Abstract
Bruton’s tyrosine kinase (Btk) is a member of the Tec-family non-receptor tyrosine kinases family. It has previously been reported to be expressed in B cells and has an important role in B-cell malignancies. While the roles of Btk in the pathogenesis of certain B-cell malignancies are well established, the functions of Btk in gastric carcinoma have never been investigated. Herein, we found that Btk is over-expressed in gastric carcinoma tissues and gastric cancer cells. Knockdown of Btk expression selectively inhibits the growth of gastric cancer cells, but not that of the normal gastric mucosa epithelial cell, which express very little Btk. Inhibition of Btk by its inhibitor ibrutinib has an additive inhibitory effect on gastric cancer cell growth. Treatment of gastric cancer cells, but not immortalized breast epithelial cells with ibrutinib results in effective cell killing, accompanied by the attenuation of Btk signals. Ibrutinib also induces apoptosis in gastric carcinoma cells as well as is a chemo-sensitizer for docetaxel (DTX), a standard of care for gastric carcinoma patients. Finally, ibrutinib markedly reduces tumor growth and increases tumor cell apoptosis in the tumors formed in mice inoculated with the gastric carcinoma cells. Given these promising preclinical results for ibrutinib in gastric carcinoma, a strategy combining Btk inhibitor warrants attention in gastric cancer.
Keywords: Btk, Ibrutinib, gastric carcinoma
Introduction
Gastric carcinoma is the most frequently diagnosed cancer and the leading cause of cancer deaths in world [1]. The risk and side effects associated with current therapies, which range from impotence and incontinence after surgery to recurrence of an androgen-independent tumor after androgen ablation therapy, are severe. Tyrosine kinase inhibitors (TKIs) are among the most promising targeted therapies, most of which are directed against receptor tyrosine kinases [2]. The outcomes of clinical trials based on TKIs as single agents have generally been modest, probably due to redundancy in receptor binding and signaling to intracellular mediators.
The Tec family of tyrosine kinases is the second largest family of cytoplasmic tyrosine kinases. It consists of six members with tissue-specific expression patterns in normal cells [3]. Btk is the prototype of this family of tyrosine kinases. Btk is reportedly expressed primarily in B cells, monocytes, macrophages and neutrophils, as well as in B-cell malignancies. In addition to being a critical effector for the B-cell receptor, Btk engages B-cell Toll-like receptors (e.g., TLR2 and TLR4) and FAS. Btk is activated by SFK (src family kinases) and Syk, and transmits signals to PI3K and PLC-gamma, resulting in a calcium flux and the activation of NF-kB and NFATc transcriptional factors [4]. The role of Btk in the immune response and hematopoietic malignancies has been well studied. Deficiency of Btk in humans leads to X-linked agammaglobulinemia (XLA). Btk has been reported as an anti-apoptotic protein in neutrophils and macrophages. Btk-deficient neutrophils have increased production of ROS and stimulation-induced apoptosis. Knockdown of Btk in macrophages led to increased LPS and TNF-induced apoptosis [5]. Btk also has an important role in arthritis, leukemia and lymphoma. Several Btk inhibitors have been reported including LFM-A13, a reversible Btk inhibitor through rational design, and ibrutinib, an irreversible Btk inhibitor. Ibrutinib has shown encouraging effect in clinical studies for treatment of chronic lymphocytic leukemia and in collagen-induced arthritis mouse model [6]. These inhibitors also exhibited potential in targeting multiple myeloma in the bone marrow microenvironment.
As described above, most reported studies of Btk focused on the hematopoietic system; however, the role of Btk in solid tumors remains unknown. Btk has been shown to be expressed in epithelial and endothelial cells, and is involved in the development or treatment resistance of several epithelial malignancies [7]. It is overexpressed in human breast cancer specimens, and provides strong survival functions in breast cancer cells. Over-expression of Btk induces breast intraepithelial neoplasia in mice, and knockout of Btk in an endothelial lineage decreases tumor angiogenesis and growth. In breast cancer cells, Btk is activated by EGFR and erbB3, as well as IL-6 and neuropeptides, leading to aberrant activation of androgen receptor [8]. In glioblastoma, Btk was found to be critical in maintaining the self-renewal and tumorigenic potential of cancer stem cells through Stat3 activation [9]. Herein, we report that Btk is also aberrantly expressed in gastric carcinoma. Moreover, Btk inhibitor induces apoptosis in gastric carcinoma cells, and inhibits gastric cancer xenograft tumor growth in vivo. To our knowledge, this is the first report of the role of Btk in gastric cancer, and also the first report of the Btk inhibitor lbrutinib with an application as an anti-gastric cancer agent.
