Abstract
Hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) is one of the most common cancers in HBV-endemic regions, with irreversible progression and poor prognosis. HBV-related HCC patients lack effective antiviral/antitumor B cell antibody responses. We hypothesize that dysregulation of PD-1-expressing follicular helper T (Tfh) cell, induced by intrahepatic/intratumoral PD-L1 expression in HCC, could contribute to the defects in B cell immunity. The Tfh responses in healthy control (HC) subjects, chronic hepatitis B (HepB) patients, and HBV-related HCC patients were examined. Compared to HC and HepB individuals, HCC patients showed reduced ICOS expression, IL-10 and IL-21 secretion, and proliferation in Tfh cells. Tfh cells from stage III patients demonstrated increased impairment than those from stage I and stage II patients. Compared to Tfh cells from HC and HepB subjects, those from stage III HCC patients were significantly less effective at inducing the differentiation of naive B cells toward plasmablasts. HCC is known to upregulate hepatic PD-L1 expression, which could suppress Tfh responses. Blocking PD-1 partially rescued the Tfh functions in stage I and stage II HCC subjects but not in stage III HCC patients, while treatment with recombinant PD-L1 strongly suppressed Tfh functions in all HCC stages. Moreover, the level of IL-10 and IL-21 expression by Tfh cells was inversely correlated with the intensity of PD-L1 expression in resected tumors. Together, our results demonstrated an HCC-specific Tfh exhaustion, which might have resulted from elevated PD-1 and PD-L1 signaling.
Keywords: Follicular helper T cells, PD-L1, hepatocellular carcinoma
Introduction
Hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) is one of the most common cancers, especially in HBV-endemic regions [1,2]. An estimated 350 million people worldwide are currently infected with HBV and are therefore at risk of developing HCC, especially in developing countries where antiviral treatments are not available [3,4]. In acute HBV infections, it is generally thought that viral clearance and clinical recovery are mainly mediated by HBV-specific CD4+ and CD8+ T cell inflammation [5,6]. But in chronic HBV infection and HCC, the activities of liver- and tumor-infiltrating T cells are restricted, often resulting in persistent inflammation but not virus eradication [7,8], likely due to the presence of multiple immunosuppression mechanisms in the hepatic microenvironment [9]. In particular, the programmed death-1 (PD-1) signaling pathway negatively regulates antitumor immunity, resulting in lower proliferation, proinflammatory IFN-γ production and cytotoxicity by T cells [10,11]. In healthy individuals, the PD-1 ligand PD-L1 is expressed on hepatocytes, hepatic stellate cells (HSCs), liver sinusoidal endothelial cells (LSECs), and the liver resident macrophage Kupffer cells [12-15]. In HBV-related HCC, PD-L1 expression can be further upregulated on peritumoral stroma cells and tumor cells [16-19].
Vaccination of HBV-uninfected individuals can effectively induce B cell-secreted antibody (HBsAb) against the HBV surface antigen HBsAg, which could mediate vaccine-induced protection [20,21]. Intriguingly, the protective HBsAb does not develop in many chronic HBV infections and HBV-related HCC patients. Also, the role of B cell responses and antibody secretion in HBV-related HCC has not been extensively examined. This suggested a possible defect in CD4+CXCR5+ follicular helper T (Tfh) cells, which is the main B cell-helper in mediating B cell differentiation in the germinal center [22-24]. Interestingly, activated germinal center Tfh cells are characterized by their exceptionally high expression of PD-1 [25]. The peripheral blood CD4+CXCR5+ T cells also express a relatively higher level of PD-1 compared with other CD4+ T cell subsets [26]. Like germinal center Tfh cells, the peripheral blood Tfh cells can stimulate antibody production through the expression of IL-10 and IL-21 [26-28]. In a mouse model, IL-21 could confer effective anti-HBV immunity in exposure to HBVs to adults [29]. To the best of our knowledge, we do not yet know whether Tfh cell functions in chronic HBV infections and HBV-related HCC patients are defective due to the existence of PD-1/PD-L1-mediated suppression.
Here, we compared and contrasted the Tfh responses in healthy control (HC) subjects, chronic HBV-infected (HepB) patients, and HBV-related HCC patients. Our results demonstrated a previously unexplored suppression of Tfh cells in HCC patients, and suggested that it might have resulted from elevated PD-1 and PD-L1 signaling.
Materials and methods
Study cohort
This study enrolled 12 HC subjects, 12 HepB patients and 20 HBV-related HCC patients. All groups were age- and gender-matched. In addition, the HCC subjects were age- and gender-matched across stage I, II and III. The study procedures were approved by the ethics board of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Written informed consent was received from every subject prior to sampling. The diagnosis of HCC was based on the results from standard liver biopsies or imaging according to the guidelines published by the American Association for the Study of Liver Diseases. Clinical stages were classified according to the TNM classification system by International Union against Cancer (6th Edition). No subject received anticancer therapy prior to surgical resection. The peritumoral tissue (at least 3 cm away from the tumor) was removed from the tumor immediately after resection and was processed separately. The liver biopsies, peritumoral tissues and tumors were mechanically dissociated with the GentleMACS (Miltenyi) into homogenized single cell suspensions and stained immediately for PD-L1 expression. A portion of the tumor tissues were processed as previously published to obtain tumor-infiltrating lymphocytes (TILs) [19]. PBMCs were isolated by the standard Ficoll centrifugation protocol. All samples were stored at -80°C in 10% DMSO, and thawed in complete RPMI medium + 10% FCS and 20 µg/mL DNase (Sigma) before use.
