Abstract
Functional recovery is often unsatisfactory after severe extended nerve defects or proximal nerve trunks injuries repaired by traditional repair methods, as the long regeneration distance for the regenerated axons to reinnervate their original target end-organs. The proximal nerve stump can regenerate with many collaterals that reinnervate the distal stump after peripheral nerve injury, it may be possible to use nearby fewer nerve fibers to repair more nerve fibers at the distal end to shorten the regenerating distance. In this study, the proximal peroneal nerve was used to repair both the distal peroneal and tibial nerve. The number and location of motor neurons in spinal cord as well as functional and morphological recovery were assessed at 2 months, 4 months and 8 months after nerve repair, respectively. Projections from the intact peroneal and tibial nerves were also studied in normal animals. The changes of motor neurons were assessed using the retrograde neurotracers FG and DiI to backlabel motor neurons that regenerate axons into two different pathways. To evaluate the functional recovery, the muscle forces and sciatic function index were examined. The muscles and myelinated axons were assessed using electrophysiology and histology. The results showed that all labeled motor neurons after nerve repair were always confined within the normal peroneal nerve pool and nearly all the distribution of motor neurons labeled via distal different nerves was disorganized as compared to normal group. However, there was a significant decline in the number of double labeled motor neurons and an obvious improvement with respect to the functional and morphological recovery between 2 and 8 months. In addition, the tibial/peroneal motor neuron number ratio at different times was 2.11±0.05, 2.13±0.08, 2.09±0.12, respectively, and was close to normal group (2.21±0.09). Quantitative analysis showed no significant morphological differences between myelinated nerve fibers regenerated along the two distal nerves except for the number of nerve fibers, which was higher in the tibial nerve. The ratio of distal regenerated axon numbers to proximal donor nerve axon numbers was about 3.95±0.10, 4.06±0.19 and 3.87±0.23, respectively. This study demonstrated that fewer nerve fibers can regenerate a large number of collaterals which successfully repopulate both distal nerves and lead to the partial recovery of lost functions. It may provide a new method to repair severe extended nerve defects or proximal nerve trunks injuries.
Keywords: Spinal motor reorganization, collaterals, topographic specificity, conduit, retrograde labeling
Introduction
Peripheral nerve injury is a serious disease that can lead to severe impairment and long-standing disability [1]. Severe nerve lesions such as severe extended nerve defects or proximal nerve trunks injuries like the brachial plexus usually results in poor functional recovery. Traditionally, there are many methods for those lesions including nerve grafts, nerve transfer [2,3], artificial nerve conduit bridging [4,5]. However, because of the long distance for the regenerated axons to reinnervate the distal end-organs, the capacity of motor neurons to regenerate axons into the distal stumps is compromised [6]. With prolonged denervation, the distal nerve segments and corresponding muscles are gradually atrophied and lose the receptivity of regenerating axons [7]. Although the regenerating axons get to the neuromuscular junction, the function could not be restored as the inability of these end-organs to accept reinnervation [8].
Therefore, nerve reinnervation within a shorter time after injury is required for optimal functional recovery. When the integrity of the peripheral nerve is broken, including transection of the axon, or the rupture of the endoneurium, perineurium and epineurium, the axons will then sprout and regenerate with many collaterals [9-11]. If there are enough distal endoneurial tubes, the regenerated axon will sprout and grow into the distal nerve segment [12-14]. As the nerve fibers regenerate distally, and the axon sprouts that achieved appropriate distal connections with motor/sensory receptors will survived, that have not made such a connection may undergo a degenerative process and be pruned selectively away [10,15-18]. So, it may be possible to use nearby fewer nerve fibers or partial axons of a nearby nerve to repair more distal nerve fibers to shorten the regenerating distance and promote the functional recovery.
Although the regenerating axons can regenerate through the repair site towards distal targets after peripheral nerve injury, reinnervation of peripheral targets does not always lead to recovery of original functions. Some aberrant regeneration including misdirected reinnervation, hyperinnervation and polyinnervation, not only play important roles in the impairment of function after nerve injury and regeneration [19-22] but also may have direct and indirect impact on those parts of the central nervous system concerned with motor control [23]. The functional organization of motor neuron afferents should be reorganized according to the new peripheral pattern of connectivity [24]. Therefore, the functional recovery will depend on not only the connection between the peripheral nerve and its end-organs, but also the degree of compensatory central reorganization [25].
In this study, we focused on the possibility and reconstruction effects of using the proximal stump of the transected peroneal nerve to repair the distal stump of the same peroneal nerve and the distal stump of the transected tibial nerve. Therefore, the aim of this study was to investigate whether the proximal peroneal nerve stump fibers can grow into both the distal different nerves at the same time and can these regenerated collaterals contribute to the functional recovery of injured nerves. If yes, to see in which proportion the regenerating axons divide into the two distal nerve stumps. What is the ratio of distal stump axon numbers to proximal donor nerve axon numbers? And what kind of changes occurred in number of collaterals between 2 and 8 months? In addition, we wanted to examine the extent of spinal motor reorganization with the functional recovery of the two different target organs.
Materials and methods
Animal preparation
Forty-two female young adult Sprague Dawley rats (200-240 g), obtained from the Laboratory Animal Centre of Peking University (Beijing, China) were deeply anesthetized for all surgical procedures with sodium pentobarbital (30 mg/kg, i.p.). This study was performed in strict accordance with recommendations in the Institutional Animal Care Guidelines and approved ethically by the Administration Committee of Experimental Animals, Peking University People’s Hospital, Beijing, China (Permit Number: 2011-16). All efforts were made to minimize suffering.
Thirty-six female rats were randomly divided into 2 months group (2 M), 4 months group (4 M) and 8 months group (8 M); when evaluation of the functional and morphological recovery of the three groups, the non-operated side served as the normal control. Regeneration was assessed at 2 months, 4 months and 8 months after nerve transection and repair, respectively. Other six rats as the normal control group were used for normal nerve retrograde labeling.
Nerve injury and repair animal model
Experiments were performed under aseptic conditions on the right sciatic nerves. The surgical procedures were performed under a surgical microscope using standard microsurgical techniques. The sciatic nerve and its two main branches (the peroneal nerve and the tibial nerve) were exposed using a dorsal gluteal-splitting approach and were separated by gentle dissection to minimize tension on the subsequent repair site. The right peroneal nerve was transected with microscissor about 10 mm distal to the bifurcation point of the sciatic nerve. The proximal tibial nerve was ligated at the bifurcation site, then transected and sewn into the nearby muscle. The proximal stump of the transected peroneal nerve was used to repair the distal stump of the same peroneal nerve and the distal stump of the transected tibial nerve. 6 mm conical biodegradable chitin conduit (0.1 mm thick) was used at the repair site, a 2 mm small gap was left between the proximal and distal stumps of the repaired nerve and the nerve was fixed with a 10-0 nylon suture to the conduit (this surgical procedure has been described by Jiang Baoguo, 2006 [26]). Biodegradable chitin conduits (patented by our lab and authorized by the State Intellectual Property Office of the People’s Republic of China No. ZL01 136314.2; this conduit is now in a preclinical study) using in this study are artificial nerve grafts consisting of a polysaccharide shell that demonstrates satisfactory biocompatibility and degradation characteristics. Finally, the surgical site was then closed in layers with 4-0 nylon sutures (Figure 1).
Figure 1.
