Abstract
Silent information regulator 1 (SIRT1) plays a critical role in maintaining vascular homeostasis via modulating senescent-related signal pathway, however, the molecular mechanism remains modest clarified. The purpose of this study was to examine whether SIRT1 specific activator SRT1720 would exhibit pro-angiogenic and anti-aging properties in response to hydrogen peroxide (H2O2)-induced endothelial senescence, and determine the underlying mechanisms. We pre-treated senescent human umbilical vein endothelial cells (HUVECs) with SRT1720, senescence-associated beta-galactosidase activity, apoptosis, migration, tube formation, proliferation and angiogenic factors were quantitatively examined. The results revealed that pharmacologic activation of SIRT1 by SRT1720 rescued apoptotic HUVECs and upregulated angiogenic response through reinforcing the protein expressions of angiogenic and survival factors in vitro. Furthermore, we confirmed that the expressions of endothelial nitric oxide synthase (eNOS), vascular endothelial growth factor (VEGF) and phosphoryl-Akt were augmented in SRT1720-treated senescent HUVECs. In conclusion, our data indicated that SRT1720 could protect against endothelial senescence and maintain cell function via Akt/eNOS/VEGF axis.
Keywords: Endothelial cell senescence, SIRT1, VEGF, eNOS
Introduction
The morbidity and mortality of cardiovascular disease (CVD) have been much higher than other diseases worldwide. Aging, a primary risk factor of CVD, is dramatically attributable to vascular cell dysfunction, and thus, disrupts the normal vascular tone and leads to vascular diseases [1]. Vascular endothelial cells (VECs) are critically involved in the maintenance of vascular homeostasis by regulating vascular tone, integrity and remodeling [2]. It has been reported that aging would accelerate VECs regenerative capacity reduction and causing endothelial senescence, which has been observed in patients with atherosclerosis, hyperlipidemia, diabetes, hypertension, aging, and obesity [3-6].
Sirtuin belongs to histone deacetylases family with homologic molecular structure to saccharomyces cerevisiae silent information regulator 2 (Sir2) that requires nicotinamide-adenine dinucleotid as a cofactor for the deacetylation reaction. It has been demonstrated that sirtuin-mediated deacetylases are highly regulated by microenvironment, oxidative stress, and metabolism [7]. There are seven sirtuins in mammals, and each comprises a conserved central core deacetylase domain flanked by variable length N- and C-terminus. Among the seven human sirtuins, Sirtuin-1 (SIRT1) plays the most critical roles during the processes of cell senescence, organism longevity, stress resistance, gene silencing, apoptosis and inflammation [8-15]. SIRT1 is highly expressed in VECs no matter in artery, vein or capillary, and modulates VECs functions [16]. Hence, altered expression of endothelial SIRT1 would affect normal endothelial function and vascular physiology. Recent studies have highlighted protective roles of SIRT1 in cardiovascular diseases [17-19]. For instance, SIRT1 has been reported to increase the expression of endothelial nitric oxide synthase (eNOS) and eNOS-derived nitric oxide (NO) [20-23].
The newly-synthesized small molecule SRT1720 has been reported to specifically activate SIRT1 [24]. It has been reported that SIRT1 activation by SRT1720 harvested beneficial effects in rodent model of aging and aging-related metabolic diseases [25-27]. Given the critical role of SIRT1 in endothelial repair and regeneration, the purpose of this study was to examine whether SRT1720 could improve angiogenesis in the circumstance of hydrogen peroxide (H2O2)-induced endothelial senescence, and uncover the underlying molecular mechanisms.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs; ATCC, Cat. CRL1730) were cultured in Dulbecco modified eagle medium (DMEM, low-glucose) plus 10% FBS at 37°C under a humidified 95%: 5% (v/v) mixture of air and CO2. HUVECs in passages 2-4 were used in this study. To induce cell senescence, HUVECs were treated with different concentrations of H2O2 (Sigma, St. Louis, MO) for 4 hours. For analyzing the anti-aging effect of SRT1720 (Sellect, Shanghai, China), HUVECs were pretreated with different concentrations of SRT1720 for 24 hours before H2O2 administration. Supernatant and cell lysates were collected respectively for biological analysis.
Reagents
SRT1720, a small molecule activator of SIRT1, was dissolved in DMSO and applied to reach the final concentration of 0, 5, 10, 15, 20 μM, respectively. H2O2 was dissolved in phosphate-buffered saline (PBS) and used at different concentration of 0, 100, 200, 300 μM.
Galactosidase (β-gal) staining
After treated with or without different concentrations of H2O2, SRT1720 was administrated to reach the indicated concentrations. HUVECs were then washed twice with PBS and then were fixed and stained by β-Galactosidase Staining Kit (Beyotime Institute of Biotechnology, Shanghai, China) for SA-β-gal activity analyses. The percentage of SA-β-gal positive cells was determined by counting the number of blue cells within a sample of 400 cells (×100 magnification).
Apoptosis analysis
HUVECs were seeded on sterile cover glasses placed in the 6-well plates and cultured in serum-free DMEM for 48 hours as previously descried [28]. HUVECs were then treated with or without different concentrations of H2O2 and SRT1720. Cells were then fixed with 4% paraformaldehyde, washed twice with PBS and stained with Hoechst 33258 staining solution according to the manufacturer instructions (Beyotime). Hoechst-stained pyknotic nuclei were counted as percentage of 200 cells in each well (×200 magnification). Each group was studied at least in triplicate.
CCK-8 assay
Cell growth was analyzed using WST-8 Cell Counting Kit-8 (CCK-8 kit, Beyotime). After treated with or without different concentrations of H2O2, and SRT1720, HUVECs (2×104/mL) were seeded on 96-well plates with 100 μL DMEM (with 10% FBS) and were incubated at 37°C for 24 hours with CCK-8 solution (10 µL). The absorbance of the reactive system was measured at 450 nm wavelength.