Flow cytometry
Cells were stained with combinations of anti-human CD3, CD4, CXCR5, ICOS, CD19, CD27, PD-1, PD-L1 and IgD monoclonal antibodies (BioLegend), as well as Aqua Dead Cell Stain (Invitrogen) for 30 min at 4°C. For live cell sorting, the cells were then washed in sterile PBS + 2% FCS twice, and were sorted in BD FACS Aria. Otherwise, the cells were washed and fixed in 2% formalin and were acquired in BD FACS LSR II.
Tfh stimulation
A total of 106 PBMCs per mL medium were labeled with CFSE (Invitrogen) and cultured without stimulation (blank), with 15-mer HBV core peptide pool (10 residues overlaping, Mimotopes) at an overall concentration of 2 µg/mL, or with 2 µg/mL endotoxin-reduced SEB (Sigma) for 72 h at 37°C and 5% CO2. At the end of stimulation, the PBMCs were harvested and split in two halves. One was directly stained for CFSE and/or ICOS expression on live CD3+CD4+CXCR5+ cells. In the other half, CD3+CD4+CXCR5+ cells were sorted in FACS Aria and counted in Trypan Blue. 105 live CD3+CD4+CXCR5+ cells per mL were then placed in the lower chamber of a transwell plate (Corning), while anti-human IL-10 and IL-21 beads (EMD Millipore) were placed at the upper chamber, separated by a 0.4 µm-pore membrane. After 12 h, the beads were harvested and examined by Luminex assay. To block PD-1 signaling, PD-1 blocking antibody clone EH12 or an irrelevant isotype control was added at 10 µg/mL [30]. To stimulate PD-1 signaling recombinant human PD-L1 (R&D systems) was added at 2 µg/mL.
Tfh coculture with naive B cells
Live-sorted CD4+CXCR5+ circulating Tfh cells and autologous CD19+CD27-IgD+ naive B cells were cultured at 1-to-1 ratio, in complete RPMI medium + 10% FCS + varying concentrations of SEB, for 12 d at 37°C and 5% CO2. Ig concentrations in the supernatant were then examined by Human IgM, IgG and IgA ELISA kits (Abcam). For Ig secretion by memory B cells, CD19+CD27+ memory B cells were sorted and cultured alone, using the same culturing conditions and ELISA procedures.
Statistical analyses
The difference between multiple groups was analyzed with one-way or two-way ANOVA tests, followed by Tukey’s multiple comparison test. RM ANOVA, followed by Sidak’s test, was used for paired data. Pearson correlation coefficient was used for correlation analyses. Two-tailed P < 0.05 was considered significant. All tests were performed in Prism 6 (GraphPad).
Results
Circulating Tfh exhaustion in HCC patients
The expression of chemokine receptor CXCR5 enables germinal center Tfh cells to traffic into B cell follicles in response to chemokine CXCL13 and provide B cell help [31,32]. Blood CD4+CXCR5+ T cells, but not CD4+CXCR5- T cells, possess the ability to induce naive B cells to produce immunoglobulins (Ig) and undergo Ig class switching ex vivo, and are thus termed circulating Tfh cells [26,27]. Dysregulated circulating Tfh frequency and function were observed in primary immunodeficiencies, autoimmune conditions, and cancer [33]. We first examined the frequencies of circulating Tfh cells in HBV-uninfected HC, HepB, and HBV-related HCC (Figure 1A). In all three categories, the frequencies of circulating Tfh cells as a percentage of total CD4+ cells were not significantly different, although higher variance were observed in the HBV-HCC group (Figure 1B).
Figure 1.
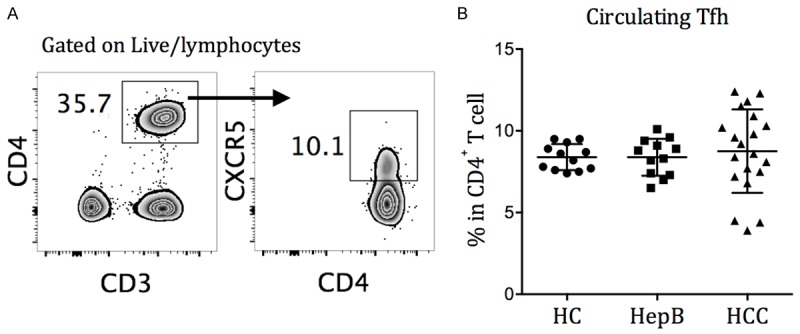
Circulating Tfh cells in HC, HepB, and HCC subjects. A. Circulating Tfh cells are identified as CD4+CXCR5+ T cells in the representative gate. PBMCs were stained with CD3, CD4 and CXCR5 mAbs and Aqua dead cell stain for 30 min at 4°C. B. Frequencies of circulating Tfh cells in healthy control (HC), chronic HBV-infected (HepB), and HBV-related hepatocellular carcinoma (HCC) patients, stained directly ex vivo. Mean ± SD.
