Abstract
Objective: To compare the effects and safety of immunotherapy using different methods to load DC-CIK cells for MDA-MB-231 breast cancer stem cells. Methods: A breast cancer model was established in BALB/c nude mice using breast cancer stem cells. All mice were randomly divided into six groups, and each group had three nude mice: the blank control group, the DC-CIK group (group D), the MDA-MB-231 CSC whole-cell lysate DC-CIK group (group L-D), the MDA-MB-231 CSC RNA DC-CIK group (group R-D), the THP DC-CIK group (group T-D) and group THP. Nude mice in groups D, L-D, R-D and T-D were injected with CSCs; 4 days later, the mice were inoculated with 1 × 106 DC-CIK cells via the tail vein. This injection was repeated 2 times a week for three weeks. The mice in groups THP and T-D were injected with a 5 mg/Kg dose of THP chemotherapeutic agents via the tail vein the day before DC-CIK injection, which was repeated one time a week for three weeks. Nude mice in the blank control group were injected with normal saline. The weights and sizes of the tumors were measured after the mice were euthanized. The expression of c-Myc, a key proto-oncogene associated with the Akt signaling pathway, was detected with RT-PCR. Results: The tumor growth rates in each group were as follows: group L-D < group R-D < group D < group T-D < blank control group < group THP. The nude mice in groups L-D, R-D and D were normal, active and had a healthy appetite. The mice in groups T-D and THP were lethargic, less active and showed loss of appetite, and their caudal vein was easy to stimulate. The mice in the blank control group were sacrificed during the third week or when their tumors developed ulceration. Compared with the blank control group, c-Myc gene expression was reduced in the tumors of the five experimental groups. Conclusion: The results showed that DC-CIK cells stimulated by different methods were highly effect against MDA-MB-231 breast cancer stem cells in nude mice in all groups, especially in group L-D. DC-CIK immunotherapy may provide a new strategy for the clinical treatment of breast cancer.
Keywords: DC-CIK, breast cancer stem cells, immunotherapy, c-Myc
Introduction
Breast cancer is one of the most common cancers of women in China and other countries; the morbidity rate of breast cancer shows an upward trend [1]. Surgical treatment combined with radiotherapy and chemotherapy has been widely used in clinical practice. However, improving the survival rates of recurrent metastatic breast cancer has been a difficult issue. The investigation of cancer stem cells (CSC) will provide a new method for the clinical diagnosis and treatment of breast cancer. Cancer stem cell theory suggests that cancer involves a subset of cells characterized by the capacity for self-renewal, differentiation and resistance to radiotherapy and chemotherapy [2]. Cancer stem cells are considered to be responsible for tumor metastasis and recurrence. Simply removing cancer cells does not cure cancer. Therefore, immunotherapy has been receiving more attention as a method to target cancer stem cells. Dendritic cells (DCs) were discovered by Steinman and Cohn in 1973 [3]. DCs are a system of antigen-presenting cells that function to stimulate several immune responses. DCs are widely considered to be the first choice of immunotherapy. The results of an immunotherapy study that combined DCs with cytokine-induced killer cells (CIKs) in vivo and in vitro indicated the effectiveness of immunotherapy in experimental animals [4]. In this study, a model of breast cancer stem cells was established in nude mice, and the immunogenicity of cancer stem cell-specific antigen was enhanced using different methods, such as loaded DC-CIK cells. Identifying the effects of killing cancer stem cells in vivo may allow the selection of an effective system to stimulate DC-CIK cells for clinical treatment. The activation of the Akt signaling pathway is closely linked with breast cancer development. c-Myc, a key proto-oncogene, plays an important dual-functional role in the transcriptional regulation (activation and repression) of cell proliferation, differentiation and apoptosis [5]. In this study, changes in c-Myc mRNA expression were tested in all groups, and the molecular mechanism of immunotherapy in cancer stem cells was explored.