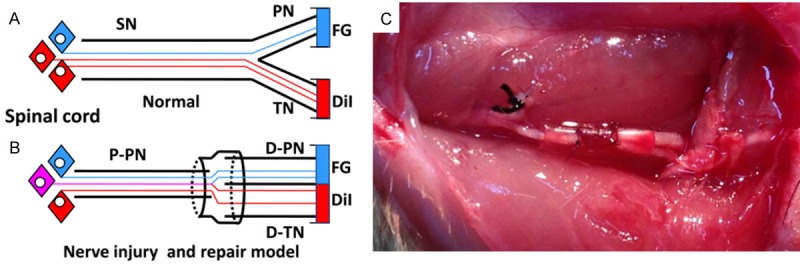
Diagrammatic representations of the following. A: The sciatic nerve with two branches, the peroneal and tibial nerve branches. All motor axons normally project to the two different branches separately. B: Axonal sprouts from transected axons in the parent nerve can regrow into both distal branches, after transection and repair, axons in the two branches are retrogradely labeled with different colored neurotracers and the motor neurons are counted. B and C: The surgical procedures for nerve injury and repair, the proximal tibial nerve was ligated at the bifurcation site, then transected and sewn into the nearby muscle. The 6 mm biodegradable chitin conduits were placed at the repair site and a 2 mm gap was left between the proximal peroneal nerve and distal peroneal and tibial nerve segments. SN: sciatic nerve; P-PN: proximal peroneal nerve; D-PN: distal peroneal nerve; D-TN: distal tibial nerve.
Sciatic function index
At the three endpoints, the SFI was used to assess functional recovery through the two-dimensional digital video analysis. Rats (2 M, 4 M and 8 M group, 12 rats in each group) were placed in a transparent runway (100 cm long, 15 cm wide, 20 cm tall) with a 45° angled mirror below the track (Figure 2). Rats were trained to walk inside the runway from one end to the other. Images were acquired with a digital camera (Panasonic DMC-LX5GK Osaka Japan) placed 100 cm from and perpendicular to the runway to prevent optical distortion. Measurements were taken from footprint images in the frame before the heel rise by manually identifying all toes. The most posterior point of the heel still in contact with the runway was determined from both the next frame (showing heel rise) and the side view of the ankle [27]. Footprint parameters for print length (from heel to toe, PL), toe spread (from first to fifth toe, TS), and intermediary toe spread (from second to fourth toe, IT) were recorded for the left normal control foot (NPL, NTS, and NIT) and the corresponding right experimental foot (EPL, ETS, and EIT) for each rat. The SFI was calculated as follows: SFI=-38.3[(EPL-NPL)/NPL]+109.5[(ETS-NTS)/NTS]+13.3[(EIT-NIT)/NIT]-8.8. An SFI of nearly 0 is normal, whereas an SFI of -100 indicates total impairment of the sciatic nerve.
Figure 2.
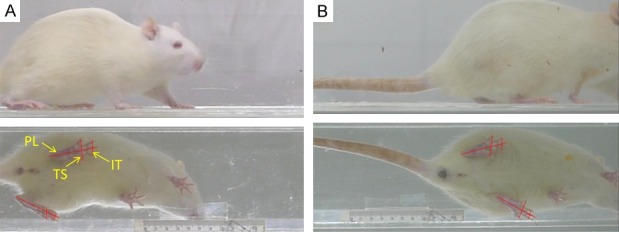
Example of frame from a digital video showing the rat in the transparent runway at 2 months (A) and 8 months (B). It also shows the mirror that was placed below the transparent track at a 45° angle to analyze the footprints for the print length (PL), toe spread (TS), and intermediate toe spread (IT).
Retrograde labeling and counting of motor neurons
For the 3 groups (2 M, 4 M and 8 M group, 6 rats in each group), the right sciatic nerve was re-exposed at the corresponding endpoints. The peroneal and tibial branches were isolated, cut, and backlabeled with neurotracers to identify the motor neurons innervating each branch (Figure 1B). The peroneal and tibial branches were cut 10 mm distal to the conduit. In each rat, one branch was labeled with FG (Fluorochrome LLC, Denver, CO, USA) and the other with DiI (1,1’-Dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate; Sigma-Aldrich 468495, Saint Louis, MO, USA) (in practice, the dye application was alternated between animals to control for possible differences in retrograde uptake and transport of the dyes). Backlabeling with FG was done by exposing the tip of the severed branch to 4% FG in cocodylic acid (pH 3) for 2 hr in a small polyethylene tube, the tube was sealed with a mixture of silicone grease and Vaseline to prevent leakage, the tube was then removed, the tip of the severed branch was extensively irrigated, sealed with silicone grease and reflected to a distant portion of the wound. The same way, backlabeling with DiI was done by exposing the tip of the severed branch to 15% DiI in 100% ethanol for 2 hr, and then irrigating the nerve and placing it in the opposite corner of the wound to prevent cross-contamination by diffusion of tracers [28]. Animals were kept for 7 days after tracer application to allow the retrograde tracers to travel back to the neuronal cell bodies.
Rats were deeply anesthetized and perfused through the left ventricle. A warm saline flush was followed by 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. After perfusion, the lumbar spinal cord L1-6 that includes all the sciatic motor neurons was removed and post-fixed for several hours in 4% paraformaldehyde and then cryoprotected in 20% sucrose overnight. The cord was frozen on dry ice and stored at -80°C until being sectioned with a cryostat. Serial 40-um frozen transverse spinal cord sections were mounted onto glass slides, air dried, and coverslipped with Prolong (P-7481, Molecular Probes) according to the manufacturer’s instructions.
The spinal cord sections were viewed by independent observers unaware of the experimental treatment under a fluorescence microscope (Olympus, BX51TR) equipped with a CCD camera (Olympus, DP70). The following Olympus mirror units were used: U-MWU2 (exciter filter 330-385 nm, dichroic beamsplitter 400 nm, barrier filter 420 nm) for FG, U-MWG2 (exciter filter 510-550 nm, dichroic beamsplitter 570 nm, barrier filter 590 nm) for DiI. Motor neurons were observed as either single labeled (FG or DiI only) or double labeled (both FG and DiI). Counting variation among the independent observers was approximately 2%. The presence of split cells in adjacent sections was corrected for by the method of Abercrombie [29]. Motor neurons were scored as projecting axons (1) to the peroneal branch, (2) to the tibial branch, or (3) simultaneously to both branches.
For the normal group (n=6), the right sciatic nerve was exposed using the same approach, the peroneal and tibial branches were cut 10 mm distal to the bifurcation point of the sciatic nerve. FG and DiI were applied to the two branches (Figure 1A) and the animals’ spinal cords prepared and analyzed as described above.
Electrophysiological tests and muscle force
The operated and non-operated sciatic nerves of three groups (2 M, 4 M and 8 M group, 6 rats in each group) were reexposed and carefully isolated from the surrounding tissue after nerve repair. The non-operated side served as the normal control. The stimulating bipolar electrodes were placed proximal and distal to the repair site in each group sequentially. The recording electrode was placed in the peroneal-innervated muscles (the anterior and lateral compartments of the lower leg) or tibial-innervated muscles (the posterior compartments of the lower leg), while the ground electrode went subcutaneously, between the stimulating and recording electrodes. The electrical stimuli (5 V in intensity, 0.1 ms in duration, 1 Hz in frequency) (MedlecSynergy; Oxford Instrument Inc, United Kingdom) was applied to the repaired peroneal or tibial nerve. The latency of Compound Muscle Action Potentials (CMAP) was recorded. The distance between the distal and proximal stimulated sites was measured to calculate the motor nerve conduction velocity (NCV) of the experimental side and control side.
Recovery of the muscle strength was determined by measuring a twitch tension and tetanic tension in peroneal-innervated muscles or tibial-innervated muscles. The muscles from the experimental side were freed from its surroundings, leaving the proximal origin intact. The knee was fixed with clamps. The distal tendon of the muscles was connected to force transducers (MLT500/D; Force Transducer, AD Instruments) using a nylon ligature. Hook-shaped stimulating electrodes were placed on the peroneal or tibial nerve trunk proximal to the repair site. While the single maximal stimulus was then delivered to the peroneal or tibial nerve, the twitch tension of the peroneal-innervated muscles or tibial-innervated muscles was recorded at the optimal muscle length. Tetanic tension was subsequently determined with a 50-Hz electronic stimulation. The monitoring data were recorded and analyzed using the Scope software (version 3.6.12). The muscle strength of the control side was measured as well.
