142(197510)36:4<1386::aid-cncr2820360430>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Gupta PC, Mehta FS, Daftary DK, Pindborg JJ, Bhonsle RB, Jalnawalla PN, Sinor PN, Pitkar VK, Murti PR, Irani RR, Shah HT, Kadam PM, Iyer KS, Iyer HM, Hegde AK, Chandrashekar GK, Shiroff BC, Sahiar BE, Mehta MN. Incidence rates of oral cancer and natural history of oral precancerous lesions in a 10-year follow-up study of Indian villagers. Community Dent Oral Epidemiol. 1980;8:283–333. doi: 10.1111/j.1600-0528.1980.tb01302.x. [DOI] [PubMed] [Google Scholar]
- 16.Hsue SS, Wang WC, Chen CH, Lin CC, Chen YK, Lin LM. Malignant transformation in 1458 patients with potentially malignant oral mucosal disorders: a follow-up study based in a Taiwanese hospital. J Oral Pathol Med. 2007;36:25–29. doi: 10.1111/j.1600-0714.2006.00491.x. [DOI] [PubMed] [Google Scholar]
- 17.Nankivell P, Mehanna H. Oral dysplasia: biomarkers, treatment, and follow-up. Curr Oncol Rep. 2011;13:145–152. doi: 10.1007/s11912-010-0150-z. [DOI] [PubMed] [Google Scholar]
- 18.Wu BH, Xiong XP, Jia J, Zhang WF. MicroRNAs: new actors in the oral cancer scene. Oral Oncol. 2011;47:314–319. doi: 10.1016/j.oraloncology.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Bandres E, Bitarte N, Arias F, Agorreta J, Fortes P, Agirre X, Zarate R, Diaz-Gonzalez JA, Ramirez N, Sola JJ, Jimenez P, Rodriguez J, Garcia-Foncillas J. microRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin Cancer Res. 2009;15:2281–2290. doi: 10.1158/1078-0432.CCR-08-1818. [DOI] [PubMed] [Google Scholar]
- 20.Kozaki K, Imoto I, Mogi S, Omura K, Inazawa J. Exploration of tumor-suppressive microRNAs silenced by DNA hypermethylation in oral cancer. Cancer Res. 2008;68:2094–2105. doi: 10.1158/0008-5472.CAN-07-5194. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Jiang L, Wang A, Yu J, Shi F, Zhou X. MicroRNA-138 suppresses invasion and promotes apoptosis in head and neck squamous cell carcinoma cell lines. Cancer Lett. 2009;286:217–222. doi: 10.1016/j.canlet.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo H, Zou J, Dong Z, Zeng Q, Wu D, Liu L. Up-regulated miR-17 promotes cell proliferation, tumour growth and cell cycle progression by targeting the RND3 tumour suppressor gene in colorectal carcinoma. Biochem J. 2012;442:311–321. doi: 10.1042/BJ20111517. [DOI] [PubMed] [Google Scholar]
- 23.Feber A, Xi L, Luketich JD, Pennathur A, Landreneau RJ, Wu M, Swanson SJ, Godfrey TE, Litle VR. MicroRNA expression profiles of esophageal cancer. J Thorac Cardiovasc Surg. 2008;135:255–260. doi: 10.1016/j.jtcvs.2007.08.055. discussion 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maru DM, Singh RR, Hannah C, Albarracin CT, Li YX, Abraham R, Romans AM, Yao H, Luthra MG, Anandasabapathy S, Swisher SG, Hofstetter WL, Rashid A, Luthra R. MicroRNA-196a is a potential marker of progression during Barrett’s metaplasia-dysplasia-invasive adenocarcinoma sequence in esophagus. Am J Pathol. 2009;174:1940–1948. doi: 10.2353/ajpath.2009.080718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bansal A, Lee IH, Hong X, Anand V, Mathur SC, Gaddam S, Rastogi A, Wani SB, Gupta N, Visvanathan M, Sharma P, Christenson LK. Feasibility of mcroRNAs as biomarkers for Barrett’s Esophagus progression: a pilot cross-sectional, phase 2 biomarker study. Am J Gastroenterol. 2011;106:1055–1063. doi: 10.1038/ajg.2011.37. [DOI] [PubMed] [Google Scholar]