Materials and methods
Cell culture
Gastric cancer cells MGC-803, BGC-823, SGC7901, MKN-45, MKN-28 and normal gastric mucosa epithelial cell lines GES-1 cells were obtained from ATCC (Manassas, VA, USA) and maintained in RPMI-1640 or DMEM medium containing 10% fetal bovine serum and 1% penicillin/streptomycin/glutamine.
Btk knockdown assay
Btk expression in MKN-45 and BGC-823 cells was knocked down using siRNA. The Btk (Smartpool) and non-targeted RNA were obtained from GenScript (Nanjing, China). The siRNAs were transfected to cells according to the manufacturer’s instruction. The expression levels of Btk were examined using a western blot after 48 h. The growth rates were examined daily using an 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay for 3 days.
MTT assay
Cells were seeded in 96-well plates and cultured overnight, followed by treatment with 0.1% DMSO, as a vehicle control, and ibrutinib at the indicated concentrations for 72 h. Growth inhibition was measured using MTT assay (Boster Biological Technology, Ltd. Wuhan, China) according to the manufacturer’s protocol.
Flow cytometry
MKN-45 and BGC-823 cells were treated with 0.1% DMSO (control) and ibrutinib at the indicated concentrations for 24 h. Cell-cycle arrest was determined by the incorporation of propidium iodide (Sigma-Aldrich) into permeabilized cells. Cells undergoing apoptosis were identified using an Annexin V-FITC kit (Abcam), following the manufacturer’s instructions. The cells were analyzed using a Coulter Epics XL flow cytometer (Beckman Coulter, Miami, FL, USA).
Colony formation assay
Cells then were plated onto a 6-well tissue culture plate in complete medium and incubated at 37°C. Cells were treated with DMSO (< 0.1%) or ibrutinib. Cells were allowed to grow in complete medium for 14 days. Then cells were fixed and stained with 1% crystal violet and counted under the microscope [10].
Western blotting
Western blotting was performed as described previously. Cells were grown in 100 mm dishes to about 50% confluence and treated with vehicle (control) and CTN06 (0.5 and 2 μM) for 12 h. Proteins were detected by the following antibodies: GAPDH (Cell Signaling Technology, # 5174S), Btk (Cell Signaling Technology, # 8547S), phospho-Btk (Tyr223) (Cell Signaling Technology, # 5082S), PLCγ2 (# 3872S), phospho-PLCγ2 (Tyr759) (Cell Signaling Technology, # 3874S), Stat3 (Cell Signaling Technology, # 12640S), Phospho-Stat3 (Tyr705 (Cell Signaling Technology, # 9145S).
Reverse transcription and quantitative real-time PCR
Reverse transcription and quantitative real-time PCR (qRT-PCR) were done as described previously. Primer sequences used to amplify Btk fragments are listed as follows: forward 5’-GGTGGAGAGCACGAGATAAA-3’; reverse 5’-CCGAGTCATGTGTTTGGAATAC-3’ (IDTDNA Inc., Coralville, IA, USA). GAPDH was used as the housekeeping gene.
Ibrutinib as a chemo-sensitizer
Growth inhibition of ibrutinib in combination with 2 ng/ml DTX to MKN-45 and BGC-823 cells was evaluated. Cells were seeded at 5000 cells/well in a 96-well plate overnight and treated with the corresponding co-treatments. The cell viability was measured using an MTT assay after 72 h.
Inhibition of MKN-45 and BGC-823 xenograft tumor growth by ibrutinib
Animal experimental conditions were in accordance with the protocol approved by the Institutional Animal Care and Use Committee at the Shaoxing Hospital of Zhejiang University, Davis. This study used 4- to 5-week-old female athymic nu/nu mice from Shanghai Slack laboratory animal co., LTD. They were acclimated for 1 week on arrival to our animal facility before testing was initiated. The local committee for animal care approved all animal studies. A total of 5 × 106 cells was suspended in 100 μl of serum-free RPMI 1640 and subcutaneously injected into the flank. When tumor volume reached 100 cm3, mice were randomly assigned to four treatment groups: vehicle, ibrutinib (10 mg/kg) and ibrutinib (20 mg/kg) (each group containing six mice). Treatments were administered every 2 days by intraperitoneal injection. The length and width of the tumor were measured using a digital caliper, and the volume of the tumor was calculated using the formula: length × (width)2/2. In addition, before each treatment, mice were weighed to monitor signs of drug toxicity [11]. On day 25 of treatment, the mice were sacrificed and tumors were harvested for molecular analyses. Western blots were performed using tumor lysates with indicated antibody.
Statistics
A one-way ANOVA was used in combination with a Tukey test for pairwise comparison. P-values of < 0.05 were considered as significant.