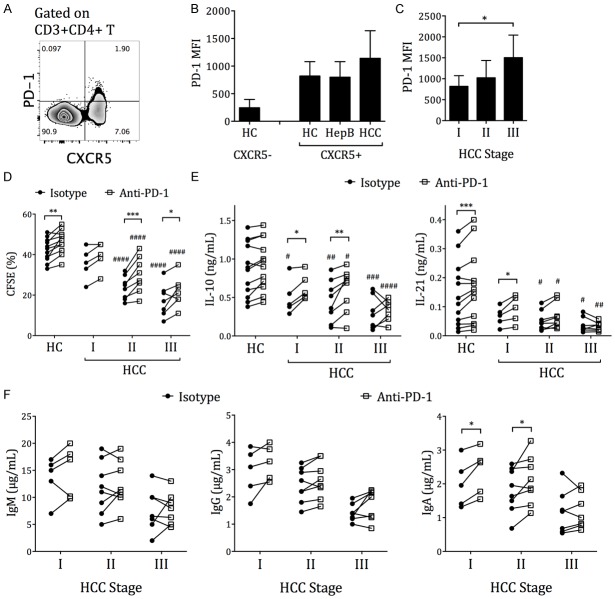
The Tfh surface molecule expression and cytokine secretion were then examined in HC, HepB, and HCC subjects after blank (unstimulated), HBV peptide, or SEB treatment. All three groups had low inducible stimulator (ICOS) expression directly ex vivo (blank) and after HBV-peptide stimulation (Figure 2A). After SEB stimulation, Tfh from HepB subjects had significantly lower ICOS expression than those from HC subjects, while Tfh from HCC subjects had further reduced ICOS expression than those from HC and HepB subjects (Figure 2B). IL-10 and IL-21 are important for B cell activation, expansion and plasmaplast differentiation cytokines expressed by circulating Tfh cells during T cell-B cell collaboration [24,26]. The level of IL-10 and IL-21 secretion by CD4+CXCR5+-sorted circulating Tfh cells were then examined directly ex vivo and after HBV-peptide or SEB stimulation. A subset of HC, HepB and HCC subjects presented elevated IL-10 and IL-21 secretion by circulating Tfh cells after HBV-peptide stimulation, suggesting the existence of antigen-specific Tfh responses (Figure 2C). The response to HBV-peptide stimulation in HC subjects was possibly due to previous vaccinations. Stimulation with SEB significantly increased IL-10 and IL-21 expression in all three groups, with a significant reduction in HCC subjects compared to the HC and HepB subjects. The proliferation of circulating Tfh cells after SEB stimulation was also significantly lower in HCC patients, compared to that in HC and HepB patients (Figure 2D and 2E).
Figure 2.
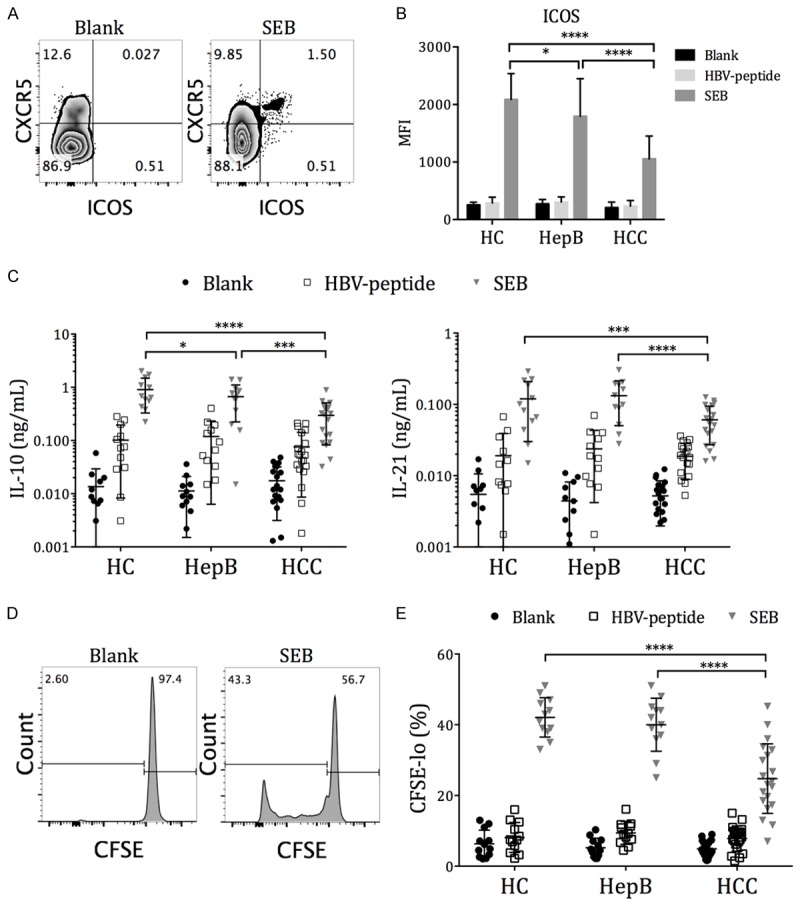
Expression of Tfh activation and function molecules in HC, HepB, and HCC subjects. PBMCs were labeled with CFSE and then cultured in plain media (blank), 2 µg/mL HBV-peptide pool (HBV-peptide), or 2 µg/mL staphylococcal enterotoxin B (SEB) for 72 h. At the end, flow cytometry of ICOS and CFSE was performed on half of the PBMCs. CD4+CXCR5+ T cells were positively selected from the other half of the PBMCs and were cultured alone with IL-10 and IL-21 luminex beads for an additional 12 h. A. Expression of ICOS on CD4+CXCR5+ circulating Tfh cells in one representative individual. B. The mean fluorescence intensity (MFI) of ICOS on circulating Tfh cells from 12 HC, 12 HepB and 20 HCC subjects, after 72 h stimulation with the stimulants. C. Secreted IL-10 and IL-21 concentration by purified CD4+CXCR5+ circulating Tfh cells, measured by luminex. D. The identification of proliferating CD4+CXCR5+ Tfh cells as CFSE-lo cells. E. The frequencies of proliferating cells in CD4+CXCR5+ Tfh cells after 72 h incubation with stimulants. Mean ± SD. Two-way ANOVA followed by Tukey’s multiple comparison test where applicable. *P < 0.05. ***P < 0.001. ****P < 0.0001.
