Abstract
Endothelial cell (EC) apoptosis is a crucial process for the development of atherosclerosis. Tanshinol is reported to protect vascular endothelia and attenuate the formation of atherosclerosis. However, the potential molecule mechanism of the protective role of tanshinol in atherosclerosis need to be further investigated. ApoE-/-mice were fed with a high-fat diet and treated with tanshinol to detect the effect of tanshinol on endothelial cells apoptosis with TUNEL staining assay. qRT-PCR and Western blot were performed to examine the expression of TUG1 and miR-26a in endothelial cells. RNA-binding protein immunoprecipitation assay was performed to verify the relationship between TUG1 and miR-26a. It has been shown that tanshinol reduced the aortic atherosclerotic lesion area in the entire aorta and aortic sinus in a concentration dependent manner, and suppressed the endothelial cells apoptosis in ApoE-/- mice. We further found that the mRNA level of TUG1 was reduced and the expression of miR-26a was up-regulated by tanshinol in endothelial cells. In addition, TUG1 down-regulated the expression of miR-26a in ECV304 cells. Finally, it was shown that overexpression of TUG1 removed the reversed effect of tanshinol on oxidized low-density lipoprotein (ox-LDL)-induced endothelial cells apoptosis. Taken together, our study reveals that tanshinol could attenuate the endothelial cells apoptosis in atherosclerotic ApoE-/- mice. Moreover, low TUG1 expression and high level of miR-26a are associated with the endothelial protecting effect of tanshinol.
Keywords: Tanshinol, lncRNA TUG1, endothelial cells, cell apoptosis, atherosclerosis, miR-26a
Introduction
Atherosclerosis (AS) is a major cause of morbidity and mortality among the cardiovascular diseases, which is initially triggered by endothelial dysfunction and characterized by an influx of atherogenic lipoprotein components [1]. It is believed that endothelial cells apoptosis results in the denudation or dysfunction of the intact endothelial monolayer, which causes lipid accumulation, monocyte adhesion, and inflammatory reactions leading to atherosclerotic lesion [2]. Tanshinol (3,4-dihydroxyphenyl lactic acid) is widely used in traditional Chinese medicine and has been reported to have vasodilatory properties and to lower methionine-induced hyperhomocysteinemia in rats [3,4]. Accumu-lating evidence has been well established that tanshinol has effective roles for the treatment of coronary heart disease, cerebrovascular disease, bone loss, hepatocirrhosis, and chronic renal failure [5]. It has been reported that tanshinol protected vascular endothelia in a rat model of hyperhomocysteinemia and attenuated the formation of atherosclerosis through inhibiting the expression of representative pro-inflammatory cytokines and adhesion molecules in arterial endothelia [4]. However, the role of tanshinol in atherosclerosis is poorly investigated.
To date, non-coding RNAs (ncRNAs) including long non-coding RNAs (lncRNAs) and microRNAs (miRNAs), have gained increasing attention in tumor malignant processes [6]. LncRNA taurine-upregulated gene 1 (TUG1) was originally identified to contribute to the forming of photoreceptors and played crucial roles in retinal development [7]. Recently, mounting evidence showed that the dysregulation of TUG1 participated in the development of several cancers, such as non-small cell lung cancer, bladder cancer, osteosarcoma and melanoma [8-10].
Recent studies were originally identified miRNAs as crucial regulators of human disease by binding to 3’-untranslated region (3’-UTR) of target messenger RNA to negatively regulate gene expression [11]. Among them, miR-26a is a highly conserved miRNA that plays essential roles in development, cell differentiation and growth. MiR-26a could regulate cortical neurite growth in Alzheimer’s disease [12]. In retinal ganglion cells, miR-26a was shown to protect RGC-5 cell against cytotoxicity and apoptosis through down-regulation of PTEN [13]. Lately, miR-26a was reported to prevent endothelial cell apoptosis by directly targeting TRPC6 in the setting of atherosclerosis [14]. TRPC6 is a calciumpermeable channel expressed in several cells, including ECs.
In the present study, the major aim was to investigate the effect of tanshinol on endothelial cells apoptosis in mice with atherosclerosis and the expression of TUG1 and miR-26a in aortic endothelial cells. Meanwhile, the interactions among TUG1, miR-26a and TRPC6 in the endothelial cells treated with tanshinol and the possible mechanism were also revealed.
Materials and methods
Preparation of tanshinol
Tanshinol was obtained from Tong Ren Tang Company (Beijing, China). The purity of tanshinol was ≥99.0%.
Animals
Eight-week-old male ApoE-/- mice were subsequently maintained on diet with a high-fat diet (0.15% cholesterol and 21% fat) for 16 weeks. During this duration, tanshinol (0, 15, 30, 60 mg/kg, respectively) was administrated intragastrically at a frequency of two days one time. C57BL/6J mice fed a high-fat diet served as control. All animals were kept in certified specific pathogen-free facilities maintained around 24°C with a 12-h light/dark cycle. All animal experiments were approved by the Animal Care and Use Committee of Shandong Qianfoshan Hospital, and all animal care and experimental procedures strictly followed the Council for International Organizations of Medical Sciences (CIOMS) guidelines.