Results
Btk is over-expressed in gastric carcinoma
Btk is mainly reported in hematopoietic cells and B-cell malignancies, and has previously been reported to be association with the growth, survival and apoptosis of chronic lymphocytic leukemia (CLL) [12]. However, the role of Btk in gastric carcinoma remains unknown. The expression level of Btk in gastric cancer cells MGC-803, BGC-823, SGC7901, MKN-45, MKN-28 and normal gastric mucosa epithelial cell lines GES-1 was examined using western blots. Btk was found to be high in all gastric cancers, especially in MKN-45 and BGC-823 cells (Figure 1A). The elevated mRNA levels of Btk in those cells were also observed, using real-time RT-PCR, which indicates the overexpression of Btk in gastric carcinoma cells at the transcriptional level (Figure 1B).
Figure 1.
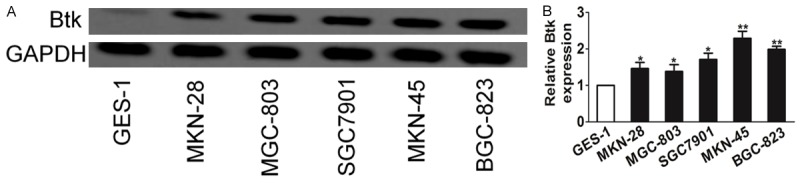
Btk is up-expressed in gastric carcinoma and cell lines. A. The expression level of Btk in gastric cancer cells was examined using western blot assay. B. Btk levels were further confirmed using RT-PCR. The data are presented as mean ± SD. For indicated comparisons, *P < 0.05, **P < 0.01.
Knock-down of Btk inhibits gastric carcinoma cell growth
To investigate the role of Btk in gastric carcinoma cell growth, the Btk level in gastric carcinoma cell lines MKN-45 and BGC-823 was knocked down using siRNA (Figure 2A). Btk knockdown in both MKN-45 cells and BGC-823 cells showed a significantly inhibitory effect on cell proliferation as compared with control cell (Figure 2B). In contrast, GES-1, the normal gastric mucosa cells line, is resistant to Btk knockdown (Figure 2C), indicating an Btk ‘addiction’ of the gastric cancer cells. The effect of Btk to gastric cancer cell growth was further examined in both cells using ibrutinib, a known Btk inhibitor. Btk inhibitor ibrutinib inhibited gastric cancer cell growth at a similar level, further confirming an important role of Btk in gastric cancer cell growth (Figure 2D).
Figure 2.
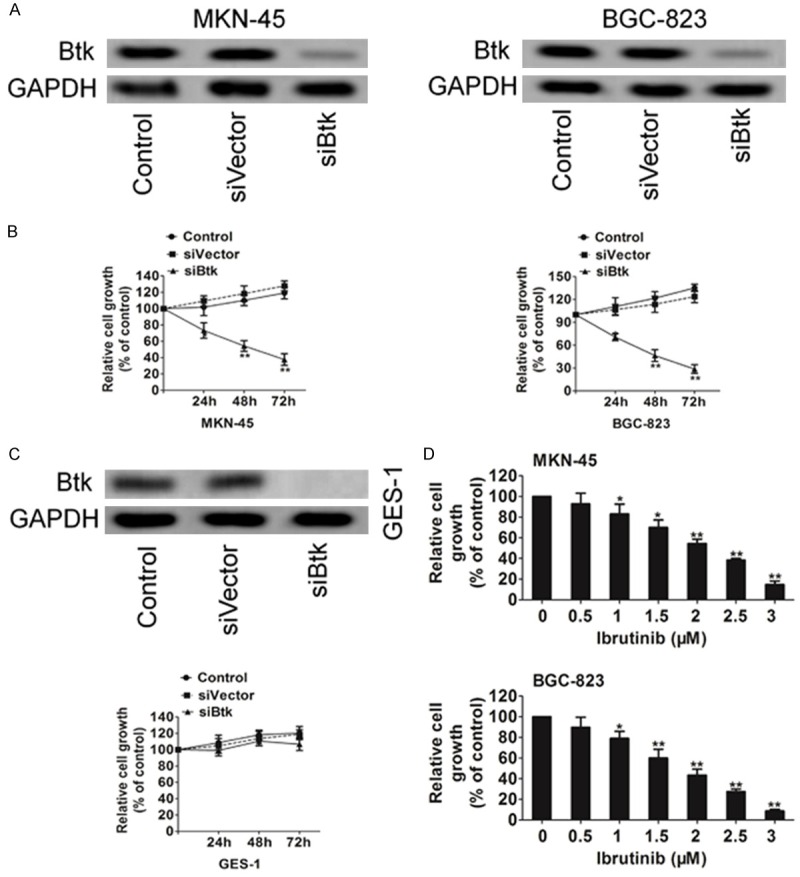
Btk contributes to proliferation of MKN-45 and BGC-823 cells. A. Cells were transiently transfected with siRNAs targeting the transcripts encoding Btk or with its siControl sequences. The expression of Btk in cells was assayed using western blot. B. The growth of the knock-down cells was measured using MTT assay. C. The growth of the knock-down GES-1 cells was measured using MTT assay. D. The effect of Btk on gastric cancer cell growth was further confirmed using ibrutinib in MKN-45 and BGC-823 cells. Data are the mean ± SD from 3 independent experiments. *P < 0.05 and **P < 0.01.