Materials and methods
Materials
Reagents
Breast cancer cell line MDA-MB-231 was provided by the Tianjin Medical University Cancer Institute & Hospital. Peripheral blood samples of healthy individuals were obtained from the Tianjin Blood Center. Culture medium RPMI1640, penicillin and streptomycin were from Gibco. Standard fetal bovine serum was from HyClone. RhGM-CSF, RhIL-4, RhIL-2, RhIL-1α, TNF-α, IFN-CD3, Mouse Anti-human PE-CD56, Mouse Anti-human APC-CD8, Mouse anti-human APC-CD40, Mouse Anti-human PE-CD80, Mouse Anti-human FITC-HLA-DR, and Mouse Anti-human PerCP-CyTM5.5-CD86 were procured from Becton Dickinson (BD). Lympholyte-H was obtained from Tianjin Meide Pacific Technology Ltd. Matrigel was obtained from Corning. The PCR primers were obtained from Beijing Aoke. The RNA extraction kit was purchased from Qiagen. The Reverse Transcription Kit was purchased from Takara. The 0.25% Trypsin Solution was from SuoLaiBao. CD44 and CD24 antibody-coated magnetic beads were obtained from Miltenyi.
Experimental animals
Eighteen SPF graded 3-4 week old female BALB/c nude mice weighing 16-18 g were obtained from Weitonglihua and raised within an IVC system.
Instruments
IVC system (company, China), biological safety cabinet (Thermo Scientific, USA), magnetic bead sorter (Miltenyi, Germany), incubator (Thermo Scientific, USA), microscope (Olympus, Japan), PCR machine (Applied Biosystems, USA), electrophoresis apparatus (Bio-Rad, USA), gel imager (Bio-Rad, USA), flow cytometer (BD, USA), and automated cell counter (Countstar, South Korea).
Methods
Cell culture
The MDA-MB-231 breast cancer cell line was cultured in DMEM supplemented with 10% Fetal Bovine Serum and 1% penicillin-streptomycin at 37°C in an atmosphere of 5% CO2. When the cells reached the logarithmic phase, they were digested with 0.25% pancreatic enzyme, washed with PBS, and then used in a continuous cell culture.
Mononuclear cells were isolated from healthy, human peripheral blood using lymphocyte separation medium. The adherent cells were collected, and the suspension cells were transferred for use. The adherent cells were cultured in RPMI1640 medium containing RhIL-4 (1000 U/ml), RhGM-CSF (1000 U/ml) and 10% calf serum. The antigen was loaded on day 5. On day 6, TNF-α (10 ng/mL) was added to the medium. The DC cells reached maturity on days 8 or 9. The suspension cells were cultured in RPMI1640 supplemented with 10% calf serum and IFN-γ (50 ng/mL). After 24 h, IL-2 (500 u/mL), IL-1α (5 ng/mL), and CD3 (50 ng/mL) were added. Half of the culture medium was replaced every 2-3 days. On day 8, CIK cells and DC cells were co-cultured. Half of the culture medium was replaced every 2 days and supplemented with IL-2 until the CIK cells reached maturity on day 14.
MDA-MB-231 cancer stem cell sorting
Logarithmic phase MDA-MB-231 cells were collected, washed with ice-cold PBS, and counted. Then, 1 × 107 cells were suspended and incubated in 40 μl of PBS with 10 μl of CD24-biotin at 4°C for 15 min. The cells were washed by centrifugation with 1-2 ml of PBS 300xg/10 min and resuspended with 80 μl of PBS. Then, 20 μl of antibiotic microspheres were added to the cell suspension and incubated at 4°C for 15 min. Next, 400 μl of PBS was added to the cell suspension. An LS column was installed on a magnetic rack and sensitized with 3 ml of PBS. After the liquid drained away, the cell suspension was added, and the uncombined cells (CD24-) were collected by centrifugation at 300 × g for 10 min. Afterward, 20 μl of CD44 beads were added and incubated at 4°C for 15 min. The cells were washed by centrifugation with 1-2 ml of PBS at 300 × g for 10 min and resuspended with 500 μl of PBS for use. The LS column was installed again. Uncombined cells were washed out thrice with 3 ml of PBS. The LS column was then removed from the magnetic rack. Upon addition of 5 ml of PBS, the CD44+ CD24- cells were immediately pushed from the column and collected.
