Abstract
Abnormal phenotypic modulation of vascular smooth muscle cells (VSMCs) is a hallmark of cardiovascular diseases such as atherosclerosis, hypertension and restenosis after angioplasty. Transcription factors have emerged as critical regulators for VSMCs function, and recently we verified inhibiting transcription factor Gax was important for controlling VSMCs proliferation and migration. This study aimed to determine its role in phenotypic modulation of VSMCs. Western blot revealed that overexpression of Gax increased expression of VSMCs differentiation marker genes such as calponin and SM-MHC 11. Then, Gax overexpression potently suppressed proliferation and migration of VSMCs with or without platelet-derived growth factor-induced-BB (PDGF-BB) stimuli whereas Gax silencing inhibited these processes. Furthermore, cDNA array analysis indicated that Rap1A gene was the downstream target of Gax in human VSMCs. And overexpression of Gax significantly inhibited expression of Rap1A in VSMCs with or without PDGF-BB stimuli. Moreover, overexpression of Rap1A decreased expression of VSMCs differentiation marker genes and increased proliferation and migration of VSMCs with or without PDGF-BB stimuli. Finally, Gax overexpression significantly inhibited the neointimal formation in carotid artery injury of mouse models, specifically through maintaining VSMCs contractile phenotype by decreasing Rap1A expression. In conclusion, these results indicated that Gax was a regulator of human VSMCs phenotypic modulation by targeting Rap1A gene, which suggested that targeting Gax or its downstream targets in human VSMCs may provide an attractive approach for the prevention and treatment of cardiovascular diseases.
Keywords: Gax, Rap1A, phenotypic modulation, vascular smooth muscle cells
Introduction
Pathological phenotype modulation of vascular smooth muscle cells (VSMCs) plays an important role in the development of many cardiovascular diseases such as postangioplasty restenosis, atherosclerosis, and hypertension [1]. Normally VSMCs within the middle layer of blood vessels reside in a quiescent (also termed contractile or differentiated) state and exhibit a very low rate of proliferation, relatively low synthetic activity, appropriate contractility to contractile cues, and express differentiation markers, such as smooth muscle α-actin (SMA), calponin, smooth muscle myosin heavy chain (SM-MHC). However, in cases of vascular injury and disease, VSMCs dedifferentiate and adopt a synthetic phenotype, which is characterized by increasing rates of proliferation, migration, and enhanced production of extracellular matrix components, as well as diminished expression of differentiation markers [2-4]. This process is called phenotypic modulation, which can drive the vascular remodeling processes leading to disease development. Previous studies have documented that numerous cellular environmental stimulus including growth factor, cytokines, mitogens, cell adhesions, cell-cell contact, mechanical influences, extracellular matrix interactions and so on could dramatically alter VSMCs phenotype [1,5]. Platelet-derived growth factor BB (PDGF-BB) has been confirmed to induce synthetic VSMCs phenotype and promote VSMCs proliferation and migration into the neointima layer after vascular injury [6]. However, the underlying mechanism for VSMCs phenotypic modulation still remains unanswered.
It is clear that VSMCs phenotypic switching is characterized by changes in gene expression patterns that underlie the changes of cell functional properties. The transformation from contractile phenotype to synthetic phenotype is associated with the silencing of differentiation marker genes expression and the upregulation of genes that facilitate other cellular functions such as cell proliferation or migration [7-9]. The transcription factor regulates genes expression by binding to response elements in the promoter regions of target genes in kinds of tissues including VSMCs. Several transcription factors, such as CREB, NFAT, SRF, MEF-2, GATA-6, HIF-1, KLF-4, MRTF-A/B, have been shown to play critical roles in VSMCs phenotype switching and modulation of function [10-14]. For example, SRF can regulate the expression of genes containing CArG boxes in their promoter regions by binding to CArG elements, and majority of genes encoding differentiation markers, such as SMA, SM-MHC, contain CArG boxes in their promoters [15]. Transcription factor NFAT is related with VSMCs phenotypic switching, proliferation and migration. Downregulation of NFAT by pharmacological inhibition or siRNA could inhibit the proliferation of serum-stimulated cultured VSMCs [16]. Moreover, studies using animal models have strongly shown that NFAT was implicated in injury-induced neointima formation of artery [17]. Together, these provide significant new insights into the pivotal importance and functional complexities of transcription factor in regulation of VSMCs phenotype.
Growth arrest-specific homeobox gene (Gax), also called MEOX2, is an inhibiting transcription factor and a member of the homeobox gene family. We and others had revealed that Gax played an important role in VSMCs proliferation and migration [18,19]. Gax is predominantly expressed in the cardiovascular system of adults. In response to PDGF in vitro and artery injury in vivo, the expression of Gax is rapidly downregulated in VSMCs, while Gax is upregulated under conditions that favor cell-cycle arrest and contractile state in VSMCs. Although the significant progress has been made in the understanding of VSMCs biology regulated by transcription factor Gax, little is known about Gax that regulates VSMCs phenotypic modulation. Therefore, clarification of Gax biological function would be fundamentally important for understanding the molecular mechanisms involved in VSMCs phenotypic transformation and identification of new therapeutic targets. In this study, we found that Gax maintains VSMCs contractile phenotype and regulates VSMCs proliferation and migration by targeting Rap1A gene.
Material and methods
Cell culture
Primary human aortic smooth muscle cells (HASMCs) (Promocell, Germany, Cat. No. C-12533) were maintained in growth media SmGM-2 (Lonza) in the presence of 10% fetal bovine serum (FBS) (Gibico) and streptomycin (100 ug/ml)/penicillin (100 U/ml) at 37°C in a humidified 5% CO2 incubator. The cells used in this study were from passages 5-6.
Cell transfection
To construct the adenoviral plasmid expressing Gax, human Gax cDNA was subcloned from the pCMV-SPORT6-Gax (Invitrogen) into the pIRES2-EGFP vector. Human Gax gene with recombination site attB1 and EGFP gene with recombination site attB2 were from pIRES2-EGFP-Gax vector by PCR, then the two segments were fused together to obtain attB1-human-Gax-EGFP-attB2 by overlap-extension PCR. The product was firstly moved into entry vector pDONR221 (Invitrogen) by BP reaction (Invitrogen) and moved into pAd/CMV/V5-DEST vector (Invitrogen) by LR reaction (Invitrogen). The objective plasmid was linear by PacI restriction enzyme and then transfected into 293A cells for packaging and amplification of recombinant virus Ad-Gax. The viruses were purified on a CsCl density gradient by ultracentrifugation and the titer was detected by using TCID50. To construct the adenoviral plasmid against Gax, siRNA against human Gax was designed by Sangon (Shanghai, China). siRNA sequences against the mRNA of human Gax were as follows: sense, 5’-GCUCCUGAAUUCUUCCUAUUU-3’; and antisense, 5’-UUCGAGGACUUAA GAAGGAUA-3’. Gax siRNA segments were cloned into the pIRES2-EGFP vector. Then, the subsequent constructional steps of Ad-Gax siRNA were the same as those of Ad-Gax constructions. Ad-EGFP vector was used as a control.