Evaluation of the peroneal-innervated or tibial-innervated muscles
After the electrophysiological tests, the peroneal-innervated or tibial-innervated muscles of the experimental side and the control side were harvested and their wet weights were measured. Muscle samples were cut from the midbelly of the harvested muscles and fixed in a buffered 4% paraformaldehyde solution. Afterwards, the muscle samples were cut and subsequently washed in water, dehydrated in a graded ethanol series, cleared in xylene, embedded in paraffin and cut into 5 um thick transverse sections. Following the H&E staining, the sample was photographed with a DFC 300FX color digital camera (Leica, Heidelberg, Germany) to measure the cross-sectional area of muscle fibers. For each in four H&E stained sections of every specimen, the images were taken from four random fields and analyzed with a Leica QWin software package Q550 IW image analysis system (Leica Imaging Systems Ltd., Cambridge, England).
Histological analysis for nerve regeneration
After the muscles were dissected out, the segments of the distal peroneal and tibial nerve at 2 mm distal to the repair site and the normal peroneal or tibial nerve at the same level were harvested. The proximal peroneal nerve at 2 mm proximal to the repair site was harvested as well. After these segments were fixed in 1% osmium tetroxide for 24 hours, they were dehydrated with ethanol and embedded in paraffin. The specimen blocks were cross-sectioned at a thickness of 5 um using an ultramicrotome. All sections were then transferred to adhesion microscope slides. The nerve sections were examined and digitized images were obtained using a DFC 300FX color digital camera (Leica, Heidelberg, Germany). The number of myelinated fiber; diameters of the myelinated axons and the thickness of myelin sheaths were examined from the digitized images.
Statistical analysis
The SPSS 17.0 software package (SPSS Inc., USA) was used for statistical analysis. Experimental data were compared using the Student’s t test and One-Way ANOVA followed by Student-Neuman-Keuls test. Differences were considered statistically significant when P < 0.05.
Results
General observations
All of the rats used in this study survived in the experiments. None of rats showed signs of systemic or regional inflammation and serious surgical complications following the surgeries. Significant muscle atrophy was observed in all rats. The distal peroneal and tibial nerve of the animals in 2 M group showed mild swelling. The proximal peroneal nerve can reinnervate the distal peroneal and tibial nerve at different times after nerve repair (Figure 3). The regenerated nerve in conduit grew smoothly, without neuroma formation at 2 months after nerve repair (Figure 3A). The biocompatibility of biodegradable chitin conduit in rats was quite good, and the conduit was almost absorbed at 8 months after nerve repair (Figure 3B).
Figure 3.
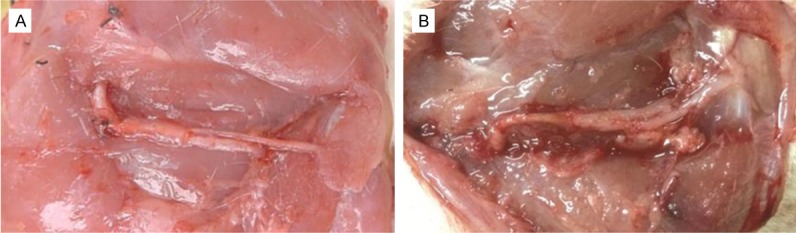
The observation of operation at 2 months (A) and 8 months (B) after nerve repair. The proximal peroneal nerve can reinnervate the distal peroneal and tibial nerve after nerve repair. The regenerated nerve in conduit grew smoothly, without neuroma formation at 2 months after nerve repair (A), and the conduit was almost absorbed at 8 months after nerve repair (B).
Sciatic functional index
Walking track analysis of rats showed that SFI in the 2 M, 4 M and 8 M groups was -76.55±4.57, -55.32±3.25, and -32.64±2.23, respectively. Figure 2 showed the footprint analysis and optimal recovery of the TFI obtained 8 months after nerve repair. The value in the 8M group was significantly higher compared with the 2 M group or 4 M group (P < 0.05) (Figure 4). Through the two-dimensional digital video finding that although the SFI had been significantly improved from 2 to 8 months, active ankle plantar flexion and dorsiflexion or motor readjustment were still limited even at 8 months after nerve repair and the plantar muscle atrophy was more obvious compared to the control side.
Figure 4.
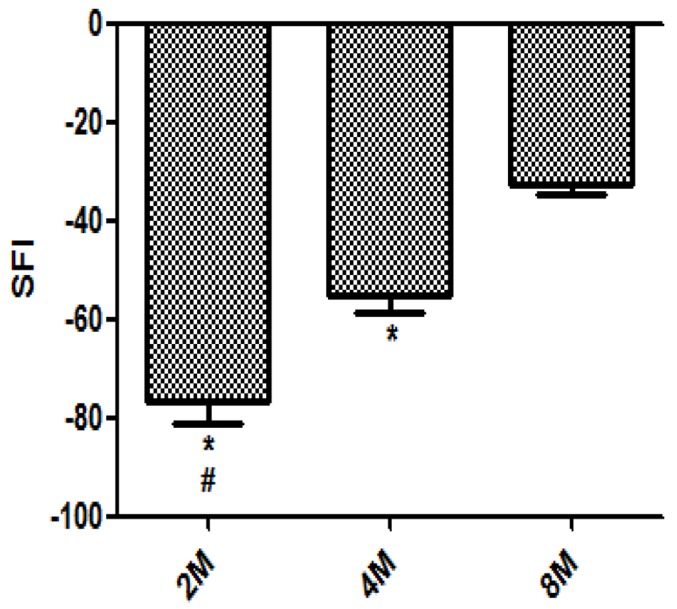
SFI: Sciatic Functional Index. *P < 0.05 versus 8 M. #P < 0.05 versus 4 M. n=12, (Bars=SD).
Retrograde labeling and counting of motor neurons
For the normal group, motor neurons were identified in the spinal cord as being labeled from peroneal or tibial branch and no double-labeled motor neurons were found in the spinal cord. Motor neurons labeled after retrograde transport of tracers from the peroneal nerve of normal animals were confined to a crescentic pool at the lateral margin of the anterior horn, and the normal tibial motor neuron pool is central and superior to that of the peroneal nerve, and extends farther caudally (Figure 5A-C). Similar localization has been observed by others [25,30,31]. The peroneal and tibial nerve, contained 515±53, 1144±163 motor neurons, respectively (Table 1), and these total motor neuron numbers are in agreement with previously published studies [30,31].
Figure 5.