Preparation of whole-cell lysate for cancer stem cells
The MDA-MB-231 CD44+ CD24- cells were collected and stored in cryogenic tubes. The cryogenic tubes were placed in liquid nitrogen for 15 min and immediately put into a 37°C water bath for 15 min. This process was repeated four times. After the cells were lysed, they were centrifuged at 12000 RPM for 10 min at 4°C. The supernatant was collected, and the protein concentration was calculated by the BCA method. The whole-cell lysate was stored at -80°C.
Extraction of total RNA from cancer stem cells
RNA was isolated according to the instructions given by the Qiagen RNeasy mini kit. The MDA-MB-231 CD44+ CD24- cells were collected, and 1 × 107 cells were counted. Next, 600 μl of Buffer RLT was added. The lysate was passed through a 0.9 mm needle attached to a 1 ml sterile plastic syringe 5-8 times until a homogenous lysate was achieved. Then, 600 μl of 70% ethanol was added and mixed well. The solution was transferred to a spin column, centrifuged at 8000 × g for 15 sec, and the flow-through was discarded. Afterward, 700 μl of RW1 Buffer was added to the spin column, which was centrifuged at 8000 × g for 15 sec to wash the spin column membrane. Next, 80 μl of Buffer RDD containing 10 μl of DNase was added to the column and incubated at 20-30°C for 15 min to digest genomic DNA. Subsequently, 350 μl of RW1 Buffer was added to the column and centrifuged at 8000 × g for 15 sec to wash the column. The column was placed in a new 1.5 ml EP tube, 30-50 μl of RNase-free deionized water was added, and the tube was centrifuged at 8000 × g for 1 min. The RNA was collected, and the concentration was determined by Nanodrop 2000.
Construction of a breast cancer stem cell model in nude mice
Eighteen BALB/c nude mice were acclimated for one week. The collected MDA-MB-231 CD44+ CD24- cells (5 × 105 cells/mL) were mixed with a mixture of serum-free medium and matrigel at a ratio of 1:1 and then preserved on ice. The BALB/c nude mice were inoculated with 0.2 mL of the cells at the right axilla. Tumor growth was recorded every two days. When nodules with an indurate texture appeared at the inoculation site, the tumor was identified.
HE staining of transplanted tumor
The tumor was removed, and the largest diameter of the tumor was sliced approximately 2 mm thick. The tissue was fixed with formaldehyde, and paraffin-embedded slices were stained with HE.
Immunotherapy of tumor-bearing nude mice
The tumor-bearing nude mice were divided into 6 groups, with three mice in each group. The mice in the blank control group were injected twice a week via the tail vein with 0.2 mL of saline. The mice in the DC-CIK group (group D) were injected twice a week with 0.2 ml of 1 × 106 non-antigen loaded DC-CIK cells via the tail vein. The mice in group L-D were injected twice a week with 0.2 ml of 1 × 106 DC-CIK cells that were loaded with total RNA from cancer stem cells via the tail vein. The mice in group T-D were injected twice a week with 0.2 ml of 1 × 106 non-antigen loaded DC-CIK cells via the tail vein but were also injected with 5 mg/Kg THP prior to the first injection day, which was repeated once a week for three weeks. The mice in the THP group were injected with 5 mg/Kg THP via the tail vein once a week for three weeks. The mice in each group received immunotherapy four days after inoculation. The tumors of the mice in each group were measured every two days over the three week treatment period. A slide gauge was used to measure the maximum diameter of the transplanted tumor as well as the two minimum diameters. Tumor volume (mm3) was calculated as length × width × width × 0.52.
Expression of the c-Myc gene
The transplanted tumor tissue (20 mg) was stored at -80°C after being ground and crushed, transferred to an RNAse-free EP tube, and briefly placed into liquid nitrogen. Total RNA was extracted from the transplanted tumor as described in the Qiagen RNeasy mini kit manual. The RNA was used as a template to synthesize cDNA by reverse transcription. The c-Myc gene sequences were obtained from NCBI, and the RT-PCR primers were designed using Oligo6 software. Primer specificity was determined with BLAST from NCBI. c-Myc upstream primer: 5‘-C C A A C A G G A G C T A T G A C C T C-3’, downstream primer: 5‘-T T G T A C C T G C A G G A T C T G A G-3’; GAPDH upstream primer: 5‘-G G T G G T C T C C T C T G A C T T C A A C A-3’, downstream primer: 5‘-G T T G C T G T A G C C A A A T T C G T T G T-3’.