To construct the lentiviral plasmid expressing Rap1A, human Rap1A cDNA with restriction enzyme cutting site Asc1 and Pme1 was cloned to lentiviral expression vector pLenti6.3-IRES-EGFP by recombinant DNA technology. The positive clones were screened. The target gene plasmid and lentiviral packaged systems were co-transfected to package virus in 293T cells by lipofectin. The reporter gene expression was observed under fluorescent microscope. Virus supernatant was collected, purified and concentrated, and the titer of recombinant lentiviral vector Lenti-Rap1A was determinate. Lenti-EGFP was used as a control.
For Gax overexpression, the cells were infected with Ad-Gax vector. For Gax knockdown, the cells were transfected with Ad-Gax siRNA. Overexpression of Rap1A was performed by using Lenti-Rap1A. HASMCs with the confluence of 70%-80% were incubated with serum-reduced medium (0.5% FBS) and viruses were added into the medium at a multiplicity of infection of 100. After 6-12 h, the medium was changed to serum-free SmGM-2 followed by different treatments or stimulus as indicated. As a control in all experiments, an identical group of HASMCs was left uninfected but incubated 6-12 h in SmGM-2 containing 0.5% FBS.
Real-time PCR analysis of target gene mRNA levels
Total RNAs were extracted from HASMCs using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions and subjected to reverse transcription using RevertAid™ First Strand cDNA Synthesis Kit (Fermentas). Quantitative real-time RT-PCR was performed with the TaqMan system (ABI-Prism 770 Sequence Detection System, Applied Biosystems) using SYBR Green Mix. The following primers were used: Gax, 5’-CCAGACTGAGGCGATACGAG-3’ (forward) and 5’-CTGTTTGCTGGAGGGTGGC-3’ (reverse); Rap1A, 5’-AAGAAGAAGCCTAA AAAGAAATCAT-3’ (forward) and 5’-TCCAGGGAACTTGTGCAAAC-3’ (reverse); GAPDH, 5’-GGGTGTGAACCATGAGAAGTATG-3’ (forward) and 5’-GATGGCAT GGACTGTGGTCAT-3’ (reverse). GAPDH was amplified as other template normalizations. For quantification, mRNA expression of target genes between groups was determined by using 2-ΔΔCt methodology.
Western blot analysis
Cell or tissue lysates were prepared in RIPA Buffer. After centrifugation, insoluble material was removed. Total cell protein concentrations were determined by using the BCA Protein Assay Kit (Qiagen). 10-40 μg of total protein was then resolved by SDS/PAGE and transferred to PVDF membranes. The membranes were blocked with 5% nonfat milk in TBS with 0.1% Tween 20 (TBST) and incubated with diluted primary antibodies to Gax (1:100 dilution; Abcam), Calponin (1:100 dilution; Abcam), smooth muscle myosin heavy chain 11 (SM-MHC 11) (1:100 dilution; Abcam), Rap1A (1:100 dilution; Santa Cruz), and GAPDH (1:1000 dilution; Santa Cruz ). The specific binding was examined with horseradish peroxidase-conjugated secondary antibodies and the membrane was developed with ECL. Blots were quantified with a Bio-Rad Gel Documentation System.
Immunofluorescence staining
HASMCs were fixed with ice cold acetone and then blocked in 10% goat serum for 1 h at 37°C. The sections were incubated with anti-Gax antibodies (1:200 dilution, Abcam) overnight, followed by fluorescein-conjugated secondary antibody at 37°C for 1 h. HASMCs nuclei were stained with DAPI.
Cell proliferation assay
Cell Counting Kit (CCK8) (Dojindo) was used to detecte cell proliferation. HASMCs (2.0×103 cells/well in 96-well plates) were transfected with either Ad-Gax, Ad-Gax siRNA, Lenti-Rap1A, or negative control, and incubated with medium containing 0.5% FBS for 24 h. HASMCs were then subjected to stimulation with or without human PDGF-BB (20 ng/ml) (R&D Systems). CCK solution was added to each well and incubated for 0.5-4 h. Optical density (OD) value was examined daily over for consecutive days at 450 nm to estimate viable cell numbers. The assay had three replicates.
Transwell assay
HASMCs migration was assessed using a Transwell system with a coated polycarbonate membrane with 8.0 μm pore size (Corning). HASMCs were seeded in 6-well plates at a concentration of 2.0×105 cells per well and transduced with Ad-Gax, Ad-Gax siRNA, Lenti-Rap1A or their negative controls. The cells were then subjected to stimulation with or without human PDGF-BB (20 ng/ml). After digesting these cells, 100 μl of cell suspension (3.0×105 cells/ml) was added to the upper chamber, and 600 μl medium containing 10% FBS which is a chemoattractant, was added to the lower chamber. The cells on upper surface were removed after 6-12 h by gentle wiping with a cotton swab. The cells on the underside (migrated cells) were stained with 0.1% crystal violet. Quantification of the number of migrated cells was counted directly in 10 random high-power fields (at 100× magnification) using an inverted microscope (Lecia) or indirectly in absorbance of eluant at 570 nm using 33% acetic acid. This experiment repeated three times.
cDNA array analysis
To explore the gene expression profile of HASMCs exposed to overexpression of transcription factor Gax, we selected the PrimeView™ Human Gene Expression Array (Affymetrix). It represented approximately 20,000 sequences that had been characterized in terms of function or disease association from the UniGEne database.
Total RNAs were isolated from 2 independently-derived groups of HASMCs transduced with Ad-Gax or control, which had been incubated for 72 h. RNAs from Gax overexpression or control sample were labeled, fragmented, hybridized, and scanned according to the manufacture’s recommendations. Data were analyzed using GeneChip 3.1 expression analysis software (Affymetrix) and imported into Microsoft Excel. Differentially expressed genes were identified by t-test at the cut-off of p-value ≤ 0.05. Gene ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were further performed for the differentially expressed genes.
Adventitial gene transfer and carotid artery wire injury model
According to the Ethical Committee of Renji Hospital of Shanghai Jiaotong University School of Medicine, all procedures of the animal experiments were performed under the institutional guidelines of animal welfare. Male C57BL/6J mice (20-25 g) were anesthetized with an intraperitoneal injection of pentobarbital (90 mg/kg). With the aid of a dissecting microscope, the left common carotid artery was dissected free of the surrounding connective tissue via a midline incision on the ventral side of the neck. The bifurcation of the carotid artery was located and two ligatures (8-0 silk suture) were placed around the external carotid artery, which was tied off distally. An angiotomy was performed between the two ligatures, and a 0.38 mm steel wire was passed towards the aortic arch and withdrawn 3 times with a rotating motion. After removal of the steel wire, the external carotid artery was tied off proximally to the incision hole with the proximal ligature. Ad-Gax (1010 pfu/ml) or Ad-EGFP (1010 pfu/ml) was suspended together in 50 μl pluronic F-127 gel solutions (1 mg/ml, Sigma) and applied around the carotid artery. The pluronic gel solidified instantaneously, generating a translucent layer that envelops the artery injury region. The skin incision was closed immediately after the application of the gel. The contralateral carotid artery served as a control group.