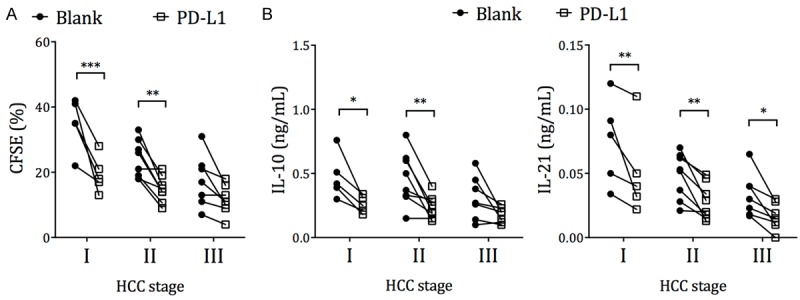
The HCC patients can be separated into stage I, stage II and stage III patients according to the TNM classification system, with progressive severity from stage I to stage III. We found that the ICOS and IL-10 expressions were comparable among the three stages (Figure 3A and 3B). The circulating Tfh cells in stage III HCC patients secreted significantly less IL-21 after SEB stimulation compared to those in stage I and stage II patients, and had significantly lower proliferation compared to those in stage I patients (Figure 3B and 3C). Together, these results demonstrated a progressive exhaustion of Tfh cells in HCC patients.
Figure 3.
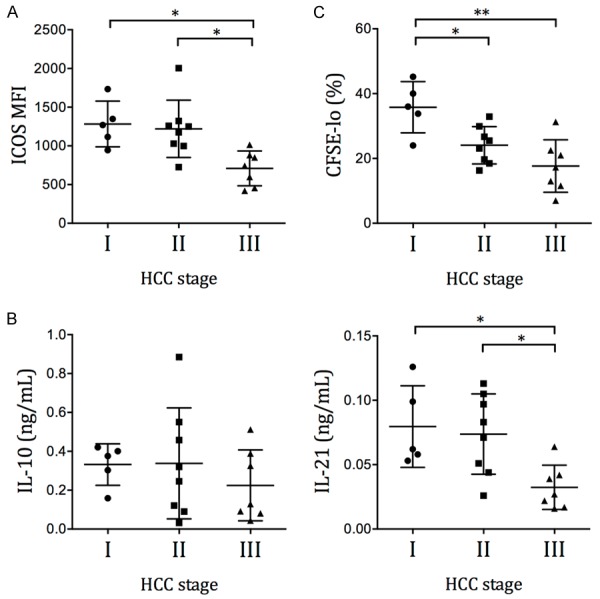
Expression of Tfh activation and function molecules in HCC patients of different stages. The (A) ICOS MFI, (B) IL-10 and IL-21 secretion, and (C) proliferation of CD4+CXCR5+ Tfh cells in HCC patients are grouped according to the tumor stage. Mean ± SD. One-way ANOVA followed by Tukey’s multiple comparison test. *P < 0.05. **P < 0.01.
Defective B cell help in HCC patients
The reduction of IL-10 and IL-21 secretion could lead to defective B cell help by Tfh cells. To examine this, CD4+CXCR5+-sorted circulating Tfh cells from HC, HepB and stage III HCC patients were incubated with autologous CD19+CD27-IgD+-sorted naive B cells, in the presence of SEB stimulation to mimic the antigen-specific interaction between T cells and B cells. The IgM, IgG and IgA production in the supernatant by day 12 were examined by ELISA. We found that Tfh cells from HC and HepB patients were significantly more efficient at stimulating IgM, IgG and IgA secretion than those from HCC patients (Figure 4A). This was not due to intrinsic defects in B cells in HCC and HepB patients, but rather reflected a defect in Tfh help, because when the antibody production from CD19+CD27+-sorted memory B cells was examined instead, no significant differences between the HC, HepB, and stage III HCC groups was observed (Figure 4B).