Statistical analysis
Statistical analysis was performed with SPSS 14.0 software. Fisher’s exact test was used to analyze cell phenotypes. P < 0.05 was considered to be statistically significant.
Results
DC-CIK cell culture
Peripheral blood monocytes were adhered for 2 h to generate DC precursors. The adherent cells were round, smooth, and gathered into small clusters. The cultured cells were treated with RhGM-CSF and RhIL-4. Dendrites were visible on the cell surface at day 5; the cells were loaded with antigen, and the DC cells were mature at day 8. A large number of dendrites appeared to have stubby, finger-like pseudopodia and mounds on their surfaces (Figure 1). The CIK cells grew in a small circle and had a uniform structure at the initial stage. The CIK cells were mixed with the DCs at day 8, and they were continuously cultured. The number of cells increased daily, and the cell shapes were irregular. The cells aggregated and formed into large suspension clusters (Figure 1).
Figure 1.
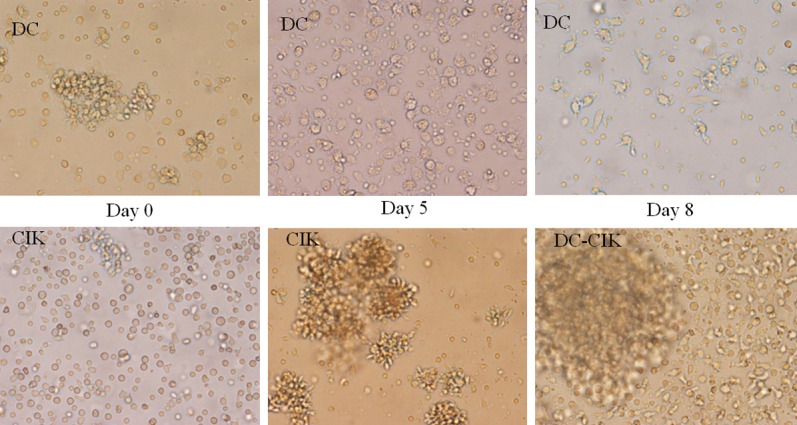
Cell morphology of DC-CIKs under an optical microscope.
Detection of DC-CIK immunity phenotype in each group
MDA-MB-231 cancer stem cell antigens were loaded with DC-CIKs, and then the expression of DC surface molecules CD40, CD80, CD86 and HLA-DR (Figure 2) and CIK surface molecules CD3, CD8, CD56 was detected by flow cytometry (Figure 3). Flow cytometry showed that highly pure mature DCs were obtained after the DCs were loaded with RNA from MDA-MB-231 cancer stem cells or whole-cell lysate (Table 1). The expression of double-positive CIK cells, CD3+ CD8+ and CD3+ CD56+, was significantly increased after the DCs of each group were mixed with homologous CIKs in culture (Table 2). The difference between the expression of DC-CIKs loaded with cancer stem cell antigen and the expression of unloaded DC-CIKs was statistically significant (P < 0.01, n = 3).
Figure 2.