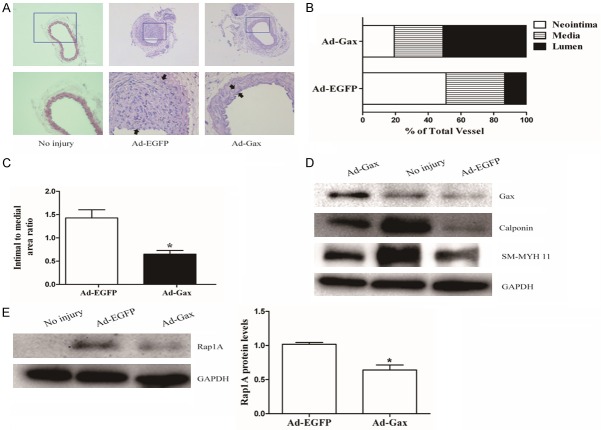
The mice were anesthetized and perfused with PBS at 14 days after injury. Then, carotid arteries were removed and fixed with 4% paraformaldehyde, and embedded in paraffinum. Vascular tissues were sectioned and stained with hematoxylin/eosin. The sections were examined under a light microscope (Nikon). The area of lumen, intima and media were measured by Image Pro Plus 6.0 software (Media Cybernetics). Neointimal formation was determined as ration of the intimal area to medial area and lumen area.
Statistical analysis
Data were presented as mean ± SD and analyzed for statistical significance by the unpaired Student t test or one-way ANOVA using SPSS 17.0 software. P<0.05 was regarded as statistical significance in all experiments.
Results
Adenovirus-mediated overexpression or knockdown of Gax in HASMCs
To explore the effects of Gax on VSMCs phenotypic modulation, HASMCs were transfected with a recombinant adenovirus encoding Gax, Gax siRNA as a control. Gene transfer was confirmed by Real-time PCR and immunofluorescent staining. Three days after adenovirus transfection, the overexpression or knockdown of Gax gene was observed in Ad-Gax- or Ad-Gax siRNA-infected HASMCs (Figure 1), demonstrating the efficacy of transgene delivery.
Figure 1.
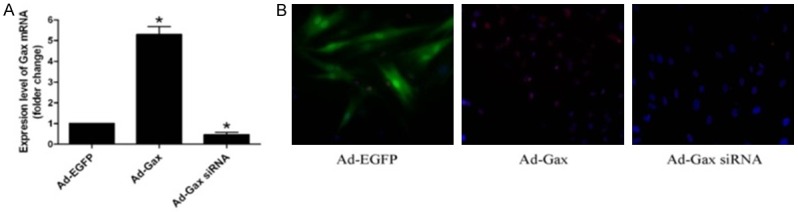
The expression levels of Gax were detected in HASMCs after transfection. A. Gax mRNA levels of HASMCs were examined by Real-time PCR. *P<0.05 versus Ad-EGFP. B. Immunofluorescence staining was used to examine Gax protein levels of HASMCs (400X).
Transcription factor Gax regulated the expression of smooth muscle maker genes in vitro
To investigate the role of transcription factor Gax in VSMCs phenotypic switch, we performed gain-of-function studies by using adenovirus-mediated delivery of Gax gene into HASMCs. As shown in Figure 2A, transduction of HASMCs with Ad-Gax resulted in increased expression of Gax. This increase was correlated with significantly increased expression of VSMCs differentiation marker genes, such as calpoin and SM-MHC 11, as determined by Western blot analysis. Next, we tested whether transcription factor Gax prevented PDGF-BB-induced downregulation of smooth muscle marker genes. As shown in Figure 2B, Gax overexpression markedly increased the protein levels of calpoin and SM-MHC 11, as determined by Western blot analysis. These results supported the notion that Gax was a regulator for VSMCs phenotypic switching.
Figure 2.
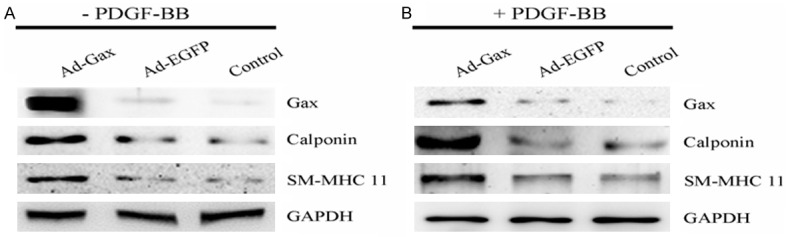
Role of transcription factor Gax in the expression of VSMCs differentiation marker genes. A. Ad-Gax increased the expression of VSMCs differentiation marker genes in HASMCs without PDGF-BB stimuli. B. The expression of VSMCs differentiation marker genes increased in Ad-Gax transfected-HASMCs in the presence of PDGF-BB treatment.
Gax was a modulator of VSMCs proliferation and migration
VSMCs differentiation was accompanied by reduced proliferation and migration of VSMCs. To evaluate the potential roles of Gax in HASMCs proliferation and migration, the cells were transfected with Ad-Gax, Ad-Gax siRNA, or negative controls. As seen in Figure 3, transfection of Ad-Gax markedly inhibited HASMCs proliferation with or without PDGF-BB stimuli, as determined by CCK8 assay. Furthermore, downregulation of Gax expression by Ad-Gax siRNA enhanced HASMCs proliferation in response to PDGF-BB or none. These findings suggested that transcription factor Gax effectively inhibited humans VSMCs proliferation.
Figure 3.
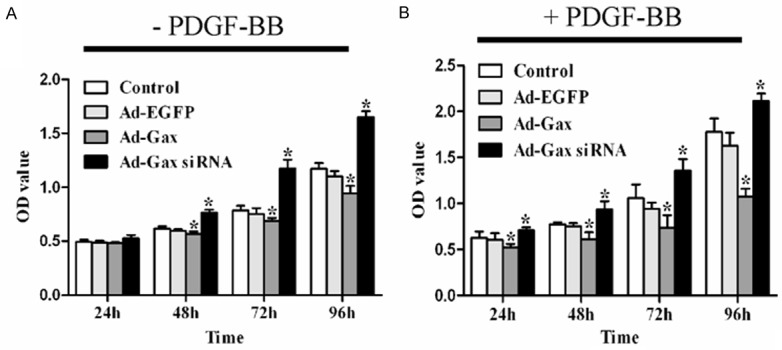
Role of transcription factor Gax in HASMCs proliferation. A. HASMCs proliferation were inhibited by Ad-Gax and promoted by Ad-Gax siRNA. *P<0.05 versus Ad-EGFP and Control. B. Ad-Gax suppressed PDGF-BB-induced HASMCs proliferation and Ad-Gax siRNA enhanced the proliferation of HASMCs with PDGF-BB stimuli. *P<0.05 versus Ad-EGFP and Control.