Migration assay
Migration assay was performed using 24-well Boyden Transwell chambers (Corning, Cambridge, MA) with 6.5-mm-diameter polycarbonate filters (8-µm pore size). Briefly, 600 µL DMEM containing 10% FBS was added into the lower compartment, HUVECs (3×105/mL) treated with different concentrations of H2O2 or SRT1720 were suspended in 100 µl serum-free DMEM and seeded in upper compartment of the Transwell chambers. After 12 hours’ incubation, upper compartments were removed, whereas the cells that migrated through the membrane to the underside were fixed with cold 4% paraformalde-hyde and stained with 0.1% crystal violet. Cell numbers were counted in 5 randomly-selected fields using light microscopy at ×100 magnification.
Tube formation assay
The tube formation assay was performed as described previously [29]. Briefly, Matrigel-Matrix (BD Biosciences) was added in the well of a 96-well cell culture plate and HUVECs (5×105/mL) treated with or without different concentrations of H2O2, SRT1720 or H2O2+SRT1720 were suspended in 50 µl DMEM (with 10% FBS) and seeded. After 6-8 hours’ incubation, images were acquired under a fluorescent microscope (IX-71; Olympus, Tokyo, Japan) with 12.8 M pixel recording digital color cooled camera (DP72; Olympus). The tube formation was calculated as fold of control. Each experiment was repeated 4 times under identified conditions, images of tube morphology were taken and tube lengths were calculated under ×100 magnification.
Western blotting
Equal amounts of total protein from the extracts of HUVECs were resolved in SDS 10% polyacrylamide gel and transferred to nitrocellulose membranes for Western blotting as described previously [30,31]. The primary antibodies used were as follows: anti-eNOS (Sigma, St. Louis, MO), anti-VEGF (Proteintech, Chicago, IL), anti-phosphor-Akt (p-Akt) and anti-GAPDH (Cell Signaling Technology, Beverly, MA). Positive signals were visualized with a FluorChem E data system (Cell Biosciences, Santa Clara, CA) and quantified by densitometry using Quantity One 4.52 (Bio-Rad, Hercules, CA). We applied GAPDH as an internal control to standardize to the protein quantity.
Statistical analysis
One-way ANOVA analysis of variance with the post-hoc Bonferroni test was applied for multiple comparisons. SPSS software version 17.0 (SPSS Inc., Chicago, IL) was used. All experiments were performed at least in triplicate. A value of P<0.05 was considered significant.
Results
H2O2 treatment led to HUVECs senescence and dysfunction
As shown in Figure 1, SA-β-gal staining indicated the ratio of senescent cells, and Hoechst staining determined the proportion of apoptotic cells by manually counting pyknotic nuclei. The proportions of senescent and apoptotic cells were dramatically increased in the HUVECs treated with H2O2 following a dose-dependent manner in comparison with control. In tube formation assay, the HUVECs-formed tube-like vasculature were shortened in response to H2O2 following a dose-dependent manner (Figure 2A). Transwell migration assay was performed to evaluate endothelial migration ability, number of migrated HUVECs were significant less in H2O2-treated groups than control (Figure 2B).
Figure 1.
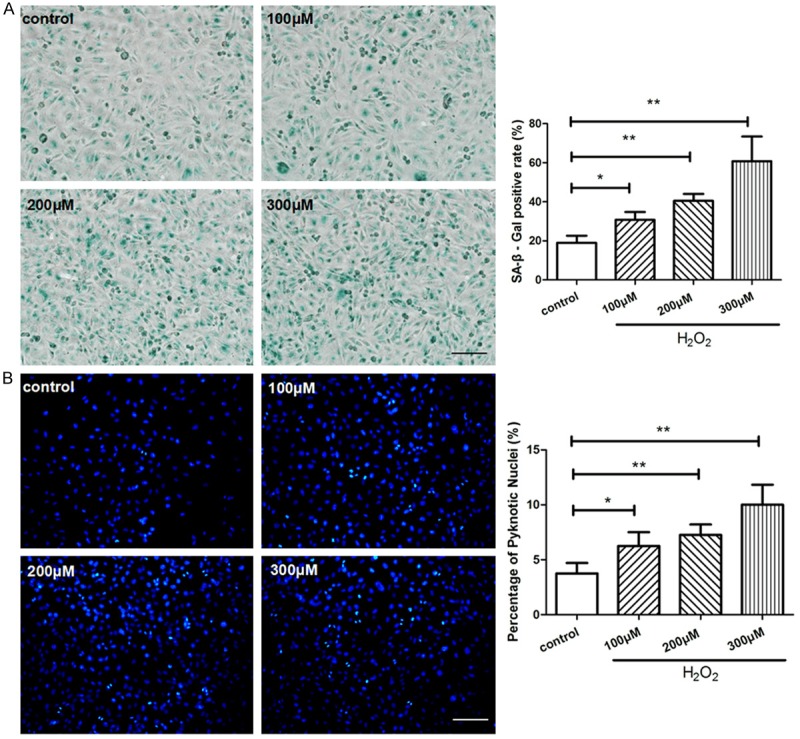
H2O2 caused HUVECs senescence and apoptosis. HUVECs were treated with 0, 100, 200, 300 μM of H2O2 respectively for 4 hours and then cultured for another 24 hours. (A) SA-β-gal staining was performed to detect senescent cells (blue color), the ratio of SA-β-gal positive cells was calculated in each group. (B) The apoptosis assay was performed by Hoechst 33258 staining, pyknotic and brighter nuclei is the feature of apoptosis. Quantitative analysis was represented as the apoptotic cells in the total cells per field. Values are mean ± SEM; n = 4 in each group, N.S. means no significant difference, *means P<0.05, **means P<0.01, v.s. control group. Scale bar indicated 100 μm in (A) and 50 μm in (B). One-way ANOVA (Bonferroni post hoc test) was used.