En face analysis of aortic lesion
The extent of aortic atherosclerotic lesions in mice was examined by en face staining of aortas with oil red O [15]. Briefly, at the end of the treatment, mice were anesthetized with pentobarbital (60 mg/kg). Aortas were removed 2 mm from the heart and excised from the aortic arch to just beyond the iliac bifurcation, cut longitudinally, fixed with 10% neutral buffered formalin, stained with oil-red O (Beyotime Biotechnology, Haimen, China), and mounted on slides with the endothelium side up. Atherosclerotic plaques in full-length aorta and aortic arch were analyzed and quantified relative to the full-length lumen area, using the updated Image-Pro Plus program (Media Cybernetics, Silver Spring, MD, USA). Lesions were expressed as positive staining percentage for oil-red O of the total aortic area.
Western blot analysis
The protein levels of TRPC6 in endothelial cells were determined by Western blotting. The cells were lysed with radioimmuneprecipitation buffer containing protease and phosphatase inhibitors. Cell lysates were subjected to SDS-PAGE and transferred to nitrocellulose membranes. After blocking with 5% non-fat milk, the membranes were probed with anti-TRPC6 (Abcam, Cambridge, MA, USA), followed by incubation with a florescence-labeled secondary antibody. Western blot bands were scanned using the Odyssey Imaging System (LICOR, Lincoln, NE, USA). β-Actin (Cell Signaling Technology, Danvers, MA, USA) was used as internal control.
Quantitative real-time PCR
Total RNA was harvested from tissues and cells using TRIzol reagent (Invitrogen, CA, USA) according to the manufacturer’s protocols. The extracted RNA was reverse transcribed into cDNA using High-Capacity cDNA Reverse Transcription Kit (Takara BioInc, China). The first strand cDNA was used for real-time PCR to quantify mRNA expressions of TUG1 and TRPC6 with GAPDH as an internal control. MiR-26a expression was detected with U6 as an internal control. The RT primer and forward and reverse primer pairs for miR-26a were designed by RiboBio Co., Ltd. (Guangzhou, Guangdong, China). TUG1, TRPC6 and miR-26a levels were presented as values of 2-ΔΔCt.
Cell culture and transfection
Human aortic endothelial cells (HAECs) and ECV304 cells were obtained from ScienCell Research Laboratories (Carlsbad, CA, USA) and cultured in Endothelial Cell Medium supplemented with endothelial cell growth factors, 5% FBS and 1% penicillin/streptomycin. The cells were maintained at 37°C with 5% CO2 and 95% air. ECV304 cells were transiently transfected with p-MIR-TUG1-WT and miR-26a mimic or p-MIR-TUG1-Mut and miR-26a mimic (RiboBio Co., Ltd., Guangzhou, Guangdong, China), using Lipofectamine 2000 reagent (Invitrogen, CA, Carlsbad, USA) according to the manufacturer’s instructions. TUG1-expressing plasmid or si-TUG1 was transfected into ECV304 Cells. After 24 h of transfection, the medium was replaced by fresh medium with or without ox-LDL and tanshinol. After drug treatment, the cells were used for immunofluorescent staining or protein/RNA extraction.
Luciferase reporter assay
MiR-26a sensor reporter was constructed using psi-CHECK2 vectors (Promega, Madison, WI, USA). Then, ECV304 cells were seeded in a 24-well plate and co-transfected with 0.5 mg plasmid and pcDNA-TUG1 or si-TUG1 or controls using Lipofectamine 2000 reagent. Renilla luciferase was used as an internal control. The cells were collected after transfection 48 h, and Renilla luciferase activities were evaluated using Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
Apoptosis detection using TUNEL staining
TUNEL staining was used to detect endothelial cells apoptosis using a TUNEL fluorescence FITC kit (Roche, Indianapolis, IN, USA). For tissues, after TUNEL staining, the aorta sections were immersed into DAPI solution to stain nuclei. For cells, endothelial cells grown on coverslips were fixed with 4% paraformaldehyde followed by permeabilization with 0.1% Triton X-100. Then, cells were incubated with TUNEL reaction mixture at 37°C for 1 h. The stained tissues and cells were examined under a confocal laser scanning microscope (FV300, Olympus, Japan).
RNA-binding protein immunoprecipitation (RIP) assay
RIP was performed using a Magna RNA-binding protein immunoprecipitation kit (Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. Briefly, ECs were lysed in complete RNA lysis buffer, and then were incubated with RIP buffer containing magnetic beads conjugated with human anti-Argonaute2 (Ago2) antibody (Millipore, Billerica, MA, USA), and negative control normal mouse IgG (Millipore, Billerica, MA, USA). Samples were incubated with Proteinase K and then immunoprecipitated RNA was isolated. Furthermore, purified RNAs extracted and analyzed by qRT-PCR to demonstrate the presence of the binding targets.