To determine the role of Gax in VSMCs migration, we performed the transwell assay. As shown in Figure 4, adenovirus-mediated overexpression of Gax inhibited HASMCs migration under both quiescent and PDGF-BB-stimulated conditions. Moreover, it was showed that transfection of HASMCs with Ad-Gax siRNA further augmented both basal and PDGF-BB-induced HASMCs migration. Together, these results suggested that transcriptional factor Gax was an inhibitor of HASMCs migration.
Figure 4.
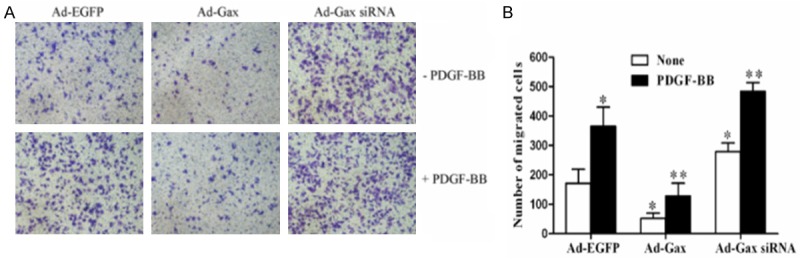
Role of transcription factor Gax in HASMCs migration. (A) Gax overexpression inhibited migration of HASMCs and Gax knockdown promoted migration of HASMCs in the presence or absence of PDGF-BB treatment. (B) The quantitative analysis of data shown in (A). *P<0.05 versus Ad-EGFP. **P<0.05 versus Ad-EGFP with PDGF-BB treatment.
cDNA array identified Rap1A as a Gax-regulated gene
To delineate a mechanism for these differences in VSMCs phenotypic modulation, we examined the gene profile by cDNA array analysis of 2 independently-derived pooled Ad-Gax and Ad-EGFP transductants. From human gene expression array data, 476 genes were found to be significantly regulated in HASMCs by Ad-Gax transfection compared with Ad-EGFP controls, as shown in Figure 5A, 5B. Because Gax was an inhibiting transcription factor, these selected down-regulated genes were pooled and subjected to further bioinformatics analysis to identify signaling networks and biological processes regulated by them. Gene Ontology analysis by DAVID indicated marked enrichment of biological processes such as cell cycle, cell proliferation, cell adhesion, and negative regulation of transcription and so on (Figure 5C). Furthermore, KEGG analysis suggested key signaling pathways including MAPK signaling, vascular smooth muscle contraction and insulin signaling, known to be involved in VSMCs phenotypic transformation (Figure 5D). These results showed that genes down-regulated by Gax might have potential functional roles in Gax overexpression inducing effects in HASMCs phenotypic switching.
Figure 5.
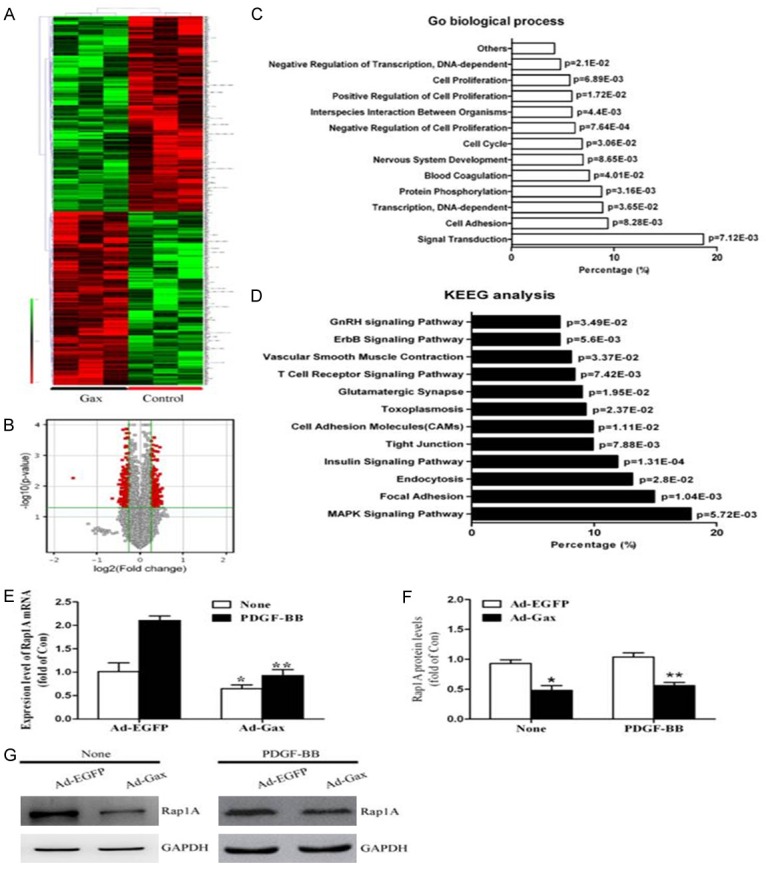
Identification of Rap1A as downstream target gene of transcription factor Gax in HASMCs. A. The unsupervised hierarchical clustering of differentially expressed genes in HASMCs transfected with Ad-Gax compared with Control samples was shown. For each gene, red represented high expression, and green indicated low expression relative to the average of all samples. B. Volcano plot showed human gene expression array data obtained from Ad-Gax -transfected HASMCs. Gax overexpression (3 experiments) altered 476 genes. The X-axis of these plots indicated the fold change in gene expression in a log2 format. Any point<0 suggested down-regulated genes. The Y-axis was the -log10 of the P value and all points in red were significant (P<0.05). The most down-regulated gene was Rap1A. C. Shown was relative distribution of the biological process highly enriched among Gax down-regulated targeted genes by GO analysis. D. Shown was KEGG analysis of potential signaling pathways highly enriched among targeted genes down-regulated by transcription factor Gax. E. Effect of Gax on Rap1A mRNA expression in None- or PDGF-induced HASMCs. *P<0.05 versus Ad-EGFP without PDGF-BB treatment. **P<0.05 versus Ad-EGFP with PDGF-BB stimuli. F. Densitometric analysis of Rap1A protein levels as examined by Western blot. G. *P<0.05 versus Ad-EGFP without PDGF-BB treatment. **P<0.05 versus Ad-EGFP with PDGF-BB stimuli.