Figure 2.
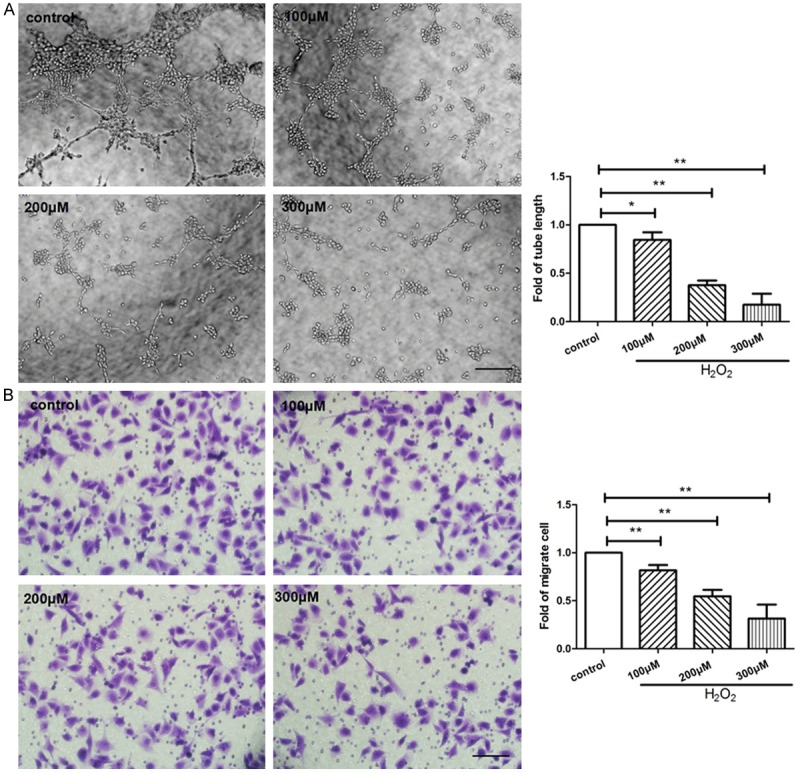
H2O2 hampered tube formation and migration of HUVECs. HUVECs were treated with 0, 100, 200, 300 μM of H2O2 respectively for 4 hours and then cultured for another 24 hours. A: (left) Representative images of tube formation in HUVECs, (right) quantitative analysis of tube length were represented as fold of control (×100 magnification). B: Migrated cells were stained and quantitative analysis of migrated cells was represented as fold of control. Values are mean ± SEM; n = 4, N.S. means no significant difference, *means P<0.05, **means P<0.01, vs. control. Scale bar indicated 100 μm. One-way ANOVA (Bonferroni post hoc test) was used.
Oxidative stress induced by H2O2 hampered angiogenic response in vitro
In present study, we confirmed that H2O2 inhibited VEGF released from HUVECs, and decreases the expressions of P-Akt and eNOS (Figure 3A). The H2O2-treated groups exhibited significantly lower proliferative capacities than control (Figure 3B). Furthermore, all the biological affects caused by H2O2 treatment showed a dose-dependent manner, and it displayed a maximum effort when the concentration of H2O2 reached 300 μM. More than 300 μM concentration of H2O2 would cause rapid apoptosis of HUVECs and failed us to continue the assay (data not shown). These results indicated that 300 μM is the optimized concentration of H2O2 in establishing endothelial senescence.
Figure 3.
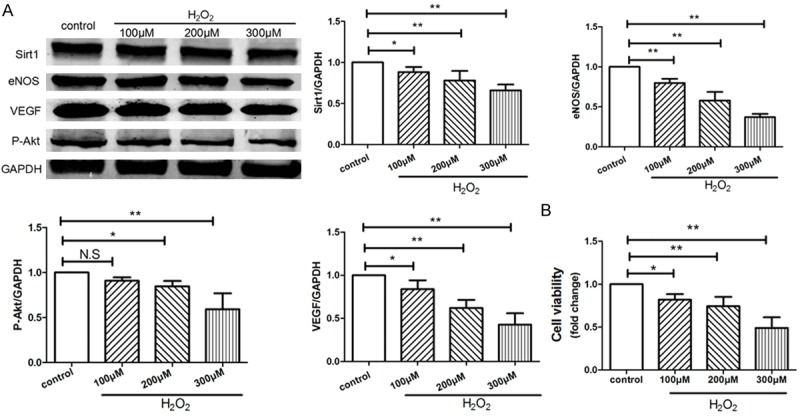
H2O2 inhibited angiogenesis and proliferation of HVUECs. HUVECs were treated with 0, 100, 200, 300 μM of H2O2 respectively for 4 hours and then cultured for another 24 hours. A: The protein expressions of SIRT1, VEGF, phospho-Akt, and eNOS in HUVECs were examined by Western blotting, data were represented as fold of control. B: The proliferation was analyzed by Cell Count Kit-8 (CCK-8) as indicated. Values are mean ± SEM; n = 4, N.S. means no significant difference, *means P<0.05, **means P<0.01, vs. control group. One-way ANOVA (Bonferroni post hoc test) was used.