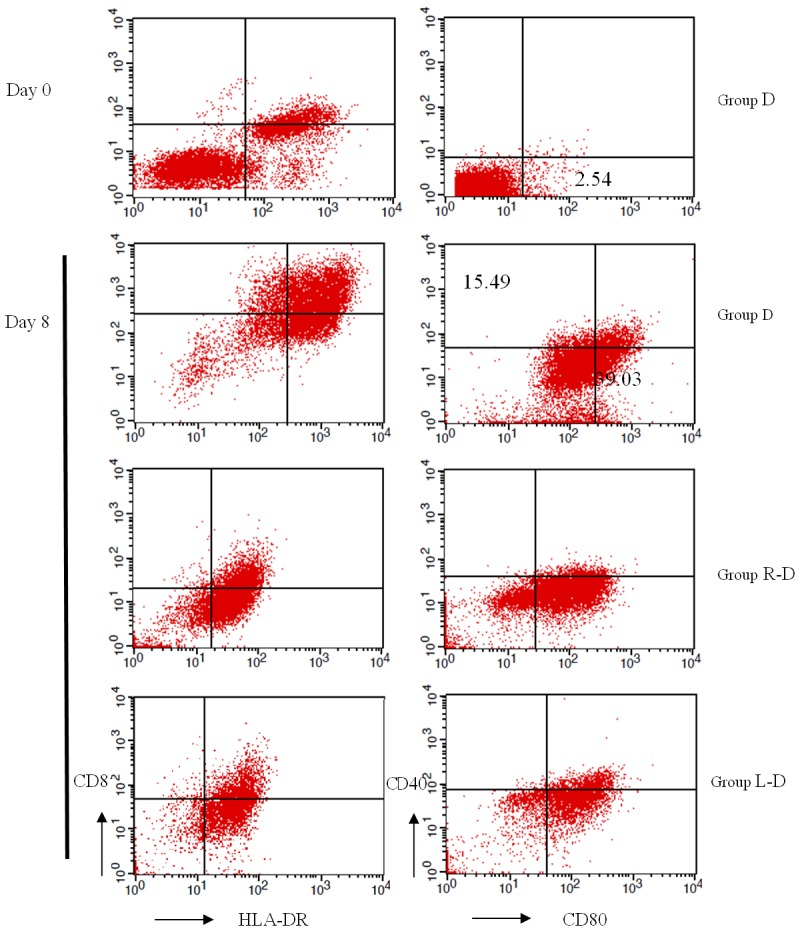
Detections of DCs immune phenotype at different times.
Figure 3.
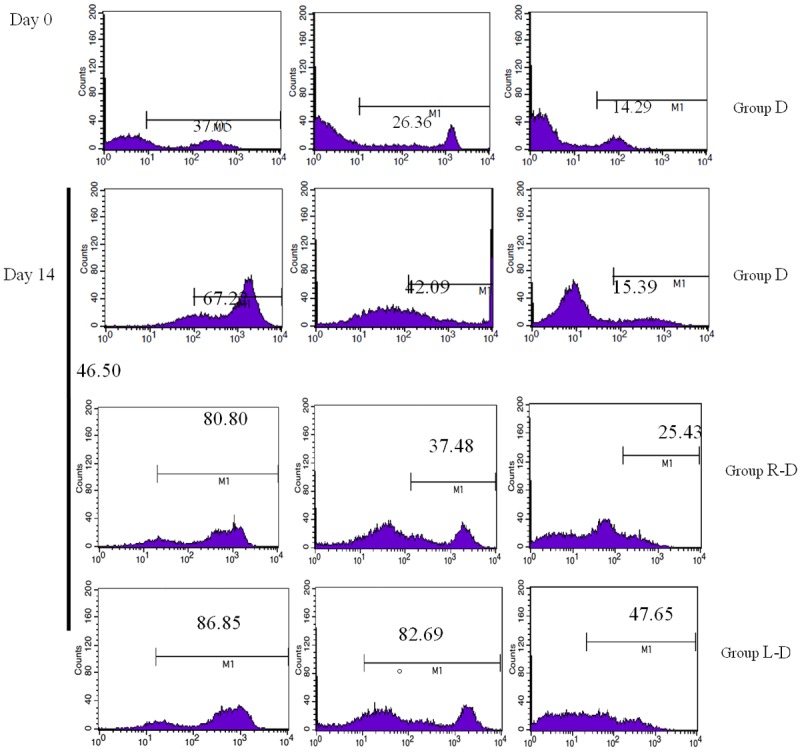
Detection of CIK immunity phenotypes at different times.
Table 1.
Analysis of DC immune phenotype (n = 3, %, x ± s)
| Group | CD40 | CD80 | CD86 | HLA-DR |
|---|---|---|---|---|
| D (day 0) | 0.59 | 2.54 | 14.03 | 36.54 |
| D (day 8) | 15.49 | 39.03 | 27.36 | 52.86 |
| R-D (day 8) | 16.23 | 66.96 | 30.54 | 83.76 |
| L-D (day 8) | 21.62 | 77.67 | 30.90 | 81.21 |
*Statistically significant difference between Group R-D/L-D and Group D (P<0.01).