Of most interest was that the most down-regulated gene was Rap1A (Fold Change (abs]=2.94, p=0.006). To further identify that Rap1A was a functional target gene of Gax in HASMCs, we transfected HASMCs with either Ad-Gax or Ad-EGFP, and the expression levels of Rap1A were detected by Real-time PCR and Western blot. As seen in Figure 5E-G, Gax overexpression markedly inhibited Rap1A expression in HASMCs under both basal and PDGF-BB-stimulated conditions. Together, these results indicated that transcription factor Gax targeted Rap1A to potently inhibit the Rap1A pathway in HASMCs.
Role of Rap1A in HASMCs proliferation and migration
To further substantiate the functional significance of Rap1A in VSMCs function, we performed gain-of-function study by using Lenti-Rap1A. As seen in Figure 6A, the transfection of HASMCs with Lenti-Rap1A markedly enhanced both basal and PDGF-BB-induced Rap1A expression. As determined by Western blot analysis (Figure 6A), Rap1A overexpression significantly inhibited the expression of VSMCs contractile genes such as calponin and SM-MHC 11. Moreover, HASMCs proliferation with PDGF-BB stimuli or none was enhanced by Rap1A overexpression (Figure 6B). Likewise, both basal and PDGF-BB-induced HASMCs migration were also significantly promoted in cells transfected with Lenti-Rap1A (Figure 6C). To further determine the importance of Rap1A in transcriptional factor Gax-mediated VSMCs function, we examined the effect of Rap1A expression on transfection of HASMCs with Ad-Gax. Overexpression of Rap1A by Lenti-Rap1A significantly attenuated the effect of Gax on HASMCs proliferation, migration, and phenotypic switching (Figure 7). Together, these results indicated that Rap1A was an important regulator for VSMCs proliferation, migration and dedifferentiation, and was implicated in Gax-mediated effect in HASMCs function.
Figure 6.
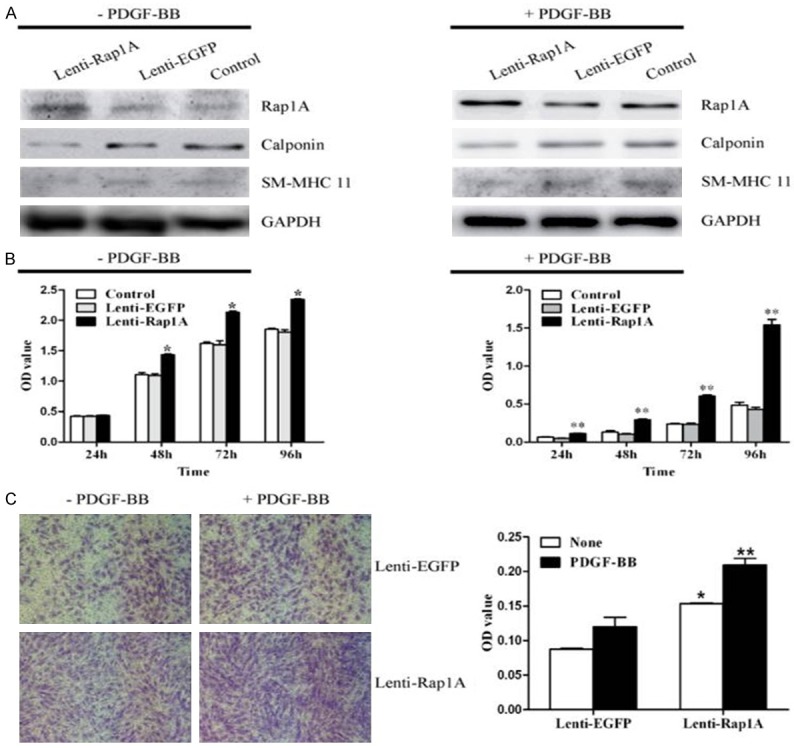
Rap1A was critically involved in VSMCs phenotypic modulation, proliferation and migration. A. Overexpression of Rap1A by Lenti-Rap1A enhanced Rap1A expression and reduced the expression of VSMCs differentiation marker genes Calponin and SM-MHC 11 in HASMCs with or without PDGF-BB treatment as determined by Western blot analysis. B. Rap1A overexpression promoted proliferation in HASMCs with or without PDGF-BB as determined by CCK8 assay. *P<0.05 versus Control and Ad-EGFP without PDGF-BB. **P<0.05 versus Control and Ad-EGFP with PDGF-BB. C. HASMCs migration was measured in presence or absence of PDGF-BB by Transwell assay and quantitation of migrated cells was performed as optical density (OD) value. *P<0.05 versus Control and Ad-EGFP without PDGF-BB. **P<0.05 versus Control and Ad-EGFP with PDGF-BB.
Figure 7.
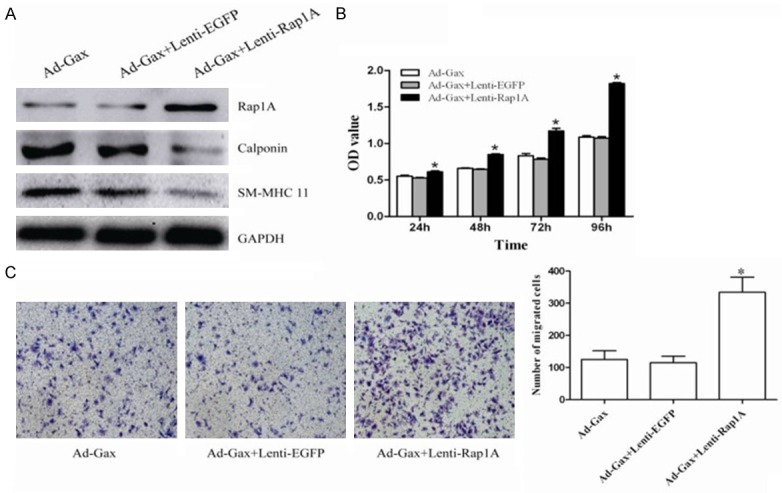
Overexpression of Rap1A attenuated Ad-Gax mediated effects in HASMCs. A. The expression of Rap1A, Calponin and SM-MHC 11 were detected by Western blot analysis at 72 h after transfection of HASMCs with either Ad-Gax, combination of both Ad-Gax and Lenti-EGFP, or combination of both Ad-Gax and Letin-Rap1A. B. HASMCs proliferation was analyzed by CCK8 assay after PDGF-BB stimulation, when HASMCs were transfected with either Ad-Gax, combination of both Ad-Gax and Lenti-EGFP, or combination of both Ad-Gax and Letin-Rap1A. *P<0.05 versus Ad-Gax and Ad-Gax+Lenti-EGFP. C. When HASMCs were transfected with either Ad-Gax, combination of both Ad-Gax and Lenti-EGFP, or combination of both Ad-Gax and Letin-Rap1A, cell migration was measured after PDGF-BB stimulation by transwell assay. And quantitative analysis of migrated cells was performed. *P<0.05 versus Ad-Gax and Ad-Gax+Lenti-EGFP.