The protective effects of SRT1720 on H2O2-treated HUVECs
For the purpose of detecting the protective effects of SRT1720 on H2O2-treated HUVECs, we pre-treated HUVECs with 0, 5, 10, 15, 20 μM SRT1720 respectively, then use 300 μM of H2O2 in this study. The proportions of senescent and apoptotic cells were dramatically decreased in the SRT1720-treated HUVECs following a dose-dependent manner compared with control (Figure 4). The HUVECs-formed micro-tubes were lengthened in response to SRT1720 (Figure 5A), and more migrated HUVECs were found in the SRT1720-treated groups (Figure 5B). SRT1720 also promotes the expressions of VEGF, P-Akt and eNOS (Figure 6A). The SRT1720-pretreated groups showed significantly more potential proliferative capacities than control (Figure 6B). More importantly, above-mentioned angiogenic effects induced by SRT1720 showed a dose dependent manner, and it reached maximum efforts at optimum concentration of 10 μM. These results indicated we could apply 10 μM SRT1720 in the following study.
Figure 4.
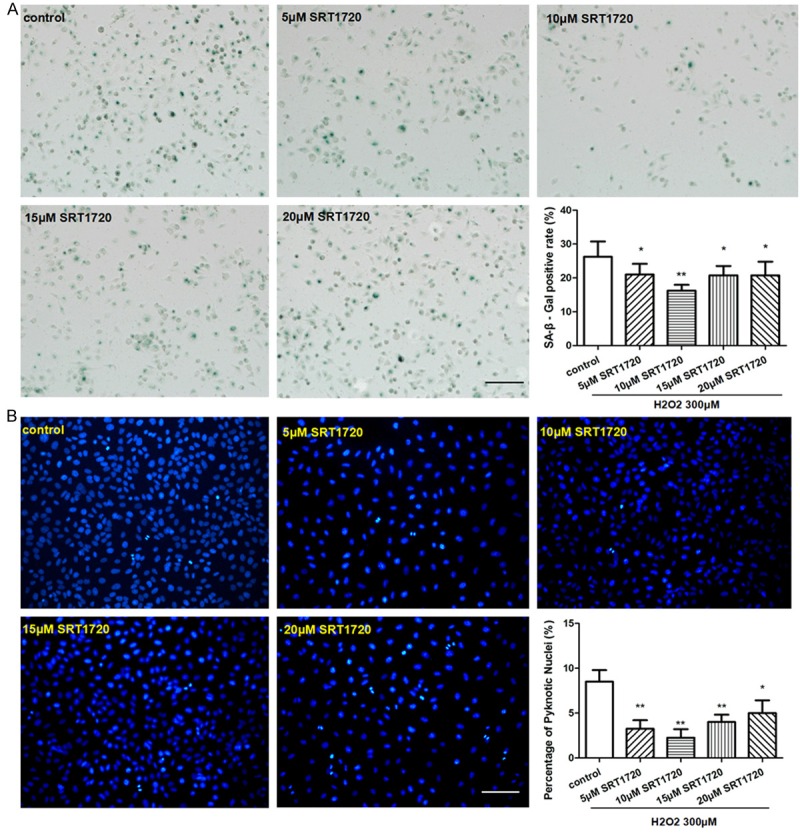
SRT1720 protected against H2O2-induced senescence and apoptosis of HUVECs. HUVECs were pre-treated with 0, 5, 10, 15, 20 μM SRT1720 respectively for 24 hours, followed by 300 μM H2O2 for additional 4 hours. (A) SA-β-gal staining was performed and senescent cells were stained with blue color, the ratio of SA-β-gal positive cells was calculated per group. (B) An analysis of apoptosis by Hoechst 33258 Staining, Quantitative analysis was represented as the apoptotic cells in the total cells per field. Values are mean ± SEM; n = 4, N.S. means no significant difference, *means P<0.05, **means P<0.01, vs. control group. Scale bar indicated 100 μm in (A) and 50 μm in (B). One-way ANOVA (Bonferroni post hoc test) was used.
Figure 5.
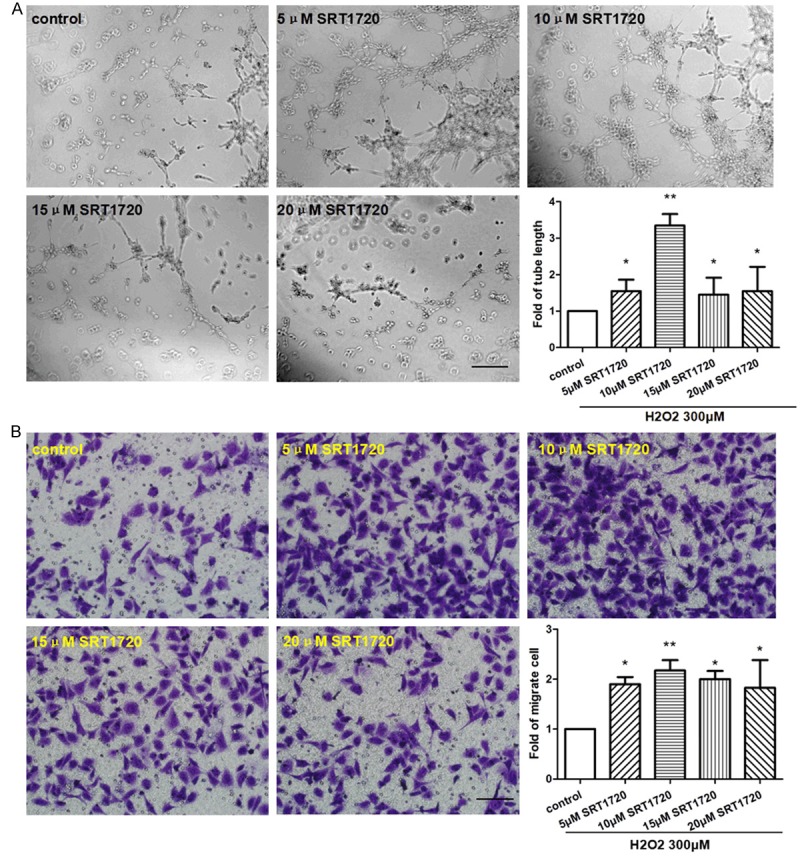
SRT1720 improved cell functions of H2O2-induced senescent HUVECs. HUVECs were pre-treated with 0, 5, 10, 15, 20 μM SRT1720 respectively for 24 hours, followed by 300 μM H2O2 for 4 hours. A: Representative images and quantitative analysis of tube formation in HUVECs (×100 magnification). B: Migrated cells were stained and quantitative analysis was represented as fold of control. Values are mean ± SEM; n = 4, N.S. means no significant difference, *means P<0.05, **means P<0.01, vs. control. Scale bar indicated 100 μm. One-way ANOVA (Bonferroni post hoc test) was used.