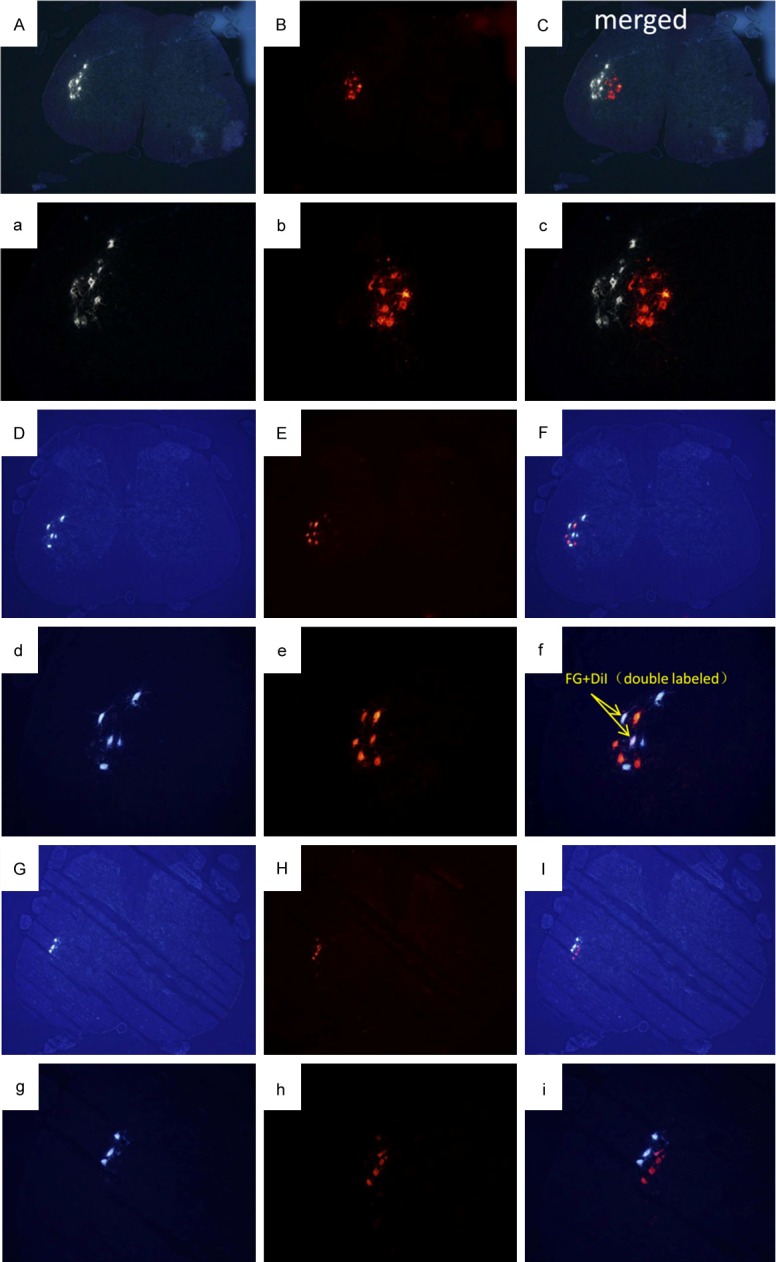
Images of transverse spinal sections of retrograde labeled motor neurons. Section demonstrating labeled cells with excitation of FG in A(a), D(d), G(g) and DiI in B(b), E(e), H(h). FG was applied to the peroneal nerve branch and DiI to the tibial nerve branch. As the merged images shown here, retrogradely labeled motor neurons that contained only one of the labels (indicating axonal distribution to a single nerve branch) or both labels (FG+DiI) were counted, indicating a simultaneous axonal distribution to both nerve branches. A(a)-C(c): Distribution of normal peroneal and tibial motor neurons, FG-labeled motor neurons are grouped in a crescentic pool at the lateral margin of the grey matter, DiI-labeled motor neurons are grouped centrally and superiorly to those of the peroneal pool. D(d)-I(i): Distribution of labeled motor neurons after nerve injury and repair, all labeled cells were confined to the normal peroneal distribution. D(d)-F(f) showed disorganized distribution of motor neurons. G(g)-I(i) showed segregated location of the two different motor neurons. Images (A-I): Magnification ×40, (a-i): Magnification ×100.
Table 1.
Measurements of retrogradely labeled motor neurons in the 2 M, 4 M, 8 M, and normal groups (Mean ± SD)
| Measurement | 2 M (n=6) | 4 M (n=6) | 8 M (n=6) | Normal (n=6) |
|---|---|---|---|---|
| Peroneal nerve branch | 130±12 | 141±25 | 148±12 | 515±53 |
| Tibial nerve branch | 273±22 | 299±43 | 311±39 | 1144±163 |
| Both branches | 51±7*,# | 36±10* | 10±3 | 0 |
| Tibial/peroneal number ratio | 2.11±0.05 | 2.13±0.08 | 2.09±0.12 | 2.21±0.09 |
P < 0.05 versus 8 M.
P < 0.05 versus 4 M.
From Figure 5D-I, finding that all labeled motor neurons in the spinal cord after nerve injury and repair were always confined within the normal peroneal motor neuron pool. Nearly all the distribution of motor neurons labeled via distal peroneal and tibial nerves was disorganized as compared to normal group (Figure 5D-F), except some profiles showed segregated location of the two different motor neurons (Figure 5G-I). However, there was a significant decline in the number of double-labeled motor neurons (P < 0.05) (Table 1). The number of motor neuron from the peroneal (or tibial) branch at three endpoints was 130±12 (273±22), 141±25 (299±43), 148±12 (311±39), respectively. The interesting finding was that the tibial/peroneal motor neuron number ratio, that in operated groups was 2.11±0.05, 2.13±0.08, 2.09±0.12, respectively, was close to the normal group (2.21±0.09)
Electrophysiological tests and muscle force
The results of nerve conduction velocity were indicated in Table 2. The motor nerve conduction velocities of distal regenerated peroneal or tibial nerve in three groups were significantly slower than that of the control sides (P < 0.05). From Table 2, it was demonstrated that the recovery level of electrophysiological properties was improved gradually, the motor nerve conduction velocities of distal regenerated nerve fibers in 8 M group was higher than that in 2 M or 4 M group (P < 0.05). No statistical differences were seen between the two distal regenerated nerves in each group (P > 0.05).
Table 2.
Measurements of contraction force on muscles and motor nerve conduction velocity (Mean ± SD)
| Measurement | 2 M (n=6) | 4 M (n=6) | 8 M (n=6) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Operated side | Control side | Operated side | Control side | Operated side | Control side | ||
| Twitch tension (N) | PN-M | 0.57±0.05*,# | 1.21±0.06 | 0.92±0.07* | 1.48±0.03 | 1.25±0.02 | 2.40±0.04 |
| TN-M | 0.82±0.07*,# | 1.72±0.04 | 1.07±0.09* | 2.06±0.08 | 1.75±0.05 | 3.25±0.07 | |
| Tetanic tension (N) | PN-M | 1.44±0.11*,# | 2.94±0.37 | 2.21±0.05* | 3.52±0.27 | 3.06±0.44 | 4.66±0.16 |
| TN-M | 2.14±0.11*,# | 4.58±0.19 | 3.17±0.29* | 5.15±0.33 | 4.62±0.23 | 6.84±0.23 | |
| MNCV (m/s) | PN | 16.37±2.18*,# | 55.03±2.22 | 20.68±1.88* | 55.56±3.07 | 28.49±2.90 | 58.55±4.87 |
| TN | 13.71±2.30*,# | 54.22±3.83 | 21.47±2.24* | 57.48±4.52 | 30.92±3.26 | 56.45±2.11 | |
PN-M (TN-M): peroneal-innervated muscles (tibial-innervated muscles). PN (TN): peroneal nerve (tibial nerve).
P < 0.05 versus 8 M.
P < 0.05 versus 4 M.
The muscle’s contraction force, containing twitch and tetanic tension, of the animals were shown in Table 2. The mean twitch and tetanic tensions of the peroneal-innervated muscles or tibial-innervated muscles on the operated sides in three groups were significantly lower than the control sides (P < 0.05). The mean twitch and tetanic tensions after nerve surgery in 8 M group were higher than that in 2 M or 4 M group (P < 0.05).
Evaluation of the muscle
The wet weight of peroneal-innervated muscles or tibial-innervated muscles after nerve surgery was significantly smaller than that on the control side. The wet weight on the operated side in 8 M group was higher than that in 2 M or 4 M group (P < 0.05) (Table 3).
Table 3.
Measurements of wet weight and cross-sectional area on muscles (Mean ± SD)
| Measurement | 2 M (n=6) | 4 M (n=6) | 8 M (n=6) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Operated side | Control side | Operated side | Control side | Operated side | Control side | ||
| Wet weight (g) | PN-M | 0.40±0.04*,# | 1.10±0.07 | 0.57±0.04* | 1.28±0.05 | 1.15±0.10 | 1.66±0.10 |
| TN-M | 1.08±0.06*,# | 2.11±0.10 | 1.33±0.08* | 2.92±0.12 | 2.43±0.07 | 3.63±0.15 | |
| Cross-sectional area (um2) | PN-M | 509.07±37.79*,# | 877.32±62.32 | 786.30±74.38* | 1101.84±136.67 | 1233.26±71.73 | 1538.48±100.88 |
| TN-M | 517.17±43.63*,# | 902.95±74.46 | 726.83±46.99* | 1135.57±113.28 | 1245.50±59.94 | 1586.54±76.41 | |
PN-M (TN-M): peroneal-innervated muscles (tibial-innervated muscles).