Table 2.
Analysis of CIK immune phenotype (n = 3, %, x̅ ± s)
| Group | CD3 | CD8 | CD56 |
|---|---|---|---|
| D (day 0) | 37.05 | 26.36 | 14.29 |
| D (day 14) | 67.22 | 42.09 | 15.39 |
| R-D (day 14) | 80.80 | 37.48 | 25.43 |
| L-D (day 14) | 86.85 | 82.69 | 47.65 |
*Statistically significant difference between Group R-D/L-D and Group D (P<0.01).
HE staining of transplantation tumors in nude mice
Pathological examination showed that the CD44+ CD24- transplantation tumor was typical of a moderately-differentiated adenocarcinoma of the breast. Tumor cells were irregular in the glandular ducts and glands, and parts of the glands were angular (Figure 4A). The pathology results indicated that the tissues of the blank control group did not show obvious degeneration necrosis. The tumor cells grew in nested patterns and polygons and were closely-packed with one another, forming brick-like structures (Figure 4B). Group D showed areas of tumor necrosis appearing as small central foci, and necrotic nuclear tissue fragments were visible (Figure 4C). The cancer cells in group L-D showed cribriform architecture around the necrotic regions (Figure 4D). Group R-D showed focal necrosis at the edge of the tumor, and the tumor cells were island-shaped (Figure 4E). Group T-D showed different stages of tumor cell apoptosis and necrosis (Figure 4F).
Figure 4.
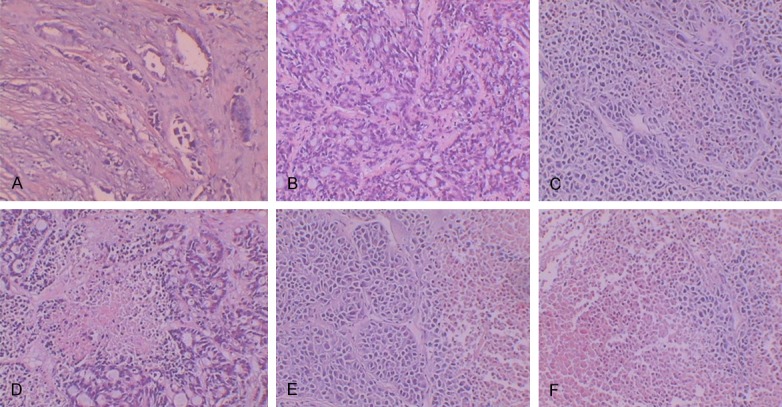
HE staining of transplantation tumors in nude mice (10 × 10).
Comparison of survival conditions in tumor-bearing nude mice
The mice in groups L-D, R-D and D were normal, active, and had a healthy appetite during immunotherapy. After being injected with chemotherapy agents, the mice in groups T-D and THP were significantly lethargic and less active. Furthermore, they displayed signs of distress, such as loss of appetite, weight loss, and back arching. The mice were moribund after two weeks of receiving chemotherapy. The mice in the blank control group were less active and moribund by the third week. After immunotherapy, the weights of the mice in the L-D, R-D, D and blank control groups decreased slightly; however, there was significant weight loss in groups T-D and THP (Figure 5).
Figure 5.
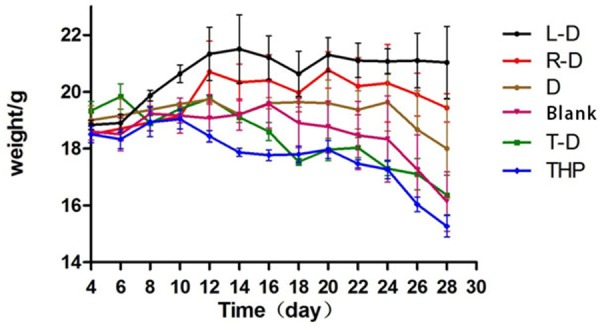
Curve of weight changes in tumor-bearing nude mice during treatment.