Btk silencing decreases gastric carcinoma cell colony growth in soft agar
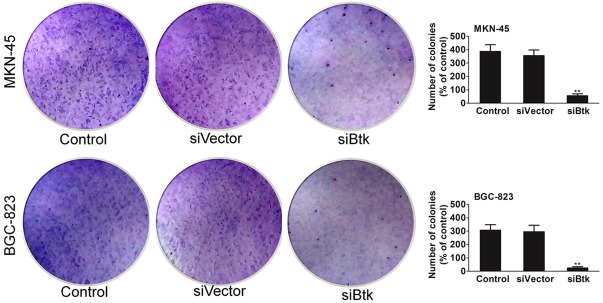
Similarly, Btk knock-down strongly inhibited the ability of MKN-45 cells and BGC-823 cells to form colonies in soft-agar (Figure 3). The impact of Btk silencing on cell proliferation and colony growth in vitro suggests that it is likely that Btk has an important function in the growth of gastric carcinoma cell derived tumors. The above results suggest that silencing of Btk induces apoptosis; therefore, we wanted to investigate the molecular.
Figure 3.
Anchorage-independent colonies growth of MKN-45 and BGC-823 cells stably silenced for Btk was shown. Cells were plated in soft agar. Two weeks later, colonies were stained with crystal violet and scored. Data are from three experiments performed in duplicate. The colonies formed in soft agar from the two respective silencing designs were photographed after two weeks. Data are the mean ± SD from 3 independent experiments. Statistical differences obtained at **P < 0.01.
lbrutinib inhibits the phosphorylation of Btk and the downstream signal PLCγ2, Stat3, AKT
To determine whether the growth inhibition induced by ibrutinib on gastric carcinoma cells was due to apoptosis, flow-cytometric analysis was carried out. Following treatment with ibrutinib for 24 h, a dose-dependent accumulation of a ‘sub-G1’ fraction was observed using propidium iodide (PI) staining. Data based on Annexin-V reactivity also indicated a dose-dependent increase in apoptosis of MKN-45 and BGC-823 cells following treatment with ibrutinib (Figure 4A). The ‘sub-G1’ fraction only measures dead cells with DNA content loss, which may explain why it was less than the percentage of apoptotic cells measured by Annexin-V. The inhibitory activity of ibrutinib against phosphorylation of Btk in intact cells was examined by western blot. Btk phosphorylation in MKN-45 and BGC-823 cells was significantly inhibited. The p-PLCγ2 inhibition is likely to result from Btk inhibition. A selective target for Btk is Stat3, whose phosphorylation was also inhibited by ibrutinib, so was Akt kinase, another important downstream effector of Btk (Figure 4B).
Figure 4.
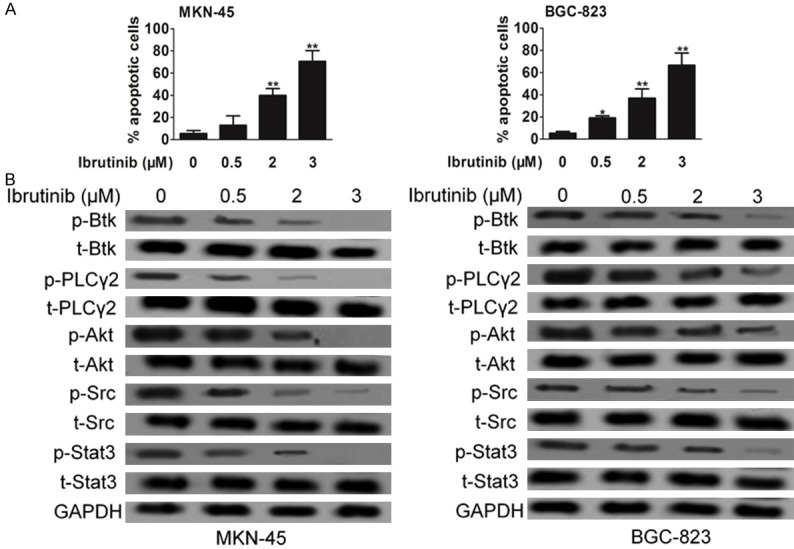
Impact of ibrutinib on Btk signaling pathway. A. Induction of apoptosis of cells following treatment with ibrutinib. Apoptosis was analyzed using PI staining as well as Annexin V-FITC apoptosis detection kit. B. Representative western blots of the expression and phosphorylation of Btk in cells treated with ibrutinib. The normalized Btk and p-Btk bands intensities were expressed as fold change in comparison to control. Data are the mean ± SD from 3 independent experiments. Statistical differences obtained at *P < 0.05, **P < 0.01.