P < 0.05 versus 8 M.
P < 0.05 versus 4 M.
The transverse sections of the muscles were displayed in Figure 6. The peroneal-innervated muscles or tibial-innervated muscles fiber boundary on the control side was clear. Sections from the operated side displayed unclear boundary. Significant muscle atrophy was observed on the operated side. The fiber cross-sectional area after nerve surgery was significantly smaller than that of the control side at different times in each group (P < 0.05). The fiber cross-sectional area on the operated side in 8 M group was higher than that in 2 M or 4 M group (P < 0.05) (Table 3). There was no significant difference on the operated side between the fiber cross-sectional area of the two different muscles in each group (P > 0.05).
Figure 6.
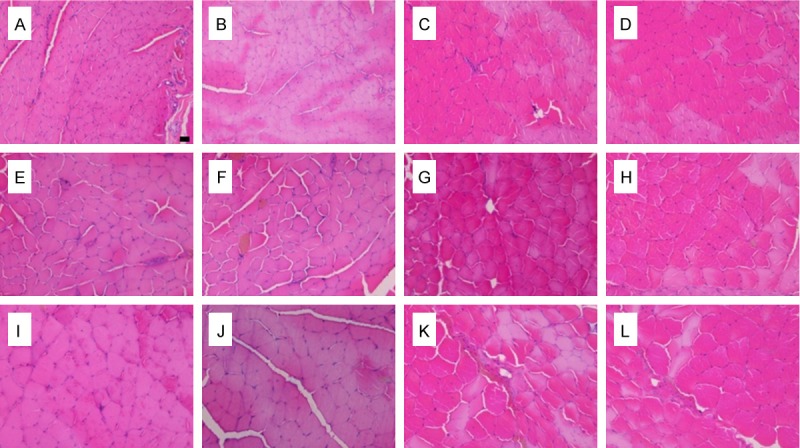
Light microscopy images of transverse sections of the peroneal-innervated and tibial-innervated muscles, A-D, E-H, I-L: Images at 2, 4 and 8 months after nerve repair, respectively; A, E and I: Peroneal-innervated muscles on the operated side; B, F and J: Tibial-innervated muscles on the operated side; C, G and K: Normal peroneal-innervated muscles; D, H and L: Normal tibial-innervated muscles. (Scale bar=10 uM).
Histological analysis for nerve regeneration
At the three endpoints after surgery, the sectioned nerves from each group were stained with osmium tetroxide. The micrographs of transverse sections of the peroneal and tibial nerve were displayed in Figure 7. The distal peroneal and tibial nerve segments at 2 mm distal to the repair site in each group revealed that the regenerated myelinated fibers occurred, with a higher density but smaller fiber size compared to the control sides. For the three groups, the regenerated motor axons were evenly distributed and the diameter of the fibers was similar.
Figure 7.
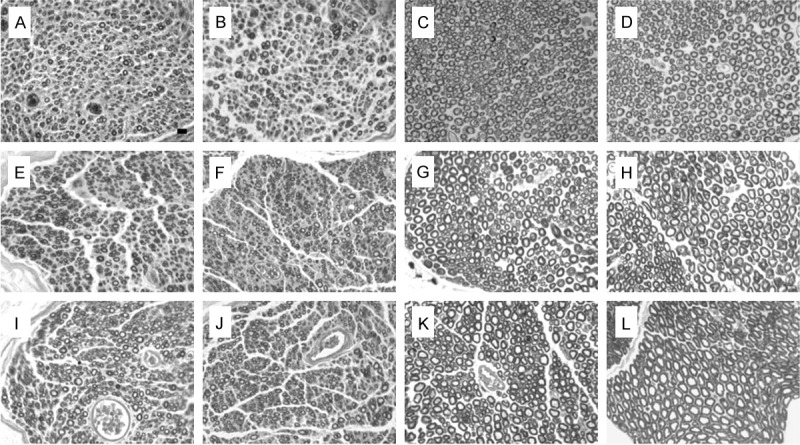
Light microscopy images of transverse sections of the peroneal and tibial nerve. A-D, E-H, I-L: Images at 2, 4 and 8 months after nerve repair, respectively; A, E and I: Regenerated peroneal nerve; B, F and J: Regenerated tibial nerve; C, G and K: Normal peroneal nerve; D, H and L: Normal tibial nerve. (Scale bar=10 uM).
The results of the morphological analysis are summarized in Figure 8. The total myelinated fiber count of the distal regenerated nerves at the three different times was 7968±469, 8052±389 and 7832±424, respectively. Although there was a decline in the number of distal regenerated myelinated axons in 8 M group compared with the 2 M or 4 M group, this difference was not statistically significant (P > 0.05). Statistical analysis showed that regenerated fibers of both distal peroneal and tibial nerves were significantly more numerous and densely packed than normal nerves. The ratio of distal regenerated axon numbers (including distal regenerated peroneal and tibial nerve fibers) to proximal donor nerve axon numbers is about 3.95±0.10, 4.06±0.19 and 3.87±0.23, respectively. On the other hand, the myelin sheath thickness as well as the axonal diameter and axonal area of myelinated nerves on the operated side in 8 M group was higher than that in 2 M or 4 M group (P < 0.05).
Figure 8.
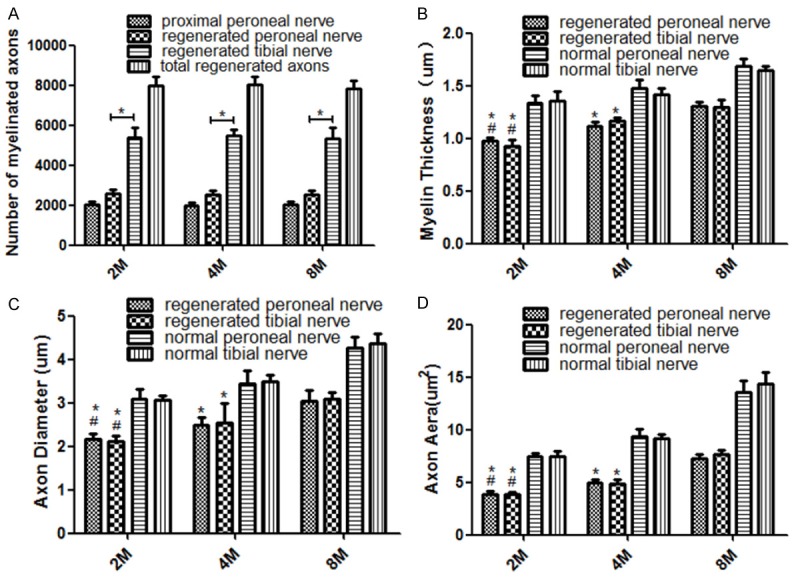
The nerve morphological parameters of the 2 M (n=6), 4 M (n=6) and 8 M (n=6) groups. A: The number of myelinated axons, most of regenerated collaterals reinnervated the distal tibial nerve, the ratio of total regenerated axon numbers to proximal peroneal nerve axon numbers was 3.95±0.10, 4.06±0.19 and 3.87±0.23, respectively. *P < 0.05; B: The myelin sheath thickness; C: The axon diameter; D: The axon area. B-D: *P < 0.05 versus 8 M, #P < 0.05 versus 4 M. (Bars=SD).
The morphological comparison between the distal regenerated peroneal and tibial nerves showed no statistically significant differences except for the total number of myelinated fibers, which was higher in the tibial nerve (P < 0.05), i.e., the larger of the two nerve trunks. The myelin sheath thickness of the distal regenerated nerve segments as well as the axonal diameter and axonal area of myelinated nerves were significantly lower than un-operated control nerve fibers (P < 0.05).