Gax inhibited neointimal formation in mice after carotid artery injury
To determine whether Gax was implicated in vascular lesion formation in vivo, the mouse carotid artery injury model was used and neointimal formation was detected at 14 days after vascular injury. The carotid arteries of mice were transduced with either Ad-Gax (1010 pfu/ml) or Ad-EGFP. Fourteen days after transfection, the expression of Gax in mouse carotid arteries was significantly increased compared with Ad-EGPF treated group, as determined by Western blot. There was no neointimal formation in the uninjured left common carotid arteries. In contrast, there was a substantial increase in neointimal formation in carotid arteries transduced with Ad-EGFP (Figure 8A). However, the transduction of carotid arteries with Ad-Gax substantially reduced the injury-induced neointimal formation by about 40%, which was related with decreases in both neointimal area (Figure 8B) and the intima to media ratio (Figure 8C) in Ad-Gax-treated carotid arteries. In addition, Western bolt analysis of Rap1A and VSMCs contractile genes such as calpoin and SM-MHC 11 was performed in injured carotid artery. As seen in Figure 8D, the expression of calpoin and SM-MHC 11 was markedly increased in Ad-Gax-transduced arteries as compared with Ad-EGFP-transduced arteries. Furthermore, the Rap1A expression was substantially inhibited in Ad-Gax-treated arteries (Figure 8E). Together, these results indicated that Ad-Gax decreased the vascular injury-induced neointimal formation in vivo by inhibiting Rap1A-mediated VSMCs phenotypic modulation.
Figure 8.
Transcription factor Gax attenuated neointimal formation in the mouse carotid artery injury model. A. Adenovirus-mediated overexpression of Gax (Ad-Gax) markedly decreased neointimal formation in vivo. Representative hematoxylin and eosin stained carotid artery slices form of mouse treated with Ad-Gax or Ad-EGFP at fourteen days after carotid artery injury. B. The effect of Gax overexpression on neointima, media, and lumen in mouse carotid arteries at fourteen days after injury. C. The effect of Ad-Gax on neointimal lesion formation in mouse model of carotid artery injury as quantitated by intima/media ratio. *P<0.05 versus Ad-EGFP. D. Representative western blot images of VSMCs differentiation markers in mouse carotid arteries treated with Ad-Gax or Ad-EGFP at fourteen days after injury. E. Representative Western blot images of Rap1A in mouse carotid arteries at fourteen days after injury, and quantification of Rap1A protein expression showed that Rap1A level was downregulated in Ad-Gax-treated vascular compared with Ad-EGFP-treated vascular. *P<0.05 versus Ad-EGFP.
Discussion
Despite obvious progress in our understanding of VSMCs biology, the exact molecular mechanism underlying VSMCs phenotypic modulation still remains unknown. In this study, we verified transcription factor Gax as a regulator involved in human VSMCs proliferation, migration and differentiation. Both gain-of-function and loss-of-function studies indicated that Gax played an important role in VSMCs phenotypic modulation in vitro and in vivo. Moreover, we identified that transcription factor Gax maintained VSMCs contractile phenotype, and inhibited VSMCs proliferation and migration in vitro and neointimal formation in vivo, mostly likely through targeting Rap1A gene, a member of Ras oncogene family.
Recently the functional significance of transcription factor Gax in human disease has been investigated. Increasing evidence indicates that Gax is importantly implicated in cell differentiation and growth. In human hepatocellular carcinoma tissues, the Gax expression has been shown to be downregulated, and the upregulation of Gax could inhibit hepatocellular carcinoma cells proliferation and migration [20,21]. The miRNA family miR-130/301/721 had been shown to enhance induced pluripotent stem cells generation by repression of Gax [22]. Moreover, in vascular endothelial cells, Gax expression played a critical role in endothelial cell phenotypic changes and the process of angiogenesis [23,24]. And overexpression of Gax could inhibit vascular adventitial fibroblasts proliferation and inflammatory cytokines of adventitial fibroblasts [25]. In addition, previous studies showed that the overexpression of Gax could inhibit rat VSMCs proliferation and migration, and neointimal hyperplasia in animal model [26-28]. Angiotensin II, which had an important role in vascular remodeling, and miR-130a mediated proliferation of rat VSMCs via suppressing Gax expression [29,30]. More importantly, the expression of Gax decreased in quiescent porcine VSMCs after serum inducing phenotype transition [31]. And MEF2, known to be a regulator of VSMCs phenotypic modulation [14], could transactivate the mouse Gax promoter via binding to specific sequences in a site-dependent manner [32]. Therefore, it would be interesting to elucidate whether Gax was implicated in maintain the differentiation effect on human VSMCs. However, at present, little is known about the role of Gax in human VSMCs phenotypic switching.
Mechanistically, we identified Rap1A gene as a critical downstream target of transcription factor Gax in VSMCs phenotypic modulation. Rap1A is a ras superfamily small GTPase which is the master regulator of a diverse range of cellular processes and acts via downstream effector molecules [33]. For example, Raf-1 was a major component of the Rap1A complex, and inhibition of Raf-1 had been shown to inhibit VSMCs proliferation and migration in vitro and neointimal formation in vivo [34,35]. In addition, Raf-1 played an important role in the regulation of vascular contractility [36]. Furthermore, Rap1A had been involved in tumor cells proliferation and cancer cells growth based on studies using genetic deletion or overexpression of molecules that modulated Rap1A activation [37,38]. Rap1A had been a well documented role in ERK/MAPK signaling and intergrin activation, which could contribute cell migration and inhibit cell differentiation [37,39]. Importantly, Rap1A expression could be upregulated by PDGF, thrombin and downregulated by TGF-β [37,40], and these factors were associated with VSMCs phenotypic modulation. In accordance with these observations, we found that overexpression of Gax significantly reduced the PDGF-BB-induced expression of Rap1A, contributing to its inhibitory effects on VSMCs proliferation and migration. Although Gax was expressed exclusively in cardiovascular tissues in adults, the binding site of Gax in target genes was conserved among different species such as human, mouse and rat levels, which prompted us to explore the functional significance of Gax in vivo by using mouse carotid artery injury model. Strikingly, Gax overexpression significantly suppressed the neointimal formation, which was accompanied by decreasing expression of Rap1A and maintaining VSMCs contractile phenotype. Our results were the first to identify transcription factor Gax as an important modulator in regulating VSMCs phenotypic switching, at least in part, through targeting the Rap1A pathway.