Different development of tumors in tumor-bearing nude mice
During immunotherapy, the tumor volumes of mice in groups L-D, R-D and D developed slowly such that L-D < R-D < D. In the blank control, THP and T-D groups, tumor volumes developed fast at the beginning of treatment such that THP > blank control > T-D (Figure 6A). The mice were moribund, and the tumors festered. After immunotherapy, the volumes and weights of the tumors in each group were as follows: L-D < R-D < D < T-D < Blank control < THP (Figure 6A, 6C).
Figure 6.
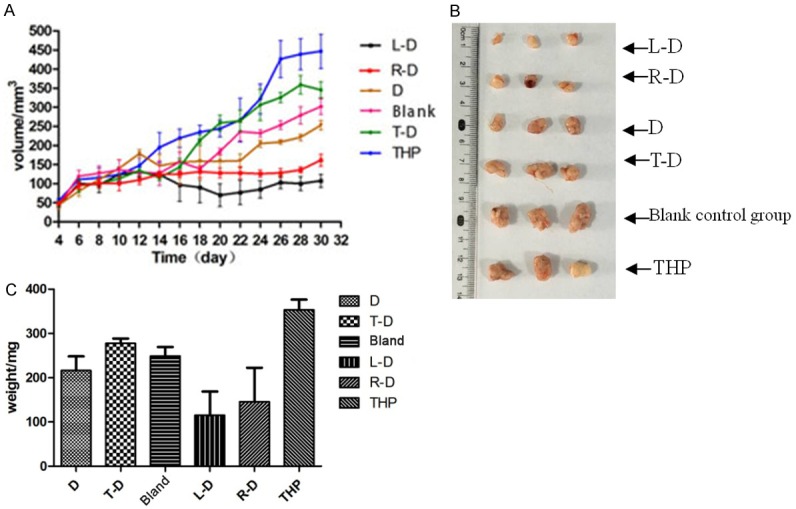
Development of tumors in tumor-bearing nude mice. A: Curves of tumor volume changes during immunotherapy. B: Volumes of tumors in mice of each group at the end of the treatment. C: Tumor weights in mice of each group at the end of treatment.
Expression of c-Myc, a key gene of the Akt signaling pathway, after immunotherapy
The blank control group was compared with groups THP, L-D, R-D, D and T-D. The expression of c-Myc mRNA was not different in group THP; however, in groups L-D and R-D, c-Myc mRNA expression was significantly reduced, whereas in groups T-D and D, c-Myc expression was slightly reduced (Figure 7).
Figure 7.
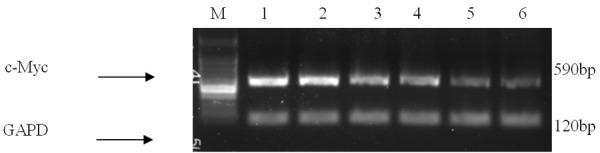
Different expression levels of c-Myc. M: Standards of DNA molecular weight (100 bp-1 KB ladder, interval 100 bp); 1: the blank control group; 2: group THP; group 3: group T-D; 4: group D; 5: group R-D: 6: group L-D.
Discussion
Cancer stem cells (CSCs) are a subpopulation of cancer cells that possess the capabilities of self-renewal and resistance to chemo-radiation therapy. CSCs are considered to be the cause of tumor recurrence and metastasis. Therefore, the development of specific therapeutic targets of CSCs may improve the survival and quality of life of cancer patients, especially those with metastatic disease [6]. Dendritic cells (DCs) are potent antigen-presenting cells and play an important role in inducing primary immune responses against tumor-associated antigens. Some strategies have been developed to modify DCs with tumor-specific antigens to generate anti-tumor immune responses. DC-based immunotherapy (Provenge ®) has achieved a successful outcome in the clinical treatment of melanoma [7]. In this study, two types of mature methods, including whole-cell freeze-thaw antigen preparation and extraction of total RNA, were used to treat CSCs. These two methods of loading DC cells contribute to the maturation of DC cells such that they possess a stronger ability to present antigens. These methods exert consistent treatment effects in tumor-bearing nude mice.