Discussion
Peripheral nerve injuries such as severe extended nerve defects or proximal nerve trunks injuries present a complex reconstructive challenge. Those injuries always result into poor outcomes because of the long regeneration distance for the regenerated axons to reinnervate their original target end-organs. As the low rate of axon regeneration [6], effective recovery for nerve regeneration over a long distance was difficult to achieve [32]. The prolonged denervation of distal nerve segments and target organs heavily influenced the restoration of muscle function [33]. Thus, if the injured location was too far away from its target organs, no good prognosis could be obtained, even though the best repair technology was used. Typically, the denervated muscles had degenerated to an unrecoverable level before the nerve reinnervation [8] over several months. Therefore, the time for the reconstruction of an injured nerve is an important factor that can greatly affect the reconstructive effects. Previous work including from this laboratory, had shown that outgrowth of collateral sprouts from both afferent and motor axons was a natural process that occurred during development of and regeneration in the peripheral nervous system [9-11]. The proximal stump of damaged axons will regenerate many sprouts into the distal endoneurial tubes after peripheral nerve injury [10,13,34]. In order to shorten the regeneration time, a smaller donor nerve not far from the denervated muscles may be used to repair the distal injured nerves. In the present study, the proximal peroneal nerve was used to repair both the distal peroneal and tibial nerve.
HOWE et al. found numerous fibers having three or four branches in the facial nerve of the monkey 44 days after operation [35]. Other studies reported that the total number of regenerated nerve fibers in the distal regenerated nerves was 3-5 fold the proximal donor nerve [13,18,36]. Here, the ratio of distal regenerated axon numbers to the proximal donor axon numbers was about 4.0, and the number of regenerating axons at the distal peroneal nerve or tibial nerve was greater than the normal control. The number of axons that regenerate is related to the length of the proximal and distal nerve ends when the gap is made [9]. With the longer the gap, the less likely axons were to cross, when the gap was increased to 15 mm, no regeneration occurred [37]. Jenq and Coggeshall also demonstrated that the number of axons that regenerate into the distal nerve stump at a particular time after transection was partially dependent on the type of lesion and proportionally fewer axons regenerate into the distal nerves following the 8 mm gap transection [9]. In this study, the gap between the proximal and distal segments was 2 mm, Jiang and Zhang reported that the suitable regeneration gap for rat peripheral nerve was 1-2 mm, and it could not facilitate the nerve regeneration effects when the gap exceeded 5 mm [38]. The number of endoneurial tubes in the distal stump is another important factor influencing the sprouting of regenerative axons. If there were enough distal endoneurial tubes into which the proximal regenerative axons can grow, the regenerated axon will sprout and maintain the most possible collaterals [18].
However, due to limitations of cell metabolism, the cell body may be unable to maintain an adequate supply of necessary nutrient constituents to many collaterals. Possibly only those fibers making contact with the periphery can survive, and the fact that many of the new axons do not become myelinated may also have some bearing on their fate [13,18]. Once the axons enter the denervated muscle, they sprout and form several new branches which ultimately send out multiple synapses on the same fiber, and with the mechanical activity and consequent recovery of muscle efficiency, the newly-formed synapses will go through a period of successive maturation and elimination of the redundant innervations [12]. Cass et al. reported that regenerating axons inhibited the function of the collaterals in axolotl leg muscles [39]. That inhibition of the collaterals had been consistent with other studies [40,41]. Mackinnon et al. demonstrated that the number of nerve fibers in the distal nerve increase as early as 1 month following the nerve repair, reached the maximum at 3 months and would subsequently decrease to normal values after 2 years [10]. While Shawe found that the fiber count of the nerve was about twice higher than normal and remained even 200 days after the lesion [13]. Horch and Lisney indicated that one and a half years after transaction of cutaneous nerves, the regenerating neurons supported multiple sprouts in the distal stump of the nerve [34]. In our study, the results demonstrated that although there was a decline in number of regenerated nerve fibers between 2 and 8 months, this difference was not significant. This study did not evaluate time periods after 8 months, what about the number of regenerated nerve fibers and how about the functional recovery after 8 months will be studies in the future.
Our data showed that most of regenerated collaterals reinnervate into the distal tibial nerve, which means that the topographic specificity of regeneration at the level of the nerve trunk has not been established, this finding was confirmed by earlier studies indicating a nonspecific reinnervation of muscles [25,30,36], however some studies have demonstrated topographic specificity [42,43]. The discrepancy among those studies may be explained by several factors. The size of the distal nerve must be firstly considered [36,44]. The higher percentage of nonspecific regenerated collaterals may be explained by the relatively larger size of the tibial nerve in this study. With the larger size, it may provide more endoneurial tubes for regenerative axons to reinnervate or provides the greater amount of trophic support [45]. Second, later correcting for misdirected axon collaterals [12,19], as the nerve fibers regenerate distally, and appropriate distal connections with sensory/motor receptors are achieved, the axon sprouts that have not made such a connection may go through a degenerative process and be prune selectively away [10,17]. Third, retrograde tracing technique must be considered. The retrograde tracers DiI and FG using in our study have the highest and similar labeling efficacy and their combinations are also most suitable for double retrograde labeling studies [46]. The number of DiI labeled motor neurons in normal animals was not statistically significantly different from the number of FG labeled motor neurons in preliminary experiments, in addition, the recovery period of 7 days was chosen in this study to allow enough time for retrograde transport and tracer accumulation in the neuron body. Other factors including the animal model and age of the animal may influence the specificity of regeneration [45,47].
In this study, although the distal injured nerves showed a good functional and morphological recovery, it could only be a proof of structural reinnervation. The functional recovery will depend on not only the connection between the peripheral nerve and its end-organs, but also the effective control from the central nerve system on the peripheral reinnervated end-organs [25]. The failure of many anterior horn motor neurons to regain peripheral connections regardless of repair technology must reflect inherent limitations in the response to peripheral nerve injury [25]. de Ruiter et al. reported that central adaptation may be another mechanism that may later correct for misdirection, finding that after sciatic nerve crush injury different labeled motor neurons were more organized in the anterior horn than they were after epineurial neurorrhaphy and nerve autograft [44]. In this study, all labeled motor neurons in the spinal cord after nerve injury and repair were always confined within the normal peroneal nerve projection, in addition, an interesting finding was that the peroneal/tibial motor neuron number ratio in operated groups was close to the ratio of normal group; however, nearly all the distribution of motor neurons labeled via distal different nerves was disorganized as compared to normal group. This disarrangement of the spinal motor reorganization could result from non-specificity of regenerating axons at the repair site [48] and may have a serious impact on voluntary motor performance and control [23]. The axonal pruning is commonly considered to be at the basis of the functional adaptation to the new connections. In our study, there was a significant decline in the number of double labeled motor neurons [19]. It is obvious that when a donor nerve was used to repair the distal two different injured nerves innervating antagonistic muscles that have opposite functions simultaneously, the donor nerve would control the two different end-organs [19,36]. Although the SFI has been significantly improved between 2-8 months, active ankle plantar flexion and dorsiflexion or motor readjustment was still obviously limited at 8 months after nerve repaired. Earlier studies indicated that motor readjustment may occur after crossing of nerves to antagonistic muscles in higher mammals, but were difficult to achieve in lower animals [49,50]. One explanation for this was that a cortical impulse to flex the ankle would result in the simultaneous contraction of antagonistic muscles and thus in repression of motor control [25]. Therefore, spinal reorganization is needed for recovery of peripheral nerve injury, and brain reorganization related to major changes in the peripheral connections is a more important factor for the real function recovery of the reconstructed nerves [25].