Statistical methods
All data were from at least three independent experiments. Results were given as mean ± SD. Statistical comparison between two groups was carried out using the Student’s t-test. Differences among groups were carried out by One-way ANOVA followed by LSD tests using Graphpad Prism version 6. P<0.05 was considered statistically significant.
Results
Tanshinol ameliorates the atherosclerotic lesions in ApoE-/- mice and relieved the endothelial cells apoptosis
To examine the impact of tanshinol on atherosclerosis, we fed ApoE-/- mice with high-fat diet with different dose of tanshinol treatment (0, 15, 30, 60 mg/kg) for 16 weeks, and then we evaluated the atherosclerotic lesion area in the aorta and aortic arch. The en face oil-red O staining of aorta was performed to determine the lesion area covering the aortic surface. In ApoE-/- mice, apparent atherosclerotic lesion were observed comparing to that of the control. Moreover, tanshinol induced a great reduction in atherosclerotic plaques in the aorta and aortic arch areas (Figure 1A and 1B). This attenuated effect was more dramatic with the higher dose of tanshinol. Next, the effect of tanshinol on endothelial cell survival was evaluated using TUNEL assay. The percentage of cells with positive TUNEL staining was markedly decreased in the tanshinol-treated group in a dose-dependent manner compared with that in the no treatment group (Figure 1C). Meanwhile, as shown in Figure 1D, tanshinol had no effect on body weight in atherosclerotic ApoE-/- mice.
Figure 1.
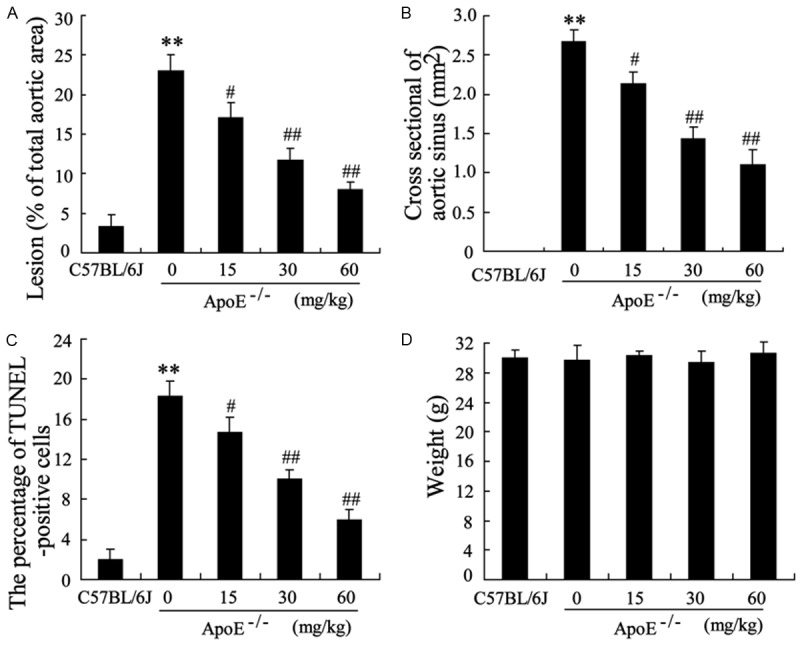
Effect of tanshinol on atherosclerotic lesions in ApoE-/- mice. ApoE-/- mice were fed with high-fat diet with different dose of tanshinol treatment (0, 15, 30, 60 mg/kg) for 16 weeks, C57BL/6J mice fed with high-fat diet served as control. ApoE-/- mice were treated with tanshinol for 2 days/time. A. The aortic atherosclerotic lesions in these groups were examined by en face oil red O staining in aortas and expressed as the percentage of positive staining for oil-red O of total aortic area (%). B. The atherosclerotic plaques in the aortic sinus in these groups were examined by oil red O staining and the area of positive staining for oil-red O (μm2) was calculated. C. TUNEL staining and the averaged data of apoptotic (TUNEL-positive) cell ratio in these groups. D. Weight was measured in these mice on the last day of treatment. The data are presented as the mean ± SD, n = 10 mice. **VS C57BL/6J, P<0.01; #VS ApoE-/-, P<0.05; ##VS ApoE-/-, P<0.01.
Tanshinol reduces the expression of TUG1 and up-regulates the expression of miR-26a in endothelial cells
We further detected the expression of TUG1 and miR-26a in endothelial cells separated from above mice with qRT-PCR. As shown in Figure 2A, significantly higher mRNA level of TUG1 was observed in ApoE-/- mice than that in C57BL/6J mice. While, the mRNA level of TUG1 was reduced in tanshinol-treated group in a dose-dependent manner. As for the expression of miR-26a, we observed that tanshinol up-regulated the expression of miR-26a in endothelial cells (Figure 2B). In addition, the mRNA and protein level of TRPC6 which was a downstream molecule of miR-26a were down-regulated by tanshinol (Figure 2C). These data indicated that TUG1 and miR-26a might involve in the effect of tanshinol on endothelial cells.
Figure 2.