Figure 6.
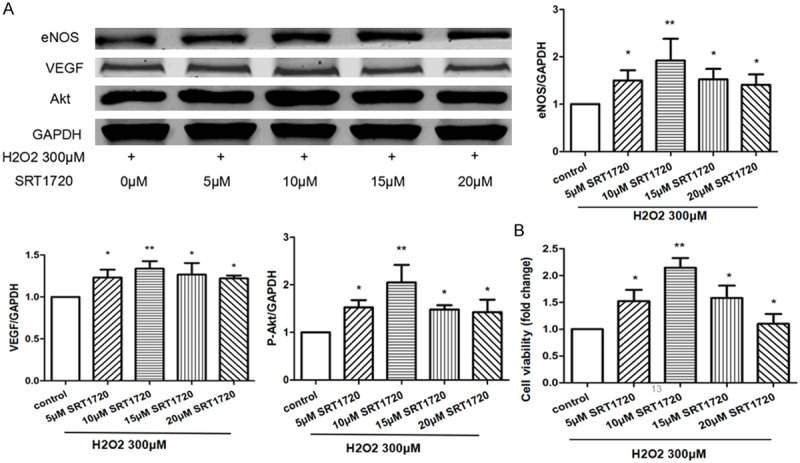
SRT1720 promoted the expressions of angiogenic factors and proliferation of senescent HVUECs. HUVECs were pre-treated with 0, 5, 10, 15, 20 μM SRT1720 respectively for 24 hours, followed by 300 μM H2O2 for additional 4 hours. A: The expressions of VEGF, p-Akt, and eNOS in HUVECs were examined by Western blotting, data were represented as fold of control. B: The proliferation was analyzed by Cell Count Kit-8 (CCK-8) as indicated. Values are mean ± SEM; n = 4, N.S. means no significant difference, *means P<0.05, **means P<0.01, vs. control group. One-way ANOVA (Bonferroni post hoc test) was used.
The effects of SRT1720 on normal and H2O2-treated HUVECs
HUVECs were pre-treated with or without 10 μM SRT1720 for 24 hours, followed by 300 μM H2O2 or PBS for 4 hours and then culture for additional 24 hours. Surprisingly, we found that, though SRT1720 rescued the senescent HUVECs, SRT1720 had little biological effects on non-senescent HUVECs, whatever on the cell apoptosis (Figure 7B), tube formation (Figure 8A), migration (Figure 8B), the expression of eNOS (Figure 9A) and cell proliferation (Figure 9B), in comparison with control. To explore the role of the SRT1720 in the H2O2-induced senescence in HUVECs, cells were pretreated with SRT17207 for 24 hours and then subjected to H2O2. Pre-treatment with SRT1720, however, significantly prevented the senescence and apoptosis (Figure 7A and 7B), reinforced tube formation and migration (Figure 8A and 8B), and augmented the expressions of angiogenic factors of the HUVECs.
Figure 7.
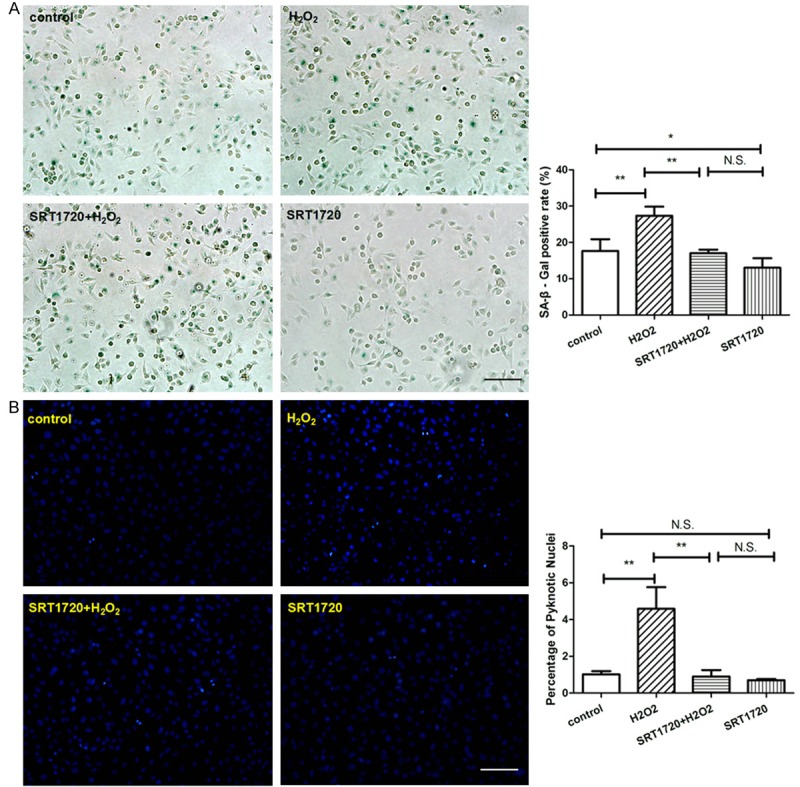
SRT1720 protected against senescence and apoptosis. HUVECs were pre-treated with or without 10 μM SRT1720 for 24 hours, followed by 300 μM H2O2 or PBS for additional 4 hours. (A) SA-β-gal staining was performed and senescent cells were stained with blue color, the ratio of SA-β-gal positive cells was calculated per group. (B) Quantitative analysis of apoptosis by Hoechst 33258 Staining was represented per field. Values are mean ± SEM; n = 4, N.S. means no significant difference,*means P<0.05,**means P<0.01, vs. control group. Scale bar indicated 100 μm in (A) and 50 μm in (B). One-way ANOVA (Bonferroni post hoc test) was used.