In this study, the interesting finding of this study is that it is possible to use one donor nerve to repair the donor itself and the injured nerve. Here, the peroneal nerve served as the donor nerve to restore the distal peroneal nerve and tibial nerve simultaneously. Our data revealed that the proximal peroneal nerve can grow into both the distal tibial nerve at the same time and establish two different neural conduction pathways [19]. Both recoveries of the two different nerves which have opposite functions were observed in the experiment. Therefore, with this kind of reconstruction method the regenerated axons can reinnervate both the distal donor nerve and the injured nerve. That means separating a nearby intact nerve as donor nerve to repair the injured nerve and donor itself simultaneously can not only get good reconstructive effects from the injured nerve but also restore partial function of the donor nerve. However, although both donor and injured nerve can be restored to a certain degree, there is a risk when cutting an intact nerve trunk nearby as a donor nerve. So, the donor nerve which had been injured or had relatively less important function would be the best choice. A partial transection of the donor nerve’s fibers can also be used to reconstruct the injured nerves. Partial denervation of a target organ may not influence its physiological functioning greatly [51,52].
In summary, the results of this study indicate that, in the rat peroneal/tibial nerve injury and repair model, fewer nerve fibers, can regenerate a large number of collaterals which successfully repopulate both distal nerves and lead to the partial recovery of lost functions. It may provide a new method to repair severe extended nerve defects or proximal nerve trunks injuries.
Acknowledgements
This research project was funded by the Chinese National Ministry of Science and Technology 973 Project Planning (No. 2014CB-542200), the ministry of education innovation team (IRT1201), the National Natural Science Fund (31271284, 31171150, 81171146, 3097-1526, 31040043, 31371210, 81372044, 31471144), the Beijing Natural Science Foundation: 7142164.
Disclosure of conflict of interest
None.
References
- 1.Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008;87:381–385. doi: 10.1097/PHM.0b013e31815e6370. [DOI] [PubMed] [Google Scholar]
- 2.Sungpet A, Suphachatwong C, Kawinwonggowit V, Patradul A. Transfer of a single fascicle from the ulnar nerve to the biceps muscle after avulsions of upper roots of the brachial plexus. J Hand Surg Br. 2000;25:325–328. doi: 10.1054/jhsb.2000.0367. [DOI] [PubMed] [Google Scholar]
- 3.Tung TH, Mackinnon SE. Flexor digitorum superficialis nerve transfer to restore pronation: two case reports and anatomic study. J Hand Surg Am. 2001;26:1065–1072. doi: 10.1053/jhsu.2001.28427. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto K, Ohnishi K, Kiyotani T, Sekine T, Ueda H, Nakamura T, Endo K, Shimizu Y. Peripheral nerve regeneration across an 80-mm gap bridged by a polyglycolic acid (PGA)-collagen tube filled with laminin-coated collagen fibers: a histological and electrophysiological evaluation of regenerated nerves. Brain Res. 2000;868:315–328. doi: 10.1016/s0006-8993(00)02207-1. [DOI] [PubMed] [Google Scholar]
- 5.Vasconcelos BC, Gay-Escoda C. Facial nerve repair with expanded polytetrafluoroethylene and collagen conduits: an experimental study in the rabbit. J Oral Maxillofac Surg. 2000;58:1257–1262. doi: 10.1053/joms.2000.16626. [DOI] [PubMed] [Google Scholar]
- 6.Sulaiman OA, Gordon T. Effects of short- and long-term Schwann cell denervation on peripheral nerve regeneration, myelination, and size. Glia. 2000;32:234–246. doi: 10.1002/1098-1136(200012)32:3<234::aid-glia40>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Borisov AB, Dedkov EI, Carlson BM. Interrelations of myogenic response, progressive atrophy of muscle fibers, and cell death in denervated skeletal muscle. Anat Rec. 2001;264:203–218. doi: 10.1002/ar.1155. [DOI] [PubMed] [Google Scholar]
- 8.Aydin MA, Mackinnon SE, Gu XM, Kobayashi J, Kuzon WM Jr. Force deficits in skeletal muscle after delayed reinnervation. Plast Reconstr Surg. 2004;113:1712–1718. doi: 10.1097/01.prs.0000118049.93654.ca. [DOI] [PubMed] [Google Scholar]
- 9.Jenq CB, Coggeshall RE. Numbers of regenerating axons in parent and tributary peripheral nerves in the rat. Brain Res. 1985;326:27–40. doi: 10.1016/0006-8993(85)91381-2. [DOI] [PubMed] [Google Scholar]
- 10.Mackinnon SE, Dellon AL, O’Brien JP. Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve. 1991;14:1116–1122. doi: 10.1002/mus.880141113. [DOI] [PubMed] [Google Scholar]
- 11.Morris JH, Hudson AR, Weddell G. A study of degeneration and regeneration in the divided rat sciatic nerve based on electron microscopy. II. The development of the “regenerating unit”. Z Zellforsch Mikrosk Anat. 1972;124:103–130. [PubMed] [Google Scholar]
- 12.Gorio A, Carmignoto G, Finesso M, Polato P, Nunzi MG. Muscle reinnervation--II. Sprouting, synapse formation and repression. Neuroscience. 1983;8:403–416. doi: 10.1016/0306-4522(83)90188-4. [DOI] [PubMed] [Google Scholar]
- 13.Shawe GD. On the number of branches formed by regenerating nerve-fibres. Br J Surg. 1955;42:474–488. doi: 10.1002/bjs.18004217505. [DOI] [PubMed] [Google Scholar]
- 14.Zhang P, Kou Y, Yin X, Wang Y, Zhang H, Jiang B. The experimental research of nerve fibers compensation amplification innervation of ulnar nerve and musculocutaneous nerve in rhesus monkeys. Artif Cells Blood Substit Immobil Biotechnol. 2011;39:39–43. doi: 10.3109/10731199.2010.494583. [DOI] [PubMed] [Google Scholar]
- 15.Brushart TM. Preferential reinnervation of motor nerves by regenerating motor axons. J Neurosci. 1988;8:1026–1031. doi: 10.1523/JNEUROSCI.08-03-01026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brushart TM. Motor axons preferentially reinnervate motor pathways. J Neurosci. 1993;13:2730–2738. doi: 10.1523/JNEUROSCI.13-06-02730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brushart TM, Seiler WA 4th. Selective reinnervation of distal motor stumps by peripheral motor axons. Exp Neurol. 1987;97:289–300. doi: 10.1016/0014-4886(87)90090-2. [DOI] [PubMed] [Google Scholar]
- 18.Jiang BG, Yin XF, Zhang DY, Fu ZG, Zhang HB. Maximum number of collaterals developed by one axon during peripheral nerve regeneration and the influence of that number on reinnervation effects. Eur Neurol. 2007;58:12–20. doi: 10.1159/000102161. [DOI] [PubMed] [Google Scholar]
- 19.Hennig R, Dietrichs E. Transient reinnervation of antagonistic muscles by the same motoneuron. Exp Neurol. 1994;130:331–336. doi: 10.1006/exnr.1994.1211. [DOI] [PubMed] [Google Scholar]
- 20.Streppel M, Angelov DN, Guntinas-Lichius O, Hilgers RD, Rosenblatt JD, Stennert E, Neiss WF. Slow axonal regrowth but extreme hyperinnervation of target muscle after suture of the facial nerve in aged rats. Neurobiol Aging. 1998;19:83–88. doi: 10.1016/s0197-4580(97)00163-2. [DOI] [PubMed] [Google Scholar]
- 21.Sumner AJ. Aberrant reinnervation. Muscle Nerve. 1990;13:801–803. doi: 10.1002/mus.880130905. [DOI] [PubMed] [Google Scholar]