Figure 4.
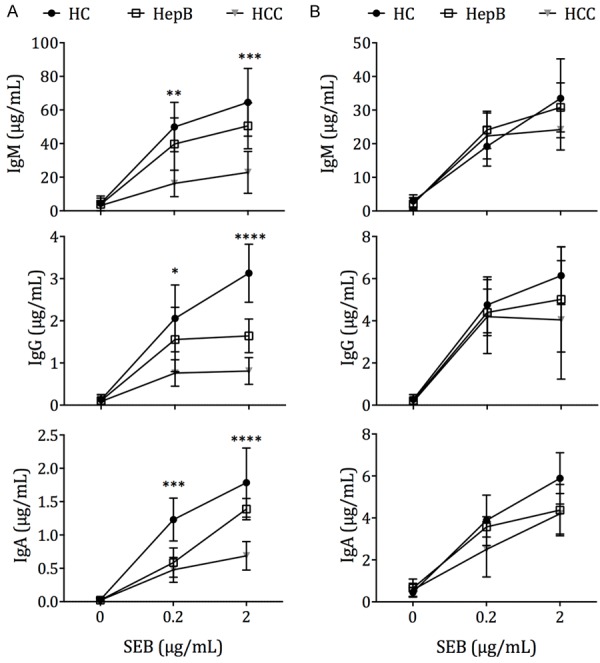
B cell help by circulating Tfh cells in HC, HepB and HCC patients. A. CD4+CXCR5+ Tfh cells sorted from PBMCs were cocultured with autologous CD19+CD27-IgD+ naive B cells for 12 d in varying concentrations of SEB (0-2 µg/mL). Ig concentrations in the supernatant were measured by ELISA. Experiment was performed on 7 HC and HepB subjects, as well as 7 stage III HCC patients. B. CD19+CD27+ memory B cells were cultured alone in varying concentrations of SEB for 12 d. Ig concentrations were measured in the supernatant by ELISA. Mean ± SD. Differences between HC, HepB and HCC samples at each SEB concentration were tested computed by one-way ANOVA. *P < 0.05. **P < 0.01. ***P < 0.001. ****P < 0.0001.
PD-1 blockade partially rescued the Tfh exhaustion in stage I and II HCC patients
In many chronic virus infections, such as HIV, HCV and HBV, as well as in several tumors, CD4+ and CD8+ T cell exhaustions can be rescued by PD-1/PD-L1 blockade [10,34-37]. In germinal center Tfh cells, PD-1 expression not only restricts excessive Tfh proliferation but is also critical for enhanced antibody responses [25]. Here, we found that circulating Tfh cells from HCC subjects expressed slightly higher average PD-1 level than those from HC and HepB subjects (Figure 5A and 5B). Stage III HCC patients’ Tfh cells presented higher PD-1 level than stage I patients’ (Figure 5C). Blocking PD-1 increased the frequencies of proliferating Tfh cells in stage II and stage III HCC patients (Figure 5D). Also blocking PD-1 elevated the IL-10 and IL-21 expression by circulating Tfh cells in all stage I HCC patients and 7 out of 8 stage II patients (Figure 5E). While in stage III HCC patients, blocking PD-1 increased IL-10 in 4 out of 7 patients and IL-21 in 3 out of 7 patients. The level of proliferation and cytokine production in HCC patients after PD-1 blockade was still lower than that in HC subjects (Figure 5D and 5E). In the Tfh cell-naive B cell coculture experiment, PD-1 blockade elevated the IgM secretion in 4 out of 5 stage I, 5 out of 8 stage II, and 2 out of 7 stage III patients. PD-1 blockade increased the IgG secretion in 4 out of 5 stage I, 7 out of 8 stage II, and 6 out of 7 stage III patients. And PD-1 blockade elevated the IgA secretion in all stage I, 7 out of 8 stage II, and 5 out of 7 stage III patients (Figure 5F). Overall, these data demonstrated that PD-1 blockade could partially rescue, but not fully restore, the Tfh exhaustion in a subset of stage I and stage II HBV-HCC patients, and to a lesser extent in stage III HBV-HCC patients.
Figure 5.