Figure 8.
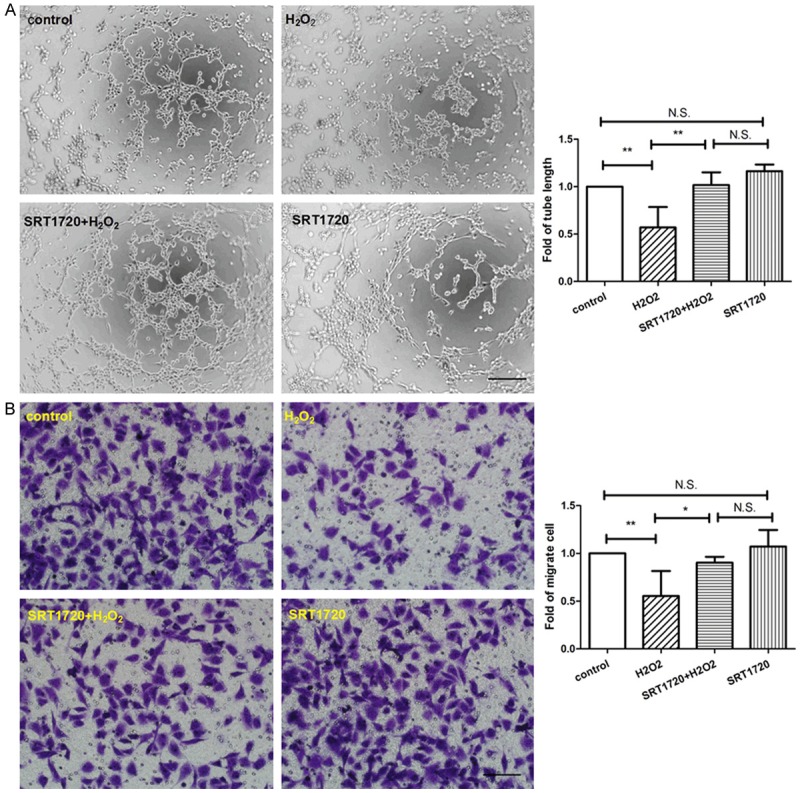
SRT1720 reinforced tube formation and migration of HUVECs. HUVECs were pre-treated with or without 10 μM SRT1720 for 24 hours, followed by 300 μM H2O2 or PBS for additional 4 hours. A: Representative images of tube formation and quantitative analysis of tube length were represented as fold of control (×100 magnification). B: Migrated cells were stained and quantitative analysis of migrated cells was represented as fold of control. Values are mean ± SEM; n = 4, N.S. means no significant difference, *means P<0.05, **means P<0.01, vs. control. Scale bar indicated 100 μm. One-way ANOVA (Bonferroni post hoc test) was used.
Figure 9.
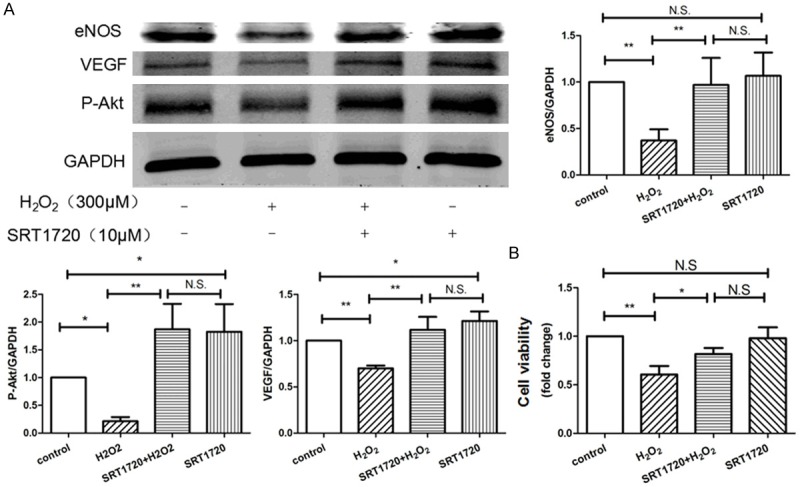
SRT1720 augmented the expression of angiogenic factors and cell viability of HUVECs. HUVECs were pre-treated with or without 10 μM SRT1720 for 24 hours, followed by 300 μM H2O2 or PBS for additional 4 hours. A: The expressions of VEGF, p-Akt, and eNOS in HUVECs were examined by Western blotting, data were represented as fold of control. B: The proliferation was analyzed by Cell Count Kit-8 (CCK-8) as indicated. Values are mean ± SEM; n = 4, N.S. means no significant difference, *means P<0.05, **means P<0.01, vs. control group. One-way ANOVA (Bonferroni post hoc test) was used.
Discussion
Endothelial senescence results in vascular dysregulation, atherosclerosis and forthcoming cardiovascular diseases. Because H2O2-mediated damage best mimics oxidative stress in aging population, H2O2 is widely applied as a stressor for oxidative stress and cellular senescence induction [32]. In our study, H2O2 effectively induced HUVECs senescence following a dose-dependent manner and 300 μM of H2O2 displayed a maximum anti-angiogenic effect followed by remarkable endothelial dysfunction.