Ibrutinib as a chemo-sensitizer in gastric carcinoma cell
Our initial studies indicated that ibrutinib has good cytotoxicity toward gastric cancer cells. To examine whether Btk inhibitor works effectively as a chemo-sensitizer, MKN-45 and BGC-823 cells were co-treated with ibrutinib (2 μM) and docetaxel (DTX) (2 ng/ml). Growth inhibition was determined using an MTT assay after 72 h. Interestingly, a synergistic effect of ibrutinib was observed when MKN-45 and BGC-823 cancer cells were co-treated with DTX (Figure 5A). Annexin-V and PI staining further confirmed that combination of ibrutinib and DTX induced apoptosis (Figure 5B).
Figure 5.
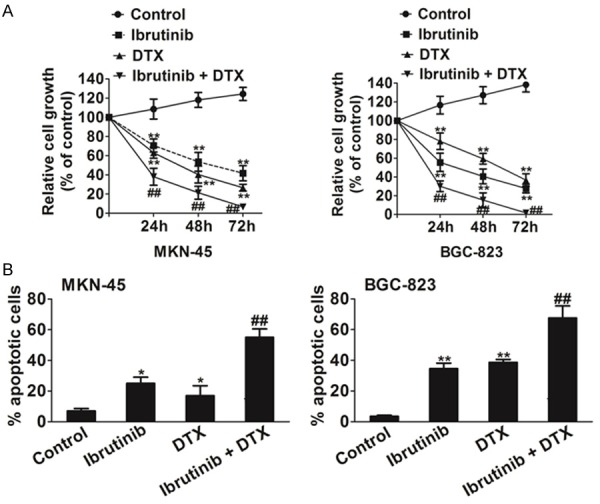
Ibrutinib as a chemo-sensitizer. A. Cells were seeded at 5000 cells/well in 96-well plates overnight and co-treated with ibrutinib (2 μM) and DTX (2 ng/ml). The cell viability was measured using MTT assay after 24 h, 48 h and 72 h. B. The apoptotic effect of ibrutinib) in combination with DTX to MKN-45 and BGC-82 cells was further measured using Annexin-V and PI staining and flow cytometry. Data are the mean ± SD from 3 independent experiments. Statistical differences obtained at *P < 0.05, **P < 0.01. ##P <0.05, combination compared to DTX.
Ibrutinib inhibits gastric carcinoma cells xenograft tumor growth in vivo
Given the in vitro activity of ibrutinib against gastric carcinoma cells, it is important to validate these results in vivo. Cells were injected subcutaneously to nude mice. The mice were treated with vehicle (control) or ibrutinib at 10 mg/kg daily or 20 mg/kg daily via IP injection. As shown in Figure 6A and 6B, ibrutinib decreased MKN-45 and BGC-82 xenograft tumor growth without significant toxicity. Furthermore, western blot assay indicated that ibrutinib inhibited Btk, Akt and Stat3 (Figure 6C).
Figure 6.