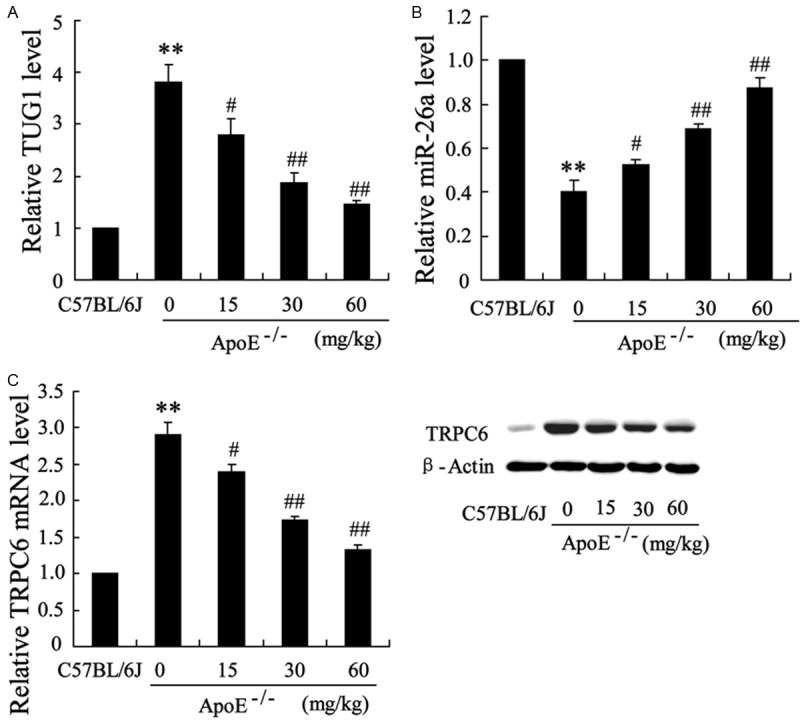
The expression of TUG1 and miR-26a in endothelial cells separated from ApoE-/- mice. ApoE-/- mice were fed with high-fat diet with different dose of tanshinol treatment (0, 15, 30, 60 mg/kg) for 16 weeks, C57BL/6J mice fed with high-fat diet served as control. ApoE-/- mice were treated with tanshinol for 2 days/times. Endothelial cells were separated from these ApoE-/- mice. A. The mRNA level of TUG1 in these endothelial cells. B. The mRNA level of miR-26a in these endothelial cells. C. The mRNA and protein level of TRPC6 in these endothelial cells. The data are presented as the mean ± SD. **VS C57BL/6J, P<0.01; #VS ApoE-/-, P<0.05; ##VS ApoE-/-, P<0.01.
Tanshinol reversed the effect of ox-LDL on the expression of TUG1 and miR-26a in human aortic endothelial cells
Oxidized low-density lipoprotein (ox-LDL) was a well-known atherogenic factor that can induce endothelial cell apoptosis, we next treated HAECs with ox-LDL (25 μg/ml) for 48 h after pretreatment with different concentrations (0, 0.25, 0.5, 1.0 mg/L) of tanshinol for 2 h. The expression of TUG1 was enhanced by ox-LDL, while tanshinol reversed this effect in a dose-dependent manner (Figure 3A). To investigate whether tanshinol mediates the effect of ox-LDL on the expression of miR-26a, we measured the mRNA level of miR-26a in these HAECs. It has been shown that lower mRNA level of miR-26a was observed in HAECs without the treatment of tanshinol comparing to the control. Furthermore, the expression of miR-26a was enhanced by tanshinol although HAECs exposed to ox-LDL (Figure 3B). TRPC6 was also regulated by ox-LDL and tanshinol. Tanshinol reversed the up-regulation of TRPC6 induced by ox-LDL in a dose-dependent manner (Figure 3C).
Figure 3.
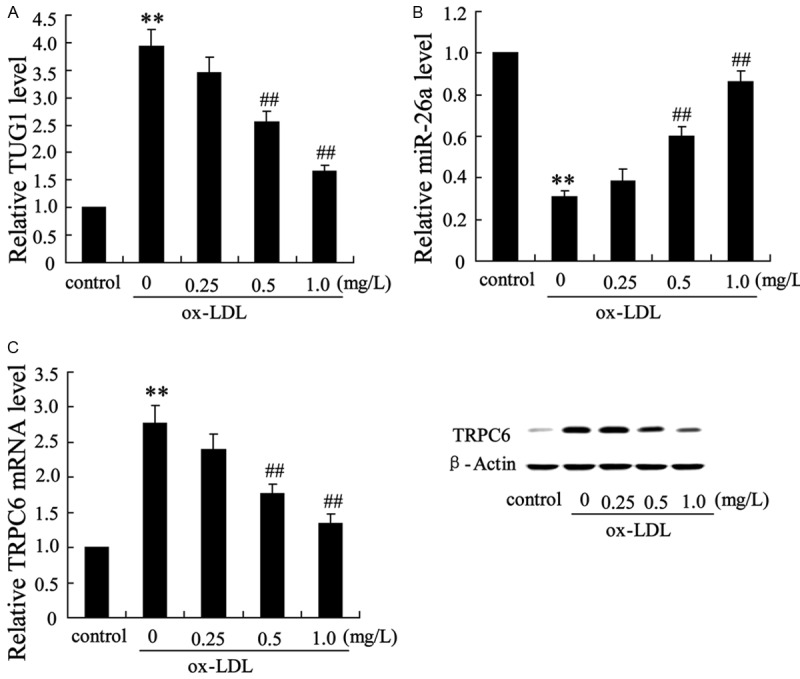
Tanshinol reversed the effect of ox-LDL on the expression of TUG1 and miR-26a in human aortic endothelial cells. Human aortic endothelial cells were treated with ox-LDL (25 μg/ml) for 48 h after pretreatment with different concentrations (0, 0.25, 0.5, 1.0 mg/L) of tanshinol for 2 h. A. The mRNA level of TUG1 in these endothelial cells. B. The mRNA level of miR-26a in these endothelial cells. C. The mRNA and protein level of TRPC6 in these endothelial cells. The data are presented as the mean ± SD. **VS control, P<0.01; ##VS ox-LDL, P<0.01.