Endothelial Akt/eNOS/VEGF signal pathway is closely associated with VECs activity and viability [33]. Recent studies reported that SIRT1 and eNOS colocalized and coprecipitated in VECs, and SIRT1 deacetylated eNOS, stimulating eNOS activity and increasing NO production [21]. In our study, we demonstrated that SRT1720, small molecular activator of SIRT1, significantly improved migration and proliferation in vitro via Akt/eNOS/VEGF signaling pathway with or without the existence of H2O2. Moreover, by determining the expressions of P-Akt, eNOS and VEGF, we demonstrated the anti-aging and anti-apoptotic beneficial of SRT1720 in vitro.
Furthermore, SIRT1 is highly expressed in endothelial progenitor cells (EPCs) during vasculature incorporation and controls the angiogenic activity [34,35]. EPCs can be recruited to ischemic area through chemotaxis, differentiate into VECs, and eventually participate in the ischemia-induced neovascularization after ischemic attack [36-38]. SIRT1 plays a critical role in EPCs-mediated re-endothelialization in response to vascular injury via mitotic pathway [39]. Moreover, SIRT1 is also involved in vascular development through augmenting vascular endothelial growth factor expression in vitro [40,41], and participated in the vascular growth in developing zebrafish [42]. Our study demonstrated that SRT1720 significantly augmented cell viability and activity of H2O2-treated HUVECs, suggesting its potential anti-aging and anti-apoptotic activity.
In conclusion, our study demonstrated that H2O2-mediated endothelial senescence dramatically decreased SIRT1 expression in HUVECs. SRT1720, a specific SIRT1 activator, exhibited protective effects to the HUVECs exposed to H2O2, as indicated by the improved cell viability, tube formation, migration, and survival. SRT1720 rescued the impaired angiogenic potential of HUVECs via activation of Akt/eNOS/VEGF pathway. Future studies would verify the angiogenic and regenerative potential of SRT1720 in animal study.
Acknowledgements
This work was supported by the China National Natural Science Foundation (11374213, 11574210, 81500372 and 81500523), and Shanghai Pujiang Program (15PJ1405000).
Disclosure of conflict of interest
None.
References
- 1.Valtcheva N, Primorac A, Jurisic G, Hollmén M, Detmar M. The orphan adhension G protein-coupled receptor GPR97 regulates migration of lymphatic endothelial cells via the small GTPases RhoA and Cdc42. J Biol Chem. 2013;288:35736–35748. doi: 10.1074/jbc.M113.512954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrendt D, Ganz P. Endothelial function. From vascular biology to clinical applications. Am J Cardiol. 2002;90:40L–48L. doi: 10.1016/s0002-9149(02)02963-6. [DOI] [PubMed] [Google Scholar]
- 3.Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Duan W, Liang Z, Yi W, Yan J, Wang N, Li Y, Chen W, Yu S, Jin Z, Yi D. Curcumin attenuates endothelial cell oxidative stress injury through Notch signaling inhibition. Cell Signal. 2013;25:615–629. doi: 10.1016/j.cellsig.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res. 2005;66:286–294. doi: 10.1016/j.cardiores.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 7.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung S, Yao H, Caito S, Hwang JW, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch Biochem Biophys. 2010;501:79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman I, Kinnula VL, Gorbunova V, Yao H. SIRT1 as a therapeutic target in inflammaging of the pulmonary disease. Prev Med. 2012;54:S20–28. doi: 10.1016/j.ypmed.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao H, Rahman I. Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem Pharmacol. 2012;84:1332–1339. doi: 10.1016/j.bcp.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao H, Chung S, Hwang JW, Rajendrasozhan S, Sundar IK, Dean DA, McBurney MW, Guarente L, Gu W, Ronty M, Kinnula VL, Rahman I. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J Clin Invest. 2012;122:2032–2045. doi: 10.1172/JCI60132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Annu Rev Biochem. 2006;75:435–465. doi: 10.1146/annurev.biochem.74.082803.133500. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa A, Tada-Oikawa S, Kawanishi S, Oikawa S. H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell Physiol Biochem. 2007;20:45–54. doi: 10.1159/000104152. [DOI] [PubMed] [Google Scholar]
- 14.Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:861–870. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarzuelo MJ, Lopez-Sepulveda R, Sanchez M, Romero M, Gomez-Guzman M, Ungvary Z, Pérez-Vizcaíno F, Jiménez R, Duarte J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol. 2003;85:1288–1296. doi: 10.1016/j.bcp.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Arunachalam G, Yao H, Sundar IK, Caito S, Rahman I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem Biophys Res Commun. 2010;393:66–72. doi: 10.1016/j.bbrc.2010.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinciguerra M, Santini MP, Martinez C, Pazienza V, Claycomb WC, Giuliani A, Rosenthal N. mIGF-1/JNK1/SirT1 signaling confers protection against oxidative stress in the heart. Aging Cell. 2012;11:139–149. doi: 10.1111/j.1474-9726.2011.00766.x. [DOI] [PubMed] [Google Scholar]