VSMCs phenotypic transformation is of great importance for the resolution of vascular remodeling. Recent studies had involved transcription factors such as MEF-2, GATA-6, CREB, KLF-4, as important targets of multiple growth factor signaling pathways that adjusted VSMCs phenotypic switching [11,12,14]. The exact molecular mechanisms underlying the regulation of VSMCs phenotypic modulation by these transcription factors were variable. However, they mainly implicated in the indirect or direct regulation of the target genes expression. In this study, we screened the target genes of Gax in VSMCs phenotypic modulation using cDNA array analysis, and found that hundreds of genes such as Rap1A, TRPS1, ASAH1, DLX1, SBNO1, SAV1, ZEFP28, were downregulated. Rap1A gene was selected for the follow-up study because different expression of Rap1A gene between two groups was most significant and recent studies reported the role of Rap1A in biological function of cells. This study suggested that Rap1A regulated expression of VSMCs differentiation marker genes, possible via a mechanism yet to be determined.
In summary, we identified Gax as an important regulator in human VSMCs proliferation, migration and differentiation by targeting, at least partly, the Rap1A pathway. Moreover, VSMCs phenotypic modulation is known to play a pivotal role in the pathogenesis of a variety of cardiovascular diseases such as restenosis and atherosclerosis. Therefore, modulation of Rap1A expression by transcription factor Gax may have potential as a therapeutic target for prevention and treatment of cardiovascular diseases.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81500366) and the Scientific Seed Funding of Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (NO.RJZZ13-018).
Disclosure of conflict of interest
None.
References
- 1.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 2.Rangrez AY, Massy ZA, Metzinger-Le Meuth V, Metzinger L. miR-143 and miR-145: molecular keys to switch the phenotype of vascular smooth muscle cells. Circ Cardiovasc Genet. 2011;4:197–205. doi: 10.1161/CIRCGENETICS.110.958702. [DOI] [PubMed] [Google Scholar]
- 3.Salmon M, Gomez D, Greene E, Shankman L, Owens GK. Cooperative binding of KLF4, pELK-1, and HDAC2 to a G/C repressor element in the SM22alpha promoter mediates transcriptional silencing during SMC phenotypic switching in vivo. Circ Res. 2012;111:685–696. doi: 10.1161/CIRCRESAHA.112.269811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li P, Zhu N, Yi B, Wang N, Chen M, You X, Zhao X, Solomides CC, Qin Y, Sun J. MicroRNA-663 regulates human vascular smooth muscle cell phenotypic switch and vascular neointimal formation. Circ Res. 2013;113:1117–1127. doi: 10.1161/CIRCRESAHA.113.301306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis-Dusenbery BN, Wu C, Hata A. Micromanaging vascular smooth muscle cell differentiation and phenotypic modulation. Arterioscler Thromb Vasc Biol. 2011;31:2370–2377. doi: 10.1161/ATVBAHA.111.226670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 2004;15:205–213. doi: 10.1016/j.cytogfr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Sung HJ, Eskin SG, Sakurai Y, Yee A, Kataoka N, McIntire LV. Oxidative stress produced with cell migration increases synthetic phenotype of vascular smooth muscle cells. Ann Biomed Eng. 2005;33:1546–1554. doi: 10.1007/s10439-005-7545-2. [DOI] [PubMed] [Google Scholar]
- 8.Tharp DL, Wamhoff BR, Turk JR, Bowles DK. Upregulation of intermediate-conductance Ca2+-activated K+ channel (IKCa1) mediates phenotypic modulation of coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2006;291:H2493–2503. doi: 10.1152/ajpheart.01254.2005. [DOI] [PubMed] [Google Scholar]
- 9.Zhang QJ, Goddard M, Shanahan C, Shapiro L, Bennett M. Differential gene expression in vascular smooth muscle cells in primary atherosclerosis and in stent stenosis in humans. Arterioscler Thromb Vasc Biol. 2002;22:2030–2036. doi: 10.1161/01.atv.0000042206.98651.15. [DOI] [PubMed] [Google Scholar]
- 10.Shan F, Li J, Huang QY. HIF-1 alpha-induced up-regulation of miR-9 contributes to phenotypic modulation in pulmonary artery smooth muscle cells during hypoxia. J Cell Physiol. 2014;229:1511–1520. doi: 10.1002/jcp.24593. [DOI] [PubMed] [Google Scholar]
- 11.Kudryavtseva O, Aalkjaer C, Matchkov VV. Vascular smooth muscle cell phenotype is defined by Ca2+-dependent transcription factors. FEBS J. 2013;280:5488–5499. doi: 10.1111/febs.12414. [DOI] [PubMed] [Google Scholar]
- 12.Davis-Dusenbery BN, Chan MC, Reno KE, Weisman AS, Layne MD, Lagna G, Hata A. Down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J Biol Chem. 2011;286:28097–28110. doi: 10.1074/jbc.M111.236950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagna G, Ku MM, Nguyen PH, Neuman NA, Davis BN, Hata A. Control of phenotypic plasticity of smooth muscle cells by bone morphogenetic protein signaling through the myocardin-related transcription factors. J Biol Chem. 2007;282:37244–37255. doi: 10.1074/jbc.M708137200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon JW, Pagiatakis C, Salma J, Du M, Andreucci JJ, Zhao J, Hou G, Perry RL, Dan Q, Courtman D, Bendeck MP, McDermott JC. Protein kinase A-regulated assembly of a MEF2{middle dot}HDAC4 repressor complex controls c-Jun expression in vascular smooth muscle cells. J Biol Chem. 2009;284:19027–19042. doi: 10.1074/jbc.M109.000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol. 2003;35:577–593. doi: 10.1016/s0022-2828(03)00110-x. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson LM, Sun ZW, Nilsson J, Nordstrom I, Chen YW, Molkentin JD, Wide-Swensson D, Hellstrand P, Lydrup ML, Gomez MF. Novel blocker of NFAT activation inhibits IL-6 production in human myometrial arteries and reduces vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2007;292:C1167–1178. doi: 10.1152/ajpcell.00590.2005. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z, Zhang C, Dronadula N, Li Q, Rao GN. Blockade of nuclear factor of activated T cells activation signaling suppresses balloon injury-induced neointima formation in a rat carotid artery model. J Biol Chem. 2005;280:14700–14708. doi: 10.1074/jbc.M500322200. [DOI] [PubMed] [Google Scholar]
- 18.Zheng H, Xue S, Hu ZL, Shan JG, Yang WG. Overexpression of the growth arrest-specific homeobox gene Gax inhibits proliferation, migration, cell cycle progression, and apoptosis in serum-induced vascular smooth muscle cells. Genet Mol Res. 2014;13:1993–2008. doi: 10.4238/2014.March.24.4. [DOI] [PubMed] [Google Scholar]