PD-1 blockade partially increased circulating Tfh function in HCC patients. A. Expression of PD-1 on CD4+CXCR5+ cells compared to CD4+CXCR5- cells, in one representative individual. B. PD-1 MFI on CD4+CXCR5+ circulating Tfh cells in HC, HepB, and HCC patients. The PD-1 expression on CD4+CXCR5- cells from HC is also shown for comparison. C. Expression of PD-1 by circulating Tfh cells in different stages of HCC patients. Mean± SD. One-way ANOVA and Tukey’s multiple comparison test. *P < 0.05. D and E. PBMCs from HC or HCC patients were labeled with CFSE and then cultured with 2 µg/mL staphylococcal enterotoxin B (SEB) for 72 h, with anti-PD-1 blocking antibody or isotype control. At the end, CFSE was measured on half of the PBMCs, while the CD4+CXCR5+ T cells were positively selected from the other half and were cultured alone with IL-10 and IL-21 luminex beads for an additional 12 h. D. The frequencies of proliferating Tfh cells, with isotype control or with PD-1 blockade. E. The IL-10 and IL-21 production by purified Tfh cells, with isotype control or with PD-1 blockade. RM two-way ANOVA and Sidak’s test. Asterisk (*) indicates the P value between the isotype and the anti-PD-1 treatments of the same sample, connected by a solid line. Pound sign (#) indicates the P value between that set of data with the corresponding set from the HC group. One symbol: P < 0.05. Two symbols: P < 0.01. Three symbols: P < 0.001. Four symbols: P < 0.0001. F. CD4+CXCR5+ circulating Tfh cells from different staged of HCC patients were cocultured with autologous CD19+CD27-IgD+ naive B cells for 12 d with 2 µg/mL SEB, with isotype control or with PD-1 blockade. Ig secretion in the supernatant was measured by ELISA. RM two-way ANOVA and Sidak’s test. *P < 0.05.
We also treated circulating Tfh cells with the PD-1 ligand PD-L1, and found that the proliferation and IL-10 and IL-21 production was significantly downregulated in nearly all HCC patients of all stages (Figure 6A and 6B), thus demonstrating that the PD-1/PD-L1 pathway could potently downregulate Tfh functions.
Figure 6.
Increased suppression of Tfh function in HCC patients by PD-L1. PBMCs from HCC patients were labeled with CFSE and cultured with plain media (blank) or recombinant PD-L1, together with 2 µg/mL staphylococcal enterotoxin B (SEB) for 72 h. A. The proliferation of CD4+CXCR5+ T cells by the end of the 72 h incubation. B. The CD4+CXCR5+ T cells were sorted and counted with Trypan Blue. 105 live cells per mL were cultured without PD-L1 for an additional 12 h, in the presence of IL-10 and IL-21 capture beads. The IL-10 and IL-21 secretion during this time was measured by luminex assay. RM two-way ANOVA and Sidak’s test. *P < 0.05. **P < 0.01. ***P < 0.001.
Circulating Tfh exhaustion is associated with PD-L1 expression in HCC tumor
The liver in healthy subjects is generally considered immunosuppressive. The PD-1 ligand PD-L1 is expressed in healthy adults’ intrahepatic environment and can be further upregulated in chronically inflamed livers, peritumoral Kupffer cells, and cancer cells [16-19]. Here, we examined whether PD-L1 expression in hepatocellular carcinoma contributed to the Tfh exhaustion. We first observed that PD-L1 expression was significantly higher in the peritumor cells in stage III HCC patients, than that in the liver biopsies of HepB patients (Figure 7A). Moreover, HCC stage III tumors expressed significantly higher levels of PD-L1, than stage I and stage II tumors (Figure 7B). We also found that the Tfh responsiveness to SEB stimulation, in terms of IL-10 and IL-21 production and proliferation, was inversely correlated with the level of PD-L1 expression in resected tumors (Figure 7C). Together, these data suggested that the circulating Tfh exhaustion in HCC patients was associated with PD-L1 expression in HCC. The frequency of CXCR5+ cells in liver- or tumor-infiltrating CD4+ T cells in HepB livers and HCC tumors was highly variable from patient to patient (Figure 7D), and was generally reduced compared to that in the peripheral blood (Figure 1B). The total numbers of CD4+CXCR5+ T cells isolated from HepB liver or HCC tumor were also low (Figure 7E). No correlation between the frequencies of liver-/tumor-infiltrating CD4+CXCR5+ T cells and the intrahepatic/intratumoral PD-L1 expression was found (data not shown).
Figure 7.
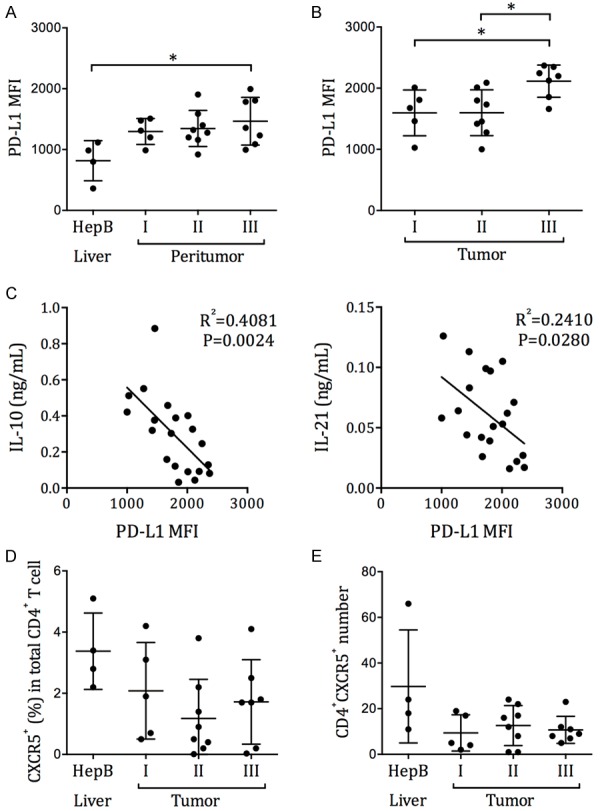
The correlation between circulating Tfh exhaustion and intrahepatic/intratumoral PD-L1 expression. Single cell preparations were obtained from the liver biopsies of HepB patients, or from the peritumor and tumor samples of HCC patients who had undergone resection. PD-L1 expression was examined by flow cytometry. (A) The MFI of PD-L1 in HepB patients’ liver cells and in HCC patients’ peritumor cells. (B) The MFI of PD-L1 in HCC patients’ tumor cells. Mean ± SD. One-way ANOVA and Tukey’s test. (C) The correlation between the IL-10 and IL-21 secretion by circulating Tfh cells after SEB stimulation, and the PD-L1 expression level in HCC tumor. P represent the Pearson correlation coefficient. (D) The frequency of CXCR5+ cells in CD4+ T cells and (E) the total number of CD4+CXCR5+ T cells in HepB liver biopsies or HCC tumor tissues.