- 18.Nadtochiy SM, Yao H, McBurney MW, Gu W, Guarente L, Rahman I, Brookes PS. SIRT1-mediated acute cardioprotection. Am J Physiol Heart Circ Physiol. 2011;301:H1506–1512. doi: 10.1152/ajpheart.00587.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadtochiy SM, Redman E, Rahman I, Brookes PS. Lysine deacetylation in ischaemic preconditioning: the role of SIRT1. Cardiovasc Res. 2011;89:643–649. doi: 10.1093/cvr/cvq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci U S A. 2010;107:10268–10273. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caito S, Rajendrasozhan S, Cook S, Chung S, Yao H, Friedman AE, Brookes PS, Rahman I. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB J. 2010;24:3145–3159. doi: 10.1096/fj.09-151308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin YJ, Zhen YZ, Wei J, Liu B, Yu ZY, Hu G. Effects of Rhein lysinate on H2O2-induced cellular senescence of human umbilical vascular endothelial cells. Acta Pharmacol Sin. 2011;32:1246–1252. doi: 10.1038/aps.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell SJ, Martin-Montalvo A, Mercken EM, Palacios HH, Ward TM, Abulwerdi G, Minor RK, Vlasuk GP, Ellis JL, Sinclair DA, Dawson J, Allison DB, Zhang Y, Becker KG, Bernier M, de Cabo R. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 2014;6:836–843. doi: 10.1016/j.celrep.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 27.Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin YK, Canto C, Scheibye-Knudsen M, Krawczyk M, Irusta PM, Martin-Montalvo A, Hubbard BP, Zhang Y, Lehrmann E, White AA, Price NL, Swindell WR, Pearson KJ, Becker KG, Bohr VA, Gorospe M, Egan JM, Talan MI, Auwerx J, Westphal CH, Ellis JL, Ungvari Z, Vlasuk GP, Elliott PJ, Sinclair DA, de Cabo R. SRT1720 improves survival and healthspan of obese mice. Sci Rep. 2011;1:70. doi: 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hao C, Shintani S, Shimizu Y, Kondo K, Ishii M, Wu H, Murohara T. Therapeutic angiogenesis by autologous adipose-derived regenerative cells: comparison with bone marrow mononuclear cells. Am J Physiol Heart Circ Physiol. 2014;307:H869–879. doi: 10.1152/ajpheart.00310.2014. [DOI] [PubMed] [Google Scholar]
- 29.Huang JJ, Shi YQ, Li RL, Hu A, Lu ZY, Weng L, Wang SQ, Han YP, Zhang L, Li B, Hao CN, Duan JL. Angiogenesis effect of therapeutic ultrasound on HUVECs through activation of the PI3K-Akt-eNOS signal pathway. Am J Transl Res. 2015;7:1106–1115. [PMC free article] [PubMed] [Google Scholar]
- 30.Hao CN, Huang ZH, Song SW, Shi YQ, Cheng XW, Murohara T, Lu W, Su DF, Duan JL. Arterial baroreflex dysfunction impairs ischemia-induced angiogenesis. J Am Heart Assoc. 2014;3:e000804. doi: 10.1161/JAHA.114.000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li RL, Huang JJ, Shi YQ, Hu A, Lu ZY, Weng L, Wang SQ, Han YP, Zhang L, Hao CN, Duan JL. Pulsed electromagnetic field improves postnatal neovascularization in response to hindlimb ischemia. Am J Transl Res. 2015;7:430–444. [PMC free article] [PubMed] [Google Scholar]
- 32.Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000;35:927–945. doi: 10.1016/s0531-5565(00)00180-7. [DOI] [PubMed] [Google Scholar]
- 33.Davis PA, Pagnin E, Dal Maso L, Caielli P, Maiolino G, Fusaro M, Paolo Rossi G, Calo LA. SIRT1, heme oxygenase-1 and NO-mediated vasodilation in a human model of endogenous angiotensin II type 1 receptor antagonism: implications for hypertension. Hypertens Res. 2013;36:873–878. doi: 10.1038/hr.2013.48. [DOI] [PubMed] [Google Scholar]
- 34.Napoli C, Hayashi T, Cacciatore F, Casamassimi A, Casini C, Al-Omran M, Ignarro LJ. Endothelial progenitor cells as therapeutic agents in the microcirculation: an update. Atherosclerosis. 2011;215:9–22. doi: 10.1016/j.atherosclerosis.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 35.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 36.Cho HJ, Kim HS, Lee MM, Kim DH, Yang HJ, Hur J, Hwang KK, Oh S, Choi YJ, Chae IH, Oh BH, Choi YS, Walsh K, Park YB. Mobilized endothelial progenitor cells by granulocyte macrophage colony-stimulating factor accelerate reendothelialization and reduce vascular inflammation after intravascular radiation. Circulation. 2003;108:2918–2925. doi: 10.1161/01.CIR.0000097001.79750.78. [DOI] [PubMed] [Google Scholar]
- 37.Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, Isner JM, Asahara T, Losordo DW. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation. 2003;108:3115–3121. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 38.Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, Nickenig G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;93:e17–24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Zhang HN, Chen HZ, Gao P, Zhu LH, Li HL, Lv X, Zhang QJ, Zhang R, Wang Z, She ZG, Wei YS, Du GH, Liu DP, Liang CC. SIRT1 acts as a modulator of neointima formation following vascular injury in mice. Circ Res. 2011;108:1180–1189. doi: 10.1161/CIRCRESAHA.110.237875. [DOI] [PubMed] [Google Scholar]
- 40.Borradaile NM, Pickering JG. Nicotinamide phosphoribosyltransferase imparts human endothelial cells with extended replicative lifespan and enhanced angiogenic capacity in a high glucose environment. Aging Cell. 2009;8:100–112. doi: 10.1111/j.1474-9726.2009.00453.x. [DOI] [PubMed] [Google Scholar]
- 41.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volkmann I, Kumarswamy R, Pfaff N, Fiedler J, Dangwal S, Holzmann A, Batkai S, Geffers R, Lother A, Hein L, Thum T. MicroRNA-mediated epigenetic silencing of sirtuin1 contributes to impaired angiogenic responses. Circ Res. 2013;113:997–1003. doi: 10.1161/CIRCRESAHA.113.301702. [DOI] [PubMed] [Google Scholar]