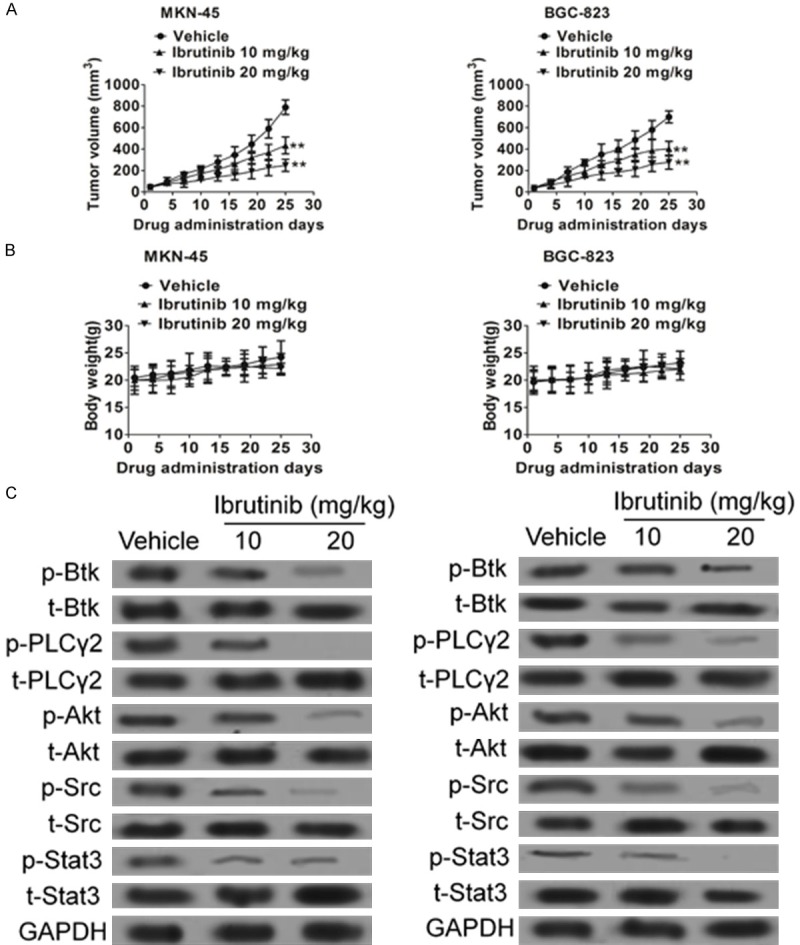
Inhibition of MKN-45 and BGC-82 xenograft tumor growth by ibrutinib. MKN-45 or BGC-82 cells were injected subcutaneously to nude mice. The tumors were grown to the indicated size and the mice were randomly divided into three groups (6 mice/group). The control group was treated with vehicle. The treatment group was treated with ibrutinib via intraperitoneal injection. A and B. The tumor size and body weight were measured once every three days. Error bars represent mean ± SD (n = 6). **P < 0.01. C. The tumor samples were further analyzed for Btk, Akt, PLCγ2 and Stat3 using western blot.
Discussion
BTK is a kinase that functions downstream of multiple receptors in various hematologic cells. BTK-dependent pathways are likely to be involved in maintaining the malignant phenotype in B-cell lymphomas and leukemia [13]. Survival of various B-cell malignancies requires BTK-dependent signals from the B-cell antigen receptor and Btk’s role in leukemia is well established. The Btk can mediate downstream signaling pathways of G-protein coupled receptors, antigen receptors, and integrins to regulate cell growth, differentiation, and apoptosis. B lymphocytes and myeloid cells with low Btk activity tend to undergo apoptosis and exhibit decreased proliferation, suggesting the importance of Btk in cell survival and growth pathways [14]. Moreover, Btk inhibits Fas-ligand-mediated apoptosis, but induces Akt and NF-κB activation. In addition, another major pathway downstream of Btk that increases cancer progression is mediated by activation of Stat3. Furthermore, Btk induction then triggers activation of phospholipase C and Ca+2 mobilization, leading in turn to downstream events (e.g., proliferation, differentiation) mediated through multiple transcription factors (e.g., nuclear factor NF-κB, NF-AT) and survival signaling cascades e.g., Ras/Raf/MEK/ERK and PI3K/Akt [15]. All these findings point to Btk as an appropriate therapeutic target. Powerful combinatorial chemistry approaches and high-throughput screening assays have led to successful identification of Btk kinase inhibitors. Several Btk inhibitors have been reported including LFM-A13, a reversible Btk inhibitor through rational design, and PCI32765 (ibrutinib), an irreversible Btk inhibitor. Ibrutinib has shown encouraging effect in clinical studies for treatment of chronic lymphocytic leukemia and in collagen-induced arthritis mouse model [16]. These inhibitors also exhibited potential in targeting multiple myeloma in the bone marrow microenvironment.
Recently, Btk has recently been implicated in the self-renewal and tumorigenic potential of glioblastoma stem cells. Btk, highly expressed in endothelial cells, has been shown to be a critical tyrosine kinase for angiogenesis and tumor growth. Ovarian cancer stem-like cell (CSC) which highly resistant to cisplatin is attributable to the over-expression of Btk signaling. Btk silencing effectively reduced the expression of the Janus kinase 2 (JAK2)/STAT3 pathway, which in turn suppresses the survival of ovarian cancer cells through Sox-2 and BCL-XL genes and, finally, restored responsiveness to cisplatin [17]. Btk is over-expressed in prostate cancer tissues and prostate cancer cells. The level of Btk in prostate cancer tissues correlates with cancer grades. Knockdown of Btk expression selectively inhibits the growth of prostate cancer cells, but not that of the normal prostate epithelial cells, which express very little Btk [18]. Although Btk has been proven to involving in ovarian cancer, prostate cancer, and other solid cancers, Btk signaling in gastric carcinoma remains unproven. Here, we report that Btk is aberrantly expressed in gastric carcinoma tissues and cells. Knockdown of Btk kinase attenuates the growth of gastric carcinoma cells MKN-45 and BGC-823. Ibrutinib belongs to a novel class of irreversible Btk inhibitor and clinical study reported that ibrutinib is associated with little toxicity [19]. Treatment of MKN-45 and BGC-823 cells with ibrutinib results in proliferation inhibition and decreasing in phosphorylation of Btk, as well as the downstream signals PLCγ2, Stat3 and Akt. Significantly, ibrutinib has much less effect on the growth of the normal gastric mucosa cells line GES-1, consistent with the Btk knockdown experimental results. Given the anti-apoptotic role of Btk in B-cell malignancies, it is likely that inhibition of Btk also contributes to the induction of apoptosis in gastric cancer cells. Indeed, we found ibrutinib induces a high level of apoptosis. While MKN-45 has much higher expression level of Btk expresses, yet MKN-45 cells appears to be more sensitive to ibrutinib than BGC-823, suggesting that gastric carcinoma cells have adapted to the Btk pathway for growth and survival. This provides a strong rationale for targeting Btk pathway in gastric carcinoma cells.