Discussion
In this study, we examined a previously unexplored Tfh exhaustion mechanism in patients with HBV-related HCC. Despite a presence of circulating Tfh cells at near-normal levels, we observed a series of functional alterations in Tfh cells from HCC patients that suggested the presence of exhaustion. Compared to the circulating Tfh cells from HC and HepB subjects, those from HCC patients presented significantly lower ICOS expression, reduced proliferation, and decreased IL-10 and IL-21 production. This exhaustion was associated with HCC severity, since circulating Tfh cells from patients of more advanced stages tended to demonstrate further reduced functions. Furthermore, the Tfh cells from stage III HCC patients presented reduced potency in helping naive B cells to secrete IgM, IgG and IgA antibodies. Because HCC is known to upregulate hepatic PD-L1 expression, we suspected that the PD-1/PD-L1-mediated signaling contributed to Tfh suppression. To demonstrate this, we first showed that the circulating Tfh cells from HCC patients had, on average, elevated PD-1 expression than those from HC and HepB patients. Circulating Tfh from stage III HCC subjects presented elevated PD-1 expression level than those from stage I. Blocking PD-1 could increase, but not fully restore, the proliferation and cytokine production by circulating Tfh cells in most stage I and stage II HCC subjects. Blocking PD-1 in stage III HCC patients tended to be less effective. Addition of PD-L1, on the other hand, had significantly suppressed the proliferation and cytokine expression by Tfh cells. To demonstrate the connection between Tfh exhaustion and HCC, we showed that the level of IL-10 and IL-21 secretion after SEB stimulation was inversely correlated with the level of PD-L1 expression in resected tumors. Together, our results demonstrated an HCC-specific suppression of Tfh cells, and suggested that it might have resulted from the interactions between intrahepatic/intratumoral PD-L1 and CD4+CXCR5+ Tfh-expressed PD-1.
These results also raised many new questions that require further examination. First, although we showed an association between more advanced stage III HCC with exacerbated Tfh exhaustion, we do not yet know how exactly these two factors are correlated. It is possible that stronger Tfh exhaustion is a simple corollary of more PD-L1 expression in stage III HCC patients, who usually have larger tumors than stage I and stage II patients and thus contained more PD-L1-high tumor cells. On the other hand, defects in Tfh responses could contribute to increased HCC progression, as suggested by the previous discoveries that IL-21 could mediate anti-HBV protection, and that lower Tfh frequency was associated with worse prognosis [29,38].
It has been observed that antibodies specific to HBV antigens could be detected in HBV-related HCC patients [39], and that B cell infiltration could be detected in some HCC tumors [40,41]. But unlike in breast cancers and ovarian cancers, where B cells was shown to promote favorable prognosis in breast and ovarian cancers [43,44], in HBV-related HCC, evidence for the existence of an effective B cell-mediated antitumor immunity is lacking. This is somewhat unusual since antibodies could offer anti-HBV protection against new infections [20,21]. Also, B cells could act as effective antigen-presenting cells and organize tertiary lymphoid structures for sustained T cell immunity in tumor [42]. Based on the results from this study, it is possible that Tfh exhaustion resulted in the lack of B cell and antibody responses in HCC. Whether rescuing Tfh functions can enable better B cell responses and improve the antitumor immunity in HCC is still unknown. Also, the trafficking of circulating Tfh examined in our study is unknown, such that the correlation between PD-L1 expression in tumor and Tfh suppression might be a coincidence rather than a causal relationship. All these questions would require further animal experiments and clinical trials for elucidation.
Disclosure of conflict of interest
None.