TUG1 regulated the expression of miR-26a in ECV304 cells
To understand the mechanisms by which TUG1 and miR-26a might involve in the protective effect of tanshinol, we employed pcDNA-TUG1 and si-TUG1 to overexpress or suppress the expression of TUG1 in ECV304 cells. The miR-26a sensor reporter were constructed and co-transfected with pcDNA-TUG1 or si-TUG1 into ECV304 cells. We found that pcDNA-TUG1 markedly inhibited the luciferase activity of the miR-26a sensor reporter, whereas si-TUG1 increased luciferase activity (Figure 4). Moreover, the mRNA level of miR-26a was also reduced by pcDNA-TUG1 and increased by si-TUG1 (Figure 4).
Figure 4.
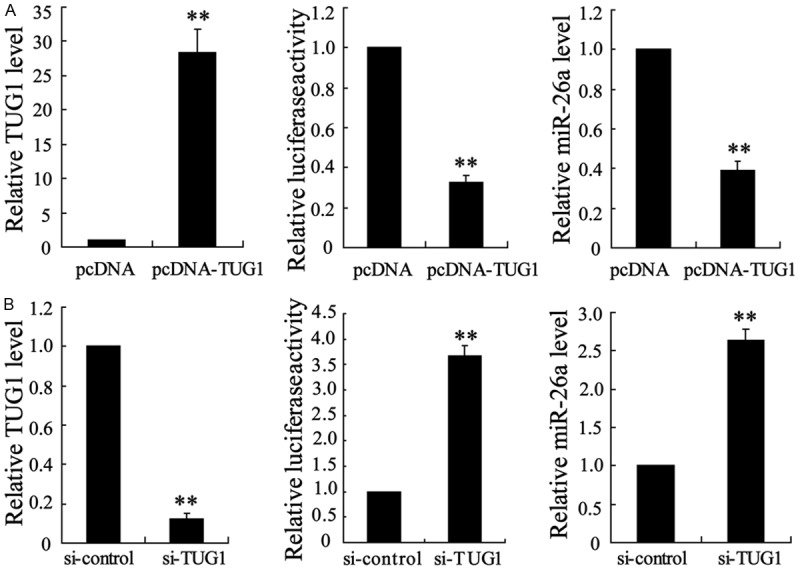
The regulation of TUG1 on the expression of miR-26a in ECV304 cells. A. The mRNA level and activity of 3’-UTR of miR-26a in ECV304 cells overexpressing TUG1. B. The mRNA level and activity of 3’-UTR of miR-26a in ECV304 cells down-regulating TUG1. The data are presented as the mean ± SD. **VS control, P<0.01.
TUG1 could directly bind to miR-26a in ECV304 cells
To confirm the regulatory effect of TUG1 on the expression of miR-26a in ECV304 cells, we examined the changes in luciferase activity in ECV304 cells co-transfected with p-MIR-TUG1-WT and miR-26a mimic or p-MIR-TUG1-Mut and miR-26a mimic. The results clearly indicated that miR-26a mimic significantly suppressed the activity of p-MIR-TUG1-WT, but has no effect on the activity of p-MIR-TUG1-Mut (Figure 5A). RNA immunoprecipitation (RIP) was performed using anti-Ago2 to examine the expression of TUG1 and miR-26a. In Figure 5B, the relative RNA enrichment of Ago2 RIP and IgG RIP showed that the expression of TUG1 and miR-26a in Ago2 RIP were significantly higher than that in IgG RIP.
Figure 5.