We further show that induction of growth inhibition and apoptosis in gastric carcinoma cells by ibrutinib was due to the down-regulation of Btk and target proteins, which are pro-proliferation proteins overexpressed in gastric cancer cells and associated with resistance to apoptosis. Overexpression of Btk correlates with proliferation and tumor growth rate, which lead to poor patient prognosis in a variety of malignancies [20]. We provide first evidence that Btk inhibitor ibrutinib effectively abrogated gastric carcinoma cells proliferation in vitro. In the present study, we found that ibrutinib blocked the activity of Btk (Tyr223) and down-regulated phosphorylation of the downstream signals PLCγ2, Stat3 and Akt. Ibrutinib is also a chemo-sensitizer and showed synergistic effect with DTX, indicating its potential role in combination therapy for gastric carcinoma. To evaluate the antitumor activity of ibrutinib in vivo, nude mice transplanted with MKN-45 cells were treated with ibrutinib. We found that ibrutinib significantly suppressed tumor volume and tumor weight without any side effects on mice, and remarkably reduced expression of phosphorylation-Btk. In addition to the effective anti-proliferation of ibrutinib in vitro, we presume that ibrutinib suppresses gastric carcinoma growth in vivo through not only directly tumor cell proliferation inhibition but also tumor apoptosis acceleration.
In summary, we found that Btk is aberrantly expressed in gastric carcinoma, which present suitable target for therapy. We have identified Btk inhibitor, ibrutinib, with good selectivity toward gastric carcinoma cells. Further evaluation of its pharmacokinetic and pharmacodynamic properties in gastric cancer is underway. Btk inhibition holds exceptional promise as a novel treatment strategy for gastric cancer.
Disclosure of conflict of interest
None.
References
- 1.Hase K, Naomoto Y, Ninomiya M, Watanabe M, Omoto T, Wang H. Staging of gastric cancer. Asian Pac J Surg Oncol. 2016;2:75–86. [Google Scholar]
- 2.Wu H, Wang A, Zhang W, Wang B, Chen C, Wang W, Hu C, Ye Z, Zhao Z, Wang L, Li X, Yu K, Liu J, Wu J, Yan XE, Zhao P, Wang J, Wang C, Weisberg EL, Gray NS, Yun CH, Liu J, Chen L, Liu Q. Ibrutinib selectively and irreversibly targets EGFR (L858R, Del19) mutant but is moderately resistant to EGFR (T790M) mutant NSCLC Cells. Oncotarget. 2015;6:31313–22. doi: 10.18632/oncotarget.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herman SE, Mustafa RZ, Jones J, Wong DH, Farooqui M, Wiestner A. Treatment with Ibrutinib Inhibits BTK- and VLA-4-Dependent Adhesion of Chronic Lymphocytic Leukemia Cells In Vivo. Clin Cancer Res. 2015;21:4642–51. doi: 10.1158/1078-0432.CCR-15-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zucha MA, Wu AT, Lee WH, Wang LS, Lin WW, Yuan CC, Yeh CT. Bruton’s tyrosine kinase (Btk) inhibitor ibrutinib suppresses stem-like traits in ovarian cancer. Oncotarget. 2015;6:13255–68. doi: 10.18632/oncotarget.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding N, Li X, Shi Y, Ping L, Wu L, Fu K, Feng L, Zheng X, Song Y, Pan Z, Zhu J. Irreversible dual inhibitory mode: the novel Btk inhibitor PLS-123 demonstrates promising anti-tumor activity in human B-cell lymphoma. Oncotarget. 2015;6:15122–36. doi: 10.18632/oncotarget.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinha S, Boysen J, Nelson M, Secreto C, Warner SL, Bearss DJ, Lesnick C, Shanafelt TD, Kay NE, Ghosh AK. Targeted Axl Inhibition Primes Chronic Lymphocytic Leukemia B Cells to Apoptosis and Shows Synergistic/Additive Effects in Combination with BTK Inhibitors. Clin Cancer Res. 2015;21:2115–26. doi: 10.1158/1078-0432.CCR-14-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin XL, Guo Y, Ye M, Shibasaki H, Davidson LE. Outcomes of gastric cancer Resection in elderly. Asian Pac J Surg Oncol. 2016;2:143–152. [Google Scholar]