References
- 1.Xu C, Zhou W, Wang Y, Qiao L. Hepatitis B virus-induced hepatocellular carcinoma. Cancer Lett. 2014;345:216–222. doi: 10.1016/j.canlet.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1907. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;2:395–403. doi: 10.1016/s1473-3099(02)00315-8. [DOI] [PubMed] [Google Scholar]
- 4.Lok ASF, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 5.Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 6.Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, King AS, Herberg J, Gilson R, Alisa A, Williams R, Vergani D, Naoumov NV, Ferrari C, Bertoletti A. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269–1280. doi: 10.1084/jem.191.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Protzer U, Maini MK, Knolle PA. Living in the liver: hepatic infections. Nat Rev Immunol. 2012;12:201–213. doi: 10.1038/nri3169. [DOI] [PubMed] [Google Scholar]
- 9.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 10.McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med. 2013;2:662–673. doi: 10.1002/cam4.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. doi: 10.1038/nri2858. [DOI] [PubMed] [Google Scholar]
- 12.Mühlbauer M, Fleck M, Schütz C, Weiss T, Froh M, Blank C, Schölmerich J, Hellerbrand C. PD-L1 is induced in hepatocytes by viral infection and by interferon-α and -γ and mediates T cell apoptosis. J Hepatol. 2006;45:520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, Lu L, Qian S. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 14.Kassel R, Cruise MW, Iezzoni JC, Taylor NA, Pruett TL, Hahn YS. Chronically inflamed livers up-regulate expression of inhibitory B7 family members. Hepatology. 2009;50:1625–37. doi: 10.1002/hep.23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diehl L, Schurich A, Grochtmann R, Hegenbarth S, Chen L, Knolle PA. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology. 2008;47:296–305. doi: 10.1002/hep.21965. [DOI] [PubMed] [Google Scholar]
- 16.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang BJ, Bao JJ, Wang JZ, Wang Y, Jiang M, Xing MY, Zhang WG, Qi JY, Roggendorf M, Lu MJ, Yang DL. Immunostaining of PD-1/PD-Ls in liver tissues of patients with hepatitis and hepatocellular carcinoma. World J Gastroenterol. 2011;17:3322–3329. doi: 10.3748/wjg.v17.i28.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 19.Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69:8067–8075. doi: 10.1158/0008-5472.CAN-09-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Bulkow L, Fiore AE, Bell BP, Hennessy TW. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis. 2009;200:1390–396. doi: 10.1086/606119. [DOI] [PubMed] [Google Scholar]
- 21.Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179:489–492. doi: 10.1086/314578. [DOI] [PubMed] [Google Scholar]
- 22.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular Helper T Cells: Lineage and Location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 25.Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, Haddad EK International AIDS Vaccine Initiative Protocol C Principal Investigators. Poignard P, Crotty S. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, Lavecchio EM, Punaro M, Pascual V, Banchereau J, Ueno H. Human Blood CXCR5+CD4+ T Cells Are Counterparts of T Follicular Cells and Contain Specific Subsets that Differentially Support Antibody Secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinuesa CG, Cook MC. Blood Relatives of Follicular Helper T Cells. Immunity. 2011;34:10–12. doi: 10.1016/j.immuni.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Publicover J, Goodsell A, Nishimura S, Vilarinho S, Wang ZE, Avanesyan L, Spolski R, Leonard WJ, Cooper S, Baron JL. IL-21 is pivotal in determining age-dependent effectiveness of immune responses in a mouse model of human hepatitis B. J Clin Invest. 2011;121:1154–12562. doi: 10.1172/JCI44198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, Altman JD, Rouse BT, Freeman GJ, Ahmed R, Grakoui A. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–53. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Förster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma CS, Deenick EK. Human T follicular helper (Tfh) cells and disease. Immunol Cell Biol. 2014;92:64–71. doi: 10.1038/icb.2013.55. [DOI] [PubMed] [Google Scholar]
- 34.Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, Missale G, Ferrari C. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588–601. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- 35.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, Bertoletti A, Ferrari C. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 37.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia Y, Zeng Z, Li Y, Li Z, Jin L, Zhang Z, Wang L, Wang FS. Impaired function of CD4+ T follicular helper (Tfh) cells associated with hepatocellular carcinoma progression. PLoS One. 2015;10:e0117458. doi: 10.1371/journal.pone.0117458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moriarty AM, Alexander H, Lerner RA, Thornton GB. Antibodies to peptides detect new hepatitis B antigen: serological correlation with hepatocellular carcinoma. Science. 1985;227:429–433. doi: 10.1126/science.2981434. [DOI] [PubMed] [Google Scholar]
- 40.Hsiao YH, Kuo SJ, Tsai HD, Chou MC, Yeh GP. Clinical Application of High-intensity Focused Ultrasound in Cancer Therapy. J Cancer. 2016;7:225–231. doi: 10.7150/jca.13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi JY, Gao Q, Wang ZC, Zhou J, Wang XY, Min ZH, Shi YH, Shi GM, Ding ZB, Ke AW, Dai Z, Qiu SJ, Song K, Fan J. Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin Cancer Res. 2013;19:5994–6005. doi: 10.1158/1078-0432.CCR-12-3497. [DOI] [PubMed] [Google Scholar]
- 42.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185:4977–4982. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, Nelson BH. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18:3281–3292. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 44.Hansen MH, Nielsen H, Ditzel HJ. The tumor-infiltrating B cell response in medullary breast cancer is oligoclonal and directed against the autoantigen actin exposed on the surface of apoptotic cancer cells. Proc Natl Acad Sci U S A. 2001;98:12659–12664. doi: 10.1073/pnas.171460798. [DOI] [PMC free article] [PubMed] [Google Scholar]