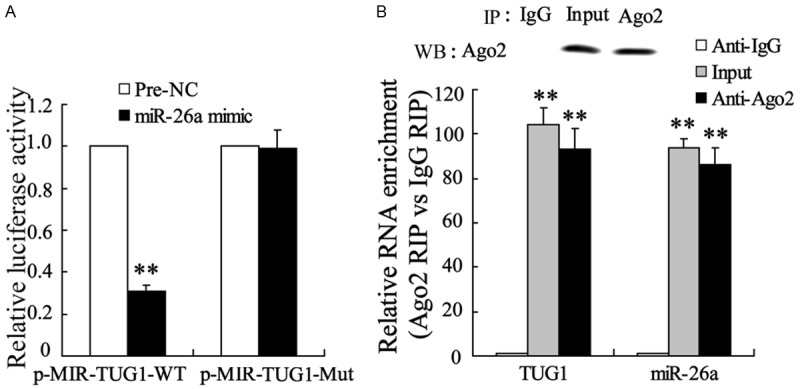
TUG1 could bind to miR-26a directly. A. ECV304 cells were co-transfected with p-MIR-TUG1-WT and miR-26a mimic or p-MIR-TUG1-Mut and miR-26a mimic. The activity of TUG1 was detected. B. ECV304 cell lysis solution was treated with Anti-Ago2 for RNA immunoprecipitation. The expression of TUG1 and miR-26a were detected. The data are presented as the mean ± SD. **VS control, P<0.01.
Overexpression of TUG1 removed the reversed effect of tanshinol on ox-LDL for endothelial cells
To validate the role of TUG1 in mediating the anti-apoptotic action of tanshinol in HAECs, we performed a rescue experiment. Endothelial cells were divided into six groups: control (group 1), treat with ox-LDL (25 μg/ml) (group 2), treat with ox-LDL and DMSO (group 3), treat with ox-LDL and tanshinol (1.0 mg/L) (group 4), treat with ox-LDL and tanshinol and pcDNA (group 5), treat with ox-LDL and tanshinol and pcDNA-TUG1 (group 6). qRT-PCR analyses indicated that tanshinol reversed the downregulation of miR-26a induced by ox-LDL, which further reversed by overexpression of TUG1, as shown in Figure 6A. Correspondingly, the expression of TRPC6 was enhanced by pcDNA-TUG1, which was originally decreased by tanshinol in endothelial cells (Figure 6B). TUNEL staining further confirmed that TUG1 overexpression reversed the ability of tanshinol to suppress apoptosis as indicated by the increase of TUNEL-positive cells with TUG1 overexpression (Figure 6C). These data suggest that tanshinol inhibits ox-LDL-induced apoptosis through down-regulating the expression of TUG1 and the subsequent anti-apoptotic molecule.
Figure 6.
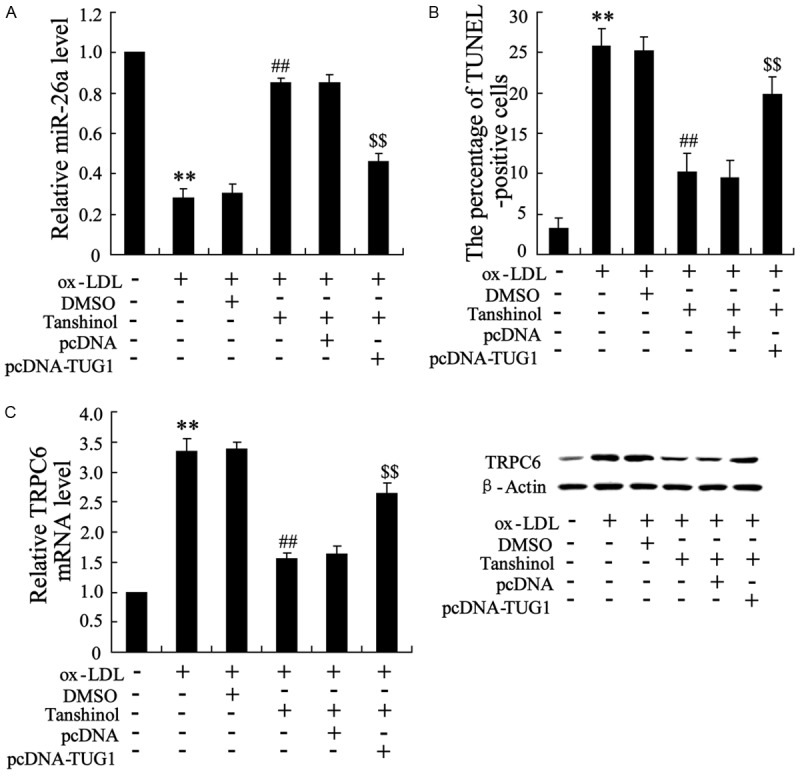
Overexpression of TUG1 removed the reversed effect of tanshinol on ox-LDL for endothelial cells. Endothelial cells were divided into six groups: control (group 1), treat with ox-LDL (25 μg/ml) (group 2), treat with ox-LDL and DMSO (group 3), treat with ox-LDL and tanshinol (1.0 mg/L) (group 4), treat with ox-LDL and tanshinol and pcDNA (group 5), treat with ox-LDL and tanshinol and pcDNA-TUG1 (group 6). A. The mRNA level of miR-26a were detected. B. The mRNA and protein level of TUG1 were detected. C. The averaged data of a poptotic (TUNEL-positive) cell ratio in endothelial cells. The data are presented as the mean ± SD. **VS control, P<0.01; ##VS ox-LDL, P<0.01; $$VS ox-LDL + pcDNA, P<0.01.