- 26.Pereira PM, Marques JP, Soares AR, Carreto L, Santos MA. MicroRNA expression variability in human cervical tissues. PLoS One. 2010;5:e11780. doi: 10.1371/journal.pone.0011780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascaux C, Laes JF, Anthoine G, Haller A, Ninane V, Burny A, Sculier JP. Evolution of microRNA expression during human bronchial squamous carcinogenesis. Eur Respir J. 2009;33:352–359. doi: 10.1183/09031936.00084108. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Li Z, Gao C, Chen P, Chen J, Liu W, Xiao S, Lu H. miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab Invest. 2008;88:1358–1366. doi: 10.1038/labinvest.2008.94. [DOI] [PubMed] [Google Scholar]
- 29.Cervigne NK, Reis PP, Machado J, Sadikovic B, Bradley G, Galloni NN, Pintilie M, Jurisica I, Perez-Ordonez B, Gilbert R, Gullane P, Irish J, Kamel-Reid S. Identification of a microRNA signature associated with progression of leukoplakia to oral carcinoma. Hum Mol Genet. 2009;18:4818–4829. doi: 10.1093/hmg/ddp446. [DOI] [PubMed] [Google Scholar]
- 30.Zhu G, He Y, Yang S, Chen B, Zhou M, Xu XJ. Identification of Gene and MicroRNA Signatures for Oral Cancer Developed from Oral Leukoplakia. Biomed Res Int. 2015;2015:841956. doi: 10.1155/2015/841956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maimaiti A, Abudoukeremu K, Tie L, Pan Y, Li X. MicroRNA expression profiling and functional annotation analysis of their targets associated with the malignant transformation of oral leukoplakia. Gene. 2015;558:271–277. doi: 10.1016/j.gene.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 32.De Sarkar N, Roy R, Mitra JK, Ghose S, Chakraborty A, Paul RR, Mukhopadhyay I, Roy B. A quest for miRNA bio-marker: a track back approach from gingivo buccal cancer to two different types of precancers. PLoS One. 2014;9:e104839. doi: 10.1371/journal.pone.0104839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy R, De Sarkar N, Ghose S, Paul RR, Ray A, Mukhopadhyay I, Roy B. Association between risk of oral precancer and genetic variations in microRNA and related processing genes. J Biomed Sci. 2014;21:48. doi: 10.1186/1423-0127-21-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brito JA, Gomes CC, Guimaraes AL, Campos K, Gomez RS. Relationship between microRNA expression levels and histopathological features of dysplasia in oral leukoplakia. J Oral Pathol Med. 2014;43:211–216. doi: 10.1111/jop.12112. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Li YX, Yang X, Jiang L, Zhou ZJ, Zhu YQ. Progress risk assessment of oral premalignant lesions with saliva miRNA analysis. BMC Cancer. 2013;13:129. doi: 10.1186/1471-2407-13-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao W, Bao ZX, Zhang CY, Zhang XY, Shi LJ, Zhou ZT, Jiang WW. Upregulation of miR-31* is negatively associated with recurrent/newly formed oral leukoplakia. PLoS One. 2012;7:e38648. doi: 10.1371/journal.pone.0038648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weng L, Wu X, Gao H, Mu B, Li X, Wang JH, Guo C, Jin JM, Chen Z, Covarrubias M, Yuan YC, Weiss LM, Wu H. MicroRNA profiling of clear cell renal cell carcinoma by whole-genome small RNA deep sequencing of paired frozen and formalin-fixed, paraffin-embedded tissue specimens. J Pathol. 2010;222:41–51. doi: 10.1002/path.2736. [DOI] [PubMed] [Google Scholar]