- 22.Valero-Cabre A, Navarro X. Functional impact of axonal misdirection after peripheral nerve injuries followed by graft or tube repair. J Neurotrauma. 2002;19:1475–1485. doi: 10.1089/089771502320914705. [DOI] [PubMed] [Google Scholar]
- 23.Valero-Cabre A, Tsironis K, Skouras E, Navarro X, Neiss WF. Peripheral and spinal motor reorganization after nerve injury and repair. J Neurotrauma. 2004;21:95–108. doi: 10.1089/089771504772695986. [DOI] [PubMed] [Google Scholar]
- 24.Gruart A, Streppel M, Guntinas-Lichius O, Angelov DN, Neiss WF, Delgado-Garcia JM. Motoneuron adaptability to new motor tasks following two types of facial-facial anastomosis in cats. Brain. 2003;126:115–133. doi: 10.1093/brain/awg008. [DOI] [PubMed] [Google Scholar]
- 25.Brushart TM, Tarlov EC, Mesulam MM. Specificity of muscle reinnervation after epineurial and individual fascicular suture of the rat sciatic nerve. J Hand Surg Am. 1983;8:248–253. doi: 10.1016/s0363-5023(83)80152-x. [DOI] [PubMed] [Google Scholar]
- 26.Jiang B, Zhang P, Zhang D, Fu Z, Yin X, Zhang H. Study on small gap sleeve bridging peripheral nerve injury. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:55–74. doi: 10.1080/10731190500430149. [DOI] [PubMed] [Google Scholar]
- 27.de Ruiter GC, Spinner RJ, Alaid AO, Koch AJ, Wang H, Malessy MJ, Currier BL, Yaszemski MJ, Kaufman KR, Windebank AJ. Two-dimensional digital video ankle motion analysis for assessment of function in the rat sciatic nerve model. J Peripher Nerv Syst. 2007;12:216–222. doi: 10.1111/j.1529-8027.2007.00142.x. [DOI] [PubMed] [Google Scholar]
- 28.Novikova L, Novikov L, Kellerth JO. Persistent neuronal labeling by retrograde fluorescent tracers: a comparison between Fast Blue, Fluoro-Gold and various dextran conjugates. J Neurosci Methods. 1997;74:9–15. doi: 10.1016/s0165-0270(97)02227-9. [DOI] [PubMed] [Google Scholar]
- 29.Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 30.Brushart TM, Mesulam MM. Alteration in connections between muscle and anterior horn motoneurons after peripheral nerve repair. Science. 1980;208:603–605. doi: 10.1126/science.7367884. [DOI] [PubMed] [Google Scholar]
- 31.Swett JE, Wikholm RP, Blanks RH, Swett AL, Conley LC. Motoneurons of the rat sciatic nerve. Exp Neurol. 1986;93:227–252. doi: 10.1016/0014-4886(86)90161-5. [DOI] [PubMed] [Google Scholar]
- 32.Kawabuchi M, Zhou CJ, Wang S, Nakamura K, Liu WT, Hirata K. The spatiotemporal relationship among Schwann cells, axons and postsynaptic acetylcholine receptor regions during muscle reinnervation in aged rats. Anat Rec. 2001;264:183–202. doi: 10.1002/ar.1159. [DOI] [PubMed] [Google Scholar]
- 33.Hoke A, Gordon T, Zochodne DW, Sulaiman OA. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp Neurol. 2002;173:77–85. doi: 10.1006/exnr.2001.7826. [DOI] [PubMed] [Google Scholar]
- 34.Horch KW, Lisney SJ. On the number and nature of regenerating myelinated axons after lesions of cutaneous nerves in the cat. J Physiol. 1981;313:275–286. doi: 10.1113/jphysiol.1981.sp013664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howe HA, Tower SS, duel AB. Facial tic in relation to injury of the facial nerve: an experimental study. Archives of Neurology & Psychiatry. 1937;38:1190–1198. [Google Scholar]
- 36.Tos P, Calcagni M, Gigo-Benato D, Boux E, Geuna S, Battiston B. Use of muscle-vein-combined Y-chambers for repair of multiple nerve lesions: experimental results. Microsurgery. 2004;24:459–464. doi: 10.1002/micr.20064. [DOI] [PubMed] [Google Scholar]
- 37.Lundborg G, Dahlin LB, Danielsen N, Gelberman RH, Longo FM, Powell HC, Varon S. Nerve regeneration in silicone chambers: influence of gap length and of distal stump components. Exp Neurol. 1982;76:361–375. doi: 10.1016/0014-4886(82)90215-1. [DOI] [PubMed] [Google Scholar]
- 38.Jiang B, Zhang P. Advances in small gap sleeve bridging peripheral nerve injury. Artif Cells Blood Substit Immobil Biotechnol. 2010;38:1–4. doi: 10.3109/10731190903495652. [DOI] [PubMed] [Google Scholar]
- 39.Cass DT, Sutton TJ, Mark RF. Competition between nerves for functional connexions with axolotl muscles. Nature. 1973;243:201–203. doi: 10.1038/243201a0. [DOI] [PubMed] [Google Scholar]
- 40.Ribchester RR. Activity-dependent and -independent synaptic interactions during reinnervation of partially denervated rat muscle. J Physiol. 1988;401:53–75. doi: 10.1113/jphysiol.1988.sp017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson W. Reinnervation of partially denervated rat soleus muscle. Acta Physiol Scand. 1978;103:81–91. doi: 10.1111/j.1748-1716.1978.tb06193.x. [DOI] [PubMed] [Google Scholar]
- 42.Politis MJ, Steiss JE. Electromyographic evaluation of a novel surgical preparation to enhance nerve-muscle specificity that follows mammalian peripheral nerve trunk transection. Exp Neurol. 1985;87:326–333. doi: 10.1016/0014-4886(85)90223-7. [DOI] [PubMed] [Google Scholar]
- 43.Seckel BR, Ryan SE, Gagne RG, Chiu TH, Watkins E Jr. Target-specific nerve regeneration through a nerve guide in the rat. Plast Reconstr Surg. 1986;78:793–800. doi: 10.1097/00006534-198678060-00014. [DOI] [PubMed] [Google Scholar]
- 44.de Ruiter GC, Malessy MJ, Alaid AO, Spinner RJ, Engelstad JK, Sorenson EJ, Kaufman KR, Dyck PJ, Windebank AJ. Misdirection of regenerating motor axons after nerve injury and repair in the rat sciatic nerve model. Exp Neurol. 2008;211:339–350. doi: 10.1016/j.expneurol.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madison RD, Robinson GA, Chadaram SR. The specificity of motor neurone regeneration (preferential reinnervation) Acta Physiol (Oxf) 2007;189:201–206. doi: 10.1111/j.1748-1716.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 46.Zele T, Sketelj J, Bajrovic FF. Efficacy of fluorescent tracers in retrograde labeling of cutaneous afferent neurons in the rat. J Neurosci Methods. 2010;191:208–214. doi: 10.1016/j.jneumeth.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 47.Robinson GA, Madison RD. Developmentally regulated changes in femoral nerve regeneration in the mouse and rat. Exp Neurol. 2006;197:341–346. doi: 10.1016/j.expneurol.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Brushart TM, Henry EW, Mesulam MM. Reorganization of muscle afferent projections accompanies peripheral nerve regeneration. Neuroscience. 1981;6:2053–2061. doi: 10.1016/0306-4522(81)90043-9. [DOI] [PubMed] [Google Scholar]
- 49.Leffert RD, Meister M. Patterns of neuromuscular activity following tendon transfer in the upper limb: a preliminary study. J Hand Surg Am. 1976;1:181–189. doi: 10.1016/s0363-5023(76)80036-6. [DOI] [PubMed] [Google Scholar]
- 50.Mendell LM, Scott JG. The effect of peripheral nerve cross-union on connections of single Ia fibers to motoneurons. Exp Brain Res. 1975;22:221–234. doi: 10.1007/BF00234765. [DOI] [PubMed] [Google Scholar]
- 51.Cederna PS, Kalliainen LK, Urbanchek MG, Rovak JM, Kuzon WM Jr. “Donor” muscle structure and function after end-to-side neurorrhaphy. Plast Reconstr Surg. 2001;107:789–796. doi: 10.1097/00006534-200103000-00021. [DOI] [PubMed] [Google Scholar]
- 52.Yin XF, Kou YH, Wang YH, Zhang P, Zhang HB, Jiang BG. Portion of a nerve trunk can be used as a donor nerve to reconstruct the injured nerve and donor site simultaneously. Artif Cells Blood Substit Immobil Biotechnol. 2011;39:304–309. doi: 10.3109/10731199.2011.574636. [DOI] [PubMed] [Google Scholar]