Discussion
The present study demonstrated that tanshinol could reduce the aortic atherosclerotic lesion area in the entire aorta and aortic sinus in a concentration dependent manner, and suppress the endothelial cells apoptosis, thus alleviating the development of atherosclerotic plaques in ApoE-/- mice, which suggested it may have potential for the treatment of atherosclerosis. We further found that TUG1 and miR-26a were involved in the protective effect of tanshinol on endothelial cells. Specifically, the mRNA level of TUG1 was reduced and the expression of miR-26a was up-regulated by tanshinol in endothelial cell. In addition, TUG1 down-regulated the expression of miR-26a in ECV304 cells. Finally, it was shown that overexpression of TUG1 removed the reversed effect of tanshinol on ox-LDL-induced endothelial cells apoptosis. Taken together, this study provides evidence that TUG1 and miR-26a may be the therapeutic target for vascular diseases, such as atherosclerosis.
It has been known that endothelial cells are the critical players in the development of atherosclerosis, which apoptosis may result in endothelial losing the ability to regulate lipid homeostasis, immunity and inflammation. Proatherosclerotic factors such as high glucose, angiotensin II and reactive species are all able to induce apoptosis of endothelial cells [16-18]. Ox-LDL, which plays a crucial role in lesion formation, stimulates endothelial cell suicide death program through several caspase-dependent or -independent pathways [19]. The use of herbal therapies is escalating worldwide. In this study, tanshinol was found to attenuate the endothelial cells apoptosis in ApoE-/- mice fed with high-fat diet.
To further investigate the endothelial protecting mechanism of tanshinol, we detect the dysregulation of TUG1 and miR-26a in endothelial cells treated with tanshinol. TUG1 was first identified as a full-length 6.7 kilobase untranslated RNA molecule and was necessary for retinal development. Recent, TUG1 was observed to be highly expressed in endothelial cells. In addition, TUG1 was accumulated and acted as an oncogenic in tumor growth and development in various tumor tissues [20]. For example, Heng Cai et al. reported that TUG1 was highly expressed in glioma vascular endothelial cells from glioma tissues [20]. TUG1 was also found to be overexpressed in urothelial carcinoma of the bladder, as well as osteosarcoma tissues [8,10]. But in another study, knockdown of TUG1 promoted tumor cell proliferation in non-small cell lung carcinoma via regulation of the expression of homeobox B7 (HOXB7) [9]. In this study, it has been shown that the mRNA level of TUG1 was reduced in tanshinol-treat group in a dose-dependent manner. As for the expression of miR-26a, we observed that tanshinol up-regulated the expression of miR-26a in endothelial cells. In addition, the mRNA and protein level of TRPC6 which was a downstream molecule of miR-26a were down-regulated by tanshinol. Tanshinol also reversed the effect of ox-LDL on the expression of TUG1 and miR-26a in human aortic endothelial cells.
Emerging evidence suggests that lncRNA may function as a molecular sponge in modulating the expression and biological functions of miRNA such as post-transcriptional regulation [21]. To find out whether TUG1 serves as a miRNA sponge, we performed dual-luciferase reporter assay and demonstrated that upregulated expression of TUG1 could suppress the miR-26a expression, whereas down-regulated TUG1 induced a reverse result. To better clarify the underlying mechanism of the lncRNA/miRNA regulatory function, we performed RIP assay and indicated that TUG1 could directly bind to miR-26a. Down-regulated miR-26a was reported to play important roles in the progression of tumor and severed as a potential tumor suppressor in several distinct cancer types. In addition, Yong Zhang found that the expression of miR-26a was substantially reduced in the aortic intima of ApoE-/- mice fed with a high-fat diet (HFD) [14], which was consistent with our results.
In conclusion, we demonstrated that tanshinol could attenuate the endothelial cells apoptosis in atherosclerotic ApoE-/- mice. Moreover, low TUG1 expression and high level of miR-26a are associated with the endothelial protecting effect of tanshinol, indicating that the therapeutic potential of TUG1 and miR-26a in ameliorating atherosclerotic lesion.
Acknowledgements
This study was supported by the grants from National Natural Science Foundation of China (No. 81302940 and No. 30873323) and Project of Traditional Chinese Medicine and Technology Development in Shandong Province (No. 2013ZDZK-090) and Shandong Provincial Natural Science Foundation, China (No. ZR2015PH046).
Disclosure of conflict of interest
None.
Authors’ contribution
GC performed the experiments and analyzed the data. CC contributed reagents/materials/analysis tools. NZ and RS wrote the manuscript. XY and CL conceived and designed the experiments and analyzed the data. All authors read and approved the final manuscript.