- 8.Wang NN, Li ZH, Zhao H, Tao YF, Xu LX, Lu J, Cao L, Du XJ, Sun LC, Zhao WL, Xiao PF, Fang F, Su GH, Li YH, Li G, Li YP, Xu YY, Zhou HT, Wu Y, Jin MF, Liu L, Ni J, Wang J, Hu SY, Zhu XM, Feng X, Pan J. Molecular targeting of the oncoprotein PLK1 in pediatric acute myeloid leukemia: RO3280, a novel PLK1 inhibitor, induces apoptosis in leukemia cells. Int J Mol Sci. 2015;16:1266–92. doi: 10.3390/ijms16011266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mei XL, Yang Y, Zhang YJ, Li Y, Zhao JM, Qiu JG, Zhang WJ, Jiang QW, Xue YQ, Zheng DW, Chen Y, Qin WM, Wei MN, Shi Z. Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo. Am J Cancer Res. 2015;5:3311–3324. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang ZD, Wei SQ, Wang QY. Targeting oncogenic KRAS in non-small cell lung cancer cells by phenformin inhibits growth and angiogenesis. Am J Cancer Res. 2015;5:3339–3349. [PMC free article] [PubMed] [Google Scholar]
- 11.Li PP, Yao QM, Zhou H, Feng LL, Ge XL, Lv X, Chen N, Lu K, Wang X. Metadherin contribute to BCR signaling in chronic lymphocytic leukemia. Int J Clin Exp Pathol. 2014;7:1588–94. [PMC free article] [PubMed] [Google Scholar]
- 12.Liu K, Chen XZ, Nakamura I, Ohki S, Eslick GD. Laparoscopic surgery for gastric cancer: survival outcome and prognostic factor. Asian Pac J Surg Oncol. 2016;2:135–142. [Google Scholar]
- 13.Pillinger G, Abdul-Aziz A, Zaitseva L, Lawes M, MacEwan DJ, Bowles KM, Rushworth SA. Targeting BTK for the treatment of FLT3-ITD mutated acute myeloid leukemia. Sci Rep. 2015;5:12949. doi: 10.1038/srep12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhuo W, Zhang L, Zhu Y, Zhu B, Chen Z. Fisetin, a dietary bioflavonoid, reverses acquired Cisplatin-resistance of lung adenocarcinoma cells through MAPK/Survivin/Caspase pathway. Am J Transl Res. 2015;7:2045–2052. [PMC free article] [PubMed] [Google Scholar]
- 15.Hoa NT, Ge L, Tajhya RB, Beeton C, Cornforth AN, Abolhoda A, Lambrecht N, DaCosta-Iyer M, Ouyang Y, Mai AP, Hong E, Shon J, Hickey MJ, Erickson KL, Kruse CA, Jadus MR. Small cell lung cancer cells express the late stage gBK tumor antigen: a possible immunotarget for the terminal disease. Am J Transl Res. 2014;6:188–205. [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada N, Maeda K, Sawada T, Jeong ID, Noh SH, Zhao Y. Surgical management of gastric cancer. Asian Pac J Surg Oncol. 2016;2:121–134. [Google Scholar]
- 17.Rushworth SA, Bowles KM, Barrera LN, Murray MY, Zaitseva L, MacEwan DJ. BTK inhibitor ibrutinib is cytotoxic to myeloma and potently enhances bortezomib and lenalidomide activities through NF-κB. Cell Signal. 2013;25:106–12. doi: 10.1016/j.cellsig.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Winer ES, Ingham RR, Castillo JJ. PCI-32765: a novel Bruton’s tyrosine kinase inhibitor for the treatment of lymphoid malignancies. Expert Opin Investig Drugs. 2012;21:355–61. doi: 10.1517/13543784.2012.656199. [DOI] [PubMed] [Google Scholar]
- 19.Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, Keating MJ, O’Brien S, Chiorazzi N, Burger JA. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–9. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiliotis J, Efstathiou E, Matsubara A, Osman MM, Choo SP. Molecular biology of gastric cancer. Asian Pac J Surg Oncol. 2016;2:86–100. [Google Scholar]