- 38.Yoon AJ, Wang S, Shen J, Robine N, Philipone E, Oster MW, Nam A, Santella RM. Prognostic value of miR-375 and miR-214-3p in early stage oral squamous cell carcinoma. Am J Transl Res. 2014;6:580–592. [PMC free article] [PubMed] [Google Scholar]
- 39.Catuogno S, Esposito CL, Quintavalle C, Cerchia L, Condorelli G, De Franciscis V. Recent Advance in Biosensors for microRNAs Detection in Cancer. Cancers (Basel) 2011;3:1877–1898. doi: 10.3390/cancers3021877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu P, Wu D, You Y, Sun J, Lu L, Tan J, Bie P. miR-208-3p promotes hepatocellular carcinoma cell proliferation and invasion through regulating ARID2 expression. Exp Cell Res. 2015;336:232–241. doi: 10.1016/j.yexcr.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Zheng D, Zhang B, Liu L, Ou J, Chen W, Xiong S, Gu Y, Yang J. Mir-208 promotes cell proliferation by repressing SOX6 expression in human esophageal squamous cell carcinoma. J Transl Med. 2014;12:196. doi: 10.1186/1479-5876-12-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watahiki A, Wang Y, Morris J, Dennis K, O’Dwyer HM, Gleave M, Gout PW, Wang Y. MicroRNAs associated with metastatic prostate cancer. PLoS One. 2011;6:e24950. doi: 10.1371/journal.pone.0024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang YW, Liu JC, Deatherage DE, Luo J, Mutch DG, Goodfellow PJ, Miller DS, Huang TH. Epigenetic repression of microRNA-129-2 leads to overexpression of SOX4 oncogene in endometrial cancer. Cancer Res. 2009;69:9038–9046. doi: 10.1158/0008-5472.CAN-09-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu X, Luo L, Wu Y, Yu X, Liu Y, Yu X, Zhao X, Zhang X, Cui L, Ye G, Le Y, Guo J. Gastric juice miR-129 as a potential biomarker for screening gastric cancer. Med Oncol. 2013;30:365. doi: 10.1007/s12032-012-0365-y. [DOI] [PubMed] [Google Scholar]
- 45.Lu CY, Lin KY, Tien MT, Wu CT, Uen YH, Tseng TL. Frequent DNA methylation of MiR-129-2 and its potential clinical implication in hepatocellular carcinoma. Genes Chromosomes Cancer. 2013;52:636–643. doi: 10.1002/gcc.22059. [DOI] [PubMed] [Google Scholar]
- 46.Ha PK, Califano JA. Promoter methylation and inactivation of tumour-suppressor genes in oral squamous-cell carcinoma. Lancet Oncol. 2006;7:77–82. doi: 10.1016/S1470-2045(05)70540-4. [DOI] [PubMed] [Google Scholar]
- 47.Li W, Jin X, Zhang Q, Zhang G, Deng X, Ma L. Decreased expression of miR-204 is associated with poor prognosis in patients with breast cancer. Int J Clin Exp Pathol. 2014;7:3287–3292. [PMC free article] [PubMed] [Google Scholar]
- 48.Bao W, Wang HH, Tian FJ, He XY, Qiu MT, Wang JY, Zhang HJ, Wang LH, Wan XP. A TrkB-STAT3-miR-204-5p regulatory circuitry controls proliferation and invasion of endometrial carcinoma cells. Mol Cancer. 2013;12:155. doi: 10.1186/1476-4598-12-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin Y, Zhang B, Wang W, Fei B, Quan C, Zhang J, Song M, Bian Z, Wang Q, Ni S, Hu Y, Mao Y, Zhou L, Wang Y, Yu J, Du X, Hua D, Huang Z. miR-204-5p inhibits proliferation and invasion and enhances chemotherapeutic sensitivity of colorectal cancer cells by downregulating RAB22A. Clin Cancer Res. 2014;20:6187–6199. doi: 10.1158/1078-0432.CCR-14-1030. [DOI] [PubMed] [Google Scholar]
- 50.Schee K, Lorenz S, Worren MM, Gunther CC, Holden M, Hovig E, Fodstad O, Meza-Zepeda LA, Flatmark K. Deep Sequencing the MicroRNA Transcriptome in Colorectal Cancer. PLoS One. 2013;8:e66165. doi: 10.1371/journal.pone.0066165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