References
- 1.Dimmeler S, Hermann C, Zeiher AM. Apoptosis of endothelial cells. Contribution to the pathophysiology of atherosclerosis? European Cytokine Network. 1998;9:697–698. [PubMed] [Google Scholar]
- 2.Menghini R, Casagrande V, Marino A, Marchetti V, Cardellini M, Stoehr R, Rizza S, Martelli E, Greco S, Mauriello A. MiR-216a: a link between endothelial dysfunction and autophagy. Cell Death Dis. 2014;5:e1029. doi: 10.1038/cddis.2013.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan K, Chui SH, Wong DY, Ha WY, Chan CL, Wong RN. Protective effects of Danshensu from the aqueous extract of Salvia miltiorrhiza (Danshen) against homocysteine-induced endothelial dysfunction. Life Sciences. 2004;75:3157–3171. doi: 10.1016/j.lfs.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Yang R, Huang S, Yan F, Lu X, Xing Y, Liu Y, Liu Y, Zhao Y. Danshensu protects vascular endothelia in a rat model of hyperhomocysteinemia. Acta Pharmacol Sin. 2010;31:1395–1400. doi: 10.1038/aps.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi CG, Yang YS, Li H, Zhang Y, Wang N, Wang SM, Wang JD, Zhang SC. Tanshinol protects hippocampus and attenuates vascular dementia development. J Asian Nat Prod Res. 2014;16:667–676. doi: 10.1080/10286020.2014.930131. [DOI] [PubMed] [Google Scholar]
- 6.Gibb E, Brown C, Lam W. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young TL, Matsuda T, Cepko CL. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Curr Biol. 2005;15:501–512. doi: 10.1016/j.cub.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Han Y, Liu Y, Gui Y, Cai Z. Long intergenic non-coding RNA TUG1 is overexpressed in urothelial carcinoma of the bladder. J Surg Oncol. 2013;107:555–559. doi: 10.1002/jso.23264. [DOI] [PubMed] [Google Scholar]
- 9.Zhang EB, Yin DD, Sun M, Kong R, Liu XH, You LH, Han L, Xia R, Wang KM, Yang JS. P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis. 2014;5:e1243–e1243. doi: 10.1038/cddis.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Geng P, Yin P, Wang X, Jia J, Yao J. Down-regulation of long non-coding RNA TUG1 inhibits osteosarcoma cell proliferation and promotes apoptosis. Asian Pac J Cancer Prev. 2013;14:2311–2315. doi: 10.7314/apjcp.2013.14.4.2311. [DOI] [PubMed] [Google Scholar]
- 11.Bartel DP. MicroRNAs: Target Recognition and Regulatory Functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li B, Sun H. miR-26a promotes neurite outgrowth by repressing PTEN expression. Mol Med Rep. 2013;8:676–680. doi: 10.3892/mmr.2013.1534. [DOI] [PubMed] [Google Scholar]
- 13.Kang Y, Jia P, Zhao H, Hu C, Yang X. MicroRNA-26a overexpression protects RGC-5 cells against H2O2-induced apoptosis. Biochem Biophys Res Commun. 2015;460:164–169. doi: 10.1016/j.bbrc.2015.02.164. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Qin W, Zhang L, Wu X, Du N, Hu Y, Li X, Shen N, Xiao D, Zhang H, Li Z, Zhang Y, Yang H, Gao F, Du Z, Xu C, Yang B. MicroRNA-26a prevents endothelial cell apoptosis by directly targeting TRPC6 in the setting of atherosclerosis. Sci Rep. 2015;5:9401. doi: 10.1038/srep09401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palinski W, Ord V, Plump A, Breslow J, Steinberg D, Witzrum J. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994;14:605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- 16.Baumgartner-Parzer SM, Wagner L, Pettermann M, Grillari J, Gessl A, Waldhäusl W. High-glucose--triggered apoptosis in cultured endothelial cells. Diabetes. 1995;44:1323–1327. doi: 10.2337/diab.44.11.1323. [DOI] [PubMed] [Google Scholar]
- 17.Dimmeler S, Rippmann V, Weiland U, Haendeler J, Zeiher AM. Angiotensin II Induces Apoptosis of Human Endothelial Cells. Circ Res. 1997;81:970–976. doi: 10.1161/01.res.81.6.970. [DOI] [PubMed] [Google Scholar]
- 18.Hermann C, Zeiher A, Dimmeler S. Shear stress inhibits H2O2-induced apoptosis of human endothelial cells by modulation of the glutathione redox cycle and nitric oxide synthase. Arterioscler Thromb Vasc Biol. 1997;17:3588–3592. doi: 10.1161/01.atv.17.12.3588. [DOI] [PubMed] [Google Scholar]
- 19.Dimmeler S, Haendeler J, Galle J, Zeiher A. Oxidized low-density lipoprotein induces apoptosis of human endothelial cells by activation of CPP32-like proteases. A mechanistic clue to the ‘response to injury’ hypothesis. Circulation. 1997;95:1760–1763. doi: 10.1161/01.cir.95.7.1760. [DOI] [PubMed] [Google Scholar]
- 20.Cai H, Xue Y, Wang P, Wang Z, Li Z, Hu Y, Li Z, Shang X, Liu Y. The long noncoding RNA TUG1 regulates blood-tumor barrier permeability by targeting miR-144. Oncotarget. 2015;6:19759–19779. doi: 10.18632/oncotarget.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]