- 19.Xia S, Tai X, Wang Y, An X, Qian G, Dong J, Wang X, Sha B, Wang D, Murthi P, Kalionis B, Bai C. Involvement of Gax gene in hypoxia-induced pulmonary hypertension, proliferation, and apoptosis of arterial smooth muscle cells. Am J Respir Cell Mol Biol. 2011;44:66–73. doi: 10.1165/rcmb.2008-0442OC. [DOI] [PubMed] [Google Scholar]
- 20.Zhou P, Jiang W, Wu L, Chang R, Wu K, Wang Z. miR-301a is a candidate oncogene that targets the homeobox gene Gax in human hepatocellular carcinoma. Dig Dis Sci. 2012;57:1171–1180. doi: 10.1007/s10620-012-2099-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhou P, Chen Z, Chang RM, Jiang W, Wu LL, Wang ZM. Growth arrest-specific homeobox is associated with poor survival in patients with hepatocellular carcinoma. Med Oncol. 2012;29:3063–3069. doi: 10.1007/s12032-012-0258-0. [DOI] [PubMed] [Google Scholar]
- 22.Pfaff N, Fiedler J, Holzmann A, Schambach A, Moritz T, Cantz T, Thum T. miRNA screening reveals a new miRNA family stimulating iPS cell generation via regulation of Meox2. EMBO Rep. 2011;12:1153–1159. doi: 10.1038/embor.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Gorski DH. Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood. 2008;111:1217–1226. doi: 10.1182/blood-2007-07-104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel S, Leal AD, Gorski DH. The homeobox gene Gax inhibits angiogenesis through inhibition of nuclear factor-kappaB-dependent endothelial cell gene expression. Cancer Res. 2005;65:1414–1424. doi: 10.1158/0008-5472.CAN-04-3431. [DOI] [PubMed] [Google Scholar]
- 25.Liu P, Zhang C, Zhao YX, Feng JB, Liu CX, Chen WQ, Yao GH, Zhang M, Wang XL, Zhang Y. Gax gene transfer inhibits vascular remodeling induced by adventitial inflammation in rabbits. Atherosclerosis. 2010;212:398–405. doi: 10.1016/j.atherosclerosis.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Perlman H, Luo Z, Krasinski K, Le Roux A, Mahfoudi A, Smith RC, Branellec D, Walsh K. Adenovirus-mediated delivery of the Gax transcription factor to rat carotid arteries inhibits smooth muscle proliferation and induces apoptosis. Gene Ther. 1999;6:758–763. doi: 10.1038/sj.gt.3300893. [DOI] [PubMed] [Google Scholar]
- 27.Witzenbichler B, Kureishi Y, Luo Z, Le Roux A, Branellec D, Walsh K. Regulation of smooth muscle cell migration and integrin expression by the Gax transcription factor. J Clin Invest. 1999;104:1469–1480. doi: 10.1172/JCI7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maillard L, Van Belle E, Tio FO, Rivard A, Kearney M, Branellec D, Steg PG, Isner JM, Walsh K. Effect of percutaneous adenovirus-mediated Gax gene delivery to the arterial wall in double-injured atheromatous stented rabbit iliac arteries. Gene Ther. 2000;7:1353–1361. doi: 10.1038/sj.gt.3301255. [DOI] [PubMed] [Google Scholar]
- 29.Saito T, Itoh H, Yamashita J, Doi K, Chun TH, Tanaka T, Inoue M, Masatsugu K, Fukunaga Y, Sawada N, Sakaguchi S, Arai H, Tojo K, Tajima N, Hosoya T, Nakao K. Angiotensin II suppresses growth arrest specific homeobox (Gax) expression via redox-sensitive mitogen-activated protein kinase (MAPK) Regul Pept. 2005;127:159–167. doi: 10.1016/j.regpep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Wu WH, Hu CP, Chen XP, Zhang WF, Li XW, Xiong XM, Li YJ. MicroRNA-130a mediates proliferation of vascular smooth muscle cells in hypertension. Am J Hypertens. 2011;24:1087–1093. doi: 10.1038/ajh.2011.116. [DOI] [PubMed] [Google Scholar]
- 31.Markmann A, Rauterberg J, Vischer P, Robenek H, Echtermeyer F, Will H, Seidler DG, Young MF, Kresse H. Expression of transcription factors and matrix genes in response to serum stimulus in vascular smooth muscle cells. Eur J Cell Biol. 2003;82:119–129. doi: 10.1078/0171-9335-00309. [DOI] [PubMed] [Google Scholar]
- 32.Andres V, Fisher S, Wearsch P, Walsh K. Regulation of Gax homeobox gene transcription by a combination of positive factors including myocyte-specific enhancer factor 2. Mol Cell Biol. 1995;15:4272–4281. doi: 10.1128/mcb.15.8.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loirand G, Sauzeau V, Pacaud P. Small G proteins in the cardiovascular system: physiological and pathological aspects. Physiol Rev. 2013;93:1659–1720. doi: 10.1152/physrev.00021.2012. [DOI] [PubMed] [Google Scholar]
- 34.Pan CH, Chen CW, Sheu MJ, Wu CH. Salvianolic acid B inhibits SDF-1alpha-stimulated cell proliferation and migration of vascular smooth muscle cells by suppressing CXCR4 receptor. Vascul Pharmacol. 2012;56:98–105. doi: 10.1016/j.vph.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Dong LH, Wen JK, Liu G, McNutt MA, Miao SB, Gao R, Zheng B, Zhang H, Han M. Blockade of the Ras-extracellular signal-regulated kinase 1/2 pathway is involved in smooth muscle 22 alpha-mediated suppression of vascular smooth muscle cell proliferation and neointima hyperplasia. Arterioscler Thromb Vasc Biol. 2010;30:683–691. doi: 10.1161/ATVBAHA.109.200501. [DOI] [PubMed] [Google Scholar]
- 36.Sathishkumar K, Yallampalli U, Elkins R, Yallampalli C. Raf-1 kinase regulates smooth muscle contraction in the rat mesenteric arteries. J Vasc Res. 2010;47:384–398. doi: 10.1159/000277726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sayyah J, Bartakova A, Nogal N, Quilliam LA, Stupack DG, Brown JH. The Ras-related protein, Rap1A, mediates thrombin-stimulated, integrin-dependent glioblastoma cell proliferation and tumor growth. J Biol Chem. 2014;289:17689–17698. doi: 10.1074/jbc.M113.536227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang J, Bian C, Wang H, Huang S, Wu D. MiR-203 down-regulates Rap1A and suppresses cell proliferation, adhesion and invasion in prostate cancer. J Exp Clin Cancer Res. 2015;34:8. doi: 10.1186/s13046-015-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujita H, Fukuhara S, Sakurai A, Yamagishi A, Kamioka Y, Nakaoka Y, Masuda M, Mochizuki N. Local activation of Rap1 contributes to directional vascular endothelial cell migration accompanied by extension of microtubules on which RAPL, a Rap1-associating molecule, localizes. J Biol Chem. 2005;280:5022–5031. doi: 10.1074/jbc.M409701200. [DOI] [PubMed] [Google Scholar]
- 40.Quarck R, Berrou E, Magnier C, Bobe R, Bredoux R, Tobelem G, Enouf J, Bryckaert M. Differential up-regulation of Rap1a and Rap1b proteins during smooth muscle cell cycle. Eur J Cell Biol. 1996;70:269–277. [PubMed] [Google Scholar]