Another advantage of immunotherapy entails removing the tumor without injuring normal tissue [8]. In this study, the tumor-bearing nude mice receiving immunotherapy, groups R-D and L-D, had good survival and did not show an agonal state at the end of treatment. In contrast, the quality of life of the animals in groups THP and T-D decreased significantly after chemotherapy. The nude mice showed loss of appetite, lethargy and limited activity. Therefore, the nude mice in groups THP and T-D showed an agonal state after immunotherapy.
The PI3K/Akt signaling pathway plays an important role in the development of tumors. The proto-oncogene c-Myc can inhibit cell apoptosis and promote tumor cell proliferation. c-Myc has been found to be overexpressed in colon, breast and prostate cancers [9]. c-Myc is mainly regulated by the PI3K/Akt signal pathway [10]. In this study, c-Myc RNA expression did not significantly change in group THP compared with the blank control group. However, down-regulation of c-Myc was observed in groups L-D and R-D, while a slight down-regulation occurred in groups D and T-D.
In conclusion, total RNA loaded DC-CIK immunotherapy is highly effective against MDA-MB-231 breast cancer stem cells and may provide a new option for the clinical treatment of breast cancer.
Acknowledgements
Tianjin application foundation and research in cutting-edge technologies in the plan (14JCYBJC26900); Tianjin health industry key research projects (13KG116).
Disclosure of conflict of interest
None.
References
- 1.Wang QJ, Zhu WX, Xing XM. Analysis of the incidence and survival of female breast cancer in Beijing during the last 20 years. Zhonghua Zhong Liu Za Zhi. 2006;28:208–210. [PubMed] [Google Scholar]
- 2.Alamgeer M, Peacock CD, Matsui W, Ganju V, Watkins DN. Cancer stem cells in lung cancer: Evidence and controversies. Respirology. 2013;18:757–764. doi: 10.1111/resp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duriancik DM, Hoag KA. The identification and enumeration of dendritic cell populations from individual mouse spleen and Peyer’s patches using flow cytometric analysis. Cytometry A. 2009;75:951–959. doi: 10.1002/cyto.a.20794. [DOI] [PubMed] [Google Scholar]
- 4.Ning N, Pan Q, Zheng F, Teitz-Tennenbaum S, Egenti M, Yet J, Li M, Ginestier C, Wicha MS, Moyer JS, Prince ME, Xu Y, Zhang XL, Huang S, Chang AE, Li Q. Cancer stem cell vaccination confers significant antitumor immunity. Cancer Res. 2012;72:1853–1864. doi: 10.1158/0008-5472.CAN-11-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corzo C, Corominas JM, Tusquets I, Salido M, Bellet M, Fabregat X, Serrano S, Solé F. The MYC oncogene in breast cancer progression: from benign epithelium to invasive carcinoma. Cancer Genet Cytogenet. 2006;165:151–156. doi: 10.1016/j.cancergencyto.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Ochsenbein AF, Sierro S, Odermatt B, Pericin M, Karrer U, Hermans J, Hemmi S, Hengartner H, Zinkernagel RM. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411:1058–1064. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 7.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 8.Riether C, Schurch C, Ochsenbein AF. From “magic bullets” to specific cancer immunotherapy. Swiss Med Wkly. 2013;143:w13734. doi: 10.4414/smw.2013.13734. [DOI] [PubMed] [Google Scholar]
- 9.Li CM, Margolin AA, Salas M, Memeo L, Mansukhani M, Hibshoosh H, Szabolcs M, Klinakis A, Tycko B. PEG10 is a c-MYC target gene in cancer cells. Cancer Res. 2006;66:665–672. doi: 10.1158/0008-5472.CAN-05-1553. [DOI] [PubMed] [Google Scholar]
- 10.Galmozzi E, Casalini P, Iorio MV, Casati B, Olgiati C, Ménard S. HER2 signaling enhances 5’UTR-mediated translation of c-Myc mRNA. J Cell Physiol. 2004;200:82–88. doi: 10.1002/jcp.20012. [DOI] [PubMed] [Google Scholar]


